Abstract
An overarching challenge of the electrochemical carbon dioxide reduction reaction (eCO2RR) is finding an earth-abundant, highly active catalyst that selectively produces hydrocarbons at relatively low overpotentials. Here, we report the eCO2RR performance of two-dimensional transition metal carbide class of materials. Our results indicate a maximum methane (CH4) current density of −421.63 mA/cm2 and a CH4 faradic efficiency of 82.7% ± 2% for di-tungsten carbide (W2C) nanoflakes in a hybrid electrolyte of 3 M potassium hydroxide and 2 M choline-chloride. Powered by a triple junction photovoltaic cell, we demonstrate a flow electrolyzer that uses humidified CO2 to produce CH4 in a 700-h process under one sun illumination with a CO2RR energy efficiency of about 62.3% and a solar-to-fuel efficiency of 20.7%. Density functional theory calculations reveal that dissociation of water, chemisorption of CO2 and cleavage of the C-O bond—the most energy consuming elementary steps in other catalysts such as copper—become nearly spontaneous at the W2C surface. This results in instantaneous formation of adsorbed CO—an important reaction intermediate—and an unlimited source of protons near the tungsten surface sites that are the main reasons for the observed superior activity, selectivity, and small potential.
Subject terms: Heterogeneous catalysis, Electrocatalysis, Electrocatalysis
It is of high interests to develop new catalysts for selective CO2 electroreduction. Here the authors investigate two-dimensional transition metal carbides for CO2 to methane conversion with superior activity, selectivity and low overpotentials.
Introduction
The electrocatalytic carbon dioxide reduction reaction (eCO2RR) driven by renewable energy has great potential for the sustainable production of chemicals and fuels at the gigaton scale that can be used any time, any place1–4. It also offers a promising way to store energy in chemical bonds due to having nearly two orders of magnitude higher energy density compared to the most advanced battery technologies5. However, reducing CO2 to value-added chemicals is both costly and slow based on intrinsic thermodynamics and kinetics, making the goal of an effective and feasible process a real challenge6–9.
Conventional pure metal catalysts such as gold (Au), palladium (Pd), silver (Ag), and newly developed transition metal dichalcogenides (TMDCs)8,10–18 are known to exhibit high activities for the CO2RR in different electrolyte solutions19–26. However, these catalysts are mainly selective for carbon monoxide (CO), known as an intermediate product8,27. Other catalysts such as copper (Cu) and Cu-based catalysts have the ability to reduce CO2 to various chemicals such as methane (CH4), ethylene (C2H4), formic acid (HCOOH), methanol (CH3OH), and ethanol (C2H5OH)28–36. Despite their good selectivity, these catalysts require high potentials—excess energy—to achieve suitable current densities—reaction rates—impeding their use for effective production of chemicals and fuels37,38. Therefore, an effective catalyst needs to be developed to selectively produce hydrocarbons at high rates at relatively low potentials.
Heteroatomic transition metal carbide (TMC) catalysts, also known as MXenes, have recently received great attention for various electrocatalytic reactions due to their unique structural and electronic properties39–42. In particular, M2C (M denotes transition metals) stoichiometry of this class of two-dimensional materials forms layered structures of M-C-M where a plane of carbon atoms is sandwiched between two hexagonal planes of metal atoms. This structure provides a high density of active metal atoms at the surface breaking conventional scaling relationships that limit the electrocatalytic performance of their counterparts such as TMDCs and pure metals43. However, there is limited knowledge of their performance and characteristics as eCO2RR catalysts under actual experimental conditions.
In this work, we investigate the performance of di-tungsten carbide (W2C), di-molybdenum carbide (Mo2C), diniobium carbide (Nb2C), and divanadium carbide (V2C) nanoflakes (NFs) as inexpensive, non-precious members of TMCs for eCO2RR. Our electrochemical results indicate that W2C NFs work remarkably well for eCO2RR by achieving a maximum CH4 formation current density of −421.63 mA/cm2 and faradaic efficiency of 82.7% ± 2 that are the highest values yet reported. These results suggest a catalytic activity higher than Au, product selectivity similar to Cu in the CO2RR for W2C NFs. Our DFT calculations also reveal that dissociation of water, chemisorption of CO2, and cleavage of the C–O bond, known as the most energy-consuming elementary steps in other catalysts, become nearly spontaneous at the W2C surface and are the main reason for the observed superior activity, selectivity, and small overpotential for CH4 production.
Results and discussion
The TMC NFs i.e., W2C, Mo2C, Nb2C, and V2C were synthesized using a carburization process followed by the liquid exfoliation technique (Supplementary section 1)27,44,45. The electrocatalytic performance of TMC NFs with similar crystallite sizes (25.4 ± 5 nm) were then studied in a three-electrode cell and compared with Au and Cu nanoparticles (NPs), conventional catalysts for this reaction,46 under identical experimental conditions (Supplementary section 2). To improve the CO2RR performance in competing with hydrogen evolution reaction (HER), we have employed a mixture of 3 M potassium hydroxide (KOH) and 2 M choline chloride (CC) solution (KOH:CC 3 M:2 M) as the electrolyte in this study47.
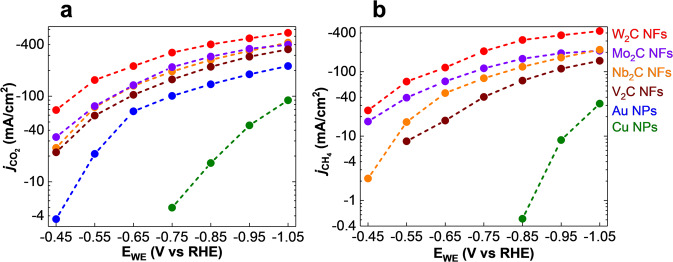
The linear sweep voltammetry (LSV) experiments and a real-time product stream analysis show that CO2RR on the W2C surface starts at a potential of −122.7 mV vs reversible hydrogen electrode (RHE) by producing CO and H2 and reach maximum CO2RR current density (jCO2RR) of −548.9 mA/cm2 at −1.05 V vs RHE (Supplementary Figs. 2–4 and Fig. 1a). As shown in Fig. 1a, jCO2RR of −419.9, −381.9, and −350.8 mA/cm2 were observed for Mo2C, Nb2C, and V2C NFs, respectively, at this potential (Supplementary section 3). However, Au and Cu NPs exhibit a jCO2RR of −208.11 and −89.53 mA/cm2 at −1.05 V vs RHE (Fig. 1a). The selectivity analysis also indicates that TMC NFs produce hydrocarbons (i.e., CH4, C2H4, CH3OH, and C2H5OH) at a potential range of −0.45 to −1.05 V vs RHE for W2C, Mo2C, and Nb2C NFs and a potential range of −0.55 to −1.05 V vs RHE for V2C NFs where CH4 is identified as the main product (Supplementary section 3).
Fig. 1. Electrocatalytic performance of TMCs i.e., W2C, Mo2C, Nb2C, V2C NFs in the two-compartment three-electrode electrochemical cell using CO2 saturated KOH:CC (3 M:2 M) electrolyte.
a CO2 reduction reaction current densities (jCO2) of TMCs compared to Au and Cu NPs at different cathodic potentials (EWE) under identical experimental condition. b CH4 formation current densities (jCH4) of TMCs compared to Cu NPs at different potentials (EWE) under identical experimental condition.
Figure 1b illustrates CH4 formation current densities (jCH4, mA/cm2) of the TMC NFs compared to Cu NPs, a conventional catalyst for hydrocarbon production. The partial current densities of different products (i.e., H2, CO, CH4, C2H4, CH3OH, and C2H5OH) were calculated by multiplying FEs and total current densities at different potentials (Supplementary section 3 and Supplementary Fig. 3). As shown in Fig. 1b, a maximum jCH4 of −421.63 mA/cm2 is obtained for W2C NFs at a potential of −1.05 V vs RHE where Nb2C NFs, Mo2C NFs, and V2C NFs show values of −219.16, −211.33, and −147.56 mA/cm2, respectively, at this potential. We also compared the CH4 formation activity of TMCs i.e., W2C, Nb2C, Mo2C, and V2C NFs with state-of-the-art catalysts in the literature by calculating their maximum CH4 formation current densities (jmax.CH4, Supplementary Table 2)46,48–54. Supplementary Table 2b indicates that the jmax.CH4 of W2C NFs is 3.6 and 4.2 times higher than recently studied La2CuO4 (−117 mA/cm2 at −1.4 V vs RHE)51 and Cu–N (−100 mA/cm2 at −1.0 V vs RHE)48, respectively. The partial current densities of other hydrocarbon products i.e., C2H4, CH3OH, and C2H5OH are also shown in Supplementary Fig. 3 (Supplementary section 3).
