Abstract
The study aimed to describe the epidemiological, virological and clinical features of sporadic HEV infection in eastern China. A total of 6112 patient sera were tested for anti-HEV IgG or anti-HEV IgM during one consecutive year (between August 2018 and July 2019). HEV RNA presence was evaluated by RT-PCR and HEV sequences were phylogenetically analyzed. Clinical features of confirmed HEV-infected patients were delineated. The sero-positivity rate of anti-HEV IgG maintained stable around 40%, while an obvious winter spike of anti-HEV IgM prevalence was observed. A total of 111 patients were confirmed of HEV viremia by molecular diagnosis. Subtype 4d was predominant. Phylogenetic analyses suggest that certain strains circulate across species and around the country. Subjects with confirmed current HEV infection had a high median age (58 years) and males were predominant (62.2%). Most patients presented with jaundice (75.7%) and anorexia (68.0%). Significantly elevated levels of liver enzymes and bilirubin were observed. Remarkably, the baseline bilirubin level was positively correlated with illness severity. Pre-existing HBV carriage may deteriorate illness. The clinical burden caused by locally acquired HEV infection is increasing. Surveillance should be enforced especially during the transition period from winter to spring. Patients with higher level of bilirubin at disease onset had slower recovery from HEV infection.
Keywords: Hepatitis E virus (HEV), Seroprevalence, Genotyping, Epidemiology, Surveillance
Introduction
Hepatitis E virus (HEV) infection is the major cause of acute hepatitis and was thought to be restricted in developing countries with poor sanitation (Khuroo 2011). In recent years, sporadic cases of hepatitis E have been reported in developed countries (Capai et al. 2018). It was initially attributed to travelling to endemic regions (Schwartz et al. 1999). Later, consumption of or close contact with HEV-contaminated meat (Yazaki et al. 2003) and blood transfusion (Khuroo et al. 2004) were found to cause HEV infection. As a zoonosis pathogen, HEV is hard to eliminate due to the existence of animal reservoir (Lanini et al. 2018).
Exposure to HEV can lead to subclinical infection, self-limiting hepatitis, and fulminant hepatitis in immunocompetent individuals. The prognosis of hepatitis E is generally good except in pregnant woman (Khuroo et al. 1981) and in patients with pre-existing chronic liver diseases (Hamid et al. 2002). HEV can establish chronic infection in immunosuppressed patients (Kamar et al. 2008; Dalton et al. 2009).
HEV is a positive-stranded RNA virus (Tam et al. 1991). It consists of a short 5′-untranslated region (UTR), three open reading frames (ORF1, ORF2 and ORF3) and a short 3′-UTR terminated by a poly (A) tail. ORF1, located at the 5′ end of the genome, encodes a large nonstructural protein with multiple functional domains (Kenney and Meng 2019). ORF2 is located at the 3′ end and encodes the capsid protein. ORF3 partially overlaps ORF2 and encodes an ion channel (Ding et al. 2017).
HEV has extensive genetic diversity. Genotype 1 and 2 can infect only human, while genotype 3 and 4 can infect both human and swine (Schlauder and Mushahwar 2001). Geographically, genotype 1 was isolated from Asia and Africa. Genotype 2 was found from a few regions, including Mexico, Nigeria, and Chad. Genotype 3 was identified worldwide. Genotype 4 was first identified in Asia (Wang et al. 1999) and emerging indigenous cases have been reported in Europe lately (Hakze-van der Honing et al. 2011; Tessé et al. 2012). The majority of endemic HEV infection in developing countries was caused by genotype 1 or 2, while isolated cases of infection in industrialized countries were usually caused by genotype 3 or 4 (Khuroo et al. 2016). Recently, it was reported that camelid and rat HEV could infect human, indicating the virus has the potential to jump over the species barrier (Lee et al. 2016; Sridhar et al. 2018; Andonov et al. 2019).
The information of actual incidence of HEV at a global level is missing, partly because it can be overlooked or misdiagnosed (Webb and Dalton 2019). Routine diagnosis of HEV infection is dependent on the detection of HEV-specific immunoglobulin (Ig) G and M. Anti-HEV IgG is considered the best marker of past infection, whereas positivity of anti-HEV IgM indicates current infection (Dawson et al. 1992). However, since anti-HEV IgM may persist for 6–9 months (Huang et al. 2010), judgement of acute infection solely based on serological and biochemical evidences may lead to misdiagnosis. Thus, a combination of serology and nucleic acid amplification techniques has been recommended for diagnosis of HEV infection (EASL 2018).
In China, HEV has been the most common cause of acute viral hepatitis (Zhang et al. 2019). Genotype 4 has overtaken genotype 1 to be the dominant genotype in China since 2000 (Aye et al. 1992; Wang et al. 1999; Liu et al. 2012). Although studies have documented the distribution of HEV genotypes in eastern China (Zhang et al. 2010; Dai et al. 2013), there is a paucity of data regarding the clinical profiles of human HEV infection, probably because the majority of infections are unrecognized or misdiagnosed.
The current study is dedicated to characterize the epidemiological and clinical phenotypes of HEV infection in eastern China. The prevalence of anti-HEV IgG and anti-HEV IgM was measured consecutively over one year, which depicts an overall demographic and temporal feature of HEV infection. Then, the genetic diversity of HEV strains circulating regionally was determined. Finally, the clinical features of laboratory-confirmed cases of HEV infection were described.
Materials and Methods
Study Design
A total of 6112 serum samples from subjects who underwent serological tests for anti-HEV antibodies between August 2018 and July 2019 were included in the study, including those presenting with symptoms of hepatitis and also those coming for disease screening purpose. Biological materials and clinical data were obtained only via standard viral diagnostics following a physician's order. Information regarding sample collection date, age, and gender was retrieved from hospital information system.
Subjects were defined as laboratory-confirmed HEV infection if positive HEV RNA was detected in serum. Medical records of confirmed HEV-infected patients, including age, gender, liver function test (LFT) results, other hepatitis viruses (hepatitis A, B and C viruses, or HAV, HBV and HCV) infection status, epidemiological risk factors, symptoms and pre-existing liver diseases were recorded.
