Abstract
Background
We conducted a multicenter clinical study to examine the prognostic value of the systemic inflammation response index (SIRI) in renal cell carcinoma (RCC) patients.
Methods
We collected patients who underwent nephrectomy from 2014 to 2019 at three centers (343 in the training group and 100 in the validation group). SIRI was created based on hemoglobin and lymphocyte to monocyte ratio (LMR). Kaplan–Meier curves and receiver operating characteristic (ROC) curves were used to analyze the effect of LMR, hemoglobin and SIRI on overall survival (OS) and cancer-specific survival (CSS) effects.
Results
In both the training and validation groups, SIRI was a better predictor of OS and CSS than LMR and hemoglobin. A total of 192 (56.0%) patients were included in grade 1, 108 (31.5%) in grade 2, and 43 (12.5%) in grade 3 based on SIRI in the training group. Higher SIRI was associated with worse prognosis. Multivariate cox regression analysis showed that SIRI was an independent prognostic risk factor for OS (grade 3 vs grade 1: HR=4.93; 95% CI 2.21–11.00, p < 0.001) and CSS (grade 3 vs grade 1: HR=6.29; 95% CI 2.28–17.39, p < 0.001) in patients with RCC. In addition, SIRI-based prognostic nomograms were able to better predict OS and CSS in RCC patients.
Conclusion
SIRI is an independent prognostic factor for patients undergoing laparoscopic nephrectomy for RCC, and a prognostic nomogram covering SIRI can better predict survival of RCC patients.
Keywords: renal cell carcinoma, systemic inflammation response index, nomogram, prognostic indicator
Introduction
Renal cell carcinoma (RCC), also known as renal cancer, is one of the most common malignancies in the urinary tract, and its incidence has been increasing at a rapid rate of 2% per year over the past two decades.1,2 400,000 new cases and 170,000 deaths of renal cancer were reported worldwide in 2018; approximately 70,000 new cases and 43,000 deaths of renal cancer were reported in China.3,4 Surgery is still the main treatment for RCC, and about 30% of RCC patients have metastases at the time of initial diagnosis, and approximately 25% of patients with localized RCC will develop local recurrence or distant metastases after surgery.5,6 Due to recurrence or distant metastasis, the 5-year survival rate of patients with advanced RCC is extremely low, approximately 5%–10%.7,8
The development of malignant tumors was related to the malignant characteristics of tumor cells, and also related to the tumor microenvironment.9 Studies have confirmed the importance of systemic inflammation and local immune response in the progression of malignant tumors and patient prognosis.10 Inflammatory cells (neutrophils, lymphocytes, monocytes) are an important part of the tumor microenvironment, and their mediated inflammatory responses can promote tumor cell proliferation, angiogenesis, apoptosis, invasion and metastasis, and suppress anti-tumor immunity.11 In addition, combined metrics based on multiple inflammatory cells, such as neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR) and lymphocyte to monocyte ratio (LMR), have been confirmed to be independently associated with tumor outcome in various cancers.12,13 In addition, preoperative hemoglobin and serum albumin levels have been identified as tumor-related prognostic predictors.14,15
In this study, we aimed to assess the prognostic value of systemic inflammatory biomarkers in patients with RCC. We created a systemic inflammatory response index (SIRI) based on hemoglobin and LMR and evaluated the prognostic ability of SIRI for overall survival (OS) and cancer-specific survival (CSS) in patients undergoing laparoscopic nephrectomy in a multicenter clinical study.
Materials and Methods
Study Design and Patients
In this study, we retrospectively collected clinical data from 590 RCC patients from January 2014 to December 2019 at three centers in Zhongda Hospital Southeast University, Shanghai Tenth People’s Hospital and Shidong Hospital. All patients were pathologically diagnosed with RCC and underwent partial or radical nephrectomy. We excluded patients who received other preoperative anti-cancer treatments, or had other malignancies in combination, or lacked complete medical records or were lost to follow-up. After screening, we excluded 147 patients, and finally 443 patients were included in the study.
Three hundred and forty-three patients from Zhongda Hospital Southeast University were included in the training group, and 100 patients from Shanghai Tenth People’s Hospital and Shidong Hospital were categorized in the validation group. All patients or relatives signed the written informed consent. The methodology of this study followed the criteria outlined in the Declaration of Helsinki (as revised in 2013) and was ethically approved by the Ethics Committees and Institutional Review Boards of all participating institutions.
