Abstract
Current pharmacological treatments for chronic obstructive pulmonary disease (COPD) are mostly limited to inhaled bronchodilators and corticosteroids. Azithromycin can contribute to exacerbation prevention. Roflumilast, a phosphodiesterase (PDE) 4 inhibitor administered orally, also prevents exacerbations in selected patients with chronic bronchitis, recurrent exacerbations, severe airflow limitation and concomitant therapy with long-acting inhaled bronchodilators. This outcome likely results from anti-inflammatory effects since PDE4 is expressed by all inflammatory cell types involved in COPD. The use of this agent is, however, limited by side-effects, particularly nausea and diarrhea. To address remaining unmet needs and enrich therapeutic options for patients with COPD, inhaled dual PDE3/4 inhibitors have been developed, with the aim of enhancing bronchodilation through PDE3 inhibition and modulating inflammation and mucus production though PDE4 inhibition, thus producing a potentially synergistic effect on airway calibre. Experimental preclinical data confirmed these effects in vitro and in animal models. At present, RPL554/ensifentrine is the only agent of this family in clinical development. It decreases sputum markers of both neutrophilic and eosinophilic inflammation in patients with COPD. Clinical Phase II trials confirmed its bronchodilator effect and demonstrated clinically meaningful symptom relief and quality of life improvements in these patients. The safety profile appears satisfactory, with less effects on heart rate and blood pressure than salbutamol and no other side effect. Altogether, these data suggest that ensifentrine could have a role in COPD management, especially in addition to inhaled long-acting bronchodilators with or without corticosteroids since experimental studies suggest potentiation of ensifentrine effects by these agents. However, results from ongoing and future Phase III studies are needed to confirm both beneficial effects and favourable safety profile on a larger scale and assess other outcomes including exacerbations, lung function decline, comorbidities and mortality.
Keywords: COPD, phosphodiesterase inhibitors, bronchodilation, inflammation, mucus, epithelium
Introduction
As a consequence of improved prevention and healthcare in many countries, age-standardized chronic obstructive pulmonary disease (COPD) prevalence, mortality rates and disability-adjusted life-years have been decreasing during the last 3 decades.1 However, the crude prevalence, morbidity and mortality related to this disease have been increasing and remain extremely burdensome worldwide. In 2017, COPD was the most frequent chronic respiratory disease (55% of all cases) with a 3.9% crude prevalence, which had increased by 5.9% since 1990. It accounted for about 80% of all deaths related to chronic respiratory diseases, which represented the 3rd leading cause of death.1
The definition of COPD is based on the coexistence of chronic respiratory symptoms and fixed (ie, persistent, or in other words not fully reversible) airflow limitation,2 which can relate to a loss of airways and/or obstruction of remaining airways, ie decreased airway calibre.3,4 Airways obstruction results from various mechanisms involved in emphysema and airway wall thickening (see below).5,6 The respective contribution of these mechanisms is markedly variable between patients and remains difficult to determine on an individual basis using routinely available tools. Inflammation and innate and adaptive immunity are thought to play an important role in the development and aggravation of COPD.7,8 As the disease in general, lung inflammation is heterogenous, involving mostly two categories of mechanisms: T2 eosinophil-predominant, and inflammasome and T1/T17 neutrophil predominant inflammation. Eosinophilic inflammation is found in up to 40% of patients, and is more corticosteroid-sensitive than its neutrophilic counterpart.9 Neutrophil-associated inflammation is the result of both innate and adaptive immune mechanisms and is associated with bacterial dysbiosis, autoimmunity and the development of lymphoid follicles.9
COPD treatment aims mostly at relieving symptoms (chronic mucus hypersecretion, dyspnea, exercise intolerance), preventing exacerbations, identifying and managing comorbidities, limiting disease progression and increasing survival, with minimal constraints and side-effects from treatments.2 To reach these goals, nonpharmacological approaches represent the first step of COPD care and include smoking cessation aids, physical activity and rehabilitation, and vaccination against influenza and Streptococcus pneumoniae. At present, the therapeutic landscape available for COPD pharmacotherapy remains mostly limited to inhaled bronchodilators (beta2 agonists and anticholinergics) and corticosteroids (ICS). A large number of (predominantly long-acting) drugs belonging to these families have been released, differing more in terms of inhalation device than of pharmacological properties and clinical effects. Agents available through the oral route include phosphodiesterase (PDE) 4 inhibitors (roflumilast), where available, and azithromycin, which can both help preventing exacerbations in some patients. Theophylline is not indicated any more except in countries with highly limited access to other medications.
Although some inhaled maintenance treatments may influence FEV1 decline and mortality,10–13 it has proved difficult to firmly demonstrate a tangible effect on the natural history of the disease. In addition, the magnitude of effects of COPD medications on clinical and lung function outcomes is relatively limited. This relates to the lack of treatment able to reverse airway wall remodelling and/or stimulate lung regeneration, and to the limited sensitivity of airways inflammation in COPD to available therapy including corticosteroids.14–16 Given the potential of these agents to induce local and systemic side-effects even when administered through the inhaled route,17 identifying the best responders appears crucial. The same is true for other available agents with anti-inflammatory properties involved in exacerbation prevention, ie, azithromycin and roflumilast, a PDE4 inhibitor. Accurate identification of the optimal target populations could come from increasingly performant approaches to phenotyping, endotyping and identification of treatable traits.18,19 However, at present biomarker-based individualized therapy in COPD is limited to the use of blood eosinophil count to guide the decision to prescribe or withdraw ICS,2,20,21 and of symptoms (chronic bronchitis) and lung function (FEV1) to define indications of roflumilast.
Given the above-mentioned limitations of current COPD medications, many pathways are explored to identify new strategies to fight airways inflammation in COPD and/or to restore corticosteroid sensitivity. These include inhaled PDE4 and PDE3/4 inhibitors, as well as dissociated steroids, PI3-kinase inhibitors, histone deacetylase-2 (HDAC-2) activators, p38 mitogen-activated protein (MAP)-kinase inhibitors, antioxidants22 … For now, drugs specifically targeting components of neutrophil-associated mechanisms, ie, the inflammasome, tumor necrosis factor (TNF) and interleukin (IL)-17, have proven ineffective,9 although this could be how azithromycin exerts its effects in COPD.23,24
In this clinically focused short narrative review, we will summarize the role of phosphodiesterases in COPD pathophysiology, describe COPD-oriented preclinical data on phosphodiesterase inhibitors, and present clinical trials on dual PDE3/4 inhibitors before briefly discussing what the role of these agents in COPD management strategies could be.
