Abstract
OBJECTIVE
This study aimed to report the spectrum of placental pathology findings in pregnancies complicated by SARS-CoV-2 infection.
DATA SOURCES
MEDLINE, Embase, Google Scholar, and the Web of Science databases were searched up to August 11, 2021.
STUDY ELIGIBILITY CRITERIA
Histopathologic anomalies included maternal vascular malperfusion, fetal vascular malperfusion, acute inflammatory pathology, chronic inflammatory pathology, increased perivillous fibrin, and intervillous thrombosis. Moreover, subanalyses of symptomatic women only and high-risk pregnancies were performed. METHODS: Histopathologic analysis of the placenta included gross examination, histopathology on hematoxylin and eosin, immunohistochemistry, fluorescence in situ hybridization, quantitative reverse transcription-polymerase chain reaction on placental tissue, and transmission electron microscope. Random-effect meta-analyses were used to analyze the data.
RESULTS
A total of 56 studies (1008 pregnancies) were included. Maternal vascular malperfusion was reported in 30.7% of placentas (95% confidence interval, 20.3–42.1), whereas fetal vascular malperfusion was observed in 27.08 % of cases (95% confidence interval, 19.2–35.6). Acute and chronic inflammatory pathologies were reported in 22.68% (95% confidence interval, 16.9–29.0) and 25.65% (95% confidence interval, 18.4–33.6) of cases, respectively. Increased perivillous fibrin was observed in 32.7% (95% confidence interval, 24.1–42.0) of placentas undergoing histopathologic analysis, whereas intervillous thrombosis was observed in 14.6% of cases (95% confidence interval, 9.7–20.2). Other placental findings, including a basal plate with attached myometrial fibers, microscopic accretism, villous edema, increased circulating nucleated red blood cells, or membranes with hemorrhage, were reported in 37.5% of cases (95% confidence interval, 28.0–47.5), whereas only 17.5% of cases (95% confidence interval, 10.9–25.2) did not present any abnormal histologic findings. The subanalyses according to maternal symptoms owing to SARS-CoV-2 infection or the presence of a high-risk pregnancy showed a similar distribution of the different histopathologic anomalies to that reported in the main analysis. Moreover, the risk of placental histopathologic anomalies was higher when considering only case-control studies comparing women with SARS-CoV-2 infection with healthy controls.
CONCLUSION
In pregnant women with SARS-CoV-2 infection, a significant proportion of placentas showed histopathologic findings, suggesting placental hypoperfusion and inflammation. Future multicenter prospective blinded studies are needed to correlate these placental lesions with pregnancy outcomes.
Key words: COVID-19, fetal vascular malperfusion, maternal vascular malperfusion, perinatal infection, placental histopathology, pregnancy outcomes, SARS-CoV-2
AJOG MFM at a Glance.
Why was this study conducted?
This systematic review aimed to quantify the prevalence of placental histopathologic abnormalities in women with SARS-CoV-2 infection in pregnancy.
Key findings
A significant proportion of women with SARS-CoV-2 infection in pregnancy showed placental histopathologic abnormalities, suggesting placental hypoperfusion and inflammation. The findings from this study might explain the higher risk of stillbirth observed in women with SARS-CoV-2 infection in pregnancy.
What does this add to what is known?
The results from this systematic review showed a high rate of maternal and fetal vascular malperfusion associated with acute and chronic inflammatory pathologies, potentially linking the observed increased risk of stillbirth with placental anomalies.
SARS-CoV-2 infection started spreading toward the end of 2019 and is still a major issue of public health, with new cases of infection, hospitalization, admission to the intensive care unit, and death increasing daily, worldwide.1 Pregnancy has been reported to be an independent risk factor for adverse outcomes in women with SARS-CoV-2 infection, especially if other comorbidities, such as diabetes mellitus or preeclampsia, coexist. The peculiar changes occurring in the cardiorespiratory system during pregnancy may be partially responsible for the increased burden of maternal morbidities observed in these women compared with the nonpregnant general population.2, 3, 4, 5 Currently, although vaccinal programs are open to pregnant women, there are still reports of a poor acceptance rate in this category of patients.6 , 7
Indeed, SARS-CoV-2 infection has been reported to potentially affect the placenta.8 Several reports suggest an increased risk of placental lesions because of hypoperfusion and inflammation in women with SARS-CoV-2 infection.9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64 The potential association between SARS-CoV-2 and impaired placental function is crucial because it might lead to fetal decompensation and increased risk of perinatal mortality and morbidity.65 , 66 Despite this, the occurrence of placental histopathologic abnormalities in pregnancies complicated by SARS-CoV-2 infection is yet to be fully explored. The small sample size of previously published studies, the heterogeneity in outcome assessment, and the inclusion criteria did not allow to extrapolate objective evidence on the actual risk of placental inflammatory and vascular anomalies in women with SARS-CoV-2 infection during pregnancy.8 , 54
This systematic review aimed to quantify the prevalence of placental histopathologic abnormalities in women with SARS-CoV-2 infection in pregnancy.
Methods
Protocol, information sources, and literature search
This systematic review was performed according to an a priori designed protocol and recommended for systematic reviews and meta-analysis. MEDLINE, Embase, Google Scholar, and the Web of Science databases were searched electronically up to August 11, 2021, using the following search terms (as words in the title or abstract), using combinations of the relevant Medical Subject Headings terms, key words, and word variants for “histopathology,” “placenta,” and “COVID-19.” The search and selection criteria were restricted to the English language. The reference lists of relevant articles and reviews were hand searched for additional reports. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines were followed.67
Outcomes measures, study selection, and data collection
According to the Amsterdam criteria, the histopathologic abnormalities assessed were divided into the following subcategories68:
-
1.
Maternal vascular malperfusion (MVM), including central villous infarction, peripheral villous infarction, villous agglutination, accelerated villous maturation, decidual arteriopathy (any atherosis and fibrinoid necrosis, mural hypertrophy of membrane arterioles, or absence of spiral artery remodeling), or retroplacental hematoma
-
2.
Fetal vascular malperfusion (FVM), including clustered avascular villi, fetal vessel mural fibrin, delayed villous maturation, hypercoiled umbilical cord, or chorioangiosis
-
3.
Acute inflammatory pathology (AIP), including maternal or fetal inflammatory response stage 2
-
4.
Chronic inflammatory pathology (CIP), including chronic villitis or low-grade chronic deciduitis with plasma cells
-
5.
Increased perivillous fibrin
-
6.
Intervillous thrombosis
-
7.
Other placental findings, including a basal plate with attached myometrial fibers, microscopic accretism, villous edema, increased circulating nucleated red blood cells, or membranes with hemorrhage.
Histopathologic anomalies were assessed in the overall population of pregnancies complicated by SARS-CoV-2 infection. Furthermore, subanalyses of symptomatic women only and those with a high-risk pregnancy (defined as those with a medical complication occurring in or preexisting pregnancy) were performed. Only studies where SARS-CoV-2 infection was confirmed by polymerase chain reaction were included. Histopathologic analysis of the placenta included gross examination, hematoxylin and eosin, immunohistochemistry, fluorescence in situ hybridization, transmission electron microscope, reverse transcriptase–polymerase chain reaction (RT-PCR), and qualitative RT-PCR.