Clinical Data Collection and Follow-Up
Basic clinical information and laboratory test data for all patients were obtained from the patients’ electronic cases. Included study variables were age, gender, body mass index (BMI, calculated by weight (kg)/height2 (m2)), hypertension, diabetes, cardiovascular disease, smoking, surgery type, laterality, AJCC stage, T stage, N stage, M stage, Fuhrman grade, neutrophils, lymphocytes, monocytes, platelets, albumin and hemoglobin. The patient’s basic clinical information was determined at the date of first diagnosis, and laboratory test data were measured two days prior to surgery or closest to the time of surgery. LMR was defined as lymphocyte to monocyte ratio. OS was calculated from the date of surgical treatment to the date of death or the last follow-up. CSS was calculated from the date of therapeutic resection to the date of death due to RCC.
Statistical Analysis
Continuous data were expressed as mean ± standard deviation (SD) and categorical data were presented as number (%). Continuous variables were analyzed using t-test and categorical variables were analyzed using chi-square test. The cutoff value for hemoglobin has been divided into male (<137 g/L) and female (<116 g/L).16,17 The optimal cutoff values for LMR, albumin, NLR and PLR were determined based on receiver operating characteristic (ROC) curves and patients were divided into high LMR and low LMR groups, high hemoglobin and low hemoglobin group. SIRI was determined based on LMR and hemoglobin. SIRI was defined as follows: high LMR and high hemoglobin patients were included in grade 1, low LMR and low hemoglobin patients were included in grade 3, and the remaining patients were included in grade 2.
Kaplan–Meier curves were used to assess the effects of LMR, hemoglobin and SIRI on OS and CSS. ROC curves, which were calculated using the area under the curve (AUC), were used to compare the predictive ability of LMR, hemoglobin and SIRI on OS and CSS. Univariate and multivariate Cox regressions were used to assess the relationship among SIRI and OS and CSS and to calculate the associated adjusted hazard ratio (aHR) and 95% confidence interval (CI). Based on the results of multivariate Cox regression analysis, we included independent risk factors for OS and CSS in the prognostic nomogram. Statistical analyses for this study were performed using SPSS software (version 26.0) and GraphPad Prism (version 8.3.0), and nomograms were constructed using R software (version 3.6.2). P value <0.05 was considered statistically significant.
Results
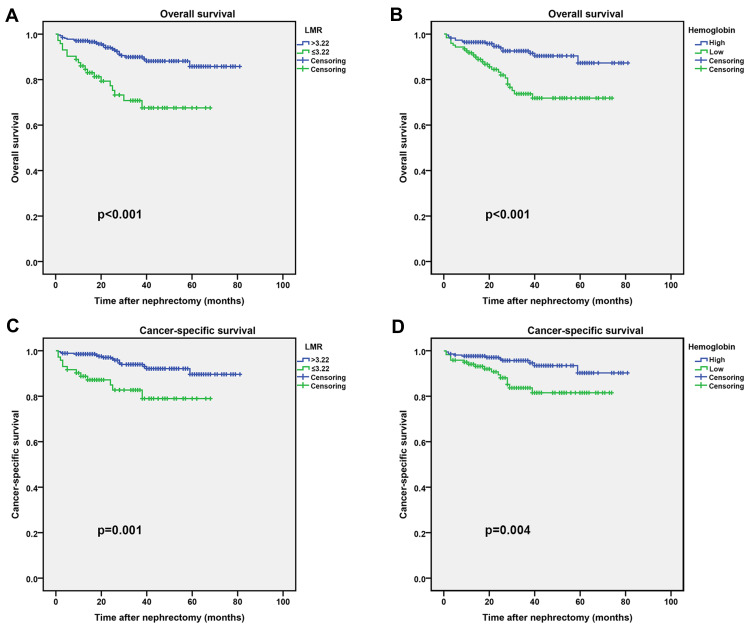
According to the ROC curve, the optimal cutoff values for LMR, albumin, NLR and PLR were 3.22, 37.7, 2.88 and 163.13, respectively (Figure S1). Based on the critical values, we divided the patients into high LMR group (>3.22) and low LMR group (≤3.22), high hemoglobin group and low hemoglobin group. The clinicopathological characteristics of all patients in the training and validation groups are shown in Table 1 and Table S1. In the training group, we found that LMR was associated with surgery type, AJCC stage, T stage, M stage and Fuhrman grade, whereas hemoglobin was associated with age, BMI, type of surgery, AJCC stage, TNM stage and Fuhrman grade. In the validation group, LMR was correlated with age, and hemoglobin was correlated with age, gender, BMI, smoking and M stage. In addition, Kaplan–Meier curves showed that high LMR and high hemoglobin were associated with longer OS and CSS in both the training and validation groups (Figure 1 and Figure S2).
