Abstract
Cardiovascular diseases such as myocardial infarction (MI) are among the major causes of death worldwide. Although intramyocardial injection of hydrogels can effectively enhance the ventricular wall, this approach is limited because of its restriction to the poor vascularization in the infarcted myocardium. Here, we reported a new type of hydrogel composed of alginate (ALG) and hyaluronic acid (HA) with lyophilized platelet-rich fibrin (Ly-PRF) for releasing abundant growth factors to realize their respective functions. The results of in vitro studies demonstrated favorable mechanical property and release ability of ALG-HA with Ly-PRF. When injected into the infarcted myocardium, this composite hydrogel preserved heart function and the Ly-PRF within the hydrogel promoted angiogenesis and increased vascular density in both infarcted and border zone, which rescued the ischemic myocardium. These beneficial effects were also accompanied by macrophage polarization and regulation of myocardial fibrosis. Moreover, the autologous origin of Ly-PRF with ALG-HA hydrogel offers myriad advantages including safety profile, easiness to obtain and cost-effectiveness. Overall, this study demonstrated the versatile therapeutic effects of a novel composite hydrogel ALG-HA with Ly-PRF, which optimizes a promising vascularized substitution strategy for improving cardiac function after MI.
Keywords: Myocardial infarction, Injectable hydrogel, Drug delivery, Vascularization, Lyophilized platelet-rich fibrin
Abbreviations: ALG, alginate; HA, hyaluronic acid; PRP, platelet rich plasma; PRF, platelet-rich fibrin; Ly-PRF, lyophilized platelet-rich fibrin; MI, myocardial infarction; LVEF, left ventricular ejection fractions; LVFS, left ventricular fractional shortening; LVESd, left ventricular end-systolic diameter; LVEDd, left ventricular end-diastolic diameter; PET-CT, positron emission tomography-computerized tomography; SUV, standardized uptake value; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labeling; IZ, infarcted zone; BZ, border zone; iNOS, inducible nitric oxide synthase
Graphical abstract
Highlights
-
•
Alginate (ALG) alone has a limited vascularized ability in vivo.
-
•
ALG with 20% hyaluronic acid (HA) could attenuate LV remodeling and promote angiogenesis.
-
•
ALG-HA with lyophilized platelet-rich fibrin (Ly-PRF) could make further improvement in angiogenesis.
-
•
The Ly-PRF is promising for further clinical application because of autologous source and definite therapeutic effects.
1. Introduction
Myocardial infarction (MI) arising from occluded coronary arteries accounts for the primary cause of cardiovascular mortality worldwide [1]. The loss of myocardium and limited regenerative ability of cardiomyocytes underlie detrimental myocardial remodeling and scar tissue formation, resulting in severe cardiac dysfunction. Hydrogels, one of the intramyocardial injectable biomaterials, have been widely applied in the modulation of the reparative process post-MI because it could substitute the infarcted myocardium and provide sustained mechanical support for debilitated ventricular wall [2,3]. Over the past decades, a variety of hydrogels with multitudinous functions has been developed and abundant studies from both experimental models and clinical trials have been established, confirming safety and effectiveness of intramyocardial substitution strategy in improving heart function post-MI [4].
However, the available hydrogels often suffer from limitations related to unsatisfactory neovascularization [5]. Growing evidence has suggested neovascularization is crucially important for cardiac repair post-MI. A great deal of effort in recent years has been focused on the utilization of growth factors in tissue regeneration. Platelets are one of the major resources of autologous growth factors involved in angiogenesis and wound healing events [6]. Platelet-rich fibrin (PRF), the second generation of platelet concentrates, is extracted from whole blood and has already been widely applied in tissue repair. Compared with earlier introduced platelet-rich plasma (PRP), which is prepared by adding anticoagulants and bovine thrombin, PRF is derived from centrifuged blood and is strictly autologous, avoiding the risk of coagulation disorders and rejection reaction [[7], [8], [9]]. Previous experiments have revealed that several key growth factors including platelet derived growth factor (PDGF), vascular endothelial growth factor (VEGF), and transforming growth factor beta 1 (TGFβ-1) were surely concentrated in PRF, significantly promoting angiogenesis and modulating wound healing process [[10], [11], [12]]. However, the gelatinous morphology of the PRF lacks stable shape and resistance to compression or stretching because of its strong tensile strength [[13], [14], [15]], consequently limiting the translational potential of PRF to clinics [16]. Lyophilization is a commonly used process and has been a widely utilized technique to prepare proteins and platelets [17]. Lyophilized platelet-rich fibrin (Ly-PRF) not only has the advantage of lowing the difficulty in application, but also provides newly grown tissue with straightforward access to multiple growth factors [18]. Moreover, data from a previous study indicated that Ly-PRF could release growth factors sustainably, presenting a better tissue regeneration process [19,20].
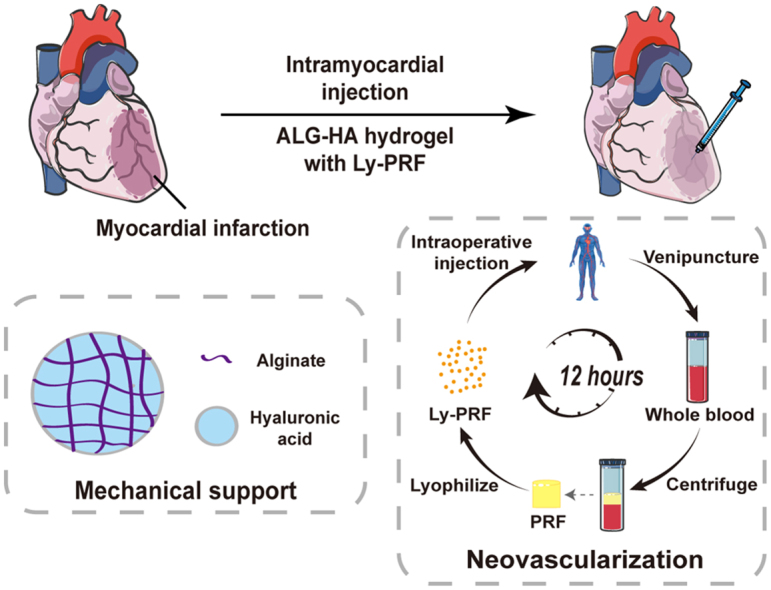
In this study, we delivered alginate and hyaluronic acid (ALG-HA) hydrogel incorporated with Ly-PRF into the infracted myocardium in rats. The hydrogel provides mechanical support and serves as a platform for controlled drug release, while the Ly-PRF shows therapeutic efficacy by promoting cardiac recovery. Thus, we developed a novel therapeutic strategy that can simultaneously provide suitable mechanical support and foster new vessel formation in ischemic myocardial tissue, as well as promote restoration of cardiac function.
2. Materials and methods
2.1. Preparation of ALG-HA hydrogels
Sodium alginate powder (Aladdin, Shanghai, China) and calcium alginate powder (Ningbo Diochange Medical Technology, Ningbo, China) were dissolved in sterilized water to form 2% (wt/vol) sodium alginate solution and 1.5% (wt/vol) calcium alginate suspension at room temperature. Different proportions of hyaluronic acid (HA) were added to alginate (ALG) solution to form ALG-HA hydrogels (Supplementary Table S1). Mannitol (Aladdin, Shanghai, China) was utilized to regulate osmotic pressure.
2.2. Preparation of platelet concentrates
The platelet concentrates including PRP, PRF and Ly-PRF were prepared according to a previously published protocol [9,21]. Briefly, 10 mL of whole blood was centrifuged for 7 min at 1000 rpm (45 g) at room temperature without brake (Centrifuge 5804 R; Eppendorf, Germany). The plasma rum was decanted up to the red blood cell sediment and then centrifuged again for 10 min at 3000 rpm (400 g) at 4 °C. Finally, the PRP was decanted and the final PRP sediment was suspended. For preparation of fresh PRF, 10 mL of whole blood was immediately centrifuged at 3000 rpm for 10 min at 4 °C and the fresh PRF clots identified as the middle layer (Supplementary Fig. S2a). Fresh PRF clots were removed and frozen at −80 °C and then freeze-dried using a Labconco lyophilizer (Free Zone, Labconco, Kansas City, MO, USA) at −51 °C for 12 h for preparation of Ly-PRF.
