Abstract
In the effort to control SARS-CoV-2 transmission, public health agencies in the United States and globally are aiming to increase population immunity. Immunity through vaccination and acquired following recovery from natural infection are the two means to build up population immunity, with vaccination being the safe pathway. However, measuring the contribution to population immunity from vaccination or natural infection is non-trivial. Historical COVID-19 case counts and vaccine coverage are necessary information but are not sufficient to approximate population immunity. Here, we consider the nuances of measuring each and propose an analytical framework for integrating the necessary data on cumulative vaccinations and natural infections at the state and national level. To guide vaccine roll-out and other aspects of control over the coming months, we recommend analytics that combine vaccine coverage with local (e.g. county-level) history of case reports and adjustment for waning antibodies to establish local estimates of population immunity. To do so, the strategic use of minimally-biased serology surveys integrated with vaccine administration data can improve estimates of the aggregate level of immunity to guide data-driven decisions to re-open safely and prioritize vaccination efforts.
Keywords: Herd Immunity, Vaccines, SARS-CoV-2, Covid-19, Serology
Quelling community transmission of SARS-CoV-2 while facilitating a return to social and economic normalcy requires building up to population immunity.[1] Epidemiological theory provides a basis for calculating a target for population immunity, suggesting we will need to reach, if not exceed,70%–80% protection against infection to curb sustained transmission. But how do we measure progress towards that threshold? Immunity to SARS-CoV-2 infection arises in two basic ways – through natural infection and through vaccination. Natural infection is perilous; for example, large-scale transmission in the United States could still lead to tens of millions of infections and hundreds of thousands of fatalities. Vaccination provides the safer route. In the United States, as of August 23, 2021 approximately 73% of all adults have received at least one vaccine dose. Estimates of cumulative incidence from natural infection vary, but likely upwards of 30% of the U.S. population has been infected. However, these values taken together should not indicate that the United States has ∼100% protection against infection. Likewise, the fact that ∼50% of all adults had received at least one dose as of April 20, 2021 should not indicate that the United States had ∼80% protection against infection a few months ago.
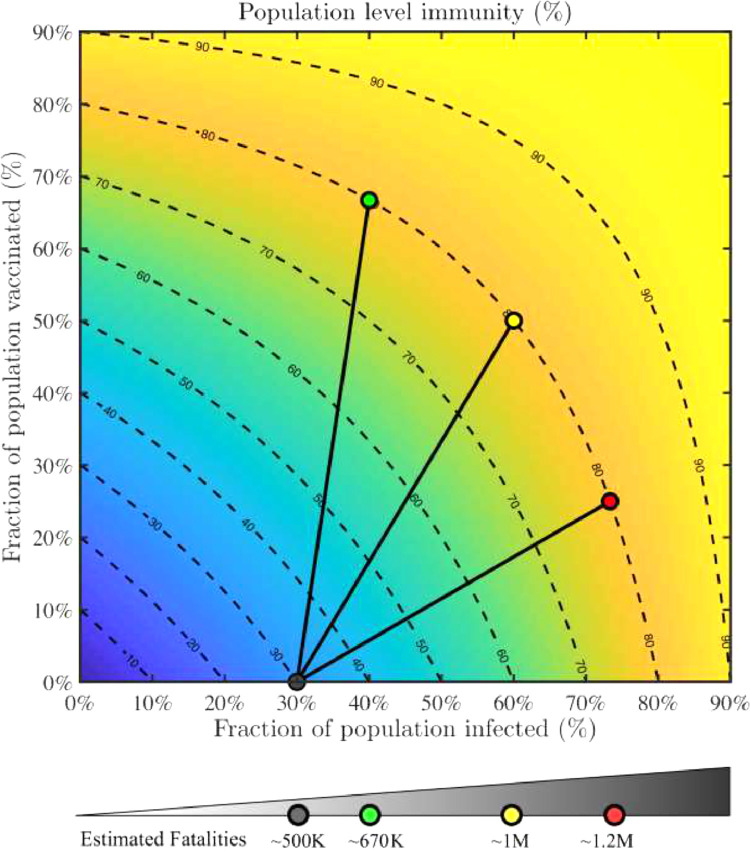
Developing an estimate of progress towards herd immunity involves, in theory, enumerating people who have experienced and survived natural infection, have been vaccinated, or both. But, in practice, limitations in available data on cumulative incidence, uncertainties about the durability of protection conferred by either natural infection or vaccination (against circulating variants), and lack of systematic data on how many people have been vaccinated who were already naturally infected complicate developing a confident estimate of our progress toward exceeding herd immunity thresholds of population-level immunity. Here we propose a framework to combine natural infection data, accounting for waning antibodies (which is not synonymous with waning immunity), with vaccine coverage data by race/ethnicity and other key demographics, to monitor movement towards population-level immunity overall and in key groups (Fig. 1 ). Such data being publicly available and/or integrated into such a framework by public health agencies is needed to allow up-to-date assessment of population-level immunity.
