Abstract
The primary aim of this experiment was to critically explore the relationship between the different levels of mixed organic acids (MOA) and growth performance, serum antioxidant status and intestinal health of weaned piglets, as well as to investigate the potential possibility of MOA alternative to antibiotics growth promoters (AGP). A total of 180 healthy piglets (Duroc × [Landrace × Yorkshire]; weighing 7.81 ± 1.51 kg each, weaned at d 28) were randomly divided into 5 treatments: 1) basal diet (CON); 2) CON + chlorinomycin (75 mg/kg) + virginiamycin (15 mg/kg) + guitaromycin (50 mg/kg) (AGP); 3) CON + MOA (3,000 mg/kg) (OA1); 4) CON + MOA (5,000 mg/kg) (OA2); 5) CON + MOA (7,000 mg/kg) (OA3). This study design included 6 replicates per treatment with 6 piglets per pen (barrow:gilt = 1:1) and the experiment was separated into phase 1 (d 1 to 14) and phase 2 (d 15 to 28). In phases 1, 2 and overall, compared with the CON, the feed conversion ratio (FCR) was reduced (P < 0.01) and the average daily gain (ADG) was increased (P < 0.05) in piglets supplemented with AGP, OA1 and OA2. The concentration of serum immunoglobulins G (IgG) was improved (P < 0.05) in piglets supplemented with OA2 in phase 2. In the jejunum and ileum, the villus height:crypt depth ratio was significantly increased (P < 0.01) in piglets fed AGP and OA1. The mRNA expression level of claudin-1 and zonula occludens-1 (ZO-1) (P < 0.01) was up-regulated in piglets supplemented with OA1 and OA2. The piglets fed AGP, OA1 and OA2 showed an increase (P < 0.05) in the content of acetate acid and total volatile fatty acids (TVFA) in the cecum, and butyric acid and TVFA in the colon compared with CON. Also, OA1 lowered (P < 0.05) the content of Lachnospiraceae in piglets. These results demonstrated that MOA at 3,000 or 5,000 mg/kg could be an alternative to antibiotics due to the positive effects on performance, immune parameters, and intestinal health of weaned piglets. However, from the results of the quadratic fitting curve, it is inferred that MOA at a dose of 4,000 mg/kg may produce a better effect.
Keywords: Weaned piglet, Organic acid, Growth performance, Intestinal health
1. Introduction
With new rearing regimes being practiced in modern swine production, the weaning period of piglets has changed from the original 7 – 8 wk to 4 wk. The piglets are faced with stress from nutrition, environment, physiology and other aspects in advance, which has led to increased diarrhea, decreased digestive enzyme ability, and stunted growth of piglets (Heo et al., 2013). Therefore, antibiotics have often been added to the feed of weaned piglets to prevent diarrhea and improve growth performance (Liu et al., 2018). However, the overuse of antibiotics has increased resistance to bacteria, and resulted in residual accumulation of drugs in the body (Suiryanrayna and Ramana 2015). In 2006, the European Union banned the addition of antibiotics to feed, and the United States and China have gradually begun to ban the use of antibiotics. Consequently, safe and green feed additives replacing antibiotics were found to solve the negative effect of antibiotics and also improve performance in pigs.
At present, the commonly used alternative feed additives are plant essential oils, organic acids, antibacterial peptides, prebiotics, enzyme preparations, etc. Organic acids are widely used in livestock production because of their advantages such as lowering the pH value of the gastrointestinal tract, improving the structure of the intestinal microbial flora, immune function and performance (Ahmed et al., 2014; Liu et al., 2014; Yang et al., 2019). Several researchers have demonstrated that the application of a small amount of mixed organic acids (MOA) has had a wide range of effects compared with that of a single organic acid in pigs (Kim et al., 2005; Partanen et al., 2007a).
Currently, there are still differences concerning the optimum concentration of MOA as a substitute to antibiotics on improving performance and intestinal health of weaned piglets, such as blood biochemistry, the abundance of microbiota, the relative mRNA expression of tight junction protein and so on. To better explore the optimum concentration of MOA, we investigated 3 levels of MOA to explore the potential possibility of MOA as an antibiotic substitute for improving the performance and health status of weaned piglets as well as to identify the optimum concentration for MOA in weaned piglets.
2. Materials and methods
All the procedures used in the animal experiment were authorized and approved by the Institutional Animal Care and Use Committee of China Agricultural University (Beijing, China).
2.1. Mixed organic acid product and antibiotic mixture
The commercial products of MOA used in this experiment were sourced from the Netherlands (Nutreco, Amsterdam, Netherlands). The MOA product mainly included formic acid (11%), ammonium formate (13%), propionic acid (10%), acetic acid (5.1%) and citric acid (3.7%) and the carrier was silica. The antibiotic mixture (chlorinomycin, virginiamycin and guitaromycin) was provided by Beijing Tongli XingKe Agricultural Technology Company Limited (Beijing, China).
2.2. Animals, diets and experimental design
A total of 180 healthy piglets (Duroc × [Landrace × Yorkshire]; weighing 7.81 ± 1.51 kg each, weaned at d 28) were randomly divided into 5 treatments: 1) basal diet (CON); 2) CON + chlorinomycin (75 mg/kg) + virginiamycin (15 mg/kg) + guitaromycin (50 mg/kg) (antibiotics growth promoters [AGP]); 3) CON + MOA (3,000 mg/kg) (OA1); 4) CON + MOA (5,000 mg/kg) (OA2); 5) CON + MOA (7,000 mg/kg) (OA3). This study designed 6 replicates per treatment with 6 piglets per pens (barrow:gilt = 1:1) and the experiment was separated into phase 1 (d 1 to 14) and phase 2 (d 15 to 28). The diet formula and the composition of nutrition are presented in Table 1, which refer to the recommendations of the NRC (2012).
Table 1.
Composition and nutritional content of the experimental diets (%, as fed basis).
| Item | Phase 1 (d 1 to 14) | Phase 2 (d 15 to 28) |
|---|---|---|
| Ingredients | ||
| Corn, 8.2% CP | 55.18 | 61.80 |
| Soybean meal, 46% CP | 18.00 | 19.00 |
| Extruded soybean, 35.6% CP | 12.00 | 7.00 |
| Fish meal, 64.7% CP | 4.00 | 2.00 |
| Whey powder, 3.8% CP | 4.00 | 4.00 |
| Soy oil | 3.04 | 2.64 |
| Dicalcium phosphate | 1.00 | 0.81 |
| Limestone | 0.82 | 0.92 |
| Salt | 0.30 | 0.30 |
| l-Lysine HCl, 78% | 0.44 | 0.48 |
| dl-Methionine, 98% | 0.09 | 0.10 |
| l-Threonine, 98% | 0.15 | 0.16 |
| l-Tryptophan | 0.03 | 0.04 |
| Zinc oxide, 85% | 0.20 | 0.00 |
| Chromic oxide | 0.25 | 0.25 |
| Vitamin-mineral premix1 | 0.50 | 0.50 |
| Nutritional levels | ||
| Digestible energy, kcal/kg | 3542 | 3490 |
| Crude protein | 20.07 | 18.04 |
| Calcium | 0.80 | 0.70 |
| Digestible phosphorus | 0.40 | 0.33 |
| Standardized ileal digestible lysine | 1.35 | 1.23 |
| Standardized ileal digestible methionine | 0.39 | 0.36 |
| Standardized ileal digestible threonine | 0.79 | 0.73 |
| Standardized ileal digestible tryptophan | 0.22 | 0.20 |
Premix provided the following per kilogram of feed: vitamin A, 12,000 IU; vitamin D3, 2,500 IU; vitamin E, 30 IU; vitamin K3, 30 mg; vitamin B12, 12 μg; riboflavin, 4 mg; pantothenic acid, 15 mg; niacin, 40 mg; choline chloride, 400 mg; folic acid, 0.7 mg; vitamin B1, 1.5 mg; vitamin B6, 3 mg; biotin, 0.1 mg; Mn, 40 mg; Fe, 90 mg; Zn, 100 mg; Cu, 8.8 mg; I, 0.35 mg; Se, 0.3 mg.
The piglets were housed at the piglet facility which has 1.5 m × 1.5 m experimental pens with a leaky plastic floor, stainless steel adjustable trough, and duckbill waterer in each pen. The temperature was maintained at 25 ± 1 °C with 60% to 70% humidity. Pigsties were disinfected once a month to maintain health and hygiene and to prevent the spread of certain diseases in all pigs. Moreover, to decrease the stress, the feed was transferred step by step for the first 3 d post-weaning based on below procedure: the first day, 75% creep feed +25% nursery feed (trial feed); the second day, 50% creep feed + 50% nursery feed; the third day, 25% creep feed + 75% nursery feed. All experimental piglets had free access to water and feed overall. Feed consumption and diarrhea were recorded daily.
2.3. Experimental procedures and sampling
On d 1, 14 and 28 of the experiment, the piglets and feed were weighed and recorded, respectively before calculated the average daily gain (ADG), average daily feed intake (ADFI), and feed conversion rate (FCR).
Blood samples of 10 mL were collected from 1 piglet of each replicate from all groups through the jugular vein to vacutainer (Becton Dickinson Vacutainer Systems, Franklin Lakes, NJ) on the morning of d 14 and 28. The samples were centrifuged at 3,000 × g at 4 °C for 10 min, and stored at − 20 °C until analysis. The immunoglobulins (IgG, IgM and IgA) and tumor necrosis factor-α (TNF-α) were determined using an ELISA kit (IgG, IgM, IgA and TNF-α quantitation kit; Bethyl Laboratories, Texa). Serum total antioxidant capacity (T-AOC), malondialdehyde (MDA), hydroxyl radical (.OH), glutathione peroxidase (GSH-PX) and superoxide dismutase (SOD) were detected by Spectrophotometry (Leng GuangSFZ1606017568, Shanghai, China) following the kit manufacturer's operating instructions. (Jiancheng Institute of Bioengineering, Nanjing, China).