Biochemical parameters including alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), total bilirubin (TBIL) were measured by a multichannel Auto Analyzer (Beckman, USA) at Ruijin Hospital, with normal range of 10–64 IU/L (ALT), 8–40 IU/L (AST), 38–126 IU/L (ALP), 7–64 IU/L (GGT) and 4.7–24 μmol/L (TBIL), respectively. The anti-HAV IgM, serum hepatitis B surface antigen (HBsAg) and anti-HCV antibodies were tested using the enzyme-linked immunosorbent assay (ELISA) from Abbott Laboratories (Chicago, IL).
HEV Serologic Assays
HEV antibodies were detected in 50 μL of serum samples using Chemiluminescence Microparticle Immuno Assay (CMIA) developed by Wantai BioPharm (Beijing, China). Patient serum was sent to the laboratory for routine testing on a daily basis. The assay uses a recombinant antigen corresponding to ORF2 of the HEV genome (Zhang et al. 2003). The results were expressed as cut-off index (COI). Positivity was defined as COI > 1.1, while negativity was defined as COI < 0.9, and COI between 0.9 and 1.1 was considered as gray zone result, or ( ±).
HEV RNA Detection
HEV RNA was detected with Promotor® HEV RNA detection kit (ACON, Hangzhou, China). Briefly, viral nucleic acids were extracted from 100 μL of serum and eluted with 50 μL of TE buffer. The reverse transcription polymerase chain reaction (RT-PCR) included 20 μL of elute product, 18 μL of RT-PCR mix, 1.4 μL of enzyme, 0.6 μL of primers and fluorescence probe mixture. RT-PCR condition included a RT step of 50 °C for 20 min and 70 °C for 15 min, followed by 45 cycles of pre-denaturation step at 94 °C for 15 s and extension at 60 °C for 30 s. The amplification was carried on a ViiA 7 Real-Time PCR System (Thermo Fisher, USA) according to the manufacturer’s instructions. The lower detection limit is 500 copies/mL.
HEV Genome Amplification and Sequencing
HEV genotyping was done on all PCR-positive samples. After nucleic acids extraction, cDNA was synthesized from 10 μL of purified RNA using PrimeScript RT Reagent Kit (Takara Bio, China) with random hexamers. The C-terminal region of HEV ORF2 was amplified in the 1st round with external forward primer HEV-LJF1 (5′-CCGACAGAATTGATTTCGTCGGC-3′) and external reverse primer HEV-LJR (5′-CCGGGTTTTACCCACCTTCA-3′), then amplified in the nested round with internal forward primer HEV-LJF2 (5′-GTCTCAGCCAATGGCGAGC-3′) and HEV-LJR (same as the external reverse primer) to generate a 770-bp DNA fragment. Another set of primers published previously were also applied to increase the detection rate (Dai et al. 2013). PCR reaction was catalyzed by PrimeSTAR GXL DNA polymerase (Takara Bio, China). PCR conditions included a pre-denaturation step at 94 °C for 3 min, followed by 35 cycles of denaturation at 98 °C for 10 s, annealing at 57 °C for 15 s, and extension at 68 °C for 45 s. PCR products with the expected size were purified and sequenced from both directions with the internal primers. Chromatograms were analyzed using Vector NTI Software (Thermo Fisher Scientific, US).
Phylogenetic Analysis
The phylogenetic analysis was performed using 83 unique partial HEV ORF2 sequences determined from the present study and genotype reference sequences as proposed previously (Smith and Simmonds 2018): 1_FJ457024, 2a_M74506, 3a_AF082843, 3b_AP003430, 3c_FJ705359, 3e_AB248521, 3f_AB369687, 3g_AF455784, 3h_JQ013794, 3i_FJ998008, 3j_AY115488, 3ra_FJ906895, 4a_AB197673, 4b_DQ279091, 4c_AB074915, 4d_AJ272108, 4e_AY723745, 4f_AB220974, 4g_AB108537, 4h_GU119961, and 4i_DQ450072. These sequences align with ORF2 at nucleotides 6403–7047 (4d_AJ272108 was used as the reference strain for numbering). Another set of randomly selected references sequences with available information of isolation regions (mainly from China) and host information was also included, with details provided in the respective figure legends. The evolutionary history was inferred by using the maximum likelihood (ML) method based on the Tamura-Nei model with MEGA, version 7.0 (Tamura and Nei 1993; Kumar et al. 2016).
Data Access
All 83 HEV partial ORF2 sequences determined by Sanger dideoxynucleotides sequencing were deposited in GenBank with accession numbers MN581748-581830.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism software version 7.0 (GraphPad Software Inc., San Diego, CA) or Statistical Analysis System (SAS) software package version 9.4 (SAS Institute Inc., Cary, NC). Categorical variables were described as frequencies and percentages (%), and continuous variables were described as mean ± standard error (SEM) or median (range). Comparisons among multiple groups or between two groups were performed using Chi-square test or t-test as appropriate. Univariate analyses were performed to identify variables significantly associated with the length of hospitalization stay. A multivariate linear regression model was built using the LASSO (least absolute shrinkage and selection operator) selection with Schwarz Bayesian information criterion by step further performed to select and eliminate variables. For all statistical tests, a two-sided P value of less than 0.05 was considered statistically significant.
Results
Seroprevalence of anti-HEV IgG and anti-HEV IgM
A total of 6112 consecutive serum samples were tested for anti-HEV IgG or anti-HEV IgM at Department of Infectious Diseases, Ruijin Hospital between August 2018 and July 2019. Samples with missing information of age or gender were excluded from further analysis. For the patient who was tested repeatedly and showed no change of result category (positivity/negativity/gray zone result), the earliest result was kept and the following repeated result was excluded. Finally, 4956 results of anti-HEV IgG test and 5289 results of anti-HEV IgM test were analyzed, respectively (Fig. 1). Most of the samples were tested for anti-HEV IgG and anti-HEV IgM simultaneously (n = 4838). The median age of subjects tested for anti-HEV IgG and anti-HEV IgM was both 45 years, and around 53% of them were males (Table 1).
Fig. 1.
The flowchart of HEV antibodies examination.
Table 1.
