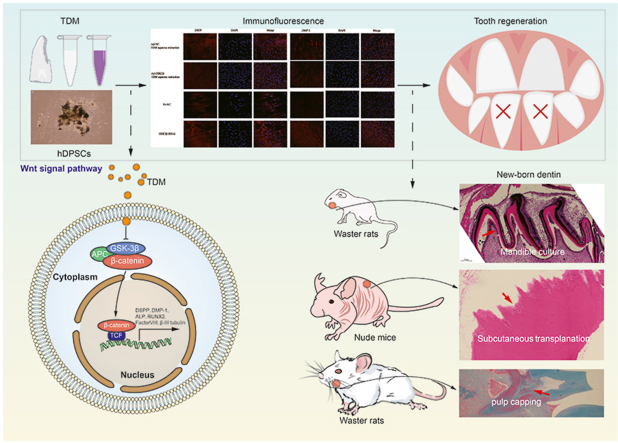
Abstract
Treated dentin matrix (TDM) is an ideal scaffold material containing multiple extracellular matrix factors. The canonical Wnt signaling pathway is necessary for tooth regeneration. Thus, this study investigated whether the TDM can promote the odontogenic differentiation of human dental pulp stem cells (hDPSCs) and determined the potential role of Wnt/β-catenin signaling in this process. Different concentrations of TDM promoted the dental differentiation of the hDPSCs and meanwhile, the expression of GSK3β was decreased. Of note, the expression of the Wnt/β-catenin pathway-related genes changed significantly in the context of TDM induction, as per RNA sequencing (RNA seq) data. In addition, the experiment showed that new dentin was visible in rat mandible cultured with TDM, and the thickness was significantly thicker than that of the control group. In addition, immunohistochemical staining showed lower GSK3β expression in new dentin. Consistently, the GSK3β knockdown hDPSCs performed enhanced odotogenesis compared with the control groups. However, GSK3β overexpressing could decrease odotogenesis of TDM-induced hDPSCs. These results were confirmed in immunodeficient mice and Wistar rats. These suggest that TDM promotes odontogenic differentiation of hDPSCs by directly targeting GSK3β and activating the canonical Wnt/β-catenin signaling pathway and provide a theoretical basis for tooth regeneration engineering.
Keywords: Dental pulp stem cells, Treated dentin matrix, GSK3β, Odontogenic differentiation, Wnt/β-catenin signaling pathway
Graphical abstract
Highlights
-
•
TDM promotes odontogenic differentiation of hDPSCs by targeting GSK3β and activating the canonical Wnt/β-catenin signaling.
1. Introduction
Odontogenic defects or loss caused by caries, trauma, and abrasion such as wedge-shaped defects, are highly prevalent in the general population and can be harmful to oral and even overall human health [1]. These defects may lead to dentin damage and the consequent pulp exposure. The reduced dental support and pulpitis can give rise to pulp necrosis, and ultimately to odontogenic fractures and ultimate dental loss [2]. Dental pulp is a tissue rich in nerves and blood vessels and which provides nutrition to the entire tooth. When subjected to constant external stimulation, the internal dental pulp stem cells can differentiate into odontoblasts to form the second and third stage dentin to maintain the physiological activities of normal teeth [[3], [4], [5]]. Human dental pulp mesenchymal stem cells (hDPSCs), with a rapid proliferation potential, are able to differentiate into a variety of cells including odontoblasts, osteoblasts/osteoclasts, adipocytes, chondrocytes, and nerve cells [6]. The odontogenic differentiation not only requires collagen, but also the mediation by some specific proteins and minerals ratio to guide the differentiation of the hDPSCs along the odontogenic lineage [7].
Clinically, the exposed part of the pulp after mechanical or iatrogenic trauma must be capped so the vital pulp can be preserved. Such intervention seals the pulp damage in time, promotes pulp healing, and forms a restorative dentin bridge; of note, the material used for capping comprises calcium hydroxide, mineral trioxide aggregate (MTA), and dentin adhesive [8]. However, pulp inflammation, the weakness of newly formed dentin-like tissue, and pulp cavity occlusion without a dentin-like structure limit long-term success rates of these drugs. As a result, they do not meet the ideal requirements of clinical application [9]. Therefore, it is necessary to develop new dental materials.
The ideal dental materials must meet the biocompatibility standards, be easy to produce, and exhibit the appropriate mechanical properties and a multi-void structure. In recent years, the application of materials in tissue regeneration has attracted widespread attention. Polyisoprene (PI) is able to promote the odontogenic differentiation of hDPSCs, and its modulus can be optimized by changing the coating thickness or adding inorganic particles [10]; Functionalized gelatin is hydrophilic and enables sustained drug release, which can induce the differentiation of hDPSCs into dentin-like tissue [11]; Importantly, it is essential to deeply understand the materials, to develop strategies that allow their optimization. For instance, the insertion of RDG or RGD peptide sequences into dental materials can change its structure and even induce the activation of specific pathways in cells [12,13]. However, the differentiation of hDPSCs to form restorative dentin is a complex process that requires interactions among multiple factors, and a single material is not expected to achieve this.
As an acellular matrix material, treated dentin matrix (TDM) is rich in natural proteins and bioactive molecules, which has broad application prospects in dentin regeneration [14,15]. Our previous studies have exhibited that the liquid extract of TDM contain a variety of bioactive odontogenic proteins as well as growth factors such as Decorin、Biglycan、TGF-β1、COL-1、DMP-1 and DSPP. Moreover, TDM extract were proved to have good biocompatibility [16]. As a natural substance extracted from human teeth, TDM has a loose, porous structure and can be used to form a paste for pulp capping [16,17]. However, the mechanism of odontogenic regeneration induced by TDM remains unclear.
Dentin regeneration requires accurate regulation of Wnt/β-catenin pathway, one of the most important signaling pathways that regulate the regenerative process in several systems [[18], [19], [20]]. In the absence of Wnt ligands, β-catenin acts as the main effector molecule [21]. Recent studies have confirmed that the canonical Wnt/β-catenin pathway (or the Wnt signaling pathway), regulates the differentiation of dental cells during root formation [22]. This pathway also plays a role in bone formation induced by BMP-9 and the odontogenic process of dental papillary stem cells [23]. It is also necessary for the formation of dentin [24]. Of note, glycogen synthesis kinase (GSK) 3β, a multifunctional serine/threonine kinase, is an important negative regulator of the canonical Wnt pathway. It continuously phosphorylates β-catenin, resulting in its degradation by ubiquitin and proteasomes. In contrast, inhibition of GSK 3β activity via Wnt stimulation allows β-catenin to accumulate in the cytoplasm and enter the nucleus [25].
hDPSCs are an important source of stem cells for tooth regeneration. Besides, the canonical Wnt signaling pathway was demonstrated to negatively regulate the odontoblast-like differentiation of hDPSCs [1]. However, the exact role of the Wnt signaling pathway in odontogenic differentiation of hDPSCs induced by TDM remains unclear. Thus, we hypothesized that TDM could regulate the odontogenic differentiation of the hDPSCs by mediating the expression of GSK3β.
To address this hypothesis, in this study, we evaluated the effect of TDM on the odontogenic differentiation of hDPSCs, with a special focus on the expression of GSK3β.
2. Materials and methods
2.1. Isolation of hDPSCs
This study was approved by the Zhengzhou University Hospital Medical Ethics Committee. As described previously [27], human dental pulp was isolated from extracted healthy permanent teeth (donors aged 18–25 years) after the informed consent was obtained. Dental pulp tissues were isolated from the teeth, rinsed repeatedly with phosphate-buffered saline (PBS), and then digested in 3 mg/mL collagenase type I (Solarbio, Zhengzhou, China) for 30 min at 37 °C in a humidified atmosphere containing 5% CO2. Then, digested cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) at 37 °C with 5% CO2. At 80% confluence, the cells were trypsinized and subcultured.
2.2. Multipotential differentiation
Passage 3 hDPSCs were seeded into a 6-well plate and respectively cultured with adipogenic induction liquid (Solarbio, Zhengzhou, China) and osteogenic medium containing 10% FBS, 10 mM β-glycerol phosphate, 50 mg/mL ascorbic acid, and 100 nM dexamethasone (Solarbio, Zhengzhou, China) for induction. The Oil red O and alizarin red staining methods were performed on day 14 and 21, respectively. The cells were routinely observed and photographed under a phase-contrast inverted microscope (Nikon, Japan).
