Dear Editor,
Parkinson’s disease (PD) is one of the most common neurodegenerative diseases. Here, we suggest that beyond iron deposition, emerging evidence further supports the involvement of ferroptosis in PD. A detailed understanding of ferroptosis in PD will help to predict the onset and progression of disease and establish personalized treatments.
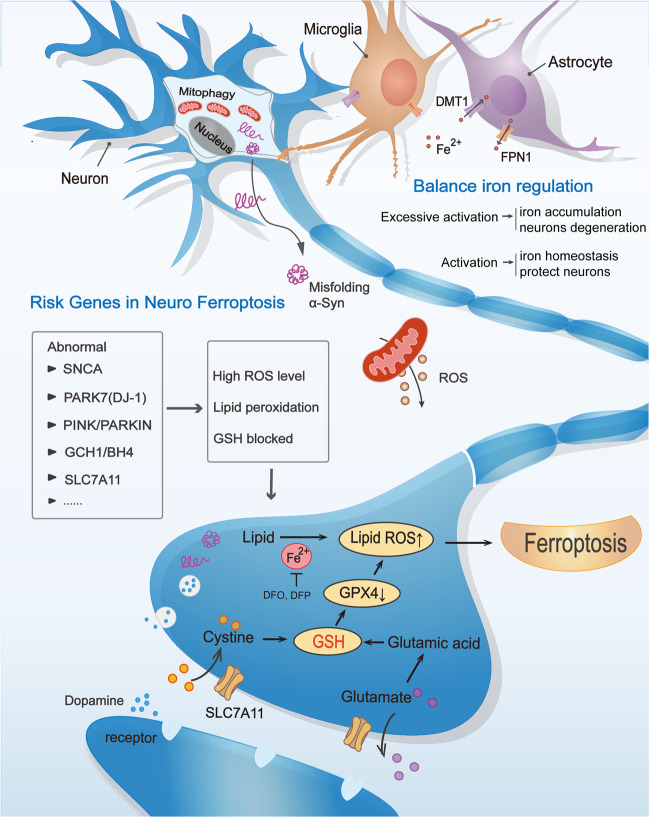
A basic axis of cellular antioxidant defense depends on the non-ribosomal tripeptide glutathione. Although controllable ROS signaling may be beneficial, when ROS and iron co-accumulate, highly toxic free radicals and lipids peroxidation reaction produced eventually trigger cell death. Within the cell, cystine is reduced to cysteine, and the inhibition of system Xc− leads to the consumption of GSH and the inactivation of GPX4, which can cause or sensitize to ferroptosis (Fig. 1). It has been noted that PD has a close connection with ferroptosis as evidenced by iron deposition in the brain causing degeneration of dopaminergic neurons. However, this degeneration always occurs in the substantia nigra pars compacta; other iron-rich areas are not affected, and extensive neocortical changes constitute a much more complex pattern of iron dysregulation than was expected, emphasizing that is a complex biological process, and more factors are yet to be discovered.
Fig. 1. Ferroptosis and cell death mechanisms in Parkinson’s disease.
Some normal functions of PD risk genes and relationship of cell–cell crosstalk maintain neuronal activity, while the specific dysfunctions will cause the higher levels of lipid peroxidation and/or lower levels of glutathione (GSH), finally may induce neuronal network ferroptosis. BH4 tetrahydrobiopterin, DFO defetoxamine, DFP deferiprone, DMT1 divalent metal transporter 1, FPN1 ferroportin-1, GPX4 glutathione peroxidase 4, GSH glutathione, Lipid ROS lipid reactive oxygen species, ROS reactive oxygen species, α-syn alpha-synuclein.
It is exciting that increasing evidence shows that PD risk genes have a potential link with ferroptosis (Fig. 1). For example, misfolding or excess alpha-synuclein (α-syn) accumulation is a primary pathological hallmark of PD, and its encoding gene SNCA has multiple mutations, ultimately impairing neuronal function in specific regions of the brain. Recently, one study has investigated that in human iPSC-derived neurons with SNCA triplication, excessive α-synuclein oligomers into the cell membrane cause abnormal calcium signaling. The inhibitor Ferrostatin-1 (Fer-1) can prevents the interaction of aggregate-membrane, reduces iron-dependent free radical accumulation, and further prevents humans neuron death [1], suggesting that ferroptosis might be the missing piece of the puzzle explaining the vicious cycle between α-syn and subsequent cell death in PD. However, in neurodegenerative ferroptosis, the interdependence of protein aggregation, membrane damage, and oxidative stress may be the reason why models based only on a single factor do not show neurodegeneration. Further research is essential to establish whether α-syn regulate both iron and lipid homeostasis are also implicated in the ferroptosis pathway in PD.