Digestive juices from the cecum and colon were collected, immersed in liquid nitrogen immediately, and stored at −80 °C for subsequent analysis of volatile fatty acid (VFA). Gently scraping with a sterile scalpel, the mucosal tissue of jejunum, ileum and pancreas was collected, and liquid nitrogen was used to freeze the mucous membrane mixture, which was preserved at −80 °C. The digestive enzymes were analyzed via spectrophotometry (PU 8720 UV/VIS Scanning Spectrophotometer, Pye Unicam, UK) according to the methods of Lee et al. (2003).
At the 28th day of the experiment, one piglet with average BW was selected in each pen to collect aseptic intestinal samples for detection of morphology. The histological specimens were immobilized with 10% neutral buffer formalin. The small intestine was sliced at a 6-μm thickness, dehydrated, paraffin-embedded, and cut into 4 cross-sections, stained with hematoxylin and eosin. Then random measurements were taken of the heights of 10 oriented villi and their crypts on small covers by optical microscope using an eyepiece scale.
On the 28th day of the experiment, the 1-cm jejunum of the three piglets with the medium weight of each treatment was taken, frozen and washed with cold phosphate-buffered saline (PBS), persevered at −80 °C till protein mRNA extraction, and the mRNA expressions of tight junction protein were detected by RT-PCR. Sample RNA was extracted with Trizol, and 20-μL RNase-free water was added to dissolve the RNA according to the protocol of the supplier. The cDNA was synthesized by 1 μg total RNA treated with RNase-free DNase I, in which the main reaction contained 1-μL oligo (DT) 18, 1-μg total RNA, 0.5-μL recombinant RNase inhibitor, 4 μL of 5 × Reverse Transcriptase M-MLV Buffer, 1-μL deoxy-ribonucleoside triphosphate (dNTP) mixture, and 1-μL Reverse Transcriptase M-MLV. This was quantified by measuring the absorbance at 260 and 280 nm. The comparative cycle threshold was compared with the level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) to compute the relative mRNA expression of tight junction protein. Table 2 shows the primer sequences of GAPDH and the tight junction protein.
Table 2.
The primer sequences of GAPDH and claudin-1, occludin and ZO-1.
| Name | Sequences (5′ to 3′) | Length, bp |
|---|---|---|
| GAPDH | CTGCCGCCTGGAGAAACCT | 226 |
| GCTGTAGCCAAATTCATTGTCG | ||
| Claudin-1 | CAAAACCTTCGCCTTCCAG TCCCCACATTCGAGATGATTAC |
293 |
| Occludin | ATGCTTTCTCAGCCAGCGTA AAGGTTCCATAGCCTCGGTC |
176 |
| ZO-1 | GAGGATGGTCACACCGTGGT GGAGGATGCTGTTGTCTCGG |
169 |
GAPDH = glyceraldehyde-3-phosphate dehydrogenase; ZO-1 = zonula occludens-1.
On the 28th day of the experiment, VFA analysis was performed on fresh chyme samples collected from the cecum, colon, and rectum. Approximately 1.5-g sample was mixed with 1.5-mL sterile water and placed in the centrifuge tube before centrifuge at 15,000 × g at 4 °C for 10 min. This was later transferred to a sample bottle of a gas chromatograph, and 200 μL metaphosphoric acid was added. The bottle was immersed in ice for 30 min, centrifuge at 4 °C at 15,000 × g for 10 min. The contents of VFA were tested using Hewlett Packard 5890 gas chromatography (HP, Pennsylvania) (Pan et al., 2016).
On the 28th day of the experiment, fresh and clean cecal chyme samples of 3 piglets with the best growth performance in each treatment were collected and stored at −80 °C. Total bacterial genomic DNA from cecal samples was extracted by E.Z.N.A. Stool DNA kit. (Omega BioTek, Norcross, USA) and total DNA was detected by Thermo NanoDrop 2000 ultraviolet microspectrophotometer (USA) and 1% agarose gel electrophoresis. The extracted DNA was used as a template to amplify the variable region of 16s rDNA V3~V4 by PCR. The primer sequence: 338F 5'-ACTCCTACGGGAGCAGCACAGCACAMUM-3', 806R 5'-GGACTACHVGGGTWTCTAAT-3'. The specific primers were designed by adding the index sequence and junction sequence suitable for HiSeq2500PE250 sequencing and were purified by a QIAquick PCR purification kit. The quality of the quantitative library was checked by a Qubit 2.0 fluorometer (Thermo Fisher Scientific, USA) to achieve a uniform concentration. Then the paired-end reading of 250 bp was obtained by sequencing on the Illumina HiSeq PE250 platform (Illumina, San Diego, USA) according to the standard protocol. The Paired-end reads were assembled into longer tags, and the quality was filtered to remove the tags with length <220 nucleotide (nt) and average quality score < 20, as well as the tags containing >3 ambiguous bases used by PANDAseq software (version 2.9) to clean the reads. All clean reads were compared with the operational taxonomy unit (OTU) sequence to acquire the final mapped reads (Edgar 2013). After discarding the singleton, the high-quality tags were clustered into OTU for species classification by using the Usearch (version 7.0.1090) in Quantitative Insights Into Microbial Ecology (QIIME) pipeline software (version 1.8.0) with a similarity threshold of 97% and the OTU were further taxonomically analyzed using the Ribosomal Database Project (RDP) database, using the RDP classifier at an 0.80 confidence level. Alpha diversity and beta diversity were tested by QIIME (University of California, San Diego, USA). Linear discriminant analysis (LDA) and effect size (LEfSe) analyses were operated via the LEfSe tool (Segata et al., 2011).
2.4. Statistical analysis
SAS statistical software (SAS version 9.2, SAS Institute Inc., Cary, NC, USA) was used for data analysis with each pen of pigs considered as an experimental unit, and measurement data were expressed as standard errors of the least squares mean (SEM). The treatments were considered as fixed effects and the piglets as a random effect. The optimal requirement of MOA was evaluated for performance of piglets by using the quadratic curve model in nonlinear regression (NLIN): y = a × x2 + b × x + c, maximum = -b/(2 × a), where y is ADG or FCR; x is concentration of MOA (Robbins et al., 2006). The differences and composition of bacterial abundance in cecal samples were analyzed by Kruskal–Wallis rank-sum test. P ≤ 0.05 was considered statistically significant different; when 0.05 < P < 0.10, the trends were noted.
3. Results
3.1. Growth performance, diarrhea rate and the optimum dosage of MOA
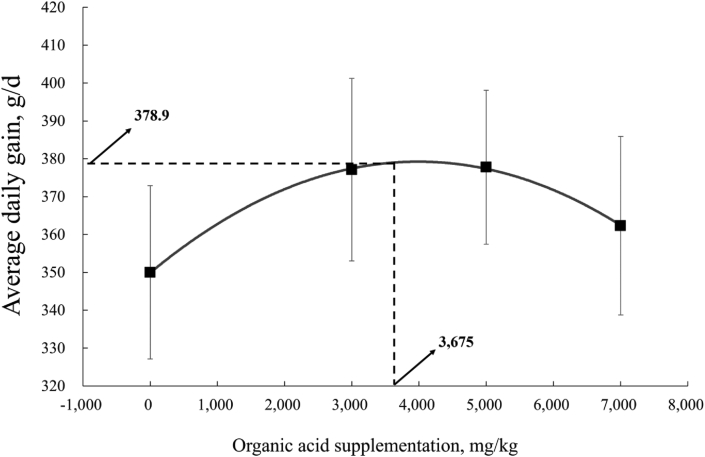
In phase 1 (d 1 to 14), compared with the CON, the piglets supplemented with AGP could improve (P < 0.05) the ADG (Table 3), piglets fed OA1, AGP had decreased (P < 0.05) the FCR, and the diarrhea rate of piglets fed OA2 was lower (P < 0.05). The fitted quadratic indicated that the supplementation level of MOA for the maximal ADG of weaned piglets in phase 1 was 3,675 mg/kg (Fig. 1).
Table 3.
Growth performance of piglets as affected by dietary mixed organic acids (MOA) and antibiotic growth promoter (AGP) supplementation.1
| Item | Dietary treatments2 |
SEM | P-value | ||||
|---|---|---|---|---|---|---|---|
| CON | AGP | OA1 | OA2 | OA3 | |||
| d 1 to 14 | |||||||
| ADG, g | 350b | 391a | 377ab | 378ab | 362ab | 7.73 | 0.02 |
| ADFI, g | 631 | 612 | 612 | 613 | 595 | 18.06 | 0.75 |
| FCR, g/g | 1.80a | 1.56b | 1.61b | 1.63ab | 1.64ab | 0.04 | <0.01 |
| Diarrhea rate, % | 5.36a | 3.21ab | 2.50ab | 1.07b | 2.86ab | 0.81 | 0.03 |
| d 15 to 28 | |||||||
| ADG, g | 532c | 602a | 583ab | 555bc | 528c | 8.38 | <0.01 |
| ADFI, g | 954 | 910 | 901 | 873 | 924 | 21.11 | 0.14 |
| FCR, g/g | 1.79a | 1.51c | 1.55bc | 1.58bc | 1.75ab | 0.05 | <0.01 |
| d 1 to 28 | |||||||
| ADG, g | 441d | 497a | 480ab | 466c | 445cd | 4.90 | <0.01 |
| ADFI, g | 792 | 761 | 756 | 743 | 760 | 15.96 | 0.31 |
| FCR, g/g | 1.79a | 1.53c | 1.57bc | 1.60bc | 1.70ab | 0.03 | <0.01 |
ADG = average daily gain; ADFI = average daily feed intake; FCR = feed conversion ratio.
a, b, c, d Different letters within a row mean a statistical difference (P < 0.05).
No piglets developed diarrhea during d 15 to d 28.
CON: control; AGP: chlorinomycin at 75 mg/kg + virginiamycin at 15 mg/kg + guitaromycin at 50 mg/kg; OA1: MOA at 3,000 mg/kg; OA2: MOA at 5,000 mg/kg; OA3: MOA at 7,000 mg/kg.
Fig. 1.