Brief Summary of the Involvement of Phosphodiesterases 3 and 4 in the Pathophysiology of COPD
As mentioned above, persistent airflow limitation is the cornerstone of COPD definition.25–27 Small airways alterations associated with disease progression27 include airway narrowing due to bronchial wall thickening (related to peribronchiolar fibrosis, smooth muscle modifications, squamous metaplasia, goblet cell hyperplasia), bronchial lumen obstruction (mucus hypersecretion, altered ciliogenesis),5,6 and the decrease in elastic lung recoil resulting from the loss of alveolar attachments characterizing emphysema. Inflammatory cells involved in COPD include macrophages and neutrophils as well as T and B-lymphocytes that can be organized in peribronchial tertiary lymphoid structures.6
The phosphodiesterases superfamily comprises 11 families of enzymes, among which PDE3 and PDE4 are of particular interest. In the lungs, they are highly expressed in airway smooth muscle.28,29 PDE4 is also present in airway epithelial cells and in most inflammatory cell types involved in COPD.30 PDE3 and PDE4 both have the ability to hydrolyze cAMP.30,31 PDE3 activity is dependent on cGMP levels.32 Enhancing cyclic AMP activity in bronchial cells appears promising to treat COPD since cAMP mediates bronchial smooth muscle relaxation, inhibition of inflammatory mediators production, downregulation of neutrophils and macrophages, cystic fibrosis transmembrane regulator (CFTR) up-regulation leading to improved airway clearance, and ultimately inhibition of bronchial wall remodelling.33,34 Furthermore, cigarette smoke exposure in mice airways and in human bronchial cells decreases cAMP cytosol levels through specific PDE3 and PDE4 up-regulation,35 which provides an additional argument supporting a potential therapeutic role for PDE3 and 4 inhibitors in COPD.
Preclinical Data with PDE3, PDE4 and Dual PDE3/4 Inhibitors and Their Relationship with Other COPD Treatments
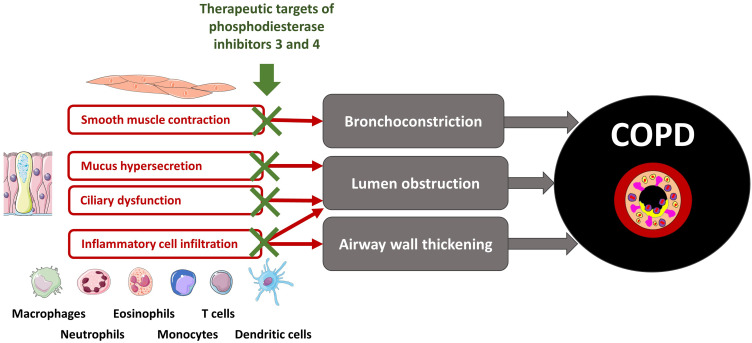
Phosphodiesterase inhibitors have bronchodilator and anti-inflammatory effects36 through a variety of cellular effects summarized in Figure 1. Many experimental studies found that PDE3 inhibition contributes more than PDE4 inhibition to bronchodilation.34,37 Conversely, airway inflammation and epithelial cell functions (mucus secretion, ciliary activity) are more responsive to PDE4 inhibition.35,38 Altogether, targeting simultaneously PDE 3 and 4 is a tempting strategy, which led to the development of dual PDE3/4 inhibitors. Among these, RPL554/Ensifentrine is the only agent currently undergoing clinical development, administered through the inhaled route.39
Figure 1.
Simplified view of the main cellular targets of dual PDE3/4 inhibition relevant to COPD.
Bronchodilation
Venkatasamy et al have studied tracheal guinea pig smooth muscle relaxation under PDE3, PDE4 or dual PDE3/4 inhibition, alone or with muscarinic receptors antagonists and beta-2 receptor agonists and demonstrated that dual PDE3/4 inhibition alone produced significant smooth muscle relaxation with a cumulative effect when added to another bronchodilator.40 The same synergy with other bronchodilators was found in human bronchial explants41 regardless of airway size and therefore including small airways,42 suggesting a potential to modulate air-trapping.
Inflammation and Airway Remodelling
PDE4 is present in many inflammatory cell types present in the lung and involved in COPD: neutrophils, eosinophils, lung macrophages, dendritic cells, lymphocytes, airway epithelial and endothelial cells.30 Lung macrophages and blood monocytes isolated from smokers overexpress PDE4.43 PDE4 inhibition inhibits neutrophilic inflammation, as shown in mice acutely exposed to cigarette smoke.44,45 Similarly, an inhibition of lipopolysaccharide (LPS)-induced neutrophil recruitment has been observed following inhalation of PDE4 and/or dual PD3/4 inhibitors in rats, ferrets and pigs.46,47 Other studies have shown that PDE4 inhibition decreased neutrophil activation and the release of reactive oxygen species, neutrophil elastase, MMP-9 and myeloperoxidase in vitro.48 In a mouse model of LPS-induced lung injury, matrix metalloproteinase (MMP)-9 and transforming growth factor (TGF)-beta release were reduced.49 Altogether, these data suggest possible effects of PDE4 inhibitors on airway remodelling. PDE4 inhibitors have also been shown to promote neutrophil apoptosis and thereby inflammation resolution in a murine model of LPS-induced neutrophil recruitment to the pleural cavity.50 There again, inflammatory response to LPS is modulated by PDE4 inhibition with a marked decrease in TNF-alpha release by human blood monocytes,51 blood leucocytes of PDE4B knock-out mice52 and in vitro-generated human dendritic cells.53 Tannheimer et al assessed the effect of roflumilast (a PDE4 inhibitor) added to a beta-2 agonist or a glucocorticosteroid on LPS-stimulated human peripheral blood monocytes; in both situations, roflumilast increased anti-inflammatory drug activity.54
Airway epithelial cells are involved in primary responses to infection and express PDE4. Mata et al demonstrated that roflumilast decreases respiratory syncytial virus infection of human airway epithelial cells all and prevents epithelial alterations (loss of ciliated cells, MUC5AC overexpression) and production of inflammatory cytokine (IL-13, IL-6, IL-8, TNFalpha and intercellular adhesion molecule (ICAM)-1) and oxidative species.55 More recently, Turner et al studied the effect to the dual PDE3/4 inhibitor ensifentrine in human bronchial epithelial cells in baseline conditions and after a pro-inflammatory challenge, with or without adjunction of a beta-2 agonist or a glucocorticosteroid. They demonstrated that dual inhibition of PDE3 and 4 by ensifentrine diminished the release of proinflammatory mediators, and that this effect was enhanced when ensifentrine was associated to a beta-2 agonist or a glucocorticosteroid.56