Table 1.
Clinical Characteristics of the Patients According to LMR and Hemoglobin in the Training Group
| Characteristic | LMR | P value | Hemoglobin | P value | |||
|---|---|---|---|---|---|---|---|
| All | High Group | Low Group | High Group | Low Group | |||
| Patients | N=271 | N=72 | N=221 | N=122 | |||
| Age categorized, y | 0.873 | 0.003 | |||||
| ≤65 | 255 (74.3) | 202 (74.5) | 53 (73.6) | 176 (79.6) | 79 (64.8) | ||
| >65 | 88 (25.7) | 69 (25.5) | 19 (26.4) | 45 (20.4) | 43 (35.2) | ||
| Gender | 0.876 | 0.116 | |||||
| Male | 226 (65.9) | 178 (65.7) | 48 (66.7) | 139 (62.9) | 87 (71.3) | ||
| Female | 117 (34.1) | 93 (34.3) | 24 (33.3) | 82 (37.1) | 35 (28.7) | ||
| BMI categorized, kg/m2 | 0.169 | 0.011 | |||||
| <25 | 185 (53.9) | 141 (52.0) | 44 (61.1) | 108 (48.9) | 77 (63.1) | ||
| ≥25 | 158 (46.1) | 130 (48.0) | 28 (38.9) | 113 (51.1) | 45 (36.9) | ||
| Hypertension | 0.980 | 0.262 | |||||
| No | 191 (55.7) | 151 (55.7) | 40 (55.6) | 128 (57.9) | 63 (51.6) | ||
| Yes | 152 (44.3) | 120 (44.3) | 32 (44.4) | 93 (42.1) | 59 (48.4) | ||
| Diabetes | 0.844 | 0.863 | |||||
| No | 288 (84.0) | 227 (83.8) | 61 (84.7) | 185 (83.7) | 103 (84.4) | ||
| Yes | 55 (16.0) | 44 (16.2) | 11 (15.3) | 36 (16.3) | 19 (15.6) | ||
| Cardiovascular diseases | 0.697 | 0.659 | |||||
| No | 300 (87.5) | 238 (87.8) | 62 (86.1) | 192 (86.9) | 108 (88.5) | ||
| Yes | 43 (12.5) | 33 (12.2) | 10 (13.9) | 29 (13.1) | 14 (11.5) | ||
| Smoking | 0.469 | 0.601 | |||||
| No | 286 (83.4) | 228 (84.1) | 58 (80.6) | 186 (84.2) | 100 (82.0) | ||
| Yes | 57 (16.6) | 43 (15.9) | 14 (19.4) | 35 (15.8) | 22 (18.0) | ||
| Surgery type | <0.001 | <0.001 | |||||
| Partial nephrectomy | 187 (54.5) | 168 (62.0) | 19 (26.4) | 148 (67.0) | 39 (32.0) | ||
| Radical nephrectomy | 156 (45.5) | 103 (38.0) | 53 (73.6) | 73 (33.0) | 83 (68.0) | ||
| Laterality | 0.615 | 0.968 | |||||
| Left | 171 (49.9) | 134 (49.4) | 38 (52.8) | 110 (49.8) | 61 (50.0) | ||
| Right | 172 (50.1) | 137 (50.6) | 34 (47.2) | 111 (50.2) | 61 (50.0) | ||
| AJCC stage | <0.001 | <0.001 | |||||
| I | 256 (74.6) | 220 (81.2) | 36 (50.0) | 183 (82.8) | 73 (59.8) | ||
| II | 19 (5.5) | 10 (3.7) | 9 (12.5) | 10 (4.5) | 9 (7.4) | ||