2.3. Mechanical property
Prior to the measurement, the hydrogel precursor solutions were mixed and applied to a temperature control stage at 37 °C. Rheological properties of hydrogels were measured using Haake MARS40 (Thermo Fisher Scientific Inc., Germany) via oscillation frequency sweep test at 37 °C in an oscillatory mode.
2.4. Angiogenic assay and growth factor release experiment
HUVECs were seeded in six-well dishes coated with 0.5 mm thick growth factor-reduced basement membrane extract Matrigel (4 × 104 cells/cm2) and allowed to attach for 3 h. The seeded HUVECs were subjected to four different conditions: PBS, ALG-HA, ALG-HA (PRP) and ALG-HA (Ly-PRF). After incubation at 37 °C with 5% CO2 for 6 h, the plates were washed with PBS and HUVEC cells were imaged. The tubular numbers, tubular length and tubular intersecting nodes were counted to evaluate the tube formation ability via Image-Pro Plus software (Media Cybernetics Inc., Bethesda, MD, USA) [22]. Each experiment was repeated twice. The growth factor release experiment was performed via ELISA assay. Please refer to the supplementary methods for further details.
2.5. Rat model of MI and hydrogel injection
The animal study ethics and in vivo Study design was declared in the supplementary methods. The Rat model of MI was constructed as described previously [23]. Briefly, animals were anaesthetized with inhaled 2% isoflurane, intubated and ventilated with a respirator at a respiratory rate of 90 times/min. After a left thoracotomy was finished, pericardium was removed and the left anterior descending artery (LAD) was permanently ligated for induction of acute myocardial infarction (AMI) model with a suture (6–0 Prolene, Ethicon). Successful AMI procedure was confirmed by ST-segment elevation on an electrocardiogram (ECG) device. The chest was closed, and the skin was sutured (3–0 Prolene, Ethicon). Animals in the sham group underwent all surgical procedures except for the LAD artery ligation. Baseline was determined at day 0 (3 days after MI). Subsequently, the chests were reopened, and syringes with PBS, ALG-HA hydrogel, ALG-HA (PRP) hydrogel (PRP derived from 10 mL whole blood in 1 mL sterile well-mixed ALG-HA hydrogel), and ALG-HA (Ly-PRF) hydrogel (Ly-PRF derived from 10 mL whole blood in 1 mL sterile well-mixed ALG-HA hydrogel) were prepared during the surgery. A total volume of 180 μL was injected directly into 3 locations, and the needle was withdrawn slowly after injection to avoid leakage. The thorax was then closed, and the rat was placed on a warm plate.
2.6. Cardiac echocardiography
Assessment of the LV cardiac function and structure was performed by the Vevo 2100 ultrasound Imaging System (VisualSonics, Canada) as previously described [23]. Briefly, rats were anaesthetized with 2% inhaled isoflurane and then echocardiograms were collected from short-axis 2-dimensional imaging at the midaxillary level. The following echocardiographic data including LVIDs, LVIDd, LVEF, and LVFS was collected and analyzed 2 days after ligation (baseline), as well as at day 7, day 14 and day 28. All parameters were measured based on the mean of three consecutive cardiac cycles.
2.7. Histological assessment
At 4 weeks post-injection, the rats (n = 8 for each group) were euthanized. The hearts were collected, perfused with 4% formaldehyde for 20 min and then rinsed with PBS for approximately 10 min. Then, the hearts were fixed, paraffin embedded and consecutively sectioned into 5 μm slices on the short axis at 2 mm intervals for histopathological and morphometric analyses. Masson's trichrome staining and sirius red staining were conducted. Histological images were scanned on a Pannoramic Digital Slide Scanner (MIDI II, 3D HISTECH, Hungary). The following data including ventricular wall thickness, scar thickness, septum thickness, LV cavity area, and whole LV area was measured via Fiji software.
2.8. Immunocytochemistry staining
Immunofluorescence was performed to identify blood vessel formation and collagen subtype. Slices were incubated with several primary antibodies (Supplementary Table S2) overnight at 4 °C, subsequently incubated with respective secondary antibodies for 1 h at room temperature, and finally counterstained with DAPI. An LSM880 Meta confocal microscope (Carl Zeiss, Feldbach, Switzerland) was utilized for confocal images. For quantification, five random fields in each section were counted.
2.9. TUNEL staining
For assessment of myocardial apoptosis TUNEL staining was carried out according to the manufacturer's instructions (Roche, 11684795910). Sections were counterstained with α-actinin antibody (Sigma, A7811, 1:500) and DAPI for cardiomyocytes and nuclei respectively. The percentage of apoptotic cardiomyocytes was identified by the number of TUNEL+, α-actinin + double positive nuclei versus the total number of α-actinin + cell nuclei.
2.10. Statistical analysis
All experimental data are expressed as the mean ± standard deviation and were analyzed using SPSS version 25.0 (SPSS, Chicago, IL) and GraphPad (GraphPad Prism version 8.0; GraphPad Software). Statistical comparisons were performed by unpaired Student's t-test or one-way analysis of variance (ANOVA). Survival analyses were performed using the Kaplan-Meier method, and differences were determined by a log-rank test. For all comparisons, P values < 0.05 were considered statistically significant.
3. Results
3.1. Characterization of ALG-HA hydrogels
To determine the optimal mechanical property for ALG-HA hydrogel system, we examined the rheological property of ALG hydrogels with different proportions of HA, and the results indicated that the series of the ALG-HA hydrogels exhibited suitable storage modulus (G′) and the loss modulus (G″) for drug delivery and tissue regeneration (Supplementary Figs. S1a and b) [24,25], especially the ALG with 20% HA. The in vivo study also demonstrated that this type of hydrogel achieved the most satisfactory therapeutic effects including attenuated left ventricular remodeling (Supplementary Fig. S3), improved cardiac function (Supplementary Fig. S4), and increased angiogenesis (Supplementary Fig. S5). Therefore, we selected the ALG with 20% HA as a candidate for the follow-up experiments.