Mucus Production, Ciliary Function and Airway Clearance
The two main mucins secreted in the airways are MUC5AC and MUC5B.57 Although mucin production is a protective mechanism against pathogens and other inhaled hazards, mucus hypersecretion is believed to contribute to the pathogenesis of COPD.57 In the study by Mata et al described above, the authors demonstrated an inhibition of RSV infection-induced MUC5AC expression by roflumilast in airway epithelial cells.55
Another potential therapeutic target in COPD is the cystic fibrosis transmembrane conductance regulator (CFTR) which is a transmembrane, cAMP-activated chloride channel expressed at the apical surface of airway epithelial cells and involved in mucus hydration. In addition to inherited CFTR gene mutations, several conditions including cigarette smoke exposure can lead to acquired CFTR dysfunction. Therefore, an involvement of CFTR dysfunction in COPD pathogenesis has been hypothesized.58 PDE3 and PDE4 inhibitors activate chloride secretion in epithelial cell lines through CFTR stimulation.59,60 A recent study confirmed cAMP-mediated CFTR up-regulation by dual PDE3/4 inhibitors in human bronchial epithelial cells expressing wild-type CFTR or F508del CFTR.56 Previously, Lambert et al had described restoration of CFTR function with a PDE4 inhibitor alone in human bronchial epithelial cells exposed to cigarette smoke; this effect was greater when the PDE4 inhibitor was associated to a CFTR potentiator (Ivacaftor).61,62
Finally, PDE 4 inhibitors can enhance ciliary function, possibly through effects on CFTR activity.39 In vitro studies demonstrated that PDE4 inhibition increases ciliary beat frequency in bronchial epithelial cells exposed to cigarette smoke.35,38 Co-administration of PDE4-inhibitors could be a valuable complement to some glucocorticosteroids and anticholinergics, which could theoretically have a negative impact on ciliary mobility.63
Clinical Data on Dual PDE3/4 Inhibitors in Patients with COPD
At present, the only phosphodiesterase inhibitor available for COPD treatment is roflumilast, an orally administered PDE4 inhibitor. Its clinical effects have been extensively reviewed elsewhere.64,65 The main benefit is exacerbation prevention. This agent also improves lung function although with a relatively modest magnitude of effect, and effects on symptoms and quality of life are small. The likelihood of beneficial effects is greater in patients with symptoms of chronic bronchitis, recurrent exacerbations, severe to very severe airflow limitation and concomitant long-acting bronchodilator therapy. Some studies also suggest that PDE4 inhibition could reduce the risk of major cardiovascular events, maybe through decreased systemic inflammation, and enhance insulin sensitivity.66 The use of roflumilast is limited by gastrointestinal side-effects (especially diarrhea and nausea). Other side effects include weight loss, insomnia and depressive symptoms. To overcome tolerance issues, the development of inhaled PDE4 inhibitors has been considered.66 Dual PDE3/4 molecules that can be administered by inhalation offer an opportunity to add more bronchodilation to the anti-inflammatory potential of phosphodiesterase inhibition.
Efficacy of Inhaled PDE3/4 Inhibition
Here, we will focus only on RPL554/ensifentrine, since other dual PDE3/4 inhibitors that have been previously tested have been abandoned, presumably due to concerns regarding their efficacy and/or their safety.39
The clinical program for the development of RPL554/ensifentrine in patients with chronic respiratory diseases started more than 10 years ago67 and has been somewhat slow. Studies evaluating the efficacy of RPL554/ensifentrine in healthy volunteers and in patients with COPD or asthma are summarized in Table 1.
Table 1.
Summary of Clinical Studies Assessing the Efficacy of the Dual Phosphodiesterase 3 and 4 Inhibitor RPL554/Ensifentrine
| First Author | Patient Groups/Disease | Study Design | n | Study Duration | RPL554/Other Drugs | Outcomes | Results |
|---|---|---|---|---|---|---|---|
| Franciosi67 | Men with mild allergic asthma | Double-blind, crossover, placebo-controlled, randomised | 10 | Single dose | RPL 0.018 mg/kg Placebo |
Other outcomes Change in FEV1 PC20 MCh |
FEV1 increase at 1h of 520 mL (+14%) Increase in the PC20MCh by 1·5 doubling doses compared with placebo |
| Men with clinically stable asthma | Single-blind (patients were masked), nonrandomised, single arm, placebo (day –1) followed by RPL554 | 12 | Six days | RPL554 0.018 mg/kg Placebo |
Primary outcome maximum mean increase in FEV1 during 6h after dosing vs placebo |
FEV1 increased in asthmatics Day 1: +555mL Day 3: +505 mL Day 6: +485 mL |
|
| Men with mild to moderate COPD | Open label, randomized placebo- controlled crossover | 12 | Single dose | RPL554 0.018 mg/kg Placebo |
Other outcomes Increase from baseline in FEV1 |
Increase in FEV1 by 194 mL at 60 min | |
| Healthy men | Double-blind randomized placebo-controlled crossover | 21 | Single dose | RPL554 0.018 mg/kg Placebo |
Primary outcome LPS-induced inflammatory cell infiltration in induced sputum (% neutrophils in sputum) |
Did not reduce %neutrophils in sputum but reduced neutrophil numbers (p=0·002) and total cell numbers (p=0·002) | |
| Singh68 | COPD with short acting bronchodilator reversibility ≥150 mL increase in FEV1 | Six-way, placebo-controlled crossover study | 36 | Single dose | RPL554 6 mg Salbutamol 200µg Ipratropium 40 µg RPL554+salbutamol RPL554+ipratropium RPL554+placebo |
Primary outcome: change from baseline in peak and average FEV1 over 8 h comparing RPL554+salbutamol vs salbutamol and RPL554+ipratropium vs ipratropium Other outcomes onset of action forced vital capacity (FVC), body plethysmography measurements (lung volumes and sGaw) |
