Abstract
The effect of uridine on the myocardial ischemic and reperfusion injury was investigated. A possible mechanism of its cardioprotective action was established. Two rat models were used: (1) acute myocardial ischemia induced by occlusion of the left coronary artery for 60 min; and (2) myocardial ischemia/reperfusion with 30-min ischemia and 120-min reperfusion. In both models, treatment with uridine (30 mg/kg) prevented a decrease in cell energy supply and in the activity of the antioxidant system, as well as an increase in the level of lipid hydroperoxides and diene conjugates. This led to a reduction of the necrosis zone in the myocardium and disturbances in the heart rhythm. The blocker of the mitochondrial ATP-dependent potassium (mitoKATP) channel 5-hydroxydecanoate limited the positive effects of uridine. The data indicate that the cardioprotective action of uridine may be related to the activation of the mitoKATP channel. Intravenously injected uridine was more rapidly eliminated from the blood in hypoxia than in normoxia, and the level of the mitoKATP channel activator UDP in the myocardium after uridine administration increased. The results suggest that the use of uridine can be a potentially effective approach to the management of cardiovascular diseases.
Subject terms: Biochemistry, Cardiology, Pathogenesis
Introduction
Despite some progress in the prevention and treatment of myocardial coronary artery disease, this pathology remains one of the main causes of disability and mortality in the world1. Hypoxia occurring in acute myocardial infarction leads to the reduction of mitochondrial respiratory chain complexes due to the lack of molecular oxygen, which is accompanied by a rapid depletion of high-energy phosphates2 and the accumulation of reactive oxygen species (ROS) in cardiomyocytes3. As a result, the dysfunction of energy-dependent ion pumps, metabolic acidosis, and oxidative stress can develop in the heart tissue4,5. The latter occurs when antioxidants are unable to remove excessive ROS, which leads to the destruction of integrity of cellular membranes. Prolonged and severe ischemia can induce irreversible damage, which culminates in cell death and the loss of viable myocardium2,4.
The European Society of Cardiology guidelines emphasize the importance of early reperfusion therapy of myocardial infarction6. Thrombolytic therapy and primary percutaneous coronary intervention made it possible to reduce significantly the risk of acute myocardial ischemic injury and decrease mortality in the case of the acute coronary syndrome. However, these interventions on their own can trigger metabolic alterations, such as intracellular calcium overloading and oxidative stress, which result in pathological changes in the structure and functioning of cardiomyocytes, a decrease in the myocardial contractile function, and the development of reperfusion arrhythmias2,4. The reperfusion injury of myocardium in itself can be the cause of death and requires novel therapeutic interventions.
Due to the high relevance of the problem of preserving the myocardial function, considerable efforts are aimed at the development of new pharmacological approaches to improve functional recovery and to limit the extent of tissue injury caused by ischemia/reperfusion (I/R). In this context, a problem of particular importance is the development of an integrated approach to the treatment of acute myocardial infarction and its reperfusion complications. Since acute myocardial infarction is a multifactor disease that causes cardiomyocyte death via multiple intracellular mechanisms7, the multitarget therapeutic strategy may be most effective. Under this approach, it is of great interest to find the ways of pharmacological control of the activity of the mitochondrial ATP-dependent potassium (mitoKATP) channel, which plays an important role in the protection of the myocardium from ischemic damage and can be the final effector in the cardioprotective effects of ischemic preconditioning5,8,9. Our previous studies have demonstrated that the intermittent hypoxic training of animals significantly increased the functional activity of this channel in isolated mitochondria10. Many synthetic activators of the mitoKATP channel were found to display cardioprotective effects in experimental research5,9. Their use in animal models promotes the preservation of the structural and functional integrity of mitochondria and heart tissue, the maintenance of ATP synthesis and, correspondingly, an increase in myocardial resistance under conditions of ischemia and reperfusion11. However, the most promising therapeutic approach would be the use of natural metabolic compounds, which are regulators of endogenous defense mechanisms and can be involved in the adaptation of the myocardium to pathological conditions. Among these activators are, for example, the sex hormones β-estradiol and testosterone12,13.
Earlier, we have found that uridine-5′-diphosphate (UDP) is an effective metabolic activator of the mitoKATP channel14. This phosphonucleotide at micromolar concentrations activates both the channel protein reconstructed into artificial membranes and the native channels localized in the inner mitochondrial membrane. However, the use of UDP for the channel activation is ineffective, since it is incapable of penetrating the cell membrane15 and, hence, affect the channels located in mitochondria. Therefore, a potential tool for the prevention and treatment of myocardial ischemic and I/R injury may be the metabolic precursor of UDP, pyrimidine nucleoside uridine, as it is transported into the cell where it serves as a source of UDP synthesis16.
The pharmacological activity of uridine and its nucleotides toward cardiovascular diseases is still poorly understood17. We have shown previously that uridine prevents the depression of the contractile function of the ischemic myocardium in isolated hearts18 and the development of myocardial stunning after postischemic reperfusion19. In addition, uridine exhibits antihypoxic activity20,21, reduces the posthypoxic pulmonary edema22, prevents oxidative stress during acute bacterial inflammation23, and increases the physical endurance of rats with low resistance to acute hypobaric hypoxia24.
The aim of the present work was to investigate the cardioprotective effect of uridine in vivo in the models of acute myocardial ischemia (AMI) and myocardial I/R in rats and to reveal whether the mitoKATP channel can be involved in the mechanism of action of the nucleoside.
Results
Elimination of exogenous uridine from the rat blood serum
Figure 1 shows that the background levels of uridine in control rats and rats with AMI were the same (5.92 ± 0.31 μmol/L and 5.93 ± 0.19 μmol/L, correspondingly). It should be noted that the results obtained are consistent with the literature data, which demonstrate that serum uridine is normally maintained at a relatively constant level in a range of 5.4–5.7 µmol/L25,26. Five minutes after the i.v. administration of the nucleoside to rats of both groups, its level in blood serum increased 11 times compared to the background level and amounted to 65.3 ± 3.6 μmol/L in the control and 63.9 ± 2.3 μmol/L in the AMI group (Fig. 1). In both groups, after a sharp increase in the concentration of serum uridine, its gradual elimination from the blood was observed. Within the first 15 min after the injection, the rate of uridine elimination was the same in two groups, and as a result, its level in the blood serum of animals in both groups did not differ. However, by 20 min of the experiment, the serum uridine level in rats with AMI sharply decreased to 10.3 ± 0.9 μmol/L; and by 35 min, it was even lower than the background level and remained approximately the same until the end of the experiment. In control rats, 20 min after the administration of uridine, its level was two times higher than in rats with the LCA occlusion. The elevated level of uridine in the control remained up to 65 min. Thus, intravenously administered uridine is more rapidly removed from the blood during AMI than under normal conditions and, hence, can be consumed by tissues at a higher rate.
Figure 1.
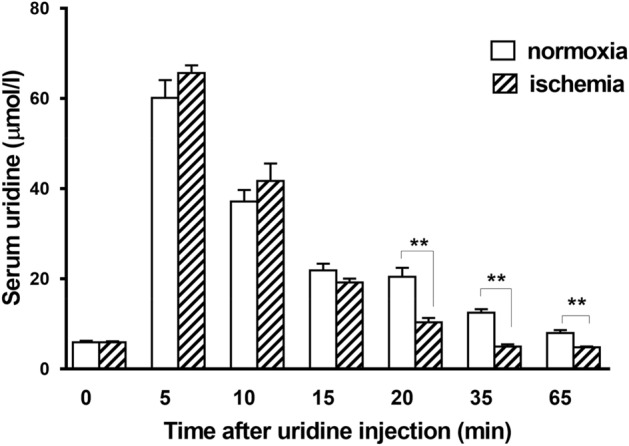
Levels of serum uridine after the intravenous administration of the nucleoside to rats in the control group (normoxia, n = 6) and in the group of animals with acute myocardial ischemia (AMI, n = 6). Uridine was given at a dose of 30 mg/kg five min before AMI. The determination of uridine in rat blood serum by HPLC was carried out at the following time points: 0 (background level), 5, 10, 15, 30, and 65 min after uridine administration. Data shown are the mean ± SD (n = 6). **p < 0.01 (vs. normoxia group).
