Abstract
Cardiovascular and metabolic diseases are the leading causes of death and disability worldwide and impose a tremendous socioeconomic burden on individuals as well as the healthcare system. Fibronectin type III domain-containing 5 (FNDC5) is a widely distributed transmembrane glycoprotein that can be proteolytically cleaved and secreted as irisin to regulate glycolipid metabolism and cardiovascular homeostasis. In this review, we present the current knowledge on the predictive and therapeutic role of FNDC5 in a variety of cardiovascular and metabolic diseases, such as hypertension, atherosclerosis, ischemic heart disease, arrhythmia, metabolic cardiomyopathy, cardiac remodeling, heart failure, diabetes mellitus, and obesity.
Keywords: cardiovascular and metabolic diseases, FNDC5, biomarker, therapeutic target
Introduction
Cardiovascular and metabolic diseases, including obesity, diabetes mellitus, and multiple cardiovascular injuries, are the leading causes of death and disability worldwide due to unhealthy behaviors, environmental toxins and genetic variants, and these conditions impose a tremendous socioeconomic burden on individuals and the healthcare system [1]. Despite improvements in health consciousness and medical practice, the prognosis of cardiovascular and metabolic diseases remains unsatisfactory, and effective interventions are still lacking.
Fibronectin type III domain-containing 5 (FNDC5) functions as a type I transmembrane glycoprotein to be proteolytically cleaved at the carboxy terminal to release irisin, which is mainly composed of the fibronectin III domain of FNDC5 [2]. FNDC5 was initially identified by two independent laboratories in 2002 and has attracted considerable attention since its definition as the precursor of an exercise-induced polypeptide myokine 10 years later [2–4]. Emerging studies have determined the wide distribution of FNDC5 in different body compartments, especially in those with high energy demand (e.g., the heart, adipose tissue, brain, liver, and skeletal muscle). Most of these studies demonstrated that FNDC5 is mainly expressed in skeletal muscle and adipose tissue; nevertheless, we recently detected a higher abundance of Fndc5 mRNA in the heart than in skeletal muscle [2, 5–7]. In general, ~72% of circulating irisin is attributable to muscle secretion, while the remaining 28% appears from adipose tissue [2, 5]. Previous studies by us and other laboratories also revealed the pleiotropic roles of FNDC5 in regulating inflammation, oxidative stress, apoptosis, autophagy, angiogenesis, and mitochondrial function [6, 8–11]. However, its specific receptors remain elusive to date. Kim et al. reported that αV integrins are potential irisin receptors in osteocytes and fat cells and that chemical inhibition of αV integrins remarkably blocks signaling and function by irisin [12]. Irisin also interacts with transforming growth factor-beta type II receptor (TGFBR2) to activate the downstream mitogen-activated protein kinases, indicating TGFBR2 as a probable receptor for irisin [13]. In addition, some investigators proved that the biological functions of irisin might not depend on a receptor-mediated mode. Extracellular irisin can be taken up from circulation to lung cells via lipid raft-mediated endocytosis to prevent pulmonary ischemia–reperfusion (I/R) injury [10]. Moreover, the typical fibronectin III domain and plasma membrane distribution of FNDC5 strongly suggest its potential as a receptor for unidentified ligands [3]. With these findings in mind, FNDC5 was reported to be essential for regulating glycolipid metabolism and cardiovascular homeostasis under either basal or stress conditions [2, 14]. Liu et al. determined that FNDC5 deficiency causes lipid accumulation in the liver and increases serum nonesterified fatty acid levels under normal conditions [11]. FNDC5 overexpression or irisin treatment stimulate lipid oxidation and energy expenditure and decrease circulating free fatty acid concentrations at baseline [2, 15]. Li et al. found that neither overexpression nor knockout of FNDC5 affects growth or body weight and that plasma glucose and insulin levels are unaffected [16].
In this review, we characterize FNDC5 alterations in response to different cardiovascular and metabolic stresses to evaluate the predictive roles of these alterations in these diseases and summarize the biological functions of FNDC5 to determine its therapeutic value in cardiovascular and metabolic diseases.
FNDC5 and hypertension
Hypertension is a set of clinical and pathological syndromes and is characterized by the elevation of systolic and/or diastolic blood pressure [17]. Previous studies detected lower serum irisin levels in hypertensive patients and found that serum irisin levels negatively correlated with systolic/diastolic blood pressure [18–20]. In addition, the FNDC5 single-nucleotide polymorphism (SNP) rs1746661 T-allele variant was reported to be associated with higher systolic blood pressure, while no correlation was found between the other two SNPs (rs16835198 and rs3480) and hypertension [21, 22]. Intriguingly, the antihypertensive drugs valsartan and amlodipine notably increased serum irisin levels after 12 weeks of treatment [20]. The hypothalamic paraventricular nucleus (PVN) is an important autonomic control center in the brain that regulates neurohormonal output and sympathetic tone. Balancing the inhibitory and excitatory neuronal inputs within the PVN is crucial to prevent sympathetic overdrive and hypertension [23, 24]. Huo et al. found that central irisin restored neurotransmitter balance in the PVN and decreased plasma norepinephrine concentrations, thereby reducing blood pressure [25]. Wang et al. also demonstrated the inhibitory effect of peripheral irisin on blood pressure in both control and spontaneously hypertensive rats. However, they stated that central administration of irisin into the third brain ventricle activates neurons in the PVN and increases blood pressure [22]. The endothelium, vascular smooth muscle, and adventitial fibroblasts coordinately orchestrate to maintain vascular homeostasis, whose dysfunction contributes to arterial stiffness and hypertension. Previous studies showed that circulating irisin positively correlates with endothelium-dependent vasodilation [26, 27]. Mechanistically, irisin activates endothelial nitric oxide synthase to increase nitric oxide production and stimulates transient receptor potential vanilloid subtype 4 channel-dependent extracellular calcium influx [28–32]. In addition, irisin promotes endothelium-independent vasodilation by inhibiting extracellular calcium influx and intracellular release in mesenteric arteries [33]. Phenotypic activation of vascular smooth muscle cells (VSMCs) and adventitial fibroblasts promotes extracellular matrix deposition, vascular remodeling, and arterial stiffness. Song et al. and Ling et al. revealed that irisin treatment significantly decreases the synthesis and secretion of matrix components, thereby preventing vascular remodeling and hypertension [9, 34]. Hyperhomocysteinemia is a crucial risk factor for arterial stiffness and hypertension, whereas circulating irisin is inversely associated with the serum homocysteine concentration [35]. Collectively, these data reveal FNDC5 as a novel biomarker and promising therapeutic target for hypertension.
FNDC5 and atherosclerosis
Atherosclerosis is considered as a chronic inflammatory disease that is due to lipid metabolism disorder, endothelial injury, VSMC proliferation, monocyte adhesion, and foam cell formation, which then boosts matrix synthesis, artery calcification, wall thickening, luminal narrowing, and fibrous cap rupture and eventually causes thrombosis [36]. Circulating irisin levels are proven to be associated with the progression of vascular atherosclerosis and are regarded as an independent predictor for subclinical atherosclerosis [37–41]. Hisamatsu et al. observed a reverse correlation between serum irisin levels and artery calcification, and they also revealed that higher serum irisin levels correlated with a lower burden of coronary atherosclerosis [42]. These findings indicate a predictive role for FNDC5 expression in atherosclerosis. Dyslipidemia is one of the most important initiators of vascular injury and atherosclerosis, and FNDC5 plays an indispensable role in maintaining lipid homeostasis (reviewed in the “FNDC5 and obesity” section) [2, 11, 43]. In particular, apolipoprotein E (ApoE) is an important component of plasma lipoproteins required for normal lipoprotein metabolism and antiatherogenic regulation [44, 45]. Fuku et al. reported the association between FNDC5 rs16835198 and the APOE ε2/ε4 allele; however, whether this association directly causes atherosclerosis needs further clarification [46]. Endothelial damage is another key determinant for atherosclerosis and a prerequisite for lipid deposition, monocyte adhesion, and intimal thickening [36]. FNDC5 directly promotes endothelial cell proliferation and suppresses cell apoptosis and vascular inflammation, thereby sustaining endothelial homeostasis [47, 48]. In addition, Zhu et al. found that FNDC5 improved the function of endothelial progenitor cells and helped with endothelial repair [49]. The accumulation of oxidized low-density lipoprotein (oxLDL) is the main pathogenic factor for endothelial injury, monocyte recruitment and foam cell formation [36]. A previous study showed that FNDC5 alleviates oxLDL-induced endothelial inflammation, oxidative stress, and apoptosis, thereby preventing the development of atherosclerosis [50]. Monocytes, the predominant inflammatory cells during the development of atherosclerosis, are recruited to the subendothelial layer of the arterial wall, where they differentiate into macrophages and engulf oxidized lipoproteins to form foam cells [36]. In addition to proinflammatory cytokines, adhesion molecule upregulation is an important prerequisite for monocyte docking and infiltration. The results from Zang et al. indicated that FNDC5 suppressed the expression of vascular cell adhesion molecule-1 and inhibited monocyte adhesion to VSMCs [51]. However, higher irisin levels also caused E-selectin and intracellular adhesion molecule-1 upregulation in primary human umbilical vein endothelial cells [52]. VSMCs are another important source for foam cells, and a previous study showed that 45% of foam cells have a VSMC phenotype in advanced atherosclerosis lesions [53]. Zang et al. showed that FNDC5 treatment significantly inhibited oxLDL-mediated foam cell formation in VSMCs [51]. These studies clearly determine the antiatherogenic functions of FNDC5 and propose it as a potential therapeutic target.
FNDC5 and ischemic heart disease
Ischemic heart disease remains the primary cause of death worldwide due to the induction of congestive heart failure and life-threatening arrhythmias. Timely blood flow restoration by percutaneous coronary intervention is an effective intervention to rescue viable cardiomyocytes, but it also causes additional I/R damage [54]. Previous studies indicated that circulating irisin is decreased in patients with either stable coronary artery disease (CAD) or myocardial infarction but gradually increases after coronary bypass surgery [55–59]. Moreover, Aydin et al. found a completely negative correlation between circulating irisin and “gold standard” levels (serum troponin I, creatine kinase isoenzymes, and creatine kinase). In addition to serum irisin, they revealed that saliva irisin also correlated with serum “gold standard” levels, defining irisin as a new and perhaps noninvasive biomarker for patients with acute myocardial infarction [60]. Infarcted patients with lower serum irisin levels had more severe fibrotic remodeling, cardiac dysfunction and an increased risk for adverse cardiovascular outcomes [61–63]. In contrast, two studies reported a positive association between serum irisin and the risk for major adverse cardiovascular events. The authors explained that the higher serum irisin levels represent an irisin-resistant status with increased cardiovascular and metabolic risks or are a direct result due to severe muscle damage [64, 65]. Reactive oxygen species (ROS) overproduction promotes oxidative damage to biomacromolecules and triggers cardiomyocyte apoptosis [66]. Mitochondria are the major source of ROS within the myocardium. Previous studies suggested that FNDC5 targets mitochondria and interacts with antioxidant molecules to prevent ROS overproduction and cardiomyocyte apoptosis during myocardial I/R injury [10, 67, 68]. Surprisingly, excessive irisin increases mitochondrial ROS generation and aggravates cardiomyocyte apoptosis in a hypoxic environment [69]. Microvessel angiogenesis is a possible cardioprotective mechanism for ischemic heart disease, and we previously showed that revascularization is essential for the repair of infarcted myocardium [70]. FNDC5 markedly promotes the proliferation of vascular endothelial cells and increases microvessel density, thereby protecting against ischemic heart disease [8, 71]. Cardiac progenitor cells have been show to be the specific regenerative cell source within the heart and have beneficial effects on cardiac regeneration and neovascularization [72]. Zhao et al. found that irisin pretreatment enhances the protective capacity of cardiac progenitor cells in terms of myocardial repair and functional improvement in the infarcted heart [73]. In a randomized controlled trial, the investigators demonstrated that omega-3 fatty acid supplementation elevates serum irisin and then attenuates the inflammatory response as well as lipid metabolism dysfunction in male CAD patients [74]. Iloprost and sildenafil are the two pharmacological agents for blood supply restoration and reoxygenation via vasodilatation during ischemic conditions. Aydin et al. proved that irisin elevation by individual or combined administration of iloprost and sildenafil contributes to wound recovery and cardioprotection in myocardial infarction [75]. Overall, targeting FNDC5 may provide therapeutic value to patients with myocardial infarction.
FNDC5 and arrhythmia
Arrhythmia is characterized by an abnormal heart rate and/or rhythm due to a disorder in electrical impulse origination and/or propagation [76]. Considering that athletes have higher irisin levels and lower resting heart rates, it is reasonable to conclude that FNDC5 is required to maintain a normal heart beat. The physical cardiac conduction depends on stable electrocardial activity, normal cardiac structure, and balanced autonomic nervous system. Sundarrajan et al. showed that exogenous irisin increases the heart rate, while irisin knockdown has the opposite effect [77]. Calcium participates in regulating cell membrane potential, which is essential for the repolarization of myocardial action potential and neuronal excitatory conduction. Xie et al. found that irisin treatment significantly increases intracellular calcium concentration via an irisin-specific membrane receptor in H9C2 cells [78]. In addition, irisin treatment induces an increase in cytosolic calcium levels and neuronal depolarization of nucleus ambiguus neurons, thereby evoking bradycardia in conscious rats [79]. FNDC5 also activates the NF-E2-related factor 2 (Nrf2) pathway in the hypothalamic PVN, decreases circulating norepinephrine levels, and effectively prevents sympathetic overdrive [25]. Normal cardiac architecture is pivotal for electrocardial conduction, whereas structural remodeling triggers electrical remodeling and the occurrence of arrhythmia. As mentioned later (“FNDC5 and cardiac remodeling”), various studies have revealed the protective effect of FNDC5 on cardiac hypertrophy and fibrotic remodeling. These data support the involvement of FNDC5 in arrhythmia, and further studies are needed to characterize its exact role and potential molecular basis under pathological conditions.
