Abstract
Ptaquiloside (PTA) is a natural carcinogen present in many ferns. Brackens (Pteridium sp.) contain PTA and are classified by WHO/IARC as ‘ … possibly carcinogenic to humans’, however, these ferns are used in food, traditional medicine and as food supplements around the world. This study aimed to outline the presence of PTA in different human exposure routes by using and validating an LC-MS based protocol to test the contents of PTA in commercial products, the degradation product Pterosin B (PtB) and wild specimens from Europe, Asia and North America. The Limit of Detection of the protocol was 0.024 μg g−1 for PTA and 0.028 μg g−1 for PtB. PTA and PtB were present in most wild specimens (PTA: BD – 6300 ± 520 μg g−1; PtB: BD - 449 ± 1 μg g−1) while commercial products made from fronds, as well as fronds prepared as traditional Chinese medicine, were in the range 44 ± 3 to 666 ± 33 μg g−1 for PTA and BD to 1653 ± 184 μg g−1 for PtB. This study did not find PTA/PtB in rhizomes and products made thereof nor in homoeopathic products based on bracken. Boiling or drying bracken showed to reduce PTA some degree but cannot remove it completely. Interestingly, crosiers with no PTA/PtB were found in the USA, indicating a potential for commercial production of PTA-free fronds.
Keywords: Cancer, Natural toxins, LC-MS, Pterosin B, Cooking
Graphical abstract
Highlights
-
•
Ptaquiloside is a natural carcinogen found in fern-based food products and natural remedies.
-
•
Bracken ferns collected in the wild contain ptaquiloside after processing.
-
•
Commercial dried bracken fronds (leaves) contain high levels of ptaquiloside.
-
•
Commercial products based on rhizomes and some natural remedies contain no ptaquiloside.
-
•
Presence of no-ptaquiloside ferns indicate potential of growing non-toxic cultivars.
1. Introduction
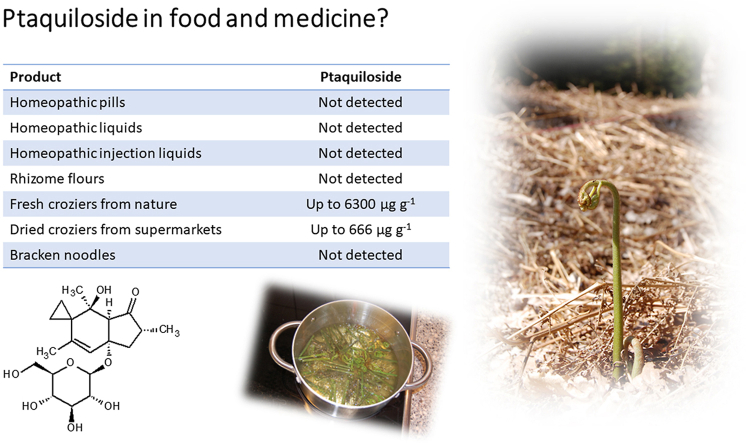
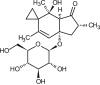
Ptaquiloside (PTA) is the most prominent illudane-type glycoside (Table 1), which are found in a variety of ferns, but the toxicology and health effects have been most intensively studied in bracken ferns, a group of large perennial ferns with a global distribution. Farm animals tend to browse only on bracken ferns in times of food scarcity, as the ferns are neither nutrient-rich nor very palatable. Bracken is known to cause a variety of diseases among farm animals such as Bovine Enzootic Haematuria, Acute Bracken Poisoning and Bright Blindness, and the two first syndromes affect cattle and buffaloes in paddocks or open grazing areas with bracken, while the latter is commonly encountered with free-ranging sheep in bracken infested land. The diseases are generally reported in countries like Brazil, Venezuela, Australia, New Zealand, Italy, Spain, Portugal, England and Scotland (GBIF.org., 2021; Marrs and Watt, 2006; O'Connor et al., 2019).
Table 1.
Properties of ptaquiloside and pterosin B.
 |
 |
|
|---|---|---|
| Compound: | Ptaquiloside | Pterosin B |
| Molecular mass (avg., Da): | 398.447 | 218.292 |
| LogKowa: | −0.95 | 3.19 |
| Water solubility (25 °C, mg/L)a: | 20,400 | 213 |
| Bioaccumulation estimate (logBCF)a: | 0.50 | 0.27 |
| Bioactivity (Yahara et al., 2016; O'Connor et al., 2019) | Acute toxic; genotoxic. | Sik3 pathway inhibitor. |
Estimate from US Environmental Protection Agency's EPISuite™ (EPA, 2018).
Bracken is suspected for causing human cancers and has therefore been classified as ‘possibly carcinogenic to humans’ by the WHO/IARC. Humans are exposed to the compounds when consuming bracken based traditional medicine (e.g. traditional Chinese medicine) or via ingesting bracken contaminated milk, meat, spores or drinking water, which is common in Asia (e.g. China, Korea and Japan) and North and South America (e.g. Brazil, USA and Canada) (Alonso-Amelot et al., 1993; Fletcher et al., 2011; GLIFWC, 2014; Kristanc and Kreft, 2016; Marliere et al., 2002; O'Driscoll et al., 2016; Skrbic, 2020; Virgilio, 2015). Due to the adverse health effects of bracken, the genus is placed on many national lists of plants not to be consumed by humans, and in Denmark, all parts of Bracken are classified as not acceptable for use as dietary supplements, in herbal teas or food (Gry et al., 1998). Bracken and PTA have also been placed on the WHO/IARC urgency list of potentially carcinogenic compounds/products to be re-evaluated (WHO/IARC, 2021).
Ptaquiloside is present in all parts of bracken ferns; fronds (leaves), rhizomes (below-ground stems), spores and roots, but the specific amount relies on the annual growth cycle and growing conditions. The fronds are known to have the highest concentration in spring, with contents reaching from 1000 to 50,000 μg g−1 (dry-weight), while the content in rhizomes is negligible most of the year. The content may however increase in autumn due to nutrient translocation from fronds to storage rhizomes (bracken is a deciduous perennial fern). Bracken stands with no PTA have been found in New Zealand. There is a substantial variation in the distribution between illudane glycosides within, as well as between species of bracken. The content of PTA in spores and roots found in this is considered low compared to what is found in the fronds (Kisielius et al., 2019; Rasmussen et al., 2008, 2013, 2015).