| III | 45 (13.1) | 29 (10.7) | 16 (22.2) | 18 (8.1) | 27 (22.1) | ||
| IV | 23 (6.7) | 12 (4.4) | 11 (15.3) | 10 (4.5) | 13 (10.7) | ||
| T-stage | <0.001 | <0.001 | |||||
| T1 | 260 (75.8) | 223 (82.3) | 37 (51.4) | 185 (83.7) | 75 (61.5) | ||
| T2 | 23 (6.7) | 13 (4.8) | 10 (13.9) | 12 (5.4) | 11 (9.0) | ||
| T3 | 51 (14.9) | 28 (10.3) | 23 (31.9) | 17 (7.7) | 34 (27.9) | ||
| T4 | 9 (2.6) | 7 (2.6) | 2 (2.8) | 7 (3.2) | 2 (1.6) | ||
| N-stage | 0.613 | 0.010 | |||||
| N0 | 330 (96.2) | 260 (95.9) | 70 (97.2) | 217 (98.2) | 113 (92.6) | ||
| N1 | 13 (3.8) | 11 (4.1) | 2 (2.8) | 4 (1.8) | 9 (7.4) | ||
| M-stage | <0.001 | 0.005 | |||||
| M0 | 327 (95.3) | 264 (97.4) | 63 (87.5) | 216 (97.7) | 111 (91.0) | ||
| M1 | 16 (4.7) | 7 (2.6) | 9 (12.5) | 5 (2.3) | 11 (9.0) | ||
| Fuhrman grade | 0.004 | 0.001 | |||||
| I | 55 (16.0) | 47 (17.3) | 8 (11.1) | 44 (19.9) | 11 (9.0) | ||
| II | 216 (63.0) | 176 (64.9) | 40 (55.6) | 136 (61.5) | 80 (65.6) | ||
| III | 64 (18.7) | 45 (16.6) | 19 (26.4) | 40 (18.1) | 24 (19.7) | ||
| IV | 8 (2.3) | 3 (1.1) | 5 (6.9) | 1 (0.5) | 7 (5.7) | ||
Abbreviations: LMR, lymphocyte to monocyte ratio; BMI, body mass index; AJCC, American Joint Committee on Cancer.
Figure 1.
Kaplan–Meier curves for OS and CSS stratified by LMR and hemoglobin in the training group. (A and C), LMR OS and CSS; (B and D), Hemoglobin OS and CSS.
Abbreviations: LMR, lymphocyte to monocyte ratio; OS, overall survival; CSS, cancer-specific survival.
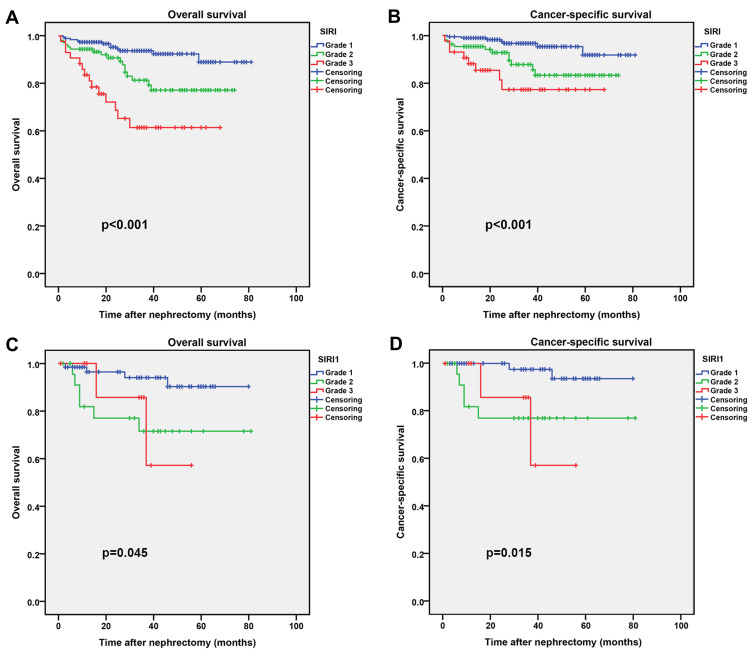
According to SIRI, the training group 192 (56.0%) patients were included in the grade 1 group, 108 (31.5%) patients in the grade 2 group, and 43 (12.5%) patients in the grade 3 group in (Table 2), and in the validation group 64 (64.0%) patients were included in the grade 1 group, 25 (25.0%) patients in the grade 2 group, and 11 (11%) patients in the grade 3 group (Table S2). We found that SIRI was associated with BMI, smoking, surgery type, AJCC stage, T stage, M stage and Fuhrman grade in the training group, while SIRI was associated with age, smoking and M stage in the validation group. Moreover, Kaplan–Meier survival curve analysis showed that low SIRI was associated with better OS and CSS both in the training and validation groups (Figure 2).
