Abstract
This 10-year retrospective observational study investigated longitudinal losses in psoas major and paraspinal muscle area in 1849 healthy individuals (1690 male, 159 female) screened using computed tomography. Logistic regression analysis revealed significant decreases in psoas major and paraspinal muscle area at 10 years relative to the baseline area regardless of age or sex, starting at 30 years of age. Only aging [≥ 50 s (odds ratio [OR]: 1.72; 95% confidence interval [CI] 1.05–2.84; p = 0.03) and ≥ 60 s (OR: 2.67; 95% CI 1.55–4.60; p < 0.001)] was a risk factor for decreases in psoas major area. Age ≥ 60 years (OR: 2.05; 95% CI 1.24–3.39; p = 0.005), body mass index ≥ 25 kg/m2 (OR: 1.32; 95% CI 1.01–1.73; p = 0.04), and visceral fat ≥ 100 cm2 (OR: 1.61; 95% CI 1.20–2.15; p = 0.001) were risk factors for decreases in paraspinal muscle area. Physical activity ≥ 900 kcal/week (OR: 0.68; 95% CI 0.50–0.94; p = 0.02) attenuated paraspinal muscle area loss in male. Our study demonstrated that walking > 45 min daily (Calories = METs (walking: 3.0) × duration of time (h) × weight (60 kg) × 1.05) can reduce paraspinal muscle loss, which may in turn decrease the risk of falls, low-back pain, and sarcopenia.
Subject terms: Orthopaedics, Epidemiology
Introduction
Sarcopenia is characterized by the progressive loss of skeletal muscle mass and strength and presents a risk for adverse outcomes, including physical disability, poor quality of life (QOL), and death1,2. This condition increases the risk of falls in older individuals, often resulting in fractures in areas such as the femoral neck3–5. These fractures negatively affect QOL and increase the number of bedridden patients and medical costs6,7. Tinetti et al.8 reported that walking disability and the risk of falls would be caused by the decrease of paraspinal muscle strength. Indeed, several studies have reported an association between paraspinal muscle size or morphology and low back pain. Ranger et al.9 longitudinally investigated the relationship between paraspinal muscle cross-sectional area and both the intensity of low back pain and related disability at 12-months. The authors concluded that paraspinal muscle cross-sectional area could predict disability from low back pain but not pain intensity. A systematic review regarding the association between paraspinal muscle cross-sectional area and low back pain reported that the area of the multifidus muscle—but not of the erector spinae, psoas, or quadratus lumborum—was negatively associated with the risk of low back pain10. Thus, preventing the decrease of paraspinal muscle strength is important for reducing adverse events, especially in countries with rapidly aging populations.
Current research shows an association between skeletal muscle area and specific characteristics. Landi et al. found that cerebrovascular disease and osteoarthritis were specific risk factors of sarcopenia in older patients11. Raval et al. determined that higher body mass index (BMI) in individuals with peripheral artery disease was associated with more adverse changes in calf muscle characteristics in a 2-year longitudinal study12.
Few longitudinal studies in healthy populations have reported psoas major and paraspinal muscle changes except for a small survey or the young generation, despite losses being associated with falls and back pain13,14. Therefore, this study aimed to longitudinally compare psoas major and paraspinal muscle area in large number of healthy individuals and determine risk factors associated with given changes. We hypothesized that risk factors, such as obesity and lack of exercise, would cause psoas major and paraspinal muscle area loss, starting in individuals in their 40 s.
Results
Individuals
Of 9749 individuals, 3931 were excluded based on preliminary criteria. Specifically, 2010 participants underwent computed tomography (CT) screenings twice from 2004 to 2006, and the second set of data was excluded. In 1867 individuals, cross-sectional CT did not include the area between the L4 upper edge and L4/5 disc. Individuals with a history of lumbar surgery were excluded (n = 54). Individuals who did not undergo a CT screening of visceral fat at 10 years and those with a CT scan that did not measure the area between the L4 upper edge and L4/5 disc 10 years after their initial screening (n = 3908 and n = 61, respectively) were also excluded. In total, 1849 individuals (1690 male, 159 female) met the inclusion criteria. The recruitment process and application of the exclusion criteria are described in Fig. 1.
Figure 1.
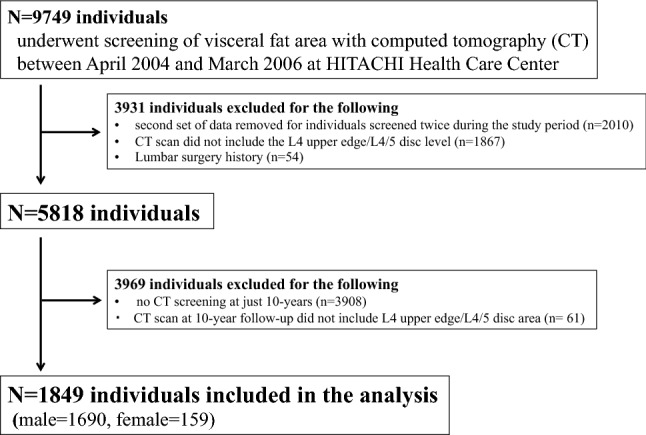
Study flow diagram.
Baseline demographic variables
Demographic data according to age groups are summarized in Table 1. No significant differences in BMI were observed between age groups for both sexes, while body fat and subcutaneous fat in male participants decreased with age. Conversely, despite no change in BMI, visceral fat area increased until participants were in their 50 s for both sexes, most notably in male participants in their 30 s and 40 s. Physical activity in male participants increased with age.