Ptaquiloside is relatively stable in bracken processed as comestibles despite undergoing acid, as well as alkaline hydrolysis, to form pterosin B (PtB; Table 1). The rate of hydrolysis is pH-dependent, leaving a window of stability for PTA in the range pH4.5 to 6.5. Degradation of PTA is strongly temperature-dependent and boiling for a long time will cause degradation of PTA (Ayala-Luis et al., 2006). Glycosidase enzymes can also cause PTA degradation. PtB becomes the end-product of the above-mentioned reactions, but other intermediates exist: Ptaquilosin and the so-called Bracken Dienone (O'Connor et al., 2019). PtB is present at similar levels as PTA in bracken fronds and can be used an indicator of an earlier presence of PTA in the compound (Rasmussen and Pedersen, 2017). Ptaquilosin and Bracken Dienone are believed to be the ultimate carcinogens (alkylating metabolites) and are highly unstable under acid and neutral pH. They are only found in neutral to alkaline matrices such as milk (Aranha et al., 2019).
Most studies addressing PTA in wild food products have been carried out in Japan or South America, but there is a lack of knowledge concerning the content of PTA in commercial products such as dried bracken and traditional medicines. The purpose of this study was to outline the presence of PTA in different human exposure routes by testing the hypothesis: Food products, natural remedies and traditional medicine made with bracken fern contain the carcinogen PTA. An LC-ESI-MS based dilute-and-shoot method has been validated to explore four exposure scenarios: Case 1) Using fresh bracken crosiers for food (fresh or after blanching); Case 2) Preparing traditional Chinese medicinal products using fresh bracken; Case 3) Collecting wild bracken in North America; Case 4) Exposure to PTA from commercial food products and European natural remedies obtained from online shops. The study is limited to Common Bracken (Pteridium aquilinum (L.) Kuhn) and PTA, as this is the dominant illudane-type glycoside in this bracken species (Kisielius et al., 2019). PtB was included as it is formed from PTA under natural conditions as well as due to sample handling.
2. Materials and methods
2.1. Chemicals and essential laboratory consumables
HPLC grade acetonitrile (Hiperpur HPLC hypergradient grade; Panreac, Barcelona, Spain). Sodium hydroxide, sodium acetate, trifluoroacetic acid (TFA) and hydrochloric acid (pro analysis; Sigma-Aldrich, Copenhagen, Denmark). Deionised water was used for preparing reagents and plant extractions (except otherwise stated in the protocol below). LCMS-grade water was used for preparing eluents (ELGA Purelab® Classic, ELGA VEOLIA, Kruger Aquacare, Glostrup, Denmark). PTA was obtained from the University of Copenhagen (purity: 103 ± 10%; Professor Hans Christian Bruun Hansen, Denmark). Analytical standards of PtB were made from pure PTA (Rasmussen and Pedersen, 2017). PTA/PtB standards/samples were kept at −18 °C when not in use. 0.45 μm Q-Max Syringe Filter (cellulose acetate, sterile, Frisenette ApS, Knebel, Denmark). Polyamide 6 resin (analytical grade; Fluka, Steinheim, Switzerland). Whatman filter vials (0.20 μm filter vial; Whatman Mini-UniPrep PTFE filter vial, GE Healthcare UK Limited, United Kingdom).
2.2. Chemical compounds studied in this article
Ptaquiloside (PubChem CID: 53297436; Table 1); Pterosin B (PubChem CID: 115049; Table 1).
2.3. Method development and validation
The analytical method was based on Rai et al. (2017) and further optimised for use in food science using loganine as an internal standard. The method was validated according to the ICH guidelines (ICH, 2005) for PTA and PtB in terms of linear range, linearity, limit of detection (LOD), limit of quantification (LOQ), precision (analytical standards; samples) and matrix effects for different sample types (accuracy; PTA only). The ruggedness/storage stability of PTA/PtB standards and samples (−20 °C), as well as the accuracy of the aqueous extraction method, have been demonstrated elsewhere (Kisielius et al., 2020; Rasmussen and Pedersen, 2017).
The precision of the analytical method was reported as the RSD of 7–11 repetitive peak area measurements for 3 analytical standards (cPTA = 4.4/48/88 μg L−1; cPtB = 4.4/44/88 μg L−1) and a single bracken extract (cPTA = 76 μg L−1; cPtB = 68 μg L−1). In addition, the precision of loganine, used as an internal standard, was also measured (cLOG = 100 μg L−1), and the precision of retention time and peak asymmetry were recorded as part of the peak area measurements. The linear range was explored in the expected relevant concentration range for PTA and PtB. Linearity was determined using 8 calibration levels in triplicate for PTA/PtB and singular for LOG (cPTA: 4.4–8.8 - 107.6–26.4 - 35.2–44–66–88 μg L−1; cPtB: 4.4–8.8 - 17.6–26.4 - 35.2–44–66–88 μg L−1; cLOG: 10–50 - 100–150–200 μg L−1). LOD/LOQ were calculated as 3/10 times the RSD of the resulting concentration from 7 repetitive injections (4.4 μg L−1; concentration calculated from the response factor of the external/internal calibration curves). Analytical accuracy and check for matrix effects for PTA were determined using a representative sample of bracken fern extract spiked with PTA at 4 levels, corresponding to 0, 33, 66 and 100% of the PTA concentration (0–60 μg L−1). The same setup was used to spike extracts of homoeopathic ampoules, globuli and rhizome flour.
The extraction protocol was optimised for extracting PTA and PtB with respect to: Extraction solution (water; 50% methanol); pH of extraction solution (pH5.5; natural pH of extract); extraction time (20/40/60 min); sample-solute ratio (40 mg–40 mL; 100 mg to 40 mL); dilution factor of extract prior to analysis (2.3; 25).
The precision of the complete method was reported as the RSD of 7 repetitive extractions of a representative bracken sample at 2 sample-solute ratios (40/100 mg to 40 mL), using 2 extract dilution factors (2.3/25). The ranges were chosen to represent expected sample analytical concentrations.
2.4. Analytical set-up and identification protocol, LC-ESI-MS
The PTA and PtB were made on an Agilent 1260 Infinity HPLC System in combination with an Agilent 6130 Single Quadrupole (LC-MS). PTA and PtB were separated at 1.0 mL min−1 using 53% MeOH and 47% 0.5 mM sodium acetate on an Agilent ZORBAX Eclipse Plus C18 column (100 × 4.6 mm, 3.5 μm; 35 °C; Opti-SOLV-TM 0.5 μm guard column; isocratic mode) with an injection volume at 10 μl. The LC-MS settings were set at (electrospray ionisation): 4,000V; Drying gas flow (N2): 3.0 L min−1 and 300 °C; Nebulizer pressure: 20PSI. Quantification took place at single-ion mode using: PTA [M+Na]+: 421.1 m/z; PtB [M+Na]+: 241.3 m/z and loganin [M+Na]+: 413.1 m/z. In addition, UV254nm and TIC-scan 100–500 m/z were obtained and monitored to obtain a qualitative insight into the general sample composition and chromatography. The identification of analytes in the samples was based on retention time and confirmed by spiking the sample with an analyte, which was then compared to MS spectra of pure samples (Figs. S1–2).
2.5. Supporting methods
pH was measured in plant extracts, soil suspensions and water using an ordinary pH combination electrode (section 2.8).