To evaluate the intrinsic activity of W2C NFs, we measured CH4 formation turnover frequency (TOFCH4) by normalizing its activity to the number of active atoms at the surface using the roughness factor method and compared it with the other catalysts in this study (Supplementary section 5). Our calculations indicate a TOFCH4 of 10.42 s−1 at a potential of −1.05 V vs RHE for W2C NFs; by comparison, TOFCH4 of 4.54, 3.74, and 2.79 s−1 were calculated for Mo2C NFs, Nb2C NFs, and V2C NFs, respectively. The calculated TOFCH4 of W2C NFs at the potential of −1.05 V vs RHE is about two orders of magnitude higher than that of Cu NPs (0.0736 s−1) under identical experimental conditions (Supplementary Fig. 7a). Moreover, total CO2RR turnover frequencies (TOFCO2RR) of 19.09, 19.36, 17.82, and 17.55 s−1 were calculated for W2C NFs, Mo2C NFs, Nb2C NFs, and V2C NFs, respectively, where Au NPs and Cu NPs exhibit TOFCO2RR of 4.35 and 0.1956 s−1, respectively (Supplementary Fig. 7f). These results suggest the superior CH4 selectivity of TMC catalysts compared to state-of-the-art catalysts48–51,54–57.
Furthermore, we performed a comparative mechanistic study by calculating Tafel slopes for different products to gain insight about the eCO2RR mechanism of the TMCs i.e., W2C, Mo2C, Nb2C, and V2C NFs in the two-compartment three-electrode electrochemical cell (Supplementary section 6 and Supplementary Fig. 8)58. Our Tafel plot analyses show that the TMC NFs possess steeper Tafel slopes, and therefore a weaker potential dependence compared with Cu NPs for the formed products (i.e., CO, CH4, and C2H4) (Supplementary Fig. 8)58. The Tafel plot analyses suggest a different CO2RR mechanism for TMC NFs than that of Cu catalysts where C–O bond scission is the rate-determining step58.
To gain more insight to the remarkable performance of these catalysts for electrocatalytic CO2RR, the structural and physicochemical properties of TMC NFs were characterized at molecular and atomic scales by performing X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and scanning transmission electron microscopy (STEM) (Supplementary sections 7–9). At first, we have performed XPS experiments to analyze the surface chemistry of TMC NFs. XPS analysis (Supplementary Fig. 9) indicates that our NF samples contain metallic TMCs, with little or no evident surface oxidation. The results show that the chemical composition of the surface, the empirical formula of M2C (M: transition metal, C: Carbide), and the oxidation state of +2 for the transition metals i.e., W, Mo, Nb, and V are similar in all synthesized TMCs (Supplementary section 7). The lattice structure and crystallite size of the TMC NFs were then studied by performing XRD experiments. The XRD pattern of W2C NFs shows a sharp peak at 39.91° along with three pronounced peaks at 34.84°, 38.54°, and 52.65° corresponding to (101), (100), (002), and (102) crystal surfaces of W2C, respectively. The XRD spectra of the TMCs show all Bragg peaks of W2C, Mo2C, Nb2C, and V2C NFs; verifying their homogenous and pure structures. The XRD results indicate a constant dominant lattice plane of (101) and a similar average crystallite size of 25.4 ± 5 nm for all synthesized TMCs (Supplementary Fig. 10)59–61.
Furthermore, we performed atomic-scale STEM experiments to study surface atom coordination, crystallite sizes, and dominant plane structures of TMC NFs (Supplementary Figs. 11–18). Figure 2a–d shows STEM results of W2C NFs. Figure 2a, b indicate high-angle annular dark-field (HAADF) image and corresponding fast Fourier transforms (FFT) of W2C NFs in the <101> zone axis. The atomic models of the <101> zone axis and bright-field (BF) image of W2C NFs are represented in Fig. 2c and d, respectively. Figure 2d indicates the carbon atomic columns in the red box and the intensity profile across the red box region showing that the distance between two carbon atoms is 2.55 Å. The STEM results of other TMCs i.e., Mo2C, Nb2C, and V2C NFs are explained in Supplementary section 9. The STEM and XRD results of synthesized TMC NFs confirm that the structure of these materials is a perfect match with the standard 1T structure, suggesting a tetragonal symmetry and octahedral coordination of the atoms (Fig. 2e)43,62. Figure 2e indicates the schematic of 1T structure TMC NFs showing tetragonal symmetry, one layer per repeat unit with octahedral coordination. The lattice constant a is in the range of 3.07 to 3.15 Å for synthesized TMC NFs. The stacking index b indicates the interlayer spacing which is in the range of 4.53 to 5 Å for studied TMCs. As shown in Fig. 2e, 1T atomic coordination provides metal-terminated surface atoms that are known to be favorable binding sites of adsorbed intermediates in eCO2RR43,62. Our atomic and molecular scale structural analyses indicate that the synthesized TMC NFs have fairly similar structural properties e.g., (1T) crystalline structure with a dominant plane of (101), crystallite sizes, and atomic coordination.
Fig. 2. Structural and electrochemical characterization of TMC catalysts.
a High-angle annular dark-field (HAADF) of W2C NFs in <101> zone axis. b FFT corresponding to the HAADF image of W2C NFs showing the diffraction spots from <101> zone axis. c Atomic model of W2C NFs in <101> zone axis. W atoms are shown as blue and carbon atoms as white spheres. d Bright field (BF) of W2C NFs in <101> zone axis. It shows the carbon atomic columns in a red box. The inset is intensity profile across red box region showing the distance between two carbon atoms is 2.55 Å. e Schematic of 1T structure TMCs showing tetragonal symmetry, one layer per repeat unit with octahedral coordination. The transition metal atoms (W, Mo, Nb, and V) are red and the carbon atoms are blue. The lattice constant a is in the range of 3.07 to 3.15 Å for synthesized TMCs. The stacking index b indicates the interlayer spacing which is in the range of 4.53 to 5 Å for studied TMCs. f Electrochemical impedance spectroscopy (EIS) for studied catalysts at a potential of −310 mV vs RHE in the two-compartment three-electrode electrochemical cell using KOH:CC (3 M:2 M) electrolyte. g Work function measurements for synthesized TMCs using ultraviolet photoelectron spectroscopy (UPS) method. h Bader charges of W2C NFs indicate that surface W-atoms are contributing significantly to the catalytic activity of the W2C (101) surface.
To further discern the difference between the observed electrocatalytic performance of the TMCs, we have studied their electronic properties by performing electrochemical impedance spectroscopy (EIS) (Supplementary section 11) and, work function measurements using ultraviolet photoelectron spectroscopy (UPS) (Supplementary section 12)8. At first, we have employed the EIS experiments to compare the overall electron-transfer properties of the TMC catalysts in the double layer region (Supplementary section 11). To do this, TMC NFs with similar structural and physical properties e.g., sizes, shapes, and mass loadings (0.1 mg/cm2) coated on glass carbon were used as the working electrodes. This results in similar roughness, morphology, intrinsic capacitance, and exposed surface area of the studied samples confirmed by our characterization results (Supplementary sections 5–10). The EIS experiments have been performed at a potential of −310 mV vs RHE for all TMCs under identical experimental conditions (Supplementary section 11). Figure 2f shows the fitted EIS spectra of each TMC catalyst using Randles circuit model, indicating a smaller charge transfer resistance (Rct) for W2C NFs (~17 ohm) compared to the other TMCs, i.e., Mo2C NFs (~25 ohm), Nb2C NFs (~33 ohm), and V2C NFs (~38 ohm)63. The UPS method also was used to compare the surface work function of TMCs (Fig. 2g). The results indicate a lower work function for W2C NFs (0.2 to 0.84 eV) compared to Mo2C NFs (3.92 eV), Nb2C NFs (4.44 eV), and V2C NFs (4.55 eV). The charge transfer resistance obtained by EIS experiments and the surface work function value measured by UPS experiments suggests the superior activity of W2C NFs compared to other TMCs in this study i.e., Mo2C, Nb2C, and V2C NFs.