3.2. Characterization of ALG-HA hydrogels with PRP or Ly-PRF
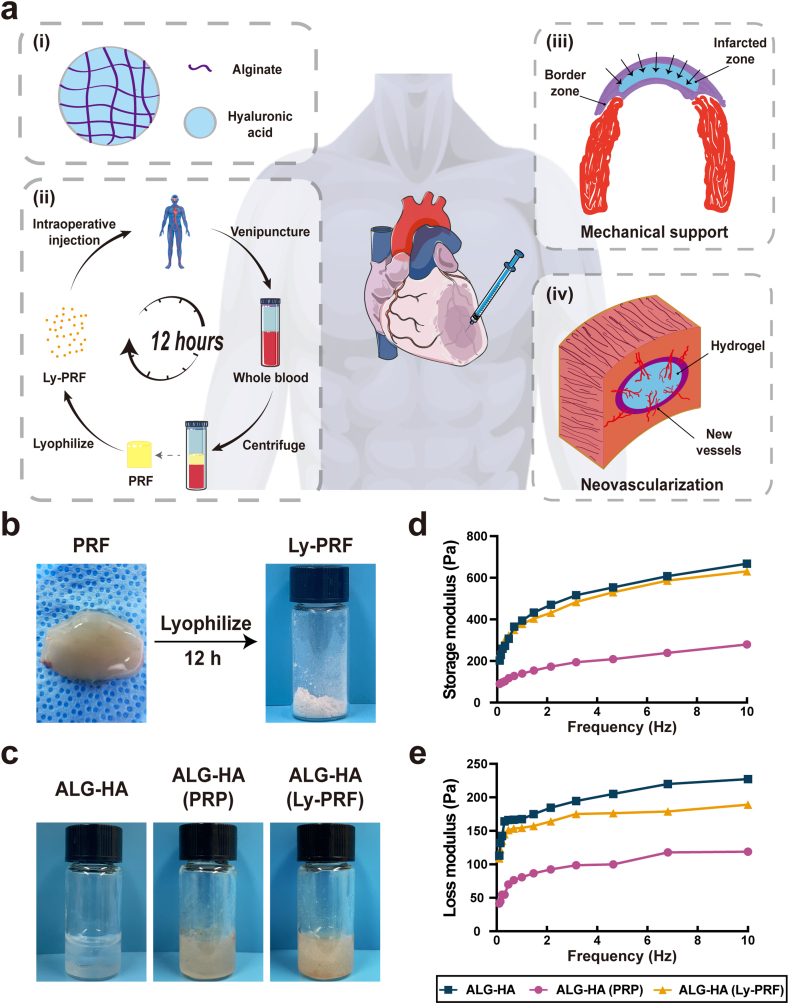
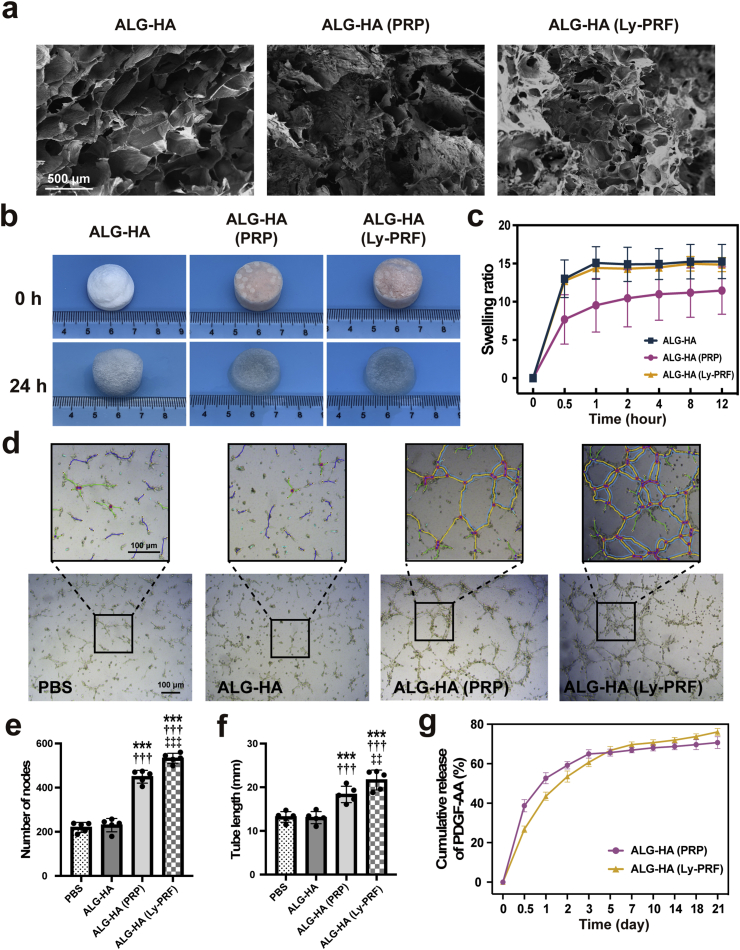
The PRF was present as a fibrin clot in the middle of the tube after centrifugation (Supplementary Fig. S1b). As shown in Fig. 1b, the macrophotographs of fresh PRF and Ly-PRF were presented. Then we examined the mechanical property of PRP and Ly-PRF with the mixture of ALG-HA and the result demonstrated the solution of PRP reduced the G′ and G″ of ALG-HA hydrogel, while the Ly-PRF showed no significant effect (Fig. 1d and e). The scanning electron microscope (SEM) revealed the sponge-like microstructure of ALG-HA, ALG-HA with PRF or Ly-PRF (Fig. 2a). Furthermore, we examined the swelling ratio of ALG-HA with PRP or Ly-PRF in PBS solution (pH 7.4) at 37 °C for property for the determination of drug releasing ability (Fig. 2b). The high swelling ratio of ALG-HA with Ly-PRF could provide a good basis for drug loading and release (Fig. 2c).
Fig. 1.
Application principle and mechanical property of the ALG-HA hydrogels. (a) Schematic of the preparation process of the composite hydrogel and MI therapy. (i) The composition of hydrogel: ALG and HA; (ii) The preparation of Ly-PRF: venipuncture, centrifuge and lyophilize for 12 h; (iii) Mechanical support for weak ventricular wall; (iv) Inducing infiltration of microvasculature in both IZ and BZ. (b) Fabrication of Ly-PRF via lyophilization. (c) The macrophotographs of ALG-HA hydrogels with PRP or Ly-PRF. (d) Dynamic rheology curves for the ALG-HA hydrogels with PRP or Ly-PRF. ALG, alginate; HA, hyaluronic acid; PRP, platelet rich plasma; Ly-PRF, lyophilized platelet-rich fibrin; IZ, infarcted zone; BZ, border zone.
Fig. 2.
Microstructure and drug delivery ability of the ALG-HA hydrogels. (a) SEM images of ALG-HA hydrogels with PRP or Ly-PRF. (b, c) The representative images (b) and the swelling ratio (c) of ALG-HA hydrogels with PRP or Ly-PRF; n = 5 per group. (d) Tube formation assay for all groups. (e, f) Quantitative analysis of nodes (e) and tube length (f); n = 5 per group. (g) The cumulative release of PDGF-AA from ALG-HA hydrogels with PRP or Ly-PRF; n = 5 per group. The data in (e, f) are expressed as mean ± standard deviation and analyzed using One-way ANOVA followed by Bonferroni post hoc test. ***p < 0.001 compared with PBS group; †††p < 0.001 compared with ALG-HA group; ‡‡‡p < 0.001 and ‡‡p < 0.01 compared with ALG-HA (PRP) group. ALG, alginate; HA, hyaluronic acid; PRP, platelet rich plasma; Ly-PRF, lyophilized platelet-rich fibrin.
3.3. Quantification of PDGF-AA release kinetics and tubular formation in HUVECs
To demonstrate the utility of the ALG-HA hydrogels for protein delivery, the release profiles of therapeutic protein from Ly-PRF were measured using PDGF-AA as a model protein. The cumulative protein release profiles from ALG-HA with PRP showed a rapid release in the first 3 days, while the ALG-HA with Ly-PRF showed a preliminary burst in the first 3 days and followed by a sustained release period of up to 7 days (Fig. 2g). The result indicated that ALG-HA with Ly-PRF released 10638.55 ± 1577.43 pg/mL of total PDGF-AA after 21 days, significantly more than ALG-HA with PRP 7940.06 ± 910.28 pg/mL (P = 0.011). The release kinetics of VEGF and TGFβ-1 was demonstrated in the Supplementary Fig. S2. The tube formation assay provides a tool for the assessment of angiogenesis in vitro, and endothelial cells obtained from the human umbilical vein (HUVECs) were used in the current study. After 8 h incubation, each treatment group was observed and counted under a light microscope (Fig. 2d). The ALG-HA (Ly-PRF) group significantly promoted the tubular formation of HUVECs compared to the PBS, ALG-HA hydrogel control group as well as ALG-HA (PRP) in terms of tubular number (Fig. 2e) and tubular length (Fig. 2f).