Table 2.
Baseline Characteristics of the Patients According to SIRI in the Training Group
| Characteristic | SIRI | P value | ||
|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | ||
| No. (%) | No. (%) | No. (%) | ||
| All patients | 192 (56.0) | 108 (31.5) | 43 (12.5) | |
| Age, y | 0.069 | |||
| ≤65 | 152 (79.2) | 74 (68.5) | 29 (67.4) | |
| >65 | 40 (20.8) | 34 (31.5) | 14 (32.6) | |
| Gender | 0.450 | |||
| Male | 121 (63.0) | 75 (69.4) | 30 (69.8) | |
| Female | 71 (37.0) | 33 (30.6) | 13 (30.2) | |
| BMI, kg/m2 | 0.038 | |||
| <25 | 94 (49.0) | 61 (56.5) | 30 (69.8) | |
| ≥25 | 98 (51.0) | 47 (43.5) | 13 (30.2) | |
| Hypertension | 0.464 | |||
| No | 112 (58.3) | 55 (50.9) | 24 (55.8) | |
| Yes | 80 (41.7) | 53 (49.1) | 19 (44.2) | |
| Diabetes | 0.918 | |||
| No | 161 (83.9) | 90 (83.3) | 37 (86.0) | |
| Yes | 31 (16.1) | 18 (16.7) | 6 (14.0) | |
| Cardiovascular diseases | 0.614 | |||
| No | 169 (88.0) | 92 (85.2) | 39 (90.7) | |
| Yes | 23 (12.0) | 16 (14.8) | 4 (9.3) | |
| Smoking | 0.042 | |||
| No | 159 (82.8) | 96 (88.9) | 31 (72.1) | |
| Yes | 33 (17.2) | 12 (11.1) | 12 (27.9) | |
| Surgery type | <0.001 | |||
| Partial nephrectomy | 134 (69.8) | 48 (44.4) | 5 (11.6) | |
| Radical nephrectomy | 58 (30.2) | 60 (55.6) | 38 (88.4) | |
| Laterality | 0.885 | |||
| Left | 96 (50.0) | 55 (50.9) | 20 (46.5) | |
| Right | 96 (50.0) | 53 (49.1) | 23 (53.5) | |
| AJCC stage | <0.001 | |||
| I | 165 (85.9) | 73 (67.6) | 18 (41.9) | |
| II | 6 (3.1) | 8 (7.4) | 5 (11.6) | |
| III | 16 (8.3) | 15 (13.9) | 14 (32.6) | |
| IV | 5 (2.6) | 12 (11.1) | 6 (14.0) | |
| T-stage | <0.001 | |||
| T1 | 167 (87.0) | 74 (68.5) | 19 (44.2) | |
| T2 | 7 (3.6) | 11 (10.2) | 5 (11.6) | |
| T3 | 13 (6.8) | 19 (17.6) | 19 (44.2) | |
| T4 | 5 (2.6) | 4 (3.7) | 0 (0.0) | |
| N-stage | 0.152 | |||
| N0 | 188 (97.9) | 101 (93.5) | 41 (95.3) | |
| N1 | 4 (2.1) | 7 (6.5) | 2 (4.7) | |
| M-stage | <0.001 | |||
| M0 | 190 (99.0) | 100 (92.6) | 37 (86.0) | |
| M1 | 2 (1.0) | 8 (7.4) | 6 (14.0) | |
| Fuhrman grade | <0.001 | |||
| I | 39 (20.3) | 13 (12.0) | 3 (7.0) | |
| II | 119 (62.0) | 74 (68.5) | 23 (53.5) | |
| III | 33 (17.2) | 19 (17.6) | 12 (27.9) | |
| IV | 1 (0.5) | 2 (1.9) | 5 (11.6) | |
Abbreviation: SIRI, systemic inflammation response index; BMI, body mass index; AJCC, American Joint Committee on Cancer.
Figure 2.
Kaplan–Meier curves for OS and CSS stratified by SIRI. (A and B), SIRI OS and CSS in the training group; (C and D), SIRI OS and CSS in the validation group.