The levels of UDP and UTP in the rat myocardium after uridine administration
The content of UDP and UTP in the myocardium of normal rats was significantly lower than the level of ATP. As follows from Table 1, the concentration of UTP was three times higher than that of UDP. At 65 min after the intravenous administration of uridine to control rats, the concentration of UDP and UTP in the myocardium increased 2.0 and 2.2 times, respectively. At the same time, the quantitative UDP/UTP ratio in the tissue remained approximately the same.
Table 1.
Concentration of UDP and UTP (nmol/g wet tissue) in the myocardium of control rats and rats with AMI 65 min after administration of uridine.
| Groups | n | UDP | UTP | UDP + UTP |
|---|---|---|---|---|
| Control (intact) | 8 | 37.8 ± 1.9 | 113.0 ± 7.9 | 150.8 ± 12.8 |
| Control + uridine | 6 | 74.4 ± 3.6*** | 248.6 ± 18.4*** | 323.0 ± 21.9*** |
| AMI | 5 | 52.0 ± 6.1*,§ | 67.8 ± 8.4**,§§§ | 119.8 ± 9.7*,§§§ |
| AMI + uridine | 5 | 76.6 ± 6.8***,## | 301.2 ± 32.7***,### | 377.8 ± 27.1***,### |
AMI, acute myocardial ischemia; UDP, uridine-diphosphate; UTP, uridine-triphosphateAll data are the mean ± SD (n = 5–8).
*p < 0.05 versus the control group; **p < 0.01 versus the control group, ***p < 0.001 versus the control group; §p < 0.05 versus the control + uridine group, §§§p < 0.001 versus the control + uridine group; ##p < 0.01 versus AMI group; ###p < 0.001 versus AMI group. Significance of differences was determined by a one-way ANOVA followed by the Turkey’s multiple comparison Post Hoc test.
At 60 min after the onset of AMI, the UDP level in the myocardium increased by 38%, and the UTP content decreased by 34% compared with the corresponding concentrations in control animals. One can assume that the increase in the UDP concentration is the result of enhanced decay of UTP, which occurs during ischemia and can play an adaptive role. In addition, intracellular UTP was found to be released in the circulation during cardiac ischemia27.
Uridine administration 5 min before the onset of AMI led to an increase in the UDP level in the myocardium by a factor of 1.5 compared to that observed 60 min after the onset of AMI without the injection of the drug. At the same time, the content of UTP increased 4.4 times, which can be related to its resynthesis from uridine and the restriction of its decomposition during AMI due to the anti-ischemic action of uridine.
The energy-saving effect of uridine
The occlusion of the LCA for 60 min led to the disorder of energy metabolism in the ischemic myocardium (Table 2). The ATP content decreased by 35% compared with that in sham-operated animals. A more pronounced decrease (by 59%) in the CrP concentration was observed. The treatment with uridine prevented a deficiency of macroergic compounds in the myocardium of rats with AMI, and the concentration of ATP and CrP became similar to that in sham-operated rats. The blocker of the mitoKATP channel 5-HD administered 5 min before uridine prevented the energy-saving effect of the nucleoside, and the levels of ATP and CrP were the same as in rats with AMI.
Table 2.
Effect of uridine on the level of macroergic compounds, lipid peroxidation and antioxidant status in the rat myocardium in acute ischemia (60 min).
| Groups | n | ATP (µmol/g wet tissue) | CrP (µmol/g wet tissue) | LPOs (OD532) | SOD activity (U/mg pr) | GSH (μmol/g wet tissue) |
|---|---|---|---|---|---|---|
| Sham-operated | 8 | 2.54 ± 0.15 | 6.63 ± 0.18 | 0.070 ± 0.003 | 4.54 ± 0.04 | 34.37 ± 0.62 |
| AMI | 10 | 1.65 ± 0.15*** | 2.75 ± 0.13*** | 0.138 ± 0.014*** | 3.26 ± 0.02*** | 23.99 ± 1.02*** |
| AMI + uridine | 10 | 2.81 ± 0.13### | 6.56 ± 0.26### | 0.077 ± 0.003### | 3.98 ± 0.10***,### | 33.61 ± 1.73### |
| AMI + uridine + 5-HD | 10 | 1.41 ± 0.05***,††† | 2.89 ± 0.29***,††† | 0.124 ± 0.005***,††† | 3.30 ± 0.08***,††† | 20.75 ± 1.25***,††† |
AMI acute myocardial ischemia, 5-HD 5-hydroxydecanoate, CrP creatine phosphate, LPOs lipid hydroperoxides, SOD superoxide dismutase, GSH reduced glutathione. All data are mean ± SEM (n = 8–10).
***p < 0.001 versus sham-operated group; ##p < 0.01 versus AMI group, ###p < 0.001 versus AMI group; †††p < 0.001 versus AMI + uridine group. Significance of differences was determined by a one-way ANOVA followed by the Turkey’s multiple comparison Post Hoc test.
Myocardial I/R also caused a sharp decrease in the level of macroergic compounds in the tissue (Table 3). The ATP level in the myocardium 120 min after the onset of reperfusion was 60% lower than that in sham-operated animals. Treatment with uridine preserved ATP in the myocardium, and its concentration after reperfusion was close to that in sham-operated rats. The positive effect of uridine was partially eliminated by 5-HD. The CrP level in control rats with I/R decreased by 67% by 120 min of reperfusion compared with that in sham-operated animals. Uridine treatment increased the amount of CrP twofold in comparison with the control (I/R) group. 5-HD decreased the effect of uridine by 39%.
Table 3.
Effect of uridine on ATP and CrP concentrations, lipid peroxidation and the antioxidant status in the rat myocardium after 120 min of reperfusion.
| Groups | n | ATP (µmol/g wet tissue) | CrP (µmol/g wet tissue) | LPOs (OD532) | DC (OD232) | SOD activity (U/mg pr) | GSH (μmol/g wet tissue) |
|---|---|---|---|---|---|---|---|
| Sham-operated | 8 | 2.77 ± 0.26 | 6.22 ± 0.36 | 0.068 ± 0.002 | 6.50 ± 0.41 | 4.72 ± 0.11 | 33.48 ± 0.63 |
| I/R | 10 | 1.15 ± 0.24*** | 2.04 ± 0.62*** | 0.096 ± 0.004*** | 10.55 ± 0.61*** | 4.03 ± 0.12*** | 25.49 ± 0.78*** |
| I/R + uridine | 10 | 2.50 ± 0.14### | 4.71 ± 0.29 ### | 0.063 ± 0.005### | 7.69 ± 0.52## | 4.67 ± 0.09### | 31.65 ± 0.97### |
| I/R + uridine + 5-HD | 9 | 1.46 ± 0.12***,†† | 2.89 ± 0.29***,† | 0.084 ± 0.004†† | 11.12 ± 0.68***, ††† | 4.10 ± 0.20**, †† | 28.28 ± 0.78***,† |
I/R ischemia/reperfusion, 5-HD 5-hydroxydecanoate, CrP creatine phosphate, LPOs lipid hydroperoxides, DC diene conjugates, SOD superoxide dismutase, GSH reduced glutathione. All data are mean ± SEM (n = 8–10).
**p < 0.01 versus sham-operated group; ***p < 0.001 versus sham-operated group; ##p < 0.01 versus I/R group, ###p < 0.001 versus I/R group; †p < 0.05 versus I/R + uridine group; ††p < 0.01 versus I/R + uridine group; †††p < 0.001 versus I/R + uridine group. Significance of differences was determined by a one-way ANOVA followed by the Turkey’s multiple comparison Post Hoc test.
Effect of uridine on lipid peroxidation and the antioxidant system
A deficiency of macroergic compounds in the ischemic myocardium was accompanied by enhanced lipid peroxidation. At 60 min after the occlusion, the level of lipid peroxides (LPOs) increased by 97% (Table 2). At the same time, the antioxidant system (AOS) was suppressed: the activity of superoxide dismutase (SOD) decreased by 28% and the amount of reduced glutathione (GSH) decreased by 30%.
The administration of uridine to animals 5 min before the ligation of the LCA completely prevented an increase in the production of LPOs and a decline in the level of GSH. The drug also limited a decrease in SOD activity. The data obtained indicate that uridine reduces the disorders in oxidative metabolism in the ischemic myocardium and restores the balance between the process of lipid peroxidation and the activity of AOS, which is important for maintaining the intracellular redox homeostasis during ischemia. 5-HD administered 5 min before uridine eliminated the protective effect of the nucleoside.