Fitted quadratic of ADG of piglets given diets with different supplemental levels of mixed organic acids (MOA) during phase 1. The formula of forecasting broken line: y = −2 × 10 - 6 x2 + 0.0147 × x + 351.9, R2 = 0.49. The fitted quadratic showed the breakpoint as 3,675 mg/kg, indicating that the maximal ADG (378.9 g/d) could be obtained by supplementing MOA at 3,675 mg/kg (P < 0.05).
In phase 2 (d 15 to 28), compared with the CON, the piglets supplemented with OA1 and AGP increased (P < 0.05) the ADG, and FCR was reduced (P < 0.05) in piglets fed AGP, OA1 and OA2. In the entire experimental period, piglets fed AGP, OA1 and OA2 increased the ADG and reduced (P < 0.05) the FCR. Additionally, no piglets developed diarrhea in phase 2.
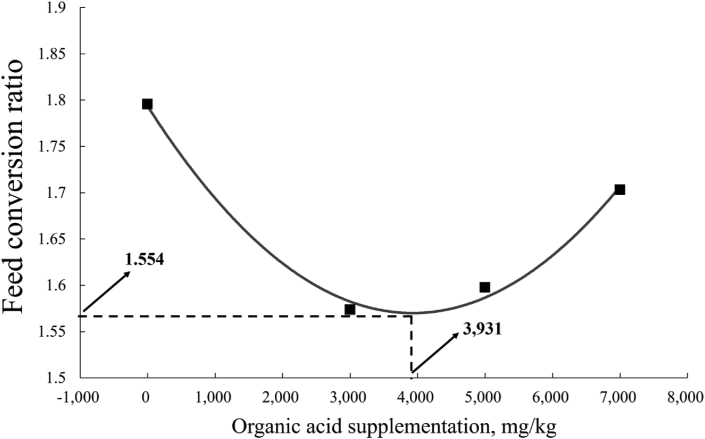
In the entire experimental period, compared with the CON, piglets fed AGP, OA1 and OA2 increased (P < 0.05) ADG by 12.7%, 8.8%, and 5.7%, respectively. Further, piglets supplemented with AGP, OA1 and OA2 lowered FCR by 14.5%, 12.3%, and 10.6% (P < 0.05) respectively. The fitted quadratic indicated that supplementation level of MOA for the optimum FCR of weaned piglets was 3,931 mg/kg during the entire study period (Fig. 2).
Fig. 2.
Fitted quadratic of FCR of piglets given diets with different supplemental levels of mixed organic acids (MOA) overall (d 1 to 28). The formula of forecasting broken line: y = 1.45 × 10−8x2 + 1.14 × 10−4x +1.793, R2 = 0.53 and the fitted quadratic analysis showed the breakpoint as 3,931 mg/kg, indicating that the optimal FCR could be obtained by supplementing MOA at 3,931 mg/kg (P < 0.05).
3.2. Digestive enzyme activity
There was no effect of dietary supplementation of organic acids or AGP on digestive enzyme activity compared with the CON (Table 4), but the piglets fed OA1 tended to promote the digestive enzyme activity of pancreas in weaned piglets.
Table 4.
Digestive enzyme activity of piglets as affected by dietary mixed organic acids (MOA) and antibiotic growth promoter (AGP) supplementation (U/L).
| Item | Dietary treatments1 |
SEM | P-value | ||||
|---|---|---|---|---|---|---|---|
| CON | AGP | OA1 | OA2 | OA3 | |||
| Jejunum | |||||||
| Trypsin | 21.10 | 21.53 | 19.25 | 20.26 | 16.19 | 1.61 | 0.23 |
| Lipase | 36.55 | 36.68 | 34.10 | 40.87 | 32.11 | 3.43 | 0.45 |
| Amylase | 67.26 | 72.07 | 63.54 | 64.52 | 55.71 | 5.99 | 0.46 |
| Ileum | |||||||
| Trypsin | 20.44 | 19.05 | 17.05 | 19.38 | 23.11 | 1.50 | 0.16 |
| Lipase | 38.53 | 37.24 | 33.33 | 38.78 | 50.20 | 4.89 | 0.25 |
| Amylase | 71.54 | 65.57 | 56.38 | 63.21 | 79.25 | 7.18 | 0.30 |
| Pancreas | |||||||
| Trypsin | 15.48ab | 17.88ab | 21.02a | 18.80ab | 12.74b | 1.31 | 0.02 |
| Lipase | 32.31ab | 35.19ab | 42.94a | 37.51ab | 26.08b | 2.44 | 0.01 |
| Amylase | 52.44ab | 57.19ab | 66.89a | 55.44ab | 43.99b | 4.07 | 0.04 |
a, b Different letters within a row mean a statistical difference (P < 0.05).
CON: control; AGP: chlorinomycin at 75 mg/kg + virginiamycin at 15 mg/kg + guitaromycin at 50 mg/kg; OA1: MOA at 3,000 mg/kg; OA2: MOA at 5,000 mg/kg; OA3: MOA at 7,000 mg/kg.
3.3. Immune parameters in serum
In phase 1, compared with the CON, the content of GSH-Px was increased (P < 0.05) in piglets fed OA2 and OA3 (Table 5). In phase 2, the dietary supplementation of OA2 showed higher concentrations of IgG compared with the CON.
Table 5.
Serum biochemical index of weaned piglets as affected by dietary mixed organic acids (MOA) and antibiotic growth promoter (AGP) supplementation.
| Item | Dietary treatments1 |
SEM | P-value | ||||
|---|---|---|---|---|---|---|---|
| CON | AGP | OA1 | OA2 | OA3 | |||
| d 1 to 14 | |||||||
| IgG, μg/mL | 13.18 | 12.49 | 11.59 | 8.95 | 12.73 | 2.11 | 0.64 |
| IgA, μg/mL | 28.11 | 34.25 | 27.90 | 23.20 | 32.04 | 4.01 | 0.38 |
| IgM, μg/mL | 14.00 | 17.00 | 13.93 | 10.78 | 14.95 | 2.18 | 0.41 |
| ·OH, ng/mL | 2.87 | 2.30 | 2.36 | 2.23 | 2.16 | 0.33 | 0.56 |
| T-AOC, U/mL | 13.10 | 14.23 | 13.57 | 13.26 | 13.73 | 0.97 | 0.93 |
| GSH-Px, μmol/L | 13.00c | 13.25bc | 13.15c | 15.48ab | 15.62a | 0.52 | <0.01 |
| MDA, nmol/mL | 1.16 | 1.10 | 1.21 | 1.28 | 1.17 | 0.08 | 0.61 |
| SOD, U/mL | 45.71 | 47.53 | 44.10 | 45.27 | 45.76 | 0.91 | 0.17 |
| TNF-α, ng/L | 97.37 | 90.04 | 90.90 | 75.84 | 101.83 | 13.31 | 0.70 |
| d 15 to 28 | |||||||
| IgG, μg/mL | 5.26b | 8.00ab | 8.14ab | 10.07a | 8.69ab | 0.93 | 0.03 |
| IgA, μg/mL | 19.88 | 24.05 | 19.64 | 26.27 | 21.16 | 2.99 | 0.47 |
| IgM, μg/mL | 9.47 | 11.94 | 10.17 | 11.52 | 11.28 | 1.21 | 0.60 |
| ·OH, ng/mL | 2.32 | 2.73 | 2.17 | 2.19 | 2.43 | 0.20 | 0.31 |
| T-AOC, U/mL | 13.35 | 14.28 | 13.63 | 14.82 | 13.73 | 0.64 | 0.53 |
| GSH-Px, μmol/L | 16.44 | 16.70 | 15.91 | 15.16 | 16.79 | 0.83 | 0.23 |
| MDA, nmol/mL | 1.06 | 1.18 | 1.09 | 1.11 | 1.11 | 0.05 | 0.44 |
| SOD, U/mL | 44.64 | 45.64 | 44.92 | 44.44 | 43.85 | 0.83 | 0.65 |
| TNF-α, ng/L | 66.96 | 82.96 | 65.19 | 88.88 | 82.39 | 8.13 | 0.20 |
a, b, c Different letters within a row mean a statistical difference (P < 0.05).
CON: control; AGP: chlorinomycin at 75 mg/kg + virginiamycin at 15 mg/kg + guitaromycin at 50 mg/kg; OA1: MOA at 3,000 mg/kg; OA2: MOA at 5,000 mg/kg; OA3: MOA at 7,000 mg/kg.
3.4. Intestinal morphology
In the duodenum, the crypt depth of the piglets supplemented with AGP and OA1 was decreased (P < 0.01) compared with those fed OA2 and OA3 (Table 6). Also, the villus height:crypt depth ratio was improved (P < 0.01) for the piglets fed AGP and OA1 compared to the piglets fed OA2 and OA3.
Table 6.
Intestinal morphology of weaned piglets as affected by dietary mixed organic acids (MOA) and antibiotic growth promoter (AGP) supplementation.
| Item | Dietary treatments1 |
SEM | P-value | ||||
|---|---|---|---|---|---|---|---|
| CON | AGP | OA1 | OA2 | OA3 | |||
| Duodenum | |||||||
| Villus height, μm | 302.56 | 301.65 | 300.41 | 306.56 | 292.20 | 3.25 | 0.11 |
| Crypt depth, μm | 204.89ab | 194.61b | 187.95b | 222.59a | 216.83a | 4.33 | <0.01 |
| Villus height:crypt depth ratio | 1.48ab | 1.55a | 1.60a | 1.38bc | 1.35bc | 0.03 | <0.01 |
| Jejunum | |||||||
| Villus height, μm | 283.01b | 311.15ab | 347.42a | 326.06ab | 275.2b | 12.07 | 0.01 |
| Crypt depth, μm | 198.69 | 191.48 | 183.32 | 198.69 | 189.21 | 7.89 | 0.72 |
| Villus height:crypt depth ratio | 1.46d | 1.62bc | 1.90a | 1.64b | 1.45d | 0.03 | <0.01 |
| Ileum | |||||||
| Villus height, μm | 249.02 | 223.40 | 259.09 | 242.16 | 218.26 | 15.08 | 0.35 |
| Crypt depth, μm | 183.69 | 149.69 | 160.65 | 165.81 | 163.30 | 10.25 | 0.31 |
| Villus height:crypt depth ratio | 1.36cd | 1.49b | 1.61a | 1.46bc | 1.34d | 0.02 | <0.01 |
a, b, c, d Different letters within a row mean a statistical difference (P < 0.05).