FNDC5 and metabolic cardiomyopathy
Metabolic cardiomyopathy develops under the context of systemic metabolic disorders that are characterized by structural and functional alterations without CAD or hypertension. Hyperglycemia or hyperlipidemia-induced metabolic disturbance triggers chronic low-grade inflammation within the heart, which subsequently provokes oxidative injury, endoplasmic reticulum stress, mitochondrial dysfunction, cardiomyocyte apoptosis, and fibrotic remodeling, thereby leading to impairment of both diastolic and systolic functions [80]. Consistently, our previous studies revealed that the attenuation of inflammation, oxidative stress, and cardiomyocyte apoptosis by either genetic or pharmacological methods significantly improved cardiac dysfunction in response to diabetes mellitus or obesity [81–83]. The results from Stratigou et al. showed that serum irisin levels are independently related to inflammation, insulin resistance, and other cardiovascular and metabolic risk factors [84]. Moreover, FNDC5 overexpression remarkably diminished obesity-induced cardiac inflammation, oxidative stress, and hypertrophic remodeling [85]. Excessive lipid accumulation within the myocardium causes lipotoxicity and promotes myocardial inflammation, oxidative damage, and cardiomyocyte apoptosis. Interestingly, irisin incubation counteracts lipotoxicity and cardiomyocyte apoptosis [86]. Cardiac fibrosis is a key feature of metabolic cardiomyopathy and contributes to congestive heart failure and lethal arrhythmia. Endothelial-to-mesenchymal transition functions as an important source of cardiac fibroblasts and plays an indispensable role in fibrotic remodeling in the context of metabolic dysfunction [87]. We previously showed that inhibiting endothelial-to-mesenchymal transition significantly ameliorates fibrotic remodeling and cardiac dysfunction [88, 89]. Liu et al. proved the inhibitory role of FNDC5 in the high glucose-induced endothelial-to-mesenchymal transition and the cardioprotective effect during diabetic cardiomyopathy. Unexpectedly, they also found that high-dose irisin treatment accelerates the proliferation and migration of cardiac fibroblasts, thereby resulting in excessive collagen deposition and cardiac dysfunction [90]. Autophagy disorder is responsible for the initiation and progression of diabetic cardiomyopathy, and we previously showed that autophagy restoration notably decreases inflammation, oxidative stress, apoptosis, and cardiac dysfunction in diabetic hearts [91, 92]. Liu et al. demonstrated that Fndc5 overexpression prevents and that Fndc5 deficiency exacerbates autophagy impairment and lipid accumulation [11]. In contrast to the extensive effects of visceral adipose tissue (VAT) on the whole body, epicardial fat is physically next to the myocardium and shares the same microcirculation that is important for cardiac regulation [93]. Increased epicardial fat volume independently correlates with myocardial fat accumulation, fibrosis, and cardiac dysfunction in the context of metabolic disturbance [94, 95]. Previous studies have shown a significant association between circulating irisin and epicardial fat; however, more thorough studies are required to further dissect the underlying mechanisms [96, 97]. These results reveal the beneficial effect of FNDC5 against metabolic cardiomyopathy.
FNDC5 and cardiac remodeling
Cardiac remodeling is characterized by cardiomyocyte hypertrophy and interstitial fibrosis upon various cardiac stresses, leading to both enhanced myocardial stiffness and compromised cardiac contractility [98]. Under pathological conditions, cardiomyocyte hypertrophy occurs as an adaptive response to incremental biomechanical loads after the activation of a series of hypertrophic pathways. Beyond the cardiomyocyte-centric view, cardiac fibroblasts are involved in orchestrating fibrotic remodeling of the heart by regulating myocardial collagen turnover [87, 98]. Cardiac remodeling is an independent risk factor for heart failure, arrhythmia, and sudden death and serves as a key determinant of the clinical course and long-term outcome of patients with cardiovascular diseases [98]. Our previous studies provide further understanding of the pathogenesis of cardiac remodeling, and these data suggest that inhibiting cardiac remodeling by either pharmacological or genetic methods notably improves cardiac dysfunction and survival [99–103]. Bashar et al. revealed a negative correlation between the serum irisin level and myocardial collagen volume in infarcted hearts [62]. Further studies revealed that irisin treatment activates Nrf2 and suppresses the ROS/TGF-β/Smad pathway in cardiac fibroblasts, which subsequently blunts collagen synthesis and fibroblast-myofibroblast transformation [104]. In addition, irisin administration promotes angiogenesis in the infarct border zone and thus reduces cardiac fibrosis and ventricular dilation [8]. Smad3 is the best-known fibrogenic transcriptional factor that directly mediates myofibroblast activation and fibrotic response, and we previously showed that Smad3 inhibition significantly alleviates myofibroblast activation and cardiac fibrosis [105, 106]. Tiano et al. identified a putative Smad3 binding site in the Fndc5 promoter, and Smad3 activation decreased FNDC5 expression, indicating the connection between FNDC5 and Smad3 [107, 108]. A further study by Peng et al. proved that irisin interacts with TGFBR2 to interfere with its recruitment to TGFBR1, thereby preventing Smad3 activation and fibrogenesis [13]. In addition, irisin treatment inhibits the high glucose-induced endothelial-to-mesenchymal transition and has a cardioprotective effect on fibrotic remodeling in diabetic hearts [90]. FNDC5 also participates in the regulation of pathological cardiac hypertrophy. Li et al. proved that FNDC5 provokes a protective autophagy flux by activating 5′ AMP-activated protein kinase (AMPK)/Unc-51-like kinase 1 signaling to alleviate pressure overload-induced cardiac hypertrophy, whereas Yu et al. found that the antihypertrophic effect of FNDC5 might be attributed to AMPK-mediated mammalian target of rapamycin suppression in a pressure overload-induced hypertrophic model [16, 109]. In addition, Geng et al. suggested that FNDC5 prevents obesity-induced cardiac hypertrophy by decreasing intramyocardial inflammation and oxidative stress [85]. These studies reveal FNDC5 as a promising therapeutic target for cardiac remodeling.
FNDC5 and heart failure
Heart failure is the common end stage of various cardiovascular diseases and places a heavy burden on individuals, families and society as a whole. Currently, no reliable biomarkers (except N-terminal pro-brain natriuretic peptide, NT-proBNP) or effective therapeutic strategies are available for failing hearts [110]. As indicated in the above context, FNDC5 significantly improves cardiac dysfunction under different pathological conditions and prevents undesirable cardiac remodeling. Previous studies suggested that patients with heart failure have decreased serum irisin levels, especially in heart failure with reduced ejection fraction [63, 111]. Some investigators explained that the reduced irisin levels might be ascribed to inflammation or protein energy wasting instead of volume overload [112, 113]. A previous study demonstrated that infarcted patients with higher circulating irisin were more likely to develop heart failure and adverse cardiovascular outcomes [65]. In addition, serum irisin levels positively correlate with circulating BNP, the New York Heart Association class and 1-year all-cause mortality [114, 115]. More importantly, the results from Shen et al. confirm that serum irisin concentrations have a better prognostic value for acute heart failure than NT-proBNP [115]. Considering the beneficial effects of FNDC5, we thought higher circulating irisin might indicate an irisin-resistant status or a more severe cardiac injury. In addition, FNDC5 expression in skeletal muscle also correlates with the aerobic performance of heart failure patients [116]. These data suggest that FNDC5 could be regarded as a promising biomarker and therapeutic target for heart failure.
FNDC5 and other cardiovascular diseases
Doxorubicin remains the cornerstone of tumor chemotherapy regimens; however, its therapeutic value is extremely hampered by its cardiotoxicity. Inflammation, oxidative stress, and cardiomyocyte apoptosis are responsible for doxorubicin-elicited cardiac dysfunction [117]. Our previous studies confirmed that inhibiting these pathogenic factors markedly protects against doxorubicin-induced acute or chronic cardiotoxicity [66, 118–121]. Aydin et al. found that myocardial and serum irisin levels are upregulated in doxorubicin-treated rats, while we recently proved that doxorubicin treatment notably suppresses FNDC5 expression in murine hearts and H9C2 cells. Moreover, cardiomyocyte-specific FNDC5 overexpression or irisin infusion alleviates oxidative stress and cardiomyocyte apoptosis, thereby preventing doxorubicin-induced cardiac dysfunction [6, 122].
Septic cardiomyopathy is a common complication in patients with severe sepsis, manifesting as reversible cardiac depression and ventricular dilation [123]. We previously reported that restraining inflammation, apoptosis, and pyroptosis significantly prevent the progression of septic cardiomyopathy [124–126]. However, mitochondrial fission also contributes to septic cardiomyopathy by provoking mitochondrial fragmentation, oxidative damage, and apoptosis. Tan et al. observed that irisin treatment prevents mitochondrial fission and dysfunction, thereby ameliorating septic cardiomyopathy [127]. Lipopolysaccharide (LPS) is a main component of the outer membrane in gram-negative bacteria and functions as the major inducer of septic cardiomyopathy. A previous study observed that individuals with high circulating irisin have significantly lower serum LPS concentrations [128].
In addition, FNDC5 might also be implicated in the pathogenesis of cardiac dysfunction among hemodialysis patients. Kałużna et al. observed that serum irisin level negatively correlates with right ventricular diameter and identified it as a potential prognostic biomarker of cardiac status in hemodialysis patients [129].
FNDC5 and diabetes mellitus
Diabetes mellitus is a clinically heterogeneous endocrine/metabolic disorder caused by insulin deficiency or resistance, and no specific biomarkers or therapeutic strategies are available to date [130]. Many studies detected lower serum irisin levels in patients with type 2 diabetes mellitus (T2DM) that were further reduced with the progression of glucose intolerance [27, 129, 131–134]. In addition, Choi et al. demonstrated that higher irisin levels were associated with lower odds of prevalent newly diagnosed T2DM, suggesting a protective effect of irisin against T2DM [135]. However, a small number of researchers observed an increased serum irisin level in T2DM patients, and they attributed this elevation to a probable irisin-resistant status or a compensatory mechanism to improve glucose intolerance [52, 136–138]. In contrast to the decreased irisin level in T2DM patients, circulating irisin was increased in T1DM patients compared with nondiabetic control subjects [139–141]. This discrepancy was deemed to be a result of the distinct insulin levels in different types of diabetes mellitus. They demonstrated a negative correlation between circulating irisin and serum insulin levels and that increased irisin concentrations compensated for the reduced insulin levels [136, 139, 142, 143]. Some investigators also proved that serum irisin levels predict the status of inflammation, oxidative stress, glucose homeostasis, and insulin sensitivity in diabetic animals or patients, which confirms the predictive role of FNDC5 in diabetes mellitus [128, 137, 144, 145].
Many direct or indirect effects of FNDC5 on glucose regulatory mechanisms in different organs are required for its protection against insulin resistance and diabetes mellitus, which is reviewed in detail by Perakakis et al. [14]. In this review, we briefly summarized the role of FNDC5 in insulin regulation and glycometabolic processes. Previous studies determined that circulating irisin correlates with pancreatic β-cell function and that irisin treatment significantly improves glucolipotoxicity-associated β-cell apoptosis and insulin reduction [146–148]. In addition, Guo et al. showed that plasma free fatty acid-mediated FNDC5 downregulation elicits insulin resistance, whereas irisin supplementation can restore insulin sensitivity [15, 149]. Glucose uptake is the first step and a prerequisite for normal glycometabolic processes. It is well known that glucose uptake depends on the upregulation and membrane translocation of glucose transporter 4 (GLUT4). Many studies have suggested that FNDC5 increases GLUT4 expression and membrane translocation, thereby promoting glucose uptake in adipocytes and muscle cells [150–153]. In addition, FNDC5 enhances the abundance of lactate dehydrogenase A and pyruvate dehydrogenase kinase 1 (two crucial enzymes in glycolysis) to accelerate aerobic glycolysis [13, 154]. Hepatic gluconeogenesis is a key source of blood glucose, and some investigators observed an inhibitory effect of FNDC5 on gluconeogenesis [153, 155]. FNDC5 also suppresses the enzymes for glycogenolysis (glycogen phosphorylase) and enhances the enzymes for glycogen synthesis (glycogen synthase), thereby decreasing blood glucose [150, 155]. Moreover, several studies have reported the synergistic effects of irisin and other hormones in maintaining glucose homeostasis, such as betatrophin (also known as angiopoietin-like protein 8) and leptin [156, 157].
There are some specific types of diabetes mellitus, including gestational diabetes mellitus (GDM) and polycystic ovarian syndrome (PCOS). Previous studies observed that serum irisin concentrations are markedly increased in pregnant women but decreased in GDM patients, as is irisin in breast milk [158–163]. The authors of those studies suggest that irisin upregulation is a compensatory response to energy imbalance and hormone disorder during pregnancy and that decreased irisin exacerbates GDM progression [164, 165]. Of note, serum irisin downregulation in pregnant women correlates with an increased risk for GDM progression and can be identified as an early biomarker to predict later development of GDM [166, 167]. PCOS is a complex and heterogeneous disease that is frequently accompanied by insulin resistance [168]. In PCOS patients, most investigators detected an elevation in circulating irisin that might be secondary to insulin-resistant conditions or hyperandrogenism [143, 169–172]. Accordingly, Li et al. demonstrated that metformin treatment for 6 months improved insulin resistance and subsequently decreased serum irisin levels in PCOS patients [173]. Several studies observed that circulating irisin is downregulated in PCOS patients and that the reduction in granulosa cells exacerbates metabolic disturbance [174–176]. Circulating irisin in PCOS patients is also closely correlated with body fat content, bone mineral density, endometrial receptivity, and metabolic homeostasis [175, 177–179].