It has been well documented that there is a large deletion and missense mutation in the DJ-1 (also known as PARK7) gene in PD patients. Our findings prove that DJ-1 depletion markedly inhibits GSH levels only when cystine import is blocked. Glutamate is a natural inhibitor of SLC7A11, which is increased in PD [2], further suggesting that neuronal cells with DJ-1 mutations experience high levels of ferroptosis. Actually, Vallerga et al. analyzed thousands of PD patients and this recent high-profile study found that a high degree of cg06690548 methylation on chromosome 4 is associated with downregulation of the SLC7A11 gene, thereby identifying SLC7A11 as a reasonable biological target in PD [3]. SLC7A11 has been considered a key regulator of ferroptosis for decades and the activation of transsulfuration pathway can protect dopaminergic neurons. We hypothesize by these evidence that the GSH metabolic network may be a bridge connecting ferroptosis and PD.
Emerging research has found that autophagy plays an important role in the induction of ferroptosis. Recent studies have provided evidence that, autophagy promotes HMGB1 release, and targeting HMGB1 can limit the inflammatory response to increased ferroptosis [4]. Although the direct link between HMGB1 gene mutations and PD development is unknown, elevated protein levels of HMGB1 have been detected in cerebrospinal fluid (CSF) and serum from PD patients [5]. In addition, the PINK1/PARKIN pathway has been implicated in HMGB1-induced mitophagy dysfunction in humans. This suggests understanding how autophagy promotes ferroptosis sensitivity in the pathological environment can improve therapeutic interventions by targeting ferroptosis pathways. On the other hand, ferroptosis can promote apoptosis at the early stage of PD, which might be associated with the phosphorylation of p53 signaling pathway. This is the first time that linked ferroptosis and p53 directly in PD. Ferroptosis inhibition could abolish subsequent apoptosis, while not vice versa [6]. Lipid peroxidation caused by ROS accumulation can induce apoptosis through NF-κB, MAPK, and PKC pathways; while ferroptosis is iron-dependent, higher levels of iron transporters increase iron-mediated ROS and subsequently lead to ferroptosis. The connectivity between ROS-induced lipid peroxidation and apoptosis and ferroptosis suggests that they help balance the life and death of neuro cellular stress.
Aside from these known PD risk genes, many studies aimed to identify other novel genes that are potential targets in PD treatment. Upon imbalance between the transcription of protective genes and iron-mediated damage accumulation, BACH1 stimulates ferroptosis at the transcriptional level [7]. A lack of BACH1 partially prevents the loss of the substantia nigra, which suggests that BACH1 represents a target for ferroptosis-related disease therapeutics, including PD. In addition, tyrosine hydroxylase is a tetrahydrobiopterin (BH4)-requiring monooxygenase that catalyzes the first step in the dopamine biosynthesis. Studies have shown that PD is associated with a decrease in BH4 in the CSF. Recently it have been shown that the GCH1-BH4-phospholipid axis can be used as the main regulator of ferroptosis, suppressing ferroptosis by selectively preventing consumption of phospholipids with two polyunsaturated fatty acyl tails [8]. This provides a unique protection mechanism that is independent of the GPX4/glutathione system by which some neuronal cells prevent death through ferroptosis.
In PD patients, the level of iron is significantly increased in the dense parts of the substantia nigra of the brain. The level of iron is positively correlated with the severity of the disease; thus, the therapeutic role of iron chelators in PD has received much attention. Ferroptosis inhibitors such as Fer-1 are used to inhibit ferroptosis occurrence in animal models of PD, and clinical trial showed improvements in iron deposition in the substantia nigra and the exercise ability of patients treated with deferiprone, but the ability of these small-molecule inhibitors to cross the blood-brain barrier may be one of the prerequisites for their mitigation of PD.