Postischemic reperfusion, similarly to acute ischemia, markedly increased lipid peroxidation and suppressed the antioxidant activity in the myocardium. At 120 min of reperfusion, the concentrations of the markers of oxidative stress LPOs and diene conjugates (DC) increased by 41% and 62%, respectively (Table 3). In parallel, a significant decrease in the activity of SOD and GSH was observed. The treatment with uridine completely prevented a reperfusion-induced increase in the amount of LPOs and reduced the formation of DC by 27% compared with the control. The nucleoside abolished the decline in SOD activity and increased the GSH level in the myocardium of rats with I/R to the level in sham-operated animals. The protective effect of uridine towards lipid peroxidation and antioxidant activity was diminished by simultaneous treatment with the inhibitor of the mitoKATP channel 5-HD.
Anti-ischemic action of uridine
Effect of uridine on the size of the ischemic zone in AMI
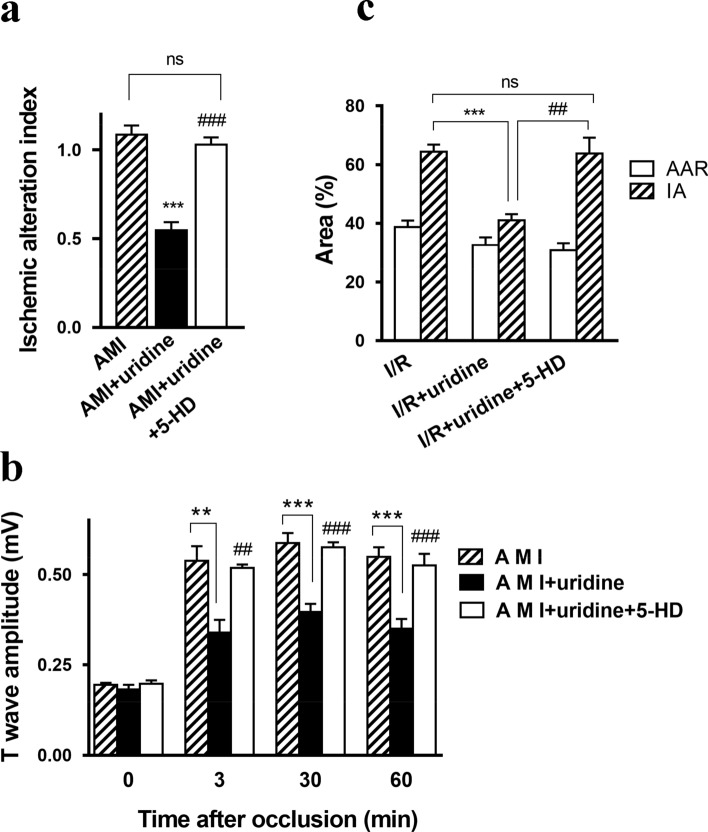
By 60 min after the LCA occlusion, the activity of glycogen phosphorylase in cardiomyocytes markedly decreased, indicating damage to the left myocardial ventricle and the formation of the ischemic alteration zone. The size of the zone at this time point was 30–60% of the total volume of the left ventricle. The ischemic alteration index (IAI) in control animals with AMI was 1.085 ± 0.071 (Fig. 2a). The treatment with uridine 5 min before the onset of ischemia induced a reduction in the IAI to 0.588 ± 0.056. A preliminary blockade of the mitoKATP channel with 5-HD almost completely eliminated the positive effect of the nucleoside. According to the literature data28, 5-HD alone does not affect the size of the myocardial alteration zone.
Figure 2.
Anti-ischemic effect of uridine treatment in the AMI and I/R models. (a) Size of the ischemic alteration zone and (b) T-wave amplitude on the EEG in the AMI model. AMI (n = 15), AMI + uridine (n = 6), AMI + uridine + 5-HD (n = 6). (c) Infarct area in the rat myocardium after 120 min of reperfusion in the I/R model. Abbreviations: AMI, acute myocardial ischemia; I/R, ischemia/reperfusion; 5-HD, 5-hydroxydecanoate; IA, infarct area; AAR, area at risk; LCA, the left coronary artery. The AAR was expressed as a percentage of the total area of the left ventricle, and the IA, as a percentage of the AAR. I/R (n = 9), I/R + uridine (n = 5), I/R + uridine + 5-HD (n = 6). All data are the mean ± SD. **p < 0.01 versus AMI (or I/R) group, ***p < 0.001 versus AMI (or I/R) group, ##p < 0.01 versus AMI (or I/R) + uridine group, ###p < 0.001 versus AMI (or I/R) + uridine group.
Effect of uridine on the amplitude of the T-wave
AMI induced a pronounced increase in the amplitude of the T-wave on the ECG (Fig. 2b, Supplementary Fig. S1 online), which reflects the extent of ischemic myocardial injury. After 3-min occlusion of the LCA, the amplitude increased 2.5 times compared to the initial value and remained at this level until the end of the observation period (60 min). Preliminary administration of uridine decreased the ischemia-induced elevation of the T-wave amplitude. Its positive effect was completely blocked by 5-HD.
Effect of uridine on the formation of the infarct area during myocardial I/R
I/R led to the formation of an area at risk (AAR) in all experimental animals, which was approximately 32–39% of the volume of the left ventricle. In control animals with I/R, the infarct area (IA) was 64.4 ± 2.5% of the AAR (Fig. 2c). The double administration of uridine 30 min before ischemia and 5 min before reperfusion decreased the IA by 36%. 5-HD given 5 min before each injection of uridine completely prevented the manifestation of its positive effect.
Anti-arrhythmic effect of uridine
Influence of uridine on early occlusion-induced arrhythmias
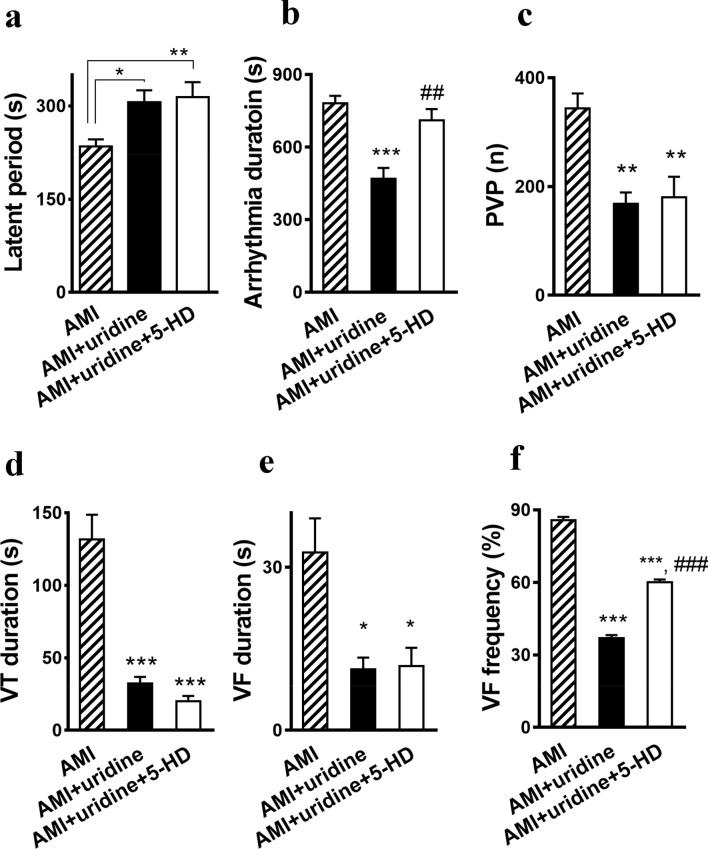
The LCA occlusion led to the development of early post-occlusion arrhythmias in all experimental groups. In control (AMI), the disturbances of the heart rhythm were observed in 100% of cases. The first episode of arrhythmia occurred 4–5 min after the LCA ligation (Fig. 3a). The average duration of arrhythmias was 14–16 min after which the normal sinus rhythm was restored. The arrhythmias were characterized by the occurrence of premature ventricular beats (PVB), ventricular tachycardia (VT), and ventricular fibrillation (VF) (Supplementary Fig. S2 online).
Figure 3.