2.6. Case 1: Using fresh bracken crosiers for food - fresh or after blanching
Crosiers (46 unfolded young fronds; 15–40 cm; P. aquilinum) were harvested in May 2017 in Ravnsholt Forest (North of Copenhagen, Denmark). The identity of the ferns has previously been confirmed as Common Bracken in the mature state according to Frederiksen et al. (2012) and GBIF.org (2021). The fronds were kept cool (by a cooling box) and transported to the laboratory to be processed by blanching within 5 h (short time blanching or steaming are used worldwide with the crosiers). In addition, the Japanese method of adding wood ash/baking powder to the process water to increase alkalinity and its effect was also explored. The fronds were cut into approximately 5 cm long pieces and thoroughly mixed before randomly split into 5 sub-batches (each of approximately 50 g). Two batches were kept in reserve while the remaining material was processed as follows: 1) Reference; 2) Blanched for 2 min in approximately 0.5 L boiling 0.005 M CaCl2; 3) Blanched for 2 min by boiling 0.005 M CaCl2 plus commercial baking powder. The blanched material was cooled to room temperature immediately after cooking by soaking in cold tap water. pH was measured twice in all batches. PTA and PtB were extracted using a modified method by Cáceres-Peña et al. (2013): Approximately 20 g fresh crosier was extracted with 100 mL 90 °C deionised water for 5 min in a kitchen blender, following centrifugation of 3 times 10 mL aliquots for 10 min at 9000 rpm and PTA/PtB measured in the supernatant (triplicate; final validated method). The samples were prepared within 15 min of cooking and kept at −18 °C until analysis.
2.7. Case 2: Preparing traditional Chinese medicinal products using fresh bracken
In March 2016, a sampling trip to the Shatin District of Hong Kong was conducted to collect crosiers and young unfurling ferns in nature. The identity of the specimens was confirmed to be Common Bracken (Frederiksen et al., 2012). The ferns were airdried for five days immediately after collection at approximately 25 °C in a well-ventilated room, mimicking a traditional way of drying ferns in private homes. The dried ferns were brought back to the University College Copenhagen and processed, from which the content of PTA/PtB was determined in triplicate using the final validated method. Samples were kept at −18 °C until analysis.
2.8. Case 3: Collecting wild bracken in North America
Eight sites used for collecting crosiers for consumption were located and bracken identified by staff from Great Lakes Indian Fish & Wildlife Commission (USA). Each site was divided into 1–3 homogeneous subsites (A-C) based on sun exposure (sunny; partial shade; full shade) and the soil texture, which were estimated based on field determination (Soil Survey Staff, 2014). A total of 7–10 young, unfolding crosiers were collected from each subsite (May 2017), cut into 2–5 cm long pieces and dried for 24–32 h at 35–38 °C in a vegetable drier (9-tray Excalibur Food Dehydrator; Sacramento, California). The samples were subsequently ground in a Magic Bullet grinder (NutriBullet, LLC; USA). In addition, soil samples were collected from each subsite and dried at air temperature before pH was measured in a 1:2.5 w/v 0.01 M CaCl2-suspension. The dried ferns were shipped to the University College Copenhagen, and the content of PTA/PtB was determined using the final method. Samples were kept at −18 °C until analysis. The pH of the soil samples was determined by Great Lakes Indian Fish & Wildlife Commission (USA; single determination; Soil Survey Staff (2014)).
2.9. Case 4: Exposure to PTA from commercial food products and European natural remedies obtained from online shops
A list of Asian supermarkets and grocery shops in the Copenhagen region of Denmark, in addition to the major cities of Denmark, was outlined (autumn 2015/spring 2016), all of whom were visited or contacted by phone or email to inquire about bracken-based food products. From here, only noodles made of bracken rhizomes were obtained as it was the only product available. A variety of bracken-based food products were obtained from shops in the USA, Japan and China through international online shops (dried crosiers, rhizome flour and warabi sprinkle; 10 products in total). The products were produced in either Korea by Sam Heung Trade, Doo Me San, Assi and Choripdong, in Japan by Ohsawa, Itoku Food or Uji Maccha or in China from ZishuThe content of PTA/PtB in the products was determined using the final method in triplicate. Samples were kept at −18 °C until analysis.
Internet searches were used to identity European natural remedies containing bracken, and two providers were identified: WALA Heilmittel GmBH (Germany) and Remedia Homeopathy (Austria). From both providers, a variety of homoeopathic solutes and pills (globules) were obtained using Remedia Homeopathy's online shop (www.remedia-homeopathy.com) and the web-pharmacy, Apo-Rot (www.apo-rot.dk), and a total of 13 different products were purchased. As low concentrations of PTA and PtB were expected, solutes were analysed in triplicate according to the protocol using minimal dilution, and maximum sample injections were completed for qualitative screening (one sample out of each set of triplicates). Globules were dissolved in deionised water according to the protocol and analysed in triplicate according. Samples were kept at −18 °C until analysis.
2.10. In silico estimates and statistical analysis
The octanol-water partition coefficient, water solubility and bioaccumulation estimate were estimated from US Environmental Protection Agency's EPISuite™ (EPA, 2018) and retrieved using ChemSpider (Royal Society of Chemistry (RSC, 2018)). Linear regression and descriptive statistics were performed in Microsoft® Excel® for Office 365 MSO (incl. data analysis package), while one-way analysis of variance and Tukey post hoc test were performed in Pisces Conservation Ltd® QED vs. 1.5.5.503.
A PTA-transformation ratio was calculated for all products to indicate how pre-processing and cooking affected the PTA-related toxicity:
| (1) |
The concentration of PTA was divided by two, as this is the approximate ratio of the molar weight between PTA and PtB. A PTAproc value close to 1.0 indicated the limited transformation of PTA into PtB (change of molar balance) without taking intermediates or other transformation products into account. The value must be interpreted along with the processing effect regarding the total content of PTA/PtB, as complete destruction of the compounds may take place. It must be noticed that PtB may be present in unprocessed samples as well.
3. Results
3.1. Validation of analytical method incl. sample extraction protocol
The performances of the developed analytical methods are presented in Tables 2 and S1. The full chromatographic resolution was obtained using a 53% MeOH and 47% 0.5 mM sodium acetate eluent at 1 mL min−1, from which no interfering compounds were observed for any of the analytes in the chromatograms (Figs. S1–2). The performance of the external standard method for quantification of PTA and PtB was found to be equivalent to the internal standard method using loganine (LOG) as an internal standard.