Improvement in peak FEV1 comparable between salbutamol, ipratropium and RPL Peak FEV1 higher for RPL+ipratropium vs ipratropium and for RPL+salbutamol vs salbutamol Lung volumes and sGaw showed greater RPL554 combination treatment effects versus monotherapy |
| COPD with short acting bronchodilator reversibility ≥150 mL increase in FEV1 | Three-way crossover study | 30 | 3 days | Tiotropium 18 µg +RPL554 1.5 mg or RPL554 3 mg or Placebo | Primary outcome: Change from baseline (pre-dose day 1) in peak and average FEV1 over 12 h on day 3 comparing RPL554 versus placebo Other outcomes onset of action forced vital capacity (FVC), body plethysmography measurements (lung volumes and sGaw) |
Both RPL554 doses caused greater peak FEV1 effects than placebo. Average FEV1 (0–12 h) increase was greater with RPL554 6 mg only versus placebo (+65 mL) Lung volumes and sGaw showed greater RPL554 combination treatment effects versus monotherapy |
|
| Singh70 Watz71 |
COPD ppFEV1 40–80 |
Randomized, double-blind, placebo-controlled, parallel-group study | 405 | 4 weeks | Nebulized ensifentrine 0.75 to 6mg twice daily | Primary outcome placebo-adjusted difference in peak FEV1 (assessed over 3 h) at Week 4. Other outcomes E-RS™:COPD total score and subscores SGRQ-C TDI focal score |
Statistically significant increase in peak FEV1 for all doses: +146 mL (0.75 mg) +153 mL (1.5 mg) +200mL (3mg) +139mL (6 mg) Improvement in symptoms: E-RS™: COPD total score and subscores for dyspnea and cough and sputum TDI focal score: improvement≥1 point with all doses at week 2 and 4 SGRQ-C: numerical improvement at week 4 (not statistically significant) |
| Ferguson72 | COPD ppFEV1 30–70 |
Randomized, double-blind, placebo-controlled, parallel-group, dose-ranging study | 416 | 4 weeks | Open-label tiotropium once daily (QD) (+) blinded escalating doses of nebulized ensifentrine (0.375 mg to 3 mg) or placebo twice daily (BID). | Primary outcome peak FEV1 from baseline to Week 4 Other outcomes average FEV1 (0–12h) at week four SGRQ-C |
Increase in peak FEV1: +77.5 mL (0.375mg) +129 mL (3 mg) Average FEV1 (0–12h) at week four +87.5mL (3mg) Improvements in SGRQ-C≥ 4 points at week 4 for 1.5 mg and 3 mg |
| Bjermer73 | Asthma Pre BD ppFEV1 60–90 and increase ≥15 following inhalation of salbutamol |
Randomised, placebo-controlled, double-blind crossover study | 29 | Single dose | Single nebulised doses of ensifentrine 0.4, 1.5, 6 and 24 mg, salbutamol 2.5 and 7.5 mg, and placebo | Co-primary outcomes: peak and average FEV1 over 12 h for ensifentrine vs placebo and salbutamol. |
Improvement in peak and average FEV1 vs placebo for all doses Effects on FEV1 were less than with both doses of salbutamol except at 24 mg |
Abbreviations: COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 sec; ppFEV1, percent predicted forced expiratory volume in 1 sec; sGaw, specific airway conductance; LPS, lipopolysaccharide; PC20MCh, provocative concentration of methacholine causing a 20% fall in FEV1; SGRQ-C, St George Respiratory Questionnaire COPD; TDI, transitional dyspnea index.
Initial studies in COPD have confirmed that a single dose of nebulized RPL554/ensifentrine has a bronchodilator effect.67,68 When administered on top of other nebulized short-acting bronchodilators (eg, salbutamol or ipratropium), nebulized RPL554/ensifentrine resulted in an additive bronchodilation.68 Nebulized RPL554/ensifentrine at the dose of 6 mg/day, but not at 1.5 mg/day, was further shown to increase peak, trough and average (0–12h) FEV1 when administered with tiotropium (18µg/d) for 3 days.68 Studies using body plethysmography showed that nebulized RPL554/ensifentrine reduced hyperinflation/gas trapping (assessed by measuring residual volume -RV-, functional residual capacity -FRC-, and specific airway conductance -sGaw-) to a greater extent than salbutamol, ipratropium or tiotropium alone.68 These latter studies were performed in patients with COPD and FEV1 reversibility ≥ 150 mL, which questioned the generalizability of these findings to all patients with COPD.69
In a recent randomized placebo-controlled study, Singh et al evaluated the effects of various doses of nebulized RPL554/ensifentrine (0.75 mg, 1.5, 3 and 6 mg) twice daily over 4 weeks in 405 patients with mild to moderate COPD (FEV1 40–80% predicted); there was no inclusion criteria relative to reversibility. All four doses of RPL554/ensifentrine resulted in an increased peak FEV1 assessed over 3h at week 4 (from 146 mL at 0.75 mg to 200 mL at 3 mg); there was no further improvement at the 6 mg dose, suggesting that 3 mg is already at the top of the dose-response curve. An important aspect of this study was the evaluation of symptoms related to COPD, including dyspnea and cough and sputum production.70,71 Importantly, both prespecified and post hoc analyses indicated a notable early and meaningful effect on dyspnea, evaluated using TDI focal score and E-RS™:COPD breathlessness subscale.70,71 These data suggest that RPL554/ensifentrine has symptomatic effects associated with its bronchodilator effects.
A limitation of most of these studies is the lack of concomitant treatment with long-acting bronchodilators and/or inhaled corticosteroids in included patients. For example, in the latter study,70,71 most patients reported a high burden of symptoms (with approximately 95% had mMRC≥2 at baseline), so that long-acting bronchodilators would have been indicated. As patients treated with RPL554/ensifentrine were not receiving such agents in that study, the role of this agent in patients receiving usual recommended therapy remains elusive. Furthermore, the duration of the study was relatively short (4 weeks), which did not allow evaluating other relevant endpoints (eg, exacerbations).