In addition, FNDC5 SNPs play an important role in regulating multiple metabolic processes, especially glucose homeostasis. Despite being rarely seen in patients with T2DM or diabetic nephropathy, the rs16835198 T allele significantly decreases T2DM prevalence among Egyptians [180]. Conversely, the rs16835198 G allele has insulin desensitizing action and causes increases in glycated hemoglobin and fasting plasma glucose [180–182]. FNDC5 SNP rs16835198 was reported to correlate with pancreatic β-cell function and glycometabolism; however, it does not affect fasting insulin levels [182, 183]. Moreover, the rs3480 G allele and rs726344 A allele were found to be linked to severe insulin resistance, whereas the T allele of rs1746661 correlates with higher systolic blood pressure and dyslipidemia among women with T2DM [21, 181, 182, 184]. A previous study found that the FNDC5 rs157069 T allele notably decreases insulin sensitivity by downregulating serum irisin levels and further promotes the development of proliferative diabetic retinopathy in a Chinese population [137, 185]. These results support the important role of FNDC5 expression and genetic SNPs in diabetes mellitus, including GDM and PCOS.
FNDC5 and obesity
Obesity has been shown to be an increasing global public health concern and serves as a predisposing factor for various cardiovascular and metabolic diseases [82, 186]. FNDC5 is widely distributed in different human tissues, especially adipose tissue, and helps to maintain normal lipid metabolism [5, 187]. Most studies found that circulating irisin levels and tissue FNDC5 expression are higher in obese patients but lower in normal-weight control subjects or anorexic individuals [188–192]. Human adipose tissue is a primary source of FNDC5, and adipocytes in VAT or subcutaneous adipose tissue (SAT) from overweight patients produce more irisin than those from healthy control subjects [5, 193, 194]. In addition, Gao et al. reported that irisin incubation further promotes irisin secretion from adipocytes by upregulating FNDC5 expression in an autocrine manner [195]. We reasonably speculate that this positive feedback helps to elevate circulating irisin levels in obese patients. However, some other studies reported conflicting results. Moreno-Navarrete et al. showed that circulating irisin and FNDC5 gene expression in adipose tissue or muscle are significantly decreased in association with obesity [196]. In addition, a reduction in serum irisin levels and FNDC5 expression within the brain has been observed in animals fed a high-fat diet [197, 198]. The discrepancy might be ascribed to the heterogeneity of subjects in these studies, as some studies included obese patients with metabolic syndrome who had decreased circulating irisin levels due to impaired beige adipogenesis [199, 200].
Generally, increased circulating irisin independently predicts a higher risk of obesity and positively correlates with adiposity indices, including body mass index, waist circumference, fat mass, and lipid profile [201–204]. Interestingly, previous studies indicated that higher irisin expression helps to maintain the homeostasis of lipid metabolism [2, 11, 205, 206]. These studies proved that FNDC5 overexpression or irisin treatment drives adipocyte browning and lipid oxidation and suppresses adipogenesis and cholesterol synthesis, thereby improving whole body energy expenditure and obesity [43, 207–211]. In addition, FNDC5 overexpression delays the accumulation and M1 polarization of macrophages in adipose tissue and reduces local inflammation to inhibit obesity progression [212–214]. Moreover, central or peripheral irisin treatment significantly suppresses food intake and energy input [215–217]. FNDC5 SNPs are also involved in the pathogenesis of obesity and its complications. The results from Todendi et al. and others demonstrated that the rs726344 G allele and rs16835198 T-allele variants increase susceptibility to obesity [218, 219]. Two additional studies reported an association of the FNDC5 rs3480 G allele with hepatic steatosis and fibrotic remodeling [220, 221]. Considering these beneficial effects of FNDC5 on obesity, we speculate that FNDC5 upregulation acts as a compensatory mechanism for the increased fat storage and energy expenditure impairment.
Various pharmacological, nonpharmacological, or even surgical interventions are advised to increase circulating or local FNDC5 production and then improve abnormal lipid profiles together with obesity. Consistent with its powerful effect on FNDC5 upregulation and weight loss, kinesitherapy exercise has been proposed as a first-line strategy against obesity and relevant complications. Previous studies observed an increased circulating irisin level in obese subjects with different training modes, and Kartinah et al. further determined that exercise enhances irisin uptake from circulation into adipose tissue, where it maintains lipid homeostasis [222–225]. Obesity is an independent risk factor for other cardiovascular and metabolic diseases that are notably prevented by exercise-induced FNDC5 in our aforementioned parts. Kang et al. also reported that swimming effectively elevates circulating irisin and bone FNDC5 levels and improves obesity-elicited osteoporosis [226]. Intriguingly, compensatory upregulation of FNDC5 in overweight subjects is reduced in parallel with body weight after hypocaloric dietary treatment, indicating an improved lipid profile [227–229]. Furthermore, oral administration of resveratrol upregulates FNDC5 expression in human SAT and promotes adipocyte energy expenditure [230]. Of note, some investigators observed an alteration in circulating irisin in fatty patients after bariatric surgery, which is accompanied by significant weight loss and improved metabolic status [231–233]. These data provide a safe and efficacious intervention strategy that involves regulating FNDC5 expression to mitigate obesity in human subjects.
Conclusion and perspective
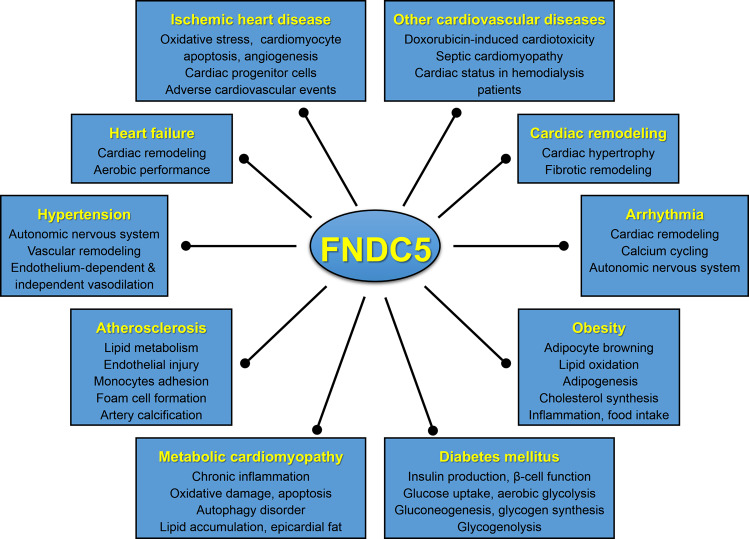
FNDC5 has received considerable attention since its first description as the precursor of an exercise-induced polypeptide myokine, irisin, which shares 100% identity between mice and humans. FNDC5 is widely distributed in different body compartments, especially in tissues with high energy demand, and has protective effects against cardiovascular and metabolic disturbance. The current review summarizes the potential involvement of FNDC5 in cardiovascular and metabolic homeostasis, thereby defining FNDC5 as a promising biomarker and therapeutic target for cardiovascular and metabolic diseases (Fig. 1). Despite the detailed understanding of the role of FNDC5 in various pathophysiological processes, several questions remain to be answered. First, special antibodies for distinguishing FNDC5 and irisin are urgently required. Second, identifying the specific receptors for irisin and dissecting the possible action modes will be a large leap forward in this field. Third, an accurate splicing procedure from FNDC5 to irisin demands further clarification. Fourth, whether the functions of FNDC5 are realized by cleaved irisin or by FNDC5 itself should be thoroughly explored. Finally, inconsistencies in reported data highlight the necessity for more accurate and well-designed clinical studies to demonstrate the predictive and therapeutic role of FNDC5 in cardiovascular and metabolic diseases.
Fig. 1. FNDC5 has pleiotropic effects on cardiovascular and metabolic diseases.
FNDC5 protects against these diseases by regulating multiple pathophysiological processes and serves as a promising biomarker and therapeutic target.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No.: 81470516 and 81700254), Key Project of the National Natural Science Foundation (No. 81530012), National Key R&D Program of China (2018YFC1311300), Fundamental Research Funds for the Central Universities (No. 2042017kf0085 and 2042018kf1032), and Development Center for Medical Science and Technology National Health and Family Planning Commission of the People’s Republic of China (The Prevention and Control Project of Cardiovascular Disease, 2016ZX-008-01) and Science and Technology Planning Projects of Wuhan (2018061005132295).
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
These authors contributed equally: Xin Zhang, Can Hu
Contributor Information
Zhen-guo Ma, Email: zhengma@whu.edu.cn.
Qi-zhu Tang, Email: qztang@whu.edu.cn.
References
- 1.Mechanick JI, Farkouh ME, Newman JD, Garvey WT. Cardiometabolic-based chronic disease, adiposity and dysglycemia drivers: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:525–38. doi: 10.1016/j.jacc.2019.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–8. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teufel A, Malik N, Mukhopadhyay M, Westphal H. Frcp1 and Frcp2, two novel fibronectin type III repeat containing genes. Gene. 2002;297:79–83. doi: 10.1016/S0378-1119(02)00828-4. [DOI] [PubMed] [Google Scholar]
- 4.Ferrer-Martinez A, Ruiz-Lozano P, Chien KR. Mouse PeP: a novel peroxisomal protein linked to myoblast differentiation and development. Dev Dyn. 2002;224:154–67. doi: 10.1002/dvdy.10099. [DOI] [PubMed] [Google Scholar]
- 5.Roca-Rivada A, Castelao C, Senin LL, Landrove MO, Baltar J, Belen CA, et al. FNDC5/irisin is not only a myokine but also an adipokine. PLoS One. 2013;8:e60563. doi: 10.1371/journal.pone.0060563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X, Hu C, Kong CY, Song P, Wu HM, Xu SC, et al. FNDC5 alleviates oxidative stress and cardiomyocyte apoptosis in doxorubicin-induced cardiotoxicity via activating AKT. Cell Death Differ. 2020;27:540–55. doi: 10.1038/s41418-019-0372-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X, Hu C, Yuan YP, Ma ZG, Tang QZ. A brief overview about the physiology of fibronectin type III domain-containing 5. Cell Signal. 2020;76:109805. [DOI] [PubMed]
- 8.Liao Q, Qu S, Tang LX, Li LP, He DF, Zeng CY, et al. Irisin exerts a therapeutic effect against myocardial infarction via promoting angiogenesis. Acta Pharmacol Sin. 2019;40:1314–21. doi: 10.1038/s41401-019-0230-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ling L, Chen D, Tong Y, Zang YH, Ren XS, Zhou H, et al. Fibronectin type III domain containing 5 attenuates NLRP3 inflammasome activation and phenotypic transformation of adventitial fibroblasts in spontaneously hypertensive rats. J Hypertens. 2018;36:1104–14. doi: 10.1097/HJH.0000000000001654. [DOI] [PubMed] [Google Scholar]
- 10.Chen K, Xu Z, Liu Y, Wang Z, Li Y, Xu X, et al. Irisin protects mitochondria function during pulmonary ischemia/reperfusion injury. Sci Transl Med. 2017;9:eaao6298. doi: 10.1126/scitranslmed.aao6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu TY, Xiong XQ, Ren XS, Zhao MX, Shi CX, Wang JJ, et al. FNDC5 alleviates hepatosteatosis by restoring AMPK/mTOR-mediated autophagy, fatty acid oxidation, and lipogenesis in mice. Diabetes. 2016;65:3262–75. doi: 10.2337/db16-0356. [DOI] [PubMed] [Google Scholar]
- 12.Kim H, Wrann CD, Jedrychowski M, Vidoni S, Kitase Y, Nagano K, et al. Irisin mediates effects on bone and fat via αV integrin receptors. Cell. 2018;175:1756–68. doi: 10.1016/j.cell.2018.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng H, Wang Q, Lou T, Qin J, Jung S, Shetty V, et al. Myokine mediated muscle-kidney crosstalk suppresses metabolic reprogramming and fibrosis in damaged kidneys. Nat Commun. 2017;8:1493. doi: 10.1038/s41467-017-01646-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perakakis N, Triantafyllou GA, Fernandez-Real JM, Huh JY, Park KH, Seufert J, et al. Physiology and role of irisin in glucose homeostasis. Nat Rev Endocrinol. 2017;13:324–37. doi: 10.1038/nrendo.2016.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiong XQ, Chen D, Sun HJ, Ding L, Wang JJ, Chen Q, et al. FNDC5 overexpression and irisin ameliorate glucose/lipid metabolic derangements and enhance lipolysis in obesity. Biochim Biophys Acta. 2015;1852:1867–75. doi: 10.1016/j.bbadis.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 16.Li RL, Wu SS, Wu Y, Wang XX, Chen HY, Xin JJ, et al. Irisin alleviates pressure overload-induced cardiac hypertrophy by inducing protective autophagy via mTOR-independent activation of the AMPK-ULK1 pathway. J Mol Cell Cardiol. 2018;121:242–55. doi: 10.1016/j.yjmcc.2018.07.250. [DOI] [PubMed] [Google Scholar]