CON: control; AGP: chlorinomycin at 75 mg/kg + virginiamycin at 15 mg/kg + guitaromycin at 50 mg/kg; OA1: MOA at 3,000 mg/kg; OA2: MOA at 5,000 mg/kg; OA3: MOA at 7,000 mg/kg.
In the jejunum, compared with the CON, the villus height of piglets fed OA1 was increased (P < 0.05). Also, the villus height:crypt depth ratios in piglets fed AGP, OA1 and OA2 were improved (P < 0.01).
In the ileum, compared with the CON, the villus height:crypt depth ratio in piglets supplemented with AGP and OA2 was improved (P < 0.05).
3.5. Relative mRNA expression of tight junction protein
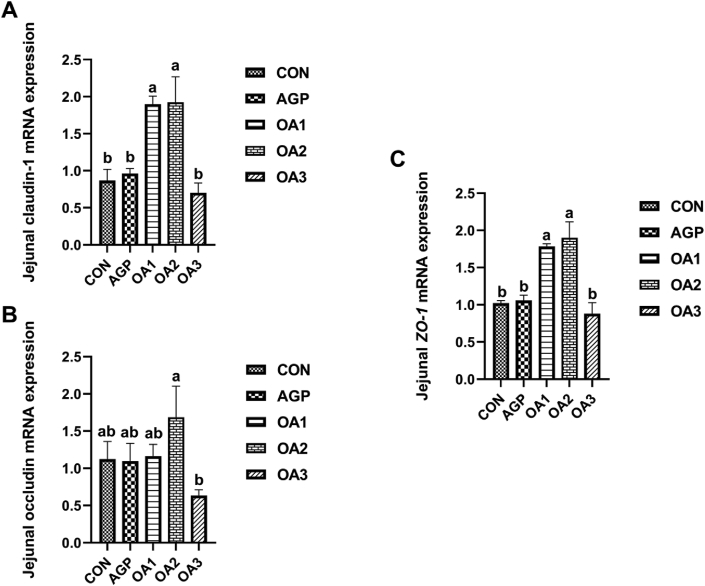
Compared with the CON, the relative mRNA expression of claudin-1 and ZO-1 were up-regulated (P < 0.05) in the jejunum of piglets supplemented with OA1 and OA2 (Fig. 3).
Fig. 3.
The relative mRNA expression of claudin-1 (A), occludin (B), and ZO-1 (C) in the jejunum of piglets as affected by dietary mixed organic acids (MOA) and antibiotic growth promoter (AGP) supplementation (n = 3). CON: control; AGP: chlorinomycin at 75 mg/kg + virginiamycin at 15 mg/kg + guitaromycin at 50 mg/kg; OA1: MOA at 3,000 mg/kg; OA2: MOA at 5,000 mg/kg; OA3: MOA at 7,000 mg/kg. Data are expressed as means ± SEM. a, b Different letters mean a statistical difference (P < 0.05).
3.6. Volatile fatty acids
In the cecum, compared with the CON, the content of acetate acid and total volatile fatty acids (TVFA) were enhanced (P < 0.01) in piglets fed AGP, OA1 and OA2 (Table 7). Also, the piglets supplemented with OA1 and OA2 showed an enhanced (P < 0.05) content of propionic acid.
Table 7.
Volatile fatty acids in the cecum, colon and rectum of piglets as affected by dietary mixed organic acids (MOA) and antibiotic growth promoter (AGP) supplementation (mg/g).
| Item | Dietary treatments1 |
SEM | P-value | ||||
|---|---|---|---|---|---|---|---|
| CON | AGP | OA1 | OA2 | OA3 | |||
| Cecum | |||||||
| Acetic acid | 4.37c | 4.84b | 5.62a | 5.51a | 4.28c | 0.09 | <0.01 |
| Propionic acid | 2.60b | 3.00ab | 3.58a | 3.44a | 3.01ab | 0.14 | 0.01 |
| Butyric acid | 0.81 | 0.96 | 1.31 | 1.46 | 1.08 | 0.14 | 0.07 |
| Valeric acid | 0.10 | 0.13 | 0.19 | 0.21 | 0.16 | 0.05 | 0.58 |
| Total volatile fatty acid | 7.91c | 8.95b | 10.71a | 10.63a | 8.54bc | 0.20 | <0.01 |
| Colon | |||||||
| Acetic acid | 5.60 | 5.68 | 6.46 | 6.19 | 5.68 | 0.19 | 0.05 |
| Propionic acid | 2.83 | 3.00 | 3.67 | 2.96 | 2.87 | 0.23 | 0.15 |
| Butyric acid | 1.00b | 1.12b | 1.65a | 1.45ab | 1.37ab | 0.10 | 0.01 |
| Valeric acid | 0.20 | 0.25 | 0.39 | 0.32 | 0.21 | 0.04 | 0.05 |
| Total volatile fatty acid | 9.70b | 10.11b | 12.28a | 11.00ab | 10.15ab | 0.44 | 0.02 |
| Rectum | |||||||
| Acetic acid | 4.62 | 4.64 | 5.16 | 4.77 | 4.61 | 0.31 | 0.69 |
| Propionic acid | 1.62 | 1.79 | 2.01 | 1.77 | 1.87 | 0.15 | 0.50 |
| Butyric acid | 0.62 | 0.67 | 0.70 | 0.70 | 0.68 | 0.08 | 0.95 |
| Valeric acid | 0.08 | 0.09 | 0.16 | 0.13 | 0.14 | 0.03 | 0.36 |
| Total volatile fatty acid | 7.52 | 7.80 | 8.85 | 8.04 | 7.92 | 0.37 | 0.22 |
a, b, c Different letters within a row mean a statistical difference (P < 0.05).
CON: control; AGP: chlorinomycin at 75 mg/kg + virginiamycin at 15 mg/kg + guitaromycin at 50 mg/kg; OA1: MOA at 3,000 mg/kg; OA2: MOA at 5,000 mg/kg; OA3: MOA at 7,000 mg/kg.
In the colon, compared with the CON, OA1 enhanced (P < 0.05) the content of butyrate acid and TVFA of piglets.
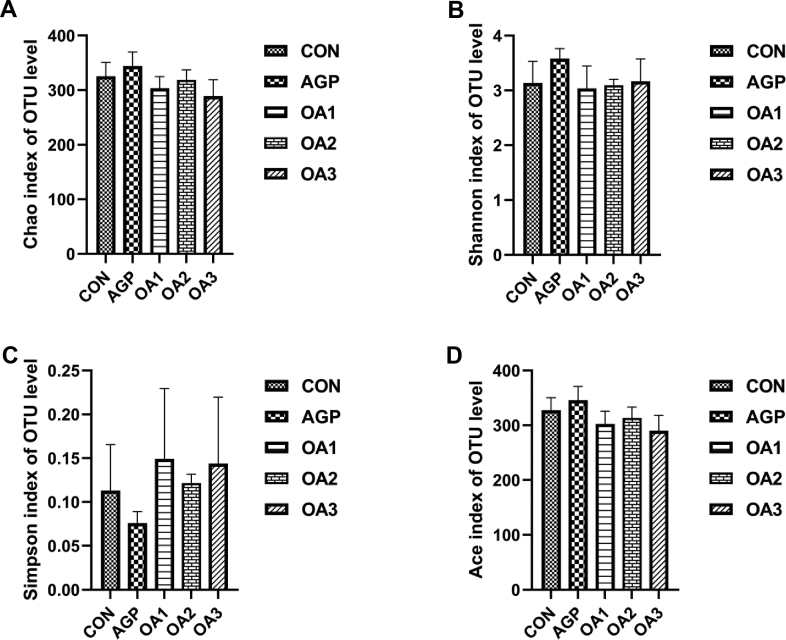
3.7. Cecal microbiota
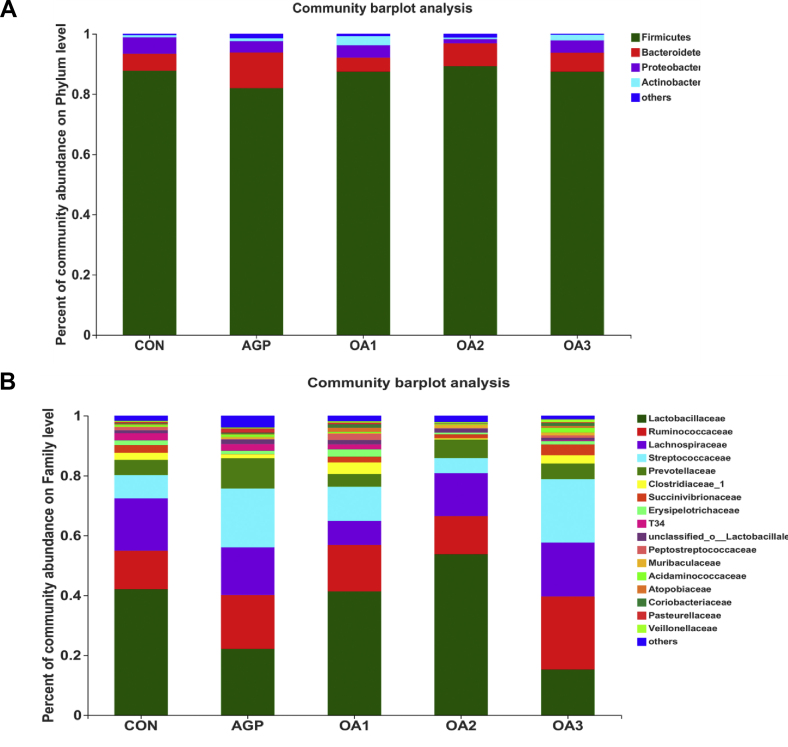
There was no significant effect on α-diversity (Fig. 4). At the phylum level, the cecum contents of the piglets were mainly composed of Firmicutes, Bacteroidetes, Proteobacteria and Actinobacteria, which accounted for more than 98.5% of the total cecal flora. The proportions of Firmicutes in the OA1 group and the AGP group were 87.38% and 81.96%, respectively, which were lower than the CON (87.73%) (Fig. 5A, Fig. 6A).