Table 1.
Summary demographic and health characteristics of the individuals at baseline.
| Male (n = 1690) | Female (n = 159) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 30 s (n = 135) | 40 s (n = 424) | 50 s (n = 855) | 60 s (n = 276) | F | p value | 40 s (n = 27) | 50 s (n = 101) | 60 s (n = 31) | F | p value | |
| BMI (kg/m2) | 24.4 ± 3.4 (17.9–35.8) | 24.6 ± 3.2 (17.1–36.1) | 23.9 ± 2.6 (16.7–33.4) | 23.7 ± 2.3 (17.6–31.7) | 1.60 | 0.188 | 23.0 ± 3.5 (16.5–32.4) | 23.3 ± 3.0 (18.3–33.0) | 22.2 ± 2.0 (19.2–28.0) | 1.93 | 0.149 |
| Body fat (%) | 23.4 ± 4.7 (12.1–37.2) | 23.1 ± 4.9 (0–39.2) | 21.9 ± 4.1 (10.0–38.6) | 21.2 ± 4.2 (10.0–32.2) | 14.97 | < 0.001 | 28.4 ± 5.8 (17.5–39.1) | 29.1 ± 5.5 (16.2–44.5) | 27.6 ± 4.2 (19.7–35.2) | 1.44 | 0.240 |
| Visceral fat (cm2) | 108.3 ± 54.0 (10.8–244.0) | 127.9 ± 52.0 (9.6–302.1) | 131.8 ± 54.2 (2.9–317.7) | 128.2 ± 53.6 (0–279.0) | 7.55 | < 0.001 | 62.6 ± 36.9 (13.8–151.0) | 87.9 ± 49.2 (0–227.0) | 81.9 ± 34.3 (20.0–140.0) | 3.20 | 0.044 |
| Subcutaneous fat (cm2) | 146.6 ± 76.5 (15.2–417.0) | 143.3 ± 61.5 (11.0–402.8) | 124.9 ± 45.6 (7.0–272.1) | 113.8 ± 40.5 (18.5–260.0) | 25.17 | < 0.001 | 155.6 ± 72.1 (61.7–317.0) | 183.1 ± 73.3 (33.0–381.0) | 162.4 ± 48.8 (61.0–278.0) | 2.65 | 0.074 |
| Physical activity (kcal/week) | 501.8 ± 393.6 (0–1950.0) | 606.8 ± 514.4 (0–4620.0) | 760.2 ± 1,025.9 (0–14,375.0) | 960.5 ± 1,220.0 (0–9562.5) | 11.27 | < 0.001 | 610.4 ± 962.7 (0–4800.0) | 537.6 ± 975.2 (0–4890.0) | 697.6 ± 958.4 (0–3750.0) | 0.49 | 0.612 |
| Smoker (%) | 85 (63.0) | 243 (57.3) | 387 (45.3) | 85 (30.8) | – | – | 4 (14.3) | 3 (2.9) | 1 (2.9) | – | – |
| Drinker (%) | 103 (76.3) | 338 (79.7) | 665 (77.8) | 202 (73.2) | – | – | 10 (35.7) | 21 (20.6) | 4 (11.4) | – | – |
| Area of psoas major (cm2) | 29.2 ± 4.7 (14.7–44.0) | 28.7 ± 5.1 (15.2–45.8) | 25.7 ± 4.7 (9.3–43.1) | 24.2 ± 4.3 (12.5–39.7) | 74.85 | < 0.001 | 17.3 ± 3.5 (10.0–26.8) | 14.7 ± 3.3 (7.4–23.7) | 14.0 ± 2.5 (8.0–18.6) | 8.10 | < 0.001 |
| Fat area of psoas major (cm2) | 0.8 ± 0.6 (0.1–3.1) | 1.0 ± 0.6 (0–3.1) | 0.9 ± 0.6 (0–4.8) | 1.0 ± 0.7 (0–3.3) | 1.99 | 0.114 | 0.7 ± 0.6 (0.2–2.4) | 0.8 ± 0.6 (0–3.1) | 0.7 ± 0.6 (0.1–2.5) | 0.55 | 0.580 |
| Area of paraspinal muscle (cm2) | 50.2 ± 7.7 (33.2–78.0) | 48.0 ± 7.2 (29.5–71.9) | 46.1 ± 6.6 (18.4–69.8) | 44.8 ± 6.5 (28.4–63.7) | 26.15 | < 0.001 | 38.1 ± 5.6 (28.6–52.8) | 35.6 ± 4.8 (23.4–47.6) | 35.0 ± 5.1 (21.2–44.8) | 2.59 | 0.078 |
| Fat area of paraspinal muscle (cm2) | 1.7 ± 1.3 (0.1–10.2) | 1.9 ± 1.3 (0.1–11.3) | 2.2 ± 1.5 (0–11.4) | 2.7 ± 1.9 (0–11.2) | 20.12 | < 0.001 | 2.5 ± 1.4 (0.4–5.6) | 3.6 ± 2.2 (0.2–9.2) | 3.8 ± 2.5 (0.7–13.2) | 3.40 | 0.036 |
| Fat rate of psoas major area (%) | 2.9 ± 1.9 (0.2–9.8) | 3.3 ± 2.1 (0.1–11.6) | 3.7 ± 2.4 (0–26.6) | 4.2 ± 2.8 (0–12.7) | 11.55 | < 0.001 | 4.3 ± 3.0 (1.1–12.5) | 5.6 ± 4.0 (0.2–24.2) | 5.4 ± 5.0 (0.9–24.9) | 1.28 | 0.282 |
| Fat rate of paraspinal muscle area (%) | 3.3 ± 2.6 (0.2–18.2) | 3.9 ± 2.7 (0.2–21.7) | 4.8 ± 3.5 (0.1–25.3) | 6.0 ± 4.6 (0.1–26.5) | 28.49 | < 0.001 | 6.7 ± 3.9 (1.1–15.9) | 10.4 ± 6.8 (0.6–29.6) | 11.4 ± 9.1 (0.7–13.2) | 3.78 | 0.025 |
Data are expressed as mean ± standard deviation (range) except smoker and drinker.