2.3. Immunofluorescence cell staining
Passage 3 hDPSCs were seeded in 24-well plates for routine culture. When cells reached approximately 80% confluence, the medium was discarded, cells were rinsed and subsequently fixed with 4% of Paraformaldehyde (Solarbio, Zhengzhou, China) at room temperature. The following primary antibodies were added to the cells at the indicated dilution: anti-STRO-1 (1:100; R&D Systems, Minneapolis, MN, USA), anti-Vimentin (1:500; Abcam, Cambridge, UK), anti-Nestin (1:100; Santa Cruz Biotechnology, Houston, TX, USA), anti-c-Kit (1:400; Cell Signaling Technology, Danvers, MA, USA), anti-RUNX2 (1:100; Santa Cruz Biotechnology), and anti-ALP (1:100; Abcam). After a washing step, the cell nuclei were counter-stained with 4′,6-Diamidino-2′-phenylindole (DAPI, Invitrogen, Carlsbad, CA, USA). Cells were analyzed under a confocal microscope (LSM800, Zeiss, Oberkochen, Germany) and the mean fluorescence intensity was determined using the ZEN 2.4 software.
2.4. Flow cytometry
For identification of specific cell surface molecules, hDPSCs were trypsinized and subsequently incubated with anti-CD3 (1:100), anti-CD31 (1:100), anti-CD34 (1:100), anti-CD73 (1:100), anti-CD90 (1:100), and anti-CD105 (1:100) antibodies (all from, BD Biosciences, Franklin Lakes, NJ, USA) for 1 h, at 4 °C. Cell populations were resolved on an Accuri C6 Flow cytometer (BD Biosciences).
2.5. TDM fabrication
Periodontal tissues, crowns, pulp and dentin were collected from patients in the First Affiliated Hospital of Zhengzhou University. The patients and their families gave informed consent, and the experiment was approved by the Hospital Ethics Committee. The inclusion criteria were as follows: no caries, no root canal treatment, or teeth that needed removal for orthodontic reasons. The human dentin matrix was cleaned with deionized water and an ultrasonic cleaner, and then soaked in ethylene diamine tetra-acetic acid (EDTA) at different concentrations (17 mol/L for 10 min, 10 mol/L for 5 min, and 5 mol/L for 10 min). Wash the human dentin matrix with sterile water after each EDTA demineralization. After freeze-drying, the samples were sterilized using Co-60 irradiation. TDM was obtained according to the protocol proposed by the International Standardization Organization (ISO10093). DMEM (100 mL) was added to the pulverized TDM (20 g) as previously described [19]; the suspension was then placed in an incubator at 37 °C for 3 days. Samples were filtered using a 0.22 μm filter and stored at −80 °C until use.
2.6. TDM-induced hDPSCs
hDPSCs were cultured in the absence, or presence of different concentrations of TDM (40%, 60%, 80%, 100% ). The medium, DMEM containing 10% FBS, was changed every 3 days. Cell proliferation was detected by EdU and CCK-8 (Dojindo Molecular Technologies, Kumamoto, Japan) assays. According to the manufacturer's instructions. Quantitative real-time PCR (q RT-PCR) and western blotting assays were performed to measure the expression of dentin-related genes and proteins. The experiments were repeated for 3 times. Primer sequences used for (q RT-PCR) are listed in Table 1.
Table 1.
Sequences of primers used for the q RT-PCR.
| Gene | Forward primer | Reverse Primer |
|---|---|---|
| Runx2 | 5′-AACAGCAGCAGCAGCAGCAG | 5′-GCACCGAGCACAGGAAGTTGG |
| ALP | 5′-TAAGGACATCGCCTACCAGCTC | 5′-TCTTCCAGGTGTGTCAACGAGGT |
| DSPP | 5′-CTGTTGGGAAGAGCCAAGATAAG | 5′-CCAAGATCATTCCATGTTGTCCT |
| DMP-1 | 5′-AGTTCCAAGCCTCCAACATCATGC | 5′-TTTTGAGTGGGAGAGTGTGTGC |
| Factor VIII | 5′-AGTTCCAAGCCTCCAACATCATGC | 5′-GGAAGTCAGTCTGTGCTCCAATGC |
| β-III tubulin | 5′-AGCAAGGTGCGTGAGGAGTATC | 5′-GCATAGGTCTCATCCGTGTTCT |
| GAPDH | 5′-CTTTGGTATCGTGGAAGGACTC-3ʹ | 5′-GTAGAGGCAGGGATGATGTTCT |
2.7. Lentiviral transduction
Lentiviral particles purchased from Gene Chem (Shanghai, China) were used to establish GSK3β stable knockdown (GSK3β-RNAi) or overexpressing cells (Ad-GSK3β). Transduction with lentiviral particles encoding scrambled sequences was used as a negative control (Sh-NC or Ad-NC). The infection multiplicity (MOI) was 40, and the transfection efficiency was over 70%. When cell confluency reached 80%, normal cells were used as control and transduced cells were selected with 0.5 μg/mL puromycin.
2.8. Cell migration assay
Passage 3 hDPSCs were placed in the upper chambers of Transwell plates. The lower chambers were filled with either 0.5% FBS (negative control), 10% FBS (positive control) or TDM. Cells were incubated at 37 °C for 24 h, and then washed with PBS. After fixation with 4% paraformaldehyde and staining with 0.1% crystal violet, hDPSCs of each filter were counted in six fields under a high-power microscope ( × 200). These fields were randomly selected in the context of blind evaluation, and the data were processed and analyzed using the ImageJ and GraphPad software.
2.9. RNA isolation and q RT-PCR
Trizol was used to extract RNA from hDPSCs followed by cDNA synthesis using the Prime Script RT kit (Thermo Fischer Scientific, Waltham, MA, USA) according to the manufacturer's instructions. cDNA samples were then quantified by q RT-PCR using the SYBR Premix Ex Taq™ II kit (Takara, Shiga, Japan). The PCR was performed on a ABI 7500 PCR system using the following program: 95 °C for 30 s, followed by 45 cycles at 95 °C for 5 s, 55 °C for 30 s, and 72 °C for 30 s. Changes in gene expression were measured using the 2−ΔΔCT method; GAPDH was used as the endogenous control. Determinations was repeated at least three times.
2.10. RNA sequencing
6 samples (from both the Control and TDM groups) were subjected to. RNA- sequencing (RNA-seq). Briefly, total RNA was extracted from the samples (at least 106 cells) using trizol reversely transcribed into double-stranded cDNA using the N6 random primer. Libraries were then constructed using the traditional methods [21], and sequenced.
The sequencing data was filtered with SOAP nuke (v1.5.2) by the following steps: (1) reads the adapter sequences were removed; (2) low-quality reads (base quality less than or equal to 20) were removed; (3) reads with an unknown base (‘N' base) ratio higher than 5% were also removed. Essentially, differentially expressed genes were those with a False Discovery Rate (FDR) ≤0.001 and a |Log2Ratio| >1. Additionally, GO (http://www.geneontology.org/) and KEGG (https://www.kegg.jp/) enrichment analysis were performed using Phyper (https://en.wikipedia.org/wiki/Hypergeometric_distribution) based on the Hypergeometric test. The significant levels of terms and pathways were corrected using Q value with a rigorous threshold (Q value ≤ 0.05) as per the Bonferroni test.
2.11. Western blotting assay
Western blotting assay was performed as previously described [5]. An equal amount of total protein (20 μg) from each sample was loaded in each well and proteins were separated by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE). The resolved proteins were subsequently transferred to a polyvinylidene fluoride membrane. The membranes were blocked with 5% BSA for about 1 h.
Dentin-related proteins were detected with 1/1000 diluted, primary antibodies against DSPP, DMP-1, ALP, β–III–Tubulin, Factor VIII and RUNX2. For β-catenin detection, hDPSCs were stimulated with or without Wnt3a (100 ng/mL), and then 1/1000 primary antibodies against GSK3β, P- GSK3β, β-catenin and Active β-catenin were used.
After wash with TBST (50 mM Tris-HCl, pH 7.4, 100 mM NaCl and 0.2% Tween-20), membranes were incubated with secondary antibodies (Goat anti-Mouse IgG and Goat anti-rabbit IgG, 1:10,000, Abcam, Cambridge, UK). Protein bands were visualized with an enhanced chemiluminescence (ECL) system (Invitrogen, Carlsbad, CA, USA). Western Blotting assay was repeated 3 times for each marker. The expression of odontogenic-related proteins was analyzed using the ImageJ software.
2.12. Pulp-capping in Wistar rats
To further study the effect of TDM on the ability of hDPSCs to induce reparative dentin formation, a paste was prepared with TDM powder and its extract in a ratio of 1:1, and 26 Wistar rats were pulp capped. After 1 week, the animals were anesthetized via intraperitoneal injection of sodium pentobarbital. A quarter-size ball drill was used to drill a hole in the center of the first maxillary molar of rats, and the operation was terminated when the depth was about 1/4 of the diameter of the ball drill. The probe was inserted into the pulp and a sterile cotton ball was used to stop pulp bleeding and dry the cavity. Subsequently, the cavity was filled with TDM paste or calcium hydroxide (CH). After 10 weeks, the rats were sacrificed by cardiac perfusion. The maxilla of the rats was extracted and a bone mass containing bilateral first molars was prepared. The bone tissue was placed in 4% paraformaldehyde for 48 h and decalcified in 100 g/L EDTA solution (pH 7.4) for 12 weeks. After conventional paraffin embedding, proximal and distal sections (5 μm thick) were continuously made in parallel with the long axis of the tooth. Routine hematoxylin and eosin (H&E) staining was performed to detect odontogenesis of cells in vivo.
