Highlights
-
•
Our study investigated the safety and tolerability of palliative radiation therapy (RT) with concurrent Dabrafenib and Trametinib in patients with metastatic BRAF mutant melanoma, and we found that none of the patients enrolled in this study experienced grade 3 or 4 RT related toxicities.
-
•
We suggest that Dabrafenib and Trametinib may be safely used concurrently with common palliative RT doses to extracranial sites.
Keywords: Melanoma, Radiation therapy, BRAF inhibitors, MEK inhibitors, Dabrafenib, Trametinib, Skin toxicity
Abstract
Background
Concurrent treatment with BRAF inhibitors and palliative radiation therapy (RT) could be associated with increased toxicity, especially skin toxicity. Current Eastern Cooperative Oncology Group (ECOG) consensus guideline recommend ceasing BRAF inhibitors during RT. There is a lack of data regarding concurrent RT with combined BRAF and MEK inhibitors. This single-arm phase I/II trial was designed to assess the safety and tolerability of palliative RT with concurrent Dabrafenib and Trametinib in patients with BRAF-mutant metastatic melanoma.
Materials and methods
Patients received Dabrafenib and Trametinib before and during palliative RT to soft tissue, nodal or bony metastases. The RT dose was escalated stepwise during the study period. Toxicity data including clinical photographs of the irradiated area was collected for up to 12 months following completion of RT.
Results
Between June 2016 to October 2019, ten patients were enrolled before the study was stopped early due to low accrual rate. Six patients were treated at level 1 (20 Gy in 5 fractions, any location) and 4 patients at level 2a (30 Gy in 10 fractions with no abdominal viscera exposed). All alive patients completed one year of post-RT follow-up. Of the 82 adverse events (AEs) documented, the majority (90%) were grade 1 and 2. Eight grade 3 events (10%) occurred in five patients, only one was treatment-related (grade 3 fever due to Dabrafenib and Trametinib). No patients experienced grade 3 or 4 RT related toxicities, including skin toxicities. One serious AE was documented in relation to a grade 3 fever due to Dabrafenib and Trametinib requiring hospitalisation.
Conclusions
The lack of grade 3 and 4 RT-related toxicities in our study suggests that Dabrafenib and Trametinib may be continued concurrently during fractionated non-visceral palliative RT to extracranial sites.
Introduction
BRAF mutations have been identified in approximately half of patients with metastatic cutaneous melanoma [1], and BRAF inhibitors represented a crucial targeted treatment for BRAF mutant advanced stage melanoma over the last decade. Palliative radiation therapy (RT) can also be of benefit for patients with metastatic melanoma and symptomatic disease involving bone, soft tissue or viscera. However, concurrent use of BRAF inhibitors during RT was associated with increased acute radiation toxicity, especially involving the skin [2], [3], [4]. A consensus guideline from the Eastern Cooperative Oncology Group (ECOG) [5] recommended withholding BRAF inhibitors for at least 3 days before and after fractionated RT.
The addition of MEK inhibitors to BRAF inhibitors provide more effective inhibition of the RAS/RAF/MEK proliferation pathway. In a phase III randomised controlled trial [6], the combination of Dabrafenib and Trametinib improved survival compared to Dabrafenib alone and also reduced skin toxicities including dry skin, pruritis, alopecia, hand-foot syndrome, hyperkeratosis, skin papilloma and cutaneous squamous-cell carcinoma. In this context, there is a paucity of data regarding the safety of RT with concurrent combined BRAF and MEK inhibitors. Withholding BRAF inhibitors for prolonged period may lead to tumour flare up, as there are some preclinical and clinical data indicating antiproliferative effects of continued mitogen-activated protein kinase pathway inhibition following acquired resistance to BRAF and/or MEK inhibition in melanoma [7], [8]. We therefore designed a single-arm phase I/II trial to assess the toxicity of palliative RT with concurrent Dabrafenib and Trametinib in patients with metastatic BRAF mutant melanoma.