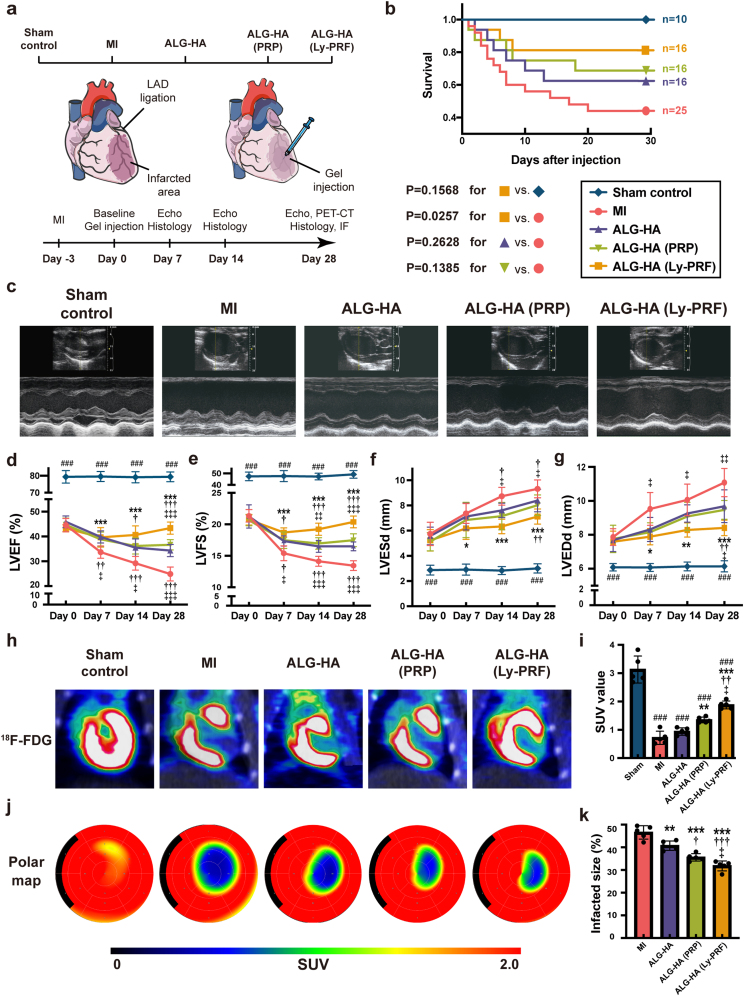
3.4. Evaluation of heart functions and cardiac metabolism after hydrogel injection
Echocardiography was used to evaluate the LV dimensions and cardiac function for each animal for 0 day (baseline, 3 days after LAD ligation), 7 days, 14 days, and 28 days after hydrogel injection (Fig. 3a, Supplementary Fig. S5). The application of the ALG-HA (Ly-PRF) improved survival but not in the ALG-HA or ALG-HA (PRP) compared to the MI group (Fig. 3b). The parameters including left ventricular ejection fraction (LVEF), fractional shortening (LVFS), left ventricular end-systolic diameter (LVESd), and left ventricular end-diastolic diameter (LVEDd) were similar in all groups at baseline, suggesting a comparable degree of initial cardiac dysfunction (Fig. 3d–g). LVEF is a parameter of the percentage of blood ejecting each cardiac circle, and a decrease was defined by the American Heart Association as a predictive factor for heart failure [26]. At 4 weeks post injection, ALG-HA (Ly-PRF) group made a marked increase on the LVEF in comparison to the MI (43.51 ± 2.48% vs. 24.86 ± 2.82%, P < 0.001), ALG-HA (43.51 ± 2.48% vs. 34.30 ± 2.46%, P < 0.001), and ALG-HA (PRP) (43.51 ± 2.48% vs. 24.86 ± 2.82%, P < 0.001) groups (Fig. 3d). For further evaluation of the heart function, the LVFS was calculated and LVFS was moderately prevented in the ALG-HA (16.50 ± 0.70% vs. 13.44 ± 0.77% in MI, P < 0.001) and ALG-HA (PRP) groups (17.41 ± 1.02% vs. 13.44 ± 0.77% in MI, P < 0.001), and significantly prevented in the ALG-HA (Ly-PRF) group (20.38 ± 0.93% vs. 13.44 ± 0.77% in MI, P < 0.001) compared to the MI group (Fig. 3e).
Fig. 3.
The ALG-HA hydrogel with Ly-PRF promoted the recovery of cardiac function. (a) Schematic of groups and the experimental design for 28-day animal study. (b) Kaplan–Meier survival analysis at different time points after hydrogel injection. Survival distributions were estimated by the Kaplan-Meier method and compared by log-rank test. (c) The representative images of echocardiography at day 28. (d-g) LVEF (d), LVFS (e), LVESd (f) and LVEDd (g) were assessed by echocardiography at day 0, day 7, day 14 and day 28; n = 8 animals per group. (h, i) The representative images of PET-CT with 18 F-FDG at day 28 (h) and assessment of cardiomyocyte metabolism by SUV (i); n = 5 animals per group (j, k) The representative analysis of PET-CT (j) and assessment of infarcted size (k) via SUV at day 28; n = 5 animals per group. The data were expressed as mean ± standard deviation and analyzed using One-way ANOVA followed by Bonferroni post hoc test. The data in (d, e, f, g, i and k) were expressed as mean ± standard deviation and analyzed using One-way ANOVA followed by Bonferroni post hoc test. ###p < 0.001 compared with sham control group; ***p < 0.001, **p < 0.01 and *p < 0.05 compared with MI group; †††p < 0.001, ††p < 0.01 and †p < 0.05 compared with ALG-HA group; ‡‡‡p < 0.001, ‡‡p < 0.01 and ‡p < 0.05 compared with ALG-HA (PRP) group. MI, myocardial infarction; ALG, alginate; HA, hyaluronic acid; PRP, platelet rich plasma; Ly-PRF, lyophilized platelet-rich fibrin; LVEF, left ventricular ejection fractions; LVFS, left ventricular fractional shortening; LVESd, left ventricular end-systolic diameter; LVEDd, left ventricular end-diastolic diameter; PET-CT, positron emission tomography-computerized tomography; SUV, standardized uptake value.
Next, in order to evaluate cardiac metabolism post injection, we assessed the viability and necrosis of the cardiomyocytes via positron emission tomography (PET), derived from the standardized uptake value (SUV) (Fig. 3h, j, Supplementary Figs. S6 and S7). The ALG-HA (Ly-PRF) group showed a marked decrease in the defect size in comparison to the MI (31.80 ± 2.20% vs. 46.57 ± 2.97 in MI, P < 0.001), ALG-HA (31.80 ± 2.20% vs. 40.71 ± 2.05% in ALG-HA, P < 0.001, and ALG-HA (PRP) (31.80 ± 2.20% vs. 35.56 ± 1.64% in the ALG-HA (PRP), P < 0.05) groups (Fig. 3j and k).
3.5. Assessment of histology and morphology for heart structure
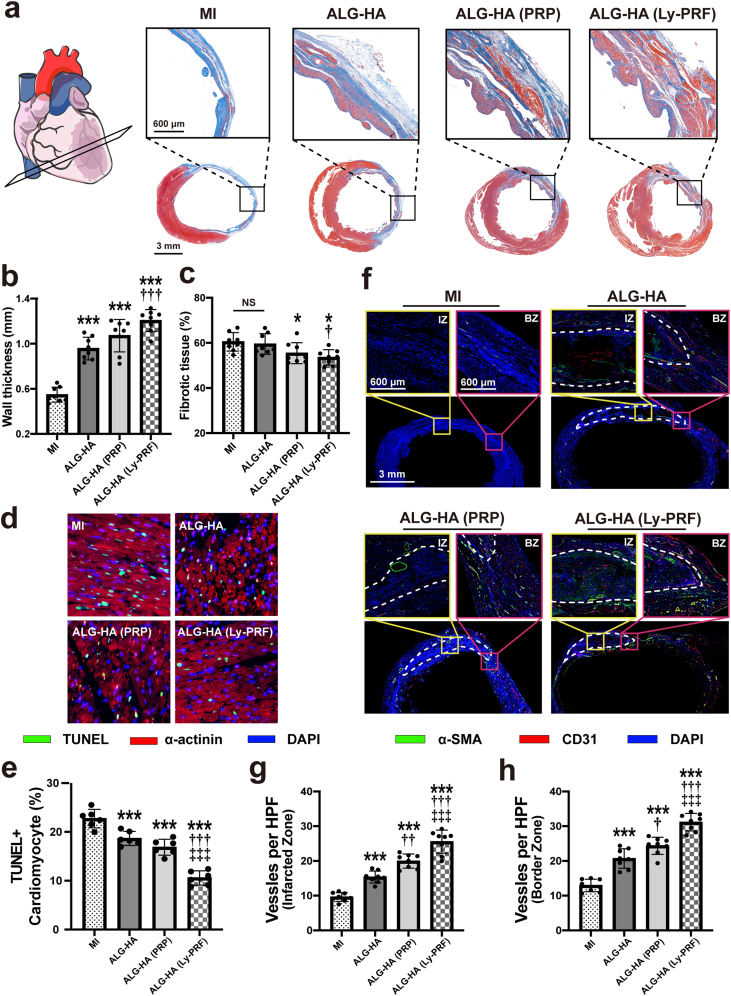
Masson's trichrome staining and magnified images revealed the histology and morphology of heart structure. LV anterior wall thickness showed a sharp increase in the ALG-HA (0.96 ± 0.10 mm vs. 0.54 ± 0.07 mm in MI, P < 0.001), ALG-HA (PRP) (1.07 ± 0.14 mm vs. 0.54 ± 0.07 mm in MI, P < 0.001), and ALG-HA (Ly-PRF) (1.21 ± 0.10 mm vs. 0.54 ± 0.07 mm in MI, P < 0.001) groups compared with the MI group at 4 weeks post injection (Fig. 4a and b). Furthermore, Masson trichrome staining demonstrated that ALG-HA (Ly-PRF) also led to a decreasing percentage of fibrosis in the LV (1.21 ± 0.10 mm vs. 0.54 ± 0.07 mm in MI, P < 0.001), appeared to limit adverse LV remodeling (Fig. 4c).