Abbreviations: OS, overall survival; CSS, cancer-specific survival; SIRI, systemic inflammation response index.
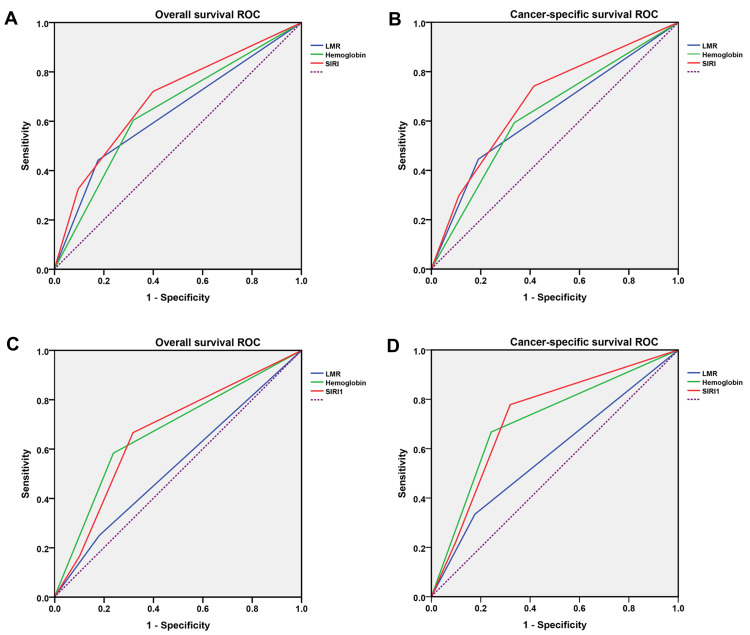
ROC curves were used to assess the prognostic ability of LMR, hemoglobin and SIRI in patients with RCC undergoing laparoscopic nephrectomy (Table 3). We found a higher predictive ability of SIRI for OS (Training set: AUC=0.691, 95% CI 0.603–0.779, p < 0.001; Test set: AUC=0.667, 95% CI 0.504–0.829, p=0.062) and CSS (Training set: AUC=0.683, 95% CI 0.578–0.789, p=0.002; Test set: AUC=0.726, 95% CI 0.559–0.894, p=0.025) than LMR and hemoglobin in both the training and test sets (Figure 3).
Table 3.
Analysis of Predictive Accuracy Through the Evaluation of the Area Under the Curve (AUC)
| Overall Survival | Cancer-Specific Survival | |||||
|---|---|---|---|---|---|---|
| AUC | 95% CI | Pvalue | AUC | 95% CI | Pvalue | |
| Training Set | ||||||
| SIRI | 0.691 | 0.603–0.779 | <0.001 | 0.683 | 0.578–0.789 | 0.002 |
| LMR | 0.633 | 0.537–0.729 | 0.005 | 0.627 | 0.509–0.746 | 0.028 |
| Hemoglobin | 0.642 | 0.552–0.732 | 0.003 | 0.629 | 0.509–0.746 | 0.027 |
| Test Set | ||||||
| SIRI | 0.667 | 0.504–0.829 | 0.062 | 0.726 | 0.559–0.894 | 0.025 |
| LMR | 0.534 | 0.354–0.714 | 0.703 | 0.579 | 0.370–0.787 | 0.437 |
| Hemoglobin | 0.672 | 0.500–0.845 | 0.054 | 0.712 | 0.526–0.899 | 0.036 |
Abbreviation: AUC, area under the curve; CI, confidence interval; OS, overall survival; CSS, cancer-specific survival; LMR, lymphocyte to monocyte ratio; SIRI, systemic inflammation response index.
Figure 3.
Comparison of area under ROC curves for LMR, hemoglobin and SIRI in predicting OS and CSS. (A) OS ROC curves in the training group; (B) CSS ROC curves in the training group; (C) OS ROC curves in the validation group; (D) CSS ROC curves in the validation group.
Abbreviations: ROC, receiver operating characteristic; OS, overall survival; CSS, cancer-specific survival; LMR, lymphocyte to monocyte ratio; SIRI, systemic inflammation response index.