Fig 1.
Paths to population immunity through recovery from natural infection and vaccination.Population immunity as a combined function of fraction recovered (x) and fraction vaccinated (y). The heat map (with contours) denotes the estimated fraction of the population that is likely immune to severe infection (f = x + (1-x)*y). Contour lines denote equivalent levels of population level immunity, f. Vaccination initiatives that start with an estimated 30% of population recovered (estimated via serosurveys when accounting for seroreversion) reach 80% population level immunity given an intensive campaign (green; 2/3 vaccinated, 10% more infected); intermediate campaign (yellow; 1/2 vaccinated, 30% more infected), and an incomplete campaign (red; 1/4 vaccinated, 43% more infected). Estimated fatalities associated with start of vaccination campaign and after reaching target population level immunity, albeit with different coverage (fatalities are scaled with the increase in infected population for each scenario). A dashboard estimating population immunity amongst US states is available at https://popimmunity.biosci.gatech.edu. (For interpretation of the references to colour in their figure legend, the reader is referred to the web version of this article.)
First, there still remains considerable uncertainty regarding how many people have been infected with SARS-CoV-2. A substantial number of infected individuals do not seek care or get tested and the proportion of cases that are diagnosed has changed over time as testing became more accessible. In addition, COVID-19 cases only represent a fraction of infections, because many are asymptomatic.[2] Even this diagnosed fraction likely changed over time as different age groups were affected, with children much more likely to be asymptomatic (and undiagnosed) than older adults. For these reasons, sero-epidemiological studies are a valuable tool to calculate the proportion of the population who have been infected.[3] In reality, however, these studies estimate the current prevalence of antibodies in the population. For some pathogens, antibodies are persistent for years or decades and are therefore an effective proxy for previous infection and/or immunity, but the situation is more complicated for SARS-CoV-2.
Relatedly, antibodies to SARS-CoV-2 wane below detectable levels at a time-scale of multiple months [4,5] so seroprevalence studies, no matter how well conducted, will underestimate the proportion of the population who have recovered from infection. The degree of underestimation will depend on the rate that antibodies decline, the assay characteristics, when the serology study was performed and the historical infection patterns in the population surveyed. Consider, for example, two distinct communities that suffered similarly-sized outbreaks: one in March 2020 and the other in December 2020. After a few months antibodies wane and become undetectable for a substantial part of the previously-infected population,[5] a serosurvey conducted in February 2021 would show lower prevalence in community A than B, despite a similar number of cumulative infections. This issue is of practical concern; seroprevalence declined throughout Summer and Fall 2020 in New York City, Connecticut and other places that had widespread outbreaks in Spring 2020 and low-incidence summers. To account for the influence of waning on seroprevalence estimates of disease burden, we have developed a statistical method to calculate cumulative incidence from seroprevalence that accounts for antibody waning.[6] This method estimates how many people have been infected to date, which can be substantially higher than the observed cross-sectional seroprevalence. We can expect the gap between seroprevalence and cumulative incidence to widen as the time since infection grows.
Of course, one really wants to know the level of population immunity, not just how many have been infected. Importantly for population serological studies, nearly all individuals infected with SARS-CoV-2 produce detectable antibodies.[7] Observational data are emerging that show strong protection for those with anti-spike IgG antibodies, [8] a finding consistent with laboratory studies that find antibody binding correlates strongly with neutralization activity.[9] Fortunately, even when antibodies to SARS-CoV-2 wane below detectable levels, a person may still be immune to infection and/or disease through cellular immunity. T cell immunity, which may play a key role in medium- to long-term protection and memory B cells that may generate rapid antibody response upon re-exposure are not measured by serological testing alone. And, while the duration of protection remains unknown, the duration seems likely to be substantially longer than a pessimistic scenario based on antibody waning alone.[8] If immune-escape variants become widespread, the correlation between seroprevalence and population immunity will be diminished. These issues require further study and ongoing assessment of the spread of variants and cross-protection. But, it is clear that loss of detectable antibodies does not equate to loss of immunity since cellular immunity persists following the decay of antibodies.