Fig. 4.
The ALG-HA hydrogel with Ly-PRF attenuate LV remodeling, improved angiogenesis and reduces apoptosis of cardiomyocyte. (a) Masson's trichrome staining for all groups at day 28. (b, c) Quantitative analysis of wall thickness (b) and fibrotic tissue (c); n = 8 animals per group. (d, e) Representative images of TUNEL staining (d) and assessment of cardiomyocyte apoptosis in BZ (e) at day 7; n = 8 animals per group. (f) Sections were stained with α-SMA (green), CD31 (red) and DAPI (blue) to visualize the arteries in IZ and BZ at day 28. The white dotted line indicated the edge of the hydrogel. (g, h) The number of arteries was counted in IZ (g) and BZ (h) at day 28; n = 8 animals per group. The data in (b, c, e, g and h) were expressed as mean ± standard deviation and analyzed using One-way ANOVA followed by Bonferroni post hoc test. NS indicates not significant. ***p < 0.001 and *p < 0.05 compared with MI group; †††p < 0.001, ††p < 0.01 and †p < 0.05 compared with ALG-HA group; ‡‡‡p < 0.001 compared with ALG-HA (PRP) group. MI, myocardial infarction; ALG, alginate; HA, hyaluronic acid; PRP, platelet rich plasma; Ly-PRF, lyophilized platelet-rich fibrin; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labeling; IZ, infarcted zone; BZ, border zone. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.6. Assessment of functional neovascularization
Regeneration of the neovascular network is crucial to restore blood and nutrient supply in ischemic tissue to maintain the vitality of the cardiomyocytes and is associated closely with ventricular remodeling. Therefore, we evaluated the therapeutic angiogenesis process in the infarcted zone (IZ) and border zone (BZ) via double staining of the heart tissue sections for α-SMA (vascular smooth muscle cell marker) and CD31 (endothelial cell marker) at 4 weeks post injection (Fig. 4f). The result demonstrated that numbers of α-SMA and CD31-positive vascular structures per high-powered field (HPF) were significantly higher in the ALG-HA (Ly-PRF) group in both IZ and BZ when compared with the MI (IZ: 9.61 ± 1.28 vs. 25.54 ± 3.33 per HPF, P < 0.001; BZ: 12.95 ± 1.81 vs. 31.17 ± 2.50 per HPF, P < 0.001), ALG-HA (IZ: 15.36 ± 1.72 vs. 25.54 ± 3.33 per HPF, P < 0.001; BZ: 20.69 ± 2.81 vs. 31.17 ± 2.50 per HPF, P < 0.001), and ALG-HA (PRP) (IZ: 19.95 ± 2.04 vs. 25.54 ± 3.33 per HPF, P < 0.001; BZ: 24.36 ± 2.47 vs. 31.17 ± 2.50 per HPF, P < 0.001) groups (Fig. 4g and h).
3.7. Assessment of cardiomyocyte apoptosis
Cardiomyocyte apoptosis was measured by terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labeling (TUNEL) within the BZ at 7 days post injection (Fig. 4d). The result indicated there were fewer apoptotic cells within the BZ of the ALG-HA (Ly-PRF) group when compared with MI (10.58 ± 1.46 vs. 22.73 ± 1.89, P < 0.001), ALG-HA (10.58 ± 1.46 vs. 18.68 ± 1.43, P < 0.001), and ALG-HA (PRP) (10.58 ± 1.46 vs. 16.87 ± 1.62, P < 0.001) groups (Fig. 4e). The number of apoptotic cells in the ALG-HA (PRP) group was slightly less than that in the ALG-HA group; however, this difference was not statistically significant (P = 0.2404).
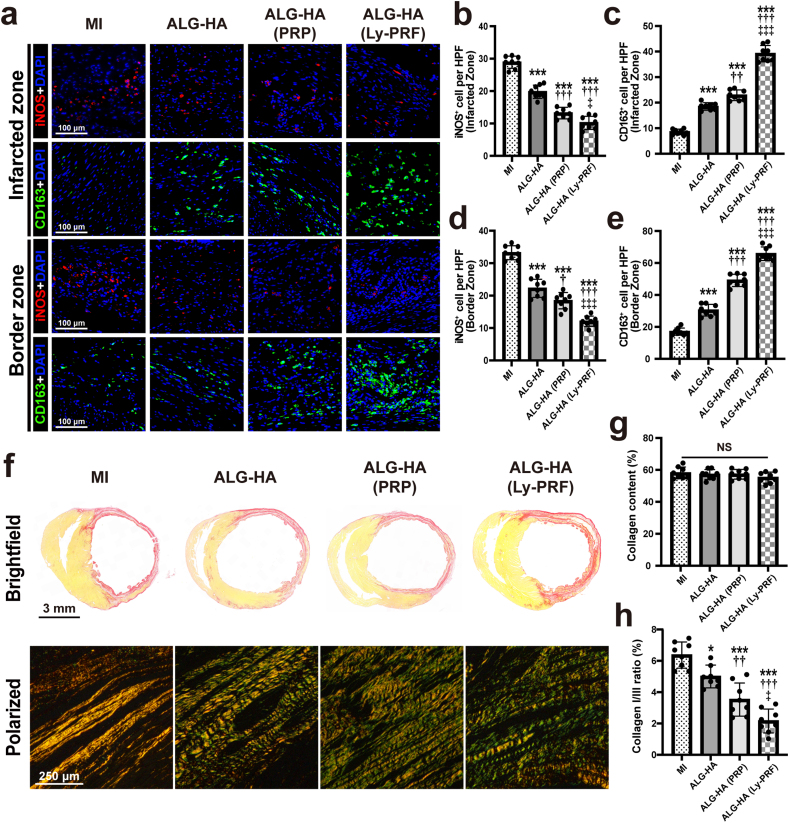
3.8. Assessment of macrophage polarization
Macrophages play an important role in wound healing. Given the diverse cell function of classical macrophages (pro-inflammatory, M1) and alternative macrophages (wound-healing, M2) [27], we examined the macrophages infiltrated into the IZ and BZ via iNOS and CD163 staining, respectively (Fig. 5a). Quantitatively, the ALG-HA (Ly-PRF) group showed a significantly lower number of iNOS-positive cells (Fig. 5b) and a significantly higher number of CD163-positive cells (Fig. 5c) within the IZ than the MI (iNOS-positive cells: 28.90 ± 1.88 vs. 10.21 ± 2.03 per HPF, P < 0.001; CD163-positive cells: 8.56 ± 1.07 vs. 39.17 ± 3.17 per HPF, P < 0.001), ALG-HA (iNOS-positive cells: 19.80 ± 2.03 vs. 10.21 ± 2.03 per HPF, P < 0.001; CD163-positive cells: 18.49 ± 1.47 vs. 39.17 ± 3.17 per HPF, P < 0.001), and ALG-HA (PRP) (iNOS-positive cells: 13.20 ± 1.77 vs. 10.21 ± 2.03 per HPF, P = 0.0215; CD163-positive cells: 22.89 ± 2.09 vs. 39.17 ± 3.17 per HPF, P < 0.001) groups, respectively. Similarly, fewer iNOS-positive cells were present in the BZ of the ALG-HA (Ly-PRF) group than MI, ALG-HA, and ALG-HA (PRP) groups (Fig. 5d). The ALG-HA (Ly-PRF) group also showed a significantly increased number of CD163-positive cells in the BZ versus other groups (Fig. 5e).
Fig. 5.