Characteristics of study population tested for anti-HEV IgG or anti-HEV IgM.
| Anti-HEV IgG (n = 4956) | Anti-HEV IgM (n = 5289) | |
|---|---|---|
| Age, median (range) | 45 (0–99) | 45 (0–99) |
| Male, n (%) | 2666 (53.8) | 2788 (52.7) |
| COI, median (range) | 0.28 (0–24.92) | 0.1 (0.01–68.49) |
Among the samples tested for anti-HEV IgG, 40.2% (1994/4956) was positive, 57.6% (2856/4956) was negative, and the remaining 2.1% (106/4956) had gray zone results. The sero-positivity rate of anti-HEV IgG increased with age (Fig. 2A), from 0% in age group [0–10 years] to 61.4% in subjects older than 80 years. Males had higher sero-positivity rate than females throughout the age groups. Males over the age of 20 had higher mean level of anti-HEV IgG (represented by COI) than females and the differences gradually expanded with age (Fig. 2B).
Fig. 2.
The seroprevalence and distribution of HEV antibodies in study population. The prevalence and level of anti-HEV IgG (A, B) or anti-HEV IgM (C, D) by age and gender. M, male. F, female. Subjects were divided into 10-year age groups. E The overall level of anti-HEV IgG or anti-HEV IgM by gender. M, male. F, female. ****P < 0.0001. ***P < 0.001. F The prevalence of anti-HEV IgG and anti-HEV IgM across the year. The prevalence was calculated by dividing the number of seropositive subjects by the total number of subjects tested in each age or time group. The level of HEV antibodies was shown as mean ± SEM.
Among the samples tested for anti-HEV IgM, 6.0% (319/5289) was positive, 93.2% (4931/5289) was negative, and the remaining 0.7% (39/5289) had gray zone results. The prevalence of anti-HEV IgM increased until age of 70 years and dropped subsequently, which peaked in the age group of [61–70] among males and [71–80] among females, respectively (Fig. 2C). The mean level of anti-HEV IgM in males increased with age and peaked in the age group of [61–70 years]. Females showed a parallel pattern to males except that women older than 70 years had higher mean level of anti-HEV IgM than counterparts (Fig. 2D). Both anti-HEV IgG and anti-HEV IgM levels were significantly higher in males than in females (Fig. 2E).
The prevalence of anti-HEV IgG maintained stable around 40% perennially, while potential heterogeneity was observed in anti-HEV IgM. The sero-positivity rate of anti-HEV IgM bottomed in November (3.9%) and June (3.6%), but peaked in February (8.6%) (Fig. 2F).
HEV RNA Prevalence and Genotype Variability
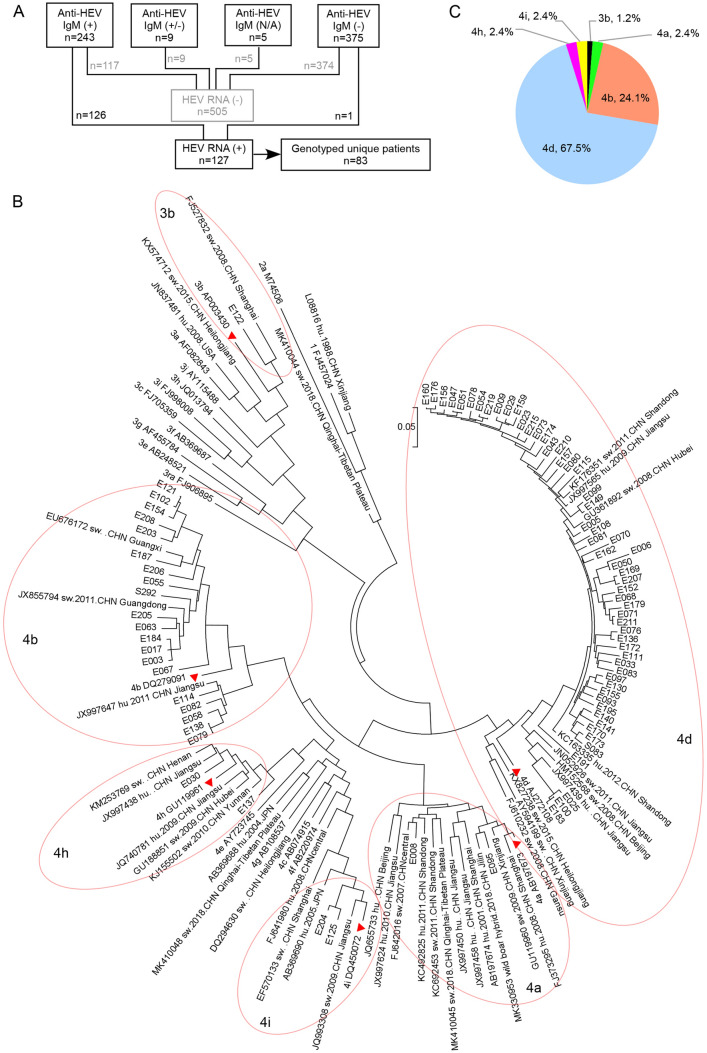
HEV RNA presence was tested in patients with evidence of active or recent HEV infection (defined by positivity of anti-HEV IgM) and patients who could not be ruled out of HEV infection (with unexplained elevated LFT results). A total of 632 samples were tested, including 243 available anti-HEV IgM-positive samples, 375 anti-HEV IgM-negative samples, 9 samples with gray zone results and 5 samples that were not tested for anti-HEV IgM (Fig. 3A). These anti-HEV IgM non-positive samples came from patients with unknown reason of hepatitis, reflected by their abnormal ALT levels (Table 2). Totally, one anti-HEV IgM-negative sample and 126 anti-HEV IgM-positive samples were positive for HEV RNA.
Fig. 3.
The flowchart of HEV RNA detection and genotyping of HEV sequences. A The composition of samples tested for HEV RNA. N/A: not available. B The subtype composition of HEV sequences determined from the study. C The phylogenetic tree inferred using the Maximum likelihood method. The analysis involved 146 nucleotide sequences, including 21 genotype references, 42 regional references and 83 unique sequences determined from this study. The vertical scale bar represents 0.05 nucleotide substitutions per site. Available information of isolation host, year and region were provided beside the 42 regional references. “hu” means the strain was isolated from human, while “sw” means swine. For the strains isolated from China, the information of isolating province or municipality was provided if available. The tree with the highest log likelihood (−17,368.42) was shown. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach, and then selecting the topology with superior log likelihood value. The tree was drawn to scale, with branch lengths measured in the number of substitutions per site. All positions containing gaps and missing data were eliminated. There were a total of 630 positions in the final dataset. The subtype reference strains (3b_AP003430, 4a_AB197673, 4b_DQ279091, 4d_AJ272108, 4h_GU119961 and 4i_DQ450072) were labeled with red triangular symbols next to the isolate, and the clusters of 3b, 4a, 4b, 4d, 4 h and 4i were circled for easy identification.