In addition to our experimental observations, we have performed density functional theory (DFT) calculations to gain more insight to the electronic and catalytic properties of M2C compounds. The aim is to address the enhanced activity and selectivity of these TMCs and to explore both electrochemical (i.e., driven) and chemical (i.e., favorable or spontaneous) processes that distinguish them from other catalysts, such as Au and Cu.
With respect to activity, the electronic density of states (DOS) indicate that transition metal d states dominate at the Fermi level of these TMCs, much more so than elemental Au, another high activity catalyst. Bader charge calculations indicate that metal atoms at the TMC surface are significantly more reduced compared to the bulk atoms (Fig. 2h and Supplementary Fig. 24). These results indicate the increased availability of electrons at metal-rich TMC surfaces, which may increase the catalytic activity of TMC NFs.
With respect to the increased selectivity of TMC NFs, especially for CH4 production, we have explored the CO2RR pathway on the W2C (101) surface in detail by using DFT calculations. Focusing initially on electrochemical processes, we employed the computational hydrogen electrode (CHE) model64–66 (Supplementary Tables 7–10) to explore the stepwise electronic reduction and protonation of adsorbed species in the low molecular coverage limit. The lowest free energy pathway to produce CH4 with only electrochemical steps is shown in Fig. 3 and Supplementary Fig. 27. The same steps with only a slight adjustment for experimental Faradaic efficiencies at the potential for optimal CH4 production is provided in Supplementary Fig. 26).
Fig. 3. Minimum energy path for the electrochemical CO2 conversion into CH4 on the surface of W2C NFs.
Only electrochemical steps are shown. The parts of the reaction where chemical and electrochemical steps are essential are highlighted by colors. The favorable reactions of key chemical steps are provided (for free energies of these reactions, see Table S9). The intermediates are indicated. Gibbs free energies for reaction at zero potential vs RHE are given in eV.
This pathway indicates that limiting steps (at zero potential with respect to RHE) are protonation of adsorbed CO2 and O, and, most importantly, the desorption of H2O following protonation of adsorbed OH. The difficulty of this final step is not surprising, as W2C (101) strongly adsorbs and spontaneously dissociates water (, ) without electrochemical assistance. Similarly, our calculations indicate that W2C (101) strongly chemisorbs CO2 (, bond length ) in contrast to normally weak physisorption on Cu () and other catalyst surfaces28,67–69. Furthermore, the (101) surface of W2C enables favorable and unassisted dissociation of adsorbed CO2 (, , Supplementary Table 10) suggesting that C–O bond scission may take place in the early stages of CO2 reduction, skipping the uphill production of adsorbed carboxyl. Based on these findings, we propose that W2C (101) distinguishes itself as a catalyst due to an interplay between surface-assisted chemical steps, whose energetics will depend on the local chemical equilibrium at the surface and electrochemical steps that reduce preexisting surface reagents and open up new pathways for the overall reaction to proceed. More detailed studies of such cooperative catalytic processes and their limiting steps may be encouraged based on the promise of W2C as a high-performance CO2 reduction catalyst. Here, we highlight the plausible cooperative effects of these steps, which set apart W2C from conventional noble metal catalysts and the other TMCs, specially for CH4 production. The immediate benefit of the favorable chemical processes mentioned above should be a higher surface coverage of CO2 (and consequently CO) and an excess of surface protons. This may explain the high Faradaic efficiencies for the production of both H2 and CO at low potentials (see Supplementary section 3 and Supplementary Table 1). However, once a limiting potential (−0.74 V estimate) is reached, the readily protonated products of adsorbed CO that produce CH4 are no longer hindered by a build-up of adsorbed byproducts (O* then OH*), which can now be protonated and released from the surface.
We can divide the complex, multistep reaction into two key parts: initial conversion of adsorbed CO2 to adsorbed CO, followed by conversion of adsorbed CO to CH4 with the release of H2O (see Fig. 3 and Supplementary Fig. 27). As indicated in Fig. 3, the first part, generation of adsorbed CO, can be achieved by chemical or electrochemical means. We have direct and favorable chemical conversion of adsorbed CO2 to adsorbed CO and O on W2C (101), but also two electrochemical pathways: production of HO–CO* in a single step (+0.66 eV, Fig. 3) or an alternative initially favorable protonation to OCHO* followed by two uphill electrochemical steps producing the first OCH2O* followed by the release of H2 and the final product of HO–CO* with a similar free energy cost (+0.68 eV, Supplementary Fig. 27 and Supplementary Table 10). A final electrochemically driven protonation of HO–CO* favorably releases H2O and leaves CO*.
With chemically or electrochemically generated adsorbed CO, we can proceed to the second part of the overall reaction to produce CH4 from CO2, which involves multiple favorable protonation steps. The W2C catalyst distinguishes itself here. The electrochemical activation of remains thermodynamically favorable (ΔG = −0.26 eV) on W2C (101), whereas on other catalysts, such as Cu, this process is usually uphill with the potential ranging from −0.74 to −0.97 V vs RHE66,70. Moreover, due to the spontaneous water dissociation, the direct H* transfer step on W2C could be even more favorable with a resultant ΔG = −0.433 eV (Supplementary Table 10). The next two electrochemical steps are downhill (ΔG = −0.04 and −0.58 eV): the first forming the unstable methoxy radical with oxygen attached to a surface W atom; the second leading to spontaneous dissociation into the methyl radical and a surface oxygen atom . The electrochemical conversion of the surface into CH4 is favorable (ΔG = −0.43 eV) and the protonation of the surface oxygen is only slightly uphill (ΔG = +0.03 eV). As we already stated, for the overall reaction on W2C (101) it is the final protonation of to release H2O that is the limiting step (ΔG = +0.74 eV).
We also compared W2C with the other TMCs studied by calculating the energies of adsorption of water and CO2 as well as the potentials of the rate-determining step (i.e., protonation of ) for Nb2C, Mo2C, and V2C (Supplementary Table 11). Our calculations indicate that these TMCs also strongly chemisorb CO2 with adsorption energies of −1.32, −1.62, and −0.96 eV, respectively. Moreover, Nb2C also shows favorable C–O bond scission of adsorbed CO2. Additionally, Nb2C, Mo2C, and V2C strongly adsorb water with the energies of −1.87, −1.23, and −0.59 eV, respectively, where Nb2C is the only other catalyst that dissociates water. In contrast to W2C, the energies required for the protonation of are higher: +1.17, +1.25, and +0.85 eV for Nb2C, Mo2C, and V2C, respectively (Supplementary Table 11). Therefore, we can conclude that, within this set of four TMCs, W2C possesses the optimal characteristics for efficient completion of CO2RR: (1) sufficiently strong adsorption of CO2, (2) spontaneous dissociation of water, and (3) the lowest limiting potential for OH* protonation. We conclude that the performance of Nb2C is reduced due to its stronger water adsorption, resulting in the protonation of requiring more energy. We would expect Mo2C to have a lower surface coverage of protons and higher costs for the protonation of . The weakest CO2 adsorption on V2C decreases its surface coverage, making it the worst TMC catalyst here, despite its relatively small limiting reaction potential of protonation of OH*.
As we mentioned before, for W2C the realistic network of pathways towards CH4 consists of a potential-dependent combination of competing chemical and electrochemical steps with the actual limiting potential being in the range from −0.483 to −0.744 V vs RHE (see the full path. Supplementary Fig. 27), which is consistent with our three-electrode electrochemical experimental results (Supplementary section 3 and Supplementary Table 1). A steeper Tafel slope for CH4 formation on W2C than other TMCs and Cu, (Supplementary Fig. 8) also indicates the competition between reactions for the active sites on the catalyst surface. Specifically, the spontaneous water dissociation on W2C (101) explains the ease of the HER in our nonacidic electrolyte where the source of protons is normally water. A weak potential dependence of the partial CO current and its small overpotential also originate from the interplay between chemical and electrochemical steps (see Supplementary Information for details).