2.13. Rat mandible culture
As described earlier, the mandible of rats was cultured [29] in a system set up by Atelocollagen membrane (CM-6, Atelocell®, Japan). Briefly, 12 rats were anesthetized with isoflurane and sacrificed. The mandibles were harvested and placed in 6-well plates with CM-6 and cultured in medium containing DMSO or TDM for 10 days. After the mandibles were fixed with 4% paraformaldehyde, the expression level and location of GSK3β were detected by H&E staining and immunohistochemistry.
2.14. Subcutaneous transplantation in immunodeficient mice
All immuno-deficient mice were purchased from Beijing Victory. In the preliminary experiment, we implanted columnar TDM into the dorsal subcutaneous site of 3 immunodeficient mice. After 10 weeks, we observed and recorded the results of H&E staining. Then, 24 immuno-deficient mice were divided into four groups (6 per group on average) namely GSK3β-RNAi group, Sh-NC group, Ad-NC group and Ad-GSK3β group. The cells of each group were combined with TDM respectively and incubated at 37 °C for 3 days. Stent implantation was performed under deep anesthesia. After 10 weeks, the samples were removed from the mice. Three implants of each group were used to detect the formation of mineralization by Scanning electron microscopy (SEM) and Energy Dispersive X-ray detection (EDS). Other implants were then fixed in 4% paraformaldehyde, demineralized with 10% EDTA (pH 8.0), embedded in paraffin and sliced. Paraffin sections were subjected to H&E, Masson and immunohistochemical stainings. Finally, a digital tissue slice scanner was used to collect immunohistochemical images, and the results of histochemistry were quantitatively analyzed by Seville image analysis system. The following indicators were analyzed: a positive area ratio (positive area ratio: positive area/tissue area) and Histochemistry score (H-SCORE = ∑(pi × i) = (percentage of weak intensity area x 1) + (percentage of moderate intensity area x 2) + (percentage of strong intensity area x 3), where, pi represents the percentage of the positive signal pixel area, and i represents the positive level. All animal experiments were conducted in accordance with the guidelines of the Zhengzhou University Animal Experimental Committee. The guidelines are also compliant with the National Institutes of Health guidelines of Zhengzhou University for laboratory animal care and use.
2.15. Statistical analysis
The SPSS 16.0 statistical software was used to analyze relevant data. The independent sample t-test was used for inter-group comparison. The Bonferroni correction was also applied, when applicable. Results with p < 0.05 were considered statistically significant.
3. Results
3.1. Characteristics of isolated hDPSCs
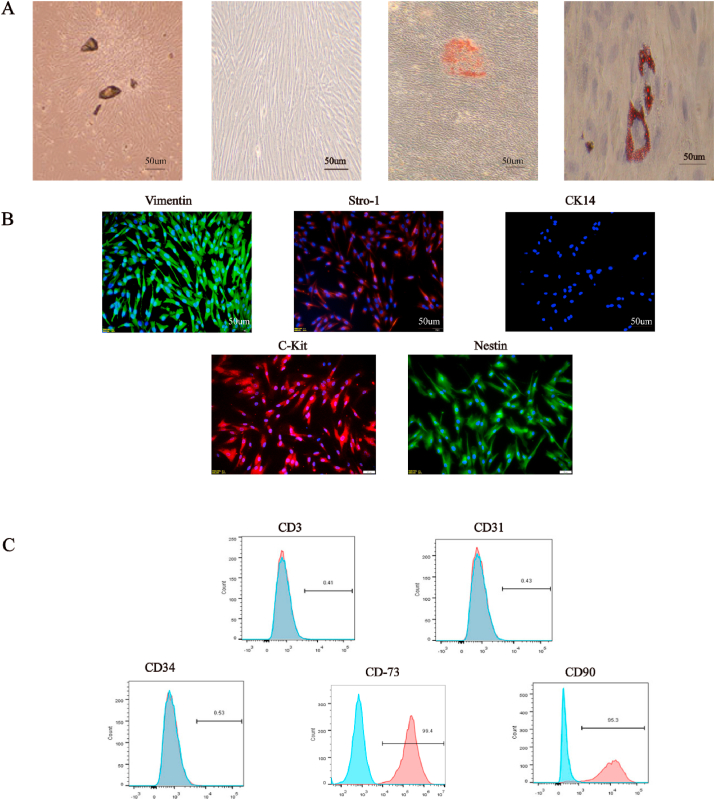
About 3 days after pulp tissue culture, cells emerged from the tissue mass and presented a fibroblast-like morphology corresponding to primary hDPSCs. About 7 days later, hDPSCs spread out completely in the culture flask, and retained the mesenchymal cell morphology during passage. hDPSCs were induced in osteogenic medium for 21 days and mineralized nodules were observed after alizarin red staining; besides, hDPSCs were cultured in adipogenic medium for 14 days, lipid droplets were observed by Oil Red O staining, indicating that hDPSCs have the potential for osteogenic and adipogenic differentiation (Fig. 1A). Further results showed that isolated hDPSCs were positive for Vimentin, STRO-1, c-Kit, Nestin, CD90 (95.3%), and CD73 (99.4%), but negative for CD31 (0.43%), CD34 (0.53%), epithelial origin marker CK14 and T cell marker CD3 (0.41%). Overall, the data indicated that the isolated hDPSCs were mesenchymal and had multidifferentiation potential.
Fig. 1.
Charactrization of hDPSCs. (A) After pulp tissue culture for 3 days, spindle-shaped cells spread out in the culture flask. P3 generation cells were spindle-shaped. Mineralized nodules and lipid droplets were found when hDPSCs were cultured in osteogenic or adipogenic medium respectively. (B) Human dental pulp stem cells (hDPSCs) were positive for Vimentin、STRO-1、C-Kit and Nestin and negative for CK14. (C) Flow cytometric analyses showed that hDPSCs were positive for CD73 and CD90 and negative for CD3, CD31 and CD34. The experiments were performed in triplicates.
3.2. Effect of TDM on the odontogenic differentiation of hDPSCs
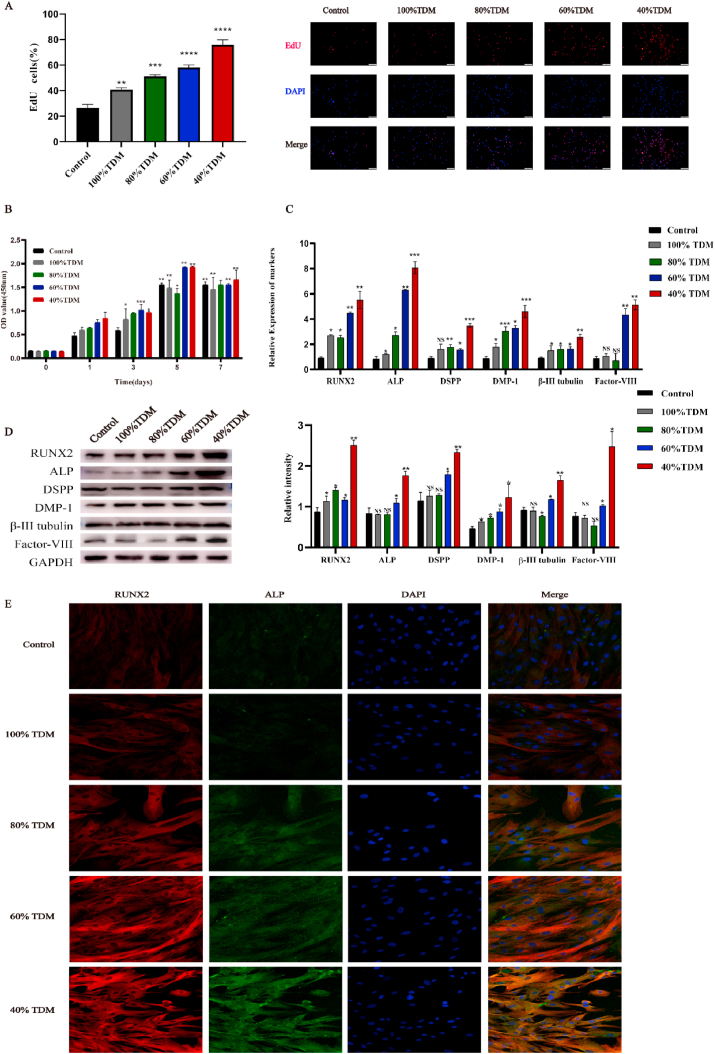
The effect of different TDM concentrations on the proliferation of hDPSCs was evaluated by EdU and CCK-8 assays. EdU assay revealed that after 48 h, the proportion of hDPSCs in proliferation phage in 40% TDM induced group was significantly higher than that in the control group, indicating that the cytotoxicity of 40% TDM was the least (Fig. 2A). CCK-8 assay showed that on day 1, the cell proliferation rate of each group was similar to that of the control group. However, over the next 5 days, the difference in cell proliferation rate increased. At 40%, 60% and 80% TDM concentrations, the proliferation rate of hDPSCs was higher than that of other concentrations. However, on day 7, the advantage of high concentration TDM was not significant. Overall, 40% and 60% TDM were the most favorable for cell growth (Fig. 2B).