Table 2.
Characteristics of samples tested for HEV RNA by real time RT-PCR.
| Anti-HEV IgM category (n) | HEV RNA detected, n (%) | ALT, × ULN1 |
|---|---|---|
| Positive (243) | 126 (51.9) | 11.2 ± 1.1 |
| Negative (375) | 1 (0.3) | 8.4 ± 0.5 |
| Gray zone (9) | 0 (0.0) | 1.0 ± 0.5 |
| Not available (5) | 0 (0.0) | 5.3 ± 2.2 |
1 Results are presented as mean ± SEM.
The 127 HEV RNA-positive samples came from 111 patients, 15 of whom had sera collected at sequential time points. Partial ORF2 region was successfully amplified from 83 unique patients. Sequence analysis showed 82 (98.8%) patients were infected with HEV genotype 4 and one (1.2%) patient was infected with genotype 3. Subtypes were determined by creating a Maximum likelihood phylogenetic tree from all patient sequences and reference sequences obtained from GenBank (Fig. 3B). Subtype 4d was the most frequently detected (67.5%), followed by subtypes 4b (24.1%). Subtypes of 4a, 4 h, 4i and 3b were rarely detected (Fig. 3C).
There existed close phylogenetic relation between HEV sequences isolated from distant areas. For example, genotype 4b sequences identified from the current study were clustered with sequences isolated from Jiangsu (JX997647), Guangxi (EU676172) and Guangdong (JX855794) provinces, the latter two of which are more than 1200 km from Shanghai. We only detected one patient of genotype 3b infection (E122), who had travel history to Japan ten days before illness onset, but denied consumption of undercooked meat or seafood there. The identification of homologous swine sequence (FJ527832) to E122 argues the indigenous circulation of this subtype in Shanghai.
Clinical Features of Laboratory-Confirmed cases of HEV Infection
Subjects with positive HEV RNA were defined as laboratory-confirmed cases of HEV infection. Distinguishing features were addressed between confirmed HEV-infected subjects and subjects with positive anti-HEV IgM but undetected HEV RNA. The latter group may consist of subjects who recovered from recent infection or had viral load below detection limit. Overall, subjects with low level of anti-HEV IgM were often PCR-negative. Anti-HEV IgM, ALT and AST levels (P < 0.0001), as well as ALP, GGT and TBIL levels (P < 0.05), were significantly higher in double positive group (Table 3). This suggests that clearance or lowering of HEV viremia was accompanied by alleviation of symptoms and recovery of liver function.
Table 3.
Comparison of anti-HEV IgM (+) patients with or without HEV RNA detected1.
| HEV RNA (+) (n = 126) | HEV RNA (-) (n = 117) | P value | |
|---|---|---|---|
| Anti-HEV IgG (COI) | 10.3 ± 0.4 | 11.7 ± 0.6 | 0.0674 |
| Anti-HEV IgM (COI) | 31.9 ± 1.5 | 11.1 ± 1.3 | < 0.0001 |
| ALT, × ULN | 18.2 ± 1.8 | 2.1 ± 0.4 | < 0.0001 |
| AST, × ULN | 18.4 ± 2.7 | 2.4 ± 0.3 | < 0.0001 |
| ALP, × ULN | 1.5 ± 0.1 | 1.1 ± 0.1 | 0.0133 |
| GGT, × ULN | 3.5 ± 0.2 | 2.2 ± 0.5 | 0.0052 |
| TBIL, × ULN | 5.6 ± 0.5 | 3.8 ± 0.7 | 0.0243 |
1Results are presented as mean ± SEM; 2ULV: Upper limit of normal value.
Understanding demographic and clinical features of cases with HEV infection may help us identify patients at high risk in the future. Confirmed cases of HEV infection included 8 outpatients and 103 inpatients. The majority of them were males (62.2%). None of the females were pregnant at infection. About 77% were aged > 40 years. Thirty-nine cases (35%) occurred in January and February. Among patients who had available LFT results at presentation, 100 out of 106 had elevated ALT, AST or GGT above normal range; 78 out of 106 had elevated ALP; 90 out of 105 had elevated TBIL. The median level and range of LFT were listed in Table 4. As for outcome, 45 patients had normal ALT level at discharge, 33 patients had ALT level restored during follow up, 10 patients had improvement of ALT level at discharge, 15 patients had negative HEV RNA detected during follow up, 1 patient died during treatment and 7 outpatients were lost to follow-up.
Table 4.
Characteristics of laboratory-confirmed cases of HEV infection.
| Parameter | Value |
|---|---|
| Male, n (%) | 69 (62.2) |
| Age (years), median (range) | 58 (25–86) |
| Baseline laboratory tests1, median (range) | |
| ALT, × ULN | 13.6 (0.6–96.8) |
| AST, × ULN | 8.3 (0.6–185.7) |
| ALP, × ULN | 1.4 (0.5–4.2) |
| GGT, × ULN | 3 (0.5–12.5) |
| TBIL, × ULN | 4.2 (0.3–26.8) |
| Symptoms2, n (%) | |
| Abdominal pain | 21 (20.4) |
| Anorexia | 70 (68.0) |
| Fever | 29 (28.2) |
| Jaundice | 78 (75.7) |
| Malaise | 49 (47.6) |
| Nausea | 44 (42.7) |
| Vomiting | 29 (28.2) |
| Concomitant liver disease2, n (%) | |
| Alcoholic fatty liver disease | 5 (4.9) |
| Non-alcoholic fatty liver disease | 17 (16.5) |
| HBV carriage | 13 (12.6) |
1106 patients had available ALT, AST, ALP and GGT test results and 105 patients had available TBIL result at presentation to our hospital
2Information of symptoms and concomitant liver disease was available for 103 inpatients.