BMI body mass index.
Baseline muscle, fat area composition, and fat rate of muscles
Baseline psoas major and paraspinal muscle composition (total for the right and left sides together) for each age group is presented in Table 1. Areas of both the psoas major and the paraspinal muscles decreased with age in male participants. Although no significant differences in the fat area of the psoas major were observed among age groups, paraspinal muscle fat area increased with age in both sexes.
Changes in the muscle area between baseline and 10 years
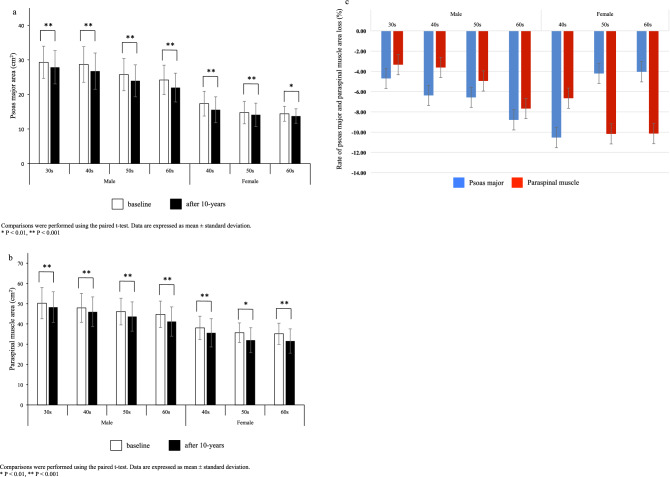
The 10-year changes in psoas major and paraspinal muscle area by age group and sex are shown in Fig. 2a,b. At the 10-year follow up, psoas major and paraspinal muscle areas were significantly smaller than those at baseline regardless of age or sex. For individuals in the 30–39-year age group, significant area loss was already present in the psoas major and paraspinal muscle. The rate of both the psoas major and paraspinal muscle area loss were 4–6% per decade for male participants aged 30–59 years and approximately 8% for male participants in their 60 s. The rate of paraspinal muscle area loss was approximately 6% for female participants in their 40 s and approximately 10% per decade for female participants in their 50 s and 60 s. In contrast, the highest reduction of the psoas muscle area was in female participants aged 40–49 years. Although male participants in their 50 s and 60 s had larger psoas major area reduction rates than female participants of the same age, female participants in their 50 s and 60 s had the largest paraspinal muscle area loss (Fig. 2c).
Figure 2.
Differences in psoas major and paraspinal muscle areas over a 10-year period. (a) Differences in psoas major muscle area over 10 years. (b) Differences in paraspinal muscle area over 10 years. (c) Rate of psoas major and paraspinal muscle areas loss over 10 years.
Multivariate analysis
The mean loss in the highest quartile of psoas major area was − 20.0 ± 6.2% (range − 45.1 to − 13.1%) (n = 423), and paraspinal muscle area loss was − 19.6 ± 9.7% (range − 67.1 to − 11.2%) (n = 423). The mean loss in the lower three quartiles of psoas major area was − 2.3 ± 7.1% (range − 13.0 to 21.1%) (n = 1267), and paraspinal muscle area loss was 0.1 ± 7.1% (range − 11.2 to 20.9%) (n = 1267). The results of a multivariate analysis evaluating risk factors of both psoas major and paraspinal muscle area loss are presented in Table 2. Only aging [≥ 50 s (odds ratio [OR], 1.72; 95% confidence interval [CI] 1.05–2.84; p = 0.03) and ≥ 60 s (OR, 2.67; 95% CI 1.55–4.60; p < 0.001)] was a risk factor for decreases in the psoas major area. Age ≥ 60 years (OR, 2.05; 95% CI 1.24–3.39; p = 0.005), BMI ≥ 25 kg/m2 (OR, 1.32; 95% CI 1.01–1.73; p = 0.04), and visceral fat ≥ 100 cm2 (OR, 1.61; 95% CI 1.20–2.15; p = 0.001) were risk factors for decreases in paraspinal muscle area, while physical activity ≥ 900 kcal/week (OR, 0.68; 95% CI 0.50–0.94; p = 0.02) reduced paraspinal muscle area loss in male participants.
Table 2.