PTA and PtB were linear with up to 1500 and 1250 μg L−1 respectively regarding the external and internal standard. Moreover, LOG proved linear in the range test (up to 200 μg L−1) with linearity (r) ranging from 0.9991 to 0.9997. The calibration range used for quantification of PTA and PtB was set to 4.4–88.0 μg L−1, which resulted in a LOD for PTA of 0.6, while LOD for PtB was 0.7–1.0, depending on the calibration method (Table 2). In general, external calibration proved more stable over the days of analysis, demonstrating the stability of the applied LC-MS system. The analytical precision of PTA was placed between 0.7 and 3.8%, while the analytical precision of PtB was between 1.3 and 5.0%, with the best performance in the upper range of the calibration range (Table S1). Similar precision was obtained for PTA and PtB in a bracken extract, while the analytical precision of LOG was 3.0% regarding the concentration used for the internal standards (100 μg L−1; Table S1). The instrumental performance was considered satisfactory and in range with other LC-MS or LC-MS/MS-based analytical methods used for quantifying PTA and PtB in environmental matrices (e.g., Clausson-Kaas et al., 2016). No matrix effects were observed in the sample matrices of ampoules, globuli, rhizome flour or bracken frond. The accuracy of PTA was satisfactory, with a complete analytical recovery percentage from 100.6 ± 1.9 to 101.5 ± 3.4% (Table S2).
Table 2.
Performance of the analytical methods - calibration curves, LODa and LOQb. The retention times were (representative values; minutes; ±standard deviation): Loganine, LOG) 1.347 ± 0.003; Ptaquiloside, PTA) 2.692 ± 0.007 and Pterosin B, PtB) 8.203 ± 0.004. The associated asymmetry factors (10% peak height; ±standard deviation) were: LOG) 0.74 ± 0.01; PTA) 0.79 ± 0.02 and PtB) 0.84 ± 0.02.
| Analyte | Linear range |
Calibration curve |
Linearity |
Calibration range |
LOD |
LOQ |
|---|---|---|---|---|---|---|
| (μg L−1) | (r)c | (μg L−1) | (μg L−1) | (μg L−1) | ||
| External standard methods: | ||||||
| Ptaquiloside | 0-1500 | 646.29x-213.07 | 0.9995 | 4.4–88.0 | 0.6 | 1.9 |
| Pterosin B | 0-1250 | 788.43x+228.24 | 0.9997 | 4.4–88.0 | 0.7 | 2.4 |
| Loganine | 0–200d | 916.16x+3187.6 | 0.9995 | 10.0–200.0 | ND | ND |
| Internal standard method (cLOGe100 μg L−1): | ||||||
| Ptaquiloside | 0-1500 | 0.0074x+0.0005 | 0.9991 | 4.4–88.0 | 0.6 | 2.0 |
| Pterosin B | 0-1250 | 0.0096x+0.0001 | 0.9997 | 4.4–88.0 | 1.0 | 3.2 |
Limit of detection.
Limit of quantification.
Correlation coefficient.
Only tested in relevant concentration range when used as an internal standard.
Loganine.
The extraction of PTA and PtB was optimised and validated with respect to extraction solution composition, pH and extraction time (Tables S3–7), and the maximum yield of PTA and PtB was achieved after 40 min with no apparent effect using a buffer of pH5.5 in aqueous extractions. A test using a buffered methanolic extraction solvent (the eluent: 53% MeOH, 47% 0.5 mM sodium acetate) revealed a negative effect on the PTA recovery compared to ordinary aqueous extraction. In conclusion, optimal PTA and PtB recovery is obtained using aqueous extraction for 40 min. This procedure was used for testing the sample-solvent ratio and the effect of the dilution factor on the yield (Tables S6–7). The results demonstrate that the dilution factor is important, possibly due to matrix interference in the MS detector, as approximately 25–30% lower concentration of PTA and PtB was measured using a dilution factor of 2.3 compared to 25. In contrast, no significant effects were found going from 40 mg to 40 mL to 100 mg to 40 mL.
The final extraction protocol was as follows: 40–100 mg sample was extracted using 40 mL deionised water for 40 min in a centrifuge tube, and the extraction was diluted 25 times resulting in a final composition of the analytical solution of 100 μg L−1 LOG, 0.1 M ammonium acetate and 48% MeOH. The samples were prepared and filtered in Whatman Mini-UniPrep PTFE 0.2 μm filter vials and kept at −20 °C until analysis. The LOD of the entire protocol was 0.024 μg g−1 PTA and 0.028 μg g−1 PtB. The accuracy is approximately 100%, while the precision of the complete extraction method including the instrumental analysis was 7.8–12.7% for PTA and 6.8–9.5% for PtB (depending on the sample-solvent ratio; Tables S2, S6-7).
3.2. Case 1: Using fresh bracken crosiers for food - fresh or after blanching
The fresh, unprocessed crosiers which were used as a control had a content of 6300 ± 520 μg g−1 PTA (Table 3). There were significant effects of the treatments on the content of PTA and PtB (p < 0.05 for both compounds). During simple processing (blanching for 2min), the PTA content was affected by the pH, which at pH 6.5 showed an approximately 17% reduction in PTA and an approximately 65% reduction at pH 7.9. Both treatments displayed a transformation ratio (PTAtrans) of 0.92, compared to 0.98 in the control. Hence, blanching increases the transformation of PTA to PtB and, at the same time, reduces the total content of PTA. However, the increase of PtB does not count for the decrease in PTA on a molar basis, indicating the formation of other metabolites or reaction products, like ptaquilosin, DNA-adducts or chloro-pterosin not quantified in the project.
Table 3.
Content of ptaquiloside and pterosin B in fresh and blanched fronds from Denmark (±standard deviation).
| Treatment | pH of water | Ptaquiloside (μg g−1)b | Pterosin B (μg g−1)b | PTAtrans |
|---|---|---|---|---|
| Control (not processed) | NDa | 6292 ± 521A | 68±4D | 0.98 |
| Blanched (2 min) | 6.50 ± 0.08 | 5261 ± 132B | 249±8E | 0.92 |
| Blanched (2 min) incl. baking powder |
7.91 ± 0.03 |
2196 ± 67C |
79±3D |
0.93 |
| ANOVA P-valuec | – | 9.4E-6 | 4.7E-8 | – |
A to D Mean contents with different letters are statistically different (Tukey post hoc test of each compound, α = 0.05).
Not determined (no blanching of control sample).
As pr. dry weight (95% water content).
One-way Analysis of Variance (significant effect of treatment, P-value < 0.05).
3.3. Case 2: Preparing traditional Chinese medicinal products using fresh bracken
Whole crosiers and pinnae were processed as traditional medicine after harvested in the wild, with a range of PTA from 294 to 638 μg g−1 (Table 4). The mature pinnae displayed the lowest amount of PTA, while crosiers and unfurling had approximately the same amount. No PtB was found in the mature pinnae in contrast to crosiers and unfurling pinnae, which showed rather high contents of PtB (177–775 μg g−1). Significant differences in the content of PTA and PtB was observed for the different growthstages (p < 0.05 for both compounds). The transformation ratio was lowest in the crozier and reached unity in the mature pinnae. The result is somewhat surprising as older plant parts are believed to have higher contents of pterosins, although this could also be a result of the processing. The crosiers and the unfurling pinnae had higher water content compared to the mature pinnae, which indicate that the older plants die more quickly and have lower enzymatic activity than the young plant, resulting in no formation of PtB. Assuming all PTA in the fresh crozier was present in the dried material as PTA or PtB, the original content ranged between 280 and 2030 μg g−1.