Comprehensive analyses based on detailed medical records of the 103 inpatients were carried out (Table 4). Patients presented within one day to one month after symptom onset. Ten patients recalled consumption of aquatic products, six patients reported eating takeout or dining out, one patient had definite history of consuming pork liver, one patient had close contact with swine, one patient traveled outside China within a month, and two patients’ spouses were infected by HEV recently. Jaundice was the most reported clinical symptom, followed by anorexia, malaise, nausea, fever, vomiting, and abdominal pain. No case of extrahepatic manifestation was recorded. Pre-existing liver diseases were recorded in 13 patients with HBV carriage (represented by HBsAg presence), five patients with alcoholic fatty liver disease (AFLD) and 17 patients with non-alcoholic fatty liver disease (NAFLD). Eight patients had liver cirrhosis and three of them had decompensated cirrhosis due to HBV infection.
Next, we want to address the major factors correlated to patients’ severity of illness, indicated by the length of hospital stays. All patients were discharged from hospital after improvement in LFT except that a 62-year-old male patient with late stage lung cancer died from renal failure 29 days after admission. The median hospital stay of the remaining 102 inpatients was 14 days, with a range between 5 and 128 days. Univariate analysis showed that the length of hospital stay was significantly correlated with age (P = 0.0384), anti-HEV IgG (P = 0.0408), ALP/ULN (P = 0.0166) and TBIL/ULN (P < 0.0001) level at admission. After adjusting the confounding factors, the length of hospital stay was significantly correlated with anti-HEV IgM (P = 0.0062) and TBIL/ULN (P < 0.0001) level, but not with age, HBsAg status, anti-HEV IgG, ALT, AST, ALP or GGT level by multivariate linear regression analysis. Remarkably, the association between hospitalization duration and TBIL/ULN level at admission was strongly positive (Table 5). No significant differences were found for age or any of the LFT results among patients with or without pre-existing HBV carriage (Table 6). Notably, patients co-infected with HBV had longer hospital stay (P = 0.0318) and higher level of TBIL, although not reaching statistical significance. No significant difference was found among patients with or without fatty liver diseases (data not shown).
Table 5.
Univariate and multivariate linear regression analysis for variables associating with the length of hospital stay.
| Variables | Length of hospital stay | |||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| β Coefficient | P value | β Coefficient | P value | |
| Age | 4.40 | 0.0384 | ||
| Anti-HEV IgG | 4.30 | 0.0408 | ||
| Anti-HEV IgM | 0.22 | 0.6389 | 7.83 | 0.0062 |
| HBsAg ( ±) | 1.41 | 0.2383 | ||
| ALT/ULN | 0.01 | 0.9391 | ||
| AST/ULN | 0.00 | 0.9628 | ||
| ALP/ULN | 5.94 | 0.0166 | ||
| GGT/ULN | 2.16 | 0.1447 | ||
| TBIL/ULN | 74.80 | < 0.0001 | 87.33 | < 0.0001 |
Table 6.
Demographic and baseline clinical parameters comparison of inpatients with or without HBV carriage1.
| Parameter | HBsAg (+) (n = 13) | HBsAg (-)(n = 89) | P value |
|---|---|---|---|
| Age (years) | 54.4 ± 3. 5 | 55.5 ± 1.5 | 0.7526 |
| Discharge days | 24.2 ± 4.0 | 18.4 ± 1.8 | 0.0318 |
| ALT, × ULN | 16.6 ± 5.6 | 21.3 ± 2.1 | 0.3099 |
| AST, × ULN | 25.1 ± 9.5 | 20.8 ± 3.3 | 0.7191 |
| ALP, × ULN | 1.7 ± 0.2 | 1.5 ± 0.1 | 0.5041 |
| GGT, × ULN | 3.2 ± 0.5 | 3.8 ± 0.3 | 0.6391 |
| TBIL, × ULN | 8.0 ± 2.0 | 5.4 ± 0.4 | 0.2403 |
1Results are presented as mean ± SEM.
Discussion
In the present study, we undertook a sero-epidemiologic study to determine the prevalence of HEV infection in Shanghai, which is the most populated city in eastern China. The annual anti-HEV IgG positive rate was around 40%. Though the current study was a hospital-based investigation, the prevalence of anti-HEV IgG is similar to other studies conducted in general Chinese population. A cross-sectional study conducted in 8 rural communities in southern China showed the anti-HEV IgG prevalence was 43.5% (Li et al. 2006). The seroprevalence of HEV in a community-based surveillance in rural eastern China was 38% (Zhu et al. 2014).
With comparable anti-HEV IgG prevalence, the number of HEV infected cases identified in our study is surprisingly high. A study conducted in Nanjing, a neighboring city of Shanghai, identified 210 HEV RNA-positive samples between January 2001 and April 2011 (Dai et al. 2013). That’s roughly 20 cases annually. Another study conducted more recently in Shenzhen, also a metropolitan city as Shanghai, identified 20 patients with acute hepatitis E over a 55-month period (between July 2012 and January 2017) (Sridhar et al. 2017). The potential causes for the far more cases of HEV infection identified in our study were analyzed. First, technical progress has improved the detection rate. Compared to nested RT-PCR method employed by (Dai et al. 2013), probe-based real time one-step RT-PCR has higher sensitivity. Second, more serum samples were screened. Around 5000 patients were tested for anti-HEV IgM in the 1-year study period. All available anti-HEV IgM-positive samples were evaluated for HEV RNA presence. Third, modern life styles may increase the risk of HEV infection. People are more likely to eat takeout currently, partly because people could afford less time to prepare food at home due to increased work pressure, and also because taking out can be ordered much more conveniently than before. Compared to cooking at home, it is more difficult to guarantee the dietary hygiene of takeout restaurants. Interestingly, we noticed a winter spike of anti-HEV IgM prevalence, which was reported previously (Liu et al. 2016; Sridhar et al. 2017). In our speculation, it may be related to the tradition of celebrating Chinese New Year, also around January to February, when people usually have more chances of dining out together during this holiday season.
Although the number of confirmed HEV-infected patients is far more than expected, we still notice a great gap between the number of anti-HEV IgG-positive and anti-HEV IgM-positive samples. Few of the anti-HEV IgG-positive subjects recalled past occurrence of hepatitis, which suggests that they may be exposed to HEV but no obvious symptoms developed. The high incidence of hepatitis E among middle-aged or elderly men suggests the immune surveillance of HEV among these subjects is probably suppressed and needs to be further explored.