In this study, 3 authors (R.D.G., S.A., and C.G.) reviewed all abstracts independently. An agreement regarding potential relevance was reached by consensus. Full-text copies of those articles were obtained, and the same 3 reviewers independently extracted relevant data regarding study characteristics, placental pathologic findings, and pregnancy outcomes. Inconsistencies were discussed by the reviewers, and a consensus was reached by discussion with the senior authors (M.L. and F.D.A.).
Quality assessment of the included studies was performed using the Newcastle-Ottawa Scale (NOS) for case-control or cohort studies. According to NOS, each study was judged on 3 broad perspectives: the selection of the study groups, the comparability of the groups, and the ascertainment of the outcome of interest. Assessment of the selection of a study included the evaluation of the representativeness of the exposed cohort, selection of the nonexposed cohort, ascertainment of exposure, and demonstration that the outcome of interest was not present at the start of the study. Assessment of the comparability of the study included the evaluation of the comparability of cohorts based on the design or analysis. Finally, the ascertainment of the outcome of interest included the evaluation of the type of the assessment of the outcome of interest, its length, and the adequacy of follow-up. According to NOS, a study can be awarded a maximum of 1 star for each numbered item within the selection and outcome categories. A maximum of 2 stars can be given for comparability.69 , 70
Case series were evaluated with a modified version of NOS,71 which is based on 8 questions in the domains of selection, ascertainment, causality, and reporting. Although a formal score could be assigned giving a binary response to each question, the numeric representation of the methodological quality was not considered appropriate as recommended, and the overall final judgment was made on the basis of questions 1, 2, 3, 7, and 8, which were deemed most critical in this specific clinical scenario.
Statistical analysis
We used meta-analyses of proportions to combine data and reported pooled proportions and their 95% confidence intervals (CI). Furthermore, we compared the risk of the different histopathologic anomalies in women with SARS-CoV-2 infection in pregnancy with healthy controls and expressed the results as odds ratios (ORs) with their CIs. Heterogeneity among the studies was explored using the I2 statistic, representing the percentage of variation that is because of heterogeneity rather than chance. A value of 0% indicated that no heterogeneity was observed, whereas values of >50% were associated with substantial heterogeneity. Because of the clinical heterogeneity among studies, a random-effects model was used for all meta-analyses. The Egger test was used to assess potential publication bias, and funnel plots were created for visual inspection. Tests for funnel plot asymmetry were not used when the total number of publications included for each outcome was <10, as the tests lacked the power to detect real asymmetry in this scenario. The analysis was performed using StatsDirect (version 3.0.171; StatsDirect Ltd, Merseyside, England, United Kingdom) and Review Manager (RevMan version 5.3; The Nordic Cochrane Centre, The Cochrane Collaboration, 2014, Copenhagen, Denmark) statistical softwares.
Results
Study selection and characteristics
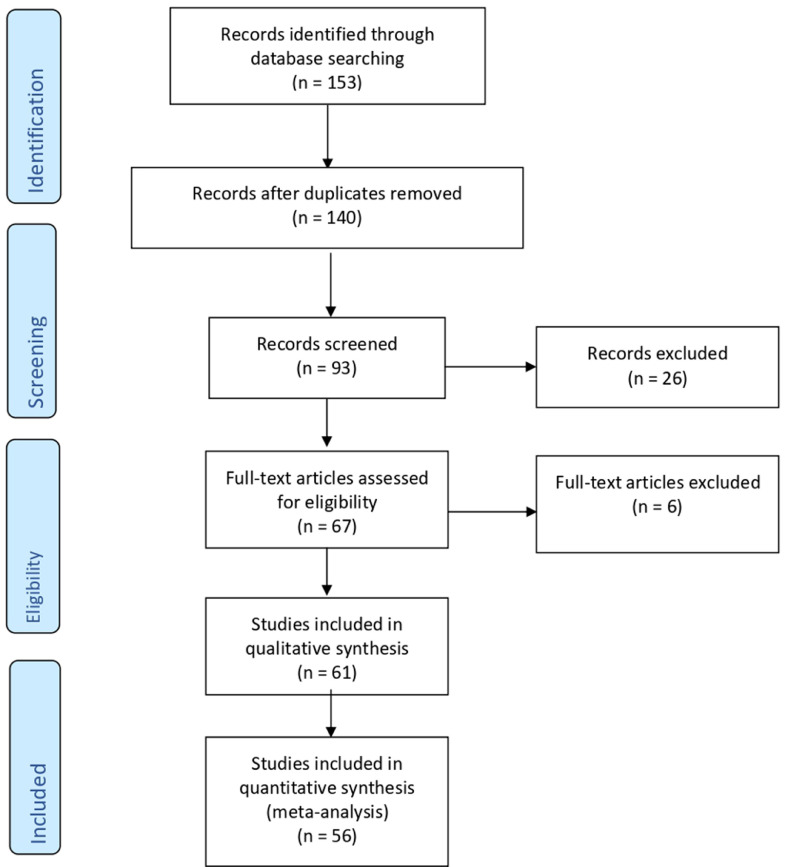
We identified 153 articles, where 80 were assessed concerning their eligibility for inclusion and 56 were included in the systematic review (Table 1 , Figure ).
Table 1.