To overcome (at least some of) these limitations and better define the role for RPL554/ensifentrine in patients with COPD, the clinical development program is ongoing. Ferguson et al recently reported the results from a randomized, double-blind, placebo-controlled, parallel-group, dose-ranging study in patients with moderate-to-severe COPD.72 Patients were randomized to open-label tiotropium once daily (QD) plus blinded escalating doses of RPL554/ensifentrine or placebo twice daily (BID). Effects on lung function, symptoms and quality of life (QoL) were assessed over 4 weeks. All RPL554/ensifentrine doses produced a significant and dose-dependent increase in peak FEV1 from baseline to Week 4,72 indicating an additive bronchodilator effects. Clinically meaningful and statistically significant improvements in the SGRQ-C additive to tiotropium were observed at Week 4, exceeding the minimally clinically important difference of 4 units with the 1.5 and 3 mg doses,72 indicating symptom improvement. A phase III randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of ensifentrine (3mg BID) over 24 weeks (with a 48-week safety subset) in subjects with moderate to severe COPD has been started with a target enrolment of 800 patients (NCT04535986). The primary outcome is the average FEV1 area under the curve (AUC) 0–12h and secondary outcomes include symptoms evaluated by the Evaluating-Respiratory Symptoms (E-RS) score, the St George Respiratory Questionnaire COPD (SGRQ-C), and the transitional dyspnea index (TDI) focal score. Patients with COPD (FEV1 30–70% predicted) and ≥2 mMRC dyspnea grade on no maintenance/background therapy or patients on stable maintenance LAMA or LABA therapy are eligible.
RPL554/ensifentrine is also being evaluated in patients with asthma,73 although the program appears less advanced. Bjermer et al showed that a single inhalation of RPL554/ensifentrine induced a dose dependent bronchodilation in asthmatic subjects; the magnitude of this effect at high doses (6 mg and even more at 24 mg) appeared comparable to what was observed using 2.5 mg and 7.5 mg of nebulized salbutamol.73 These data suggest that to pursue the development of RPL554/ensifentrine in asthmatic patients.
Because phosphodiesterase inhibitors play pleiotropic roles in cellular function and because roflumilast, an oral PDE4 inhibitor, reduced the number of neutrophils and eosinophils, as well as soluble markers of neutrophilic and eosinophilic inflammatory activity in induced sputum samples of patients with COPD,74 Franciosi et al evaluated neutrophils in the sputum of 21 healthy volunteers treated with RPL554/ensifentrine (0.018 mg/kg once daily for 6 days).67 These authors reported a reduction in the total numbers of cells and the number of neutrophils in sputum, but not the % of neutrophils in sputum. Although these data suggest that some of the effects of RPL554/ensifentrine could be related to anti-inflammatory effects, no data are currently available in patients with COPD.
Safety/Adverse Effects
A major drawback with oral phosphodiesterase 4 inhibitors is the occurrence of adverse events. As mentioned above, use of roflumilast has been limited by gastro-intestinal adverse effects including diarrhea, nausea and weight loss. By contrast clinical trials so far suggest that inhaled RPL554/ensifentrine has a favourable safety profile. Adverse effects were generally mild and not increased compared to placebo.67,68,70,72 This promising safety profile appears likely related to the inhaled nature of RPL554/ensifentrine, which likely limits the occurrence of systemic adverse effects. Of note, in the study by Bjermer et al that compared single doses of RPL554/ensifentrine (form 0.4 mg to 24 mg) to nebulized salbutamol 2.5 mg and 7.5 mg or placebo, salbutamol resulted in dose-dependent increase in heart rate and fall in blood pressure and decreases in serum potassium, all of which were milder for heart rate and blood pressure and not observed for potassium with RPL554/ensifentrine.73 In that latter study, which used high doses (up to 24 mg) of RPL554/ensifentrine, systemic exposure was increased without the occurrence of adverse effects.73
Conclusion
For now, the evidence on clinical effects of ensifentrine remains too limited to position this drug in COPD management strategies: results from the first phase III trials are still awaited. However, pathophysiological, preclinical and phase II data suggest that this dual PDE3/4 inhibitor administered through the inhaled route could have a favorable benefit-risk profile: with PDE3 inhibition targeting bronchodilation and PDE4 inhibition targeting inflammation, mucus production and ciliary function, it has been showed to improve lung function, relieve dyspnea and improve quality of life. Effects have been clinically meaningful, which supports its potential role. Preclinical data indicate that the effects of dual PDE3/4 inhibition could be potentiated by bronchodilators and corticosteroids, and clinical studies found additional effects when associated with tiotropium, altogether suggesting a role as add-on therapy. Possible benefits in terms of exacerbations, lung function decline, comorbidities and mortality need to be explored, and studies aiming at identifying the best responders need to be performed.
Disclosure
Professor Pierre-Régis Burgel reports personal fees from Astra Zeneca, personal fees from Boehringer Ingelheim, personal fees from Chiesi, grants, personal fees from Gsk, personal fees from Insmed, personal fees from Novartis, personal fees from Pfizer, grants, personal fees from Vertex, personal fees from Zambon, outside the submitted work. Professor Nicolas Roche reports grants, personal fees from Boehringer Ingelheim, Novartis, Pfizer, personal fees from Teva, GSK, AstraZeneca, Chiesi, Sanofi, Trudell, Zambon, outside the submitted work.
The authors report no other conflicts of interest in this work.
References
- 1.Soriano JB, Kendrick PJ, Paulson KR; GBD Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2020;8(6):585–596. doi: 10.1016/S2213-2600(20)30105-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive lung disease. Available from:http://www.goldcopd.com. Accessed July19, 2021.