- 17.Benetos A, Petrovic M, Strandberg T. Hypertension management in older and frail older patients. Circ Res. 2019;124:1045–60. doi: 10.1161/CIRCRESAHA.118.313236. [DOI] [PubMed] [Google Scholar]
- 18.Zhang LJ, Xie Q, Tang CS, Zhang AH. Expressions of irisin and urotensin II and their relationships with blood pressure in patients with preeclampsia. Clin Exp Hypertens. 2017;39:460–7. doi: 10.1080/10641963.2016.1273945. [DOI] [PubMed] [Google Scholar]
- 19.De Meneck F, Victorino DSL, Oliveira V, Do FM. High irisin levels in overweight/obese children and its positive correlation with metabolic profile, blood pressure, and endothelial progenitor cells. Nutr Metab Cardiovasc Dis. 2018;28:756–64. doi: 10.1016/j.numecd.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Celik HT, Akkaya N, Erdamar H, Gok S, Kazanci F, Demircelik B, et al. The effects of valsartan and amlodipine on the levels of irisin, adropin, and perilipin. Clin Lab. 2015;61:1889–95. doi: 10.7754/Clin.Lab.2015.150420. [DOI] [PubMed] [Google Scholar]
- 21.Brondani LA, Boelter G, Assmann TS, Leitao CB, Canani LH, Crispim D. Irisin-encoding gene (FNDC5) variant is associated with changes in blood pressure and lipid profile in type 2 diabetic women but not in men. Metabolism. 2015;64:952–7. doi: 10.1016/j.metabol.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Wang X, Cao Y, Han W, Guo Y, Yang G, et al. Association of polymorphisms of preptin, irisin and adropin genes with susceptibility to coronary artery disease and hypertension. Medicine. 2020;99:e19365. doi: 10.1097/MD.0000000000019365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carmichael CY, Kuwabara JT, Pascale CL, Moreira JD, Mahne SE, Kapusta DR, et al. Hypothalamic paraventricular nucleus Gαi2 (guanine nucleotide-binding protein alpha inhibiting activity polypeptide 2) protein-mediated neural control of the kidney and the salt sensitivity of blood pressure. Hypertension. 2020;75:1002–11. doi: 10.1161/HYPERTENSIONAHA.119.13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mukerjee S, Gao H, Xu J, Sato R, Zsombok A, Lazartigues E. ACE2 and ADAM17 interaction regulates the activity of presympathetic neurons. Hypertension. 2019;74:1181–91. doi: 10.1161/HYPERTENSIONAHA.119.13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huo CJ, Yu XJ, Sun YJ, Li HB, Su Q, Bai J, et al. Irisin lowers blood pressure by activating the Nrf2 signaling pathway in the hypothalamic paraventricular nucleus of spontaneously hypertensive rats. Toxicol Appl Pharmacol. 2020;394:114953. doi: 10.1016/j.taap.2020.114953. [DOI] [PubMed] [Google Scholar]
- 26.Hou N, Han F, Sun X. The relationship between circulating irisin levels and endothelial function in lean and obese subjects. Clin Endocrinol. 2015;83:339–43. doi: 10.1111/cen.12658. [DOI] [PubMed] [Google Scholar]
- 27.Xiang L, Xiang G, Yue L, Zhang J, Zhao L. Circulating irisin levels are positively associated with endothelium-dependent vasodilation in newly diagnosed type 2 diabetic patients without clinical angiopathy. Atherosclerosis. 2014;235:328–33. doi: 10.1016/j.atherosclerosis.2014.04.036. [DOI] [PubMed] [Google Scholar]
- 28.Inoue K, Fujie S, Hasegawa N, Horii N, Uchida M, Iemitsu K, et al. Aerobic exercise training-induced irisin secretion is associated with the reduction of arterial stiffness via nitric oxide production in adults with obesity. Appl Physiol Nutr Metab. 2020;45:715–22. doi: 10.1139/apnm-2019-0602. [DOI] [PubMed] [Google Scholar]
- 29.Fu J, Han Y, Wang J, Liu Y, Zheng S, Zhou L, et al. Irisin lowers blood pressure by improvement of endothelial dysfunction via AMPK-Akt-eNOS-NO pathway in the spontaneously hypertensive rat. J Am Heart Assoc. 2016;5:e003433. doi: 10.1161/JAHA.116.003433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han F, Zhang S, Hou N, Wang D, Sun X. Irisin improves endothelial function in obese mice through the AMPK-eNOS pathway. Am J Physiol Heart Circ Physiol. 2015;309:H1501–8. doi: 10.1152/ajpheart.00443.2015. [DOI] [PubMed] [Google Scholar]
- 31.Ye L, Xu M, Hu M, Zhang H, Tan X, Li Q, et al. TRPV4 is involved in irisin-induced endothelium-dependent vasodilation. Biochem Biophys Res Commun. 2018;495:41–5. doi: 10.1016/j.bbrc.2017.10.160. [DOI] [PubMed] [Google Scholar]
- 32.Zhu D, Wang H, Zhang J, Zhang X, Xin C, Zhang F, et al. Irisin improves endothelial function in type 2 diabetes through reducing oxidative/nitrative stresses. J Mol Cell Cardiol. 2015;87:138–47. doi: 10.1016/j.yjmcc.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 33.Jiang M, Wan F, Wang F, Wu Q. Irisin relaxes mouse mesenteric arteries through endothelium-dependent and endothelium-independent mechanisms. Biochem Biophys Res Commun. 2015;468:832–6. doi: 10.1016/j.bbrc.2015.11.040. [DOI] [PubMed] [Google Scholar]
- 34.Song H, Xu J, Lv N, Zhang Y, Wu F, Li H, et al. Irisin reverses platelet derived growth factor-BB-induced vascular smooth muscle cells phenotype modulation through STAT3 signaling pathway. Biochem Biophys Res Commun. 2016;479:139–45. doi: 10.1016/j.bbrc.2016.07.052. [DOI] [PubMed] [Google Scholar]
- 35.Alis R, Sanchis-Gomar F, Pareja-Galeano H, Hernandez-Mijares A, Romagnoli M, Victor VM, et al. Association between irisin and homocysteine in euglycemic and diabetic subjects. Clin Biochem. 2014;47:333–5. doi: 10.1016/j.clinbiochem.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 36.Hopkins PN. Molecular biology of atherosclerosis. Physiol Rev. 2013;93:1317–542. doi: 10.1152/physrev.00004.2012. [DOI] [PubMed] [Google Scholar]
- 37.Sesti G, Andreozzi F, Fiorentino TV, Mannino GC, Sciacqua A, Marini MA, et al. High circulating irisin levels are associated with insulin resistance and vascular atherosclerosis in a cohort of nondiabetic adult subjects. Acta Diabetol. 2014;51:705–13. doi: 10.1007/s00592-014-0576-0. [DOI] [PubMed] [Google Scholar]
- 38.Lee MJ, Lee SA, Nam BY, Park S, Lee SH, Ryu HJ, et al. Irisin, a novel myokine is an independent predictor for sarcopenia and carotid atherosclerosis in dialysis patients. Atherosclerosis. 2015;242:476–82. doi: 10.1016/j.atherosclerosis.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Saadeldin MK, Elshaer SS, Emara IA, Maged M, Abdel-Aziz AK. Serum sclerostin and irisin as predictive markers for atherosclerosis in Egyptian type II diabetic female patients: a case control study. PLoS One. 2018;13:e206761. doi: 10.1371/journal.pone.0206761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moreno-Perez O, Reyes-Garcia R, Munoz-Torres M, Merino E, Boix V, Reus S, et al. High irisin levels in nondiabetic HIV-infected males are associated with insulin resistance, nonalcoholic fatty liver disease, and subclinical atherosclerosis. Clin Endocrinol. 2018;89:414–23. doi: 10.1111/cen.13800. [DOI] [PubMed] [Google Scholar]
- 41.Icli A, Cure E, Cumhur CM, Uslu AU, Balta S, Arslan S, et al. Novel myokine: Irisin may be an independent predictor for subclinic atherosclerosis in Behcet’s disease. J Investig Med. 2016;64:875–81. doi: 10.1136/jim-2015-000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hisamatsu T, Miura K, Arima H, Fujiyoshi A, Kadota A, Kadowaki S, et al. Relationship of serum irisin levels to prevalence and progression of coronary artery calcification: a prospective, population-based study. Int J Cardiol. 2018;267:177–82. doi: 10.1016/j.ijcard.2018.05.075. [DOI] [PubMed] [Google Scholar]
- 43.Tang H, Yu R, Liu S, Huwatibieke B, Li Z, Zhang W. Irisin inhibits hepatic cholesterol synthesis via AMPK-SREBP2 signaling. EBioMedicine. 2016;6:139–48. doi: 10.1016/j.ebiom.2016.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martins IJ, Hone E, Foster JK, Sunram-Lea SI, Gnjec A, Fuller SJ, et al. Apolipoprotein E, cholesterol metabolism, diabetes, and the convergence of risk factors for Alzheimer’s disease and cardiovascular disease. Mol Psychiatry. 2006;11:721–36. doi: 10.1038/sj.mp.4001854. [DOI] [PubMed] [Google Scholar]
- 45.Heeren J, Beisiegel U, Grewal T. Apolipoprotein E recycling: implications for dyslipidemia and atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:442–8. doi: 10.1161/01.ATV.0000201282.64751.47. [DOI] [PubMed] [Google Scholar]
- 46.Fuku N, Diaz-Pena R, Arai Y, Abe Y, Zempo H, Naito H, et al. Epistasis, physical capacity-related genes and exceptional longevity: FNDC5 gene interactions with candidate genes FOXOA3 and APOE. BMC Genom. 2017;18:803. doi: 10.1186/s12864-017-4194-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song H, Wu F, Zhang Y, Zhang Y, Wang F, Jiang M, et al. Irisin promotes human umbilical vein endothelial cell proliferation through the ERK signaling pathway and partly suppresses high glucose-induced apoptosis. PLoS One. 2014;9:e110273. doi: 10.1371/journal.pone.0110273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deng X, Huang W, Peng J, Zhu TT, Sun XL, Zhou XY, et al. Irisin alleviates advanced glycation end products-induced inflammation and endothelial dysfunction via inhibiting ROS-NLRP3 inflammasome signaling. Inflammation. 2018;41:260–75. doi: 10.1007/s10753-017-0685-3. [DOI] [PubMed] [Google Scholar]
- 49.Zhu G, Wang J, Song M, Zhou F, Fu D, Ruan G, et al. Irisin increased the number and improved the function of endothelial progenitor cells in diabetes mellitus mice. J Cardiovasc Pharmacol. 2016;68:67–73. doi: 10.1097/FJC.0000000000000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y, Mu Q, Zhou Z, Song H, Zhang Y, Wu F, et al. Protective effect of irisin on atherosclerosis via suppressing oxidized low density lipoprotein induced vascular inflammation and endothelial dysfunction. PLoS One. 2016;11:e158038. doi: 10.1371/journal.pone.0158038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zang YH, Chen D, Zhou B, Chen AD, Wang JJ, Gao XY, et al. FNDC5 inhibits foam cell formation and monocyte adhesion in vascular smooth muscle cells via suppressing NF-κB-mediated NLRP3 upregulation. Vasc Pharmacol. 2019;121:106579. doi: 10.1016/j.vph.2019.106579. [DOI] [PubMed] [Google Scholar]
- 52.Rana KS, Pararasa C, Afzal I, Nagel DA, Hill EJ, Bailey CJ, et al. Plasma irisin is elevated in type 2 diabetes and is associated with increased E-selectin levels. Cardiovasc Diabetol. 2017;16:147. doi: 10.1186/s12933-017-0627-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenfeld ME, Ross R. Macrophage and smooth muscle cell proliferation in atherosclerotic lesions of WHHL and comparably hypercholesterolemic fat-fed rabbits. Arteriosclerosis. 1990;10:680–7. doi: 10.1161/01.ATV.10.5.680. [DOI] [PubMed] [Google Scholar]
- 54.Moran AE, Forouzanfar MH, Roth GA, Mensah GA, Ezzati M, Flaxman A, et al. The global burden of ischemic heart disease in 1990 and 2010: the Global Burden of Disease 2010 study. Circulation. 2014;129:1493–501. doi: 10.1161/CIRCULATIONAHA.113.004046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anastasilakis AD, Koulaxis D, Kefala N, Polyzos SA, Upadhyay J, Pagkalidou E, et al. Circulating irisin levels are lower in patients with either stable coronary artery disease (CAD) or myocardial infarction (MI) versus healthy controls, whereas follistatin and activin A levels are higher and can discriminate MI from CAD with similar to CK-MB accuracy. Metabolism. 2017;73:1–8. doi: 10.1016/j.metabol.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 56.Emanuele E, Minoretti P, Pareja-Galeano H, Sanchis-Gomar F, Garatachea N, Lucia A. Serum irisin levels, precocious myocardial infarction, and healthy exceptional longevity. Am J Med. 2014;127:888–90. doi: 10.1016/j.amjmed.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 57.Kuloglu T, Aydin S, Eren MN, Yilmaz M, Sahin I, Kalayci M, et al. Irisin: a potentially candidate marker for myocardial infarction. Peptides. 2014;55:85–91. doi: 10.1016/j.peptides.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 58.Khorasani ZM, Bagheri RK, Yaghoubi MA, Chobkar S, Aghaee MA, Abbaszadegan MR, et al. The association between serum irisin levels and cardiovascular disease in diabetic patients. Diabetes Metab Syndr. 2019;13:786–90. doi: 10.1016/j.dsx.2018.11.050. [DOI] [PubMed] [Google Scholar]