Experimentally, we have studied the effect of CC on the activity and selectivity of the TMC catalysts. To do this, we have performed electrochemical CO2RR in different CC concentrations of i.e., 0.01, 0.1, 1, and 2 M mixed with 3 M KOH (Supplementary section 14). Figure 4 shows CO2RR overall current density and different products (i.e., CH4, C2H4, CO, alcohols-CH3OH, and C2H5OH- and H2) partial current densities for W2C NFs in different CC concentration electrolytes. Figure 4a indicates that by increasing the concentration of CC in the electrolyte the CO2RR current density (jCO2RR) increases and reaches a maximum value of −548.89 mA/cm2 at a potential of −1.05 V vs RHE for 2 M of CC. The obtained value is about 32, 24, and 17, 9% higher than that of 0, 0.01, 0.1, and 1 M of CC, respectively. Moreover, a maximum CH4 formation current density (jCH4) of −421.63 mA/cm2 is obtained for 2 M CC at a potential of −1.05 V vs RHE that is about 1.41, 1.29, 1.19, and 1.1 times higher than that of 0, 0.01, 0.1, and 1 M, respectively (Fig. 4b).
Fig. 4. Effect of choline chloride in the electrochemical performance of W2C NFs for CO2RR. The values are measured using 3 M KOH and mixed 3 M KOH with different concentrations (0.1, 0.01, 1, and 2 M) of choline chloride (CC) electrolytes.
Partial current density (j) measurements for a CO2RR, b CH4, c C2H4, d CO, e Alcohols (CH3OH and C2H5OH), and f H2 as a function of potential (EWE).
The results also indicate using W2C NFs, maximum partial current densities of other products i.e., C2H4 (jC2H4 of −35.84 mA/cm2), CO (jCO of −78.48 mA/cm2), and alcohols (jAlcohols of −12.81 mA/cm2; −6.84 mA/cm2 for CH3OH and −5.97 mA/cm2 for C2H5OH) were obtained at the potential of −1.05 V vs RHE in 2 M CC (Fig. 4b–d). In contrast, the measured H2 partial current densities indicate that by adding a higher concentration of CC to the electrolyte solution the rate of H2 production decreases significantly where a minimum H2 formation current density of −4.48 mA/cm2 was obtained for 2 M CC at a potential of −0.85 V vs RHE that is 12.31, 8.97, 6.76, 3.23 times lower than that of 0, 0.01, 0.1 and 1 M CC, respectively.
These results suggest that adding CC to the 3 M KOH electrolyte suppresses the competing HER and increases the formation of CO2RR products more specifically CH427.
The stability of the CC electrolytes was studied by conducting nuclear magnetic resonance (NMR) and 13CO2 isotope experiments (Supplementary sections15 and 16)27,46,71. The 1H and 13C NMR spectra reveal similar peak areas and chemical shifts for fresh and used electrolytes indicating no generation of new diamagnetic species or change in the CC structure under an applied potential of −1.05 V vs RHE (Supplementary Figs. 38, 39). The 13CO2 isotope experiments also show that the CO2 gas present inside the electrolyte is the only source of the formed products in the electrochemical CO2RR (Supplementary Fig. 41). These results confirm that CC with different concentrations i.e., 0, 01, 0.1, 1, and 2M remains stable at the range of applied potentials in the electrochemical CO2RR experiments.
Next, we studied the performance of W2C NFs in our developed solid polymer electrolyte flow electrolyzer for continuous electrochemical CO2RR using this catalyst as the cathode (Supplementary section 17). The flow electrolyzer used in this study consists of a two-compartment electrochemical setup with an active area of 5 cm2 coated with W2C NFs at the cathode and iridium oxide nanoparticles (IrO2 NPs) as the anode and were then fed with humidified CO2 and KOH:CC (3 M:2 M) electrolyte, respectively (Supplementary section 17).
To study the CO2RR performance of W2C NFs in the flow electrolyzer, we performed chronoamperometry (CA) experiments at different cell potentials ranging from −1.5 to −2.3 V for W2C NFs (Supplementary section 17). As shown in Fig. 5a, the results show that at a cell potential of −1.5 V, hydrogen (H2, FE of 54.9% ± 1.4) and CO (FE of 40.1% ± 1.8) are the dominant products. However, our measurements indicate that by increasing the cell potential a system becomes more selective for CH4 formation with the maximum FE of 82.7% ± 2 at a cell potential of −2.1 V. At this potential, W2C NFs slightly produce other products such as C2H4, CH3OH, C2H5OH, CO, and H2 with FEs of 5.6, 1.4, 1.2, 6.1, and 1.4, respectively. Figure 5b shows the maximum CH4, C2H4, CH3OH, and C2H5OH current densities of −421.28, −27.31, −5.95, and −5.19 mA/cm2 at the cell potential of −2.3 V, respectively, confirming high selectivity of W2C NFs towards CH4 as the main product.
Fig. 5. Electrocatalytic performance and stability of W2C NFs in the solid polymer electrolyte flow electrolyzer.
a Faradaic efficiency (FE) measurements of H2, CO, CH4, C2H4, methanol (MeOH), and ethanol (EtOH) for W2C NFs at different cell potentials. The error bars represent standard deviations of four independent experiments. b Partial current density for each product as a function of cell potential. The values are obtained considering the total current density and faradaic efficiencies of products at the entire range of cell potential. c Measured total current densities and cell potentials of the solar-driven solid polymer electrolyte flow electrolyzer under one sun illumination provided by the TJ-PV cell over time. d Total sun to fuels efficiency and sun to CH4 production efficiency in the solar-driven solid polymer electrolyte flow electrolyzer over time.
Next, we coupled the electrolyzer to a triple junction photovoltaic (TJ-PV) cell with a maximum efficiency of 34.3% to determine the CO2RR performance and energy efficiency of W2C NFs in a solar-driven device (Supplementary section 18). The j-V characteristic curve of the TJ-PV cell under one sun illumination (100 mW/cm2) using a sun simulator light source is shown in Supplementary Fig. 48. The operating point is chosen to provide a photocurrent density of −394.3 mA/cm2 at a potential of −2.1 V which has the maximum FE of CH4 (82.7% ± 2) calculated in the flow electrolyzer (Supplementary Fig. 49).
Figure 5c shows the current density of the solar-driven electrolyzer for a 700-h continuous process at a potential of −2.1 V. The results shown in Fig. 5c indicate a negligible decrease (~2%) in the photocurrent density of W2C NFs over the 700-h experiment while the corresponding photo-potential fluctuates between −2.08 to −2.12 V, confirming the high stability of W2C NFs for CO2RR.
The measured sun to CO2RR products (CO, CH4, C2H4, CH3OH, and C2H5OH) as well as total solar-to-fuel efficiency (SFE) of W2C NFs over a 700-h process are shown in Fig. 5d (Supplementary section 18). As shown in this figure, an average sun to the CH4 production efficiency of 17.3% with negligible variation (2%) is achieved during the 700-h continuous process. Considering other products, W2C NFs show an SFE of 20.7%.
We also calculated the energy efficiency of CO2RR in our developed flow electrolyzer and compared it with state-of-the-art catalytic systems in the literature (Supplementary section 17)29,30,37,57,72. As shown in this figure (Supplementary Fig. 47), the maximum energy efficiency of 62.3% was obtained for our developed flow electrolyzer using W2C catalyst that is about 67 and 73% more efficient than Cuoh (37.4%)50 and recently developed Cu-CIPH (36.1%)72 catalytic systems, respectively.