Fig. 4.
The α-diversity of cecal microflora in piglets as affected by dietary mixed organic acids (MOA) and antibiotic growth promoter (AGP) supplementation: (A) Chao index. (B) Shannon index. (C) Simpson index. (D) Ace index (n = 3). CON: control; AGP: chlorinomycin at 75 mg/kg + virginiamycin at 15 mg/kg + guitaromycin at 50 mg/kg; OA1: MOA at 3,000 mg/kg; OA2: MOA at 5,000 mg/kg; OA3: MOA at 7,000 mg/kg. The results were tested by Kruskal–Wallis H test and were expressed as mean values of different bacteria. OTU = operational taxonomic unit.
Fig. 5.
Microbial community in cecal samples of piglets as affected by dietary mixed organic acids (MOA) and antibiotic growth promoter (AGP) supplementation. Microbial community barplot at (A) the phylum level and (B) the family level (n = 3). CON: control; AGP: chlorinomycin at 75 mg/kg + virginiamycin at 15 mg/kg + guitaromycin at 50 mg/kg; n = 3. OA1: MOA at 3,000 mg/kg; OA2: MOA at 5,000 mg/kg; OA3: MOA at 7,000 mg/kg. Results were expressed as mean values of different bacteria. Different colors indicated the bacteria abundance of each treatment.
Fig. 6.
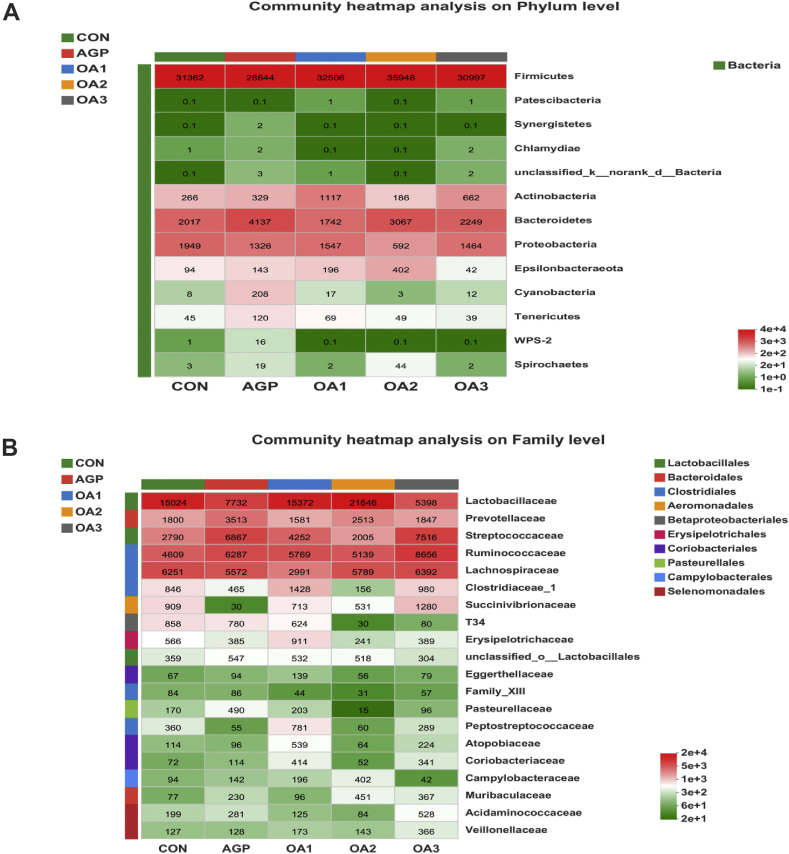
Microbial community in cecal sample of piglets as affected by dietary mixed organic acids (MOA) and antibiotic growth promoter (AGP) supplementation: (A) heatmap at the phylum level, (B) heatmap at the family level (n = 3). CON: control; AGP: chlorinomycin at 75 mg/kg + virginiamycin at 15 mg/kg + guitaromycin at 50 mg/kg; OA1: MOA at 3,000 mg/kg; OA2: MOA at 5,000 mg/kg; OA3: MOA at 7,000 mg/kg. Results were expressed as mean values of different bacteria. Different colors indicated the bacteria abundance of each treatment.
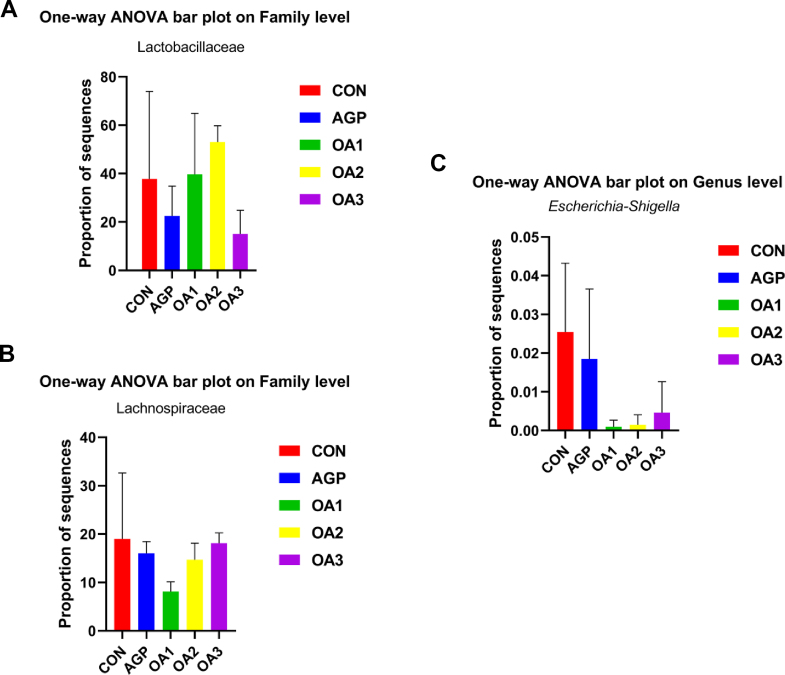
At the family level (Figs. 5B and 6B), Lactobacillaceae, Ruminococcaceae, Lachnospiraceae and Streptococcaceae were the major Firmicutes in the cecum contents of weaned piglets. Bacteroidetes were mainly composed of Prevotellaceae. Most of the cecum contents of the CON were Lactobacillaceae (42.03%), Ruminococcaceae (12.89%) and Lachnospiraceae (17.49%). Most of the cecum contents in piglets supplemented with AGP were Lactobacillaceae (22.12%), Ruminococcaceae (17.99%) Lachnospiraceae (15.94%), Streptococcaceae (19.65%) and Prevotellaceae (10.05%). The relative abundances of the cecum of the OA1 group were mainly composed of Lactobacillaceae (41.32%), Ruminococcaceae (15.51%) and Streptococcaceae (11.43%). The relative abundances of main flora in the cecum of piglets supplemented with OA2 were Lactobacillaceae (53.72%), Ruminococcaceae (12.75%) and Lachnospiraceae (14.37%). The relative abundances of the cecum of the OA3 group were mainly composed of Lactobacillaceae (15.22%), Ruminococcaceae (24.40%), Lachnospiraceae (18.02%) and Streptococcaceae (21.19%). The content of Lactobacillaceae in the cecum of piglets fed OA2 was increased (P < 0.05) compared with those fed AGP and OA3. Compared with the CON, the content of Lachnospiraceae in the cecum of piglets fed OA1 was declined (P < 0.05). Furthermore, piglets fed OA1 and OA2 had a tendency to lower (P = 0.07) the relative abundance of Escherichia-Shigella (Fig. 7).
Fig. 7.
Relative abundance of (A) Lactobacillaceae, (B) Lachnospiraceae, and (C) Escherichia-Shigella in piglets as affected by dietary mixed organic acids (MOA) and antibiotic growth promoter (AGP) supplementation (n = 3). CON: control; AGP: chlorinomycin at 75 mg/kg + virginiamycin at 15 mg/kg + guitaromycin at 50 mg/kg; OA1: MOA at 3,000 mg/kg; OA2: MOA at 5,000 mg/kg; OA3: MOA at 7,000 mg/kg. Data are indicated as means ± SEM.
4. Discussion
The application of organic acids in swine production was suggested in a review article published by Suiryanrayna and Ramana (2015). Many studies have proved the utility of MOA as a potential alternative to antibiotics in developing performance and enhancing microbial flora in pigs (Grecco et al., 2018; Li et al., 2019). However, no systematic research has been dedicated to the optimum concentration of MOA in the feed. In this research, MOA was supplemented at 3 levels based on the previous findings of Long et al. (2017), who demonstrated that piglets supplemented with MOA at 3,000 mg/kg could show improved the growth performance, with enhanced immune function of weaned piglets. The objective of this research was to find out the optimum dosage of MOA and further explore the effect of MOA on digestive enzyme activity, expression of tight junction protein and intestinal microorganisms.
In our research, piglets fed MOA at 3,000 and 5,000 mg/kg as well as dietary supplementation of AGP seemed to improve ADG and FCR during the entire experiment, which was similar to the results of Long et al. (2017) and Wang et al. (2016) who revealed that piglets fed MOA at 3,000 and 5,000 mg/kg respectively could show improved the growth performance, with a positive effect on preventing diarrhea in weaned piglets. But our results showed that the piglets fed MOA at 7,000 mg/kg did not have a positive effect on growth performance of piglets. This might be caused by the high concentration of MOA reducing the pH of feed, which may have affected the appetite for the feed of weaned piglets. Moreover, the main component of the MOA product is formic acid, which is highly irritating, and the piglets have poor palatability to formic acid, which also explains the current finding of ADFI in piglets. For suckling and nursing piglets (before and after weaning), due to their imperfect gastrointestinal development, insufficient gastric acid secretion, and poor digestion of solid feed, it is easy to cause nutritional diarrhea in piglets; the main purpose of adding acid at this stage is to make up for the lack of gastric acid secretion of young piglets. Therefore, the amount of acid addition should be determined according to the physiological characteristics of piglets and the acid power of feed (dietary type).