Effect of uridine on early postocclusion arrhythmia. (a) A latent period between the LCA occlusion and the first episode of arrhythmia and (b) the duration of arrhythmia. AMI (n = 11), AMI + uridine (n = 7), AMI + uridine + 5-HD (n = 7). Effect of uridine on the arrhythmia manifestation: number of PVP (c), duration of VT (d), duration of VF (e), frequency of VF (f). Abbreviations: AMI, acute myocardial ischemia; 5-HD, 5-hydroxydecanoate; PVP, premature ventricular beats; VT, ventricular tachycardia; VF, ventricular fibrillation. All data (except F) are the mean ± SEM. *p < 0.05 versus AMI group, **p < 0.01 versus AMI group, ***p < 0.001 versus AMI group, ##p < 0.01 versus AMI + uridine group, ###p < 0.001 versus AMI + uridine group.
Uridine reduced the total duration of arrhythmia 1.8 times (Fig. 3в) and increased the latent period until the onset of the first episode of arrhythmia (Fig. 3a). It decreased the number of PVB from 344 ± 27 in the control to 180 ± 37 (Fig. 3c) and the duration of VT from 132 ± 17 s to 32 ± 5 s (Fig. 3d). The blockade of the mitoKATP channel by 5-HD did not significantly affect the manifestation of antiarrhythmic activity of uridine toward PVB and VT.
Along with the above positive effects of uridine, a significant antifibrillatory action of the drug was found. It decreased the duration of VF 2.75 times (Fig. 3e). Since the administration of 5-HD did not abolish this effect, the antifibrillatory activity of uridine is most likely not related to the activation of the mitoKATP channel. The nucleoside also reduced the incidence of VF up to 37% compared to 86% in the control (Fig. 3f). In this case, 5-HD partially prevented the effect of uridine, and the frequency of occurrence of VF increased to 60%.
Effect of uridine on reperfusion-induced arrhythmias
The restoration of the coronary blood flow after 7-min myocardial ischemia resulted in the development of reperfusion disorders of heart rhythms in 70% of cases in the control group, in 67% of cases in the group of animals treated with uridine, and in 90% of cases in animals treated with a combination of uridine + 5-HD. Representative ECG recordings at the different time points in the I/R rat model are shown in Supplementary Fig. S3 online. The data on the onset and duration of reperfusion-induced arrhythmias are presented in Fig. 4a,b. As can be seen, uridine did not affect these parameters.
Figure 4.
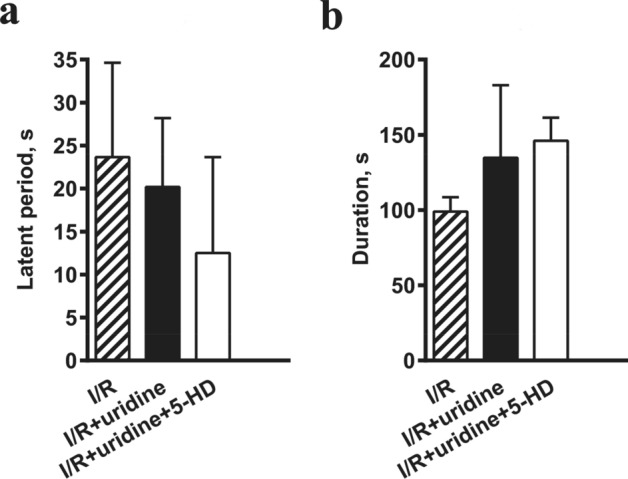
Effect of uridine on the onset of reperfusion-induced arrhythmias (a) and its duration (b). No statistical significance between I/R, I/R + uridine, and I/R + uridine + 5-HD groups was found.
The main manifestations of reperfusion tachyarrhythmia were VT and VF (Supplementary Fig. S4 online). Table 4 presents the data on the influence of uridine on the frequency of occurrence, duration, and the number of episodes of VT. One can see that uridine had no effect on the incidence of VT. At the same time, it reduced the total duration and the number of episodes of VT three and two times, correspondingly. The blockade of the mitoKATP channel significantly decreased the protective effect of uridine toward VT.
Table 4.
Effect of uridine on the ventricular tachycardia and ventricular fibrillation associated with myocardial I/R damage.
| Groups | n | Ventricular tachycardia | Ventricular fibrillation (VF) | |||
|---|---|---|---|---|---|---|
| Frequency of occurrence (%) | Duration, s | Number of episodes | Frequency of occurrence (%) | Including lethal VF (%) | ||
| I/R | 18 | 100 | 155 ± 27 | 155 ± 27 | 94% (17/18) | 59% (10/17) |
| I/R + uridine | 8 | 100 | 50 ± 12** | 50 ± 12** | 50% (4/8)* | 75% (3/4) |
| I/R + uridine + 5-HD | 10 | 100 | 118 ± 34## | 118 ± 34## | 90% (9/10)# | 56% (5/9) |
I/R ischemia/reperfusion, 5-HD 5-hydroxydecanoate. All data are the mean ± SD (n = 8–18).
*p < 0.05 versus I/R group, **p < 0.01 versus I/R group; #p < 0.05 versus I/R + uridine group, ##p < 0.01 versus I/R + uridine group. Significance of differences was determined by a one-way ANOVA followed by the Turkey’s multiple comparison Post Hoc test.
The data on the influence of uridine on the reperfusion-induced VF are presented in Table 4. It reduced the frequency of occurrence of VF by 50% but increased the number of lethal VF among the animals with this type of arrhythmia. 5-HD given prior to uridine reduced the number of lethal VF to the values in untreated animals with I/R.
An integral assessment of the severity of reperfusion-induced disturbances of heart rhythm was carried out according to a 6-score system29. The results showed that the intensity of the reperfusion tachyarrhythmia corresponds to 5.2 ± 0.2 and 3.9 ± 0.6 scores in untreated animals and animals treated with uridine, respectively (Table 4). The data obtained suggest that uridine exhibits a moderate antiarrhythmic effect toward reperfusion-induced arrhythmias.
Discussion
Using two rat models of myocardial injury, acute ischemia and ischemia–reperfusion, we have shown for the first time that treatment with uridine has a cardioprotective effect, which may be related to the activation of the mitoKATP channel by UDP. It should be noted that the use of the mitoKATP activator UDP as a pharmacological agent is complicated due to its instability and inability to penetrate through the cell membrane. At the same time, uridine is transported into the cell16 and could be a potential agent that increases the concentration of UDP in heart tissue.
In our experiments, acute ischemic injury of the myocardium resulted in the accelerated disappearance of uridine from the blood (Fig. 1). This is in agreement with the literature data that the perfusion of isolated rat hearts with a solution containing [H3] uridine shows the increased intensity of uridine uptake and its inclusion in uracil nucleotides in ischemia compared with normoxia30. It should be noted that, in our experiments, the period of the accelerated removal of uridine from the blood (15–30 min after the LCA occlusion) coincides with the most pronounced disturbances in energy metabolism in the myocardium31.
Simultaneously with the disappearance of uridine from the blood, the concentration of uridine nucleotides in the myocardial tissue changed. We found that the levels of UDP and UTP by 65 min after the administration of uridine to non-operated animals increased twofold (Table 1). In rats with AMI, the level of UDP in the myocardium increased, and the concentration of UTP decreased in comparison with the control (intact) group. Similar trends were noted in a culture of rat corticoencephalic cells under ischemic conditions32. It may be assumed that the increase in the level of UDP results from the disintegration of UTP to UDP provoked by ischemia. We do not exclude that this may be an endogenous protective mechanism leading to the activation of mitoKATP. The low level of UTP in the ischemic myocardium can be also associated with its release from cardiomyocytes due to an increase in cell membrane permeability27. In rats treated with uridine, the increase in the UDP and UTP levels in the ischemic myocardium was more pronounced than that in rats without treatment (Table 1), indicating that exogenous uridine could be a source for the synthesis of UDP and UTP in cardiomyocytes under the experimental pathology.