In summary, we have synthesized four members of TMCs with a formula of M2C, i.e., W2C, Mo2C, Nb2C, and V2C NFs using the carburization method followed by the liquid exfoliation technique and tested their catalytic performance for eCO2RR in KOH:CC (3 M:2 M) electrolyte. The electrocatalytic performance studies of TMCs shows these materials are mainly selective for CH4 formation with W2C NFs having the best CO2RR activity compared to the studied catalysts. For instance, a CO2RR current density of −548.89 mA/cm2 and a maximum CH4 current density of −421.63 mA/cm2 at the potential of −1.05 V vs RHE were observed for W2C NFs. Our electrochemical results also indicate that adding CC to the electrolyte enhances the formation of CO2RR products by suppressing the HER for all studied TMCs. Moreover, the NMR and 13CO2 isotope experiments confirm that the CC remains stable during the electrochemical experiments. Atomic and molecular scale characterizations such as XPS, XRD, and STEM indicate that all synthesized TMCs have a similar lattice structure of 1 T with a dominant plane of (101) and almost the same average crystallite size of 25.4 nm. Furthermore, the electronic property analyses of TMCs reveal superior electronic properties of W2C NFs: low work function; small charge transfer resistance in the electrochemical double layer region; and heavily reduced tungsten atoms at the surface, which may lead to the observed high activity. Computational results also indicate that the studied TMCs spontaneously chemisorb CO2 and water as compared to Cu. However, among the TMCs studied, W2C exhibits the optimal combination for CH4 production, with favorable adsorption energies of water and CO2 coupled with spontaneous dissociation, and less costly protonation of OH*, which is the limiting step, with a low limiting potential in the range of −0.483 to −0.744 V vs RHE. Using W2C NFs, we have demonstrated a solar-driven flow electrolyzer that can work up to 700 h with a solar to CH4 efficiency and a total SFE of 17.3 and 20.7%, respectively, under one sun illumination. The demonstrated solar-driven flow electrolyzer using a non-precious metal catalyst (W2C NFs) in this study achieves maximum efficiency of 62.3% making it a good candidate to approach the commercially relevant electrocatalytic CO2RR. This opens a new direction toward a low-cost, sustainable large-scale production of fuels from CO2 that can be used any time any place.
Methods
Synthesis of TMCs
TMCs were prepared by carburization process in a dual-zone tubular furnace with a controlled flow of CH4 and H2 mixture (volumetric ratio CH4:H2 of 1:9) at a temperature of 973 K. The obtained bulk powders were then collected and ground to fine powders in a mortar and pestle. Next, a certain amount of TMC powders were processed in isopropyl alcohol using an ultrasonic liquid processor (Sonics VibraCell VCX-130) to obtain a solution of TMC NFs. The resulting solution was further centrifuged and the top two-third of the solutions were collected and stored as the TMCs in a vial for cathode electrode preparation. A detailed explanation is provided in Supplementary section 1.
Electrochemical setup
A two-compartment three-electrode electrochemical cell was used to perform the fundamental study for cathodic half-cell reaction using the synthesized W2C, Mo2C, Nb2C, and V2C NFs and compared them with Au and Cu NPs. In the three-electrode cell study, the working electrode was prepared by drop-casting the catalysts (mass loading of 0.1 mg) on a glassy carbon electrode with a geometric surface area of 1 cm2. The catalyst loading on the electrode was precisely controlled to be 0.1 mg/cm2 on the glassy carbon electrode. Platinum (Pt) gauze 52 mesh (Alfa Aesar) and Ag/AgCl (BASi) were used as counter and reference electrodes, respectively. The cathode and anode parts of the cell were separated through an anion exchange membrane (Sustainion X37-50 Grade RT, Dioxide Materials). All experiments were performed in a CO2 saturated KOH:CC (3 M:2 M) electrolyte with a pH of 14.5 ± 0.1. A two-compartment zero-gap solid polymer electrolyte flow electrolyzer was used to study the electrochemical performance where the working and counter electrodes are separated using an anion exchange membrane. Working electrodes (cathode) were prepared by brush-coating the solution of studied catalysts (W2C NFs, Au NPs, and Cu NPs) on the gas diffusion layer (GDL, Sigracet 39 BC, Fuel Cell Store) electrodes with a geometrical surface area of 5 cm2. The counter electrode (anode) was prepared using a similar procedure where IrO2 powder (Sigma Aldrich) was used as the catalyst solution. The actual loadings of 0.1 ± 0.01 mg/cm2 were determined by weighing the dry GDLs before catalyst deposition and coated GDLs after being dried in a vacuum oven overnight. As a separator in our experiments, we used an anion exchange membrane (Sustainion X37-50 Grade RT, Dioxide Materials) which was treated in 1 M KOH overnight at 75 °C and then washed with deionized water prior to use. Anolyte flow of KOH:CC (3 M:2 M) with a flow rate of 20 ml/min was fed to the anode compartment using a peristaltic pump (Masterflex, Cole-Parmer). A mass flow controller (SmartTrak 50, Sierra, calibrated with CO2 gas) connected to the CO2 humidifier kit, was used to feed the cathode compartment with a flow rate of 50 ml/min.
PV cell characterization
A solar-powered flow cell was assembled by connecting the solid polymer electrolyte flow electrolyzer to a triple junction photovoltaic (TJ-PV) solar cell. The TJ-PV cell was characterized at different sun illuminations using a custom-made sun simulator light source and an InGaAs photodiode (Thorlabs, FDG03-CAL) with a known responsivity calibration curve. Our results indicated a maximum efficiency of 34.32% under one sun illumination used in our study.
Electrochemical characterization
Electrochemical experiments were performed using a Biologic Potentiostat SP-150. The CA technique was used to study the performance of TMC NFs i.e., W2C, Mo2C, Nb2C, and V2C NFs and compared them with that of Au and Cu NPs. The CA experiments were carried out in the range of −0.45 to −1.05 V vs RHE potentials. All experiments were performed under identical experimental conditions. The LSV technique was used to study the fundamentals of the cathodic half-cell reaction in the three-electrode cell setup. LSV curves were obtained by sweeping the potential between +0.2 and −1.05 V vs RHE with a scan rate of 20 mV/s. The conversion of Ag/AgCl reference electrode potential to the RHE scale was calculated using the Nernst equation considering the pH of the solution (pH = 14.5).
Product characterization
A gas chromatography system (GC, SRI, 8610 C) equipped with a flame ionization detector (FID) and a thermal conductivity detector (TCD) was used to detect and quantify the electrochemical CO2RR products. Ultra-high purity helium (He) and nitrogen (N2) gases (UHP 99.99%, Airgas) were used as the carrier gas to identify any possible type of product. the signal response of the FID and TCD to each gaseous product (e.g., H2, CO, CH4, and C2H4) was calibrated by analyzing a series of standard gas mixtures with known compositions prior to the experiments. To study the products, 1 mL sample of the headspace of the cell was injected into the GC system using a lock-in syringe (Hamilton). Moreover, an in situ differential electrochemical mass spectrometer (DEMS, Hiden Analytical, HPR-40) was used to validate the obtained information from the GC system by continuously detecting all possible products, even at trace amounts (partial pressure of 1 × 10−13 Torr), during the electrochemical CO2RR (CA experiment), resulting in more precise measurement. The signal responses of the DEMS instrument for different products (H2, CO, CH4, C2H4, CH3OH, and C2H5OH) were calibrated by feeding standard samples into the mass spectrometer. An electron energy of 70 eV was used for ionization of all species, with an emission current of 500 µA. All mass-selected product cations were detected by a secondary electron multiplier with a detector voltage of 1200 V for maximizing the signal-to-noise ratio of the products.
X-ray diffraction (XRD)
The XRD technique was used to identify the phase purity and crystallinity of all studied catalysts (W2C, Mo2C, Nb2C, V2C NFs, Au NPs, and Cu NPs) using a Bruker D2 PHASER diffractometer in Bragg–Brentano geometry employing a Ni filtered Cu Kα radiation (1.5405 Å). The XRD patterns were obtained using a LynxEye linear position-sensitive detector and a step width of 0.2 °2θ with a counting time of 1 s/step.
X-ray photoelectron spectroscopy (XPS)
A Thermo-Scientific ESCALAB 250Xi instrument equipped with an electron flood and scanning ion gun was used to identify the oxidation states of the W2C NFs. All obtained spectra were analyzed using Thermo-Avantage software, considering the standard carbon peak at 284.8 eV and relative sensitivity factors.