Vaccination is being implemented amidst this dynamic backdrop of population immunity from natural infection. Vaccination has the potential to confer immunity to the susceptible population and, depending on the rate of roll-out, can rapidly increase population immunity. This is illustrated in the Figure; high levels of immunity (yellow contours) occur through combinations of recovery natural infection and vaccine coverage. The model offers a simple framework for combining immunity from natural infection and vaccination. To estimate population immunity, we first calculate a proportion susceptible to severe infection (g), as a function of the proportion recovered from natural infection (x) and the proportion vaccinated (y) such that g = (1-x)*(1-y). This formula can be interpreted as the fraction of the population that was neither previously infected nor vaccinated. Hence, the proportion of the population (f) that is unlikely to be susceptible to severe infection is 1-g or f = 1-(1-x)*(1-y). This can be reduced to f = x+(1-x)y. This formula assumes that vaccination is independent of natural infection. If previously infected people are more likely to get vaccinated, this formula overestimates immunity and if susceptible people are more likely to get vaccinated, this formula underestimates immunity. We assume a two-week delay to recognize that immunity is not acquired instantaneously upon infection and the complete dose(s) of vaccination. Assuming 30% of the population had recovered at the time of vaccine introduction, we highlight three pathways to 80% immunity in the population: intensive, intermediate and incomplete campaigns, the latter two of which require more natural infection (and resulting illness and death) to arrive at high levels of immunity.
It should also be noted that the population immunity threshold (i..e., ‘herd immunity’) is a rough estimate and can change. The population immunity threshold is calculated, in the simplest sense as 1–1/R0, assuming a reproduction number R0 of 5; perfect life-long immunity and homogenous risk across the population. In reality, each of these factors is more nuanced. R0 is not a fixed, biological value. Rather, the reproduction number will decrease when NPIs, such as masking, are practiced and rise again when they are relaxed. Second, immunity to SARS-CoV-2 is clearly not perfect nor life long, as reinfections have been documented. Third, and perhaps most importantly, the herd immunity threshold may be affected by new variants in two ways. More transmissible variants such as Delta have higher R0-s. And, immune escape variants reduce the proportion of the population who are effectively immune. Age is another important factor that, for the sake of simplicity, is not formally accounted for in our visualization. But, immunity in all age groups contribute to population immunity. About 15% of the US population are under the age of 12 years and therefore currently age-ineligible for vaccination, highlighting how critical it will be to achieve high coverage in the rest of the population, including adolescent and young adults who are at much lower risk of severe disease but can nonetheless be infected and transmit to individuals at higher risk of severe disease.
Note that we utilize the term ‘population immunity’ to denote the fraction of the population that is not immunologically naïve, due either to prior infections or to vaccinations. This fraction of the population is expected to have significantly reduced risk of severe infection. We recognize that the term herd immunity generally refers to a critical point at which susceptible depletion leads to a reduction in expected transmission rate. Observational studies have shown that mRNA vaccines reduce risk of infection in addition to risks of severe disease,[8] suggesting that mRNA vaccines include both direct and indirect benefits.[10]
An additional complexity in our framework that some individuals have received only a single dose of the 2-dose mRNA vaccines, some of whom will not go onto receive their second dose. The online version of the framework (https://popimmunity.biosci.gatech.edu) calculates immunity for the population having received at least one dose or fully vaccinated. Both mRNA vaccines used in the US confer slightly lower protection (VE = 82%; 95% CI = 74%–87%) for one dose compared to a full-course (VE = 94%; 95% CI = 87%–97%).[11] These real-world vaccine effectiveness estimates are similar to vaccine efficacy from the pivotal trials.[12,13] Vaccine protection is not immediate. Rather, substantial protection begins about 10 days post dose 1, with full protection about 14 days post dose 2. As a result, there will be meaningful lags between the number vaccinated and the number immune. Since all current vaccines elicit a response to the SARS-CoV-2 spike protein, seroprevalence to anti-spike IgG also rises against a backdrop of seroprevalence that is falling as a result of waning antibodies. A bump in seroprevalence following roll out is likely an indication of vaccine-induced immunity, but it should be interpreted in the context of declining infection-induced seroprevalence in this population. Populations that had more recent outbreaks may not be in the midst of declining seroprevalence when vaccines are introduced.
A critical issue in interpreting post-vaccination serology studies and understanding vaccine impact is the distribution of vaccines. If random, previously infected and uninfected would be equally likely to be vaccinated. Perhaps it would be ideal to first vaccinate immunologically-susceptible people. Given limited vaccination capacity, such an approach would be more efficient than vaccinating those already protected, but we lack the capacity to identify immune individuals though individual serological testing prior to vaccination. Indeed, vaccination has not been administered evenly or equitably through the population, with white populations vaccinated at higher levels compared to Black and Hispanic populations.[14] Conversely, Black, Hispanic, Native American and Native Hawaiian and Pacific Islander populations were disproportionately infected in the early stages of the US outbreak. Other socioeconomic factors have also driven inequalities in susceptibility to exposure to transmission and vaccine accessibility. With unequal exposure to natural infection followed by unequal distribution of vaccines across population groups, measuring population immunity will be complex, and serology studies will require nuanced interpretation and careful integration with vaccination data. To facilitate further research and allow measuring of herd immunity in important subgroups, public health agencies both in the United States and globally should make time series data on COVID-19 cases, deaths, and vaccine coverage stratified by age, race/ethnicity, and geography publicly available.