Fig. 2.
Effect of different concentrations of TDM on hDPSCs. (A) The EdU assay clearly showed that 40% TDM group showed enhanced proliferation of hDPSCs. (B) CCK-8 assay corroborated these results. The expression of dentin-related genes and proteins induced by TDM was assessed by (C) q RT-PCR and (D) Western blot analysis. (E) Immunofluorescence was also performed in the context of dentin-related proteins.* means p < 0.05 and ** means p < 0.01 compared with the control group.
To explore the effect of TDM on odontogenic differentiation, We investigated genes and proteins expression of odontogenic markers, including Runx2, ALP, DSPP, DMP-1, Factor VIII, and β-III Tubulin. Of note, the groups treated with concentration lower than 40% (10% and 20%) were excluded from the analysis due to poor ability of tooth formation (Supplement Figure 1). The mRNA expression levels of the above mentione increased in TDM groups, with the highest level in 40% TDM group (Fig. 2C). Additionally, as per Western blotting assay, the expression levels of RUNX2, ALP, Factor VIII, and DSPP significantly increased in 40% TDM group (versus control group). Immunofluorescence were performed to detect the changes in the protein expression levels of several odontogenic markers in hDPSCs. The results showed that the expression levels of RUNX2 and ALP in the 40% groups were up-regulated significantly (Fig. 2E). As a result, 40% TDM was used in the subsequent experiments (TDM for short in the following experiments).
3.3. TDM-induction downregulates the expression of GSK3β during the odontogenic differentiation of hDPSCs
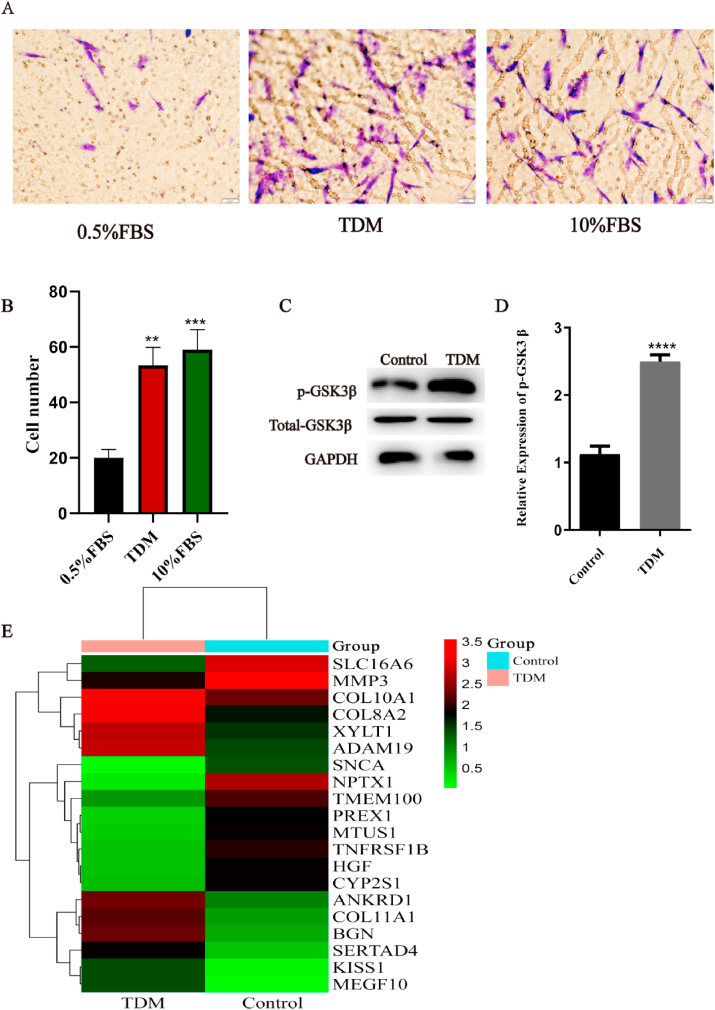
Migration of hDPSCs after TDM induction was detected by transwell assay. In the presence of the TDM, the motility of hDPSCs was similar to the positive control group (10% FBS), and significantly higher than the negative control group (0.5% FBS). These results suggest that TDM can induce cell migration and possibly cell aggregation and differentiation (Fig. 3A–B). Next, the gene and protein expression levels of GSK3β in these cells were detected by q RT-PCR and western blotting assay. As shown in Fig. 3C–D, the expression level of p-GSK3β increased while the total expression level of GSK3β remained stable during hDPSCs odontogenesis induced by TDM. Since total GSK3β consists of p-GSK3β and unphosphorylated GSK3β, our results indicate that the expression of unphosphorylated GSK3β decreased. In subsequent experiments, GSK3β specifically refers to unphosphorylated GSK3β. These findings suggest an correlation between the odontogenic differentiation of hDPSCs induced by TDM and the expression level of GSK3β.
Fig. 3.
Downregulation of the expression of GSK3β during TDM induced odontogenesis. hDPSCs odontogenesis was induced using treated dentin matrix (TDM). (A-B) Cell migration was as indicated and detected via the transwell assay. (C-D) The expression levels of p-GSK3β in hDPSCs treated or not with TDM induction was detected by Western blotting assay. (E) The differentially expressed genes between control group and TDM TDM-treated cells, as per RNA-seq analysis are shown in the heat map. * means p<0.05 and **means p<0.01 compared with the control group.
To further study the mechanism of formation of dentin-like tissue induced by TDM, we used RNA seq to detect the gene expression of hDPSCs between TDM and control groups. As the result, there were 253 up-regulated genes and 395 down-regulated genes in the TDM group. The significant genes are listed in Table 2 and Fig. 3E. We found that the expression of mineralization markers such as BGN, COL11A1, and COL10A1 were significantly increased [[30], [31], [32], [33]]. Similarly, the expression of vascular and neurological differentiation markers such as COL8A2 and ANKRD1 also increased to a certain extent [34,35]. Furthermore, these results demonstrated that the odontogenesis of hDPSCs was promoted by TDM induction. In addition, we used KEGG analysis to exhibit the changes of pathways. Specific pathways were listed in Table 3, and pathway enrichment was shown in Supplement Figure 2. We found that the Wnt/β-catenin signal pathway was one of the most significantly altered pathways directly related to osteogenesis. Moreover, consistent with our previous results, GSK3β expression was down-regulated (Fig. 3C–D). Therefore, we chose the Wnt/β-catenin signaling pathway for following research.
Table 2.
Important differentially expressed genes (DEGs) between control and TDM-treated cells.
| Gene symbol | log2FC | Gene feature | Description |
|---|---|---|---|
| BGN | 5.1005 | Up | Biglycan |
| COL8A2 | 4.7527 | Up | Collagen type VIII alpha 2 chain |
| ADAM19 | 4.6443 | Up | ADAM metallopeptidase domain 19 |
| COL11A1 | 4.5031 | Up | Collagen type XI alpha 1 chain |
| MEGF10 | 4.4955 | Up | Multiple EGF like domains 10 |
| SERTAD4 | 4.4933 | Up | SERTA domain containing 4 |
| ANKRD1 | 4.2948 | Up | Ankyrin repeat domain 1 |
| KISS1 | 4.2559 | Up | KiSS-1 metastasis-suppressor |
| COL10A1 | 4.2336 | Up | Collagen type X alpha 1 chain |
| XYLT1 | 4.1285 | Up | Xylosyltransferase 1 |
| NPTX1 | −8.18 | Down | Neuronal pentraxin 1 |
| SLC16A6 | −5.605 | Down | Solute carrier family 16 member 6 |
| MMP3 | −4.892 | Down | Matrix metallopeptidase 3 |
| TNFRSF1B | −4.784 | Down | TNF receptor superfamily member 1B |
| MTUS1 | −4.629 | Down | Microtubule associated scaffold protein 1 |
| PREX1 | −4.49 | Down | Phosphatidylinositol-3,4,5-trisphosphate dependent Rac exchange factor 1 |
| SNCA | −4.386 | Down | Synuclein alpha |
| HGF | −4.37 | Down | Hepatocyte growth factor |
| CYP2S1 | −4.209 | Down | Cytochrome P450 family 2 subfamily S member 1 |
| TMEM100 | −4.144 | Down | Transmembrane protein 100 |
Table 3.