Ultraviolet photoelectron spectroscopy (UPS)
Surface work function measurements were carried out using the UPS technique. All UPS data were acquired by a Thermo-Scientific ESCALAB 250Xi instrument using He I (21.2 eV) ultraviolet radiation and a pass energy of 8.95 eV.
Scanning transmission electron microscopy (STEM)
W2C NFs were characterized at the atomic scale using a spherical aberration-corrected JEOL JEM-ARM 200CF STEM with a cold field emission gun operating at 200 kV. HAADF detector with 22 mrd inner-detector angle and BF detector were utilized to obtain the atomic resolution images.
Theoretical study
We performed a comparative DFT analysis for the observed catalytic activity and reactivity of W2C NFs with Au and other TMCs using the SIESTA package, with Perdew–Burke–Ernzerhof functional with a double-zeta with polarization (DZP) localized basis set and the norm-conserving Troullier-Martins pseudopotentials. Calculations of DOS for bulk and slab geometries of Au and TMCs were performed using the Effective Screening Method (ESM)73 for Brillouin zones of the unit cells sampled by Monkhorst-Pack k-point grids of size 9 × 9 × 9 and 1 × 9 × 9, respectively, together with a plane-wave cutoff of 300.0 Ry. The optimization of the atomic positions and cell parameters were carried out using a conjugate-gradient algorithm until a maximum atomic force tolerance of 0.04 eV/Å and a maximum stress component along each periodic direction of lower than 1 GPa were achieved. The Vienna ab initio Simulation Package (VASP, version 5.4.4) with PAW (projector augmented wave method) and Perdew–Burke–Ernzerhof exchange-correlation functionals were used to analyze the adsorption free energies of various molecular species on the (101) surface of M2C (M = W, V, Mo, Nb). All the VASP calculations were performed for neutral non-spin-polarized systems and a dipolar electrostatic correction was used along the normal to the surface of the slab. Next, we used the tetrahedron method with Blöchl corrections and 1 × 3 x 3 Monkhorst-Pack grid k-point sampling for the calculations of total electronic energy (smearing σ = 0.1). The adsorption free energies were then used within the CHE model64–66 to evaluate the lowest free energy pathways and the limiting reaction potentials.
Supplementary information
Acknowledgements
Mohammad Asadi’s work was supported by Illinois Institute of Technology start-up funding, Wanger Institute for Sustainable Energy Research (WISER) Institute for Sustainable Energy Research (WISER) seed fund (262029 221E 2300), American Institute of Architects (AIA) Upjohn Development Research Grant (387523 240 M 2301) and the Soft and Hybrid Nanotechnology Experimental (SHyNE) Resource (NSF ECCS-152205) funding at Northwestern University. This work was also supported by the Molecular Foundry and its compute cluster (vulcan), managed by the High-Performance Computing Services Group, at Lawrence Berkeley National Laboratory (LBNL), and by the National Energy Research Scientific Computing Center (NERSC) at LBNL. LBML resources are provided by the Office of Science of the US Department of Energy under contract No. DE-AC02-05CH11231. Reza Shahbazian-Yassar efforts were supported by NSF (DMR-1809439). We acknowledge the EPIC facility (NUANCE Center, Northwestern University), which has received support from the MRSEC program (NSF DMR-1121262) at the Materials Research Center; the Nanoscale Science and Engineering Center (NSF EEC−0647560) at the International Institute for Nanotechnology; and the State of Illinois, through the International Institute for Nanotechnology. The authors acknowledge Dr. Rao Tatavarti from Micro-Link Device, Inc. at Chicago for providing the triple junction PV cell. This work made use of instruments in the Electron Microscopy Service (Research Resources Center, UIC). The acquisition of the UIC JEOL JEM-ARM200CF was supported by an MRI-R2 grant from the National Science Foundation (Award No. DMR-0959470).
Source data
Author contributions
M.A. and M.E. conceived the idea of the work. M.E. synthesized the nanostructured materials and designed and fabricated the experimental devices. M.E., A.K., A.R.B., P.N.M.D., and J.P. performed electrocatalysis experiments and data analysis. R.A. contributed to flow cell design. D.P., A.B., A.S.M., and J.Q. carried out DFT calculations and CHE model analysis. M.E., K.K., and C.U.S. did the XRD characterization and analysis. M.E. and A.K. did the XPS, UPS, DLS, and NMR characterizations. B.S., M.T.S., and R.S.Y. performed the STEM characterization. M.A. supervised M.E., A.K., A.R.B., P.N.M.D., and J.P. efforts. All authors discussed the results and assisted with manuscript preparation.
Data availability
The data supporting the findings of this study are available within the article and its Supplementary Information. Other relevant data are available from the corresponding author upon reasonable request. The Source data underlying figures of this manuscript are provided as a Source Data file which is provided with this paper. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 2089992–2089995. Source data are provided with this paper.
Competing interests
M.A., M.E., A.K., and A.R.B. filed a patent application. The remaining authors declare no competing interests.
Footnotes
Peer review informationNature Communications thanks David Willock and the other anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Mohammadreza Esmaeilirad, Artem Baskin.
Contributor Information
David Prendergast, Email: dgprendergast@lbl.gov.
Mohammad Asadi, Email: m.asadi@iit.edu.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-25295-y.
References
- 1.Ross MB, et al. Designing materials for electrochemical carbon dioxide recycling. Nat. Catal. 2019;2:648–658. doi: 10.1038/s41929-019-0306-7. [DOI] [Google Scholar]
- 2.Lewis NS. Toward cost-effective solar energy use. Science. 2007;315:798–801. doi: 10.1126/science.1137014. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Lewis NS, Xiang C. Operational constraints and strategies for systems to effect the sustainable, solar-driven reduction of atmospheric CO2. Energy Environ. Sci. 2015;8:3663–3674. doi: 10.1039/C5EE02908B. [DOI] [Google Scholar]
- 4.Esmaeili Rad F, Abbasian J, Arastoopour H. Numerical simulation of CO2 adsorption in a fluidized bed using solid-supported amine sorbent. Can. J. Chem. Eng. 2020;99:1595–1606. doi: 10.1002/cjce.24000. [DOI] [Google Scholar]
- 5.Shih CF, Zhang T, Li J, Bai C. Powering the future with liquid sunshine. Joule. 2018;2:1925–1949. doi: 10.1016/j.joule.2018.08.016. [DOI] [Google Scholar]
- 6.Birdja YY, et al. Advances and challenges in understanding the electrocatalytic conversion of carbon dioxide to fuels. Nat. Energy. 2019;4:732–745. doi: 10.1038/s41560-019-0450-y. [DOI] [Google Scholar]
- 7.Rosen BA, et al. Ionic liquid–mediated selective conversion of CO2 to CO at low overpotentials. Science. 2011;334:643–644. doi: 10.1126/science.1209786. [DOI] [PubMed] [Google Scholar]
- 8.Asadi M, et al. Nanostructured transition metal dichalcogenide electrocatalysts for CO2 reduction in ionic liquid. Science. 2016;353:467–470. doi: 10.1126/science.aaf4767. [DOI] [PubMed] [Google Scholar]
- 9.Rosen BA, Zhu W, Kaul G, Salehi-Khojin A, Masel RI. Water enhancement of CO2 conversion on silver in 1-ethyl-3-methylimidazolium tetrafluoroborate. J. Electrochem. Soc. 2012;160:H138–H141. doi: 10.1149/2.004303jes. [DOI] [Google Scholar]
- 10.Abbasi P, et al. Tailoring the edge structure of molybdenum disulfide toward electrocatalytic reduction of carbon dioxide. ACS Nano. 2017;11:453–460. doi: 10.1021/acsnano.6b06392. [DOI] [PubMed] [Google Scholar]
- 11.Asadi M, et al. Robust carbon dioxide reduction on molybdenum disulphide edges. Nat. Commun. 2014;5:4470. doi: 10.1038/ncomms5470. [DOI] [PubMed] [Google Scholar]