All except one HEV isolates obtained in this study belonged to genotype 4, which was previously shown to be the most common genotype in eastern China (Zhang et al. 2010; Dai et al. 2013). Our data suggest that subtype 4d is currently the dominant cause of human HEV infection in Shanghai. Phylogenetic analysis showed HEV isolates prevalent in the human and swine populations were classified into the same subtypes, suggesting that cross-species transmission between swine and humans had taken place. Clustering of HEV sequences isolated from distant regions suggests certain strains are circulating across the country and hard to eliminate. Interestingly, one HEV sequence (E122) belonging to genotype 3b was identified. Genotype 3 HEV strains were thought to be imported from Japan (Liu et al. 2012) and are now common in eastern China (Ning et al. 2007; Si et al. 2009; Shuai et al. 2017; Wang et al. 2020). The patient carrying E122 indeed had travel history to Japan before illness onset. However, the nucleotide homology between E122 to sequence FJ527832, which was amplified from local swine specimen in 2008 (Si et al. 2009), suggests that indigenous infection is also possible. The high degree of genetic diversity of human-pathogenic HEV strains in this area may contribute to the adaptation of HEV to other hosts.
Diagnosis of acute HEV infection used to depend on the detection of anti-HEV IgM or increase of anti-HEV IgG titers retrospectively. In the current study, we noticed that about half of the anti-HEV IgM-positive samples were negative for HEV RNA. There are several possibilities: subjects were infected by HEV recently and in the convalescent period at the time of sample collection; or subjects were reactive to anti-HEV antibodies non-specifically due to unknown reasons. Our study of clinical phenotypes of sporadic HEV infection is based on the laboratory-confirmed patients with HEV viremia, which makes the data more reliable. Only one patient had close contact with swine sewage, and 10 patients recalled consumption of aquatic products, indicating transmission is mainly through foodborne route and contaminated seafood remains a risk factor of HEV infection in Shanghai. High levels of serum bilirubin and liver enzymes were common (Wang et al. 2016; Sridhar et al. 2017), indicating severe virulence of certain HEV strains. The hospitalization duration was positively correlated with TBIL level at admission (P < 0.0001). Why patients with more severe hepatitis E had higher baseline level of TBIL is intriguing. During HEV infection, inflammation of hepatocytes may lead to their inability to remove and process bilirubin from the bloodstream, leading to a buildup of bilirubin in the blood. Meanwhile, blockage or inflammation of bile ducts, which help to drain bilirubin, can also lead to an increased level of bilirubin. HEV was detected in bile duct epithelia by immunohistochemistry (Beer et al. 2019), which suggests that cholangiocytes may be the target cell of HEV infection. Therefore, increased level of TBIL may be caused by the damage of HEV to hepatocytes and cholangiocytes as well. Moreover, it could be a reflection of the concomitant liver disease(s) of the patient (Sridhar et al. 2017). HBV carriage was not shown to affect TBIL level significantly but associated with longer hospital stay (P < 0.05). In clinical practice, special attention should be paid to patients with extremely high level of TBIL and/or HBV superinfection. Multiple factors may contribute collectively to the severity of HEV infection and need further investigation.
The study has some limitations. First, all cases were collected from one hospital and selection bias may be present. A full-scale investigation with more hospitals involved is helpful to understand the local prevalence of HEV infection. Second, follow-up data were missing for some patients. Only 15 patients were confirmed negative of HEV RNA during follow-up, while other patients had merely LFT results available or loss to follow-up. Thus, the possibility of chronic infection in some patients could not be ruled out. Third, HEV RNA was detected retrospectively. It may degrade in stored sera over time and the accuracy of detection may be impaired. Forth, the inclusion criterion of HEV infection and corresponding studies of clinical profile are based on HEV RNA presence, which may lead to missing of some real HEV infection cases, since it is widely accepted that serum HEV RNA lasts very short during the natural history of acute HEV infection.
With that said, we still believe this study has provided insight into sporadic HEV infection in Shanghai. HEV infection poses increasing challenge to public health and continuous surveillance is necessary in China.
Acknowledgements
This study was funded by grants from the National Natural Science Foundation of China (No. 81501733), the Shanghai Municipal Key Clinical Specialty (shslczdzk01103), and Key Projects in the National Science & Technology Pillar Program during the Thirteenth Five-year Plan Period (2017ZX10203201-008, 2018ZX09201016-003-001, 2017ZX10202202-005-004). The sponsors had no role in the study design, data collection, analyses or in the decision to submit the article for publication.
Author contributions
JL designed the study. JL, QL, ZL and PW performed experiments. JL and JJ performed analysis and prepared the manuscript. JL, ZS, RL, HZ, WC, HW, QG and HG collected clinical data. QG, HG and QX critically revised and finalized the manuscript. All authors had full access to and approved the final version of the manuscript.
Compliance with Ethical Standards
Conflict of interest
The authors have no interest to declare.
Animal and Human Rights Statement
This non-interventional study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki 1975 and approved by the Human Ethics Committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. Informed consent was waived since the research involved no more than minimal risk to the subjects by using abandoned serum samples of routine clinical diagnosis. All medical information was coded and kept confidential as required. This study does not contain any studies with animal subjects performed by any of the authors.
Footnotes
Jie Lu, Qing Li and Jiayuan Jiang have contributed equally to this work.
Contributor Information
Qing Guo, Email: 13901922856@163.com.
Honglian Gui, Email: lillian_ghl@163.com.
Qing Xie, Email: xieqingrjh@163.com.