Pathways impacted by TDM-treatment, as per KEGG analysis.
| KEGG pathway | Count | KEGG_ID/KO |
|---|---|---|
| Pathways in cancer | 14 | hsa:650|hsa:8322|hsa:332|hsa:3082|hsa:6387|hsa:1869|hsa:2737|hsa:8313|hsa:1870|hsa:51,176|hsa:1163|hsa:6932|hsa:2034|hsa:2252| |
| Basal cell carcinoma | 6 | hsa:650|hsa:8322|hsa:2737|hsa:8313|hsa:51,176|hsa:6932| |
| Wnt signaling pathway | 7 | hsa:8322|hsa:85407|hsa:8313|hsa:51176|hsa:27123|hsa:6932|hsa:5176| |
| Hippo signaling pathway | 7 | hsa:650|hsa:8322|hsa:85407|hsa:8313|hsa:51176|hsa:332|hsa:6932| |
| ECM-receptor interaction | 5 | hsa:3690|hsa:1292|hsa:3371|hsa:1291|hsa:6382| |
| Cytokine-cytokine receptor interaction | 8 | hsa:3592|hsa:650|hsa:55504|hsa:6387|hsa:3082|hsa:27242|hsa:7133|hsa:11009| |
| Protein digestion and absorption | 4 | hsa:1292|hsa:4311|hsa:1291|hsa:1294| |
| Mineral absorption | 3 | hsa:261,729|hsa:2495|hsa:2495| |
| PI3K-Akt signaling pathway | 7 | hsa:1291|hsa:3082|hsa:1292|hsa:3690|hsa:672|hsa:3371|hsa:2252| |
| Focal adhesion | 5 | hsa:3690|hsa:1292|hsa:3371|hsa:1291|hsa:3082| |
| Arrhythmogenic right ventricular cardiomyopathy (ARVC) | 3 | hsa:3690|hsa:51176|hsa:6932| |
| Small cell lung cancer | 3 | hsa:1870|hsa:1869|hsa:1163| |
| Rheumatoid arthritis | 3 | hsa:6387|hsa:245972|hsa:4314| |
| African trypanosomiasis | 2 | hsa:3592|hsa:2150| |
3.4. TDM regulates the odontogenic differentiation of hDPSCs via the inhibition of the expression of GSK3β
In the preliminary experiment of this research, we found activation of Wnt/β-catenin signaling pathway can promote odontogenic differentiation of hDPSCs (Supplement figure 3).
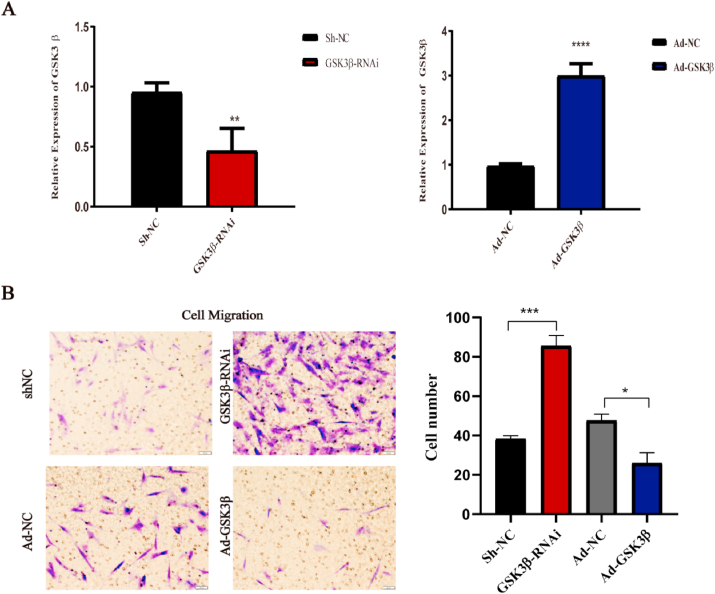
To further investigate the effect of TDM on hDPSCs, the GSK3β knockdown and overexpressing cells were generated. The transfection efficiency of GSK3β was verified by q RT-PCR (Fig. 4A). Cell migration experiments were performed to analyze the migration ability of hDPSCs. The results showed that the migration of hDPSCs increased after the down-regulation of GSK3β. Conversely, GSK3β overexpression (Ad-GSK3β) either decreased cell migration or had no effect compared to that on negative control cells (Ad-NC) (Fig. 4B). Hence, these findings demonstrate the importance of GSK3β in the TDM-induced odontogenic differentiation of hDPSCs.
Fig. 4.
Changes in the migration ability of DPSCs after transfection. (A) q RT-PCR verified the transfection efficiency of hDPSCs with GSK3β down-regulated or overexpressed. (B) Transwell test showed that the migration ability of hDPSCs changed after transfection. * means p < 0.05 and **means p < 0.01 compared with the control group.
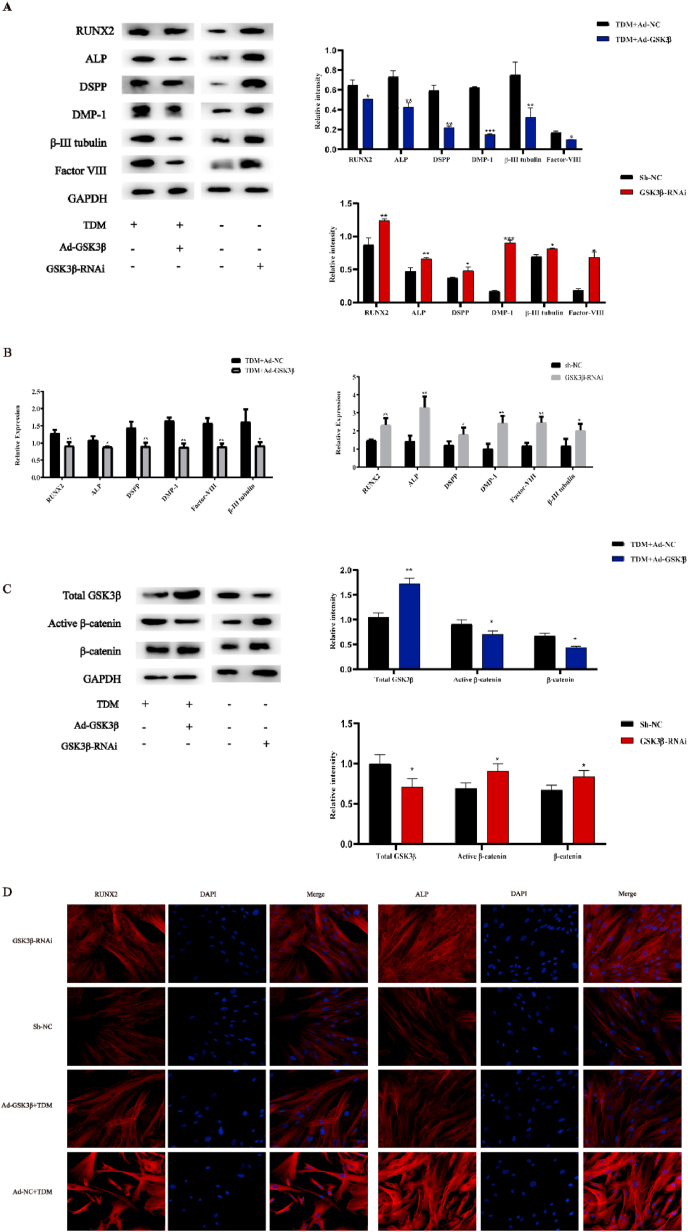
Next, q RT-PCR and western blotting assay were used to analyze the expression of odontogenic genes and proteins in GSK3β-RNAi and Sh-NC hDPSCs. The results of q RT-PCR showed that the expression of DMP-1, DSPP and RUNX2 was significantly increased in GSK3β-RNAi versus Sh-NC cells. Moreover, western blotting assay revealed that the expression levels of dentin-related proteins including DMP-1, DSPP, RUNX2, and Factor VIII were significantly increased. Also, as shown in Fig. 5 C, GSK3β and Active β-catenin expression changed as we expected. Overall, the results above suggest that GSK3β inhibition can activate the Wnt/β-catenin signaling pathway, which promotes the odontogenic differentiation of hDPSCs.
Fig. 5.
TDM regulates the odontogenic differentiation of hDPSCs via inhibition of the expression of GSK3β. The expression levels of odontogenic genes and proteins significantly increased in GSK3β-RNAi group compared with Sh-NC, as detected by (A) Western blotting assay and (B) q RT-PCR. Also, The expression of the odontogenic genes and proteins, including RUNX2, ALP, DSPP, DMP-1, β-III Tubulin and Factor VIII significantly decreased in Ad-GSKβ group compared with control group. (C) The expression of active β-catenin was significantly changed as expected in Ad- GSK3β group and GSK3β-RNAi group. (D) Immunofluorescence was also performed in the context of dentin-related proteins. * means p<0.05 and **means p<0.01 compared with the control group.