- 12.Chan K, Tsai C, Hansen HA, Nørskov JK. Molybdenum sulfides and selenides as possible electrocatalysts for CO2 reduction. ChemCatChem. 2014;6:1899–1905. doi: 10.1002/cctc.201402128. [DOI] [Google Scholar]
- 13.Hong X, Chan K, Tsai C, Nørskov JK. How doped MoS2 breaks transition-metal scaling relations for CO2 electrochemical reduction. ACS Catal. 2016;6:4428–4437. doi: 10.1021/acscatal.6b00619. [DOI] [Google Scholar]
- 14.Clark EL, et al. Influence of atomic surface structure on the activity of Ag for the electrochemical reduction of CO2 to CO. ACS Catal. 2019;9:4006–4014. doi: 10.1021/acscatal.9b00260. [DOI] [Google Scholar]
- 15.Rosen J, et al. Mechanistic insights into the electrochemical reduction of CO2 to CO on nanostructured Ag surfaces. ACS Catal. 2015;5:4293–4299. doi: 10.1021/acscatal.5b00840. [DOI] [Google Scholar]
- 16.Fang Y, Flake JC. Electrochemical reduction of CO2 at functionalized Au electrodes. J. Am. Chem. Soc. 2017;139:3399–3405. doi: 10.1021/jacs.6b11023. [DOI] [PubMed] [Google Scholar]
- 17.Zhao Y, Wang C, Liu Y, MacFarlane DR, Wallace GG. Engineering surface amine modifiers of ultrasmall gold nanoparticles supported on reduced graphene oxide for improved electrochemical CO2 reduction. Adv. Energy Mater. 2018;8:1801400. doi: 10.1002/aenm.201801400. [DOI] [Google Scholar]
- 18.Lee HE, et al. Concave rhombic dodecahedral Au nanocatalyst with multiple high-index facets for CO2 reduction. ACS Nano. 2015;9:8384–8393. doi: 10.1021/acsnano.5b03065. [DOI] [PubMed] [Google Scholar]
- 19.Zhao S, Jin R, Jin R. Opportunities and challenges in CO2 reduction by gold- and silver-based electrocatalysts: from bulk metals to nanoparticles and atomically precise nanoclusters. ACS Energy Lett. 2018;3:452–462. doi: 10.1021/acsenergylett.7b01104. [DOI] [Google Scholar]
- 20.Zhang Z, et al. Rational design of bi nanoparticles for efficient electrochemical CO2 reduction: the elucidation of size and surface condition effects. ACS Catal. 2016;6:6255–6264. doi: 10.1021/acscatal.6b01297. [DOI] [Google Scholar]
- 21.Todoroki N, et al. Surface atomic arrangement dependence of electrochemical CO2 reduction on gold: online electrochemical mass spectrometric study on low-index Au(hkl) surfaces. ACS Catal. 2019;9:1383–1388. doi: 10.1021/acscatal.8b04852. [DOI] [Google Scholar]
- 22.Back S, Yeom MS, Jung Y. Active sites of Au and Ag nanoparticle catalysts for CO2 electroreduction to CO. ACS Catal. 2015;5:5089–5096. doi: 10.1021/acscatal.5b00462. [DOI] [Google Scholar]
- 23.Kim KS, Kim WJ, Lim HK, Lee EK, Kim H. Tuned chemical bonding ability of Au at grain boundaries for enhanced electrochemical CO2 reduction. ACS Catal. 2016;6:4443–4448. doi: 10.1021/acscatal.6b00412. [DOI] [Google Scholar]
- 24.Tao Z, Wu Z, Yuan X, Wu Y, Wang H. Copper–gold interactions enhancing formate production from electrochemical CO2 reduction. ACS Catal. 2019;9:10894–10898. doi: 10.1021/acscatal.9b03158. [DOI] [Google Scholar]
- 25.Morales-Guio CG, et al. Improved CO2 reduction activity towards C2+ alcohols on a tandem gold on copper electrocatalyst. Nat. Catal. 2018;1:764–771. doi: 10.1038/s41929-018-0139-9. [DOI] [Google Scholar]
- 26.Liu M, et al. Enhanced electrocatalytic CO2 reduction via field-induced reagent concentration. Nature. 2016;537:382. doi: 10.1038/nature19060. [DOI] [PubMed] [Google Scholar]
- 27.Asadi, M. et al. Highly efficient solar-driven carbon dioxide reduction on molybdenum disulfide catalyst using choline chloride-based electrolyte. Adv. Energy Mater. 9, 1803536 (2019).
- 28.Nitopi S, et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 2019;119:7610–7672. doi: 10.1021/acs.chemrev.8b00705. [DOI] [PubMed] [Google Scholar]
- 29.Dinh CT, et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science. 2018;360:783–787. doi: 10.1126/science.aas9100. [DOI] [PubMed] [Google Scholar]
- 30.Li F, et al. Molecular tuning of CO2-to-ethylene conversion. Nature. 2020;577:509–513. doi: 10.1038/s41586-019-1782-2. [DOI] [PubMed] [Google Scholar]
- 31.Wakerley D, et al. Bio-inspired hydrophobicity promotes CO2 reduction on a Cu surface. Nat. Mater. 2019;18:1222–1227. doi: 10.1038/s41563-019-0445-x. [DOI] [PubMed] [Google Scholar]
- 32.Jiang K, et al. Metal ion cycling of Cu foil for selective C–C coupling in electrochemical CO2 reduction. Nat. Catal. 2018;1:111–119. doi: 10.1038/s41929-017-0009-x. [DOI] [Google Scholar]
- 33.Zhang X, Sun X, Guo SX, Bond AM, Zhang J. Formation of lattice-dislocated bismuth nanowires on copper foam for enhanced electrocatalytic CO2 reduction at low overpotential. Energy Environ. Sci. 2019;12:1334–1340. doi: 10.1039/C9EE00018F. [DOI] [Google Scholar]
- 34.Iijima G, Inomata T, Yamaguchi H, Ito M, Masuda H. Role of a hydroxide layer on Cu electrodes in electrochemical CO2 reduction. ACS Catal. 2019;9:6305–6319. doi: 10.1021/acscatal.9b00896. [DOI] [Google Scholar]
- 35.Liang Z, et al. Copper-on-nitride enhances the stable electrosynthesis of multi-carbon products from CO2. Nat. Commun. 2018;9:3828. doi: 10.1038/s41467-018-06311-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garza AJ, Bell AT, Head-Gordon M. Mechanism of CO2 reduction at copper surfaces: pathways to C2 products. ACS Catal. 2018;8:1490–1499. doi: 10.1021/acscatal.7b03477. [DOI] [Google Scholar]
- 37.Ringe S, et al. Understanding cation effects in electrochemical CO2 reduction. Energy Environ. Sci. 2019;12:3001–3014. doi: 10.1039/C9EE01341E. [DOI] [Google Scholar]
- 38.Li CW, Kanan MW. CO2 reduction at low overpotential on Cu electrodes resulting from the reduction of thick Cu2O films. J. Am. Chem. Soc. 2012;134:7231–7234. doi: 10.1021/ja3010978. [DOI] [PubMed] [Google Scholar]
- 39.Gogotsi Y, Anasori B. The rise of MXenes. ACS Nano. 2019;13:8491–8494. doi: 10.1021/acsnano.9b06394. [DOI] [PubMed] [Google Scholar]
- 40.Anasori B, et al. Two-dimensional, ordered, double transition metals carbides (MXenes) ACS Nano. 2015;9:9507–9516. doi: 10.1021/acsnano.5b03591. [DOI] [PubMed] [Google Scholar]
- 41.Hantanasirisakul K, Gogotsi Y. Electronic and optical properties of 2D transition metal carbides and nitrides (MXenes) Adv. Mater. 2018;30:1804779. doi: 10.1002/adma.201804779. [DOI] [PubMed] [Google Scholar]
- 42.Esmaeilirad M, Kondori A, Ruiz Belmonte A, Asadi M. Electroreduction of carbon dioxide to methane enabled by molybdenum carbide nanocatalyst. ECS Meet. Abstr. 2020;MA2020-02:3234. doi: 10.1149/MA2020-02633234mtgabs. [DOI] [Google Scholar]
- 43.Lei J, Kutana A, Yakobson BI. Predicting stable phase monolayer Mo2C (MXene), a superconductor with chemically-tunable critical temperature. J. Mater. Chem. C. 2017;5:3438–3444. doi: 10.1039/C7TC00789B. [DOI] [Google Scholar]
- 44.Lewandowski M, Szyma A, Sayag C, Beaunier P. Applied catalysis B: environmental atomic level characterization and sulfur resistance of unsupported W2C during dibenzothiophene hydrodesulfurization. Classical kinetic simulation of the reaction. Appl. Catal. B Environ. 2014;144:750–759. doi: 10.1016/j.apcatb.2013.08.011. [DOI] [Google Scholar]
- 45.Kondori A, et al. Identifying catalytic active sites of trimolybdenum phosphide (Mo3P) for electrochemical hydrogen evolution. Adv. Energy Mater. 2019;9:1900516. doi: 10.1002/aenm.201900516. [DOI] [Google Scholar]
- 46.Esmaeilirad M, et al. Oxygen functionalized copper nanoparticles for solar-driven conversion of carbon dioxide to methane. ACS Nano. 2020;14:2099–2108. doi: 10.1021/acsnano.9b08792. [DOI] [PubMed] [Google Scholar]
- 47.Vasilyev DV, Dyson P. J. The role of organic promoters in the electroreduction of carbon dioxide. ACS Catal. 2021;11:1392–1405. doi: 10.1021/acscatal.0c04283. [DOI] [Google Scholar]
- 48.Zhang, T. et al. Highly dispersed, single-site copper catalysts for the electroreduction of CO2 to methane. J. Electroanal. Chem. 875 113862 (2020).