The improvement of growth performance is closely related to digestive enzyme activity. The lack of gastric acid secretion in early-weaned piglets resulted in the acidity of a gastrointestinal tract not reaching the range of pH required by various digestive enzymes. The findings show that different enzymes have a different optimal acid-base environment, and the optimal pH range of pepsin is from 2.0 to 3.5 (Tian et al., 2016). The intervention of MOA can maintain the acid environment in the stomach, activate the pepsin and promote the secretion of trypsin, improve the digestion of diet and accelerate the decomposition of protein (Partanen et al., 2007b; Xu et al., 2018). The present study indicated that there was a tendency to increase the activity of pancreatic lipase, trypsin and amylase in piglets fed MOA at 3,000 and 5,000 mg/kg compared with the CON, which is similar to the findings of Kasprowicz-Potocka et al. (2009). Similarly, Diao et al. (2016) also revealed that the piglets which were fed benzoic acid at 5,000 mg/kg could show reduced pH value of jejunal digestion and increased activities of trypsin, lipase, amylase. It was indicated that the organic acids increased the activity of digestive enzymes of piglets via lowering the pH of the gastrointestinal tract.
The weak immunity of piglets in early weaning is mainly because when under weaning stress, free radicals are increased which can lead to protein denaturation, degradation, and loss of function by free radicals attacking proteins such as SH-containing protein, K+-ATPase, etc (Buchet et al., 2017). Glutathione peroxidase (GSH-Px) is an important peroxide-degrading enzyme which can catalyze the conversion of GSH to glutathione (oxidized), reducing toxic peroxides to non-toxic hydroxyl compounds and promoting the decomposition of H2O2, thus protecting the structure and function of the cell membrane from interference and damage by peroxides (Alverdy 1990). In our research, GSH-Px activity increased with increasing dietary MOA concentrations in phase 1. The mechanism may be that shortly after weaning, the consumption of GSH increased to eliminate a large number of free radicals produced by stress, and some components of MOA (citric acid) participated in the cycle of tricarboxylic acid, accelerated the production of α-ketoglutarate, and converted into glutamic acid. Therefore, MOA can effectively prevent the GSH synthesis obstacle caused by the lack of precursor materials, improve the feedback regulation of GSH-Px synthesis, promote the increase of GSH-Px enzyme activity, and accelerate to remove free radicals, which was demonstrated by Tezcan et al. (2009), who found that citric acid has strong antioxidant activities. Also, the activities of GSH-Px decreased at 15 to 28 d indicated that there were more free radicals and active antioxidant system in the early stage of weaning stress. Still, the adaptability of piglets to stress became stronger with the increase in age, resulting in a decrease of stress intensity and the activity of antioxidant enzymes. IgA, IgG and IgM can specifically bind to corresponding antigens and are the main substances of humoral immunity. The content of immunoglobulin in serum can measure the immune function of the body. In a previous study, organic acids were shown to improve the immune system of piglets, especially by increasing serum concentration of IgG (Ahmed et al., 2014). In our research, the piglets fed MOA tended to increase the content of IgA, IgM and IgG compared with the CON in phase 2, which is in agreement with Yang et al. (2019), who revealed that piglets which received diets supplemented with MOA increased the concentration of IgM and IgG. Ren et al. (2020) indicated that piglets fed 1% mixture of formic acid and propionic acid reduced the inflammatory response in ETEC K88 attacked weaned piglets, improved immune response and reduced fever and diarrhea. Allaire et al. (2018) also reported that there is a correlation between immunity and the number of intestinal epithelial cells. Therefore, the improvement of immune performance by MOA in piglets was probably related to the improvement of intestinal flora and intestinal morphology.
Intestinal morphology is one of the indexes used to evaluate intestinal digestive performance (Jha et al., 2019). The villus length represents the absorption capacity of nutrients in livestock and poultry. The longer the villi, the more epithelial cells and lymphocytes, and the stronger the immune response. The depth of the crypts represents the rate of cell formation. The shallower the crypt, the better the cell maturity and the higher the secretory function. The higher the villus height is, the greater the villus height:crypt depth ratio, also the larger the total villus area, the more sufficient the digestive enzyme development is, resulting in higher digestibility (Van, Leeuwen et al., 1996). Studies have shown that weaning stress can reduce the height of villi, while villi atrophy is the result of an increase in programmed cell death and a decrease in cell renewal rate (Hampson and Kidder, 1986). In our research, the piglets fed MOA at 3,000 mg/kg showed a positive effect on villus height and villus height:crypt depth ratio in the duodenum, jejunum and ileum. Diao et al. (2014) revealed that piglets fed benzoic acid at 5,000 mg/kg demonstrated improved villus height:crypt depth ratio in the duodenum, jejunum and ileum on day 14 and 42, and thus enhanced the nutrient absorption of piglets. Long et al. (2017) indicated that piglets fed MOA could significantly lower the crypt depth in the jejunum, improve the ratio of villus height and crypt depth in jejunum and ileum of piglets. Previous research has revealed that the improvement of the acidic environment (Lupton and Jacobs, 1987) and the increase of volatile fatty acid concentration both contribute to the increase of intestinal morphology (Karlsson et al., 2013). Therefore, MOA may help to improve the morphology of the intestinal tract and can alleviate intestinal stress in piglets.
Tight junction (TJ) proteins play a vital role in maintaining the difference of substances on both sides of epithelial cells and maintaining cell polarity which mainly contains claudin, occludin, and zonula occludens (ZO) proteins, that take a systematic way to modulate TJ function (Yin et al., 2015; Roxas et al., 2010). Bacteria, viruses, and endotoxins can regulate the expression of transmembrane protein occludin, claudin and ZO-1 via regulating or affecting some cytokines and protein kinase C, and reduce the barrier function of intestinal epithelial cells (Weiler et al., 2005; Zhang et al., 2010). Hu et al. (2013) indicated that early-weaning could hamper intestinal barrier function by destroying the intestinal structure, altering the expression of TJ proteins, and increasing the intestinal permeability, activating mitogen-activated protein kinases in pigs, which may be attributed to altering expression of TJ proteins in intestinal epithelial cells. The results in the present research indicated that piglets fed MOA at 3,000 and 5,000 mg/kg increased the relative mRNA expression of claudin-1 and ZO-1 in jejunal mucosal epithelial cells, which was consistent with Feng et al. (2018), who revealed that piglets fed sodium butyrate at 2,000 mg/kg facilitated expression of the TJ protein in the jejunum by acting on the protein kinase B (PKB)signaling pathway in a GPR109A dependent manner, which possibly activated or triggered a series of signaling pathways. But MOA at 7,000 mg/kg declined the expression, which may be due to the high concentration of MOA shutting down some signal pathway, and then inhibiting the expression of TJ protein. The detailed mechanism of action, therefore, need further research.
Microorganisms in the digestive tract of piglets (especially colon and cecum) can ferment carbohydrates (Canh et al., 1998) and produce a variety of VFA (propionic acid, butyric acid, etc.). These short-chain fatty acids can provide energy for piglets, resist pathogenic microorganisms and maintain animal intestinal health, among which butyric acid is particularly important. It is easily absorbed by colon epithelial cells and used as energy, and it can also reduce the incidence of colon disease (Corrier et al., 1990; Howard et al., 1995). Kathrin (2009) revealed that piglets fed organic acid showed an increase in the content of butyric acid, which was similar to our research in that dietary supplementation of MOA at 5,000 mg/kg (OA2) increased acetic acid and propionic acid in the cecum and increased the butyric acid in the colon. Likewise, the total VFA concentration was increased in the cecum and colon when piglets were fed OA2 and OA3, which is in agreement with Diao et al. (2014) who noted that piglets fed organic acids showed an enhanced content of total VFA in the cecum. Long et al. (2017) and Li et al. (2019) have demonstrated that MOA increased the content of total VFA in feces in piglets and growing-finishing pigs, respectively. This study did not find any difference between the CON and OA3 on VFA concentration, which may because the high concentration of organic acids can inhibit the secretion of gastric acid by gastric bottom gland cells, hinder the development of gastric secretion function, and make organic acids exhausted in the stomach so that the organic acid could not continue to play a role in intestinal tract. The full effects of high concentration of MOA on VFA in piglets are still unknown. Therefore, the detailed mode of MOA in the intestinal tract needs to be further researched.
Symbiotic microbiota in the gastrointestinal tract acts in a vital role on the growth and health of the host by performing metabolic, immunologic and protective functions (Duan et al., 2019). In the current research, the relative abundances of Firmicutes, Bacteroides and Proteobacteria were more than 95%, which belong to the predominant microbiota, and the results were consistent with the previous study of Chen et al. (2017) in suckling piglets. Lactobacillaceae family belongs to the Firmicutes phylum, which is a valuable microorganism for improving intestinal barrier function and immune regulation of intestinal health (Yang et al., 2018). Many studies have documented that piglet supplementation with MOA could increase the relative abundance of Lactobacillaceae and improve the microflora in the intestinal tract (Grecco et al., 2018; Sotira et al., 2020), which is in line with our findings. Xu et al. (2018) also showed that MOA modulated the microbial populations by enhancing the Lactobacillus in faeces of weaned piglets, which may be related to the promotion of proliferation of epithelial cells. Lachnospiraceae are one of the most abundant families from the order Clostridiales, and play a role in maintaining gut health (Biddle et al., 2013). Studies have confirmed that Lachnospiraceae is more abundant in NOD (non-obese diabetic) mice that develop diabetes and less abundant in mice that do not develop diabetes (Krych et al., 2015). In our research, the piglets supplemented with MOA at 3,000 mg/kg lowered the relative abundance of Lachnospiraceae, which implies that the addition of MOA inhibits the growth of some potentially harmful bacteria and increases the health of intestinal microorganisms. Moreover, we also observed that the piglets fed MOA group tended to decrease the relative abundance of Escherichia-Shigella, which was also similar to previous research (De Busser et al., 2011; Silva et al., 2020). This is mainly because the MOA provides an acidic environment and lowers the pH of the gastrointestinal tract, which inhibits the reproduction of Escherichia-Shigella (the optimal pH value was 6.0 to 8.0). Additionally, a higher relative abundance of Firmicutes and a lower population of Bacteroides was found in piglets fed OA1, which enhanced the ratio of Firmicutes to Bacteroidetes. Ley et al. (2006) have proven that the proportion of Firmicute to Bacteroidetes is positively correlated with body weight. Therefore, MOA may enhance the performance of piglets by increasing the proportion of Firmicutes/Bacteroidetes and improving the intestinal microflora. Still, a high concentration of MOA did not result in optimal performance in piglets, which probably related to the inhibition of gastric fundus gland cells due to high concentration of organic acids. In short, these findings revealed that the relative abundance and composition of the intestinal microbiota could differ in piglets fed MOA, so further research is now warranted to clarify the underlying mechanisms of MOA for intestinal microbiota, and clarify the potential prospect of replacing antibiotics with optimal concentrations of MOA.