We also noticed that the expression of GSK3β decreased in hDPSCs induced by TDM compared with control cells (Fig. 3C–D). Therefore, Ad-GSK3β hDPSCs were cultured with TDM to determine the relationship between TDM-induced odontogenic differentiation and GSK3β expression. As expected, the expression of odontogenic genes and proteins, including RUNX2, ALP, DSPP, DMP-1, β-III Tubulin and Factor VIII significantly decreased in Ad-GSKβ group compared with control group (Fig. 5A–B). Of note, these result is consistent with the immunofluorescence results (Fig. 5 D). Overall, all these results indicate that TDM activates the Wnt/β-catenin signaling pathway by inhibiting GSK3β expression, thereby promoting dental differentiation of hDPSCs; the TDM induction of hDPSCs can be mostly eliminated by the inhibitory effect of GSK3β on hDPSCs.
3.5. Expression of GSK3β during TDM-induced odontogenic differentiation of hDPSCs
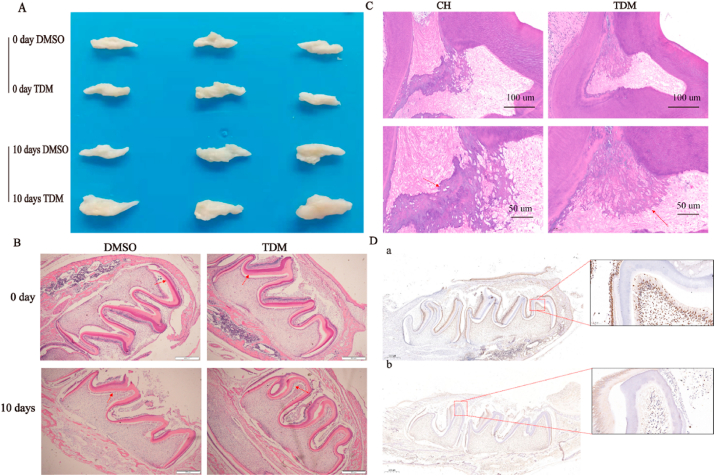
To investigate the expression changes of GSK3β during the TDM-induced odontogenic differentiation of hDPSCs, the mandibles of two-day-old rats were cultured with DMSO or TDM for 10 days (Fig. 6A). The results showed that new dentin was visible in the mandibles cultured with TDM and much thicker than that of the control group (Fig. 6B). In addition, immunohistochemical staining showed decreased GSK3β expression in the new dentin (Fig. 6D).
Fig. 6.
TDM is an effective matrix for dentin regeneration. (A)The mandibles of 2-day-old rats were cultured in the presence of DMSO or TDM for 0 day and 10 days. (B)H&E staining showed the new dentin was visible in the mandible cultured with TDM, much thicker than that of the control group (the arrow). (C)Histological analysis showed that restorative dentin formed in both groups. However, in the CH group, a portion of necrotic pulp tissue was observed between the restorative dentin and the primary dentin (the arrow). (D) In addition, immunohistochemistrycal staining showed decreased expression of GSK3β in newborn dentin (the arrow in D b, versus compared to D a).
To confirm the physiological role of TDM, cavities were prepared on the molars of rats and CH or TDM were used to cover the pulp. After 10 weeks, the molars were collected and subjected to H&E analysis. The results showed that restorative dentin formed in both groups (Fig. 6C). However, in the CH group, a portion of necrotic pulp tissue was observed between the restorative dentin and the primary dentin. Thus, our results provide evidence that both TDM and CH can be used for direct pulp capping, but CH isn't able to regenerate dentin.
3.6. Subcutaneous ectopic implantation in immunodeficient mice
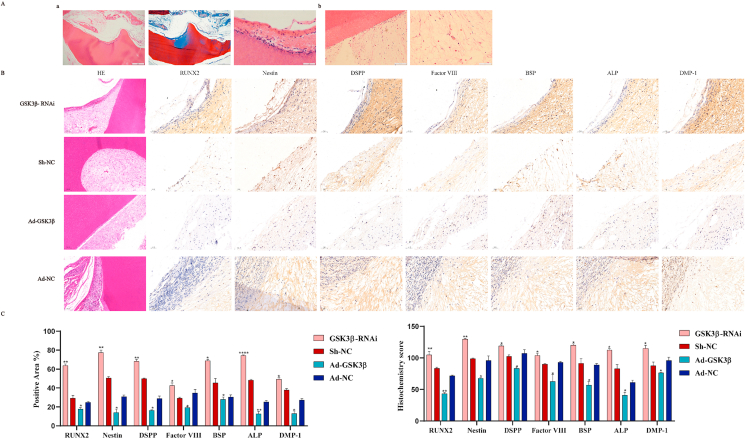
Our previous study found that TDM is a material with good biocompatibility, and the extract obtained by soaking contains a variety of growth factors [16]; in the preliminary experiment of this research, cell-free TDM was implanted subcutaneously into immunodeficient mice. After 10 weeks, no inflammatory reaction and restorative dentin layers were found around the implants (Supplement Figure 4). Therefore, next, we implanted TDM in combination with hDPSCs. Of note, immunodeficient mice recovered quickly after subcutaneous implantation of TDM combined with hDPSCs with low or overexpression of GSK3β.Moreover, there was no postoperative inflammatory response, indicating that TDM has good biocompatibility. H&E staining the transplanted cells adhered and proliferated well after TDM induction (Fig. 7). In the TDM group loaded with GSK3β-RNAi, restorative dentin layers formed, which was shallow and disorderly arranged. SEM and EDS confirmed that there were mineralized deposits on TDM surface in the GSK3β-RNAi group. The mineralized tissue was mainly composed of phosphate and calcium ions (Supplement Figure 5). In addition, after hDPSCs loading with TDM, the odontoblast-related proteins such as DMP-1, DSPP, RUNX2, and ALP were detected by immunohistology; the high expression of these proteins in GSK3β-RNAi group was not detected in Ad-GSK3β group. (Fig. 7B–C). The above results indicate that TDM may regulate odontogenic differentiation of hDPSCs via GSK3β; TDM can induce the odontogenic and neurogenic differentiation of hDPSCs.
Fig. 7.
Subcutaneous ectopic implantation in immunodeficient mice. (A)TDM combined with hDPSCs with low or overexpressed GSK3β were subcutaneously implanted in nude mice. (Aa) In the TDM group loaded with GSK3β-RNAi, restorative dentin layers formed, which was shallow and disorderly arranged. There was no such structure in other groups. However, some blood vessels newly formed in all groups. (Ab). (B) The odontoblast-related proteins such as DMP-1, DSPP, RUNX2, ALP, Nestin and Factor VIII were detected by immunohistology; the high expression of these proteins in GSK3β-RNAi group was not detected in Ad-GSK3β group. (C–D) The quantitative histochemical analysis was based the positive area and H-SCORE.
4. Discussion
Loss of teeth can negatively affect an individual's oral function and facial appearance. In theory, the best way to protect teeth is to preserve living pulp with drugs or various dentin-like matrix materials [1]. This study evaluated the regeneration potential of treated dentin matrix materials and explored the mechanism underlying their actions.
Specific tissue-derived extracellular matrix can be used as a culture substrate, under which cells can proliferate and differentiate into the parent tissue [[21], [38], [39]]. As an extracellular matrix material, TDM extract is rich in growth factors, such as transforming growth factor β, DMP-1 and DSPP, which can induce odontogenic differentiation of stem cells [8,[9], [36], [37]]. To study the TDM-induced odontogenic inducement of hDPSCs, we tested a series of TDM concentrations (10%, 20%, 40%, 60%, 80%, and 100%). All TDM leaching solution concentrations were proved to promote odontogenic differentiation of hDPSCs. Moreover, the lower TDM concentrations also induced odontogenic differentiation of hDPSCs, which may be related to their odontogenic origin [[22], [40]]. Nevertheless, only a few studies have explored the pathway modulations observed during odontogenic differentiation induced by TDM extract. Therefore, to understand the role of GSK3β during osteogenic differentiation of hDPSCs induced by TDM, a lentiviral system was established to suppress or overexpress GSK3β in hDPSCs. We observed that the expression levels of odontogenic protein and mRNA significantly increased in GSK3β-RNAi combined hDPSCs after the induction of TDM extract, compared with the control group. On the contrary, overexpressed GSK3β combined hDPSCs after the induction of TDM extract led to decreased expression levels of RUNX2, ALP, DSPP, and DMP-1. These results highlight the importance of the Wnt/β-catenin signaling pathway in GSK3β-mediated odontogenic differentiation of hDPSCs. This was further confirmed in immunodeficient mice with ectopic odontogenesis. Next, we examined the expression of GSK3β in molars of rats. As expected, GSK3β suppression was observed in the odontoblast cells 10 days after TDM culture. This was consistent with the results of normal molar development model in rats [[23], [41]], suggesting that GSK3β is involved odontogenic differentiation and dentin development of hDPSCs with TDM induction.