Materials and methods
This was a multi-centre, prospective, open-label, phase I/II study. The study protocol was approved by the St Vincent’s Hospital Human Research Ethics Committee, New South Wales (Approval Number HREC/15/SVH/36). The study was developed under the auspices of Melanoma and Skin Cancer Trials Ltd (Protocol Number 02.14). The ClinicalTrials.gov registration number is NCT02392871.
Patients
Patients aged 18 years or older with either unresectable stage III or stage IV BRAF V600E/K mutation-positive cutaneous melanoma, and symptomatic or bulky (>2cm) soft tissue, nodal or bony metastases requiring palliative RT were eligible. An ECOG performance status between 0 and 2 was required. Exclusion criteria included overlap of planned RT with previously irradiated volumes, symptomatic brain metastases or brain metastases treated less than 3 months prior to the study treatment. Patients requiring concurrent RT with immune check point inhibitors were also excluded. Patients who had isolated areas of progression on Dabrafenib and Trametinib requiring palliative RT were eligible for this study, although patients with clear evidence of widespread systemic disease progression on Dabrafenib and Trametinib were excluded.
Dabrafenib and trametinib
Patients received a combination of oral Dabrafenib (150 mg twice daily) and oral Trametinib (2 mg once daily), at least 2 weeks prior to and during RT. Drug treatment continued until further disease progression according to RECIST 1.1 criteria. Compliance with Dabrafenib and Trametinib was assessed by querying the patients at each visit throughout the study period.
Radiotherapy
RT was delivered to up to 3 sites simultaneously. Simple field arrangements using parallel opposed fields or 3D conformal technique were encouraged, reflecting standard practice at the time of the study, but intensity modulated RT (IMRT) was permitted. Gross tumour volume (GTV) was contoured on the planning CT scan and a planning target volume (PTV) was generated to account for uncertainties associated with treatment. GTV to PTV expansion margin was at the discretion of the treating radiation oncologist, with a protocol recommendation of 1 cm. The aim was for at least 95% of the PTV to receive 95% of the prescribed dose, although a reduction to 90% was permitted to respect standard constraints for normal tissues.
Toxicity assessment and radiation dose escalation
During each clinic visit, patients were reviewed systemically, and any adverse events (AE) were carefully documented. Toxicities were assessed using Common Terminology Criteria for Adverse Events (CTCAE Version 3.0). Dose limiting toxicity (DLT) was defined as any grade 4 skin reactions or any other CTCAE grade 3 or above toxicity, felt to be related to RT, in that it occurred within or in the vicinity of the irradiated volume.
The RT dose delivered with concurrent Dabrafenib and Trametinib was escalated according to a standard 3 + 3 protocol, as follows:
-
•
Level 1: 20 Gy in 5 fractions, to any location
-
•
Level 2a: 30 Gy in 10 fractions, with no abdominal viscera directly exposed to RT
-
•
Level 2b: 30 Gy in 10 fractions, with abdominal viscera directly exposed to RT
-
•
Level 3a: 40 Gy in 16 fractions, with no abdominal viscera directly exposed to RT
-
•
Level 3b: 40 Gy in 16 fractions, with abdominal viscera directly exposed to RT
RT dose was escalated to the next dose level if zero out of 3 patients at the current dose level experienced a DLT and had completed at least 4 weeks follow up post-RT. If one out of 3 patients experienced DLT, 3 more patients were treated at the same dose level. If two or more patients experienced DLT, dose escalation was stopped. The clinician had discretion to prescribe a dose of palliative RT lower than the current dose level if it had already been deemed safe by the Trial Management Committee (TMC).
Patient follow-up and evaluation
Patients were reviewed weekly during RT, then weekly until week 4 post-RT, then 8-weekly until study completion at 12 months post-RT. Blood test and CT Imaging was performed at baseline, then at week 4 post-RT, then 8-weekly prior to each visit. Treatment response was assessed using Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1 [9]. Local tumour response and the time to local progression in the irradiated lesion was evaluated, if these lesions were measurable by RECIST 1.1 criteria. Clinical photographs of the irradiated area were taken at each clinic visit.