- 59.Aydin S, Catak Z, Eren MN, Topal AE, Aydin S. Irisin in coronary bypass surgery. Cardiovasc Hematol Disord Drug Targets. 2018;18:208–14. doi: 10.2174/1871529X18666180511141151. [DOI] [PubMed] [Google Scholar]
- 60.Aydin S, Aydin S, Kobat MA, Kalayci M, Eren MN, Yilmaz M, et al. Decreased saliva/serum irisin concentrations in the acute myocardial infarction promising for being a new candidate biomarker for diagnosis of this pathology. Peptides. 2014;56:141–5. doi: 10.1016/j.peptides.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 61.Deng W. Association of serum irisin concentrations with presence and severity of coronary artery disease. Med Sci Monit. 2016;22:4193–7. doi: 10.12659/MSM.897376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bashar SM, Samir ES, Boraie MZ. Correlation between the blood level of irisin and the severity of acute myocardial infarction in exercise-trained rats. J Basic Clin Physiol Pharmacol. 2018;30:59–71. doi: 10.1515/jbcpp-2018-0090. [DOI] [PubMed] [Google Scholar]
- 63.Abd EN, Galal HM, El MK, Gadallah AI. Serum irisin level in myocardial infarction patients with or without heart failure. Can J Physiol Pharmacol. 2019;97:932–8. doi: 10.1139/cjpp-2018-0736. [DOI] [PubMed] [Google Scholar]
- 64.Aronis KN, Moreno M, Polyzos SA, Moreno-Navarrete JM, Ricart W, Delgado E, et al. Circulating irisin levels and coronary heart disease: association with future acute coronary syndrome and major adverse cardiovascular events. Int J Obes. 2015;39:156–61. doi: 10.1038/ijo.2014.101. [DOI] [PubMed] [Google Scholar]
- 65.Hsieh IC, Ho MY, Wen MS, Chen CC, Hsieh MJ, Lin CP, et al. Serum irisin levels are associated with adverse cardiovascular outcomes in patients with acute myocardial infarction. Int J Cardiol. 2018;261:12–7. doi: 10.1016/j.ijcard.2017.11.072. [DOI] [PubMed] [Google Scholar]
- 66.Hu C, Zhang X, Wei WY, Zhang N, Wu HM, Ma ZG, et al. Matrine attenuates oxidative stress and cardiomyocyte apoptosis in doxorubicin-induced cardiotoxicity via maintaining AMPKα/UCP2 pathway. Acta Pharm Sin B. 2019;9:690–701. doi: 10.1016/j.apsb.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Z, Chen K, Han Y, Zhu H, Zhou XY, Tan T, et al. Irisin protects heart against ischemia-reperfusion injury through a SOD2-dependent mitochondria mechanism. J Cardiovasc Pharmacol. 2018;72:259–69. doi: 10.1097/FJC.0000000000000608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao YT, Wang H, Zhang S, Du J, Zhuang S, Zhao TC. Irisin ameliorates hypoxia/reoxygenation-induced injury through modulation of histone deacetylase 4. PLoS One. 2016;11:e166182. doi: 10.1371/journal.pone.0166182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ho MY, Wen MS, Yeh JK, Hsieh IC, Chen CC, Hsieh MJ, et al. Excessive irisin increases oxidative stress and apoptosis in murine heart. Biochem Biophys Res Commun. 2018;503:2493–8. doi: 10.1016/j.bbrc.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 70.Liu FY, Fan D, Yang Z, Tang N, Guo Z, Ma SQ, et al. TLR9 is essential for HMGB1-mediated post-myocardial infarction tissue repair through affecting apoptosis, cardiac healing, and angiogenesis. Cell Death Dis. 2019;10:480. doi: 10.1038/s41419-019-1718-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao G, Zhang X, Xu P, Mi JY, Rui YJ. The protective effect of Irisin against ischemia-reperfusion injury after perforator flap grafting in rats. Injury. 2018;49:2147–53. doi: 10.1016/j.injury.2018.09.054. [DOI] [PubMed] [Google Scholar]
- 72.Sharma S, Mishra R, Bigham GE, Wehman B, Khan MM, Xu H, et al. A deep proteome analysis identifies the complete secretome as the functional unit of human cardiac progenitor cells. Circ Res. 2017;120:816–34. doi: 10.1161/CIRCRESAHA.116.309782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao YT, Wang J, Yano N, Zhang LX, Wang H, Zhang S, et al. Irisin promotes cardiac progenitor cell-induced myocardial repair and functional improvement in infarcted heart. J Cell Physiol. 2019;234:1671–81. doi: 10.1002/jcp.27037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Agh F, Mohammadzadeh HN, Djalali M, Nematipour E, Gholamhoseini S, Zarei M, et al. Omega-3 fatty acid could increase one of myokines in male patients with coronary artery disease: a randomized, double-blind, placebo-controlled trial. Arch Iran Med. 2017;20:28–33. [PubMed] [Google Scholar]
- 75.Aydin S, Kuloglu T, Aydin S, Yardim M, Azboy D, Temizturk Z, et al. The effect of iloprost and sildenafil, alone and in combination, on myocardial ischaemia and nitric oxide and irisin levels. Cardiovasc J Afr. 2017;28:389–96. doi: 10.5830/CVJA-2017-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Herring N, Kalla M, Paterson DJ. The autonomic nervous system and cardiac arrhythmias: current concepts and emerging therapies. Nat Rev Cardiol. 2019;16:707–26. doi: 10.1038/s41569-019-0221-2. [DOI] [PubMed] [Google Scholar]
- 77.Sundarrajan L, Yeung C, Hahn L, Weber LP, Unniappan S. Irisin regulates cardiac physiology in zebrafish. PLoS One. 2017;12:e181461. doi: 10.1371/journal.pone.0181461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xie C, Zhang Y, Tran TD, Wang H, Li S, George EV, et al. Irisin controls growth, intracellular Ca2+ signals, and mitochondrial thermogenesis in cardiomyoblasts. PLoS One. 2015;10:e136816. doi: 10.1371/journal.pone.0136816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brailoiu E, Deliu E, Sporici RA, Brailoiu GC. Irisin evokes bradycardia by activating cardiac-projecting neurons of nucleus ambiguus. Physiol Rep. 2015;3:e12419. doi: 10.14814/phy2.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nishida K, Otsu K. Inflammation and metabolic cardiomyopathy. Cardiovasc Res. 2017;113:389–98. doi: 10.1093/cvr/cvx012. [DOI] [PubMed] [Google Scholar]
- 81.Ma ZG, Yuan YP, Xu SC, Wei WY, Xu CR, Zhang X, et al. CTRP3 attenuates cardiac dysfunction, inflammation, oxidative stress and cell death in diabetic cardiomyopathy in rats. Diabetologia. 2017;60:1126–37. doi: 10.1007/s00125-017-4232-4. [DOI] [PubMed] [Google Scholar]
- 82.Ma ZG, Kong CY, Song P, Zhang X, Yuan YP, Tang QZ. Geniposide protects against obesity-related cardiac injury through AMPKalpha- and Sirt1-dependent mechanisms. Oxid Med Cell Longev. 2018;2018:6053727. doi: 10.1155/2018/6053727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang N, Yang Z, Xiang SZ, Jin YG, Wei WY, Bian ZY, et al. Nobiletin attenuates cardiac dysfunction, oxidative stress, and inflammatory in streptozotocin: induced diabetic cardiomyopathy. Mol Cell Biochem. 2016;417:87–96. doi: 10.1007/s11010-016-2716-z. [DOI] [PubMed] [Google Scholar]
- 84.Stratigou T, Dalamaga M, Antonakos G, Marinou I, Vogiatzakis E, Christodoulatos GS, et al. Hyperirisinemia is independently associated with subclinical hypothyroidism: correlations with cardiometabolic biomarkers and risk factors. Endocrine. 2018;61:83–93. doi: 10.1007/s12020-018-1550-3. [DOI] [PubMed] [Google Scholar]
- 85.Geng Z, Fan WY, Zhou B, Ye C, Tong Y, Zhou YB, et al. FNDC5 attenuates obesity-induced cardiac hypertrophy by inactivating JAK2/STAT3-associated inflammation and oxidative stress. J Transl Med. 2019;17:107. doi: 10.1186/s12967-019-1857-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moscoso I, Cebro-Marquez M, Rodriguez-Manero M, Gonzalez-Juanatey JR, Lage R. FNDC5/Irisin counteracts lipotoxic-induced apoptosis in hypoxic H9c2 cells. J Mol Endocrinol. 2019;63:151–9. doi: 10.1530/JME-19-0123. [DOI] [PubMed] [Google Scholar]
- 87.Ma ZG, Yuan YP, Wu HM, Zhang X, Tang QZ. Cardiac fibrosis: new insights into the pathogenesis. Int J Biol Sci. 2018;14:1645–57. doi: 10.7150/ijbs.28103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu Y, Hu ZF, Liao HH, Liu W, Liu J, Ma ZG, et al. Toll-like receptor 5 deficiency attenuates interstitial cardiac fibrosis and dysfunction induced by pressure overload by inhibiting inflammation and the endothelial-mesenchymal transition. Biochim Biophys Acta. 2015;1852:2456–66. doi: 10.1016/j.bbadis.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 89.Jin YG, Yuan Y, Wu QQ, Zhang N, Fan D, Che Y, et al. Puerarin protects against cardiac fibrosis associated with the inhibition of TGF-β1/Smad2-mediated endothelial-to-mesenchymal transition. PPAR Res. 2017;2017:2647129. doi: 10.1155/2017/2647129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu X, Mujahid H, Rong B, Lu QH, Zhang W, Li P, et al. Irisin inhibits high glucose-induced endothelial-to-mesenchymal transition and exerts a dose-dependent bidirectional effect on diabetic cardiomyopathy. J Cell Mol Med. 2018;22:808–22. doi: 10.1111/jcmm.13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xiao Y, Wu QQ, Duan MX, Liu C, Yuan Y, Yang Z, et al. TAX1BP1 overexpression attenuates cardiac dysfunction and remodeling in STZ-induced diabetic cardiomyopathy in mice by regulating autophagy. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1728–43. doi: 10.1016/j.bbadis.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 92.Wu QQ, Liu C, Cai ZL, Xie QW, Hu TT, Duan MX, et al. High-mobility group AT-hook 1 promotes cardiac dysfunction in diabetic cardiomyopathy via autophagy inhibition. Cell Death Dis. 2020;11:160. doi: 10.1038/s41419-020-2316-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gonzalez N, Moreno-Villegas Z, Gonzalez-Bris A, Egido J, Lorenzo O. Regulation of visceral and epicardial adipose tissue for preventing cardiovascular injuries associated to obesity and diabetes. Cardiovasc Diabetol. 2017;16:44. doi: 10.1186/s12933-017-0528-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Park HE, Choi SY, Kim M. Association of epicardial fat with left ventricular diastolic function in subjects with metabolic syndrome: assessment using 2-dimensional echocardiography. BMC Cardiovasc Disord. 2014;14:3. doi: 10.1186/1471-2261-14-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ng A, Strudwick M, van der Geest RJ, Ng A, Gillinder L, Goo SY, et al. Impact of epicardial adipose tissue, left ventricular myocardial fat content, and interstitial fibrosis on myocardial contractile function. Circ Cardiovasc Imaging. 2018;11:e7372. doi: 10.1161/CIRCIMAGING.117.007372. [DOI] [PubMed] [Google Scholar]
- 96.Sahin M, Canpolat AG, Corapcioglu D, Canpolat U, Emral R, Uysal AR. Association between circulating irisin levels and epicardial fat in patients with treatment-naive overt hyperthyroidism. Biomarkers. 2018;23:742–7. doi: 10.1080/1354750X.2018.1485056. [DOI] [PubMed] [Google Scholar]
- 97.Kaneda H, Nakajima T, Haruyama A, Shibasaki I, Hasegawa T, Sawaguchi T, et al. Association of serum concentrations of irisin and the adipokines adiponectin and leptin with epicardial fat in cardiovascular surgery patients. PLoS One. 2018;13:e201499. doi: 10.1371/journal.pone.0201499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu QQ, Xiao Y, Yuan Y, Ma ZG, Liao HH, Liu C, et al. Mechanisms contributing to cardiac remodelling. Clin Sci. 2017;131:2319–45. doi: 10.1042/CS20171167. [DOI] [PubMed] [Google Scholar]
- 99.Ma ZG, Yuan YP, Zhang X, Xu SC, Kong CY, Song P, et al. C1q-tumour necrosis factor-related protein-3 exacerbates cardiac hypertrophy in mice. Cardiovasc Res. 2019;115:1067–77. doi: 10.1093/cvr/cvy279. [DOI] [PubMed] [Google Scholar]
- 100.Ma ZG, Dai J, Yuan YP, Bian ZY, Xu SC, Jin YG, et al. T-bet deficiency attenuates cardiac remodelling in rats. Basic Res Cardiol. 2018;113:19. doi: 10.1007/s00395-018-0678-x. [DOI] [PubMed] [Google Scholar]