Activation of the canonical Wnt/β-catenin pathway requires stabilization and protection of β-catenin by inhibiting GSK3β. This promotes the accumulation of β-catenin into the nucleus, where it exerts its effects in combination with lymphoid enhancement factors and T cell factor (LEF/TCF) [19,20]. Furthermore, we noted that the GSK3β expression decreased during odontogenesis of hDPSCs induced by TDM. Therefore, the reasonable conclusion is that TDM facilitates osteogenic differentiation of hDPSCs by regulating GSK3β expression. Moreover, our results showed that the TDM-induced hDPSCs strongly expressed p-GSK3β and active β-catenin, which enhanced their translocation to the nucleus. These findings indicate that the TDM extract activates the canonical Wnt/β-catenin pathway to exert its effect. Interestingly, the relationship between the Wnt/β-catenin signaling pathway and dentin regeneration remains controversial. The activation of the Wnt/β-catenin signaling pathway inhibits the odontogenic differentiation of stem cells and promotes their proliferation. Scheller et al. demonstrated that the canonical Wnt/β-catenin signaling pathway inhibits the expression of odontoblast-specific genes during odontogenic differentiation of hDPSCs [[24], [42]]. Moreover, Wnt10a, a Wnt agonist, was reported to negatively regulate odontoblastic differentiation of hDPSCs [15,[25], [43]]. Conversely, other studies have shown that the Wnt/β-catenin signaling pathway can promote the odontogenic differentiation of the hDPSCs. The importance of β-catenin has been revealed in several studies [[30], [31], [32]]. β-catenin is strongly expressed in odontoblasts and is necessary for root formation [26,[27], [44]]. Shan et al. demonstrated that lithium chloride, the Wnt/β-catenin pathway activator, can promote the proliferation and odontoblast differentiation of hair follicle neural crest cells [[28], [29], [30], [45]]. In the present study, the expression of GSK3β decreased in TDM-induced hDPSCs, compared with the control group.
The limitation of this study is that we only focused on the effect of TDM extract on odontogenic stem cells and therefore, further research is warranted to investigate the mechanism underlying of TDM-induced differentiation of non-odontogenic cells. In addition, the correlation between the proteins, transcriptomic changes and epigenetic regulation in the odontogenic differentiation of hDPSCs induced by TDM should be further evaluated.
Ethical approval
The study was approved by the Medical Ethics Committee of the First Affiliated Hospital of Zhengzhou University, and was performed according to the institutional guidelines.
Author contribution
Sirui Liu: Conceptualization, Methodology, Software, Jingjing Sun: Validation, Data curation, Shuai Yuan: Writing – original draft, Yanyu Yang:Visualization, Investigation. Yuping Gong, Supervision. Ying Wang, Supervision. and Runying Guo: Supervision. Xue Zhang, Software and Validation, Yiming Liu, Software and Validation, Hongyan Mi; Software and Validation, Meiyue Wang; Software and Validation, Mengzhe Liu: Software and Validation, Rui Li: Writing- Reviewing and Editing
Declaration of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was funded by the National Natural Science Foundation of China (grant numbers 31670994, U1904145, and 81901039), Nature Science Foundation of Henan province (grant number 182300410340), and Union project of Medical and Technology Research Program of Henan Province (grant number LHGJ20190191).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2021.05.026.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Eramo S., Natali A., Pinna R., Milia E. Dental pulp regeneration via cell homing. Int. Endod. J. 2018;51:405–419. doi: 10.1111/iej.12868. [DOI] [PubMed] [Google Scholar]
- 2.Chaussain-Miller C., Fioretti F., Goldberg M., Menashi S. The role of matrix metalloproteinases (MMPs) in human caries. J. Dent. Res. 2006;85:22–32. doi: 10.1177/154405910608500104. [DOI] [PubMed] [Google Scholar]
- 3.Sabbagh J., Ghassibe-Sabbagh M., Fayyad-Kazan M., Al-Nemer F., Fahed J.C., Berberi A., Badran B. Differences in osteogenic and odontogenic differentiation potential of DPSCs and SHED. J. Dent. 2020;101 doi: 10.1016/j.jdent.2020.103413. [DOI] [PubMed] [Google Scholar]
- 4.Gopinath V.K., Soumya S., Jayakumar M.N. Osteogenic and odontogenic differentiation potential of dental pulp stem cells isolated from inflamed dental pulp tissues (I-DPSCs) by two different methods. Acta Odontol. Scand. 2020;78:281–289. doi: 10.1080/00016357.2019.1702716. [DOI] [PubMed] [Google Scholar]
- 5.Ching H.S., Luddin N., Rahman I.A., Ponnuraj K.T. Expression of odontogenic and osteogenic markers in DPSCs and SHED: a review. Curr. Stem Cell Res. Ther. 2017;12:71–79. doi: 10.2174/1574888x11666160815095733. [DOI] [PubMed] [Google Scholar]
- 6.Nuti N., Corallo C., Chan B.M.F., Ferrari M., Gerami-Naini B. Multipotent differentiation of human dental pulp stem cells: a literature review. Stem Cell Rev. Rep. 2016;12:511–523. doi: 10.1007/s12015-016-9661-9. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L., Yu Y., Feng K.-C., Chuang Y.-C., Zuo X., Zhou Y., Chang C.-C., Simon M., Rafailovich M. Templated dentin formation by dental pulp stem cells on banded collagen bundles nucleated on electrospun poly (4-vinyl pyridine) fibers in vitro. Acta Biomater. 2018;76:80–88. doi: 10.1016/j.actbio.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 8.Katge F.A., Patil D.P. Comparative analysis of 2 calcium silicate-based cements (biodentine and mineral trioxide aggregate) as direct pulp-capping agent in young permanent molars: a split mouth study. J. Endod. 2017;43:507–513. doi: 10.1016/j.joen.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 9.Dammaschke T., Leidinger T.J., Schafer E. Long-term evaluation of direct pulp capping--treatment outcomes over an average period of 6.1 years. Clin. Oral Invest. 2010;14:559–567. doi: 10.1007/s00784-009-0326-9. [DOI] [PubMed] [Google Scholar]
- 10.Chuang Y.-C., Yu Y., Wei M.-T., Chang C.-C., Ricotta V., Feng K.-C., Wang L., Bherwani A.K., Ou-Yang H.D., Simon M., Zhang L., Rafailovich M. Regulating substrate mechanics to achieve odontogenic differentiation for dental pulp stem cells on TiO2 filled and unfilled polyisoprene. Acta Biomater. 2019;89:60–72. doi: 10.1016/j.actbio.2019.02.040. [DOI] [PubMed] [Google Scholar]
- 11.Wang L., Fu H., Wang W., Liu Y., Li X., Yang J., Li L., Wu G., Pan Y. Notoginsenoside R1 functionalized gelatin hydrogels to promote reparative dentinogenesis. Acta Biomater. 2021;122:160–171. doi: 10.1016/j.actbio.2020.12.031. [DOI] [PubMed] [Google Scholar]
- 12.Li J., Yu Y., Myungwoong K., Li K., Mikhail J., Zhang L., Chang C.-C., Gersappe D., Simon M., Ober C., Rafailovich M. Manipulation of cell adhesion and dynamics using RGD functionalized polymers. J. Mater. Chem. B. 2017;5:6307–6316. doi: 10.1039/c7tb01209h. [DOI] [PubMed] [Google Scholar]
- 13.Li J., Yu Y., Kim M., Li K., Mikhail J., Zhang L., Chang C.-C., Gersappe D., Simon M., Ober C., Rafailovich M. Correction: manipulation of cell adhesion and dynamics using RGD functionalized polymers. J. Mater. Chem. B. 2017;5:6973. doi: 10.1039/c7tb90117h. [DOI] [PubMed] [Google Scholar]
- 14.Anneroth G., Bang G. The effect of allogeneic demineralized dentin as a pulp capping agent in Java monkeys. Odontol. Revy. 1972;23:315–328. [PubMed] [Google Scholar]
- 15.Tabatabaei F.S., Ai J., Kashi T.S.J., Khazaei M., Kajbafzadeh A.-M., Ghanbari Z. Effect of dentine matrix proteins on human endometrial adult stem-like cells: in vitro regeneration of odontoblasts cells. Arch. Oral Biol. 2013;58:871–879. doi: 10.1016/j.archoralbio.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Luo S., Pei F., Zhang W., Guo W., Li R., He W., Tian W. Bone marrow mesenchymal stem cells combine with treated dentin matrix to build biological root. Sci. Rep. 2017;7 doi: 10.1038/srep44635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li R., Guo W., Yang B., Guo L., Sheng L., Chen G., Li Y., Zou Q., Xie D., An X., Chen Y., Tian W. Human treated dentin matrix as a natural scaffold for complete human dentin tissue regeneration. Biomaterials. 2011;32:4525–4538. doi: 10.1016/j.biomaterials.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Su Y., Wen J., Zhu J., Xie Z., Liu C., Ma C., Zhang Q., Xu X., Wu X. Pre-aggregation of scalp progenitor dermal and epidermal stem cells activates the WNT pathway and promotes hair follicle formation in in vitro and in vivo systems. Stem Cell Res. Ther. 2019;10:403. doi: 10.1186/s13287-019-1504-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng L., Hong H., Zhang X., Chen D., Chen Z., Ling J., Wu L. Down-regulated lncRNA MEG3 promotes osteogenic differentiation of human dental follicle stem cells by epigenetically regulating Wnt pathway. Biochem. Biophys. Res. Commun. 2018;503:2061–2067. doi: 10.1016/j.bbrc.2018.07.160. [DOI] [PubMed] [Google Scholar]