Statistics
Summary statistics by CTCAE version 3 term and grade were used to describe safety and tolerability of the combination of Dabrafenib and Trametinib with palliative RT. The causes of adverse events were assessed by the investigators, and attributed to either RT, Dabrafenib and Trametinib, underlying melanoma, or pre-existing and unrelated medical conditions. Secondary end points included progression free survival and overall survival which were summarised using Kaplan-Meier survival plots.
Results
From June 2016 to October 2019, 10 patients were enrolled in the study. The median age was 65 years (range 32–91). All patients had stage IV melanoma. Baseline characteristics are outlined in Table 1. All patients received their planned RT; six patients were treated on dose level 1 (20 Gy in 5 fractions) and 4 patients were treated on dose level 2a (30 Gy in 10 fractions). All patients received combination of oral Dabrafenib (150 mg twice daily) and oral Trametinib (2 mg once daily), the duration of Dabrafenib and Trametinib prior to RT ranged from 2 weeks to 42 weeks, with a median of 6 weeks. Nine of ten patients had concurrent Dabrafenib and Trametinib throughout their course of RT. In one patient, Dabrafenib and Trametinib had to be stopped temporarily for 3 treatment days due to grade 3 fever, on fractions 6–8 of 10.
Table 1.
Baseline characteristics.
| Characteristic | Number or site |
|---|---|
| Median age (range), years | 65 (32, 91) |
| Gender | |
| Female: Male | 2:8 |
| ECOG performance status | |
| 0 | 4 |
| 1 | 4 |
| 2 | 2 |
| RT dose levels | |
| Level 1 (20 Gy in 5 fractions) | 6 |
| Level 2a (30 Gy in 10 fractions) | 4 |
| RT technique | |
| Anterior Posterior-Posterior Anterior | 1 |
| 3D Conformal Technique | 7 |
| Intensity Modulated Radiation Therapy | 2 |
| Site of palliative radiotherapy | |
| RT dose Level 1 | Right humerus |
| Right shoulder, left groin | |
| Left posterior neck | |
| T5-T9 vertebrae | |
| Lumbar spine | |
| Right ilium and L1 vertebra | |
| RT dose Level 2a | Right axilla |
| Left groin | |
| Right axilla | |
| Right parotid | |
All patients completed 12 months of post-RT follow up or died prior, and median follow up was 8.3 months. Within the follow up period, the collection of data on toxicity is complete. In total, 82 AEs were reported (Table 2, supplementary material): 74 (90%) grade 1 or 2 AEs were documented in 9 out of 10 patients, 8 (10%) grade 3 or higher AEs were documented in 5 patients.
With regard to grade 3 or higher AEs, one patient with pre-existing hypertension had three episodes of grade 3 hypertension during the study period. One patient had one episode of grade 3 pain relating to progression of underlying metastatic melanoma 12 months after the initial palliative RT. One patient had skin ulceration due to fungating tumour in the left groin, representing local disease progression 5 months after palliative RT at a dose of 30 Gy in 10 fractions. One patient experienced grade 3 fever, attributed to Dabrafenib and Trametinib; this resulted in hospitalization and was reported as a serious adverse event (SAE). One patient had both grade 3 febrile neutropenia and grade 3 functional decline, both attributed to underlying disease progression. No grade 3 or higher RT related toxicity was documented in any of the patients, and therefore no DLT was encountered.
The maximum radiation dose to the skin was assessed using thermoluminescent dosimeter in 5 out of the 10 patients, and was estimated from the treatment planning system in the remaining 5 patients. For the 5 cases where the skin dose was estimated from the treatment planning system, inner body rings of 5 mm was taken as surrogates of skin, and maximum point dose was documented. For the 6 patients who received 20 Gy in 5 fractions, the maximum dose to the skin were 12 Gy, 14.1 Gy, 15.0 Gy, 19.1 Gy, 21.6 Gy and 23.7 Gy. For the 4 patients who received 30 Gy in 10 fractions, the maximum dose to the skin were 19.6 Gy, 21.5 Gy, 31.2 Gy and 32.5 Gy. The maximum dose to the skin exceeded 95% of the prescribed dose in 4 out of 10 patients.