- 101.Ma ZG, Zhang X, Yuan YP, Jin YG, Li N, Kong CY, et al. A77 1726 (leflunomide) blocks and reverses cardiac hypertrophy and fibrosis in mice. Clin Sci. 2018;132:685–99. doi: 10.1042/CS20180160. [DOI] [PubMed] [Google Scholar]
- 102.Yuan Y, Yan L, Wu QQ, Zhou H, Jin YG, Bian ZY, et al. Mnk1 (Mitogen-activated protein kinase-interacting kinase 1) deficiency aggravates cardiac remodeling in mice. Hypertension. 2016;68:1393–9. doi: 10.1161/HYPERTENSIONAHA.116.07906. [DOI] [PubMed] [Google Scholar]
- 103.Zhang X, Hu C, Zhang N, Wei WY, Li LL, Wu HM, et al. Matrine attenuates pathological cardiac fibrosis via RPS5/p38 in mice. Acta Pharmacol Sin. 2020;41: in press. 10.1038/s41401-020-0473-8. [DOI] [PMC free article] [PubMed]
- 104.Chen RR, Fan XH, Chen G, Zeng GW, Xue YG, Liu XT, et al. Irisin attenuates angiotensin II-induced cardiac fibrosis via Nrf2 mediated inhibition of ROS/TGF-β1/Smad2/3 signaling axis. Chem Biol Interact. 2019;302:11–21. doi: 10.1016/j.cbi.2019.01.031. [DOI] [PubMed] [Google Scholar]
- 105.Yan L, Wei X, Tang QZ, Feng J, Zhang Y, Liu C, et al. Cardiac-specific mindin overexpression attenuates cardiac hypertrophy via blocking AKT/GSK3β and TGF-β1-Smad signalling. Cardiovasc Res. 2011;92:85–94. doi: 10.1093/cvr/cvr159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang X, Ma ZG, Yuan YP, Xu SC, Wei WY, Song P, et al. Rosmarinic acid attenuates cardiac fibrosis following long-term pressure overload via AMPKα/Smad3 signaling. Cell Death Dis. 2018;9:102. doi: 10.1038/s41419-017-0123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tiano JP, Springer DA, Rane SG. SMAD3 negatively regulates serum irisin and skeletal muscle FNDC5 and peroxisome proliferator-activated receptor γ coactivator 1-α (PGC-1α) during exercise. J Biol Chem. 2015;290:7671–84. doi: 10.1074/jbc.M114.617399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guo Q, Wei XJ, Hu HL, Yang DQ, Zhang BY, Fan XP, et al. The saturated fatty acid palmitate induces insulin resistance through Smad3-mediated down-regulation of FNDC5 in myotubes. Biochem Biophys Res Commun. 2019;520:619–26. doi: 10.1016/j.bbrc.2019.10.077. [DOI] [PubMed] [Google Scholar]
- 109.Yu Q, Kou W, Xu X, Zhou S, Luan P, Xu X, et al. FNDC5/Irisin inhibits pathological cardiac hypertrophy. Clin Sci. 2019;133:611–27. doi: 10.1042/CS20190016. [DOI] [PubMed] [Google Scholar]
- 110.Hao G, Wang X, Chen Z, Zhang LF, Zhang YH, Wei BQ, et al. Prevalence of heart failure and left ventricular dysfunction in China: the China Hypertension Survey, 2012-2015. Eur J Heart Fail. 2019;21:1329–37. doi: 10.1002/ejhf.1629. [DOI] [PubMed] [Google Scholar]
- 111.Silvestrini A, Bruno C, Vergani E, Venuti A, Favuzzi A, Guidi F, et al. Circulating irisin levels in heart failure with preserved or reduced ejection fraction: a pilot study. PLoS One. 2019;14:e210320. doi: 10.1371/journal.pone.0210320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Matsuo Y, Gleitsmann K, Mangner N, Werner S, Fischer T, Bowen TS, et al. Fibronectin type III domain containing 5 expression in skeletal muscle in chronic heart failure-relevance of inflammatory cytokines. J Cachexia Sarcopenia Muscle. 2015;6:62–72. doi: 10.1002/jcsm.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhou SJ, Han QF, Zhang AH, Tang W, Sun LH. Irisin and volume overload are associated with protein energy wasting in peritoneal dialysis patients. Kidney Blood Press Res. 2017;42:1216–24. doi: 10.1159/000485925. [DOI] [PubMed] [Google Scholar]
- 114.Kalkan AK, Cakmak HA, Erturk M, Kalkan KE, Uzun F, Tasbulak O, et al. Adropin and irisin in patients with cardiac cachexia. Arq Bras Cardiol. 2018;111:39–47. doi: 10.5935/abc.20180109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shen S, Gao R, Bei Y, Li J, Zhang H, Zhou Y, et al. Serum irisin predicts mortality risk in acute heart failure patients. Cell Physiol Biochem. 2017;42:615–22. doi: 10.1159/000477867. [DOI] [PubMed] [Google Scholar]
- 116.Lecker SH, Zavin A, Cao P, Arena R, Allsup K, Daniels KM, et al. Expression of the irisin precursor FNDC5 in skeletal muscle correlates with aerobic exercise performance in patients with heart failure. Circ Heart Fail. 2012;5:812–8. doi: 10.1161/CIRCHEARTFAILURE.112.969543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wallace KB, Sardao VA, Oliveira PJ. Mitochondrial determinants of doxorubicin-induced cardiomyopathy. Circ Res. 2020;126:926–41. doi: 10.1161/CIRCRESAHA.119.314681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yuan YP, Ma ZG, Zhang X, Xu SC, Zeng XF, Yang Z, et al. CTRP3 protected against doxorubicin-induced cardiac dysfunction, inflammation and cell death via activation of Sirt1. J Mol Cell Cardiol. 2018;114:38–47. doi: 10.1016/j.yjmcc.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 119.Zhang X, Zhu JX, Ma ZG, Wu HM, Xu SC, Song P, et al. Rosmarinic acid alleviates cardiomyocyte apoptosis via cardiac fibroblast in doxorubicin-induced cardiotoxicity. Int J Biol Sci. 2019;15:556–67. doi: 10.7150/ijbs.29907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hu C, Zhang X, Zhang N, Wei WY, Li LL, Ma ZG, et al. Osteocrin attenuates inflammation, oxidative stress, apoptosis, and cardiac dysfunction in doxorubicin-induced cardiotoxicity. Clin Transl Med. 2020;10:e124. doi: 10.1002/ctm2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hu C, Zhang X, Song P, Yuan YP, Kong CY, Wu HM, et al. Meteorin-like protein attenuates doxorubicin-induced cardiotoxicity via activating cAMP/PKA/SIRT1 pathway. Redox Biol. 2020;37:101747. doi: 10.1016/j.redox.2020.101747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Aydin S, Eren MN, Kuloglu T, Aydin S, Yilmaz M, Gul E, et al. Alteration of serum and cardiac tissue adropin, copeptin, irisin and TRPM2 expressions in DOX treated male rats. Biotech Histochem. 2015;90:197–205. doi: 10.3109/10520295.2014.977949. [DOI] [PubMed] [Google Scholar]
- 123.Merx MW, Weber C. Sepsis and the heart. Circulation. 2007;116:793–802. doi: 10.1161/CIRCULATIONAHA.106.678359. [DOI] [PubMed] [Google Scholar]
- 124.Song P, Shen DF, Meng YY, Kong CY, Zhang X, Yuan YP, et al. Geniposide protects against sepsis-induced myocardial dysfunction through AMPKα-dependent pathway. Free Radic Biol Med. 2020;152:186–96. doi: 10.1016/j.freeradbiomed.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 125.Li N, Zhou H, Wu HM, Wu QQ, Duan MX, Deng W, et al. STING-IRF3 contributes to lipopolysaccharide-induced cardiac dysfunction, inflammation, apoptosis and pyroptosis by activating NLRP3. Redox Biol. 2019;24:101215. doi: 10.1016/j.redox.2019.101215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wei WY, Ma ZG, Zhang N, Xu SC, Yuan YP, Zeng XF, et al. Overexpression of CTRP3 protects against sepsis-induced myocardial dysfunction in mice. Mol Cell Endocrinol. 2018;476:27–36. doi: 10.1016/j.mce.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 127.Tan Y, Ouyang HC, Xiao XC, Zhong JK, Dong ML. Irisin ameliorates septic cardiomyopathy via inhibiting DRP1-related mitochondrial fission and normalizing the JNK-LATS2 signaling pathway. Cell Stress Chaperones. 2019;24:595–608. doi: 10.1007/s12192-019-00992-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bonfante I, Chacon-Mikahil M, Brunelli DT, Gaspari AF, Duft RG, Oliveira AG, et al. Obese with higher FNDC5/Irisin levels have a better metabolic profile, lower lipopolysaccharide levels and type 2 diabetes risk. Arch Endocrinol Metab. 2017;61:524–33. doi: 10.1590/2359-3997000000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kaluzna M, Pawlaczyk K, Schwermer K, Hoppe K, Czlapka-Matyasik M, Ibrahim AY, et al. Adropin and irisin: new biomarkers of cardiac status in patients with end-stage renal disease? A preliminary study. Adv Clin Exp Med. 2019;28:347–53. doi: 10.17219/acem/81538. [DOI] [PubMed] [Google Scholar]
- 130.Hu C, Jia WP. Therapeutic medications against diabetes: what we have and what we expect. Adv Drug Deliv Rev. 2019;139:3–15. doi: 10.1016/j.addr.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 131.Hu WC, Wang R, Li J, Zhang J, Wang WH. Association of irisin concentrations with the presence of diabetic nephropathy and retinopathy. Ann Clin Biochem. 2016;53:67–74. doi: 10.1177/0004563215582072. [DOI] [PubMed] [Google Scholar]
- 132.Li BX, Yao Q, Guo SQ, Ma S, Dong YH, Xin HH, et al. Type 2 diabetes with hypertensive patients results in changes to features of adipocytokines: Leptin, Irisin, LGR4, and Sfrp5. Clin Exp Hypertens. 2019;41:645–50. doi: 10.1080/10641963.2018.1529779. [DOI] [PubMed] [Google Scholar]
- 133.Duran ID, Gulcelik NE, Unal M, Topcuoglu C, Sezer S, Tuna MM, et al. Irisin levels in the progression of diabetes in sedentary women. Clin Biochem. 2015;48:1268–72. doi: 10.1016/j.clinbiochem.2015.07.098. [DOI] [PubMed] [Google Scholar]
- 134.Wang LS, Song J, Wang C, Lin P, Liang K, Sun Y, et al. Circulating levels of betatrophin and irisin are not associated with pancreatic β-cell function in previously diagnosed type 2 diabetes mellitus patients. J Diabetes Res. 2016;2016:2616539. doi: 10.1155/2016/2616539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Choi YK, Kim MK, Bae KH, Seo HA, Jeong JY, Lee WK, et al. Serum irisin levels in new-onset type 2 diabetes. Diabetes Res Clin Pr. 2013;100:96–101. doi: 10.1016/j.diabres.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 136.Guilford BL, Parson JC, Grote CW, Vick SN, Ryals JM, Wright DE. Increased FNDC5 is associated with insulin resistance in high fat-fed mice. Physiol Rep. 2017;5:e13319. [DOI] [PMC free article] [PubMed]
- 137.Al-Daghri NM, Mohammed AK, Al-Attas OS, Amer OE, Clerici M, Alenad A, et al. SNPs in FNDC5 (irisin) are associated with obesity and modulation of glucose and lipid metabolism in Saudi subjects. Lipids Health Dis. 2016;15:54. doi: 10.1186/s12944-016-0224-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Garcia-Fontana B, Reyes-Garcia R, Morales-Santana S, Avila-Rubio V, Munoz-Garach A, Rozas-Moreno P, et al. Relationship between myostatin and irisin in type 2 diabetes mellitus: a compensatory mechanism to an unfavourable metabolic state? Endocrine. 2016;52:54–62. doi: 10.1007/s12020-015-0758-8. [DOI] [PubMed] [Google Scholar]
- 139.Espes D, Lau J, Carlsson PO. Increased levels of irisin in people with long-standing type 1 diabetes. Diabet Med. 2015;32:1172–6. doi: 10.1111/dme.12731. [DOI] [PubMed] [Google Scholar]
- 140.Faienza MF, Brunetti G, Sanesi L, Colaianni G, Celi M, Piacente L, et al. High irisin levels are associated with better glycemic control and bone health in children with type 1 diabetes. Diabetes Res Clin Pr. 2018;141:10–7. doi: 10.1016/j.diabres.2018.03.046. [DOI] [PubMed] [Google Scholar]
- 141.Ates I, Arikan MF, Erdogan K, Kaplan M, Yuksel M, Topcuoglu C, et al. Factors associated with increased irisin levels in the type 1 diabetes mellitus. Endocr Regul. 2017;51:1–7. doi: 10.1515/enr-2017-0001. [DOI] [PubMed] [Google Scholar]
- 142.Sanchis-Gomar F, Alis R, Pareja-Galeano H, Sola E, Victor VM, Rocha M, et al. Circulating irisin levels are not correlated with BMI, age, and other biological parameters in obese and diabetic patients. Endocrine. 2014;46:674–7. doi: 10.1007/s12020-014-0170-9. [DOI] [PubMed] [Google Scholar]
- 143.Adamska A, Karczewska-Kupczewska M, Lebkowska A, Milewski R, Gorska M, Otziomek E, et al. Serum irisin and its regulation by hyperinsulinemia in women with polycystic ovary syndrome. Endocr J. 2016;63:1107–12. doi: 10.1507/endocrj.EJ16-0249. [DOI] [PubMed] [Google Scholar]
- 144.Liu BW, Yin FZ, Qi XM, Fan DM, Zhang Y. The levels of serum irisin as a predictor of insulin resistance in Han Chinese adults with metabolically healthy obesity. Clin Lab. 2017;63:881–6. doi: 10.7754/Clin.Lab.2016.160805. [DOI] [PubMed] [Google Scholar]
- 145.Belviranli M, Okudan N, Celik F. Association of circulating irisin with insulin resistance and oxidative stress in obese women. Horm Metab Res. 2016;48:653–7. doi: 10.1055/s-0042-116155. [DOI] [PubMed] [Google Scholar]