As known, the main pathogenetic link of myocardial ischemic damage is a deficiency of macroergic compounds33. The results of our study showed that AMI and I/R markedly diminish the level of CrP and ATP in the rat myocardium (Tables 2 and 3). The treatment with uridine provided protection against the destabilization of energy metabolism by maintaining the level of ATP and CrP in the ischemic myocardium close to the norm. The combined treatment with uridine and the blocker of the mitoKATP channel 5-HD (5 mg/kg) prevented the energy-stabilizing effect of uridine, suggesting the involvement of the channel in the mechanism of cardioprotection. It should be noted that the used dose of 5-HD alone has no cardiotropic and side effects28,34,35. It may be assumed that the activation of the channel leads to the preservation of the structure and function of mitochondria and their ability to synthesize ATP. At the same time, one cannot exclude that the energy-saving effect of uridine can be related to its ability to activate glycogen synthesis and thereby the operation of the Krebs cycle through an increase in the synthesis of UTP and UDP-glucose16,17,23. This is also consistent with our earlier data indicating that exogenous uridine nucleotides increased the glycogen content and prevented the decomposition of pyruvate and at the same time did not increase ischemia-induced acidosis in the rat myocardium31.
It is well known that long-term hypoxia and I/R can lead to an excessive increase in lipid peroxidation and the depletion of the AOS, that is, the development of oxidative stress, which is an important factor in morphological and functional disorders in cardiomyocytes6,36. According to our data, the level of LPOs in the myocardium of rats with AMI and I/R increased by 97% and 41%, respectively. I/R led also to an increase in the content of DC by 62% compared with the sham-operated group. At the same time, the primary line of antioxidant defense, the activity of SOD, and the second line of defense, the level of GSH, were suppressed. Our results showed that uridine significantly limited the increase in lipid peroxidation and inhibition of AOS. The blocker of the mitoKATP channel 5-HD administered to rats 5 min before uridine eliminated (in the case of SOD) or significantly decreased (in the case of LPO, DC, GSH) the positive effect of uridine. Based on the results obtained on both models, we suggest that the antioxidant action of uridine, as well as its energy-stabilizing effect, are related to the activation of the mitoKATP channel.
As can be seen from the data obtained, the protection of heart tissue of rats with AMI and I/R from oxidative stress by uridine significantly reduces the size of the ischemic region in the myocardium (Fig. 2a,b). Furthermore, the anti-ischemic activity of the nucleoside also manifested itself in the reduction of the occlusion-induced increase in the amplitude of the T-wave, which is an electrophysiological indicator of the development of acute myocardial ischemia37. These positive effects of the drug were completely blocked by 5-HD.
It is well known that acute myocardial ischemia and postischemic restoration of the coronary blood flow are accompanied by the impairment of the electrical stability of the heart38. In our models, early ischemic arrhythmias developed in 100% of cases, and reperfusion arrhythmias in 70% of cases. They were characterized by the occurrence of PVB, VT, and VF. These heart rhythm disturbances develop due to a decrease in the duration of the membrane action potential and its regional heterogeneity. Extracellular K+ accumulation, which occurs during ischemia and ischemia/reperfusion, plays a key role in the initiation of these processes. Based on most parameters of assessing the effect of uridine on cardiac arrhythmias, we can conclude that the nucleoside has pronounced antiarrhythmic and antifibrillatory activity toward early ischemic and reperfusion arrhythmias. However, inhibitory analysis showed that uridine antiarrhythmic effect was not always associated with the activation of the mitoKATP channel. It is known that UDP is a non-selective agonist and can also affect sarcKATP channel activation, which can lead to an increase in the extracellular concentration of K+. It should be noted that the activation of the sarcKATP channel requires a higher concentration of UDP than that of the mitoKATP channel14,39. In hypoxic conditions, the positive effect of uridine can be also associated with the production of glycolytically derived ATP, which provides the functioning of ion channels and transporters in the cell membrane, including Na/K ATP-ase, the main regulator of the K/Na flow in the cell40. Therefore, the final effect of uridine on the heart rate during acute ischemia and I/R is likely to be the result of a complex integration of various processes that affect the electrical properties of cardiomyocytes. It can be explained partly by the opening of the mitoKATP channel and partly by the influence on the energy supply of the cell and the work of the ion channels of cell membranes.
A research group led by Prof. K. Garlid discovered that H2O2 is a metabolic opener of the mitoKATP channel41. It was found that the activation of the channel occurs in ischemic preconditioning, which is associated with a temporary increase in the degree of reduction of complexes of the electron transport chain and production of hydrogen peroxide in mitochondria42. The findings are also consistent with the data on the reversion of the cardioprotective effect of preconditioning by antioxidants43,44 and the mitoKATP blockers28. In addition, it was shown that intermittent hypoxic training of animals with low resistance to acute hypoxia leads both to an increase in the tolerance of rats to hypoxic exposure and the activation of mitoKATP channel10. Thus, the signaling role of ROS in cardioprotection may be mediated by an increase in the activity or expression level of mitoKATP channel45,46.
In contrast, prolonged and severe hypoxia is known to induce excessive lipid peroxidation in tissues, which leads to a depletion of the capacity of antioxidant systems and the development of oxidative stress36. Some studies indicate that an increase in the level of expression or activity of mitoKATP channel can protect tissues from oxidative damage by reducing excessive ROS production in mitochondria8,35.
It was found that succinate-driven reverse electron transport (RET) is one of the main sources of mitochondrial ROS upon ischemia/reperfusion injury47. It was shown that a driving force for RET is the mitochondrial membrane potential. Growing evidence is revealing that a slight decrease in the mitochondrial membrane potential may be applied as a therapeutic approach to diminish RET-driven ROS generation47,48. Notably, RET-induced ROS production was found to be attenuated by activation of mitochondrial potassium channels49.
In studies in vitro, the administration of pharmacological openers of mitoKATP channel was shown to induce a slight depolarization of the inner mitochondrial membrane50,51. It was also observed that mild mitochondrial uncoupling can be induced by the influx of potassium ions into mitochondria and the subsequent activation of the potassium recycling across the mitochondrial membrane52. In parallel, recent data suggest that the hypoxia-induced overexpression of mitochondrial uncoupling proteins also leads to a decrease in ROS production and protection of tissues from ischemic injury53.
At the same time, the activation of potassium recycling in mitochondria can prevent the hypoxia-induced calcium overload of the organelles, which leads to the opening of the mitochondrial permeability transition (MPT) pore and cell death54,55. The use of pharmacological openers of the mitochondrial potassium channels was reported to activate the potassium cycle in mitochondria and may prevent tissue damage under conditions of calcium overload50,54–56.
Thus, the data obtained in this work demonstrate that treatment with uridine attenuates myocardial injury and oxidative stress in acute ischemia and ischemia/reperfusion, which may be mediated by the activation of the mitoKATP channel. The findings are consistent with the data on the therapeutic effect of uridine in several pathologies associated with the development of hypoxia and oxidative stress, including cardiovascular diseases.
Methods
Animals
Male Wistar rats weighting 300–350 g were housed for two weeks before surgery under standard conditions at the room temperature of 18–22 °C, relative humidity 60–70%, and regular 12-h light–dark cycles. The animals received commercial pellets and water ad libitum. All surgery procedures were carried out on animals anesthetized with sodium pentobarbital (50 mg/kg) via intraperitoneal injection.
Compliance with ethical standards
All manipulations with animals were performed in accordance with the European Convention for the Protection of Vertebrates used for experimental and other purposes (Strasbourg, 1986) and the principles of the Helsinki Declaration (2000). All the protocols were approved by the Ethics Committees at the Institute of Experimental Medicine and the Institute of Theoretical and Experimental Biophysics, Russian Academy of Sciences (Protocol No. 09 of 05.03.2019). The study was carried out in compliance with the ARRIVE guidelines.
Acute myocardial ischemia (AMI) model
Surgical procedure
AMI was modeled by the occlusion of the descending branch of the left coronary artery (LCA) using a standard method57 with mechanical ventilation of the lungs through tracheostomy (Harvard rodent ventilator (USA), with a rate of 60 breaths/min, tidal volume of 2 ml/100 g). A significant elevation of the T-wave on electrocardiogram (ECG, lead II) indicated a successful modeling of AMI. ECG was recorded by a Schiller VET AT-1 device (Switzerland). The left femoral vein was catheterized for drug injections. Sham-operated rats underwent the same surgical procedures except that the suture around the LCA was not ligated.