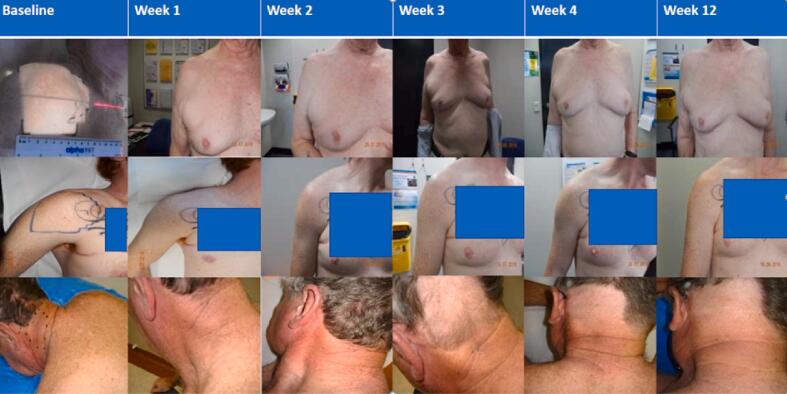
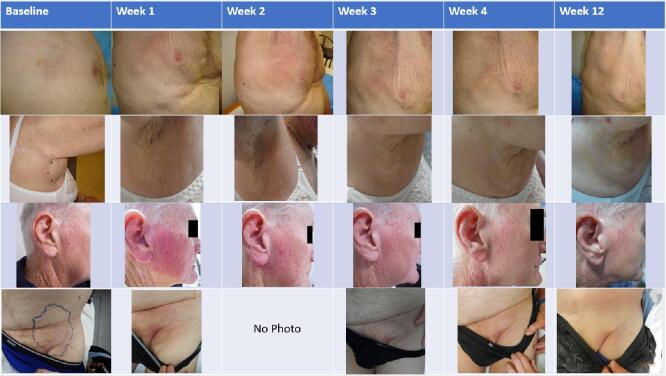
Immediate and delayed post-RT skin toxicity is summarised in Table 3. No patients experienced grade 3 or higher RT related toxicities, including skin toxicities. Grade 2 skin toxicity occurred in 3 patients (grade 2 maculopapular rash in one level 1 patient who received 20 Gy in 5 fractions, and grade 2 radiation dermatitis in two level 2a patients who received 30 Gy in 10 fractions). The acute skin toxicity appears to peak at 1 week post RT, only 2 patients still have residual G1 radiation dermatitis at week 4 post RT, and none of the patients had residual skin toxicity at week 12 post RT. Late skin toxicity (>12 weeks post-RT) was noted in 2 patients, with grade 1 hyperpigmentation and superficial soft tissue fibrosis in one patient and grade 1 superficial soft tissue fibrosis in another. Fig. 1, Fig. 2 show examples of clinical photographs of the irradiated areas for patients in level 1 and level 2a respectively, up to 12 weeks post RT.
Table 3.
RT related immediate and delayed skin toxicity (all Grade 1 or 2).
| Characteristic | Immediate (<3 months) |
Delayed (>3 months) |
||
|---|---|---|---|---|
| # Participants (N = 10) | # Events | # Participants (N = 10) | # Events | |
| Rash maculo-papular | 5 | 7 | 0 | 0 |
| Skin atrophy | 1 | 2 | 0 | 0 |
| Dermatitis radiation | 8 | 22 | 0 | 0 |
| Skin hyperpigmentation | 1 | 2 | 1 | 2 |
| Superficial soft tissue fibrosis | 2 | 2 | 2 | 2 |
Fig. 1.
Clinical photographs of irradiated area for select patients on dose level I. *Identifying tattoo has been obscured for one patient.
Fig. 2.
Clinical photographs of irradiated area for patients on dose level IIa.
Overall survival and progression free survival curves are shown in Figs. 3 and 4 (supplementary material). One-year overall survival was 60% and one-year progression free survival probability was 30%. Median time to progression was 172 days. Time to local progression of the irradiated index lesion was evaluated for lesions that were measurable according to RECIST 1.1 criteria. Local progression occurred in two out of 10 patients, at 3 months (Level 1, lumbar spine) and 5 months post-RT (Level 2a, left groin).