General characteristics of the studies included in the systematic review
| Author | Year | Country | Study design | Pregnancies (n) | Placentas examined (n) | Type of pathology assessment |
|---|---|---|---|---|---|---|
| Algarroba et al9 | 2020 | United States | Case report | 1 | 1 | H&E; TEM |
| Algeri et al10 | 2020 | Italy | Case series | 5 | 5 | H&E |
| Baergen et al11 | 2020 | United States | Case series | 20 | 20 | H&E |
| Baud et al12 | 2020 | Switzerland | Case report | 1 | 1 | H&E; RT-PCR on amniotic fluid |
| Bertero et al13 | 2021 | Italy | Retrospective case-control study | 10 | 11 | H&E; FISH |
| Chen et al14 | 2020 | China | Case series | 3 | 3 | H&E; RT-PCR on placenta samples, membranes, and umbilical cords |
| Chen et al15 | 2020 | China | Case series | 5 | 5 | H&E |
| Chen et al16 | 2020 | China | Case series | 3 | 3 | Gross examination; RT-PCR on placenta samples |
| Cribiù et al17 | 2020 | Italy | Retrospective cohort | 21 | 21 | H&E; RT-PCR on placenta samples; IHC |
| Lu-Culligan et al18 | 2021 | United States | Prospective observational case-control study | 27 | 27 | Gross examination; H&E; IHC; FISH; RT-PCR |
| Debelenko et al19 | 2021 | United States | Retrospective case-control study | 75 | 75 | H&E; IHC; FISH |
| Facchetti et al20 | 2020 | Italy | Retrospective cohort | 19 | 19 | H&E; IHC; FISH; TEM; RT-PCR on placenta samples |
| Fan et al21 | 2020 | China | Case series | 2 | 2 | Qualitative RT-PCR on placenta samples |
| Ferraiolo et al22 | 2020 | Italy | Case report | 1 | 1 | H&E; PCR on placenta samples |
| Gao et al23 | 2021 | China | Retrospective cohort | 8 | 8 | H&E; IHC; FISH |
| Gulersen et al24 | 2020 | United States | Retrospective cohort | 50 | 50 | Gross examination; H&E |
| Guo et al25 | 2021 | China | Case series | 20 | 20 | Gross examination; H&E |
| He et al26 | 2020 | China | Retrospective case-control study | 41 | 41 | H&E |
| He et al27 | 2021 | China | Case report | 1 | 1 | H&E |
| Hecht et al28 | 2020 | United States | Retrospective case-control study | 19 | 19 | H&E; IHC; FISH |
| Hosier et al29 | 2020 | United States | Case report | 1 | 1 | H&E; IHC; FISH; TEM; RT-PCR on placenta samples |
| Hsu et al30 | 2020 | United States | Case report | 1 | 1 | Gross examination; H&E; IHC |
| Huang et al31 | 2020 | China | Case report | 1 | 1 | H&E; PCR on placenta samples |
| Jani et al32 | 2021 | United States | Retrospective medical record review | 34 | 34 | IHC |
| Kirtsman et al33 | 2020 | United States | Case report | 1 | 1 | H&E; RT-PCR on placenta samples |
| Kuhrt et al34 | 2020 | United Kingdom | Case report | 1 | 1 | H&E |
| Levitan et al35 | 2021 | United Kingdom | Retrospective case-control study | 65 | 64 | IHC |
| Linehan et al36 | 2021 | Ireland | Case report | 1 | 1 | H&E; IHC |
| Lokken et al37 | 2020 | United States | Case series | 46 | 1 | H&E; PCR on placenta samples |
| Menter et al38 | 2021 | Switzerland | Case series | 3 | 3 | H&E; FISH |
| Mongula et al39 | 2020 | The Netherlands | Case report | 1 | 1 | H&E; RT-PCR on placenta samples; IHC |
| Morotti et al40 | 2021 | Italy | Case report | 1 | 1 | H&E; IHC |
| Mulvey et al41 | 2020 | United States | Case series | 5 | 5 | H&E; IHC; FISH |
| Ozer et al42 | 2021 | Turkey | Case report | 1 | 1 | H&E; IHC; FISH |
| Patanè et al43 | 2020 | Italy | Case series | 22 | 2 | H&E; IHC |
| Patberg et al44 | 2021 | United States | Retrospective cohort | 77 | 77 | Gross examination; H&E; IHC |
| Peng et al45 | 2020 | China | Case report | 1 | 1 | Gross examination; RT-PCR on placenta samples |
| Poisson and Pierone46 | 2021 | United States | Case report | 1 | 1 | H&E |
| Prabhu et al47 | 2020 | United States | Prospective observational case-control study | 70 | 29 | RT-PCR on placenta samples; IHC |
| Pulinx et al48 | 2020 | Belgium | Case report | 1 | 1 | Blinded histopathology; RT-PCR on placenta samples |
| Rebutini et al49 | 2021 | Brazil | Prospective observational case-control study | 19 | 19 | Gross examination; H&E; IHC |
| Resta et al50 | 2021 | Italy | Prospective observational case-control study | 81 | 71 | Gross examination; H&E; IHC; TEM |
| Richtmann et al51 | 2020 | Brazil | Case series | 5 | 5 | H&E; RT-PCR on placenta samples |
| Blasco Santana et al52 | 2021 | Spain | Retrospective case-control study | 29 | 32 | Gross examination; H&E; IHC; RT-PCR on placenta samples |
| Schwartz et al53 | 2021 | Italy | Retrospective cohort | 6 | 6 | H&E; IHC |
| Shanes et al54 | 2020 | United States | Retrospective case-control study | 16 | 16 | H&E |
| Singh et al55 | 2021 | United States | Retrospective case-control study | 50 | 50 | H&E |
| Sisman et al56 | 2020 | United States | Case report | 1 | 1 | TEM; H&E; IHC |
| Smithgall et al57 | 2020 | United States | Retrospective case-control study | 51 | 51 | Gross examination; IHC; FISH |
| Taglauer et al58 | 2020 | United States | Retrospective case-control study | 15 | 15 | Gross examination; IHC |
| Tasca et al59 | 2021 | Italy | Prospective observational case-control study | 64 | 64 | Gross examination; H&E; IHC; RT-PCR on placenta samples |
| Valdespino-Vázquez et al60 | 2021 | Mexico | Case report | 1 | 1 | H&E; IHC |
| Vivanti et al61 | 2020 | France | Case report | 1 | 1 | H&E; RT-PCR on placenta samples; IHC |
| Wang et al62 | 2020 | China | Case report | 1 | 1 | RT-PCR on placenta samples |
| Wang et al63 | 2020 | China | Case report | 1 | 1 | RT-PCR on placenta samples |
| Xiong et al64 | 2020 | China | Case report | 1 | 1 | H&E; FISH |
FISH, fluorescence in situ hybridization; H&E, hematoxylin and eosin; IHC, immunohistochemistry; RT-PCR, reverse transcriptase–polymerase chain reaction; TEM, transmission electron microscopy.
Di Girolamo. SARS-CoV-2 infection and placenta. Am J Obstet Gynecol MFM 2021.
Figure.
Flow diagram of the study inclusion
Di Girolamo. SARS-CoV-2 infection and placenta. Am J Obstet Gynecol MFM 2021.
These 56 studies included 1008 pregnancies complicated by SARS-CoV-2 infection. Complete histopathologic analysis of the placenta was reported in 895 cases (Table 1). The results of the quality assessment of the included studies using NOS and modified NOS are presented in Tables 2 and 3 . The included studies showed an overall good score regarding the selection and comparability of the study groups and for ascertainment of the outcome of interest. The main weaknesses of these studies were their small sample sizes, lack of blinding toward the presence of infection, and heterogeneity of histopathologic analyses reported among the included studies.
Table 2.
Quality assessment of the included studies according to the Newcastle-Ottawa Scale for cohort studies
| Author | Year | Selection | Comparability | Outcome |
|---|---|---|---|---|
| Bertero et al13 | 2021 | ★★ | ★ | ★★★ |
| Cribiù et al17 | 2020 | ★ | ★★ | ★★ |
| Lu-Culligan et al18 | 2021 | ★ | ★ | ★ |
| Debelenko et al19 | 2021 | ★★ | ★★ | ★★ |
| Facchetti et al20 | 2020 | ★ | ★ | ★ |
| Gao et al23 | 2021 | ★★ | ★ | ★★ |
| Gulersen et al24 | 2020 | ★★★ | ★★ | ★★ |
| He et al26 | 2020 | ★★ | ★ | ★★ |
| Hecht et al28 | 2020 | ★ | ★★ | ★★ |
| Jani et al32 | 2021 | ★ | ★ | ★★ |
| Patberg et al44 | 2021 | ★★ | ★★ | ★★★ |
| Prabhu et al47 | 2020 | ★★ | ★ | ★★ |
| Rebutini et al49 | 2021 | ★★ | ★★ | ★★★ |
| Resta et al50 | 2021 | ★★ | ★ | ★★ |
| Blasco Santana et al52 | 2021 | ★★ | ★★ | ★★★ |
| Schwartz et al53 | 2021 | ★ | ★ | ★★ |
| Shanes et al54 | 2020 | ★★ | ★★ | ★★★ |
| Singh et al55 | 2021 | ★ | ★ | ★★ |
| Smithgall et al57 | 2020 | ★★ | ★ | ★★ |
| Tasca et al59 | 2021 | ★★ | ★ | ★★ |
A study can be awarded a maximum of 1 star for each numbered item within the selection and outcome categories, and a maximum of 2 stars can be given for the comparability category.