- 3.Martin C, Frija J, Burgel P-R. Dysfunctional lung anatomy and small airways degeneration in COPD. Int J Chron Obstruct Pulmon Dis. 2013;8:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hogg JC, McDonough JE, Suzuki M. Small airway obstruction in COPD: new insights based on micro-CT imaging and MRI imaging. Chest. 2013;143(5):1436–1443. doi: 10.1378/chest.12-1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin C, Frija-Masson J, Burgel P-R. Targeting mucus hypersecretion: new therapeutic opportunities for COPD? Drugs. 2014;74(10):1073–1089. doi: 10.1007/s40265-014-0235-3 [DOI] [PubMed] [Google Scholar]
- 6.Hogg JC, Paré PD, Hackett T-L. The Contribution of small airway obstruction to the pathogenesis of chronic obstructive pulmonary disease. Physiol Rev. 2017;97(2):529–552. doi: 10.1152/physrev.00025.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brusselle GG, Joos GF, Bracke KR. New insights into the immunology of chronic obstructive pulmonary disease. Lancet. 2011;378(9795):1015–1026. doi: 10.1016/S0140-6736(11)60988-4 [DOI] [PubMed] [Google Scholar]
- 8.Hou J, Sun Y. Role of regulatory t cells in disturbed immune homeostasis in patients with chronic obstructive pulmonary disease. Front Immunol. 2020;11:723. doi: 10.3389/fimmu.2020.00723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brightling C, Greening N. Airway inflammation in COPD: progress to precision medicine. Eur Respir J. 2019;54(2). doi: 10.1183/13993003.00651-2019 [DOI] [PubMed] [Google Scholar]
- 10.Lipson DA, Crim C, Criner GJ, et al. Reduction in all-cause mortality with fluticasone furoate/umeclidinium/vilanterol in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2020;201(12):1508–1516. doi: 10.1164/rccm.201911-2207OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez FJ, Rabe KF, Ferguson GT, et al.; ETHOS investigators. Reduced all-cause mortality in the ethos trial of budesonide/glycopyrrolate/formoterol for COPD: a Randomized, Double-Blind, Multi-Center Parallel-Group Study. Am J Respir Crit Care Med. 2021. Mar 1;203(5):553–564. doi: 10.1164/rccm.202006-2618OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Celli B, Decramer M, Kesten S, Liu D, Mehra S, Tashkin DP. Mortality in the 4-year trial of tiotropium (UPLIFT) in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180(10):948–955. doi: 10.1164/rccm.200906-0876OC [DOI] [PubMed] [Google Scholar]
- 13.Celli BR, Thomas NE, Anderson JA, et al. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med. 2008;178(4):332–338. doi: 10.1164/rccm.200712-1869OC [DOI] [PubMed] [Google Scholar]
- 14.Boardman C, Chachi L, Gavrila A, et al. Mechanisms of glucocorticoid action and insensitivity in airways disease. Pulm Pharmacol Ther. 2014;29(2):129–143. doi: 10.1016/j.pupt.2014.08.008 [DOI] [PubMed] [Google Scholar]
- 15.Barnes PJ. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2013;131(3):636–645. doi: 10.1016/j.jaci.2012.12.1564 [DOI] [PubMed] [Google Scholar]
- 16.Jiang Z, Zhu L. Update on molecular mechanisms of corticosteroid resistance in chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2016;37:1–8. doi: 10.1016/j.pupt.2016.01.002 [DOI] [PubMed] [Google Scholar]
- 17.Price D, Yawn B, Brusselle G, Rossi A. Risk-to-benefit ratio of inhaled corticosteroids in patients with COPD. Prim Care Respir J. 2013;22(1):92–100. doi: 10.4104/pcrj.2012.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agusti A, Bel E, Thomas M, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur. Respir. J. 2016;47(2):410–419. doi: 10.1183/13993003.01359-2015 [DOI] [PubMed] [Google Scholar]
- 19.Woodruff PG, Agusti A, Roche N, Singh D, Martinez FJ. Current concepts in targeting chronic obstructive pulmonary disease pharmacotherapy: making progress towards personalised management. Lancet. 2015;385(9979):1789–1798. doi: 10.1016/S0140-6736(15)60693-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chalmers JD, Laska IF, Franssen FME, et al. Withdrawal of inhaled corticosteroids in COPD: a European Respiratory Society guideline. Eur Respir J. 2020;55(6):2000351. doi: 10.1183/13993003.00351-2020. [DOI] [PubMed] [Google Scholar]
- 21.Nici L, Mammen MJ, Charbek E, et al. Pharmacologic management of chronic obstructive pulmonary disease. An official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;201(9):e56–e69. doi: 10.1164/rccm.202003-0625ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mei D, Tan WSD, Wong WSF. Pharmacological strategies to regain steroid sensitivity in severe asthma and COPD. Curr Opin Pharmacol. 2019;46:73–81. doi: 10.1016/j.coph.2019.04.010 [DOI] [PubMed] [Google Scholar]
- 23.Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N. Engl. J. Med. 2011;365(8):689–698. doi: 10.1056/NEJMoa1104623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uzun S, Djamin RS, Kluytmans JAJW, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2014;2(5):361–368. doi: 10.1016/S2213-2600(14)70019-0 [DOI] [PubMed] [Google Scholar]
- 25.Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med. 1968;278(25):1355–1360. doi: 10.1056/NEJM196806202782501 [DOI] [PubMed] [Google Scholar]
- 26.Yanai M, Sekizawa K, Ohrui T, Sasaki H, Takishima T. Site of airway obstruction in pulmonary disease: direct measurement of intrabronchial pressure. J Appl Physiol. 1992;72(3):1016–1023. doi: 10.1152/jappl.1992.72.3.1016 [DOI] [PubMed] [Google Scholar]
- 27.Young AL, Bragman FJS, Rangelov B, et al.; COPDGene Investigators. Disease progression modeling in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2020;201(3):294–302. doi: 10.1164/rccm.201908-1600OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rabe KF, Tenor H, Dent G, Schudt C, Liebig S, Magnussen H. Phosphodiesterase isozymes modulating inherent tone in human airways: identification and characterization. Am J Physiol. 1993;264:L458–464. [DOI] [PubMed] [Google Scholar]