Historical COVID-19 case counts and vaccine coverage are necessary information but are not sufficient to approximate population immunity. While we often refer to a country or state's epidemic, transmission is local, and, to a large extent, so is population immunity. Accordingly, to guide vaccine roll-out and other aspects of control over the coming months, we recommend analytics that combine vaccine coverage with local (e.g. county-level) history of case reports and adjustment for waning antibodies to gain local estimates of population immunity. To do so, the strategic use of minimally-biased serology surveys integrated with vaccine distribution data can improve estimates of the aggregate level of immunity to guide data-driven decisions to re-open safely and prioritize vaccination efforts. Specifically, we recommend population-based, cross-sectional serological studies that cover the age range, with sufficient sampling to produce reliable estimates by age and race. Such high quality studies can supplement ongoing, convenient sampling conducted by the CDC.[15] To isolate the contribution of natural infection these studies should test for nucleocapsid antibodies, which are not produced by vaccination. If deployed at scale, these data can enhance the speed at which vaccination complements pre-existing immunity to reduce the risk of transmission for all.
Acknowledgements
Author Patrick Sullivan is the Editor in Chief of Annals of Epidemiology. This article was reviewed and handled by an independent editor. Dr. Sullivan was not involved in the editorial decision of the submission.
This work was supported by the National Science Foundation (2032082/2032084), National Institute General Medical Sciences (R01 GM124280) and the US National Institute of Allergy and Infectious Diseases (3R01AI143875-02S1).
Footnotes
Declaration of interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Sridhar D., Gurdasani D. Herd immunity by infection is not an option. Science. 2021;371:230–231. doi: 10.1126/science.abf7921. [DOI] [PubMed] [Google Scholar]
- 2.CMMID COVID-19 working group. Davies N.G., Klepac P., Liu Y., Prem K., Jit M., CMMID COVID-19 working group Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020;26:1205–1211. doi: 10.1038/s41591-020-0962-9. [DOI] [PubMed] [Google Scholar]
- 3.Krammer F., Simon V. Serology assays to manage COVID-19. Science. 2020;368:1060–1061. doi: 10.1126/science.abc1227. [DOI] [PubMed] [Google Scholar]
- 4.Iyer A.S., Jones F.K., Nodoushani A., Kelly M., Becker M., Slater D., et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abe0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel M.M., Thornburg N.J., Stubblefield W.B., Talbot H.K., Coughlin M.M., Feldstein L.R., et al. Change in antibodies to SARS-CoV-2 Over 60 days among health care personnel in Nashville, Tennessee. JAMA. 2020 doi: 10.1001/jama.2020.18796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shioda K., Lau M.S.Y., Kraay A.N.M., Nelson K.N., Siegler A.J., Sullivan P.S., et al. Estimating the cumulative incidence of SARS-CoV-2 infection and the infection fatality ratio in light of waning antibodies. Epidemiology. 2021;32(4):518–524. doi: 10.1097/EDE.0000000000001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wajnberg A., Amanat F., Firpo A., Altman D.R., Bailey M.J., Mansour M., et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370:1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall V.J., Foulkes S., Charlett A., Atti A., Monk E.J.M., Simmons R., et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN) Lancet. 2021;397:1459–1469. doi: 10.1016/S0140-6736(21)00675-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang A.T., Garcia-Carreras B., Hitchings M.D.T., Yang B., Katzelnick L.C., Rattigan S.M., et al. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat Commun. 2020;11:4704. doi: 10.1038/s41467-020-18450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallagher M.E., Sieben A.J., Nelson K.N., Kraay A.N.M., Orenstein W.A., Lopman B., et al. Indirect benefits are a crucial consideration when evaluating SARS-CoV-2 vaccine candidates. Nat Med. 2021;27:4–5. doi: 10.1038/s41591-020-01172-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pilishvili T. Interim estimates of vaccine effectiveness of Pfizer-Biotech and Moderna covid-19 vaccines among health care personnel — 33 U.S. Sites, January–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70 doi: 10.15585/mmwr.mm7020e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. New England Journal of Medicine. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Early State Vaccination Data Raise . 2021. Warning Flags for Racial Equity. KFF. https://www.kff.org/policy-watch/early-state-vaccination-data-raise-warning-flags-racial-equity/ (accessed March 11, 2021) [Google Scholar]
- 15.CDC. Cases . 2021. Data, and Surveillance. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/serology.html (accessed June 16, 2021) [Google Scholar]