Di Girolamo. SARS-CoV-2 infection and placenta. Am J Obstet Gynecol MFM 2021.
Table 3.
Tool for evaluating the methodological quality of case reports and case series68
| Domains | Leading explanatory questions |
|---|---|
| Selection | 1. Does the patient represent the whole experience of the investigator (center), or is the selection method unclear to the extent that other patients with similar presentation may not have been reported? |
| Ascertainment | 2. Was the exposure adequately ascertained? 3. Was the outcome adequately ascertained? |
| Causality | 4. Were other alternative causes that may explain the observation ruled out? 5. Was there a challenge or rechallenge phenomenon? 6. Was there a dose-response effect? 7. Was follow-up long enough for outcomes to occur? |
| Reporting | 8. Is the case described with sufficient details to allow other investigators to replicate the research or to allow practitioners to make inferences related to their own practice? |
Questions 4, 5 and 6 are mostly relevant to cases of adverse drug events.
Di Girolamo. SARS-CoV-2 infection and placenta. Am J Obstet Gynecol MFM 2021.
Synthesis of the results
MVM was reported in 30.7% of placentas (95% CI, 20.3–42.1), whereas FVM was observed in 27.08% of cases (95% CI, 19.2–35.6). AIP and CIP were reported in 22.68% (95% CI, 16.9–29.0) and 25.65% (95% CI, 18.4–33.6) of cases, respectively. Increased perivillous fibrin was observed in 32.7% of placentas (95% CI, 24.1–42.0) undergoing histopathologic analysis, whereas intervillous thrombosis was observed in 14.6% of cases (95% CI, 9.7–20.2). Other placental findings, including a basal plate with attached myometrial fibers, microscopic accretism, villous edema, increased circulating nucleated red blood cells, or membranes with hemorrhage, were reported in 37.5% of cases (95% CI, 28.0–47.5), whereas only 17.5% of cases (95% CI, 10.9–25.2) did not present any abnormal histologic findings (Table 4 ).
Table 4.
Pooled proportions (with their 95% confidence interval) for the different histopathologic anomalies detected in placentas from women with SARS-CoV-2 infection in pregnancy
| Histopathologic findings | Studies (n) | Cases (n/N) | Pooled proportions (95% CI) | I2 (%) |
|---|---|---|---|---|
| Maternal vascular malperfusion | 41 | 235/753 | 30.69 (20.3–42.1) | 88.4 |
| Fetal vascular malperfusion | 45 | 192/847 | 27.08 (19.2–35.6) | 82.1 |
| Acute inflammatory pathology | 53 | 176/819 | 22.68 (16.9–29.0) | 66.9 |
| Chronic inflammatory pathology | 49 | 152/735 | 25.65 (18.4–33.6) | 75.0 |
| Increased perivillous fibrin | 47 | 176/662 | 32.77 (24.1–42.0) | 77.3 |
| Intervillous thrombosis | 46 | 102/710 | 14.63 (9.7–20.2) | 63.0 |
| Other findings | 55 | 269/825 | 37.54 (28.0–47.5) | 84.5 |
| No placental pathology | 48 | 102/639 | 17.49 (10.9–25.2) | 74.0 |
CI, confidence interval.
Di Girolamo. SARS-CoV-2 infection and placenta. Am J Obstet Gynecol MFM 2021.
Subanalyses according to the presence of maternal symptoms of the infection or high-risk pregnancy were reported in Table 5 . MVM was reported in 40.4% of women (95% CI, 27.0–54.4) with symptoms owing to SARS-CoV-2 infection, whereas FVM, AIP, and CIP were detected in 28.6% (95% CI, 19.2–39.1), 21.1% (95% CI, 14.8–28.1), and 27.4% (95% CI, 18.8–37.0) of cases, respectively. Increased perivillous fibrin and intervillous thrombosis were reported in 32.9% (95% CI, 21.4–45.6) and 11.3% (95% CI, 6.1–17.7) of cases, respectively, whereas only 13.9% of symptomatic women (95% CI, 7.0–22.6) did not show any anomalies at histopathologic examination of the placenta.
Table 5.
Pooled proportions (with their 95% confidence interval) for the different histopathologic anomalies detected in placentas from symptomatic women with SARS-CoV-2 infection in pregnancy and in those with high-risk pregnancies
| Subanalysis of symptomatic women only | ||||
|---|---|---|---|---|
| Histopathologic findings | Studies (n) | Cases (n/N) | Pooled proportions (95% CI) | I2 (%) |
| Maternal vascular malperfusion | 26 | 222/608 | 40.36 (27.0–54.4) | 90.3 |
| Fetal vascular malperfusion | 27 | 159/689 | 28.65 (19.2–39.1) | 86.0 |
| Acute inflammatory pathology | 28 | 133/636 | 21.09 (14.8–28.1) | 69.3 |
| Chronic inflammatory pathology | 26 | 133/603 | 27.43 (18.8–37.0) | 79.7 |
| Increased perivillous fibrin | 25 | 120/482 | 32.94 (21.4–45.6) | 83.8 |
| Intervillous thrombosis | 26 | 64/546 | 11.30 (6.1–17.7) | 70.1 |
| Other findings | 30 | 212/642 | 32.34 (20.3–45.7) | 89.9 |
| No placental pathology | 27 | 87/552 | 13.90 (7.0–22.6) | 81.2 |
| Subanalysis of women with high-risk pregnancy | ||||
| Maternal vascular malperfusion | 37 | 234/745 | 31.34 (20.5–43.2) | 89.4 |
| Fetal vascular malperfusion | 41 | 167/763 | 28.0 (19.5–37.3) | 83.2 |
| Acute inflammatory pathology | 47 | 138/731 | 21.20 (15.7–27.2) | 61.8 |
| Chronic inflammatory pathology | 43 | 148/647 | 26.20 (19.7–36.0) | 74.9 |
| Increased perivillous fibrin | 41 | 165/574 | 34.16 (24.7–44.2) | 78.0 |
| Intervillous thrombosis | 40 | 101/622 | 15.62 (10.8–21.1) | 55.0 |
| Other findings | 49 | 207/737 | 36.29 (27.1–46.0) | 81.6 |
| No placental pathology | 42 | 86/551 | 16.20 (9.5–24.3) | 74.3 |
CI, confidence interval.