During the study period, there was a shift of systemic management for patients with metastatic BRAF mutant melanoma, with more patients treated first line with immune checkpoint inhibitors as opposed to BRAF and MEK inhibitors. Patients with isolated progression on BRAF and MEK inhibitors, were also switched to immune checkpoint inhibitors much earlier in their disease course. This led to very slow accrual of patients onto the study, and the TMC made the decision to stop this study early after 10 patients.
Discussion
For the ten patients in our study who received palliative RT with concurrent Dabrafenib and Trametinib, the in-field skin reaction did not appear to exceed that expected from RT alone. Grade 3 or higher RT-related adverse events were not encountered by any of the patients. This finding is in contrast to multiple cases reports and series, suggesting greater acute skin toxicity associated with concurrent RT and single agent BRAF inhibition with Vemurafenib or Dabrafenib [5], [10], [11], [12].
The explanations are potentially two-fold. Hecht et al. [3] analysed chromosomal breaks after ex vivo irradiation of blood samples from 35 patients with melanoma and found significantly increased radiosensitivity for those receiving Vemurafenib but not Dabrafenib. Secondly, some of the previously reported increased skin toxicity may simply be due to additive effects of BRAF inhibitors and RT causing skin toxicity independently, whereas the addition of Trametinib reduces some Dabrafenib-related skin toxicities [6].
Anker et al. [5] performed a comprehensive review of 40 cases of severe extracranial toxicity from combined BRAF inhibitors with RT, and showed that the vast majority were related to increased skin toxicities with or without non-dermatological toxicity. The lack of grade 3 or higher skin toxicity among our patients is reassuring. Anker et al. [5] also showed that virtually all the cases of severe toxicity related to BRAF inhibitors (Vemurafenib or Dabrafenib) and RT. The only case of severe toxicity after RT with BRAF and MEK inhibitors (Dabrafenib and Trametinib) in the Anker et al study [5] was a case of bowel perforation, although the authors acknowledged that a causal relationship could not be confirmed in that particular case, and Dabrafenib and Trametinib were commenced 10 days after the palliative RT.
With this case in mind, we designed separate RT dose levels in our study relating to whether abdominal viscera were directly exposed to RT or not. Due to slow accrual, our study was closed early before reaching level 2b (30 Gy in 10 fractions, with abdominal viscera directly exposed to RT), even though no DLTs were encountered in the lower levels. It is worth noting that two patients were treated at dose level 1 receiving 20 Gy in 5 fractions using a 3D conformal technique to the lumbar spine and right ilium/L1 vertebra without experiencing any significant gastrointestinal toxicities during the study period.
Palliative RT techniques have evolved significantly over the past few years, now with IMRT and volumetric modulated arc therapy (VMAT) frequently utilised in this setting. While the majority of our patients received 3D conformal technique without severe RT-related toxicity, IMRT or VMAT are expected to further reduce high doses to normal tissues, which may be advantageous when treating with concurrent Dabrafenib and Trametinib, especially if there are nearby abdominal viscera such as bowel or liver.
The main limitation of this study is the small sample size which could limit our ability to identify less common toxicity events, and moderately increased skin toxicity remains a possibility given our limited number of 10 patients. While acknowledging this limitation, the lack of grade 3 or higher RT-related toxicities suggests that Dabrafenib and Trametinib may be safely used concurrently with fractionated non-visceral palliative RT to extracranial sites, including 20 Gy in 5 fractions and 30 Gy in 10 fractions. We encourage other investigators to carefully report both dermatological and non-dermatological toxicities if encountered using this regimen of concurrent RT with Dabrafenib and Trametinib.
Other disclosures
MSC: consultant advisor for Amgen, BMS, Eisai, Ideaya, MSD, Nektar, Novartis, Oncosec, Pierre-Fabre, Qbiotics, Regeneron, Roche, Merck and Sanofi, and received honoraria from BMS, MSD, and Novartis.