References
- Andonov A, Robbins M, Lang J, Cao J, Hatchette T, Stueck A, Deschambault Y, Murnaghan K, Varga J, Johnston L. Rat hepatitis e virus linked to severe acute hepatitis in an immunocompetent patient. J Infect Dis. 2019;220:951–955. doi: 10.1093/infdis/jiz025. [DOI] [PubMed] [Google Scholar]
- Aye TT, Uchida T, Ma XZ, Iida F, Shikata T, Zhuang H, Win KM. Complete nucleotide sequence of a hepatitis e virus isolated from the Xinjiang Epidemic (1986–1988) of China. Nucleic Acids Res. 1992;20:3512. doi: 10.1093/nar/20.13.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer A, Holzmann H, Pischke S, Behrendt P, Wrba F, Schlue J, Drebber U, Neudert B, Halilbasic E, Kreipe H, Lohse A, Sterneck M, Wedemeyer H, Manns M, Dienes HP. Chronic hepatitis e is associated with cholangitis. Liver Int. 2019;39:1876–1883. doi: 10.1111/liv.14137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capai L, Charrel R, Falchi A (2018) Hepatitis e in high-income countries: what do we know? And what are the knowledge gaps? Viruses 10:285 [DOI] [PMC free article] [PubMed]
- Dai X, Dong C, Zhou Z, Liang J, Dong M, Yang Y, Fu J, Tian H, Wang S, Fan J, Meng J, Purdy MA. Hepatitis e virus genotype 4, Nanjing, China, 2001–2011. Emerg Infect Dis. 2013;19:1528–1530. doi: 10.3201/eid1909.130013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton HR, Bendall RP, Keane FE, Tedder RS, Ijaz S. Persistent carriage of hepatitis e virus in patients with hiv infection. N Engl J Med. 2009;361:1025–1027. doi: 10.1056/NEJMc0903778. [DOI] [PubMed] [Google Scholar]
- Dawson GJ, Mushahwar IK, Chau KH, Gitnick GL. Detection of long-lasting antibody to hepatitis e virus in a us traveller to pakistan. Lancet. 1992;340:426–427. doi: 10.1016/0140-6736(92)91507-5. [DOI] [PubMed] [Google Scholar]
- Ding Q, Heller B, Capuccino JM, Song B, Nimgaonkar I, Hrebikova G, Contreras JE, Ploss A. Hepatitis e virus orf3 is a functional ion channel required for release of infectious particles. Proc Natl Acad Sci U S A. 2017;114:1147–1152. doi: 10.1073/pnas.1614955114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Association for the Study of the Liver (EASL) (2018) Easl clinical practice guidelines on hepatitis e virus infection. J Hepatol 68:1256–1271 [DOI] [PubMed]
- Hakze-van der Honing RW, van Coillie E, Antonis AFG, van der Poel WHM. First isolation of hepatitis e virus genotype 4 in europe through swine surveillance in the netherlands and Belgium. PLoS ONE. 2011;6:e22673. doi: 10.1371/journal.pone.0022673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid SS, Atiq M, Shehzad F, Yasmeen A, Nissa T, Salam A, Siddiqui A, Jafri W. Hepatitis e virus superinfection in patients with chronic liver disease. Hepatology. 2002;36:474–478. doi: 10.1053/jhep.2002.34856. [DOI] [PubMed] [Google Scholar]
- Huang S, Zhang X, Jiang H, Yan Q, Ai X, Wang Y, Cai J, Jiang L, Wu T, Wang Z, Guan L, Shih JW, Ng MH, Zhu F, Zhang J, Xia N. Profile of acute infectious markers in sporadic hepatitis e. PLoS ONE. 2010;5:e13560. doi: 10.1371/journal.pone.0013560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamar N, Selves J, Mansuy JM, Ouezzani L, Peron JM, Guitard J, Cointault O, Esposito L, Abravanel F, Danjoux M, Durand D, Vinel JP, Izopet J, Rostaing L. Hepatitis e virus and chronic hepatitis in organ-transplant recipients. N Engl J Med. 2008;358:811–817. doi: 10.1056/NEJMoa0706992. [DOI] [PubMed] [Google Scholar]
- Kenney SP, Meng XJ (2019) hepatitis e virus genome structure and replication strategy. Cold Spring Harb Perspect Med 9:a031724 [DOI] [PMC free article] [PubMed]
- Khuroo MS. Discovery of hepatitis e: the epidemic non-a, non-b hepatitis 30 years down the memory lane. Virus Res. 2011;161:3–14. doi: 10.1016/j.virusres.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Khuroo MS, Kamili S, Yattoo GN. Hepatitis e virus infection may be transmitted through blood transfusions in an endemic area. J Gastroenterol Hepatol. 2004;19:778–784. doi: 10.1111/j.1440-1746.2004.03437.x. [DOI] [PubMed] [Google Scholar]
- Khuroo MS, Khuroo MS, Khuroo NS. Hepatitis e: discovery, global impact, control and cure. World J Gastroenterol. 2016;22:7030–7045. doi: 10.3748/wjg.v22.i31.7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khuroo MS, Teli MR, Skidmore S, Sofi MA, Khuroo MI. Incidence and severity of viral hepatitis in pregnancy. Am J Med. 1981;70:252–255. doi: 10.1016/0002-9343(81)90758-0. [DOI] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K. Mega7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanini S, Pisapia R, Capobianchi MR, Ippolito G. Global epidemiology of viral hepatitis and national needs for complete control. Expert Rev Anti Infect Ther. 2018;16:625–639. doi: 10.1080/14787210.2018.1505503. [DOI] [PubMed] [Google Scholar]
- Lee GH, Tan BH, Teo EC, Lim SG, Dan YY, Wee A, Aw PP, Zhu Y, Hibberd ML, Tan CK, Purdy MA, Teo CG. Chronic infection with camelid hepatitis e virus in a liver transplant recipient who regularly consumes camel meat and milk. Gastroenterology. 2016;150(355–357):e353. doi: 10.1053/j.gastro.2015.10.048. [DOI] [PubMed] [Google Scholar]
- Li RC, Ge SX, Li YP, Zheng YJ, Nong Y, Guo QS, Zhang J, Ng MH, Xia NS. Seroprevalence of hepatitis e virus infection, rural southern people's republic of china. Emerg Infect Dis. 2006;12:1682–1688. doi: 10.3201/eid1211.060332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Cai J, Wang S, Wu Z, Li L, Jiang T, Chen B, Cai G, Jiang Z, Chen Y, Wang Z, Zhu X, Hu L, Gu H, Jiang J. Identification of distribution characteristics and epidemic trends of hepatitis e in zhejiang province, China from 2007 to 2012. Sci Rep. 2016;6:25407. doi: 10.1038/srep25407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Li L, Wang L, Bu Q, Fu H, Han J, Zhu Y, Lu F, Zhuang H. Phylogenetic analysis of 626 hepatitis e virus (hev) isolates from humans and animals in china (1986–2011) showing genotype diversity and zoonotic transmission. Infect Gen Evolut: J Mol Epidemiol Evolut Gen Infect Dis. 2012;12:428–434. doi: 10.1016/j.meegid.2012.01.017. [DOI] [PubMed] [Google Scholar]