- 29.Dent G, Poppe H, Egerland J, et al. Effects of a selective PDE4 inhibitor, D-22888, on human airways and eosinophils in vitro and late phase allergic pulmonary eosinophilia in guinea pigs. Pulm Pharmacol Ther. 1998;11(1):13–21. doi: 10.1006/pupt.1998.0111 [DOI] [PubMed] [Google Scholar]
- 30.Abbott-Banner KH, Page CP. Dual PDE3/4 and PDE4 inhibitors: novel treatments for COPD and other inflammatory airway diseases. Basic Clin Pharmacol Toxicol. 2014;114(5):365–376. doi: 10.1111/bcpt.12209 [DOI] [PubMed] [Google Scholar]
- 31.Fertig BA, Baillie GS. PDE4-mediated cAMP signalling. J Cardiovasc Dev Dis. 2018;5(1):8. doi: 10.3390/jcdd5010008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brescia M, Zaccolo M. Modulation of compartmentalised cyclic nucleotide signalling via local inhibition of phosphodiesterase activity. Int J Mol Sci. 2016;17(10):1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beavo JA, Brunton LL. Cyclic nucleotide research – still expanding after half a century. Nat Rev Mol Cell Biol. 2002;3(9):710–718. doi: 10.1038/nrm911 [DOI] [PubMed] [Google Scholar]
- 34.Zuo H, Cattani-Cavalieri I, Valença SS, Musheshe N, Schmidt M. Function of cAMP scaffolds in obstructive lung disease: focus on epithelial-to-mesenchymal transition and oxidative stress. Br J Pharmacol. 2019;176(14):2402–2415. doi: 10.1111/bph.14605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zuo H, Han B, Poppinga WJ, et al. Cigarette smoke up-regulates PDE3 and PDE4 to decrease cAMP in airway cells. Br J Pharmacol. 2018;175(14):2988–3006. doi: 10.1111/bph.14347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matera MG, Rogliani P, Calzetta L, Cazzola M. Phosphodiesterase inhibitors for chronic obstructive pulmonary disease: what does the future hold? Drugs. 2014;74(17):1983–1992. [DOI] [PubMed] [Google Scholar]
- 37.Spina D, Page CP. Xanthines and phosphodiesterase inhibitors. Handb Exp Pharmacol. 2017;237:63–91. [DOI] [PubMed] [Google Scholar]
- 38.Milara J, Armengot M, Bañuls P, et al. Roflumilast N-oxide, a PDE4 inhibitor, improves cilia motility and ciliated human bronchial epithelial cells compromised by cigarette smoke in vitro. Br J Pharmacol. 2012;166(8):2243–2262. doi: 10.1111/j.1476-5381.2012.01929.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cazzola M, Calzetta L, Rogliani P, Matera MG. Ensifentrine (RPL554): an investigational PDE3/4 inhibitor for the treatment of COPD. Expert Opin Investig Drugs. 2019;28(10):827–833. doi: 10.1080/13543784.2019.1661990 [DOI] [PubMed] [Google Scholar]
- 40.Venkatasamy R, Spina D. Novel relaxant effects of RPL554 on guinea pig tracheal smooth muscle contractility. Br J Pharmacol. 2016;173(15):2335–2351. doi: 10.1111/bph.13512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calzetta L, Page CP, Spina D, et al. Effect of the mixed phosphodiesterase 3/4 inhibitor RPL554 on human isolated bronchial smooth muscle tone. J Pharmacol Exp Ther. 2013;346(3):414–423. doi: 10.1124/jpet.113.204644 [DOI] [PubMed] [Google Scholar]
- 42.Calzetta L, Cazzola M, Page CP, Rogliani P, Facciolo F, Matera MG. Pharmacological characterization of the interaction between the dual phosphodiesterase (PDE) 3/4 inhibitor RPL554 and glycopyrronium on human isolated bronchi and small airways. Pulm Pharmacol Ther. 2015;32:15–23. doi: 10.1016/j.pupt.2015.03.007 [DOI] [PubMed] [Google Scholar]
- 43.Barber R, Baillie GS, Bergmann R, et al. Differential expression of PDE4 cAMP phosphodiesterase isoforms in inflammatory cells of smokers with COPD, smokers without COPD, and nonsmokers. Am J Physiol Lung Cell Mol Physiol. 2004;287(2):L332–343. doi: 10.1152/ajplung.00384.2003 [DOI] [PubMed] [Google Scholar]
- 44.Leclerc O, Lagente V, Planquois J-M, et al. Involvement of MMP-12 and phosphodiesterase type 4 in cigarette smoke-induced inflammation in mice. Eur Respir J. 2006;27(6):1102–1109. doi: 10.1183/09031936.06.00076905 [DOI] [PubMed] [Google Scholar]
- 45.Martorana PA, Beume R, Lucattelli M, Wollin L, Lungarella G. Roflumilast fully prevents emphysema in mice chronically exposed to cigarette smoke. Am J Respir Crit Care Med. 2005;172(7):848–853. doi: 10.1164/rccm.200411-1549OC [DOI] [PubMed] [Google Scholar]
- 46.Kuss H, Hoefgen N, Johanssen S, Kronbach T, Rundfeldt C. In vivo efficacy in airway disease models of N-(3,5-dichloropyrid-4-yl)-[1-(4-fluorobenzyl)-5-hydroxy-indole-3-yl]-glyoxylic acid amide (AWD 12-281), a selective phosphodiesterase 4 inhibitor for inhaled administration. J Pharmacol Exp Ther. 2003;307(1):373–385. doi: 10.1124/jpet.103.053942 [DOI] [PubMed] [Google Scholar]
- 47.Banner KH, Trevethick MA. PDE4 inhibition: a novel approach for the treatment of inflammatory bowel disease. Trends Pharmacol Sci. 2004;25(8):430–436. doi: 10.1016/j.tips.2004.06.008 [DOI] [PubMed] [Google Scholar]
- 48.Jones NA, Boswell-Smith V, Lever R, Page CP. The effect of selective phosphodiesterase isoenzyme inhibition on neutrophil function in vitro. Pulm Pharmacol Ther. 2005;18(2):93–101. doi: 10.1016/j.pupt.2004.10.001 [DOI] [PubMed] [Google Scholar]
- 49.Lagente V, Martin-Chouly C, Boichot E, Martins MA, Silva PMR. Selective PDE4 inhibitors as potent anti-inflammatory drugs for the treatment of airway diseases. Mem Inst Oswaldo Cruz. 2005;100(Suppl 1):131–136. doi: 10.1590/S0074-02762005000900023 [DOI] [PubMed] [Google Scholar]
- 50.Sousa LP, Lopes F, Silva DM, et al. PDE4 inhibition drives resolution of neutrophilic inflammation by inducing apoptosis in a PKA-PI3K/Akt-dependent and NF-kappaB-independent manner. J Leukoc Biol. 2010;87:895–904. [DOI] [PubMed] [Google Scholar]
- 51.Molnar-Kimber K, Yonno L, Heaslip R, Weichman B. Modulation of TNF alpha and IL-1 beta from endotoxin-stimulated monocytes by selective PDE isozyme inhibitors. Agents Actions. 1993;39(S1):C77–C79. doi: 10.1007/BF01972726 [DOI] [PubMed] [Google Scholar]