Experimental protocol
Animals were randomly divided into 4 groups: 1—sham-operated rats (n = 9); 2—AMI + saline 5 min before occlusion (control, n = 17); 3—rats treated with uridine 30 mg/kg, intravenously (i.v.), 5 min prior to AMI (n = 18); 4—rats treated with uridine according to the same scheme + the inhibitor of the mitoKATP channel 5-hydroxydecanoate (5-HD) 5 mg/kg i.v., given 5 min prior to uridine (n = 18). Uridine and 5-HD were purchased from Sigma-Aldrich, St. Louis, MO, and dissolved in sterile saline. Sixty minutes after occlusion, the size of the ischemic alteration zone was determined, and biochemical assays of ischemic tissue were performed. Lead II ECG monitoring within the first 30 min after the LCA occlusion was fulfilled for the estimation of the heart rate. ECG measurements of T-wave elevation were taken before and 3, 30, and 60 min after the LCA occlusion.
An ischemia–reperfusion (I/R) model
Surgical procedure
After left thoracotomy and pericardiotomy, a ligature (6–0 polypropylene suture) was placed around the descending branch of the LCA, and the ends of the suture were passed through a polyethylene tube to form a snare. Tight pulling of the suture and pressing to the epicardial surface resulted in the LCA occlusion and regional ischemia of the left ventricular. The suture was removed after 30 min of ischemia to allow reperfusion for 120 min. The effect of uridine on the reperfusion-induced arrhythmias was studied in a separate experiment in which the periods of acute ischemia and reperfusion were 7 and 30 min, correspondingly. ECG monitoring was performed from the first second of ischemia until the end of heart rhythm disorders or until the full cardiac arrest for the verification of myocardial ischemia and evaluation of the ventricular arrhythmias during myocardial I/R.
Experimental protocol
Animals were randomly divided into four groups: 1—sham-operated rats (n = 9); 2—I/R with saline 30 min before ischemia and 5 min before reperfusion (control, n = 19); 3—rats treated twice with i.v. injections of uridine at a dose of 30 mg/kg 30 min before ischemia and 5 min prior to reperfusion (n = 16); 4—rats treated with uridine 30 min before ischemia and 5 min prior to reperfusion + 5-HD at the dose of 5 mg/kg i.v. 5 min prior to each injection of uridine (n = 16). After the end of reperfusion, the area at risk and the zone of necrosis in the heart or biochemical changes in the myocardium were estimated.
To study a possible effect of uridine on the reperfusion arrhythmia, rats were randomly divided into three experimental groups: 1—rats injected with saline (1 ml/kg i.v.) 5 min before the LCA occlusion (control, n = 6); 2—rats treated with uridine (30 mg/kg i.v.) 5 min before the occlusion (n = 8); 3—rats injected with uridine (30 mg/kg i.v.) 5 min before the occlusion + 5-HD (5 mg/kg i.v.) 5 min prior the administration of uridine (n = 5).
Measurement of the size of the infarct area
In the rat model of AMI, the area of early ischemic changes was visualized using the method of histochemical detection of glycogen phosphorylase activity58 60 min after the LCA occlusion. The hearts were frozen at − 20 °C for further processing. Then, the heart was sliced transversely from the apex to the basal part of the left ventricle into four slices with a thickness of 2 mm. The ischemia zone was not stained. The size of this zone was determined by the planimetric method and expressed as the ischemic alteration index (IAI). The IAI was calculated by the following formula: IAI = ∑S1-4/linear magnification2 × heart weight, where S1-4 is the sum of the areas of all zones of ischemic damage in one heart58.
The infarct area (IA) and the area at risk (AAR) in the I/R model were determined after 120 min of reperfusion using the method of double staining28. A Canon A650IS camera and an MBS microscope (LOMO, Russia) were used to detect the AAR. The images were digitized using the Adobe Photoshop CC 2017 software (Adobe Systems Incorporated, San Jose, CA, USA). The AAR was expressed as a percentage of the total area of the histological section, and the IA, as a percentage of AAR.
Oxidative stress-related parameters
The anterior wall of the left ventricle was quickly taken and frozen in liquid nitrogen. Then, a 10% homogenate in ice-cold phosphate buffer (50 mM, pH 7.4) was prepared and used for spectrophotometric assays of the levels of reduced glutathione (GSH)59, lipid hydroperoxides (LPO), diene conjugates (DC)60, and superoxide dismutase (SOD) activity61 with the use of a Unico2802S spectrophotometer (United products & Instruments, USA).
Determination of UDP, UTP, ATP, and CrP in the myocardium by HPLC
The levels of UDP and UTP were determined in neutralized protein-free tissue extracts by HPLC using a Knauer chromatography system (Germany) with a ProPac PA 1 column (4 × 250 mm, 10 μm) (Sigma-Aldrich, St. Louis, MO) and ProPac PA 1 pre-column (4 × 50 mm, 10 μm) (Sigma-Aldrich, St. Louis, MO). The detection was carried out at a wavelength of 254 nm. The content of nucleotides in samples was assessed by the external standard method using the 1.52.0.0 software program for Windows.
ATP and CrP were separated by ion-pair reverse-phase HPLC according to published protocols62 with minor modifications using Beckman System Gold (USA) with a Saphire 600 UV detector (Ecom, Czechia) and an RP 18 column. ATP was detected at 254 nm, CrP at 210 nm.
Determination of uridine in blood serum
Animals were randomly divided into 2 groups: 1—control (normoxia) rats were treated with uridine (control, n = 6); 2—rats with experimental AMI treated with uridine 5 min before the LCA occlusion (n = 6). Uridine was given i.v. at a dose of 30 mg/kg. Blood was collected from the femoral vein, and the concentration of uridine in rat blood serum was measured at the following time points: before uridine (0 min, background level), 5, 10, 15, 30, and 65 min after its administration. Serum uridine levels were determined by HPLC on an Orlant microcolumn liquid chromatograph (Medicant LLC, Russia) with a Nukleosil 5C 18 column (Macherey–Nagel GmbH & Co, Germany). Detection was fulfilled at 254 nm.
Analysis of heart rhythm
The analysis of the incidence and severity of ischemic and reperfusion ventricular tachyarrhythmias was performed in accordance with Lambeth Conventions63, which are guidelines for the recognition and evaluation of experimentally induced disturbances of the cardiac rhythm. The following parameters were measured: 1, the frequency of occurrence of ventricular tachyarrhythmia; 2, the incidence of ventricular tachycardia (VT), the total duration (s), and the number of its episodes; 3, the frequency of occurrence and the total duration of episodes of ventricular fibrillation (VF), including the number of cases of the persistent form of VF, which lead to the animal death; 4, integrated assessment of the severity of reperfusion-induced rhythm disturbances in scores according to29: 0 score = no arrhythmia; 1 score = ≤ 10 s VT, no VF; 2 scores = 11–30 s VT, no VF; 3 scores = 31–90 s VT, no VF; 4 scores = 91–180 s VT and/or ≤ 10 s reversible VF; 5 scores = ≥ 180 s VT and/or ≥ 10 s reversible VF; 6 scores = lethal VF.
Statistical analysis
The data are expressed as the mean ± standard derivation (m ± SD). Statistical analysis of the data was carried out using the GraphPad Prism version 6.0 software (GraphPad Software, CA, USA). The data were analyzed for normal distribution (Shapiro–Wilk test). The statistical significance of differences between the experimental groups was evaluated using the Fisher's Exact test or one-way repeated analysis of variance (ANOVA) followed by the Turkey multiple comparison Post Hoc test (for multiple-group comparisons). Significant differences were represented as follows: ns, not significant; *p < 0.05; **p < 0.01; ***p < 0.001 (vs. AMI or I/R group); #p < 0.05; ##p < 0.01; ###p < 0.001 (vs. AMI (or I/R) + uridine group).
Supplementary Information
Acknowledgements
This work was supported by the Ministry of Science and Higher Education of the Russian Federation (state task No. 075-00381-21-00). We are sincerely grateful to Svetlana V. Sidorova for help with language editing.
Author contributions
I.B.K. designed the project, performed surgical procedures and wrote the manuscript; E.N.S. performed HPLC analysis; V.V.B. carried out biochemical assays; O.M.R. estimated the size of the ischemic alteration zone; N.R.E. performed analysis of heart rhythm. N.V.B. and M.I.S. assessed oxidative stress biomarkers and performed statistical data analysis. G.D.M. contributed to conception and design, analysis and interpretation of the data, and the article preparation. All authors have read and approved the final submitted manuscript.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding authors on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Irina B. Krylova, Email: irinakrylova@mail.ru
Galina D. Mironova, Email: mironova40@mail.ru
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-96562-7.