The effect of AGP on piglets is mainly achieved by improving the ADG and FCR. However, it has a negative impact, to some extent, on the intestinal health of piglets (mainly affecting the structure of intestinal microflora and the expression of intestinal epithelial tight junction protein) (Francois, 1961; Visek, 1978; Thomas, 2015). In our study, dietary supplementation of MOA at 3,000 and 5,000 mg/kg had beneficial effects on performance, immune function, and intestinal health in piglets. These findings revealed that MOA could replace AGP to some extent to achieve a growth-promoting effect, and may be even better than antibiotics in improving digestive enzyme activity, intestinal morphology, immune capacity and intestinal microflora in weaned piglets.
5. Conclusions
In summary, the evidence from this research suggested that MOA at dosages of 3,000 and 5,000 mg/kg could potentially serve as AGP substitutes based on their beneficial effect on performance, immune function, the relative mRNA expression of TJ protein, and the gut microbiota community in weaned piglets. Moreover, our study found that the optimal content of MOA (3,675 mg/kg) in piglets can be obtained from the maximum ADG (378.9 g/d) in phase 1, and the optimal content of MOA (3,931 mg/kg) can be obtained from the optimum FCR (1.554) during the entire experimental period.
Author contributions
Jiayu Ma: Conceptualization, Software, Data Curation, Writhing-Original Draft Preparation, and Visualization; Jiayu Ma and Shenfei Long: Methodology; Qinghui Shang and Sujie Liu: Investigation; Xiangshu Piao: Supervision; Validation; Xiangshu Piao, Shenfei Long and Shad Mahfuz: Writing- Reviewing and Editing.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (31772612) and Beijing Municipal Natural Science Foundation (6202019). We acknowledge Nutreco Company (Amersfoort Netherland) for providing the new organic acid commercial products and Beijing Tongli XingKe Agricultural Technology Company Limited for providing the antibiotic mixture.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Ahmed S.T., Hwang J.A., Hoon J., Mun H.S., Yang C.J. Comparison of single and blend acidifiers as alternative to antibiotics on growth performance, fecal microflora, and humoral immunity in weaned piglets. Asian-Australas J Anim. 2014;27:93–100. doi: 10.5713/ajas.2013.13411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaire J.M., Crowley S.M., Law H.T., Chang S.Y., Ko H.J., Vallance B.A. The intestinal epithelium: central coordinator of mucosal immunity. Trends Immunol. 2018;39(9):677–696. doi: 10.1016/j.it.2018.04.002. [DOI] [PubMed] [Google Scholar]
- Alverdy J.C. Effects of glutamine-supplemented diets on immunology of the gut. JPEN J Parenter Enteral Nutr. 1990;14:109S–113S. doi: 10.1177/014860719001400415. [DOI] [PubMed] [Google Scholar]
- Biddle A., Stewart L., Blanchard J., Leschine S. Untangling the genetic basis of fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities. Diversity. 2013;5:627–640. [Google Scholar]
- Buchet A., Belloc C., Leblanc-Maridor M., Merlot E. Effects of age and weaning conditions on blood indicators of oxidative status in pigs. PloS One. 2017;12 doi: 10.1371/journal.pone.0178487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canh T.T., Sutton A.L., Aarnink A.J., Verstegen M.W., Schrama J.W., Bakker G.C. Dietary carbohydrates alter the fecal composition and pH and the ammonia emission from slurry of growing pigs. J Anim Sci. 1998;76:1887–1895. doi: 10.2527/1998.7671887x. [DOI] [PubMed] [Google Scholar]
- Chen L., Xu Y., Chen X., Fang C., Zhao L., Chen F. The maturing development of gut microbiota in commercial piglets during the weaning transition. Front Microbiol. 2017;8:1688. doi: 10.3389/fmicb.2017.01688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrier D.E., Hinton J.A., Ziprin R.L., DeLoach J.R. Effect of dietary lactose on Salmonella colonization of market-age broiler chickens. Avian Dis. 1990;34:668–676. [PubMed] [Google Scholar]
- De Busser E.V., Dewulf J., Zutter L.D., Haesebrouck F., Callens J., Meyns T., Maes W., Maes D. Effect of administration of organic acids in drinking water on faecal shedding of E. coli, performance parameters and health in nursery pigs. Vet J. 2011;188:184–188. doi: 10.1016/j.tvjl.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Diao H., Zheng P., Yu B., He J., Mao X.B., Yu J., Chen D.W. Effects of dietary supplementation with benzoic acid on intestinal morphological structure and microflora in weaned piglets. Livest Sci. 2014;167:249–256. doi: 10.1016/j.livsci.2014.05.029. [DOI] [Google Scholar]
- Diao H., Gao Z., Yu B., Zheng P., He J., Yu J., Huang Z., Chen D., Mao X. Effects of benzoic acid (VevoVitall(R)) on the performance and jejunal digestive physiology in young pigs. J Anim Sci Biotechnol. 2016;7:32. doi: 10.1186/s40104-016-0091-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Y., Zhong Y., Xiao H., Zheng C., Song B., Wang W., Guo Q., Li Y., Han H., Gao J., Xu K., Li T., Yin Y., Li F., Yin J., Kong X. Gut microbiota mediates the protective effects of dietary beta-hydroxy-beta-methylbutyrate (HMB) against obesity induced by high-fat diets. Faseb J. 2019;33:10019–10033. doi: 10.1096/fj.201900665RR. [DOI] [PubMed] [Google Scholar]
- Edgar R.C. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- Feng W., Wu Y., Chen G., Fu S., Li B., Huang B., Wang D., Wang W., Liu J. Sodium butyrate attenuates diarrhea in weaned piglets and promotes tight junction protein expression in colon in a GPR109A-dependent manner. Cell Physiol Biochem. 2018;47:1617–1629. doi: 10.1159/000490981. 10.1159/000490981. [DOI] [PubMed] [Google Scholar]
- Francois A.C. Mode of action of antibiotics on growth. World Rev Nutr Diet. 1961;3:21–64. [PubMed] [Google Scholar]
- Grecco H.A.T., Amorim A.B., Saleh M.A.D., M L.P.T., Telles F.G., Miassi G.M., Pimenta G.M., Berto D.A. Evaluation of growth performance and gastro-intestinal parameters on the response of weaned piglets to dietary organic acids. An Acad Bras Cienc. 2018;90:401–414. doi: 10.1590/0001-3765201820160057. 10.1590/0001-3765201820160057. [DOI] [PubMed] [Google Scholar]
- Hampson D.J., Kidder D.E. Influence of creep feeding and weaning on brush border enzyme activities in the piglet small intestine. Res Vet Sci. 1986;40:24–31. [PubMed] [Google Scholar]
- Heo J.M., Opapeju F.O., Pluske J.R., Kim J.C., Hampson D.J., Nyachoti C.M. Gastrointestinal health and function in weaned piglets: a review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J Anim Physiol Anim Nutr. 2013;97:207–237. doi: 10.1111/j.1439-0396.2012.01284.x. [DOI] [PubMed] [Google Scholar]
- Howard M.D., Gordon D.T., Pace L.W., Garleb K.A., Kerley M.S. Effects of dietary supplementation with fructooligosaccharides on colonic microbiota populations and epithelial cell proliferation in neonatal pigs. J Pediatr Gastroenterol Nutr. 1995;21:297–303. doi: 10.1097/00005176-199510000-00007. 10.1097/00005176-199510000-00007. [DOI] [PubMed] [Google Scholar]
- Hu C.H., Xiao K., Luan Z.S., Song J. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs. J Anim Sci. 2013;91:1094–1101. doi: 10.2527/jas.2012-5796. [DOI] [PubMed] [Google Scholar]
- Jha R., Fouhse J.M., Tiwari U.P., Li L., Willing B.P. Dietary fiber and intestinal health of monogastric animals. Front Vet Sci. 2019;6:48. doi: 10.3389/fvets.2019.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson F.H., Tremaroli V., Nookaew I., Bergstrom G., Behre C.J., Fagerberg B., Nielsen J., Backhed F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99–103. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- Kasprowicz-Potocka M., Frankiewicz A., Selwet M., Chilomer K. Effect of salts and organic acids on metabolite production and microbial parameters of piglets' digestive tract. Livest Sci. 2009;126:310–313. doi: 10.1016/j.livsci.2009.06.011. [DOI] [Google Scholar]
- Kathrin B. ETH.; Eth Zurich: 2009. Benzoic acid as feed additive in pig nutrition: effects of diet composition on performance, digestion and ecological aspects. PhD Diss. [Google Scholar]
- Kim Y.Y., Kil D.Y., Oh H.K., Han I.K. Acidifier as an alternative material to antibiotics in animal feed. Asian-Australas J Anim Sci. 2005;18:1048–1060. doi: 10.5713/ajas.2005.1048. [DOI] [Google Scholar]