- 146.Zhang D, Xie T, Leung PS. Irisin ameliorates glucolipotoxicity-associated β-cell dysfunction and apoptosis via AMPK signaling and anti-inflammatory actions. Cell Physiol Biochem. 2018;51:924–37. doi: 10.1159/000495395. [DOI] [PubMed] [Google Scholar]
- 147.Duan HK, Ma BC, Ma XF, Wang HS, Ni ZZ, Wang B, et al. Anti-diabetic activity of recombinant irisin in STZ-induced insulin-deficient diabetic mice. Int J Biol Macromol. 2016;84:457–63. doi: 10.1016/j.ijbiomac.2015.12.049. [DOI] [PubMed] [Google Scholar]
- 148.Yang ML, Chen PH, Jin H, Xie XM, Gao T, Yang LL, et al. Circulating levels of irisin in middle-aged first-degree relatives of type 2 diabetes mellitus-correlation with pancreatic β-cell function. Diabetol Metab Syndr. 2014;6:133. doi: 10.1186/1758-5996-6-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pang YL, Zhu HH, Xu JQ, Yang LH, Liu LJ, Li J. β-arrestin-2 is involved in irisin induced glucose metabolism in type 2 diabetes via p38 MAPK signaling. Exp Cell Res. 2017;360:199–204. doi: 10.1016/j.yexcr.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 150.Huh JY, Mougios V, Kabasakalis A, Fatouros I, Siopi A, Douroudos II, et al. Exercise-induced irisin secretion is independent of age or fitness level and increased irisin may directly modulate muscle metabolism through AMPK activation. J Clin Endocrinol Metab. 2014;99:E2154–61. doi: 10.1210/jc.2014-1437. [DOI] [PubMed] [Google Scholar]
- 151.Huh JY, Dincer F, Mesfum E, Mantzoros CS. Irisin stimulates muscle growth-related genes and regulates adipocyte differentiation and metabolism in humans. Int J Obes. 2014;38:1538–44. doi: 10.1038/ijo.2014.42. [DOI] [PubMed] [Google Scholar]
- 152.Lee HJ, Lee JO, Kim N, Kim JK, Kim HI, Lee YW, et al. Irisin, a novel myokine, regulates glucose uptake in skeletal muscle cells via AMPK. Mol Endocrinol. 2015;29:873–81. doi: 10.1210/me.2014-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Xin C, Liu JY, Zhang JL, Zhu D, Wang HC, Xiong LZ, et al. Irisin improves fatty acid oxidation and glucose utilization in type 2 diabetes by regulating the AMPK signaling pathway. Int J Obes. 2016;40:443–51. doi: 10.1038/ijo.2015.199. [DOI] [PubMed] [Google Scholar]
- 154.Zhang DD, Bae C, Lee J, Lee J, Jin ZY, Kang M, et al. The bone anabolic effects of irisin are through preferential stimulation of aerobic glycolysis. Bone. 2018;114:150–60. doi: 10.1016/j.bone.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 155.Liu TY, Shi CX, Gao R, Sun HJ, Xiong XQ, Ding L, et al. Irisin inhibits hepatic gluconeogenesis and increases glycogen synthesis via the PI3K/Akt pathway in type 2 diabetic mice and hepatocytes. Clin Sci. 2015;129:839–50. doi: 10.1042/CS20150009. [DOI] [PubMed] [Google Scholar]
- 156.Sanchis-Gomar F, Perez-Quilis C. The p38-PGC-1α-irisin-betatrophin axis: exploring new pathways in insulin resistance. Adipocyte. 2014;3:67–8. doi: 10.4161/adip.27370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gutierrez-Repiso C, Garcia-Serrano S, Rodriguez-Pacheco F, Garcia-Escobar E, Haro-Mora JJ, Garcia-Arnes J, et al. FNDC5 could be regulated by leptin in adipose tissue. Eur J Clin Invest. 2014;44:918–25. doi: 10.1111/eci.12324. [DOI] [PubMed] [Google Scholar]
- 158.Cui LL, Qiao TY, Xu F, Li ZL, Chen TT, Su HL, et al. Circulating irisin levels of prenatal and postnatal patients with gestational diabetes mellitus: a systematic review and meta-analysis. Cytokine. 2020;126:154924. doi: 10.1016/j.cyto.2019.154924. [DOI] [PubMed] [Google Scholar]
- 159.Ebert T, Stepan H, Schrey S, Kralisch S, Hindricks J, Hopf L, et al. Serum levels of irisin in gestational diabetes mellitus during pregnancy and after delivery. Cytokine. 2014;65:153–8. doi: 10.1016/j.cyto.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 160.Aydin S, Kuloglu T, Aydin S. Copeptin, adropin and irisin concentrations in breast milk and plasma of healthy women and those with gestational diabetes mellitus. Peptides. 2013;47:66–70. doi: 10.1016/j.peptides.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 161.Yuksel MA, Oncul M, Tuten A, Imamoglu M, Acikgoz AS, Kucur M, et al. Maternal serum and fetal cord blood irisin levels in gestational diabetes mellitus. Diabetes Res Clin Pr. 2014;104:171–5. doi: 10.1016/j.diabres.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 162.Zhao L, Li J, Li ZL, Yang J, Li ML, Wang GL. Circulating irisin is lower in gestational diabetes mellitus. Endocr J. 2015;62:921–6. doi: 10.1507/endocrj.EJ15-0230. [DOI] [PubMed] [Google Scholar]
- 163.Kuzmicki M, Telejko B, Lipinska D, Pliszka J, Szamatowicz M, Wilk J, et al. Serum irisin concentration in women with gestational diabetes. Gynecol Endocrinol. 2014;30:636–9. doi: 10.3109/09513590.2014.920006. [DOI] [PubMed] [Google Scholar]
- 164.Briana DD, Boutsikou M, Athanasopoulos N, Marmarinos A, Gourgiotis D, Malamitsi-Puchner A. Implication of the myokine irisin in maternal energy homeostasis in pregnancies with abnormal fetal growth. J Matern Fetal Neonatal Med. 2016;29:3429–33. doi: 10.3109/14767058.2015.1137283. [DOI] [PubMed] [Google Scholar]
- 165.Garces MF, Peralta JJ, Ruiz-Linares CE, Lozano AR, Poveda NE, Torres-Sierra AL, et al. Irisin levels during pregnancy and changes associated with the development of preeclampsia. J Clin Endocrinol Metab. 2014;99:2113–9. doi: 10.1210/jc.2013-4127. [DOI] [PubMed] [Google Scholar]
- 166.Wang P, Ma HH, Hou XZ, Song LL, Song XL, Zhang JF. Reduced plasma level of irisin in first trimester as a risk factor for the development of gestational diabetes mellitus. Diabetes Res Clin Pr. 2018;142:130–8. doi: 10.1016/j.diabres.2018.05.038. [DOI] [PubMed] [Google Scholar]
- 167.Erol O, Erkal N, Ellidag HY, Isenlik BS, Aydin O, Derbent AU, et al. Irisin as an early marker for predicting gestational diabetes mellitus: a prospective study. J Matern Fetal Neonatal Med. 2016;29:3590–5. doi: 10.3109/14767058.2016.1142967. [DOI] [PubMed] [Google Scholar]
- 168.Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14:270–84. doi: 10.1038/nrendo.2018.24. [DOI] [PubMed] [Google Scholar]
- 169.Chang CL, Huang SY, Soong YK, Cheng PJ, Wang CJ, Liang IT. Circulating irisin and glucose-dependent insulinotropic peptide are associated with the development of polycystic ovary syndrome. J Clin Endocrinol Metab. 2014;99:E2539–48. doi: 10.1210/jc.2014-1180. [DOI] [PubMed] [Google Scholar]
- 170.Li H, Xu X, Wang X, Liao X, Li L, Yang G, et al. Free androgen index and Irisin in polycystic ovary syndrome. J Endocrinol Invest. 2016;39:549–56. doi: 10.1007/s40618-015-0403-7. [DOI] [PubMed] [Google Scholar]
- 171.Bostanci MS, Akdemir N, Cinemre B, Cevrioglu AS, Ozden S, Unal O. Serum irisin levels in patients with polycystic ovary syndrome. Eur Rev Med Pharmacol Sci. 2015;19:4462–8. [PubMed] [Google Scholar]
- 172.Bacopoulou F, Athanasopoulos N, Efthymiou V, Mantzou A, Aravantinos L, Vlahopoulos S, et al. Serum irisin concentrations in lean adolescents with polycystic ovary syndrome. Clin Endocrinol. 2018;88:585–91. doi: 10.1111/cen.13555. [DOI] [PubMed] [Google Scholar]
- 173.Li MY, Yang ML, Zhou XX, Fang X, Hu WJ, Zhu W, et al. Elevated circulating levels of irisin and the effect of metformin treatment in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2015;100:1485–93. doi: 10.1210/jc.2014-2544. [DOI] [PubMed] [Google Scholar]
- 174.Abali R, Temel YI, Yuksel MA, Bulut B, Imamoglu M, Emirdar V, et al. Implications of circulating irisin and Fabp4 levels in patients with polycystic ovary syndrome. J Obstet Gynaecol. 2016;36:897–901. doi: 10.3109/01443615.2016.1174200. [DOI] [PubMed] [Google Scholar]
- 175.Wang W, Guo Y, Zhang XX, Zheng JH. Abnormal irisin level in serum and endometrium is associated with metabolic dysfunction in polycystic ovary syndrome patients. Clin Endocrinol. 2018;89:474–80. doi: 10.1111/cen.13805. [DOI] [PubMed] [Google Scholar]
- 176.Bakhshalizadeh S, Rabiee F, Shirazi R, Ghaedi K, Amidi F, Nasr-Esfahani MH. Assessment of PGC1α-FNDC5 axis in granulosa cells of PCOS mouse model. J Reprod Infertil. 2018;19:89–94. [PMC free article] [PubMed] [Google Scholar]
- 177.Pukajlo K, Laczmanski L, Kolackov K, Kuliczkowska-Plaksej J, Bolanowski M, Milewicz A, et al. Irisin plasma concentration in PCOS and healthy subjects is related to body fat content and android fat distribution. Gynecol Endocrinol. 2015;31:907–11. doi: 10.3109/09513590.2015.1065482. [DOI] [PubMed] [Google Scholar]
- 178.Li C, Zhou L, Xie Y, Guan C, Gao H. Effect of irisin on endometrial receptivity of rats with polycystic ovary syndrome. Gynecol Endocrinol. 2019;35:395–400. doi: 10.1080/09513590.2018.1529158. [DOI] [PubMed] [Google Scholar]
- 179.Gao S, Cheng Y, Zhao L, Chen Y, Liu Y. The relationships of irisin with bone mineral density and body composition in PCOS patients. Diabetes Metab Res Rev. 2016;32:421–8. doi: 10.1002/dmrr.2767. [DOI] [PubMed] [Google Scholar]
- 180.Khidr EG, Ali SS, Elshafey MM, Fawzy OA. Association of irisin and FNDC5 rs16835198 G>T gene polymorphism with type 2 diabetes mellitus and diabetic nephropathy. An Egyptian pilot study. Gene. 2017;626:26–31. doi: 10.1016/j.gene.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 181.Staiger H, Bohm A, Scheler M, Berti L, Machann J, Schick F, et al. Common genetic variation in the human FNDC5 locus, encoding the novel muscle-derived ‘browning’ factor irisin, determines insulin sensitivity. PLoS One. 2013;8:e61903. doi: 10.1371/journal.pone.0061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tanisawa K, Taniguchi H, Sun X, Ito T, Cao ZB, Sakamoto S, et al. Common single nucleotide polymorphisms in the FNDC5 gene are associated with glucose metabolism but do not affect serum irisin levels in Japanese men with low fitness levels. Metabolism. 2014;63:574–83. doi: 10.1016/j.metabol.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 183.Tang SS, Zhang R, Jiang F, Wang J, Chen M, Peng DF, et al. An interaction between a FNDC5 variant and obesity modulates glucose metabolism in a Chinese Han population. PLoS One. 2014;9:e109957. doi: 10.1371/journal.pone.0109957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ebert T, Kralisch S, Wurst U, Scholz M, Stumvoll M, Kovacs P, et al. Association of metabolic parameters and rs726344 in FNDC5 with serum irisin concentrations. Int J Obes. 2016;40:260–5. doi: 10.1038/ijo.2015.157. [DOI] [PubMed] [Google Scholar]
- 185.Tang SS, Zhang R, Jiang F, Wang J, Chen M, Peng DF, et al. Association between FNDC5 genetic variants and proliferative diabetic retinopathy in a Chinese population. Clin Exp Pharmacol Physiol. 2016;43:580–2. doi: 10.1111/1440-1681.12566. [DOI] [PubMed] [Google Scholar]
- 186.Saxton SN, Clark BJ, Withers SB, Eringa EC, Heagerty AM. Mechanistic links between obesity, diabetes, and blood pressure: role of perivascular adipose tissue. Physiol Rev. 2019;99:1701–63. doi: 10.1152/physrev.00034.2018. [DOI] [PubMed] [Google Scholar]
- 187.Aydin S, Kuloglu T, Aydin S, Kalayci M, Yilmaz M, Cakmak T, et al. A comprehensive immunohistochemical examination of the distribution of the fat-burning protein irisin in biological tissues. Peptides. 2014;61:130–6. doi: 10.1016/j.peptides.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 188.Gouda W, Mageed L, Shaker Y, Hamimy WI, Afify M. Assessment of serum vitamin D and irisin levels in obese patients. Clin Lab. 2018;64:180416. doi: 10.7754/Clin.Lab.2018.180416. [DOI] [PubMed] [Google Scholar]
- 189.Bensmaine F, Benomar K, Espiard S, Vahe C, Le Mapihan K, Lion G, et al. Irisin levels in LMNA-associated partial lipodystrophies. Diabetes Metab. 2019;45:67–75. doi: 10.1016/j.diabet.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 190.Kazeminasab F, Marandi SM, Ghaedi K, Safaeinejad Z, Esfarjani F, Nasr-Esfahani MH. A comparative study on the effects of high-fat diet and endurance training on the PGC-1α-FNDC5/Irisin pathway in obese and nonobese male C57BL/6 mice. Appl Physiol Nutr Metab. 2018;43:651–62. doi: 10.1139/apnm-2017-0614. [DOI] [PubMed] [Google Scholar]