References
- 1.World Health Organization. Cardiovascular diseases (CVDs). Fact sheet Updated May 2017, http://www.who.int/mediacentre/factsheets/fs317/en/ (2017).
- 2.Buja LM. Myocardial ischemia and reperfusion injury. Cardiovasc. Pathol. 2005;14:170–175. doi: 10.1016/j.carpath.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Cadenas S. Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim. Biophys. Acta Bioenerg. 1859;940–950:2018. doi: 10.1016/j.bbabio.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Sinning C, Westermann D, Clemmensen P. Oxidative stress in ischemia and reperfusion: Current concepts, novel ideas and future perspectives. Biomark. Med. 2017;11:1031–1040. doi: 10.2217/bmm-2017-0110. [DOI] [PubMed] [Google Scholar]
- 5.Hausenloy DJ, et al. Mitochondrial ion channels as targets for cardioprotection. Cell Mol. Med. 2020 doi: 10.1111/jcmm.15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tubaro M, Petronio AS. ST-segment elevation myocardial infarction management in Europe. J. Cardiovasc. Med. Hagerstown. 2009;Suppl 1:S3–S6. doi: 10.2459/01.jcm.0000362037.41014.e3. [DOI] [PubMed] [Google Scholar]
- 7.Davidson SM, et al. Multitarget strategies to reduce myocardial ischemia/reperfusion injury. J. Am. Coll. Cardiol. 2019;73:89–99. doi: 10.1016/j.jacc.2018.09.086. [DOI] [PubMed] [Google Scholar]
- 8.Garlid KD, Dos Santos P, Xie ZJ, Costa AD, Paucek P. Mitochondrial potassium transport: The role of the mitochondrial ATP-sensitive K(+) channel in cardiac function and cardioprotection. Biochim. Biophys. Acta. 2003;1606:1–21. doi: 10.1016/S0005-2728(03)00109-9. [DOI] [PubMed] [Google Scholar]
- 9.Gross GJ, Peart JN. KATP channels and myocardial preconditioning: An update. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H921–H930. doi: 10.1152/ajpheart.00421.2003. [DOI] [PubMed] [Google Scholar]
- 10.Mironova GD, et al. Functioning of the mitochondrial ATP-dependent potassium channel in rats varying in their resistance to hypoxia. Involvement of the channel in the process of animal’s adaptation to hypoxia. J. Bioenerg. Biomembr. 2010;42:473–481. doi: 10.1007/s10863-010-9316-5. [DOI] [PubMed] [Google Scholar]
- 11.Vanden Hoek TL, Becker LB, Shao ZH, Li CQ, Schumacker PT. Preconditioning in cardiomyocytes protects by attenuating oxidant stress at reperfusion. Circ. Res. 2000;86:541–548. doi: 10.1161/01.RES.86.5.541. [DOI] [PubMed] [Google Scholar]
- 12.Er F, Michels G, Gassanov N, Rivero F, Hoppe UC. Testosterone induces cytoprotection by activating ATP-sensitive K+ channel in the cardiac mitochondrial inner membrane. Circulation. 2004;110:3100–3107. doi: 10.1161/01.CIR.0000146900.84943.E0. [DOI] [PubMed] [Google Scholar]
- 13.Tsai CH, Su SF, Chou TF, Lee TM. Differential effects of sarcolemmal and mitochondrial KATP channels activated by 17β-estradiol on reperfusion arrhythmias and infarct sizes in canine hearts. J. Pharmacol. Exp. Ther. 2002;301:234–240. doi: 10.1124/jpet.301.1.234. [DOI] [PubMed] [Google Scholar]
- 14.Mironova GD, et al. Functional distinctions between the mitochondrial ATP-dependent K+ channel (mitoKATP) and its inward rectifier subunit (mitoKIR) J. Biol. Chem. 2004;279:32562–32568. doi: 10.1074/jbc.M401115200. [DOI] [PubMed] [Google Scholar]
- 15.Matsushita S, Fanburg BL. Pyrimidine nucleotide synthesis in the normal and hypertrophying rat heart. Relative importance of the de novo and salvage pathways. Circ. Res. 1970;27:415–428. doi: 10.1161/01.RES.27.3.415. [DOI] [PubMed] [Google Scholar]
- 16.Pizzorno G, et al. Homeostatic control of uridine and the role of uridine phosphorylase: A biological and clinical update. Biochim. Biophys. Acta. 2002;1587:133–144. doi: 10.1016/S0925-4439(02)00076-5. [DOI] [PubMed] [Google Scholar]
- 17.Connolly GP, Duley JA. Uridine and its nucleotides: Biological actions, therapeutic potentials. Trends Pharmacol. Sci. 1999;20:218–225. doi: 10.1016/S0165-6147(99)01298-5. [DOI] [PubMed] [Google Scholar]
- 18.Eliseev VV, Rodionova OM, Sapronov NS, Selizarova NO. The effect of uridine and uridine nucleotides on isolated rat heart performance in regional myocardial ischemia. Patol. Fiziol. Eksp. Ter. 2002;2:13–15. [PubMed] [Google Scholar]
- 19.Sapronov NS, Eliseev VV, Rodionova OM. Effect of uridine derivatives on myocardial stunning during postischemic reperfusion of rat heart. Bull. Exp. Biol. Med. 2000;130:964–966. [PubMed] [Google Scholar]
- 20.Krylova IB, Bulion VV, Selina EN, Mironova GD, Sapronov NS. Effect of uridine on energy metabolism, LPO, and antioxidant system in the myocardium under conditions of acute coronary insufficiency. Bull. Exp. Biol. Med. 2012;153:644–646. doi: 10.1007/s10517-012-1787-4. [DOI] [PubMed] [Google Scholar]
- 21.Krylova IB, Safonova AF, Evdokimova NR. Correction of hypoxic state by metabolic precursors of endogenous activator of mitochondrial ATP-dependent K+ channels. Rev. Clin. Pharmacol. Drug Ther. 2018;16:25–31. doi: 10.17816/RCF16325-31. [DOI] [Google Scholar]
- 22.Rozova EV, Mankovskaya IN, Belosludtseva NV, Khmil HV, Mironova GD. Uridine as a protector against hypoxia-induced lung injury. Sci. Rep. 2019;9:9418. doi: 10.1038/s41598-019-45979-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mironova GD, et al. The role of mitochondrial KATP channel in anti-inflammatory effects of uridine in endotoxemic mice. Arch. Biochem. Biophys. 2018;654:70–76. doi: 10.1016/j.abb.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Man’kovskaia IN, et al. The effect of uridine on the endurance of animals with different resistance to physical stress: The role of mitochondrial ATP-dependent potassium channel. Biofizika (Russian) 2014;59:764–767. [PubMed] [Google Scholar]
- 25.Traut TW. Physiological concentrations of purines and pyrimidines. Mol. Cell Biochem. 1994;140:1–22. doi: 10.1007/BF00928361. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto T, et al. Biochemistry of uridine in plasma. Clin. Chim. Acta. 2011;412:1712–1724. doi: 10.1016/j.cca.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Erlinge D, et al. Uridine triphosphate (UTP) is released during cardiac ischemia. Int. J. Cardiol. 2005;100:427–433. doi: 10.1016/j.ijcard.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Fryer RM, Eells JT, Hsu AK, Henry MM, Gross GJ. Ischemic preconditioning in rats: Role of mitochondrial K(ATP) channel in preservation of mitochondrial function. Am. J. Physiol. Heart Circ. Physiol. 2000;278:H305–312. doi: 10.1152/ajpheart.2000.278.1.H305. [DOI] [PubMed] [Google Scholar]
- 29.Gonca E. The effects of zileuton and montelukast in reperfusion-induced arrhythmias in anesthetized rats. Curr. Ther. Res. Clin. Exp. 2013;75:27–32. doi: 10.1016/j.curtheres.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aussedat J, Ray A, Rossi A. Uridine incorporation in normal and ischemic perfused rat hearts. J. Mol. Physiol. 1984;6:247–255. [Google Scholar]
- 31.Bulion VV, et al. Comparative study of cardioprotective effects of uridine-5′-monophosphate and uridine-5′-triphosphate during the early periods of acute myocardial ischemia. Bull. Exp. Biol. Med. 2007;144:322–325. doi: 10.1007/s10517-007-0323-4. [DOI] [PubMed] [Google Scholar]
- 32.Reinhardt R, et al. Early biochemical and histological alterations in rat corticoencephalic cell cultures following metabolic damage and treatment with modulators of mitochondrial ATP-sensitive potassium channels. Neurochem. Int. 2003;43:563–571. doi: 10.1016/S0197-0186(03)00053-6. [DOI] [PubMed] [Google Scholar]