Multivariate analysis for predicting changes in the psoas major and paraspinal muscle area.
| Risk factors | Score assigned | Psoas major | Paraspinal muscle | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | ||||
| All generations | Age | 30 s | 0 | Reference | – | – | Reference | – | – |
| 40 s | 1 | 1.52 | 0.90–2.56 | 0.12 | 0.76 | 0.46–1.24 | 0.27 | ||
| 50 s | 2 | 1.72 | 1.05–2.84 | 0.03 | 1.26 | 0.80–1.98 | 0.33 | ||
| 60 s | 3 | 2.67 | 1.55–4.60 | < 0.001 | 2.05 | 1.24–3.39 | 0.005 | ||
| Alcohol | − | 0 | Reference | – | – | Reference | – | – | |
| + | 1 | 0.91 | 0.70–1.19 | 0.49 | 0.95 | 0.72–1.24 | 0.69 | ||
| Smoking | − | 0 | Reference | – | – | Reference | – | – | |
| + | 1 | 1.01 | 0.81–1.27 | 0.92 | 0.84 | 0.67–1.06 | 0.15 | ||
| BMI (kg/m2) | < 25.0 | 0 | Reference | – | – | Reference | – | – | |
| ≥ 25.0 | 1 | 1.21 | 0.92–1.58 | 0.17 | 1.32 | 1.01–1.73 | 0.04 | ||
| Body fat (%) | < 25.0 | 0 | Reference | – | – | Reference | – | – | |
| ≥ 25.0 | 1 | 0.87 | 0.64–1.17 | 0.34 | 1.05 | 0.78–1.40 | 0.75 | ||
| Visceral fat (cm2) | < 100.0 | 0 | Reference | – | – | Reference | – | – | |
| ≥ 100.0 | 1 | 1.24 | 0.94–1.63 | 0.13 | 1.61 | 1.20–2.15 | 0.001 | ||
| Physical activity (kcal/week) | < 275.0 | 0 | Reference | – | – | Reference | – | – | |
| < 510.0 | 1 | 1.19 | 0.87–1.65 | 0.28 | 0.90 | 0.66–1.24 | 0.53 | ||
| < 900.0 | 2 | 1.06 | 0.78–1.46 | 0.70 | 0.81 | 0.59–1.10 | 0.17 | ||
| ≥ 900.0 | 3 | 1.19 | 0.86–1.63 | 0.29 | 0.68 | 0.50–0.94 | 0.02 | ||
Discussion
We longitudinally investigated changes in psoas major and paraspinal muscle area of healthy individuals and determined the risk factors for psoas major and paraspinal muscle loss according to age group and sex. Both psoas and paraspinal muscle areas significantly decreased after a 10-year period for all age groups and sexes in individuals starting in their 30 s. The rate of psoas major and paraspinal muscle area loss increased gradually with age except for psoas muscle area loss in female participants. A regression analysis revealed that only age ≥ 50 years was a risk factor for decreases in psoas major area. Age ≥ 60 years, BMI ≥ 25 kg/m2, and visceral fat ≥ 100 cm2 were risk factors for decreases in paraspinal muscle area, while physical activity ≥ 900 kcal/week reduced paraspinal muscle area loss in male participants.
Age-related muscle change in paraspinal muscle or skeletal muscle has been examined in cross-sectional studies. Sasaki et al. measured the paraspinal muscle area of 796 Japanese participants (mean age = 63.5 years, categorized as < 50, 50–59, 60–69, 70–79, and ≥ 80 years old) in a cross-sectional study and revealed that muscle area significantly decreased in individuals in their 50 s compared to those aged < 50 years15. Another cross-sectional study examined the influence of age and whole-body skeletal muscle mass in 468 participants (age range 18–88 years) using magnetic resonance imaging and found that skeletal muscle mass gradually increased until individuals were in their 30 s and started to decrease around ages of 45–50 years16. However, the present longitudinal study is the first to provide evidence of a noticeable decrease in muscle area occurring in individuals in their 30 s since it included healthy individuals from multiple age groups to calculate muscle area loss.
Although previous longitudinal studies have reported significant changes in the cross-sectional muscle area, most investigated the lower extremity muscle area and included older individuals. In a study of 1880 older adults, Goodpaster et al. reported an annual reduction of approximately 1% in the lean leg area17. Delmonico et al. reported changes in the mid-thigh muscle area in individuals in their 70 s over a 5-year period and found a 3–5% decrease18. The rate of muscle area loss per year in the present study was lower than in previous studies, and this is likely due to study differences in the measured muscle and age groups targeted. In the longitudinal study for paraspinal muscle, Mäki et al. longitudinally investigated 298 participants (mean age, 21.2 years; range 19–22) with follow-up scans conducted at a mean age of 30.6 years and found that the paraspinal muscle area, except for the area of the psoas major, increased in the young adult population (20–30 years of age)19. Therefore, our results add to the aforementioned evidence that the psoas major and paraspinal muscle area, except for the area of the psoas major, increases in individuals from their 20 s to 30 s, and both psoas major and paraspinal muscle areas decrease after that.
Our research indicated that alcohol and smoking were not leading risk factors for a decrease in psoas major and paraspinal muscle areas. A previous study found that skeletal muscle autophagy increased with alcohol consumption20. However, another study on alcohol consumption effects reported no sarcopenia correlation21. Our results support this as we found no association of alcohol consumption with psoas major and paraspinal muscle areas.