- Ning H, Niu Z, Yu R, Zhang P, Dong S, Li Z. identification of genotype 3 hepatitis e virus in fecal samples from a pig farm located in a shanghai suburb. Vet Microb. 2007;121:125–130. doi: 10.1016/j.vetmic.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Schlauder GG, Mushahwar IK. Genetic heterogeneity of hepatitis e virus. J Med Virol. 2001;65:282–292. doi: 10.1002/jmv.2031. [DOI] [PubMed] [Google Scholar]
- Schwartz E, Jenks NP, van Damme P, Galun E. Hepatitis e virus infection in travelers. Clin Infect Dis. 1999;29:1312–1314. doi: 10.1086/313430. [DOI] [PubMed] [Google Scholar]
- Shuai J-B, Li L-H, Li A-Y, He Y-Q, Zhang X-F. Full genome analysis of swine genotype 3 hepatitis e virus isolated from eastern China. J Zhejiang Univ Sci B. 2017;18:549–554. doi: 10.1631/jzus.B1600419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si FS, Zhu YM, Dong SJ, Yu SS, Yu RS, Shen SY, Yang Q, Li Z. Full genomic sequence analysis of swine genotype 3 hepatitis e virus isolated from shanghai. Virus Res. 2009;144:290–293. doi: 10.1016/j.virusres.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Smith DB, Simmonds P (2018) Classification and genomic diversity of enterically transmitted hepatitis viruses. Cold Spring Harb Perspect Med 8:a031880 [DOI] [PMC free article] [PubMed]
- Sridhar S, Lo SK, Xing F, Yang J, Ye H, Chan JF, Teng JL, Huang C, Yip CC, Lau SK, Woo PC. Clinical characteristics and molecular epidemiology of hepatitis e in shenzhen, China: a shift toward foodborne transmission of hepatitis e virus infection. Emerg Microbes Infect. 2017;6:e115. doi: 10.1038/emi.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar S, Yip CCY, Wu S, Cai J, Zhang AJ, Leung KH, Chung TWH, Chan JFW, Chan WM, Teng JLL, Au-Yeung RKH, Cheng VCC, Chen H, Lau SKP, Woo PCY, Xia NS, Lo CM, Yuen KY. Rat hepatitis e virus as cause of persistent hepatitis after liver transplant. Emerg Infect Dis. 2018;24:2241–2250. doi: 10.3201/eid2412.180937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam AW, Smith MM, Guerra ME, Huang CC, Bradley DW, Fry KE, Reyes GR. Hepatitis e virus (hev): molecular cloning and sequencing of the full-length viral genome. Virology. 1991;185:120–131. doi: 10.1016/0042-6822(91)90760-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial dna in humans and chimpanzees. Mol Biol Evol. 1993;10:512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- Tessé S, Lioure B, Fornecker L, Wendling M-J, Stoll-Keller F, Bigaillon C, Nicand E. Circulation of genotype 4 hepatitis e virus in europe: first autochthonous hepatitis e infection in france. J Clin Virol: Off Pub Pan Am Soc Clin Vir. 2012;54:197–200. doi: 10.1016/j.jcv.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Wang L, Liu L, Wei Y, Wang Q, Tian Q, Wang L, Zhuang H. Clinical and virological profiling of sporadic hepatitis e virus infection in China. J Infect. 2016;73:271–279. doi: 10.1016/j.jinf.2016.06.005. [DOI] [PubMed] [Google Scholar]
- Wang L, Yan L, Jiang J, Zhang Y, He Q, Zhuang H, Wang L. Presence and persistence of hepatitis e virus rna and proteins in human bone marrow. Emerg Micro InfECT. 2020;9:994–999. doi: 10.1080/22221751.2020.1761762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ling R, Erker JC, Zhang H, Li H, Desai S, Mushahwar IK, Harrison TJ. A divergent genotype of hepatitis e virus in chinese patients with acute hepatitis. J Gen Virol. 1999;80(1):169–177. doi: 10.1099/0022-1317-80-1-169. [DOI] [PubMed] [Google Scholar]
- Webb GW, Dalton HR. Hepatitis e: an underestimated emerging threat. Ther Adv Infect Dis. 2019;6:2049936119837162. doi: 10.1177/2049936119837162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazaki Y, Mizuo H, Takahashi M, Nishizawa T, Sasaki N, Gotanda Y, Okamoto H. Sporadic acute or fulminant hepatitis e in hokkaido, japan, may be food-borne, as suggested by the presence of hepatitis e virus in pig liver as food. J Gen Virol. 2003;84:2351–2357. doi: 10.1099/vir.0.19242-0. [DOI] [PubMed] [Google Scholar]
- Zhang J, Ge SX, Huang GY, Li SW, He ZQ, Wang YB, Zheng YJ, Gu Y, Ng MH, Xia NS. Evaluation of antibody-based and nucleic acid-based assays for diagnosis of hepatitis e virus infection in a rhesus monkey model. J Med Virol. 2003;71:518–526. doi: 10.1002/jmv.10523. [DOI] [PubMed] [Google Scholar]
- Zhang M, Wu R, Xu H, Uhanova J, Gish R, Wen X, Jin Q, Gerald MY, Nguyen MH, Gao Y, Niu J. changing incidence of reported viral hepatitis in china from 2004 to 2016: an observational study. BMJ Open. 2019;9:e028248. doi: 10.1136/bmjopen-2018-028248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, He Y, Wang H, Shen Q, Cui L, Wang X, Shao S, Hua X. Hepatitis e virus genotype diversity in eastern China. Emerg Infect Dis. 2010;16:1630–1632. doi: 10.3201/eid1610.100873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu FC, Huang SJ, Wu T, Zhang XF, Wang ZZ, Ai X, Yan Q, Yang CL, Cai JP, Jiang HM, Wang YJ, Ng MH, Zhang J, Xia NS. epidemiology of zoonotic hepatitis e: a community-based surveillance study in a rural population in china. PLoS ONE. 2014;9:e87154. doi: 10.1371/journal.pone.0087154. [DOI] [PMC free article] [PubMed] [Google Scholar]