JLS: honoraria from Bristol-Myers Squibb, MSD and Pierre Fabre.
MS: consultant advisor for MSD, Novartis, Oncosec, Pierre-Fabre, Roche, and Merck, and received honoraria from BMS, MSD, Novartis and Pierre-Fabre.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: [GlaxoSmithKline and Novartis provided initial seed funding for this clinical trial. GlaxoSmithKline or Novartis had no involvement in the data analysis and preparation of this manuscript.]
Acknowledgement
We acknowledge support by GlaxoSmithKline and Novartis for providing the initial seed funding of this clinical trial. GlaxoSmithKline or Novartis had no involvement in the data analysis and preparation of this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2021.08.006.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Davies H., Bignell G.R., Cox C., Stephens P., Edkins S., Clegg S. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 2.Pulvirenti T., Hong A., Clements A. Acute radiation skin toxicity associated With BRAF Inhibitors. J Clin Oncol. 2016;34(3):e17–e20. doi: 10.1200/JCO.2013.49.0565. [DOI] [PubMed] [Google Scholar]
- 3.Hecht M., Zimmer L., Loquai C., Weishaupt C., Gutzmer R., Schuster B. Radiosensitization by BRAF inhibitor therapy – mechanism and frequency of toxicity in melanoma patients. Ann Oncol. 2015;26(6):1238–1244. doi: 10.1093/annonc/mdv139. [DOI] [PubMed] [Google Scholar]
- 4.Strobel S.B., Pätzold S., Zimmer L., Jensen A., Enk A., Hassel J.C. Radiosensitization by BRAF inhibitors. J Dtsch Dermatol Ges. 2017;15(7):703–708. doi: 10.1111/ddg.12672. [DOI] [PubMed] [Google Scholar]
- 5.Anker C.J., Grossmann K.F., Atkins M.B., Suneja G., Tarhini A.A., Kirkwood J.M. Avoiding severe toxicity from combined BRAF inhibitor and radiation treatment: consensus guidelines from the Eastern Cooperative Oncology Group (ECOG) Int J Radiat Oncol Biol Phys. 2016;95(2):632–646. doi: 10.1016/j.ijrobp.2016.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Long G.V., Stroyakovskiy D., Gogas H., Levchenko E., de Braud F., Larkin J. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371(20):1877–1888. doi: 10.1056/NEJMoa1406037. [DOI] [PubMed] [Google Scholar]
- 7.Carlino M.S., Gowrishankar K., Saunders C.A.B., Pupo G.M., Snoyman S., Zhang X.D. Antiproliferative effects of continued mitogen-activated protein kinase pathway inhibition following acquired resistance to BRAF and/or MEK inhibition in melanoma. Mol Cancer Ther. 2013;12(7):1332–1342. doi: 10.1158/1535-7163.MCT-13-0011. [DOI] [PubMed] [Google Scholar]
- 8.Chan M.M.K., Haydu L.E., Menzies A.M., Azer M.W.F., Klein O., Lyle M. The nature and management of metastatic melanoma after progression on BRAF inhibitors: effects of extended BRAF inhibition. Cancer. 2014;120(20):3142–3153. doi: 10.1002/cncr.28851. [DOI] [PubMed] [Google Scholar]
- 9.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Euro. J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Pulvirenti T., Hong A., Clements A. Acute radiation skin toxicity associated with BRAF inhibitors. J Clin Oncol. 2014;34:e17–e20. doi: 10.1200/JCO.2013.49.0565. [DOI] [PubMed] [Google Scholar]
- 11.Anker C.J., Ribas A., Grossmann A.H. Severe liver and skin toxicity after radiation and vemurafenib in metastatic melanoma. J Clin Oncol. 2013;31:e283–e287. doi: 10.1200/JCO.2012.44.7755. [DOI] [PubMed] [Google Scholar]
- 12.Satzger I., Degen A., Asper H. Serious skin toxicity with the combination of BRAF inhibitors and radiotherapy. J Clin Oncol. 2013;31:e220–e222. doi: 10.1200/JCO.2012.44.4265. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.