The ALG-HA hydrogel with Ly-PRF modulated macrophage polarization and regulated myocardial fibrosis. (a) Sections were stained for iNOS (red), CD163 (green) and DAPI (blue) to visualize macrophage type 1 (M1) and macrophage type 2 (M2) in both IZ and BZ. (b-e) Quantification of M1 (iNOS+ cell) number (d) and M2 (CD163+ cell) number (c) in IZ; quantification of M1 (iNOS+ cell) number (d) and M2 (CD163+ cell) number (e) in BZ; n = 8 animals per group. (f) Picrosirius red staining for collagen I and collagen III. (g, h) Quantification of collagen content (g) and the type I/type III collagen ratios (h) among groups; n = 8 animals per group. The data in (b, c, d, e, g and h) were expressed as mean ± standard deviation and analyzed using One-way ANOVA followed by Bonferroni post hoc test. NS indicates not significant. ***p < 0.001 compared with MI group; †††p < 0.001, ††p < 0.01 and †p < 0.05 compared with ALG-HA group; ‡‡‡p < 0.001 and ‡p < 0.05 compared with ALG-HA (PRP) group. MI, myocardial infarction; ALG, alginate; HA, hyaluronic acid; PRP, platelet rich plasma; Ly-PRF, lyophilized platelet-rich fibrin; iNOS, inducible nitric oxide synthase; IZ, infarcted zone; BZ, border zone. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.9. Assessment of collagen remodeling
Picrosirius red staining combined with light and polarization microscopy was utilized to examine collagen fibers and to quantitatively determine their content and types at 4 weeks post injection. Briefly, type I collagen was identified by yellow or red staining, while type III collagen was identified by green staining under polarized light (Fig. 5f). The result indicated the total collagen content in the IZ was similar (Fig. 5g), showing no difference in collagen deposition among the groups (6.37 ± 0.84 in the MI; 5.00 ± 0.73 in the ALG-HA; 3.53 ± 1.06 in ALG-HA (PRP); 2.61 ± 0.76 in ALG-HA (Ly-PRF), all P < 0.05) (Fig. 5h).
4. Discussion
In this study, we investigated ALG hydrogels with different proportions of HA and provided evidence that injection of ALG-HA hydrogel encapsulated with Ly-PRF into the infarct area could lead to improved cardiac function and accelerated wound healing process, as demonstrated by the attenuation of wall thickening, the limited degree of ventricular expansion and facilitation of angiogenesis, immunoregulation, and collagen remodeling, indicating that Ly-PRF could markedly modulate the healing process post-MI. To the best of our knowledge, this is the first report to utilize intramyocardial filling strategy with Ly-PRF encapsulated hydrogel in the healing process post-MI.
Several natural and synthetic hydrogel matrices have been widely utilized in regenerative medicine and tissue engineering [4,28]. Accordingly, intramyocardial administration of hydrogels is beneficial for cardiac repair via providing structural support for infarcted tissue and a natural platform for further vascularization [29]. ALG is a representative hydrogel with good biocompatibility and has been previously received clearance as a food and pharmaceutical ingredient by the U.S. Food and Drug Administration (FDA) in the early 1970s. It should also be noteworthy that ALG has already reached the clinical trials and the AUGMENT-HF trial reported encouraging results that ALG hydrogel was effective for providing sustained 1-year benefits in exercise capacity, symptoms, clinical status, and overall improvement in quality of life for patients with advanced HF [[30], [31], [32]]. Nevertheless, our observations suggested the injection of ALG alone resulted in poor neovascularization. In this study, we attempted to promote angiogenesis via the combination of ALG and HA hydrogels. HA is a linear glycosaminoglycan polymer that is found in the extracellular matrix of mammalian tissues and plays a variety of roles in tissue structure and function. Since the HA could not be cross-linked, we sought to utilize the HA along with ALG based hydrogels to mimic the molecular tissue network in the human body. In order to obtain satisfactory functions, we fabricated the hydrogels with ALG and HA at different proportions and the results indicated ALG with 20% HA possess appropriate mechanical properties and the application of this hydrogel could attenuate LV remodeling as well as promote angiogenesis process 28 days post-MI. However, the therapeutic effects, especially the angiogenesis effect, did not reach the expectation and therefore we selected the ALG with 20% HA as a candidate for the further experiments.
Revascularization is crucially important for cardiac repair post-MI and the formation of new blood vessels in both IZ and BZ is an effective strategy to restore blood and nutrient supply in ischemic tissue. Hydrogels are by far the most extensive type of biomaterial that has been utilized to preserve growth factors and release them with spatial and temporal coordination [25]. Platelets are rich in numerous bioactive growth factors and present excellent potential in tissue regeneration. Therefore, the current study tried to combine platelet rich concentrations with ALG-HA hydrogels in order to seek delivery systems of bioactive factors for cardiac healing [33]. PRP was first utilized as a regenerative agent derived from autologous blood, containing an increased blood-derived growth factors that facilitate wound healing and tissue repair [34]. Although PRP has been proven to possess advantages on wound healing, it has been hypothesized that the added addition of anti-coagulants has potentially either caused a cytotoxic effect or significantly interfered with the natural coagulation cascade. In addition, the liquid formed PRP would influence the mechanical property of existing hydrogels which may be unpredictable for each mixed sample. PRF, a novel generation of platelet concentrate product, is prepared via a simplified preparation without the addition of biochemical additives. Previous studies also reported that PRF appears to possess more abundant growth factors than PRP [7,35,36]. However, PRF lacks stable shape and resistance to compression or stretching, limiting the development of further clinical applications. Thus, we employed freeze-drying technology addressed the issue of PRF and manufactured Ly-PRF mixed with ALG-HA. The histological and morphological analyses revealed that Ly-PRF encapsulated ALG-HA hydrogel could significantly increase the Ventricular wall thickness and attenuate adverse ventricular remodeling. These therapeutic effects may result from the supraphysiological doses of growth factors in Ly-PRF including PDGF-AA, thus increased capillaries in both IZ and BZ. PDGF-AA was proven to be an angiogenic factor that improves proliferation and differentiation of endothelial cells, rescuing the ischemic myocardium and improving the cardiac function. Moreover, our data suggested that Ly-PRF reduced the total number of M1 macrophages and shifted the macrophage polarization towards the M2 phenotype, hence improved the immunoreaction in conditions of MI, which might contribute to the downstream regulation of myocardial fibrosis [37,38]. This suggests that our Ly-PRF hydrogel may act as a protective unit limiting the loss of wound-healing macrophages [39]. These results suggested Ly-PRF encapsulated ALG-HA hydrogel may have synergistic effects in potentially modulating the immune microenvironment of the injured myocardium, while attenuating adverse cardiac remodeling post-MI.
Since the PRP and PRF have been utilized for a variety of medical procedures as a regenerative tool [[40], [41], [42]], our Ly-PRF encapsulated ALG-HA hydrogel can be easily tuned for more biological application of interest, thus offering immense potential for future clinical therapies. On one hand, this therapeutic strategy provides advantages in clinical applications due to the rapid preparation of Ly-PRF. The preparation process comprises blood collection, centrifugation and lyophilization and the whole process takes less than 12 h which is suitable for most patients undergoing elective operations (Fig. 1a). On the other hand, the autologous-derived source of Ly-PRF provide diverse physiological doses of growth factors when compared with single supra-physiological dose of recombinant growth factor [43]. The numerous growth factors including PDGF, VEGF and TGFβ-1 contained in the Ly-PRF may have synergistic effects in improving the function of the injured myocardium. Moreover, the autologous Ly-PRF is a cost-efficient therapeutic tool, thus lightning the financial burden on patients.
Although several encouraging achievements have been made in this study, there are still some limitations that should be considered. First, in this study, the therapeutic effects of ALG-HA with Ly-PRF were assessed only four weeks post injection. Thus, studies concentrating on longer time points are required to elucidate how profoundly ALG-HA and Ly-PRF impact cardiac function. Second, since there exist differences in physiological conditions between rats and humans, further evaluation of the Ly-PRF in porcine models should be performed in future studies, regarded as a suitable candidate for preclinical studies. Finally, the detailed molecular mechanisms underlying the myocardial protection effect of Ly-PRF remain unknown. Signaling pathways associated with mechanical support and angiogenesis will be investigated in future studies.