- 33.Ferrary R. Metabolic disturbances during myocardial ischemia and reperfusion. Am. J. Cardiol. 1995;76:17B–24B. doi: 10.1016/S0002-9149(99)80457-3. [DOI] [PubMed] [Google Scholar]
- 34.Piriou V, et al. Prevention of isoflurane-induced preconditioning by 5-hydroxydecanoate and gadolinium: Possible involvement of mitochondrial adenosine triphosphate-sensitive potassium and stretch-activated channels. Anesthesiology. 2000;93:756–764. doi: 10.1097/00000542-200009000-00025. [DOI] [PubMed] [Google Scholar]
- 35.Checchetto V, et al. Mitochondrial K+ channels and their implications for disease mechanisms. Pharmacol. Ther. 2021;227:107874. doi: 10.1016/j.pharmthera.2021.107874. [DOI] [PubMed] [Google Scholar]
- 36.Gao L, Laude K, Cai H. Mitochondrial pathophysiology, reactive oxygen species, and cardiovascular diseases. Vet. Clin. N. Am. Small Anim. Pract. 2008;38:137–155. doi: 10.1016/j.cvsm.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Genzlinger MA, Eberhardt M. Analyzing prominent T waves and ST-segment abnormalities in acute myocardial infarction. J. Emerg. Med. 2012;43:e81–e85. doi: 10.1016/j.jemermed.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 38.Said M, et al. Calcium-calmodulin dependent protein kinaseII (CaMKII): A main signal responsible for early reperfusion arrhythmias. J. Mol. Cell Cardiol. 2011;51:936–944. doi: 10.1016/j.yjmcc.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alekseev AE, Brady PA, Terzic A. Ligand-insensitive state of cardiac ATP-sensitive K+ channels. Basis for channel opening. J. Gen. Physiol. 1998;111:381–394. doi: 10.1085/jgp.111.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dhar-Chowdhury P, Malester B, Rajacic P, Coetzee WA. The regulation of ion channels and transporters by glycolytically derived ATP. Cell Mol. Life Sci. 2007;64:3069–3083. doi: 10.1007/s00018-007-7332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Costa AD, Garlid KD. Intramitochondrial signaling: Interactions among mitoKATP, PKCepsilon, ROS, and MPT. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H874–H882. doi: 10.1152/ajpheart.01189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forbes RA, Steenberger C, Murphy E. Diazoxide-induced cardioprotection requires signaling through a redox-sensitive mechanism. Circ. Res. 2001;88:802–809. doi: 10.1161/hh0801.089342. [DOI] [PubMed] [Google Scholar]
- 43.Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416:15–18. doi: 10.1016/S0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- 44.Vanden Hoek TL, Becker LB, Shao Z, Li C, Schumacker PT. Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. J. Biol. Chem. 1998;273:18092–18098. doi: 10.1074/jbc.273.29.18092. [DOI] [PubMed] [Google Scholar]
- 45.Lu C, Halvorsen SW. Channel activators regulate ATP-sensitive potassium channel (KIR 6.1) expression in chick cardiomyocytes. FEBS Lett. 1997;412:121–125. doi: 10.1016/S0014-5793(97)00760-6. [DOI] [PubMed] [Google Scholar]
- 46.Pain T, et al. Opening of mitochondrial KATP channels triggers the preconditioned state by generating free radicals. Circ. Res. 2000;87:460–466. doi: 10.1161/01.res.87.6.460. [DOI] [PubMed] [Google Scholar]
- 47.Onukwufor JO, Berry BJ, Wojtovich AP. Physiologic implications of reactive oxygen species production by mitochondrial complex I reverse electron transport. Antioxidants. 2019;8:285. doi: 10.3390/antiox8080285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Komlódi T, Geibl FF, Sassani M, Ambrus A, Tretter L. Membrane potential and delta pH dependency of reverse electron transport-associated hydrogen peroxide production in brain and heart mitochondria. J. Bioenerg. Biomembr. 2018;50:355–365. doi: 10.1007/s10863-018-9766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heinen A, et al. Reverse electron flow-induced ROS production is attenuated by activation of mitochondrial Ca2+-sensitive K+ channels. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H1400-7. doi: 10.1152/ajpheart.00198.2007. [DOI] [PubMed] [Google Scholar]
- 50.Holmuhamedov EL, Wang L, Terzic A. ATP-sensitive K+ channel openers prevent Ca2+ overload in rat cardiac mitochondria. J. Physiol. 1999;519:347–360. doi: 10.1111/j.1469-7793.1999.0347m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sato T, O’Rourke B, Marban E. Modulation of mitochondrial ATP-dependent K+ channels by protein kinase C. Circ. Res. 1998;83:110–114. doi: 10.1161/01.RES.83.1.110. [DOI] [PubMed] [Google Scholar]
- 52.Schonfeld P, Wieckowski MR, Wojtczak L. Long-chain fatty acid-promoted swelling of mitochondria: Further evidence for the protonophoric effect of fatty acids in the inner mitochondrial membrane. FEBS Lett. 2000;471:108–112. doi: 10.1016/S0014-5793(00)01376-4. [DOI] [PubMed] [Google Scholar]
- 53.Wu F-F, et al. Beneft of a single simulated hypobaric hypoxia in healthy mice performance and analysis of mitochondria-related gene changes. Sci. Rep. 2021;11:4494. doi: 10.1038/s41598-020-80425-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ishida H, Hirota Y, Genka C, Nakazawa H, Nakaya H, Sato T. Opening of mitochondrial K(ATP) channels attenuates the ouabain-induced calcium overload in mitochondria. Circ. Res. 2001;89:856–858. doi: 10.1161/hh2201.100341. [DOI] [PubMed] [Google Scholar]
- 55.Murata M, Akao M, O’Rourke B, Marban E. Mitochondrial ATP-sensitive potassium channels attenuate matrix Ca2+ overload during simulated ischemia and reperfusion: Possible mechanism of cardioprotection. Circ. Res. 2001;89:891–898. doi: 10.1161/hh2201.100205. [DOI] [PubMed] [Google Scholar]
- 56.Xu W, et al. Cytoprotective role of Ca2+- activated K+ channels in the cardiac inner mitochondrial membrane. Science. 2002;298:1029–1033. doi: 10.1126/science.1074360. [DOI] [PubMed] [Google Scholar]
- 57.Selye H, Bajusz E, Grasso S, Mendel P. Simple techniques for the surgical occlusion of coronary vessels in the rat. Angiology. 1960;11:398–407. doi: 10.1177/000331976001100505. [DOI] [PubMed] [Google Scholar]
- 58.Frederiks W, Schellens J, Marx F, Bosch K, Vreeling-Sindelárová H. Histochemical detection of glycogen phosphorylase activity as parameter for early ischaemic damage in rat heart. Basic Res. Cardiol. 1993;88:130–140. doi: 10.1007/BF00798261. [DOI] [PubMed] [Google Scholar]
- 59.Maron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione-s-transferase activities in rat lung and liver. Biochim. Biophys. Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 60.Arutunyan AV, Dubinina EE, Zybina NN. Methods of Free Radical Oxidation and Organism Antioxidant System Estimation: Guidelines. Izdatelistvo Foliant; 2000. [Google Scholar]
- 61.Kostyuk VA, Potapovich AI. Superoxide-driven oxidation of quercetin and a simple sensitive assay for determination of superoxide dismutase. Biochem. Int. 1989;19:1117–1124. [PubMed] [Google Scholar]
- 62.Volonte MG, Yuln G, Quiroga P, Consolini AE. Development of an HPLC method for determination of metabolic compounds in myocardial tissue. J. Pharm. Biomed. Anal. 2004;35:647–653. doi: 10.1016/j.jpba.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 63.Curtis MJ, et al. The Lambeth Conventions (II): Guidelines for the study of animal and human ventricular and supraventricular arrhythmias. Pharmacol. Ther. 2013;139:213–248. doi: 10.1016/j.pharmthera.2013.04.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding authors on reasonable request.