Di Girolamo. SARS-CoV-2 infection and placenta. Am J Obstet Gynecol MFM 2021.
In women with a high-risk pregnancy, MVM and FVM were reported in 31.3% (95% CI, 20.5–43.2) and 28.0% (95% CI, 19.5–37.3) of cases, respectively, whereas the corresponding figures for AIP and CIP were 21.2% (95% CI, 15.7–27.2) and 27.5% (95% CI, 19.7–36.0), respectively. Increased perivillous fibrin deposition and intervillous thrombosis were observed in 34.1% (95% CI, 24.7–44.2) and 15.6 % (95% CI, 10.8–21.1) of cases, respectively. Finally, no placental anomaly was reported in 16.2% of women (95% CI, 9.5–24.3) (Table 5).
Risk analysis
Assessment of the actual risk of developing histopathologic anomalies was assessed, including only case-control studies comparing women with SARS-CoV-2 infection in pregnancy with healthy controls. Unfortunately, the analysis was affected by the smaller number of studies and an even smaller number of cases included compared with the proportion of meta-analyses, thus potentially representing a source of bias. Furthermore, we could not stratify the analysis according to the presence of maternal symptoms or high-risk pregnancy.
Overall, women with SARS-CoV-2 infection in pregnancy had a higher risk of FVM (OR, 1.9; 95% CI, 1.3–2.6; P=.002), CIP (OR, 1.94; 95% CI, 1.3–2.8; P=.003), increased perivillous fibrin (OR, 6.8; 95% CI, 2.7–17.0; P<.001), intervillous thrombosis (OR, 3.2; 95% CI, 2.0–5.2; P<.001), and other histopathologic anomalies (OR, 2.0; 95% CI, 1.4–2.7), whereas there was no difference in the risk of developing histopathologic signs of MVM (P=.198) or AIP (P=.204) (Table 6 ).
Table 6.
Pooled odds ratio of the different categorical outcomes explored in this systematic review
| Histologic findings | Studies (n) | Placentas examined (n/N) | Pooled OR (95% CI) | I2 (%) | P value (P<.05) |
|---|---|---|---|---|---|
| Maternal vascular malperfusion | 10 | 161/458 vs 226/779 | 1.82 (0.7–4.6) | 84.4 | .198 |
| Fetal vascular malperfusion | 11 | 115/535 vs 187/835 | 1.85 (1.3–2.6) | 67.6 | .002a |
| Acute inflammatory pathology | 11 | 95/499 vs 235/820 | 0.67 (0.4–1.2) | 64.2 | .204 |
| Chronic inflammatory pathology | 9 | 74/417 vs 105/712 | 1.94 (1.3–2.8) | 74.0 | .003a |
| Increased perivillous fibrin | 9 | 105/397 vs 46/656 | 6.78 (2.7–17.0) | 61.7 | <.001a |
| Intervillous thrombosis | 9 | 61/433 vs 38/671 | 3.23(2.0–5.2) | 85.5 | <.001a |
| Other findings | 11 | 165/499 vs 191/820 | 2.01 (1.4–2.7) | 73.8 | <.001a |
| No placental pathology | 8 | 35/385 vs 72/429 | 0.40 (0.2–0.6) | 86.0 | <.001a |
CI, confidence interval; OR, odds ratio.
p<0.05.
Di Girolamo. SARS-CoV-2 infection and placenta. Am J Obstet Gynecol MFM 2021.
Discussion
Summary of the main findings
The findings from this systematic review showed that a large proportion of pregnancies complicated by SARS-CoV-2 infection present placental histopathologic abnormalities consistent with placental inflammation and hypoperfusion, whereas approximately 17.5% of these pregnancies did not show any placental anomalies. Subgroup analyses according to the presence of maternal symptoms or high-risk pregnancy showed similar results with most placentas from women with SARS-CoV-2 infection in pregnancy.
Interpretation of the study findings and clinical and research implications
The recently reported association between SARS-CoV-2 infection in pregnancy and stillbirth questions whether the placenta can be a targeted host to viral infection. A population study from the United Kingdom that included >3000 pregnancies with laboratory-confirmed SARS-CoV-2 infection reported that stillbirth was significantly more common in women with infection than women without the infection (8.5 per 1000 vs 3.4 per 1000) with an OR of 2.21 (95% CI, 1.58–3.11; P<.001).65
The viral agent responsible for SARS-CoV-2 infection enters the host cells by interacting with the angiotensin-converting enzyme 2 receptor (ACE2), the levels of which are increased in the uterus and placenta of a pregnant woman. This assumption has been subsequently strengthened by the reported increased prevalence of signs of decidual arteriopathy in pregnant women with SARS-CoV-2 infection, suggesting a potential connection between infection and impaired placental function.9 , 19 , 28 , 35 The potential mechanisms responsible for the higher risk of fetal death in pregnancy may be primarily explained on the basis of a secondary effect of the virus owing to placental hypoperfusion induced by the compromised hemodynamic status of the mother, as viremia in patients with SARS-CoV-2 infection is not common, thus making it unlikely a direct damage of the virus to the placenta.54 , 55 , 58 Alternatively, increase proinflammatory mediators induced by the virus may represent an alternative hypothesis. SARS-CoV-2 infection is accompanied by an aggressive inflammatory response with the release of a large number of proinflammatory cytokines in an event known as a “cytokine storm.” The host immune response to the SARS-CoV-2 virus is hyperactive, resulting in an excessive inflammatory reaction. In this scenario, inflammation may lead to placental damage and the subsequent occurrence of histopathologic anomalies related to inflammation. Furthermore, this proinflammatory effect of the infection may be triggered by a down-regulation of the renin-angiotensin system (RAS) induced by the binding of the virus to the ACE2 receptor. RAS plays an important role in regulating the uteroplacental blood flow by balancing vasodilator and vasoconstrictive pathways. Down-regulation of RAS can lead to reduced levels of angiotensin 1 and 7 vasoconstrictions and impaired uteroplacental blood flow.72 A recent systematic review that included 28 studies and assessed the risk of preeclampsia in women with SARS-CoV-2 infection during pregnancy reported an increased risk of overall preeclampsia; severe preeclampsia; hemolysis, elevated liver enzymes, and low platelet count syndrome; and eclampsia compared with pregnant women without infection.73
The Royal College of Obstetricians and Gynaecologists recommends that pregnant women recovering from SARS-CoV-2 infection should be offered at least a fetal growth scan approximately 14 days after recovery from their illness (or >21 days from previous fetal biometry ultrasound), unless there is a preexisting clinical reason for an earlier scan, thus suggesting that these pregnancies might theoretically be at higher risk of fetal growth restriction.74
Despite this, there is still a substantial lack of evidence on the actual role of SARS-CoV-2 infection in affecting fetal growth. We have previously reported that, in women with mildly symptomatic infection, there was no difference in estimated fetal weight and fetal Dopplers in pregnancies complicated by SARS-CoV-2 infection compared with those which were not, although this study was hampered by the small sample size, the lack of severely symptomatic cases, and the heterogeneity in the gestational age at infection.75
Maternal vascular hypoperfusion is potentially associated with higher risks of impaired placental function, growth restriction, and stillbirth.76 In this systematic review, we reported a high incidence of placental lesions, suggesting hypoperfusion and inflammation, thus questioning whether these women should undergo additional scans during pregnancy.