Previous studies have reported an association between physical activity and muscle strength in older people22,23. In a 5-year longitudinal analysis, Rantanen et al. demonstrated that maintaining activity levels prevents muscle strength decline with age24. Our study confirms these trends and adds evidence to the amount of physical activity required. Hao et al. reported an association between the skeletal muscle mass index (SMMI) and physical activity for 640 adolescents in short-term outcomes and suggested that SMMI was positively associated with physical activity25. However, there is limited knowledge regarding the association between skeletal muscle mass changes and the amount of physical activity for individuals aged 30–60. Our study indicates that exercise burning 900 kcal/week, including daily movement such as commuting on foot, attenuates paraspinal muscle area loss in male participants. This translates to walking at 4.0 km/h for 300 min/week for individuals weighing 60 kg. Based on our findings, we recommend that male participants walk more than 45 min daily.
Fortin et al. provided evidence that greater multifidus and erector spinae fatty infiltration at L5/S1 was associated with low back pain at the 1-year follow-up26. On the other hand, their 15-year longitudinal magnetic resonance imaging study concluded that the level of physical activity at work and leisure and low back pain were not associated with changes in paraspinal muscle morphology27. Additionally, Gelhorn et al.28 found that the cross-sectional area of paraspinal muscles could not predict low back pain at the 6- or 12-month follow-up in older adults with spinal degeneration. Hebert et al.29 explored the cross-sectional relationships between lumbar multifidus intramuscular adipose tissue infiltration and low back pain, reporting that such infiltration was consistently associated with low back pain at the age of 40 years, although these associations were no longer present at the ages of 45 or 50 years. Ito et al.30 investigated the association between the cross-sectional area of the trunk muscles (the erector spinae and multifidus) and falls in older patients with lumbar spinal stenosis, demonstrating that the cross-sectional area of the L4/5 multifidus was related to fall risk. Furthermore, other studies have reported that the sagittal alignment of spine is associated with falls. Ishikawa et al.31 reported that a lumbar lordosis angle of 3 degrees or less was associated with falls in older individuals. Additionally, one report noted that the horizontal distance between the C7 plumb line and the center of the ankle is associated with the risk of falls32. Given the lack of functional data in the present study, further studies are required to determine risk factors influencing function, low back pain, and fall risk.
The longitudinal study design and large sample size of healthy individuals are two strengths of the present study. Moreover, a multivariate analysis with variables from comprehensive health data allowed for robust analysis. However, this study has some limitations. First, participants were not randomly selected from the general Japanese population since screenings were conducted for employees of corporations in Hitachi city. Additionally, there is a possibility that the visceral fat site as measured by CT revealed differences between baseline and 10-year follow-up values as CT imaging was done at the umbilicus level; however, we accounted for this by excluding individuals whose CT scans were not between the L4 upper edge and L4/5 disc area. Third, there is a clear lack of information regarding the history of low back pain in the selected individuals. However, participants with low back pain that resulted in leaves of absence were not included in this study, as measurements were obtained at annual health check-ups for workers. Fourth, the areas of the multifidus and erector spinae muscles were not measured separately since we used CT, even though MRI is the gold-standard method for assessing paraspinal muscle area and composition. Fifth, since this study focused on changes in muscle area only, future studies should examine changes in fatty infiltration for each muscle. Additionally, the reliability of the software used in this study remains to be reported. Finally, this study did not assess the inter- and intra-observer reliability of muscle area measurements even though measurements were manually minute-adjusted in the only case when the scan seemed to be in correct.
In conclusion, the present findings demonstrate that psoas major and paraspinal muscle areas significantly decreased over a 10-year period for all age groups, starting in the 30 s. Aging was a risk factor for decreases in the psoas major area. Additionally, aging, obesity, and visceral obesity were risk factors for decreases in paraspinal muscle area. However, physical activity ≥ 900 kcal/week reduced paraspinal muscle area loss in male participants. This finding indicates that walking for > 45 min daily (Calories = METs (walking: 3.0) × duration of time (hr) × weight (60 kg) × 1.05) can reduce paraspinal muscle loss, which may in turn decrease the risk of falls, low back pain, and aid sarcopenia.
Methods
Study participants
This study was approved by the Ethics Committee of Medical Research of the University of Occupational and Environmental Health Institutional Review Board in accordance with the Declaration of Helsinki 2013. Informed consent was obtained from all participants, and all experiments were performed according to relevant guidelines and regulations.
Individuals who underwent a low-dose CT screening of the abdominal visceral fat area during annual health check-ups from April 2004 to March 2006 at the Hitachi Health Care Center were recruited for this study. For individuals screened twice within the period, the first set of data was used. CT measurements of visceral fat area were performed as previously described33–35. To minimize radiation exposure, single-slice imaging was performed at the level of the umbilicus in the supine position using a Radix Turbo CT scanner (Hitachi Medico, Tokyo, Japan). Imaging conditions were set at 120 kV and 50 mA, with a slice thickness of 5 mm. For the annual examinations, we standardized imaging at the level of the umbilicus and the most of this level was between L4 upper edge and L4/5 disc level36,37. L4 upper edge and L4/5 disc level was selected for the analysis because these levels have been used in previous studies9,15,38. We selected CT slices that included the L4 upper edge and L4/5 disc level while referring to the characteristics of the iliac bone; run of the aorta; and the shapes of the quadratus, psoas, and vertebra. These are totally different from those at the level of L3/4 or L5. Any disagreement was addressed via discussion between the two spine surgeons. Individuals whose cross-sectional CT images did not include the area between the L4 upper edge and the L4/5 disc, as well as those with history of lumbar surgery, were excluded. Individuals who did not undergo CT screening 10 years after their initial screening and whose CT images did not include the area between the L4 upper edge and L4/5 disc at the 10-year screening were excluded.