5. Conclusion
In summary, the current work focused on developing an injectable ALG-HA composite hydrogel encapsulated with Ly-PRF to realize the release of growth factors. We demonstrated that such combination therapy showed significant improvements in cardiac morphology and functionality at 4 weeks after injection. We further investigated the functional roles of the Ly-PRF within ALG-HA composite hydrogel in promoting angiogenesis and reducing cardiac cell apoptosis. Moreover, it increased M2 macrophage infiltration, which might further contribute to the regulation of myocardial fibrosis. The Ly-PRF provides advantages in clinical applications due to the autologous-derived origin, safety profile and easiness to obtain. It is also noteworthy that ALG-HA alone without Ly-PRF also showed benefits in thickening the ventricular wall and improving cardiac function after injection partially due to the sustained mechanical support for the debilitated ventricular wall. The application of this composite hydrogel with Ly-PRF may provide a powerful therapeutic tool in the treatment of MI for further clinical application.
CRediT authorship contribution statement
Bei Qian: Conceptualization, Methodology, Investigation, Writing – original draft. Qi Yang: Conceptualization, Methodology, Investigation, Writing – original draft. Mingliang Wang: Methodology, Investigation. Shixing Huang: Methodology. Chenyu Jiang: Conceptualization, Investigation. Hongpeng Shi: Investigation. Qiang Long: Methodology. Mi Zhou: Supervision, Funding acquisition, Conceptualization. Qiang Zhao: Supervision, Funding acquisition, Conceptualization. Xiaofeng Ye: Supervision, Funding acquisition, Conceptualization, Methodology, Writing – review & editing.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgments
This work was supported by the Shanghai Science and Technology Committee (18411966200, 19441906800), the Ningbo 2025 Science and Technology Major Project (2019B10068), Project on Independent Innovation and Research of Health System in Putuo District Shanghai (ptkwws201818) and Shanghai Sailing Program (21YF1426500). The author would like to thank Chao Chen from Ningbo Diochange Medical Technology, Ningbo, China for his support with this work.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2021.05.042.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Delling F.N., Djousse L., Elkind M.S.V., Ferguson J.F., Fornage M., Khan S.S., Kissela B.M., Knutson K.L., Kwan T.W., Lackland D.T., Lewis T.T., Lichtman J.H., Longenecker C.T., Loop M.S., Lutsey P.L., Martin S.S., Matsushita K., Moran A.E., Mussolino M.E., Perak A.M., Rosamond W.D., Roth G.A., Sampson U.K.A., Satou G.M., Schroeder E.B., Shah S.H., Shay C.M., Spartano N.L., Stokes A., Tirschwell D.L., VanWagner L.B., Tsao C.W., American Heart Association Council on E., Prevention Statistics C., Stroke Statistics S. Heart disease and stroke statistics-2020 update: a report from the American heart association. Circulation. 2020;141(9):e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 2.Landa N., Miller L., Feinberg M.S., Holbova R., Shachar M., Freeman I., Cohen S., Leor J. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation. 2008;117(11):1388–1396. doi: 10.1161/CIRCULATIONAHA.107.727420. [DOI] [PubMed] [Google Scholar]
- 3.Hao T., Li J., Yao F., Dong D., Wang Y., Yang B., Wang C. Injectable fullerenol/alginate hydrogel for suppression of oxidative stress damage in Brown adipose-derived stem cells and cardiac repair. ACS Nano. 2017;11(6):5474–5488. doi: 10.1021/acsnano.7b00221. [DOI] [PubMed] [Google Scholar]
- 4.Ruvinov E., Cohen S. Alginate biomaterial for the treatment of myocardial infarction: progress, translational strategies, and clinical outlook: from ocean algae to patient bedside. Adv. Drug Deliv. Rev. 2016;96:54–76. doi: 10.1016/j.addr.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 5.Rufaihah A.J., Seliktar D. Hydrogels for therapeutic cardiovascular angiogenesis. Adv. Drug Deliv. Rev. 2016;96:31–39. doi: 10.1016/j.addr.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Martinez C.E., Smith P.C., Palma Alvarado V.A. The influence of platelet-derived products on angiogenesis and tissue repair: a concise update. Front. Physiol. 2015;6:290. doi: 10.3389/fphys.2015.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dohan Ehrenfest D.M., Rasmusson L., Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF) Trends Biotechnol. 2009;27(3):158–167. doi: 10.1016/j.tibtech.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Dohan Ehrenfest D.M., Pinto N.R., Pereda A., Jimenez P., Corso M.D., Kang B.S., Nally M., Lanata N., Wang H.L., Quirynen M. The impact of the centrifuge characteristics and centrifugation protocols on the cells, growth factors, and fibrin architecture of a leukocyte- and platelet-rich fibrin (L-PRF) clot and membrane. Platelets. 2018;29(2):171–184. doi: 10.1080/09537104.2017.1293812. [DOI] [PubMed] [Google Scholar]
- 9.Dohan D.M., Choukroun J., Diss A., Dohan S.L., Dohan A.J., Mouhyi J., Gogly B. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006;101(3):e37–44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Mariani E., Pulsatelli L. Platelet concentrates in musculoskeletal medicine. Int. J. Mol. Sci. 2020;21(4) doi: 10.3390/ijms21041328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choukroun J., Diss A., Simonpieri A., Girard M.O., Schoeffler C., Dohan S.L., Dohan A.J., Mouhyi J., Dohan D.M. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006;101(3):e56–60. doi: 10.1016/j.tripleo.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J., Qi X., Luo X., Li D., Wang H., Li T. Clinical and immunohistochemical performance of lyophilized platelet-rich fibrin (Ly-PRF) on tissue regeneration. Clin. Implant Dent. Relat. Res. 2017;19(3):466–477. doi: 10.1111/cid.12473. [DOI] [PubMed] [Google Scholar]
- 13.Powell C.A., Bannister S.R., Mackey S.A., Maller S.C., McDonnell H.T., Deas D.E. Periodontal wound healing with and without platelet-rich plasma: histologic observations and assessment of flap tensile strength. J. Periodontol. 2009;80(6):985–992. doi: 10.1902/jop.2009.080626. [DOI] [PubMed] [Google Scholar]
- 14.Pascoal M., Dos Santos N.B.M., Completo A.M.G., Fernandes G.V.O. Tensile strength assay comparing the resistance between two different autologous platelet concentrates (leucocyte-platelet rich fibrin versus advanced-platelet rich fibrin): a pilot study. Int J Implant Dent. 2021;7(1):1. doi: 10.1186/s40729-020-00284-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee H.M., Shen E.C., Shen J.T., Fu E., Chiu H.C., Hsia Y.J. Tensile strength, growth factor content and proliferation activities for two platelet concentrates of platelet-rich fibrin and concentrated growth factor. J. Dent. Sci. 2020;15(2):141–146. doi: 10.1016/j.jds.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun C.K., Zhen Y.Y., Leu S., Tsai T.H., Chang L.T., Sheu J.J., Chen Y.L., Chua S., Chai H.T., Lu H.I., Chang H.W., Lee F.Y., Yip H.K. Direct implantation versus platelet-rich fibrin-embedded adipose-derived mesenchymal stem cells in treating rat acute myocardial infarction. Int. J. Cardiol. 2014;173(3):410–423. doi: 10.1016/j.ijcard.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 17.Liu Z., Jin H., Xie Q., Jiang Z., Guo S., Li Y., Zhang B. Controlled release strategies for the combination of fresh and lyophilized platelet-rich fibrin on bone tissue regeneration. BioMed Res. Int. 2019;2019:4923767. doi: 10.1155/2019/4923767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nie J., Zhang S., Wu P., Liu Y., Su Y. Electrospinning with lyophilized platelet-rich fibrin has the potential to enhance the proliferation and osteogenesis of MC3T3-E1 cells. Front Bioeng Biotechnol. 2020;8:595579. doi: 10.3389/fbioe.2020.595579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q., Reed D.A., Min L., Gopinathan G., Li S., Dangaria S.J., Li L., Geng Y., Galang M.T., Gajendrareddy P., Zhou Y., Luan X., Diekwisch T.G. Lyophilized platelet-rich fibrin (PRF) promotes craniofacial bone regeneration through Runx 2. Int. J. Mol. Sci. 2014;15(5):8509–8525. doi: 10.3390/ijms15058509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennardo F., Liborio F., Barone S., Antonelli A., Buffone C., Fortunato L., Giudice A. Efficacy of platelet-rich fibrin compared with triamcinolone acetonide as injective therapy in the treatment of symptomatic oral lichen planus: a pilot study. Clin. Oral Invest. 2021;25(6):3747–3755. doi: 10.1007/s00784-020-03702-w. [DOI] [PubMed] [Google Scholar]