Table 4.
Content of ptaquiloside and pterosin B in bracken fern from Hong Kong prepared as traditional Chinese medicine (air-dried; 5 days; approximately 25 °C; ± standard deviation).
| Sample type | Ptaquiloside (μg g−1)a | Pterosin B (μg g−1)a | PTAtrans |
|---|---|---|---|
| Croziers | 638 ± 90A | 775 ± 158C | 0.29 |
| Unfurling pinnae | 736 ± 57A | 312 ± 19D | 0.55 |
| Mature pinnae |
294 ± 12B |
BD |
1.00 |
| ANOVA P-valueb | 7.7E-6 | 1.2E-4 | – |
A to D Mean contents with different letters are statistically different (Tukey post hoc test of each compound, α = 0.05).
As pr. dry weight.#Below Limit of Detection.
One-way Analysis of Variance (significant effect of treatment, P-value < 0.05).
3.4. Case 3: Collecting wild bracken in North America
Twenty bracken stands were sampled from eight different locations used for harvesting crosiers in spring (Table 5). The crosiers are usually prepared fresh in the region, but for practical reasons, the PTA and PtB were measured in dried material. The content of PTA ranged from 0 to 273 μg g−1 while PtB ranged from 37 to 449 μg g−1, and the transformation ratio varied 0.06 to 0.48 despite using strict protocols in the laboratory, and it is, therefore, possible that a portion of PtB was produced before the harvest. The results demonstrate that crosiers may include substantial amounts of PTA, though considering that the drying process transforms PTA into PtB, the content of PTA in crosiers might be even larger. Interestingly, a high number of sites did not have a detectable content of PTA nor PtB, and they can, therefore, be used to harvest “safe” bracken, provided no other toxins of the illudane-type are present in the ferns.
Table 5.
Content of ptaquiloside and pterosin B in bracken crosiers from USA (food dehydrator; 24–32 hrs; 35–38 °C; ± standard deviation). The locations are used to collect crosiers in spring (to be used in traditional food).
| Location | Ptaquiloside (μg g−1)a | Pterosin B (μg g−1)a | PTAtrans | pH | Light exposure | Soil typec | |
|---|---|---|---|---|---|---|---|
| Site 1: | |||||||
| 01A | 128 ± 30 | 262 ± 12 | 0.20 | 6.2 | Sunny | Loam | |
| 01B | 218 ± 35 | 449 ± 1 | 0.20 | 5.3 | Partial-Shade | Clay | |
| 01C | 273 ± 72 | 405 ± 88 | 0.25 | 5.2 | Full Shade | Not recorded | |
| Site 2: | |||||||
| 02A | 24 ± 3 | 49 ± 1 | 0.20 | 6.0 | Partial-Shade | Sand/Loam/Clay | |
| 02B | 67 ± 2 | 57 ± 12 | 0.37 | 5.9 | Full Shade | Loam/Sand | |
| 02C | 43 ± 6 | 42 ± 7 | 0.34 | 6.1 | Sunny | Loam/Sand | |
| Site 3: | |||||||
| 03A | BDb | BD | – | 4.4 | Sunny | Sand/Clay | |
| 03B | BD | BD | – | 4.2 | Partial-Shade | Sand/Clay | |
| Site 4: | |||||||
| 04A | BD | BD | – | 4.0 | Full Shade | Sand/Clay | |
| Site 20: | |||||||
| 20A | BD | BD | – | 4.5 | Partial-Shade | Silt/Loam | |
| 20B | BD | BD | – | 4.5 | Sunny | Silt/Loam | |
| 20C | BD | BD | – | 4.6 | Full Shade | Silt/Loam | |
| Site 21: | |||||||
| 21A | 35 ± 2 | 276 ± 37 | 0.06 | 3.9 | Full Shade | Silt/Loam | |
| 21B | 25 ± 0 | 161 ± 20 | 0.07 | 4.3 | Sunny | Silt/Loam | |
| 21C | 71 ± 0 | 241 ± 10 | 0.13 | 4.2 | Partial-Shade | Silt/Loam | |
| Site 40: | |||||||
| 40A | 75 ± 0 | 62 ± 0 | 0.38 | 4.8 | Partial-Shade | Sand | |
| 40B | 67 ± 0 | 37 ± 0 | 0.48 | 4.9 | Full Shade | Sand | |
| 40C | BD | BD | – | 4.7 | Sunny | Sand | |
| Site 41: | |||||||
| 41A | BD | BD | – | 5.2 | Full Shade | Loam | |
| 41B | BD | BD | – | 4.9 | Partial-Shade | Sand | |
The content of PTA/PtB in crosiers with PTA/PtB was not strongly correlated with environmental co-factors such as soil pH, light exposure nor soil type. However, the average pH of stands with PTA/PtB was 5.2 ± 0.5, while the locations with no PTA/PtB had a soil pH of 4.6 ± 0.3, indicating that soil pH may influence the presence of toxins in the crosiers. Looking at the normalised PTA equivalents for the positive sites, a statistically significant difference is found between the sunny and the fully shaded sites, with the partially shaded sites in-between indicating lower contents of PTA in crosiers harvested from sunny locations compared to crosiers emerging in the shade of trees and bushes (Table S8).
3.5. Case 4: Exposure to PTA from commercial food products and European natural remedies obtained from online shops
In total, 22 commercial food products and natural remedies were tested for PTA and PtB (Table 6), though only dried bracken crosiers from Korea were found to contain the compounds. The reminding part of the products (homoeopathic solutes, pills, rhizome flour or sprinkle) contained no PTA nor PtB above the Limit of Detection and can be considered safe in relation to PTA toxicity.
Table 6.