Examinations
Self-administered demographic and health questionnaire
Individuals completed a self-administered questionnaire with lifestyle-related questions including occupation, work posture, smoking habits, alcohol consumption, family history, medical history, physical activity, and health-related QOL. For this study, data on the frequency and current or past use of cigarettes and alcohol consumption were collected. Those who never or occasionally drank alcohol were considered non-drinkers, and those who never smoked or smoked in the past were considered non-smokers.
Physical activity
Physical activity was calculated according to previous reports39. Information on physical activity was obtained from the questionnaire. When preferred activities were not listed, activities of similar exertion levels were selected. Of the 20 types of exercise listed, “other” was not used. Metabolic equivalents (METs) of each activity based on physical activity guidelines were assigned (if the MET value was not on the list, a related value was used)40. Of the 19 exercises, 13 [work and commuting, walking, swimming, golf practice, golf, baseball, softball, cycling, table tennis, badminton, strength training, light jogging (approximately 6 min/km), jogging, soccer, tennis, aerobics, and jump rope] were active activities (> 6 METs), and weekly and hourly METs were calculated. Walking time for commuting was self-reported. Calories burned during commuting were calculated by multiplying the daily walking time by five (assuming two days off a week). Total physical activity was calculated from the calories burned for regular exercise during leisure plus those for commuting.
Anthropometric measurements
Anthropometric measurements were performed. BMI was calculated by dividing weight (kg) by the square of height (m2). The percentage of body fat was measured using a bioelectrical impedance analysis where fat mass was divided by total mass and multiplied by 100. The visceral and subcutaneous fat areas at the umbilical level were measured using a CT scanner (Radix turbo; Hitachi Medico, Tokyo, Japan) and automatically estimated using fatPointer software (Hitachi Medico, Tokyo, Japan) as previously described33–35.
Psoas and paraspinal muscle area
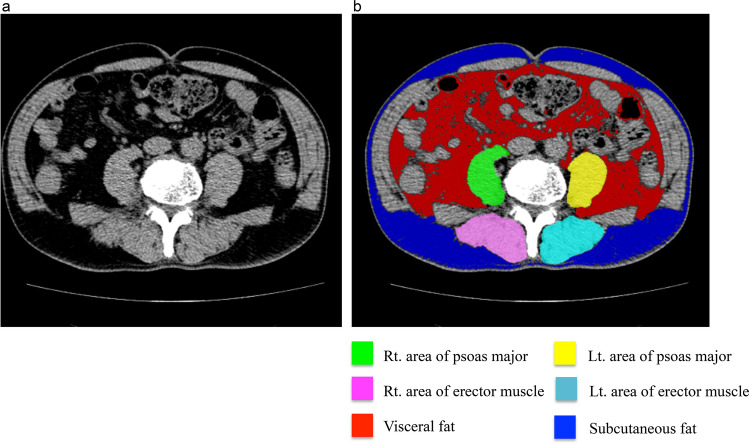
Psoas major and paraspinal muscle areas were assessed by a retrospective examination of CT scans utilizing musclePointer software (HITACHI Ltd., Tokyo, Japan). This software was developed as an evolution of fatPointer, which was used to measure cross-sectional visceral and subcutaneous fat areas and has been cited in some previous studies36,37,41. An engineer among the co-authors of the present study developed this automated software. CT images were divided by the threshold value of − 400 Hounsfield units (HU) into two cross-sectional areas: air and other tissues. The skin area was removed, and the areas of other tissues left in CT images were divided by the threshold value of -50 HU into two cross-sectional areas: fat and bone, muscle. Software automatically scanned each muscle (bilateral psoas major and paraspinal muscle). The paraspinal muscle group contains the multifidus and the erector spinae. The borders between the multifidus and erector spinae muscles are often difficult to distinguish, as mentioned in previous studies38,42 Therefore, the multifidus and erector spinae muscles were measured together and considered as the paraspinal muscle. Six orthopedic surgeons performed pilot measurements using several dozen slices to control for inter-observer variability in the interpretation of the images. Measurements were manually minute-adjusted by at least two orthopedic surgeons in the only case when the scan seemed to be incorrect. Each area was calculated, and data were entered into an Excel spreadsheet (Fig. 3).
Figure 3.
Representative view with musclePointer software. (a) An original image at the level of the umbilicus as observed using computed tomography. (b) Software calculated the area of each muscle automatically.
Statistical analysis
For descriptive variables, individuals were grouped by age in 10-year increments and by sex. Demographic and clinical characteristics are summarized as means ± standard deviations. Muscle areas between baseline and the 10-year follow-up were compared using a paired t-test. A one-way analysis of variance was used to compare baseline demographic variables, muscle area, and fat area composition according to age group for each sex. Given the small number of female participants, a logistic regression analysis was performed for male participants only. Four groups were created according to quartiles of muscle area loss. The group with the lower three quartiles (bottom 75%) was designated as the reference group. Covariates were dichotomized with reference groups of non-drinkers, non-smokers, BMI < 25 kg/m2, body fat < 25%, and visceral fat < 100 cm2, except for physical activity, which was split into quartiles. Associations between muscle area loss and potential risk factors were assessed via a multivariable logistic regression analysis, with muscle area loss dichotomized into the highest quartile of muscle area lost (top 25%) versus the lower three quartiles (bottom 75%). ORs and 95% CIs were calculated using the reference group. All statistical analyses were performed using STATA/IC 14 (StataCorp, College Station, TX, USA). The level of significance was set at a p value < 0.05.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Author contributions
E.N. and Y.M. approved the submitted version. E.N., Y.M. and A.S. contributed to the conception or design of the work. E.N., Y.M., M.T., T.N., M.T., M.K., T.K., K.N., K.S., S.U., and T.H. contributed to the acquisition, analysis and interpretation of data. E.N. and Y.M. have drafted the work and substantively revised it.