- 20.Yang Y., Ge Y., Chen G., Yan Z., Yu M., Feng L., Jiang Z., Guo W., Tian W. Hertwig's epithelial root sheath cells regulate osteogenic differentiation of dental follicle cells through the Wnt pathway. Bone. 2014;63:158–165. doi: 10.1016/j.bone.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Lybrand D.B., Naiman M., Laumann J.M., Boardman M., Petshow S., Hansen K., Scott G., Wehrli M. Destruction complex dynamics: Wnt/beta-catenin signaling alters Axin-GSK3beta interactions in vivo. Development. 2019;146 doi: 10.1242/dev.164145. dev164145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim T.H., Bae C.H., Lee J.C., Ko S.O., Yang X., Jiang R., Cho E.S. β-catenin is required in odontoblasts for tooth root formation. J. Dent. Res. 2013;92:215–221. doi: 10.1177/0022034512470137. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H., Wang J., Deng F., Huang E., Yan Z., Wang Z., Deng Y., Zhang Q., Zhang Z., Ye J., Qiao M., Li R., Wang J., Wei Q., Zhou G., Luu H.H., Haydon R.C., He T.-C., Deng F. Canonical Wnt signaling acts synergistically on BMP9-induced osteo/odontoblastic differentiation of stem cells of dental apical papilla (SCAPs) Biomaterials. 2015;39:145–154. doi: 10.1016/j.biomaterials.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bae C.H., Kim T.H., Ko S.O., Lee J.C., Yang X., Cho E.S. Wntless regulates dentin apposition and root elongation in the mandibular molar. J. Dent. Res. 2015;94:439–445. doi: 10.1177/0022034514567198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carreira-Barbosa F., Nunes S.C. Wnt signaling: paths for cancer progression. Adv. Exp. Med. Biol. 2020;1219:189–202. doi: 10.1007/978-3-030-34025-4_10. [DOI] [PubMed] [Google Scholar]
- 26.Chang C.-C., Lin T.-A., Wu S.-Y., Lin C.-P., Chang H.-H. Regeneration of tooth with allogenous, autoclaved treated dentin matrix with dental pulpal stem cells: an in vivo study. J. Endod. 2020;46:1256–1264. doi: 10.1016/j.joen.2020.05.016. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y., Yuan S., Sun J., Gong Y., Liu S., Guo R., He W., Kang P., Li R. Inhibitory effect of the TSG-6 on the BMP-4/Smad signaling pathway and odonto/osteogenic differentiation of dental pulp stem cells. Biomed. Pharmacother. 2020;128 doi: 10.1016/j.biopha.2020.110266. 110266. [DOI] [PubMed] [Google Scholar]
- 28.Chen J., Cui C., Qiao X., Yang B., Yu M., Guo W., Tian W. Treated dentin matrix paste as a novel pulp capping agent for dentin regeneration. J. Tissue Eng. Regen. Med. 2017;11:3428–3436. doi: 10.1002/term.2256. [DOI] [PubMed] [Google Scholar]
- 29.Yang Y., Li Z., Chen G., Li J., Li H., Yu M., Zhang W., Guo W., Tian W. GSK3beta regulates ameloblast differentiation via Wnt and TGF-beta pathways. J. Cell. Physiol. 2018;233:5322–5333. doi: 10.1002/jcp.26344. [DOI] [PubMed] [Google Scholar]
- 30.Yin Y., Zhou N., Zhang H., Dai X., Lv X., Chen N., Miao D., Hu Q. Bmi1 regulate tooth and mandible development by inhibiting p16 signal pathway. J. Cell Mol. Med. 2021:4195–4203. doi: 10.1111/jcmm.16468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He S.-K., Ning L.-J., Yao X., Hu R.-N., Cui J., Zhang Y., Ding W., Luo J.-C., Qin T.-W. Hierarchically demineralized cortical bone combined with stem cell-derived extracellular matrix for regeneration of the tendon-bone interface. Am. J. Sports Med. 2021 doi: 10.1177/0363546521994511. [DOI] [PubMed] [Google Scholar]
- 32.Liu Q., Li X.J., Wang Z.S. [Effects of porcine acellular cartilaginous matrix on the proliferation and differentiation of human adipose-derived stromal cells] Hua xi kou qiang yi xue za zhi. 2020;38:122–127. doi: 10.7518/hxkq.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richardson B.M., Walker C.J., Maples M.M., Randolph M.A., Bryant S.J., Anseth K.S. Mechanobiological interactions between dynamic compressive loading and viscoelasticity on chondrocytes in hydrazone covalent adaptable networks for cartilage tissue engineering. Adv. Healthc. Mater. 2021 doi: 10.1002/adhm.202002030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bao H., Li Z.-T., Xu L.-H., Su T.-Y., Han Y., Bao M., Liu Z., Fan Y.-J., Lou Y., Chen Y., Jiang Z.-L., Gong X.-B., Qi Y.-X. Platelet-derived extracellular vesicles increase Col8a1 secretion and vascular stiffness in intimal injury. Front. Cell. Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.641763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Truve K., Parris T.Z., Vizlin-Hodzic D., Salmela S., Berger E., Ågren H., Funa K. Identification of candidate genetic variants and altered protein expression in neural stem and mature neural cells support altered microtubule function to be an essential component in bipolar disorder, Transl. Psychiatry. 2020;10:390. doi: 10.1038/s41398-020-01056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holiel A.A., Mahmoud E.M., Abdel-Fattah W.M., Kawana K.Y. Histological evaluation of the regenerative potential of a novel treated dentin matrix hydrogel in direct pulp capping. Clin. Oral Invest. 2021;25:2101–2112. doi: 10.1007/s00784-020-03521-z. [DOI] [PubMed] [Google Scholar]
- 37.Bakhtiar H., Mazidi A., Mohammadi-Asl S., Hasannia S., Ellini M.R., Pezeshki-Modaress M., Ostad S.N., Galler K., Azarpazhooh A., Kishen A. Potential of treated dentin matrix xenograft for dentin-pulp tissue engineering. J. Endod. 2020;46:57–64.e1. doi: 10.1016/j.joen.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Stern M.M., Myers R.L., Hammam N., Stern K.A., Eberli D., Kritchevsky S.B., Soker S., Dyke M.V. The influence of extracellular matrix derived from skeletal muscle tissue on the proliferation and differentiation of myogenic progenitor cells ex vivo. Biomaterials. 2009;30:2393–2399. doi: 10.1016/j.biomaterials.2008.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun J., Li J., Li H., Yang H., Chen J., Yang B., Huo F., Guo W., Tian W. tBHQ suppresses osteoclastic resorption in xenogeneic-treated dentin matrix-based scaffolds. Adv. Healthc. Mater. 2017;6 doi: 10.1002/adhm.201700127. [DOI] [PubMed] [Google Scholar]
- 40.Moriguchi M., Yamada M., Miake Y., Yanagisawa T. Immunolocalization of TAK1, TAB1, and p38 in the developing rat molar. Anat. Sci. Int. 2011;86:69–77. doi: 10.1007/s12565-010-0089-z. [DOI] [PubMed] [Google Scholar]
- 41.Scheller E.L., Chang J., Wang C.Y. Wnt/beta-catenin inhibits dental pulp stem cell differentiation. J. Dent. Res. 2008;87:126–130. doi: 10.1177/154405910808700206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baksh D., Boland G.M., Tuan R.S. Cross-talk between Wnt signaling pathways in human mesenchymal stem cells leads to functional antagonism during osteogenic differentiation. J. Cell. Biochem. 2007;101:1109–1124. doi: 10.1002/jcb.21097. [DOI] [PubMed] [Google Scholar]
- 43.Qiu W., Hu Y., Andersen T.E., Jafari A., Li N., Chen W., Kassem M. Tumor necrosis factor receptor superfamily member 19 (TNFRSF19) regulates differentiation fate of human mesenchymal (stromal) stem cells through canonical Wnt signaling and C/EBP. J. Biol. Chem. 2010;285:14438–14449. doi: 10.1074/jbc.M109.052001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han N., Zheng Y., Li R., Li X., Zhou M., Niu Y., Zhang Q. β-catenin enhances odontoblastic differentiation of dental pulp cells through activation of Runx2. PloS One. 2014;9 doi: 10.1371/journal.pone.0088890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shan T., Zhou C., Yang R., Yan F., Zhang P., Fu Y., Jiang H. Lithium chloride promotes the odontoblast differentiation of hair follicle neural crest cells by activating Wnt/beta-catenin signaling. Cell Biol. Int. 2005;39:35–43. doi: 10.1002/cbin.10340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.