Content of ptaquiloside and pterosin B in commercial bracken food products and natural remedies obtained from online shops (±standard deviation).
| Product | Ptaquiloside (μg g−1)a | Pterosin B (μg g−1)a | PTAtrans |
|---|---|---|---|
| Homeopathic solutes: | |||
| WALA. SALIX RHUS COMP. Ampoules N1. Germany. | BDb | BD | – |
| WALA. AQUILINUM COMP. Ampoules N1. Germany. | BD | BD | – |
| REMEDIA HOMEOPATHY. Pteridium aquilinum Dilution C4. Austria. | BD | BD | – |
| REMEDIA HOMEOPATHY. Pteridium aquilinum Dilution C200. Austria. | BD | BD | – |
| REMEDIA HOMEOPATHY. Pteridium aquilinum Dilution LM2. Austria. | BD | BD | – |
| REMEDIA HOMEOPATHY. Pteridium aquilinum Dilution LM6. Austria. | BD | BD | – |
| Homeopathic pills: | |||
| WALA. SALIX RHUS COMP. Globuli relati N1. Germany. | BD | BD | – |
| WALA. AQUILINUM COMP. Globuli relati N1. Germany. | BD | BD | – |
| WALA. CONCHAE COMP. Globuli relati N1. Germany. | BD | BD | – |
| REMEDIA HOMEOPATHY. Pteridium aquilinum Globuli C4. Austria. | BD | BD | – |
| REMEDIA HOMEOPATHY. Pteridium aquilinum Globuli 50 MK. Austria. | BD | BD | – |
| REMEDIA HOMEOPATHY. Pteridium aquilinum Globuli LM2. Austria. | BD | BD | – |
| REMEDIA HOMEOPATHY. Pteridium aquilinum Globuli LM6. Austria. | BD | BD | – |
| Dried crosiers: | |||
| Sam Heung Trade. Dried fern brake. Korea. | 175 ± 16 | 1653 ± 184 | 0.05 |
| Doo Me San. Dried bracken. Korea. | 666 ± 33 | 1417 ± 125 | 0.19 |
| Assi dried fern bracken (pink package). Korea. | 74 ± 16 | 830 ± 217 | 0.04 |
| Assi dried fern bracken (green package). Korea. | 47 ± 3 | 473 ± 44 | 0.04 |
| Choripdong. Dried bracken. Fougére seche. Gold. Korea. | 44 ± 3 | 553 ± 47 | 0.04 |
| Rhizome flour and noodles: | |||
| Ohsawa Rhizome flour. Japan. | BD | BD | – |
| Itoku Food Warabi Mochi Kit (flour). Japan. | BD | BD | – |
| Zishu Zhuanggyuan noodles. China. | BD | BD | – |
| Warabi mochi sprinkle: | |||
| Itoku Food. Warabi Mochi Kit (powder). Japan. | BD | BD | – |
| Uji Maccha. Warabi Mochi (powder). Japan. | BD | BD | – |
As pr. dry weight.
Below Limit of Detection.
The content of PTA in the dried crosiers ranged from 44 to 666 μg g−1 with a low ptaquiloside transformation ratio (0.04–0.19), indicating the general transformation of PTA into PtB. The content of PtB was found to be in the range 473 to 1653 μg g−1. Assuming all PTA present in the crosiers were either present as PTA or transformed into PtB, the PTA content in fresh Korean crosiers ranged from approximately 900 to 3200 μg g−1.
4. Discussion
Humans and bracken ferns have co-existed for millennia, and bracken has played a prominent part in human culture in many parts of the world. A brief contemporary review of bracken ethnobotany in Europe, Asia and North America can be found in Supplementary Information (Section 3). Historical founds date the use of bracken back centuries ago, in which bracken has been found in the stomach of a 5300-year-old glacier mummy, Ötzi (Maixner et al., 2018). In Europe, the fern rhizomes have mainly been used as a food supplement during war and famine, in which flour was made from the rhizomes, while fronds have been used as bedding, in religious rituals, as bleach, thatch and a source of potash. Crosiers, however, do not seem to have been used for any purpose at all (Brøndegaard, 1978; Kristanc and Kreft, 2016; Rymer, 1976). Brackens is continually considered an ingredient in traditional medicine and the local diet for people in some parts of the world, particularly in Asia, from where numerous industrial products can be obtained (Table 5 and Supplementary Information Section 3). Bracken is, therefore, grown industrially all over the world to satisfy market needs in countries like China, Japan and Korea (Liu et al., 2012). This paper is concerned with the ingestion of bracken in North America, as well as China and Europe (traditional medicine and natural remedies), and particularly with PTA in bracken food, which only a few studies have addressed. An old study of PTA in commercial bracken products from Asia did not reveal PTA, however, the Limit of Detection (LOD) was quite high (100 μg g−1 fresh weight; approximately 1–2000 μg g−1 dry weight; Saito et al., 1989). The market for bracken and bracken products is wide in demand, and several products are easily found in Asian, European and American online shops like Amazon. In Denmark, all bracken-based food products and natural remedies are banned by regulation, which explains why there are only a few online shops that sell bracken based in Denmark. Importing the commodities from Asia and Europe was an easy feat, while products from Amazon based in the USA could not be directly shipped and were, therefore, obtained via colleagues in the USA. Internet searches revealed a high number of hits on bracken and bracken recipes, including a high number of videos on how to collect bracken in the wild and prepare different dishes (Supplementary Information Section 4). These findings indicate that bracken is collected in nature, that bracken can be obtained from industrial food suppliers and that there is a keen interest in the product mirrored in the number of recipes and videos.
The interest in bracken may cause human health concerns, as bracken is known to contain natural genotoxic illudane glycosides (O'Connor et al., 2019). Traditional treatments with bracken in many parts of the world typically include soaking and boiling/steeping, sometimes using alkaline water. This study demonstrates that fresh bracken collected in the wild and prepared as a traditional vegetable dish following Asian guides by increasing pH of boing water has a very high content of PTA compared to the fresh plant material. These findings support other studies concerned with illudane glycoside extraction and the effect of pH (Ayala-Luis et al., 2006; Cáceres-Peña et al., 2013). Hot water extraction is one of the best extraction methods for PTA, preserving the toxin for analytical methods. The alkaline hot-water treatment does, however, lack molar balance in the conversion from PTA to PtB, as a large part of the PTA cannot be accounted for in the mass balance. Hence, this fraction must have reacted into something else within the plant or in the soaking/boiling water. That could be direct reactions with other compounds or due to the formation of still genotoxic reaction intermediates as ptaquilosin. This study only addressed the effect of cooking regarding fresh crosiers, and it, therefore, warrants further studies on the effects of cooking procedures (e.g. soaking and boiling) concerning the carcinogenicity of bracken-based foods, including commercial bracken products as well as freshly harvested crosiers.
The results of this study showed high contents of PTA in several comestible products and found that all products based on dried fern fronds, including crosiers, contain PTA as starting point. These findings support results from other studies concerned with bracken, where drying is used as a preparation method for sampling (e.g., Kisielius et al., 2020; Rasmussen et al., 2015). Industrial drying processes seem to lower the content of PTA as indicated in the transformation rates found in commercial products, as well as in home-dried crosiers using kitchen-scale driers (Table 4, Table 5). Hence, drying seems to be an effective mean for lowering PTA, provided no equally carcinogenic compounds are formed during the process.
Several products, mainly those based on rhizomes, were found to contain no PTA. The content may vary depending on the season, and high levels have only been observed in autumn. Rhizomes and rhizome-based products could be considered safe concerning PTA toxicity and when prepared from rhizomes collected in winter, which seems to be the practice in China. This also applies to European natural remedies, which was not surprising, as these were all homoeopathic products (Table 5).