Funding
This work was supported in part by the Japanese Orthopaedic Association Grants-in-Aid for Scientific Research (E.N.).
Data availability
The data are not available for public access because of patient privacy concerns, but are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Goodpaster BH, et al. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J. Gerontol. A Biol. Sci. Med. Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 2.Delmonico MJ, et al. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J. Am. Geriatr. Soc. 2007;55:769–774. doi: 10.1111/j.1532-5415.2007.01140.x. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez-Montalvo JI, et al. Prevalence of sarcopenia in acute hip fracture patients and its influence on short-term clinical outcome. Geriatr. Gerontol. Int. 2016;16:1021–1027. doi: 10.1111/ggi.12590. [DOI] [PubMed] [Google Scholar]
- 4.Ho AW, et al. Prevalence of pre-sarcopenia and sarcopenia in Hong Kong Chinese geriatric patients with hip fracture and its correlation with different factors. Hong Kong Med. J. 2016;22:23–29. doi: 10.12809/hkmj154570. [DOI] [PubMed] [Google Scholar]
- 5.Di Monaco M, et al. Presarcopenia and sarcopenia in hip-fracture women: Prevalence and association with ability to function in activities of daily living. Aging Clin. Exp. Res. 2015;27:465–472. doi: 10.1007/s40520-014-0306-z. [DOI] [PubMed] [Google Scholar]
- 6.Alexiou KI, Roushias A, Varitimidis SE, Malizos KN. Quality of life and psychological consequences in elderly patients after a hip fracture: A review. Clin. Interv. Aging. 2018;13:143–150. doi: 10.2147/cia.s150067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ju DG, Rajaee SS, Mirocha J, Lin CA, Moon CN. Nationwide analysis of femoral neck fractures in elderly patients: A receding tide. J. Bone Jt. Surg. Am. 2017;99:1932–1940. doi: 10.2106/jbjs.16.01247. [DOI] [PubMed] [Google Scholar]
- 8.Tinetti ME, Kumar C. The patient who falls: "It's always a trade-off". JAMA. 2010;303:258–266. doi: 10.1001/jama.2009.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ranger TA, Cicuttini FM, Jensen TS, Heritier S, Urquhart DM. Paraspinal muscle cross-sectional area predicts low back disability but not pain intensity. Spine J. Off. J. N. Am. Spine Soc. 2019;19:862–868. doi: 10.1016/j.spinee.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Ranger TA, et al. Are the size and composition of the paraspinal muscles associated with low back pain? A systematic review. Spine J. Off. J. N. Am. Spine Soc. 2017;17:1729–1748. doi: 10.1016/j.spinee.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Landi F, et al. Prevalence and risk factors of sarcopenia among nursing home older residents. J. Gerontol. A Biol. Sci. Med. Sci. 2012;67:48–55. doi: 10.1093/gerona/glr035. [DOI] [PubMed] [Google Scholar]
- 12.Raval Z, et al. Higher body mass index is associated with more adverse changes in calf muscle characteristics in peripheral arterial disease. J. Vasc. Surg. 2012;55:1015–1024. doi: 10.1016/j.jvs.2011.10.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granacher U, Gollhofer A, Hortobagyi T, Kressig RW, Muehlbauer T. The importance of trunk muscle strength for balance, functional performance, and fall prevention in seniors: A systematic review. Sports Med. 2013;43:627–641. doi: 10.1007/s40279-013-0041-1. [DOI] [PubMed] [Google Scholar]
- 14.Sions JM, Elliott JM, Pohlig RT, Hicks GE. Trunk muscle characteristics of the multifidi, erector spinae, psoas, and quadratus lumborum in older adults with and without chronic low back pain. J. Orthop. Sports Phys. Ther. 2017;47:173–179. doi: 10.2519/jospt.2017.7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasaki T, et al. MRI-defined paraspinal muscle morphology in Japanese population: The Wakayama Spine Study. PLoS ONE. 2017;12:e0187765. doi: 10.1371/journal.pone.0187765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 years. J. Appl. Physiol. 2000;1985(89):81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- 17.Goodpaster BH, et al. Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: A randomized controlled trial. J. Appl. Physiol. 2008;1985(105):1498–1503. doi: 10.1152/japplphysiol.90425.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delmonico MJ, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am. J. Clin. Nutr. 2009;90:1579–1585. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mäki T, et al. Longitudinal analysis of paraspinal muscle cross-sectional area during early adulthood—A 10-year follow-up MRI study. Sci. Rep. 2019;9:19497. doi: 10.1038/s41598-019-56186-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thapaliya S, et al. Alcohol-induced autophagy contributes to loss in skeletal muscle mass. Autophagy. 2014;10:677–690. doi: 10.4161/auto.27918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steffl M, Bohannon RW, Petr M, Kohlikova E, Holmerova I. Alcohol consumption as a risk factor for sarcopenia—A meta-analysis. BMC Geriatr. 2016;16:99. doi: 10.1186/s12877-016-0270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Englund DA, et al. Nutritional supplementation with physical activity improves muscle composition in mobility-limited older adults, The VIVE2 Study: A randomized, double-blind, placebo-controlled trial. J. Gerontol. A Biol. Sci. Med. Sci. 2017;73:95–101. doi: 10.1093/gerona/glx141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pahor M, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: The LIFE study randomized clinical trial. JAMA. 2014;311:2387–2396. doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rantanen T, Era P, Heikkinen E. Physical activity and the changes in maximal isometric strength in men and women from the age of 75 to 80 years. J. Am. Geriatr. Soc. 1997;45:1439–1445. doi: 10.1111/j.1532-5415.1997.tb03193.x. [DOI] [PubMed] [Google Scholar]