In addition to removing iron deposits directly, currently, researchers expect glial reprogramming to become a promising approach for treating neurodegenerative diseases. It is known that glial cells have the ability to accumulate and store large amounts of iron and have high resistance to iron-mediated toxicity. In addition to store iron via ferritin efficiently, astrocytes also release iron by FPN1 to achieve iron redistribution. Meanwhile, neurotoxin 6-hydroxydopamine, inducing iron accumulation in neurons, might also be capable of promoting iron transport rate in astroglia, indicating a different response between neurons and astroglia in PD. Interestingly, recent studies have found that M1 phagocytes are more resistant to pharmacologically induced ferroptosis than M2 phagocytes due to inducible nitric oxide synthase/nitric oxide (iNOS/NO) enrichment [9]. This finding may allow us to reconsider the role of iNOS inhibitors in PD treatment. The inflammatory environment around neuronal cells exhibits two-way characteristics; expanding the clinical value of targeted cell–cell crosstalk has the opportunity to providing treatment solutions for PD.
Finally, we hope that confirming the relationship between PD risk genes and ferroptosis also benefits the future treatment of the disease. With the current knowledge and evidence, it is difficult to predict whether other known PD risk genes are linked to ferroptosis. Further experiments are urgently needed to evaluate these genes. Negative results might suggest that there are at least two subtypes of PD based on ferroptosis status (“ferroptotic PD” and “nonferroptotic PD”), which might present an opportunity to precisely predict disease progression and formulate personalized treatments, including designing rational medication regimens and diet plans, to alleviate neuronal degeneration in patients.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81402951 to JC), Leading Talent of “Ten Thousand Plan”-National High-Level Talents Special Support Plan, and the Talent Project of Zhejiang Association for Science and Technology (No. 2018YCGC002 to JC).
Author contributions
HYZ and JC wrote the manuscript; QJH, BY, and JC directed the paper.
Competing interests
The authors declare no competing interests.
References
- 1.Angelova PR, Choi ML, Berezhnov AV, Horrocks MH, Hughes CD, De S, et al. Alpha synuclein aggregation drives ferroptosis: an interplay of iron, calcium and lipid peroxidation. Cell Death Differ. 2020;27:2781–96. doi: 10.1038/s41418-020-0542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao J, Chen X, Jiang L, Lu B, Yuan M, Zhu D, et al. DJ-1 suppresses ferroptosis through preserving the activity of S-adenosyl homocysteine hydrolase. Nat Commun. 2020;11:1251. doi: 10.1038/s41467-020-15109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vallerga CL, Zhang F, Fowdar J, McRae AF, Qi T, Nabais MF, et al. Analysis of DNA methylation associates the cystine-glutamate antiporter SLC7A11 with risk of Parkinson’s disease. Nat Commun. 2020;11:1238. doi: 10.1038/s41467-020-15065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wen Q, Liu J, Kang R, Zhou B, Tang D. The release and activity of HMGB1 in ferroptosis. Biochem Biophys Res Commun. 2019;510:278–83. doi: 10.1016/j.bbrc.2019.01.090. [DOI] [PubMed] [Google Scholar]
- 5.Angelopoulou E, Piperi C, Papavassiliou AG. High-mobility group box 1 in Parkinson’s disease: from pathogenesis to therapeutic approaches. J Neurochem. 2018;146:211–8. doi: 10.1111/jnc.14450. [DOI] [PubMed] [Google Scholar]
- 6.Zhang P, Chen L, Zhao Q, Du X, Bi M, Li Y, et al. Ferroptosis was more initial in cell death caused by iron overload and its underlying mechanism in Parkinson’s disease. Free Radic Bio Med. 2020;152:227–34. doi: 10.1016/j.freeradbiomed.2020.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Nishizawa H, Matsumoto M, Shindo T, Saigusa D, Kato H, Suzuki K, et al. Ferroptosis is controlled by the coordinated transcriptional regulation of glutathione and labile iron metabolism by the transcription factor BACH1. J Biol Chem. 2020;295:69–82. doi: 10.1074/jbc.RA119.009548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kraft VAN, Bezjian CT, Pfeiffer S, Ringelstetter L, Müller C, Zandkarimi F, et al. GTP cyclohydrolase 1/Tetrahydrobiopterin counteract ferroptosis through lipid remodeling. ACS Cent Sci. 2020;6:41–53. doi: 10.1021/acscentsci.9b01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kapralov AA, Yang Q, Dar HH, Tyurina YY, Anthonymuthu TS, Kim R, et al. Redox lipid reprogramming commands susceptibility of macrophages and microglia to ferroptotic death. Nat Chem Biol. 2020;16:278–90. doi: 10.1038/s41589-019-0462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]