5. Conclusion
In this study, an LC-ESI-MS dilute-and-shoot method was validated regarding quantification of PTA and PtB in food products, traditional medicine and natural remedies, along with a novel protocol for extracting PTA and PtB. The analytical method proved as successful as other LC-MS/MS methods in relation to PTA/PtB quantification, and the combined method was used to explore human exposure to bracken toxins in four cases:
Case 1
- Using fresh bracken crosiers for food - fresh or after blanching: Fresh bracken crosiers displayed very high contents of PTA. Blanching reduced the PTA significantly, and the effect was enhanced when adding baking powder to increase the pH. The formation of carcinogenic degradation products may, however, take place.
Case 2
- Preparing traditional Chinese medicinal products using fresh bracken: PTA was found in the dried materials and could cause danger to the consumers. The preparations had PTA in range with brackens collected from other parts of the world. This is the first report on PTA in the Chinese bracken.
Case 3
- Collecting wild bracken in North America: PTA was present in many specimens, thereby posing a danger for people collecting bracken in the investigated region. However, some bracken did not contain PTA indicating a possible option for growing PTA-free safe bracken, provided other illudane glycosides are not present. This is the first extensive report on PTA in the North American bracken.
Case 4
- Exposure to PTA from commercial food products and European natural remedies obtained from online shops: PTA was found only in dried crosiers from Korea, which content was in range with similar findings of PTA in wild bracken from Asia and other parts of the world. All other products, including European natural remedies, were safe from a PTA point of view as they contained no PTA.
In conclusion, PTA can be found in a variety of food products, however, the content varies and depends on the origin, as well as the processing. From a food-safety point of view, collecting wild bracken and consuming dried bracken crosiers may cause health issues due to the high content of PTA in the materials. It must be emphasised that processing (drying and blanching) does not remove all PTA, but it can be an effective tool to lower the content of PTA.
CRediT authorship contribution statement
Lars Holm Rasmussen: Conceptualization, Project administration, Funding acquisition, Methodology, Investigation, Formal analysis, Data curation, Writing – original draft, Writing – review & editing, Visualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The fieldwork in China was covered by travel grants from The Study Fund of the Danish Association of Foresters (DK: Forstkandidatforeningens Rejsefond) and The Danish Agricultural PhD Unions Study Fund (DK: Danmarks Jordbrugsvidenskabelige Ph.D.-forenings Studiefond). The remaining part of the study was financed by University College Copenhagen with Danish state funding. The author wishes to thank Professor Hans Christian Bruun Hansen (the University of Copenhagen; Denmark) for providing the PTA that made this study possible, Associate Professor Jennifer Wan for her support during the field studies in Hong Kong (the University of Hong Kong; Hong Kong Special Administrative Region of the People's Republic of China), PhD student Vaidotas Kisielius and Laboratory technician Jimena Bertorello Martinez for their excellent laboratory work, and Dieticians Owen Maroney and LaTisha Coffin from the Great Lakes Indian Fish & Wildlife Commission for their effort in sampling crosiers in the USA. The author also wishes to thank Jane Wu for obtaining samples in the USA, Liza Rylander Krintel from University College Copenhagen for proofreading and correcting the grammar, and my colleagues Janni Sandersen and Dan Nybro Lindqvist for their collaboration on LC-MS methods development. A special thank you to my wife Karina Rasmussen: Without your long-lasting support, this study would not have been possible.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2021.08.004.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Alonso-Amelot M.E., Castillo U.F., de Jongh F. Passage of the bracken-fern carcinogen ptaquiloside into bovine milk. Lait. 1993;73:332. [Google Scholar]
- Aranha PCdR., Rasmussen L.H., Wolf-Jäckel G.A., Jensen H.M.E., Hansen H.C.B., Friis C. Fate of ptaquiloside - a bracken fern toxin - in cattle. PloS One. 2019;14(6) doi: 10.1371/journal.pone.0218628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala-Luis K.B., Hansen P.B., Rasmussen L.H., Hansen H.C.B. Kinetics of ptaquiloside hydrolysis in aqueous solution. Environ. Toxicol. Chem. 2006;27:252–259. doi: 10.1897/05-695r.1. [DOI] [PubMed] [Google Scholar]
- Brøndegaard V.J. Rosenkilde og Bagger; Copenhagen: 1978. Folk Og Flora. 1. Dansk Etnobotanik (In English: People and Flora. 1. Danish Ethnobotany) Denmark. [Google Scholar]
- Cáceres-Peña Y.C., Naya M., Calcagno-Pissarelli M.P., Alonso-Amelot M.E. Influence of bracken fern (Pteridium caudatum L. Maxon) pre-treatment on extraction yield of illudane glycosides and pterosins. Phytochem. Anal. 2013;24:290–295. doi: 10.1002/pca.2409. [DOI] [PubMed] [Google Scholar]
- Clausson-Kaas F., Hansen H.C.B., Strobel B.W. UPLC-MS/MS determination of ptaquiloside and pterosin B in preserved natural water. Anal. Bioanal. Chem. 2016;408:7981–7990. doi: 10.1007/s00216-016-9895-z. [DOI] [PubMed] [Google Scholar]
- EPA . United States Environmental Protection Agency; 2018. Predictive Models and Tools for Assessing Chemicals under the Toxic Substances Control Act (TSCA)https://www.epa.gov/tsca-screening-tools (access date: 25JAN2018) [Google Scholar]
- Fletcher M.T., Brock I.J., Reichmann K.G., McKenzie R.A., Blaney B.J. Norsesquiterpene glycosides in bracken ferns (Pteridium esculentum and Pteridium aquilinum subsp. wightianum) from Eastern Australia: reassessed poisoning risk to animals. J. Agric. Food Chem. 2011;59:5133–5138. doi: 10.1021/jf104267c. [DOI] [PubMed] [Google Scholar]
- Frederiksen S., Rasmussen F.N., Seberg O. Gyldendal. Copenhagen; Denmark: 2012. Dansk flora. (In English: Danish flora) [Google Scholar]