- 21.Gassling V.L., Acil Y., Springer I.N., Hubert N., Wiltfang J. Platelet-rich plasma and platelet-rich fibrin in human cell culture. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009;108(1):48–55. doi: 10.1016/j.tripleo.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Joki T., Machluf M., Atala A., Zhu J., Seyfried N.T., Dunn I.F., Abe T., Carroll R.S., Black P.M. Continuous release of endostatin from microencapsulated engineered cells for tumor therapy. Nat. Biotechnol. 2001;19(1):35–39. doi: 10.1038/83481. [DOI] [PubMed] [Google Scholar]
- 23.Huang S., Lei D., Yang Q., Yang Y., Jiang C., Shi H., Qian B., Long Q., Chen W., Chen Y., Zhu L., Yang W., Wang L., Hai W., Zhao Q., You Z., Ye X. A perfusable, multifunctional epicardial device improves cardiac function and tissue repair. Nat. Med. 2021;27(3):480–490. doi: 10.1038/s41591-021-01279-9. [DOI] [PubMed] [Google Scholar]
- 24.Plotkin M., Vaibavi S.R., Rufaihah A.J., Nithya V., Wang J., Shachaf Y., Kofidis T., Seliktar D. The effect of matrix stiffness of injectable hydrogels on the preservation of cardiac function after a heart attack. Biomaterials. 2014;35(5):1429–1438. doi: 10.1016/j.biomaterials.2013.10.058. [DOI] [PubMed] [Google Scholar]
- 25.Lv K., Li Q., Zhang L., Wang Y., Zhong Z., Zhao J., Lin X., Wang J., Zhu K., Xiao C., Ke C., Zhong S., Wu X., Chen J., Yu H., Zhu W., Li X., Wang B., Tang R., Wang J., Huang J., Hu X. Incorporation of small extracellular vesicles in sodium alginate hydrogel as a novel therapeutic strategy for myocardial infarction. Theranostics. 2019;9(24):7403–7416. doi: 10.7150/thno.32637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yancy C.W., Jessup M., Bozkurt B., Butler J., Casey D.E., Jr., Drazner M.H., Fonarow G.C., Geraci S.A., Horwich T., Januzzi J.L., Johnson M.R., Kasper E.K., Levy W.C., Masoudi F.A., McBride P.E., McMurray J.J., Mitchell J.E., Peterson P.N., Riegel B., Sam F., Stevenson L.W., Tang W.H., Tsai E.J., Wilkoff B.L. ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):1810–1852. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 27.Mosser D.M., Edwards J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008;8(12):958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pena B., Laughter M., Jett S., Rowland T.J., Taylor M.R.G., Mestroni L., Park D. Injectable hydrogels for cardiac tissue engineering. Macromol. Biosci. 2018;18(6) doi: 10.1002/mabi.201800079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leor J., Tuvia S., Guetta V., Manczur F., Castel D., Willenz U., Petnehazy O., Landa N., Feinberg M.S., Konen E., Goitein O., Tsur-Gang O., Shaul M., Klapper L., Cohen S. Intracoronary injection of in situ forming alginate hydrogel reverses left ventricular remodeling after myocardial infarction in Swine. J. Am. Coll. Cardiol. 2009;54(11):1014–1023. doi: 10.1016/j.jacc.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 30.Anker S.D., Coats A.J., Cristian G., Dragomir D., Pusineri E., Piredda M., Bettari L., Dowling R., Volterrani M., Kirwan B.A., Filippatos G., Mas J.L., Danchin N., Solomon S.D., Lee R.J., Ahmann F., Hinson A., Sabbah H.N., Mann D.L. A prospective comparison of alginate-hydrogel with standard medical therapy to determine impact on functional capacity and clinical outcomes in patients with advanced heart failure (AUGMENT-HF trial) Eur. Heart J. 2015;36(34):2297–2309. doi: 10.1093/eurheartj/ehv259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee R.J., Hinson A., Bauernschmitt R., Matschke K., Fang Q., Mann D.L., Dowling R., Schiller N., Sabbah H.N. The feasibility and safety of Algisyl-LVR as a method of left ventricular augmentation in patients with dilated cardiomyopathy: initial first in man clinical results. Int. J. Cardiol. 2015;199:18–24. doi: 10.1016/j.ijcard.2015.06.111. [DOI] [PubMed] [Google Scholar]
- 32.Tong S., Zhang L., Joseph J., Jiang X. A potential and novel therapeutic approach to ischemic heart failure: algisyl-LVR. Int. J. Cardiol. 2018;271:209. doi: 10.1016/j.ijcard.2018.05.052. [DOI] [PubMed] [Google Scholar]
- 33.Vu T.D., Pal S.N., Ti L.K., Martinez E.C., Rufaihah A.J., Ling L.H., Lee C.N., Richards A.M., Kofidis T. An autologous platelet-rich plasma hydrogel compound restores left ventricular structure, function and ameliorates adverse remodeling in a minimally invasive large animal myocardial restoration model: a translational approach: vu and Pal "Myocardial Repair: PRP, Hydrogel and Supplements". Biomaterials. 2015;45:27–35. doi: 10.1016/j.biomaterials.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 34.Anitua E., Nurden P., Prado R., Nurden A.T., Padilla S. Autologous fibrin scaffolds: when platelet- and plasma-derived biomolecules meet fibrin. Biomaterials. 2019;192:440–460. doi: 10.1016/j.biomaterials.2018.11.029. [DOI] [PubMed] [Google Scholar]
- 35.Giannini S., Cielo A., Bonanome L., Rastelli C., Derla C., Corpaci F., Falisi G. Comparison between PRP, PRGF and PRF: lights and shadows in three similar but different protocols. Eur. Rev. Med. Pharmacol. Sci. 2015;19(6):927–930. [PubMed] [Google Scholar]
- 36.Dohan D.M., Choukroun J., Diss A., Dohan S.L., Dohan A.J., Mouhyi J., Gogly B. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006;101(3):e45–50. doi: 10.1016/j.tripleo.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 37.Prabhu S.D., Frangogiannis N.G. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ. Res. 2016;119(1):91–112. doi: 10.1161/CIRCRESAHA.116.303577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frangogiannis N.G. The inflammatory response in myocardial injury, repair, and remodelling. Nat. Rev. Cardiol. 2014;11(5):255–265. doi: 10.1038/nrcardio.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang N., Liu C., Wang X., He T., Li L., Liang X., Wang L., Song L., Wei Y., Wu Q., Gong C. Hyaluronic acid oligosaccharides improve myocardial function reconstruction and angiogenesis against myocardial infarction by regulation of macrophages. Theranostics. 2019;9(7):1980–1992. doi: 10.7150/thno.31073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murray P.E. Platelet-rich plasma and platelet-rich fibrin can induce apical closure more frequently than blood-clot revascularization for the regeneration of immature permanent teeth: a meta-analysis of clinical efficacy. Front Bioeng Biotechnol. 2018;6:139. doi: 10.3389/fbioe.2018.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keene D.J., Alsousou J., Harrison P., Hulley P., Wagland S., Parsons S.R., Thompson J.Y., O'Connor H.M., Schlussel M.M., Dutton S.J., Lamb S.E., Willett K., group P.-t. Platelet rich plasma injection for acute Achilles tendon rupture: PATH-2 randomised, placebo controlled, superiority trial. BMJ. 2019;367:l6132. doi: 10.1136/bmj.l6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reurink G., Goudswaard G.J., Moen M.H., Weir A., Verhaar J.A., Bierma-Zeinstra S.M., Maas M., Tol J.L. I. Dutch Hamstring Injection Therapy Study, Platelet-rich plasma injections in acute muscle injury. N. Engl. J. Med. 2014;370(26):2546–2547. doi: 10.1056/NEJMc1402340. [DOI] [PubMed] [Google Scholar]
- 43.Hao X., Silva E.A., Mansson-Broberg A., Grinnemo K.H., Siddiqui A.J., Dellgren G., Wardell E., Brodin L.A., Mooney D.J., Sylven C. Angiogenic effects of sequential release of VEGF-A165 and PDGF-BB with alginate hydrogels after myocardial infarction. Cardiovasc. Res. 2007;75(1):178–185. doi: 10.1016/j.cardiores.2007.03.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.