- 52.Jin S-LC, Conti M. Induction of the cyclic nucleotide phosphodiesterase PDE4B is essential for LPS-activated TNF-alpha responses. Proc Natl Acad Sci U S A. 2002;99(11):7628–7633. doi: 10.1073/pnas.122041599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gantner F, Schudt C, Wendel A, Hatzelmann A. Characterization of the phosphodiesterase (PDE) pattern of in vitro-generated human dendritic cells (DC) and the influence of PDE inhibitors on DC function. Pulm Pharmacol Ther. 1999;12(6):377–386. doi: 10.1006/pupt.1999.0220 [DOI] [PubMed] [Google Scholar]
- 54.Tannheimer SL, Sorensen EA, Haran AC, Mansfield CN, Wright CD, Salmon M. Additive anti-inflammatory effects of beta 2 adrenoceptor agonists or glucocorticosteroid with roflumilast in human peripheral blood mononuclear cells. Pulm Pharmacol Ther. 2012;25(2):178–184. doi: 10.1016/j.pupt.2012.01.003 [DOI] [PubMed] [Google Scholar]
- 55.Mata M, Martinez I, Melero JA, Tenor H, Cortijo J. Roflumilast inhibits respiratory syncytial virus infection in human differentiated bronchial epithelial cells. PLoS One. 2013;8(7):e69670. doi: 10.1371/journal.pone.0069670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turner MJ, Dauletbaev N, Lands LC, Hanrahan JW. The phosphodiesterase inhibitor ensifentrine reduces production of proinflammatory mediators in well differentiated bronchial epithelial cells by inhibiting PDE4. J Pharmacol Exp Ther. 2020;375(3):414–429. doi: 10.1124/jpet.120.000080 [DOI] [PubMed] [Google Scholar]
- 57.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363(23):2233–2247. doi: 10.1056/NEJMra0910061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rab A, Rowe SM, Raju SV, Bebok Z, Matalon S, Collawn JF. Cigarette smoke and CFTR: implications in the pathogenesis of COPD. Am J Physiol Lung Cell Mol Physiol. 2013;305(8):L530–541. doi: 10.1152/ajplung.00039.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu S, Veilleux A, Zhang L, et al. Dynamic activation of cystic fibrosis transmembrane conductance regulator by type 3 and type 4D phosphodiesterase inhibitors. J Pharmacol Exp Ther. 2005;314(2):846–854. doi: 10.1124/jpet.105.083519 [DOI] [PubMed] [Google Scholar]
- 60.Kelley TJ, Al-Nakkash L, Drumm ML. CFTR-mediated chloride permeability is regulated by type III phosphodiesterases in airway epithelial cells. Am J Respir Cell Mol Biol. 1995;13(6):657–664. doi: 10.1165/ajrcmb.13.6.7576703 [DOI] [PubMed] [Google Scholar]
- 61.Lambert JA, Raju SV, Tang LP, et al. Cystic fibrosis transmembrane conductance regulator activation by roflumilast contributes to therapeutic benefit in chronic bronchitis. Am J Respir Cell Mol Biol. 2014;50(3):549–558. doi: 10.1165/rcmb.2013-0228OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blanchard E, Zlock L, Lao A, et al. Anchored PDE4 regulates chloride conductance in wild-type and ΔF508-CFTR human airway epithelia. FASEB J. 2014;28(2):791–801. doi: 10.1096/fj.13-240861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Workman AD, Cohen NA. The effect of drugs and other compounds on the ciliary beat frequency of human respiratory epithelium. Am J Rhinol Allergy. 2014;28(6):454–464. doi: 10.2500/ajra.2014.28.4092 [DOI] [PubMed] [Google Scholar]
- 64.Chong J, Leung B, Poole P. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;9:CD002309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shen L-F, Lv X-D, Chen W-Y, Yang Q, Fang Z-X, Lu W-F. Effect of roflumilast on chronic obstructive pulmonary disease: a systematic review and meta-analysis. Ir J Med Sci. 2018;187(3):731–738. doi: 10.1007/s11845-018-1738-9 [DOI] [PubMed] [Google Scholar]
- 66.Rhee CK, Kim DK. Role of phosphodiesterase-4 inhibitors in chronic obstructive pulmonary disease. Korean J Intern Med. 2020;35(2):276–283. doi: 10.3904/kjim.2020.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Franciosi LG, Diamant Z, Banner KH, et al. Efficacy and safety of RPL554, a dual PDE3 and PDE4 inhibitor, in healthy volunteers and in patients with asthma or chronic obstructive pulmonary disease: findings from four clinical trials. Lancet Respir Med. 2013;1(9):714–727. doi: 10.1016/S2213-2600(13)70187-5 [DOI] [PubMed] [Google Scholar]
- 68.Singh D, Abbott-Banner K, Bengtsson T, Newman K. The short-term bronchodilator effects of the dual phosphodiesterase 3 and 4 inhibitor RPL554 in COPD. Eur Respir J. 2018;52(5):1801074. doi: 10.1183/13993003.01074-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cazzola M, Page C. An inhaled “bifunctional” dual PDE3/4 inhibitor provides additional short-term improvements in lung function compared to existing classes of bronchodilator: implications for future treatment of COPD. Eur Respir J. 2018;52(5):1801675. doi: 10.1183/13993003.01675-2018 [DOI] [PubMed] [Google Scholar]
- 70.Singh D, Martinez FJ, Watz H, Bengtsson T, Maurer BT. A dose-ranging study of the inhaled dual phosphodiesterase 3 and 4 inhibitor ensifentrine in COPD. Respir Res. 2020;21(47). doi: 10.1186/s12931-020-1307-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Watz H, Rickard K, Rheault T, Bengtsson T, Singh D. Symptom improvement following treatment with the inhaled dual phosphodiesterase 3 and 4 inhibitor ensifentrine in patients with moderate to severe COPD - A detailed analysis. Int J Chron Obstruct Pulmon Dis. 2020;15:2199–2206. doi: 10.2147/COPD.S263025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ferguson GT, Kerwin EM, Rheault T, Bengtsson T, Rickard K. A dose-ranging study of the novel inhaled dual PDE 3 and 4 inhibitor ensifentrine in patients with COPD receiving maintenance tiotropium therapy. Int J Chron Obstruct Pulmon Dis. 2021;16:1137–1148. doi: 10.2147/COPD.S307160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bjermer L, Abbott-Banner K, Newman K. Efficacy and safety of a first-in-class inhaled PDE3/4 inhibitor (ensifentrine) vs salbutamol in asthma. Pulm Pharmacol Ther. 2019;58:101814. doi: 10.1016/j.pupt.2019.101814 [DOI] [PubMed] [Google Scholar]
- 74.Grootendorst DC, Gauw SA, Verhoosel RM, et al. Reduction in sputum neutrophil and eosinophil numbers by the PDE4 inhibitor roflumilast in patients with COPD. Thorax. 2007;62(12):1081–1087. doi: 10.1136/thx.2006.075937 [DOI] [PMC free article] [PubMed] [Google Scholar]