- 25.Hao G, et al. Associations between muscle mass, physical activity and dietary behaviour in adolescents. Pediatr. Obes. 2019;14:e12471. doi: 10.1111/ijpo.12471. [DOI] [PubMed] [Google Scholar]
- 26.Fortin M, Gibbons LE, Videman T, Battié MC. Do variations in paraspinal muscle morphology and composition predict low back pain in men? Scand. J. Med. Sci. Sports. 2015;25:880–887. doi: 10.1111/sms.12301. [DOI] [PubMed] [Google Scholar]
- 27.Fortin M, Videman T, Gibbons LE, Battié MC. Paraspinal muscle morphology and composition: A 15-year longitudinal magnetic resonance imaging study. Med. Sci. Sports Exerc. 2014;46:893–901. doi: 10.1249/mss.0000000000000179. [DOI] [PubMed] [Google Scholar]
- 28.Gellhorn AC, et al. Lumbar muscle cross-sectional areas do not predict clinical outcomes in adults with spinal stenosis: A longitudinal study. PM & R J. Injury Funct. Rehabil. 2017;9:545–555. doi: 10.1016/j.pmrj.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 29.Hebert JJ, Kjaer P, Fritz JM, Walker BF. The relationship of lumbar multifidus muscle morphology to previous, current, and future low back pain: A 9-year population-based prospective cohort study. Spine. 2014;39:1417–1425. doi: 10.1097/brs.0000000000000424. [DOI] [PubMed] [Google Scholar]
- 30.Ito T, Sakai Y, Yamazaki K, Oikawa M, Morita Y. Relationship between L4/5 lumbar multifidus cross-sectional area ratio and fall risk in older adults with lumbar spinal stenosis: A retrospective study. Geriatrics (Basel, Switzerland) 2019;4:38. doi: 10.3390/geriatrics4020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishikawa Y, Miyakoshi N, Kasukawa Y, Hongo M, Shimada Y. Spinal sagittal contour affecting falls: Cut-off value of the lumbar spine for falls. Gait Posture. 2013;38:260–263. doi: 10.1016/j.gaitpost.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 32.Kim J, et al. The association between whole body sagittal balance and risk of falls among elderly patients seeking treatment for back pain. Bone Jt. Res. 2017;6:337–344. doi: 10.1302/2046-3758.65.bjr-2016-0271.r2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsushita Y, et al. Effect of longitudinal changes in visceral fat area on incidence of metabolic risk factors: The Hitachi health study. Obesity (Silver Spring) 2013;21:2126–2129. doi: 10.1002/oby.20347. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto S, et al. Visceral fat area and markers of insulin resistance in relation to colorectal neoplasia. Diabetes Care. 2010;33:184–189. doi: 10.2337/dc09-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsushita Y, et al. Associations of visceral and subcutaneous fat areas with the prevalence of metabolic risk factor clustering in 6292 Japanese individuals: The Hitachi Health Study. Diabetes Care. 2010;33:2117–2119. doi: 10.2337/dc10-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamamoto S, et al. Visceral fat accumulation, insulin resistance, and elevated depressive symptoms in middle-aged Japanese men. PLoS ONE. 2016;11:e0149436. doi: 10.1371/journal.pone.0149436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuwahara K, et al. Body mass index trajectory patterns and changes in visceral fat and glucose metabolism before the onset of type 2 diabetes. Sci. Rep. 2017;7:43521. doi: 10.1038/srep43521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Honkanen T, et al. Cross-sectional area of the paraspinal muscles and its association with muscle strength among fighter pilots: A 5-year follow-up. BMC Musculoskelet. Disord. 2019;20:170. doi: 10.1186/s12891-019-2551-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Honda T, et al. Association between information and communication technology use and ocular axial length elongation among middle-aged male workers. Sci. Rep. 2019;9:17489. doi: 10.1038/s41598-019-53423-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ainsworth BE, et al. 2011 Compendium of physical activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011;43:1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 41.Matsushita Y, et al. Effect of longitudinal changes in visceral fat area and other anthropometric indices to the changes in metabolic risk factors in Japanese men: The Hitachi Health Study. Diabetes Care. 2012;35:1139–1143. doi: 10.2337/dc11-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Käser L, et al. Active therapy for chronic low back pain: Part 2. Effects on paraspinal muscle cross-sectional area, fiber type size, and distribution. Spine. 2001;26:909–919. doi: 10.1097/00007632-200104150-00014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are not available for public access because of patient privacy concerns, but are available from the corresponding author on reasonable request.