- Krych L., Nielsen D.S., Hansen A.K., Hansen C.H. Gut microbial markers are associated with diabetes onset, regulatory imbalance, and IFN-gamma level in NOD mice. Gut Microb. 2015;6:101–109. doi: 10.1080/19490976.2015.1011876. 10.1080/19490976.2015.1011876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.W., Everts H., Kappert H.J., Frehner M., Losa R., Beynen A.C. Effects of dietary essential oil components on growth performance, digestive enzymes and lipid metabolism in female broiler chickens. Br Poultry Sci. 2003;44:450–457. doi: 10.1080/0007166031000085508. 10.1080/0007166031000085508. [DOI] [PubMed] [Google Scholar]
- Ley R.E., Turnbaugh P.J., Klein S., Gordon J.I. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- Li M., Long S.F., Wang Q.Q., Zhang L.H., Hu J.X., Yang J., Cheng Z., Piao X.S. Mixed organic acids improve nutrients digestibility, volatile fatty acids composition and intestinal microbiota in growing-finishing pigs fed high-fiber diet. Asian-Australas J Anim Sci. 2019;32:856–864. doi: 10.5713/ajas.18.0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.T., Hou W.X., Cheng S.Y., Shi B.M., Shan A.S. Effects of dietary citric acid on performance, digestibility of calcium and phosphorus, milk composition and immunoglobulin in sows during late gestation and lactation. Anim Feed Sci Technol. 2014;191:67–75. doi: 10.1016/j.anifeedsci.2014.01.017. [DOI] [Google Scholar]
- Liu Y., Espinosa C.D., Abelilla J.J., Casas G.A., Lagos L.V., Lee S.A., Kwon W.B., Mathai J.K., Navarro D., Jaworski N.W., Stein H.H. Non-antibiotic feed additives in diets for pigs: a review. Anim Nutr. 2018;4:113–125. doi: 10.1016/j.aninu.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S.F., Xu Y., Pan L., Wang Q.Q., Wang C.L., Wu J.Y., Wu Y.Y., Han Y.M., Yun C.H., Piao X. Mixed organic acids as antibiotic substitutes improve performance, serum immunity, intestinal morphology and microbiota for weaned piglets. Anim Feed Sci Technol. 2017;235:23–32. doi: 10.1016/j.anifeedsci.2017.08.018. [DOI] [Google Scholar]
- Lupton J.R., Jacobs L.R. Fiber supplementation results in expanded proliferative zones in rat gastric mucosa. Am J Clin Nutr. 1987;46:980–984. doi: 10.1093/ajcn/46.6.980. [DOI] [PubMed] [Google Scholar]
- NRC . 11th revised edn. National Academy Press; Washington, DC: 2012. Nutrient requirements of swine. [Google Scholar]
- Pan L., Ma X.K., Wang H.L., Xu X., Zeng Z.K., Tian Q.Y., Zhao P.F., Zhang S., Yang Z.Y., Piao X.S. Enzymatic feather meal as an alternative animal protein source in diets for nursery pigs. Anim Feed Sci Technol. 2016;212:112–121. doi: 10.1016/j.anifeedsci.2015.12.014. [DOI] [Google Scholar]
- Partanen K., Jalava T., Valaja J. Effects of a dietary organic acid mixture and of dietary fibre levels on ileal and faecal nutrient apparent digestibility, bacterial nitrogen flow, microbial metabolite concentrations and rate of passage in the digestive tract of pigs. Animal. 2007;1:389–401. doi: 10.1017/S1751731107657838. [DOI] [PubMed] [Google Scholar]
- Partanen K., Siljander-Rasi H., Pentikainen J., Pelkonen S., Fossi M. Effects of weaning age and formic acid-based feed additives on pigs from weaning to slaughter. Arch Anim Nutr. 2007;61:336–356. doi: 10.1080/17450390701556866. 10.1080/17450390701556866. [DOI] [PubMed] [Google Scholar]
- Ren C., Wang Y., Lin X., Song H., Zhou Q., Xu W., Shi K., Chen J., Song J., Chen F., Zhang S., Guan W. A combination of formic acid and monolaurin attenuates enterotoxigenic Escherichia coli induced intestinal inflammation in piglets by inhibiting the NF-kappaB/MAPK pathways with modulation of gut microbiota. J Agric Food Chem. 2020;68:4155–4165. doi: 10.1021/acs.jafc.0c01414. [DOI] [PubMed] [Google Scholar]
- Robbins K.R., Saxton A.M., Southern L.L. Estimation of nutrient requirements using broken-line regression analysis. J Anim Sci. 2006;84(Suppl):E155–E165. doi: 10.2527/2006.8413_supple155x. [DOI] [PubMed] [Google Scholar]
- Roxas J.L., Koutsouris A., Bellmeyer A., Tesfay S., Royan S., Falzari K., Harris A., Cheng H., Rhee K.J., Hecht G. Enterohemorrhagic E. coli alters murine intestinal epithelial tight junction protein expression and barrier function in a Shiga toxin independent manner. Lab Invest. 2010;90:1152–1168. doi: 10.1038/labinvest.2010.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segata N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W.S., Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva C.D., Junior, Martins C.C.S., Dias F.T.F., Sitanaka N.Y., Ferracioli L.B., Moraes J.E., Pizzolante C.C., Budino F.E.L., Pereira R., Tizioto P., Paula V.R.C., Coutinho L.L., Ruiz U.S. The use of an alternative feed additive, containing benzoic acid, thymol, eugenol, and piperine, improved growth performance, nutrient and energy digestibility, and gut health in weaned piglets. J Anim Sci. 2020;98 doi: 10.1093/jas/skaa119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotira S., Dell'Anno M., Caprarulo V., Hejna M., Pirrone F., Callegari M.L., Tucci T.V., Rossi L. Effects of tributyrin supplementation on growth performance, insulin, blood metabolites and gut microbiota in weaned piglets. Animals. 2020;10 doi: 10.3390/ani10040726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suiryanrayna M.V., Ramana J.V. A review of the effects of dietary organic acids fed to swine. J Anim Sci Biotechnol. 2015;6:45. doi: 10.1186/s40104-015-0042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezcan F., Gültekin-Özgüven M., Diken T., Özçelik B., Erim F.B. Antioxidant activity and total phenolic, organic acid and sugar content in commercial pomegranate juices. Food Chem. 2009;115:873–877. doi: 10.1016/j.foodchem.2008.12.103. [DOI] [Google Scholar]
- Thomas F. Missing Microbes: how the overuse of antibiotics is fueling our modern plagues. Clin Infect Dis. 2015;60(8):1293. doi: 10.1093/cid/ciu1164. [DOI] [Google Scholar]
- Tian Z.M., Ma X.Y., Yang X.F., Fan Q.L., Xiong Y.X., Qiu Y.Q., Wang L., Wen X.L., Jiang Z.Y. Influence of low protein diets on gene expression of digestive enzymes and hormone secretion in the gastrointestinal tract of young weaned piglets. J Zhejiang Univ - Sci B. 2016;17(10):742–751. doi: 10.1631/jzus.B1600229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leeuwen P., Veldman A., Boisen S., Deuring K., Van Kempen G.J., Derksen G.B., Verstegen M.W., Schaafsma G. Apparent ileal dry matter and crude protein digestibility of rations fed to pigs and determined with the use of chromic oxide (Cr2O3) and acid-insoluble ash as digestive markers. Br J Nutr. 1996;76:551–562. doi: 10.1079/bjn19960062. [DOI] [PubMed] [Google Scholar]
- Visek W.J. The mode of growth promotion by antibiotics. J Anim Sci. 1978;46(5):1447–1469. doi: 10.2527/jas1978.4651447x. [DOI] [Google Scholar]
- Wang Y., Kuang Y., Zhang Y., Song Y., Zhang X., Lin Y., Che L., Xu S., Wu, Xue B., Fang Z. Rearing conditions affected responses of weaned piglets to organic acids showing a positive effect on digestibility, microflora and immunity. Anim Sci J. 2016;87:1267–1280. doi: 10.1111/asj.12544. [DOI] [PubMed] [Google Scholar]
- Weiler F., Marbe T., Scheppach W., Schauber J. Influence of protein kinase C on transcription of the tight junction elements ZO-1 and Occludin. J Cell Physiol. 2005;204(1):83–86. doi: 10.1002/jcp.20268. [DOI] [PubMed] [Google Scholar]
- Xu Y.T., Liu L., Long S.F., Pan L., Piao X.S. Effect of organic acids and essential oils on performance, intestinal health and digestive enzyme activities of weaned piglets. Anim Feed Sci Technol. 2018;235:110–119. doi: 10.1016/j.anifeedsci.2017.10.012. [DOI] [Google Scholar]
- Yang J., Qian K., Wang C., Wu Y. Roles of probiotic lactobacilli inclusion in helping piglets establish healthy intestinal inter-environment for pathogen defense. Probiotics Antimicrob Proteins. 2018;10:243–250. doi: 10.1007/s12602-017-9273-y. [DOI] [PubMed] [Google Scholar]
- Yang C., Zhang L., Cao G., Feng J., Yue M., Xu Y., Dai B., Han Q., Guo X. Effects of dietary supplementation with essential oils and organic acids on the growth performance, immune system, fecal volatile fatty acids, and microflora community in weaned piglets. J Anim Sci. 2019;97:133–143. doi: 10.1093/jas/sky426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J., Duan J., Cui Z., Ren W., Li T., Yin Y. Hydrogen peroxide-induced oxidative stress activates NF-κB and Nrf2/Keap1 signals and triggers autophagy in piglets. RSC Adv. 2015;5:15479–15486. doi: 10.1039/C4RA13557A. [DOI] [Google Scholar]
- Zhang Q., Li Q., Wang C., Liu X., Li N., Li J. Enteropathogenic Escherichia coli changes distribution of occludin and ZO-1 in tight junction membrane microdomains in vivo. Microb Pathog. 2010;48:28–34. doi: 10.1016/j.micpath.2009.10.002. [DOI] [PubMed] [Google Scholar]