- 191.Elizondo-Montemayor L, Silva-Platas C, Torres-Quintanilla A, Rodriguez-Lopez C, Ruiz-Esparza GU, Reyes-Mendoza E, et al. Association of irisin plasma levels with anthropometric parameters in children with underweight, normal weight, overweight, and obesity. Biomed Res Int. 2017;2017:2628968. doi: 10.1155/2017/2628968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Binay C, Paketci C, Guzel S, Samanci N. Serum irisin and oxytocin levels as predictors of metabolic parameters in obese children. J Clin Res Pediatr Endocrinol. 2017;9:124–31. doi: 10.4274/jcrpe.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Varela-Rodriguez BM, Pena-Bello L, Juiz-Valina P, Vidal-Bretal B, Cordido F, Sangiao-Alvarellos S. FNDC5 expression and circulating irisin levels are modified by diet and hormonal conditions in hypothalamus, adipose tissue and muscle. Sci Rep. 2016;6:29898. doi: 10.1038/srep29898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Perez-Sotelo D, Roca-Rivada A, Baamonde I, Baltar J, Castro AI, Dominguez E, et al. Lack of adipocyte-Fndc5/Irisin expression and secretion reduces thermogenesis and enhances adipogenesis. Sci Rep. 2017;7:16289. doi: 10.1038/s41598-017-16602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Gao S, Li F, Li H, Huang Y, Liu Y, Chen Y. Effects and molecular mechanism of GST-irisin on lipolysis and autocrine function in 3T3-L1 adipocytes. PLoS One. 2016;11:e147480. doi: 10.1371/journal.pone.0147480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Moreno-Navarrete JM, Ortega F, Serrano M, Guerra E, Pardo G, Tinahones F, et al. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J Clin Endocrinol Metab. 2013;98:E769–78. doi: 10.1210/jc.2012-2749. [DOI] [PubMed] [Google Scholar]
- 197.Barja-Fernandez S, Folgueira C, Castelao C, Al-Massadi O, Bravo SB, Garcia-Caballero T, et al. FNDC5 is produced in the stomach and associated to body composition. Sci Rep. 2016;6:23067. doi: 10.1038/srep23067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhao ZM, Yao MM, Wei L, Ge SJ. Obesity caused by a high-fat diet regulates the Sirt1/PGC-1α/FNDC5/BDNF pathway to exacerbate isoflurane-induced postoperative cognitive dysfunction in older mice. Nutr Neurosci. 2019;1–12. 10.1080/1028415X.2019.1581460. [DOI] [PubMed]
- 199.Yan B, Shi XL, Zhang HJ, Pan LL, Ma ZM, Liu SH, et al. Association of serum irisin with metabolic syndrome in obese Chinese adults. PLoS One. 2014;9:e94235. doi: 10.1371/journal.pone.0094235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Leung W, Yu AP, Lai C, Siu PM. Association of markers of proinflammatory phenotype and beige adipogenesis with metabolic syndrome in Chinese centrally obese adults. J Diabetes Res. 2018;2018:8956509. doi: 10.1155/2018/8956509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Jang HB, Kim HJ, Kang JH, Park SI, Park KH, Lee HJ. Association of circulating irisin levels with metabolic and metabolite profiles of Korean adolescents. Metabolism. 2017;73:100–8. doi: 10.1016/j.metabol.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 202.Eslampour E, Ebrahimzadeh F, Abbasnezhad A, Khosroshahi MZ, Choghakhori R, Asbaghi O. Association between circulating irisin and C-reactive protein levels: a systematic review and meta-analysis. Endocrinol Metab. 2019;34:140–9. doi: 10.3803/EnM.2019.34.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Palacios-Gonzalez B, Vadillo-Ortega F, Polo-Oteyza E, Sanchez T, Ancira-Moreno M, Romero-Hidalgo S, et al. Irisin levels before and after physical activity among school-age children with different BMI: a direct relation with leptin. Obesity. 2015;23:729–32. doi: 10.1002/oby.21029. [DOI] [PubMed] [Google Scholar]
- 204.Panagiotou G, Mu L, Na B, Mukamal KJ, Mantzoros CS. Circulating irisin, omentin-1, and lipoprotein subparticles in adults at higher cardiovascular risk. Metabolism. 2014;63:1265–71. doi: 10.1016/j.metabol.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Xiong Y, Wu ZH, Zhang B, Wang C, Mao FY, Liu X, et al. Fndc5 loss-of-function attenuates exercise-induced browning of white adipose tissue in mice. FASEB J. 2019;33:5876–86. doi: 10.1096/fj.201801754RR. [DOI] [PubMed] [Google Scholar]
- 206.Canivet CM, Bonnafous S, Rousseau D, Leclere PS, Lacas-Gervais S, Patouraux S, et al. Hepatic FNDC5 is a potential local protective factor against non-alcoholic fatty liver. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165705. doi: 10.1016/j.bbadis.2020.165705. [DOI] [PubMed] [Google Scholar]
- 207.Zhang HJ, Zhang XF, Ma ZM, Pan LL, Chen Z, Han HW, et al. Irisin is inversely associated with intrahepatic triglyceride contents in obese adults. J Hepatol. 2013;59:557–62. doi: 10.1016/j.jhep.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 208.Zhang Y, Xie C, Wang H, Foss RM, Clare M, George EV, et al. Irisin exerts dual effects on browning and adipogenesis of human white adipocytes. Am J Physiol Endocrinol Metab. 2016;311:E530–41. doi: 10.1152/ajpendo.00094.2016. [DOI] [PubMed] [Google Scholar]
- 209.Li H, Shen J, Wu T, Kuang JY, Liu QH, Cheng SH, et al. Irisin is controlled by farnesoid X receptor and regulates cholesterol homeostasis. Front Pharmacol. 2019;10:548. doi: 10.3389/fphar.2019.00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, Idelson C, et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014;19:302–9. doi: 10.1016/j.cmet.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zhang Y, Li R, Meng Y, Li S, Donelan W, Zhao Y, et al. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes. 2014;63:514–25. doi: 10.2337/db13-1106. [DOI] [PubMed] [Google Scholar]
- 212.Xiong XQ, Geng Z, Zhou B, Zhang F, Han Y, Zhou YB, et al. FNDC5 attenuates adipose tissue inflammation and insulin resistance via AMPK-mediated macrophage polarization in obesity. Metabolism. 2018;83:31–41. doi: 10.1016/j.metabol.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 213.Xing T, Kang Y, Xu X, Wang B, Du M, Zhu MJ. Raspberry supplementation improves insulin signaling and promotes brown-like adipocyte development in white adipose tissue of obese mice. Mol Nutr Food Res. 2018;62:1701035. doi: 10.1002/mnfr.201701035. [DOI] [PubMed] [Google Scholar]
- 214.Mazur-Bialy AI, Bilski J, Pochec E, Brzozowski T. New insight into the direct anti-inflammatory activity of a myokine irisin against proinflammatory activation of adipocytes. J Physiol Pharmacol. 2017;68:243–51. [PubMed] [Google Scholar]
- 215.Ferrante C, Orlando G, Recinella L, Leone S, Chiavaroli A, Di Nisio C, et al. Central inhibitory effects on feeding induced by the adipo-myokine irisin. Eur J Pharmacol. 2016;791:389–94. doi: 10.1016/j.ejphar.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 216.Butt ZD, Hackett JD, Volkoff H. Irisin in goldfish (Carassius auratus): effects of irisin injections on feeding behavior and expression of appetite regulators, uncoupling proteins and lipoprotein lipase, and fasting-induced changes in FNDC5 expression. Peptides. 2017;90:27–36. doi: 10.1016/j.peptides.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 217.Schlogl M, Piaggi P, Votruba SB, Walter M, Krakoff J, Thearle MS. Increased 24-hour ad libitum food intake is associated with lower plasma irisin concentrations the following morning in adult humans. Appetite. 2015;90:154–9. doi: 10.1016/j.appet.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Todendi PF, Klinger EI, Geraldo A, Brixner L, Reuter CP, Lindenau J, et al. Genetic risk score based on fat mass and obesity-associated, transmembrane protein 18 and fibronectin type III domain containing 5 polymorphisms is associated with anthropometric characteristics in South Brazilian children and adolescents. Br J Nutr. 2019;121:93–9. doi: 10.1017/S0007114518002738. [DOI] [PubMed] [Google Scholar]
- 219.Abdu AA, Hammoudah SA, Abd EGE, El-Attar LM, Shehab-Eldin WA. Obesity and its association with irisin level among individuals with FNDC5/Irisin gene variants RS16835198 and RS726344. Protein Pept Lett. 2018;25:560–9. doi: 10.2174/0929866525666180508120653. [DOI] [PubMed] [Google Scholar]
- 220.Petta S, Valenti L, Svegliati-Baroni G, Ruscica M, Pipitone RM, Dongiovanni P, et al. Fibronectin type III domain-containing protein 5 rs3480 A>G polymorphism, irisin, and liver fibrosis in patients with nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2017;102:2660–9. doi: 10.1210/jc.2017-00056. [DOI] [PubMed] [Google Scholar]
- 221.Metwally M, Bayoumi A, Romero-Gomez M, Thabet K, John M, Adams LA, et al. A polymorphism in the irisin-encoding gene (FNDC5) associates with hepatic steatosis by differential miRNA binding to the 3’UTR. J Hepatol. 2019;70:494–500. doi: 10.1016/j.jhep.2018.10.021. [DOI] [PubMed] [Google Scholar]
- 222.Shirvani H, Rahmati-Ahmadabad S. Irisin interaction with adipose tissue secretions by exercise training and flaxseed oil supplement. Lipids Health Dis. 2019;18:15. doi: 10.1186/s12944-019-0960-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Bluher S, Panagiotou G, Petroff D, Markert J, Wagner A, Klemm T, et al. Effects of a 1-year exercise and lifestyle intervention on irisin, adipokines, and inflammatory markers in obese children. Obesity. 2014;22:1701–8. doi: 10.1002/oby.20739. [DOI] [PubMed] [Google Scholar]
- 224.Lu Y, Li H, Shen SW, Shen ZH, Xu M, Yang CJ, et al. Swimming exercise increases serum irisin level and reduces body fat mass in high-fat-diet fed Wistar rats. Lipids Health Dis. 2016;15:93. doi: 10.1186/s12944-016-0263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Tine KN, Rosalyn SI, Nafi’Ah R. The effects of exercise regimens on irisin levels in obese rats model: comparing high-intensity intermittent with continuous moderate-intensity training. Biomed Res Int. 2018;2018:4708287. doi: 10.1155/2018/4708287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kang YS, Kim JC, Kim JS, Kim SH. Effects of swimming exercise on serum irisin and bone FNDC5 in rat models of high-fat diet-induced osteoporosis. J Sports Sci Med. 2019;18:596–603. [PMC free article] [PubMed] [Google Scholar]
- 227.Sajoux I, Lorenzo PM, Gomez-Arbelaez D, Zulet MA, Abete I, Castro AI, et al. Effect of a very-low-calorie ketogenic diet on circulating myokine levels compared with the effect of bariatric surgery or a low-calorie diet in patients with obesity. Nutrients. 2019;11:2368. doi: 10.3390/nu11102368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Crujeiras AB, Pardo M, Arturo RR, Navas-Carretero S, Zulet MA, Martinez JA, et al. Longitudinal variation of circulating irisin after an energy restriction-induced weight loss and following weight regain in obese men and women. Am J Hum Biol. 2014;26:198–207. doi: 10.1002/ajhb.22493. [DOI] [PubMed] [Google Scholar]
- 229.de la Iglesia R, Lopez-Legarrea P, Crujeiras AB, Pardo M, Casanueva FF, Zulet MA, et al. Plasma irisin depletion under energy restriction is associated with improvements in lipid profile in metabolic syndrome patients. Clin Endocrinol. 2014;81:306–11. doi: 10.1111/cen.12383. [DOI] [PubMed] [Google Scholar]
- 230.Andrade J, Barcala-Jorge AS, Batista-Jorge GC, Paraiso AF, Freitas KM, Lelis DF, et al. Effect of resveratrol on expression of genes involved thermogenesis in mice and humans. Biomed Pharmacother. 2019;112:108634. doi: 10.1016/j.biopha.2019.108634. [DOI] [PubMed] [Google Scholar]
- 231.Kumar S, Hossain J, Inge T, Balagopal PB. Changes in myokines in youths with severe obesity following Roux-en-Y gastric bypass surgery. JAMA Surg. 2019;154:668–9. doi: 10.1001/jamasurg.2019.0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Lee YJ, Heo Y, Choi JH, Park S, Kim KK, Shin DW, et al. Association of circulating irisin concentrations with weight loss after Roux-en-Y gastric bypass surgery. Int J Environ Res Public Health. 2019;16:660. doi: 10.3390/ijerph16040660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Huh JY, Panagiotou G, Mougios V, Brinkoetter M, Vamvini MT, Schneider BE, et al. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism. 2012;61:1725–38. doi: 10.1016/j.metabol.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]