- 49.Wu J, et al. A metal-free electrocatalyst for carbon dioxide reduction to multi-carbon hydrocarbons and oxygenates. Nat. Commun. 2016;7:13869. doi: 10.1038/ncomms13869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Gregorio GL, et al. Facet-dependent selectivity of Cu catalysts in electrochemical CO2 reduction at commercially viable current densities. ACS Catal. 2020;10:4854–4862. doi: 10.1021/acscatal.0c00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen S, et al. Highly selective carbon dioxide electroreduction on structure-evolved copper perovskite oxide toward methane production. ACS Catal. 2020;10:4640–4646. doi: 10.1021/acscatal.0c00847. [DOI] [Google Scholar]
- 52.Wang X, et al. Efficient methane electrosynthesis enabled by tuning local CO2 availability. J. Am. Chem. Soc. 2020;142:3525–3531. doi: 10.1021/jacs.9b12445. [DOI] [PubMed] [Google Scholar]
- 53.Weng Z, et al. Active sites of copper-complex catalytic materials for electrochemical carbon dioxide reduction. Nat. Commun. 2018;9:415. doi: 10.1038/s41467-018-02819-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manthiram K, Beberwyck BJ, Alivisatos AP. Enhanced electrochemical methanation of carbon dioxide with a dispersible nanoscale copper catalyst. J. Am. Chem. Soc. 2014;136:13319–13325. doi: 10.1021/ja5065284. [DOI] [PubMed] [Google Scholar]
- 55.Jones JP, Prakash GKS, Olah GA. Electrochemical CO2 reduction: recent advances and current trends. Isr. J. Chem. 2014;54:1451–1466. doi: 10.1002/ijch.201400081. [DOI] [Google Scholar]
- 56.Kuhl KP, Cave ER, Abram DN, Jaramillo TF. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 2012;5:7050–7059. doi: 10.1039/c2ee21234j. [DOI] [Google Scholar]
- 57.Hoang TTH, et al. Nanoporous copper–silver alloys by additive-controlled electrodeposition for the selective electroreduction of CO2 to ethylene and ethanol. J. Am. Chem. Soc. 2018;140:5791–5797. doi: 10.1021/jacs.8b01868. [DOI] [PubMed] [Google Scholar]
- 58.Kim SK, Zhang YJ, Bergstrom H, Michalsky R, Peterson A. Understanding the low-overpotential production of CH4 from CO2 on Mo2C catalysts. ACS Catal. 2016;6:2003–2013. doi: 10.1021/acscatal.5b02424. [DOI] [Google Scholar]
- 59.Holzwarth U, Gibson N. The Scherrer equation versus the Debye-Scherrer equation. Nat. Nanotechnol. 2011;6:534. doi: 10.1038/nnano.2011.145. [DOI] [PubMed] [Google Scholar]
- 60.Patterson AL. The Scherrer formula for X-ray particle size determination. Phys. Rev. 1939;56:978–982. doi: 10.1103/PhysRev.56.978. [DOI] [Google Scholar]
- 61.Esmaeilirad M, Zabihi M, Shayegan J, Khorasheh F. Oxidation of toluene in humid air by metal oxides supported on Γ-alumina. J. Hazard. Mater. 2017;333:293–307. doi: 10.1016/j.jhazmat.2017.03.038. [DOI] [PubMed] [Google Scholar]
- 62.Wang QH, Kalantar-Zadeh K, Kis A, Coleman JN, Strano MS. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012;7:699–712. doi: 10.1038/nnano.2012.193. [DOI] [PubMed] [Google Scholar]
- 63.Bienen F, Kopljar D, Geiger S, Wagner N, Friedrich KA. Investigation of CO2 electrolysis on tin foil by electrochemical impedance spectroscopy. ACS Sustain. Chem. Eng. 2020;8:5192–5199. doi: 10.1021/acssuschemeng.9b07625. [DOI] [Google Scholar]
- 64.Nørskov JK, et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B. 2004;108:17886–17892. doi: 10.1021/jp047349j. [DOI] [Google Scholar]
- 65.Rossmeisl J, Logadottir A, Nørskov JK. Electrolysis of water on (oxidized) metal surfaces. Chem. Phys. 2005;319:178–184. doi: 10.1016/j.chemphys.2005.05.038. [DOI] [Google Scholar]
- 66.Peterson AA, Abild-Pedersen F, Studt F, Rossmeisl J, Nørskov JK. How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels. Energy Environ. Sci. 2010;3:1311–1315. doi: 10.1039/c0ee00071j. [DOI] [Google Scholar]
- 67.Li N, et al. Understanding of electrochemical mechanisms for CO2 capture and conversion into hydrocarbon fuels in transition-metal carbides (MXenes) ACS Nano. 2017;11:10825–10833. doi: 10.1021/acsnano.7b03738. [DOI] [PubMed] [Google Scholar]
- 68.Garza AJ, Bell AT, Head-Gordon M. Is subsurface oxygen necessary for the electrochemical reduction of CO2 on copper? J. Phys. Chem. Lett. 2018;9:601–606. doi: 10.1021/acs.jpclett.7b03180. [DOI] [PubMed] [Google Scholar]
- 69.Favaro M, et al. Subsurface oxide plays a critical role in CO2 activation by Cu(111) surfaces to form chemisorbed CO2, the first step in reduction of CO2. Proc. Natl Acad. Sci. USA. 2017;114:6706–6711. doi: 10.1073/pnas.1701405114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lim DH, et al. Carbon dioxide conversion into hydrocarbon fuels on defective graphene-supported Cu nanoparticles from first principles. Nanoscale. 2014;6:5087–5092. doi: 10.1039/C3NR06539A. [DOI] [PubMed] [Google Scholar]
- 71.Kondori A, et al. Kinetically stable oxide overlayers on Mo3P nanoparticles enabling lithium–air batteries with low overpotentials and long cycle life. Adv. Mater. 2020;32:2004028. doi: 10.1002/adma.202004028. [DOI] [PubMed] [Google Scholar]
- 72.García de Arquer FP, et al. CO2 electrolysis to multicarabon products at activities greater than 1 A cm−2. Science. 2020;367:661 LP–661666. doi: 10.1126/science.aay4217. [DOI] [PubMed] [Google Scholar]
- 73.Otani M, Sugino O. First-principles calculations of charged surfaces and interfaces: A plane-wave nonrepeated slab approach. Phys. Rev. B. 2006;73:115407. doi: 10.1103/PhysRevB.73.115407. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available within the article and its Supplementary Information. Other relevant data are available from the corresponding author upon reasonable request. The Source data underlying figures of this manuscript are provided as a Source Data file which is provided with this paper. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 2089992–2089995. Source data are provided with this paper.