Although subgroup analysis according to the severity of the disease and gestational age at infection could not be performed, it might be reasonable to offer women who recovered from SARS-CoV-2 infection an additional growth scan in the third trimester of pregnancy, to rule out the possibility of reduced fetal growth because of impaired placental function and therefore reassure the parents.
More importantly, additional ultrasound scans throughout pregnancy might be required in women presenting with objective risk factors for growth restriction, such as a previous complicated pregnancy, abnormal placental biomarkers, or increased pulsatility index in the uterine arteries, because SARS-CoV-2 infection in these women may worsen an already compromised placenta.76 , 77
Strengths and limitations
A thorough literature search aimed at including all potentially relevant studies, a multitude of histopathologic anomalies, and a large numbers of included cases9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64 represented the main strengths of this systematic review. The retrospective design of most included studies, the small sample size, and the heterogeneity in histopathologic anomalies observed are the main limitations of this systematic review. Furthermore, in none of the studies, the pathologists performing the analysis of the placenta were blinded to maternal infection status. Another major limitation of this systematic review was that we considered only macroscopic histopathologic anomalies of the placenta, and we did not consider the single pathologic diagnoses contained within these categories. Finally, subgroup analysis considering only case-control studies was affected by a small number of studies. Despite these limitations, this systematic review represented the most comprehensive up-to-date critical appraisal on the occurrence of histopathologic anomalies in placentas from women who had SARS-CoV-2 infection in pregnancy.
Conclusion
A significant proportion of women with SARS-CoV-2 infection in pregnancy showed placental histopathologic abnormalities, suggesting placental hypoperfusion and inflammation. Large multicenter prospective studies where routine, blinded histopathologic assessment of the placentas is performed are needed. These studies need to plan a priori sensitivity analysis according to whether the pregnant women were symptomatic or not, gestational age at infection, and whether the pregnancy was further complicated by another comorbidity or not.
Footnotes
The authors report no conflict of interest.
The authors received no funding for this study.
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.ajogmf.2021.100468.
Appendix. Supplementary materials
References
- 1.Mohamadian M, Chiti H, Shoghli A, Biglari S, Parsamanesh N, Esmaeilzadeh A. COVID-19: virology, biology and novel laboratory diagnosis. J Gene Med. 2021;23:e3303. doi: 10.1002/jgm.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Mascio D, Khalil A, Saccone G, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020;2 doi: 10.1016/j.ajogmf.2020.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Mascio D, Sen C, Saccone G, et al. Risk factors associated with adverse fetal outcomes in pregnancies affected by coronavirus disease 2019 (COVID-19): a secondary analysis of the WAPM study on COVID-19. J Perinat Med. 2020;48:950–958. doi: 10.1515/jpm-2020-0355. [DOI] [PubMed] [Google Scholar]
- 4.D'Antonio F, Sen C, Mascio DD, et al. Maternal and perinatal outcomes in high compared to low risk pregnancies complicated by severe acute respiratory syndrome coronavirus 2 infection (phase 2): the World Association of Perinatal Medicine working group on coronavirus disease 2019. Am J Obstet Gynecol MFM. 2021;3 doi: 10.1016/j.ajogmf.2021.100329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carbone L, Esposito R, Raffone A, Verrazzo P, Carbone IF, Saccone G. Proposal for radiologic diagnosis and follow-up of COVID-19 in pregnant women. J Matern Fetal Neonatal Med. 2020 doi: 10.1080/14767058.2020.1793325. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Mappa I, Luviso M, Distefano FA, Carbone L, Maruotti GM, Rizzo G. Women perception of SARS-CoV-2 vaccination during pregnancy and subsequent maternal anxiety: a prospective observational study. J Matern Fetal Neonatal Med. 2021 doi: 10.1080/14767058.2021.1910672. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Carbone L, Mappa I, Sirico A, et al. Pregnant women's perspectives on severe acute respiratory syndrome coronavirus 2 vaccine. Am J Obstet Gynecol MFM. 2021;3 doi: 10.1016/j.ajogmf.2021.100352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharps MC, Hayes DJL, Lee S, et al. A structured review of placental morphology and histopathological lesions associated with SARS-CoV-2 infection. Placenta. 2020;101:13–29. doi: 10.1016/j.placenta.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Algarroba GN, Rekawek P, Vahanian SA, et al. Visualization of severe acute respiratory syndrome coronavirus 2 invading the human placenta using electron microscopy. Am J Obstet Gynecol. 2020;223:275–278. doi: 10.1016/j.ajog.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Algeri P, Stagnati V, Spazzini MD, et al. Considerations on COVID-19 pregnancy: a cases series during outbreak in Bergamo Province, North Italy. J Matern Fetal Neonatal Med. 2020 doi: 10.1080/14767058.2020.1791817. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Baergen RN, Heller DS. Placental pathology in Covid-19 positive mothers: preliminary findings. Pediatr Dev Pathol. 2020;23:177–180. doi: 10.1177/1093526620925569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baud D, Greub G, Favre G, et al. Second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infection. JAMA. 2020;323:2198–2200. doi: 10.1001/jama.2020.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertero L, Borella F, Botta G, et al. Placenta histopathology in SARS-CoV-2 infection: analysis of a consecutive series and comparison with control cohorts. Virchows Arch. 2021 doi: 10.1007/s00428-021-03097-3. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen S, Huang B, Luo DJ, et al. Pregnancy with new coronavirus infection: clinical characteristics and placental pathological analysis of three cases. Zhonghua Bing Li Xue Za Zhi. 2020;49:418–423. doi: 10.3760/cma.j.cn112151-20200225-00138. [DOI] [PubMed] [Google Scholar]