- GBIF.org. GBIF home page. 2021. https://www.gbif.org (access date: 25FEB2021)
- GLIFWC . Commission Press; Odanah, Wisconsin, USA: 2014. Mino Wiisinidaa! Let's Eat Good! Great Lakes Indian Fish and Wildlife. [Google Scholar]
- Gry J., Hallas-Møller T., Pedersen E., Pilegaard K., Strube M. Fødevarestyrelsen; Denmark: 1998. Drogelisten (In English: Drug list) [Google Scholar]
- ICH . The International Council for Harmonisation; 2005. ICH Harmonized Tripartite Guideline Validation of Analytical Procedures: Text and Methodology Q2 (R1)https://www.ich.org/ (access date: 04MAY2021) [Google Scholar]
- Kisielius V., Lindqvist D.N., Thygesen M.B., Rodamer M., Hansen H.C.B., Rasmussen L.H. Fast LC-MS quantification of ptesculentoside, caudatoside, ptaquiloside and corresponding pterosins in bracken ferns. J. Chromatogr. B. 2020;1138:121966. doi: 10.1016/j.jchromb.2019.121966. [DOI] [PubMed] [Google Scholar]
- Kristanc L., Kreft S. European medicinal and edible plants associated with subacute and chronic toxicity part I: plants with carcinogenic, teratogenic and endocrine-disrupting effects. Food Chem. Toxicol. 2016;92:150–164. doi: 10.1016/j.fct.2016.04.007. [DOI] [PubMed] [Google Scholar]
- Liu Y., Wujisguleng W., Long C. Food uses of ferns in China: a review. Acta Soc. Bot. Pol. 2012;81(4):263–270. doi: 10.5586/asbp.2012.046. [DOI] [Google Scholar]
- Maixner F., Turaev D., Cazenave-Gassiot A., Janko M., Krause-Kyora B., Hoopmann M.R., Kusebauch U., Sartain M., Guerriero G., O'Sullivan N., Teasdale M., Cipollini G., Paladin A., Mattiangeli V., Samadelli M., Tecchiati U., Putzer A., Palazoglu M., Meissen J., Lösch S., Rausch P., Baines J.F., Kim B.J., An H.J., Gostner P., Egarter-Vigl E., Malfertheiner P., Keller A., Stark R.W., Wenk M., Bishop D., Bradley D.G., Fiehn O., Engstrand L., Moritz R.L., Doble P., Franke A., Nebel A., Oeggl K., Rattei T., Grimm R., Zink A. The iceman's last meal consisted of fat, wild meat, and cereals. Curr. Biol. 2018;28:2348–2355. doi: 10.1016/j.cub.2018.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlière C.A., Wathern P., Castro M.C.F.M., O'Connor P., Galvao M.A. Bracken fern (Pteridium aquilinum) ingestion and oesophageal and stomach cancer. IARC (Int. Agency Res. Cancer) Sci. Publ. 2002;156:379–380. [PubMed] [Google Scholar]
- Marrs R.H., Watt A.S. Biological flora of the British isles: Pteridium aquilinum (L.) Kuhn. J. Ecol. 2006;94:1272–1321. [Google Scholar]
- O'Connor P.J., Alonso-Amelot M.E., Roberts S.A., Povey A.C. The role of bracken fern illudanes in bracken fern-induced toxicities. Mutat. Res. Rev. Mutat. Res. 2019;782:108276. doi: 10.1016/j.mrrev.2019.05.001. [DOI] [PubMed] [Google Scholar]
- O'Driscoll C., Ramwell C., Harhen B., Morrison L., Clauson-Kaas F., Hansen H.C.B., Campbell G., Sheahan J., Misstear B., Xiao L. Ptaquiloside in Irish bracken ferns and receiving waters, with implications for land managers. Molecules. 2016;21:543. doi: 10.3390/molecules21050543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai S.K., Sharma R., Kumari A., Rasmussen L.H., Patil R.D., Bhar R. Survey of ferns and clinico-pathological studies on the field cases of Enzootic bovine haematuria in Himachal Pradesh, a north-western Himalayan state of India. Toxicon. 2017;138:31–36. doi: 10.1016/j.toxicon.2017.08.010. [DOI] [PubMed] [Google Scholar]
- Rasmussen L.H., Pedersen H.Æ. Screening for ptaquiloside in ferns: using herbarium specimens for qualitative mapping purposes. Phytochem. Anal. 2017;28:575–583. doi: 10.1002/pca.2707. [DOI] [PubMed] [Google Scholar]
- Rasmussen L.H., Lauren D.R., Smith B.L., Hansen H.C.B. Variation in the ptaquiloside content in bracken (Pteridium esculentum (Forst.f.) Cockayne) in New Zealand. N. Z. Vet. J. 2008;56:304–309. doi: 10.1080/00480169.2008.36851. [DOI] [PubMed] [Google Scholar]
- Rasmussen L.H., Schmidt B., Sheffield E. Ptaquiloside in bracken spores from britain. Chemosphere. 2013;90:2539–2541. doi: 10.1016/j.chemosphere.2012.10.092. [DOI] [PubMed] [Google Scholar]
- Rasmussen L.H., Donnelly E., Strobel B.W., Holm P.E., Hansen H.C.B. Land management of bracken needs to account for bracken carcinogens. A case study from Britain. J. Environ. Manag. 2015;151:258–266. doi: 10.1016/j.jenvman.2014.12.052. [DOI] [PubMed] [Google Scholar]
- RSC . Royal Society of Chemistry; 2018. ChemSpider - Search and Share Chemistry.http://www.chemspider.com (access data: 24JAN2018) [Google Scholar]
- Rymer L. The history and ethnobotany of bracken. Bot. J. Linn. Soc. 1976;73:151–176. [Google Scholar]
- Saito K., Nagao T., Matoba M., Koyama K., Natori S., Murakami T., Saiki Y. Chemical assay of ptaquiloside, the carcinogen of Pteridium aquilinum, and the distribution of related compounds in the Pteridaceae. Phytochemistry. 1989;28:1605–1611. [Google Scholar]
- Skrbic N., Pedersen A.-K., Christensen S.C.B., Hansen H.C.B., Rasmussen L.H. A novel method for determination of the natural toxin ptaquiloside in ground and drinking water. Water. 2020;12:2852. doi: 10.3390/w12102852. [DOI] [Google Scholar]
- Soil Survey Staff . twelfth ed. USDA-Natural Resources Conservation Service; Washington, DC: 2014. Keys to Soil Taxonomy. [Google Scholar]
- Virgilio A., Sinisi A., Russo V., Gerardo S., Santoro A., Galeone A., Taglialatela-Scafati O., Roperto F. Ptaquiloside, the major carcinogen of bracken fern, in the pooled raw milk of healthy sheep and goats: an underestimated, global concern of food safety. J. Agric. Food Chem. 2015;63:4886–4892. doi: 10.1021/acs.jafc.5b01937. [DOI] [PubMed] [Google Scholar]
- WHO/IARC International agency for research on cancer (IARC) - summaries & evaluations. Bracken fern (Pteridium aquilinum) and some of its constituents. 2021;1998 http://www.iarc.fr (access date: 25FEB2021) [Google Scholar]
- Yahara Y., Takemori H., Okada M., Kosai A., Yamashita A., Kobayashi T., Fujita K., Itoh Y., Nakamura M., Fuchino H., Kawahara N., Fukui N., Watanabe A., Kimura T., Tsumaki N. Pterosin B prevents chondrocyte hypertrophy and osteoarthritis in mice by inhibiting Sik3. Nat. Commun. 2016;7:10959. doi: 10.1038/ncomms10959. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.