- 15.Chen S, Liao E, Cao D, Gao Y, Sun G, Shao Y. Clinical analysis of pregnant women with 2019 novel coronavirus pneumonia. J Med Virol. 2020;92:1556–1561. doi: 10.1002/jmv.25789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X, Li Y, Wang J, Cai H, Cao H, Sheng J. [Pregnant women complicated with COVID-19: a clinical analysis of 3 cases] Zhejiang Da Xue Bao Yi Xue Ban. 2020;49:240–244. doi: 10.3785/j.issn.1008-9292.2020.03.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cribiù FM, Croci GA, Del Gobbo A, et al. Histological characterization of placenta in COVID19 pregnant women. Eur J Obstet Gynecol Reprod Biol. 2020;252:619–621. doi: 10.1016/j.ejogrb.2020.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu-Culligan A, Chavan AR, Vijayakumar P, et al. SARS-CoV-2 infection in pregnancy is associated with robust inflammatory response at the maternal-fetal interface. medRxiv. January 26, 2021 doi: 10.1101/2021.01.25.21250452. Preprint posted online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Debelenko L, Katsyv I, Chong AM, Peruyero L, Szabolcs M, Uhlemann AC. Trophoblast damage with acute and chronic intervillositis: disruption of the placental barrier by severe acute respiratory syndrome coronavirus 2. Hum Pathol. 2021;109:69–79. doi: 10.1016/j.humpath.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Facchetti F, Bugatti M, Drera E, et al. SARS-CoV2 vertical transmission with adverse effects on the newborn revealed through integrated immunohistochemical, electron microscopy and molecular analyses of Placenta. EBiomedicine. 2020;59 doi: 10.1016/j.ebiom.2020.102951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan C, Lei D, Fang C, et al. Perinatal transmission of 2019 coronavirus disease-associated severe acute respiratory syndrome coronavirus 2: should we worry? Clin Infect Dis. 2021;72:862–864. doi: 10.1093/cid/ciaa226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferraiolo A, Barra F, Kratochwila C, et al. Report of positive placental swabs for SARS-CoV-2 in an asymptomatic pregnant woman with COVID-19. Medicina (Kaunas) 2020;56:306. doi: 10.3390/medicina56060306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao L, Ren J, Xu L, et al. Placental pathology of the third trimester pregnant women from COVID-19. Diagn Pathol. 2021;16:8. doi: 10.1186/s13000-021-01067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gulersen M, Prasannan L, Tam Tam H, et al. Histopathologic evaluation of placentas after diagnosis of maternal severe acute respiratory syndrome coronavirus 2 infection. Am J Obstet Gynecol MFM. 2020;2 doi: 10.1016/j.ajogmf.2020.100211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo Y, Yuan J, Wang M, Yu Y, Bian J, Fan C. Case series of 20 pregnant women with 2019 novel coronavirus disease in Wuhan, China. J Obstet Gynaecol Res. 2021;47:1344–1352. doi: 10.1111/jog.14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He M, Skaria P, Kreutz K, et al. Histopathology of third trimester placenta from SARS-CoV-2-Positive women. Fetal Pediatr Pathol. 2020 doi: 10.1080/15513815.2020.1828517. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.He JR, Xiao YH, Ding W, et al. Maternal, placental and neonatal outcomes after asymptomatic SARS-CoV-2 infection in the first trimester of pregnancy: a case report. Case Rep Womens Health. 2021;31:e00321. doi: 10.1016/j.crwh.2021.e00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hecht JL, Quade B, Deshpande V, et al. SARS-CoV-2 can infect the placenta and is not associated with specific placental histopathology: a series of 19 placentas from COVID-19-positive mothers. Mod Pathol. 2020;33:2092–2103. doi: 10.1038/s41379-020-0639-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hosier H, Farhadian SF, Morotti RA, et al. SARS-CoV-2 infection of the placenta. J Clin Invest. 2020;130:4947–4953. doi: 10.1172/JCI139569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu AL, Guan M, Johannesen E, et al. Placental SARS-CoV-2 in a pregnant woman with mild COVID-19 disease. J Med Virol. 2021;93:1038–1044. doi: 10.1002/jmv.26386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jani S, Jacques SM, Qureshi F, et al. Clinical characteristics of mother-infant dyad and placental pathology in COVID-19 cases in predominantly African American population. AJP Rep. 2021;11:e15–e20. doi: 10.1055/s-0040-1721673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirtsman M, Diambomba Y, Poutanen SM, et al. Probable congenital SARS-CoV-2 infection in a neonate born to a woman with active SARS-CoV-2 infection. CMAJ. 2020;192:E647–E650. doi: 10.1503/cmaj.200821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuhrt K, McMicking J, Nanda S, Nelson-Piercy C, Shennan A. Placental abruption in a twin pregnancy at 32 weeks’ gestation complicated by coronavirus disease 2019 without vertical transmission to the babies. Am J Obstet Gynecol MFM. 2020;2 doi: 10.1016/j.ajogmf.2020.100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levitan D, London V, McLaren RA, et al. Histologic and immunohistochemical evaluation of 65 placentas from women with polymerase chain reaction-proven severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Arch Pathol Lab Med. 2021;145:648–656. doi: 10.5858/arpa.2020-0793-SA. [DOI] [PubMed] [Google Scholar]
- 36.Linehan L, O'Donoghue K, Dineen S, White J, Higgins JR, Fitzgerald B. SARS-CoV-2 placentitis: an uncommon complication of maternal COVID-19. Placenta. 2021;104:261–266. doi: 10.1016/j.placenta.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lokken EM, Walker CL, Delaney S, et al. Clinical characteristics of 46 pregnant women with a severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am J Obstet Gynecol. 2020;223 doi: 10.1016/j.ajog.2020.05.031. 911.e1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Menter T, Mertz KD, Jiang S, et al. Placental pathology findings during and after SARS-CoV-2 infection: features of villitis and malperfusion. Pathobiology. 2021;88:69–77. doi: 10.1159/000511324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mongula JE, Frenken MWE, van Lijnschoten G, et al. COVID-19 during pregnancy: non-reassuring fetal heart rate, placental pathology and coagulopathy. Ultrasound Obstet Gynecol. 2020;56:773–776. doi: 10.1002/uog.22189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morotti D, Cadamuro M, Rigoli E, et al. Molecular pathology analysis of SARS-CoV-2 in syncytiotrophoblast and Hofbauer cells in placenta from a pregnant woman and fetus with COVID-19. Pathogens. 2021;10:479. doi: 10.3390/pathogens10040479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mulvey JJ, Magro CM, Ma LX, Nuovo GJ, Baergen RN. Analysis of complement deposition and viral RNA in placentas of COVID-19 patients. Ann Diagn Pathol. 2020;46 doi: 10.1016/j.anndiagpath.2020.151530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ozer E, Cagliyan E, Yuzuguldu RI, Cevizci MC, Duman N. Villitis of unknown etiology in the placenta of a pregnancy complicated by COVID-19. Turk Patoloji Derg. 2021;37:167–171. doi: 10.5146/tjpath.2020.01506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patanè L, Morotti D, Giunta MR, et al. Vertical transmission of coronavirus disease 2019: severe acute respiratory syndrome coronavirus 2 RNA on the fetal side of the placenta in pregnancies with coronavirus disease 2019-positive mothers and neonates at birth. Am J Obstet Gynecol MFM. 2020;2 doi: 10.1016/j.ajogmf.2020.100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patberg ET, Adams T, Rekawek P, et al. Coronavirus disease 2019 infection and placental histopathology in women delivering at term. Am J Obstet Gynecol. 2021;224 doi: 10.1016/j.ajog.2020.10.020. 382.e1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peng Z, Wang J, Mo Y, et al. Unlikely SARS-CoV-2 vertical transmission from mother to child: a case report. J Infect Public Health. 2020;13:818–820. doi: 10.1016/j.jiph.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poisson TM, Pierone G., Jr. Placental pathology and fetal demise at 35 weeks of gestation in a woman with SARS-CoV-2 infection: a case report. Case Rep Womens Health. 2021;30:e00289. doi: 10.1016/j.crwh.2021.e00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prabhu M, Cagino K, Matthews KC, et al. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: a prospective cohort study. BJOG. 2020;127:1548–1556. doi: 10.1111/1471-0528.16403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pulinx B, Kieffer D, Michiels I, et al. Vertical transmission of SARS-CoV-2 infection and preterm birth. Eur J Clin Microbiol Infect Dis. 2020;39:2441–2445. doi: 10.1007/s10096-020-03964-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rebutini PZ, Zanchettin AC, Stonoga ETS, et al. Association between COVID-19 pregnant women symptoms severity and placental morphologic features. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.685919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Resta L, Vimercati A, Cazzato G, et al. SARS-CoV-2 and placenta: new insights and perspectives. Viruses. 2021;13:723. doi: 10.3390/v13050723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richtmann R, Torloni MR, Oyamada Otani AR, et al. Fetal deaths in pregnancies with SARS-CoV-2 infection in Brazil: a case series. Case Rep Womens Health. 2020;27:e00243. doi: 10.1016/j.crwh.2020.e00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blasco Santana L, Miraval Wong E, Álvarez-Troncoso J, Sánchez García L, Bartha JL, Regojo-Zapata RM. Maternal and perinatal outcomes and placental pathologic examination of 29 SARS-CoV-2 infected patients in the third trimester of gestation. J Obstet Gynaecol Res. 2021;47:2131–2139. doi: 10.1111/jog.14784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwartz DA, Baldewijns M, Benachi A, et al. Chronic histiocytic intervillositis With trophoblast necrosis is a risk factor associated with placental infection from coronavirus disease 2019 (COVID-19) and intrauterine maternal-fetal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission in live-born and stillborn infants. Arch Pathol Lab Med. 2021;145:517–528. doi: 10.5858/arpa.2020-0771-SA. [DOI] [PubMed] [Google Scholar]
- 54.Shanes ED, Mithal LB, Otero S, Azad HA, Miller ES, Goldstein JA. Placental pathology in COVID-19. Am J Clin Pathol. 2020;154:23–32. doi: 10.1093/ajcp/aqaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh N, Buckley T, Shertz W. Placental pathology in COVID-19: case series in a community hospital setting. Cureus. 2021;13:e12522. doi: 10.7759/cureus.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sisman J, Jaleel MA, Moreno W, et al. Intrauterine transmission of SARS-COV-2 infection in a preterm infant. Pediatr Infect Dis J. 2020;39:e265–e267. doi: 10.1097/INF.0000000000002815. [DOI] [PubMed] [Google Scholar]
- 57.Smithgall MC, Liu-Jarin X, Hamele-Bena D, et al. Third-trimester placentas of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-positive women: histomorphology, including viral immunohistochemistry and in-situ hybridization. Histopathology. 2020;77:994–999. doi: 10.1111/his.14215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taglauer E, Benarroch Y, Rop K, et al. Consistent localization of SARS-CoV-2 spike glycoprotein and ACE2 over TMPRSS2 predominance in placental villi of 15 COVID-19 positive maternal-fetal dyads. Placenta. 2020;100:69–74. doi: 10.1016/j.placenta.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tasca C, Rossi RS, Corti S, et al. Placental pathology in COVID-19 affected pregnant women: a prospective case-control study. Placenta. 2021;110:9–15. doi: 10.1016/j.placenta.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Valdespino-Vázquez MY, Helguera-Repetto CA, León-Juárez M, et al. Fetal and placental infection with SARS-CoV-2 in early pregnancy. J Med Virol. 2021;93:4480–4487. doi: 10.1002/jmv.26965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vivanti AJ, Vauloup-Fellous C, Prevot S, et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun. 2020;11:3572. doi: 10.1038/s41467-020-17436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang S, Guo L, Chen L, et al. A case report of neonatal 2019 coronavirus disease in China. Clin Infect Dis. 2020;71:853–857. doi: 10.1093/cid/ciaa225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang X, Zhou Z, Zhang J, Zhu F, Tang Y, Shen X. A Case of 2019 novel coronavirus in a pregnant woman with preterm delivery. Clin Infect Dis. 2020;71:844–846. doi: 10.1093/cid/ciaa200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xiong X, Wei H, Zhang Z, et al. Vaginal delivery report of a healthy neonate born to a convalescent mother with COVID-19. J Med Virol. 2020;92:1657–1659. doi: 10.1002/jmv.25857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gurol-Urganci I, Jardine JE, Carroll F, et al. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection at the time of birth in England: national cohort study. Am J Obstet Gynecol. 2021 doi: 10.1016/j.ajog.2021.05.016. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rizzo G, Mappa I, Maqina P, et al. Effect of SARS-CoV-2 infection during the second half of pregnancy on fetal growth and hemodynamics: a prospective study. Acta Obstet Gynecol Scand. 2021;100:1034–1039. doi: 10.1111/aogs.14130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zorzela L, Loke YK, Ioannidis JP, et al. PRISMA harms checklist: improving harms reporting in systematic reviews. BMJ. 2016;352:i157. doi: 10.1136/bmj.i157. [DOI] [PubMed] [Google Scholar]
- 68.Khong TY, Mooney EE, Ariel I, et al. Sampling and definitions of placental lesions: Amsterdam placental workshop group consensus statement. Arch Pathol Lab Med. 2016;140:698–713. doi: 10.5858/arpa.2015-0225-CC. [DOI] [PubMed] [Google Scholar]
- 69.Centre for Reviews and Dissemination. Systematic reviews: CRD's guidance for undertaking reviews in health care. 2009. Available at:https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf. Accessed December 3, 2016.
- 70.Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14:45. doi: 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23:60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Seymen CM. Being pregnant in the COVID-19 pandemic: effects on the placenta in all aspects. J Med Virol. 2021;93:2769–2773. doi: 10.1002/jmv.26857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Conde-Agudelo A, Romero R. SARS-COV-2 infection during pregnancy and risk of preeclampsia: a systematic review and meta-analysis. Am J Obstet Gynecol. 2021 doi: 10.1016/j.ajog.2021.07.009. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Benski C, Di Filippo D, Taraschi G, Reich MR. Guidelines for pregnancy management During the COVID-19 pandemic: a public health conundrum. Int J Environ Res Public Health. 2020;17:8277. doi: 10.3390/ijerph17218277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Iacovelli A, Liberati M, Khalil A, et al. Risk factors for abnormally invasive placenta: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2020;33:471–481. doi: 10.1080/14767058.2018.1493453. [DOI] [PubMed] [Google Scholar]
- 76.Di Mascio D, Buca D, Berghella V, et al. Counseling in maternal-fetal medicine: SARS-CoV-2 infection in pregnancy. Ultrasound Obstet Gynecol. 2021;57:687–697. doi: 10.1002/uog.23628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.WAPM (World Association of Perinatal Medicine) Working Group on COVID-19 Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection. Ultrasound Obstet Gynecol. 2021;57:232–241. doi: 10.1002/uog.23107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.