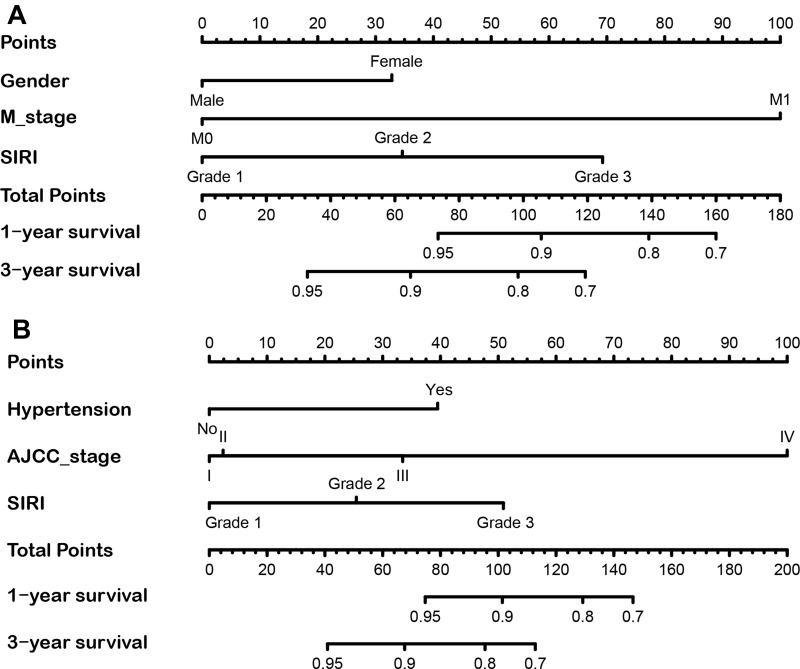
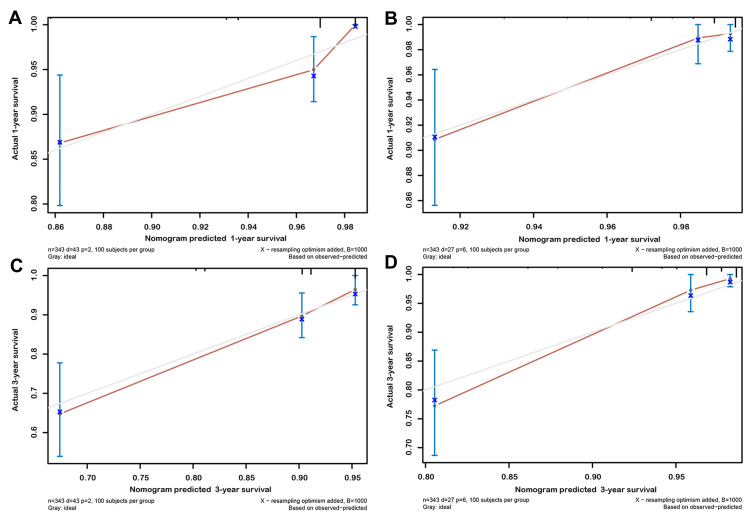
Subsequently, in the training group, we used multivariate Cox regression model to assess the correlation of SIRI with OS and CSS (Table 4). The results showed that SIRI was an independent risk factor for OS (grade 3 vs grade 1: HR=4.93; 95% CI 2.21–11.00, p < 0.001) and CSS (grade 3 vs grade 1: HR=6.29; 95% CI 2.28–17.39, p < 0.001), and that SIRI grade 3 was associated with the worst prognosis. Based on the results of the above multivariate regression analysis, we constructed prognostic nomograms for OS and CSS and validated in the validation group (Figure 4). The results showed that the constructed prognostic nomograms covering SIRI were able to predict 1-year and 3-year OS and CSS readily in both the training and validation groups (Figure 5 and Figure S3).
Table 4.
Multivariate Analyses of Factors Associated with Overall Survival (OS) and Cancer-Specific Survival (CSS) in the Training Group
| Characteristics | Overall Survival | Cancer-Specific Survival | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P value | Hazard Ratio (95% CI) | P value | |
| Age, y | ||||
| ≤65 | Reference | Reference | ||
| >65 | - | 0.657 | 0.402 | |
| Gender | ||||
| Male | Reference | Reference | ||
| Female | 2.25 (1.14–4.44) | 0.020 | 0.284 | |
| BMI, kg/m2 | ||||
| <25 | Reference | Reference | ||
| ≥25 | - | 0.457 | 0.497 | |
| Hypertension | ||||
| No | Reference | Reference | ||
| Yes | - | 0.484 | 2.95 (1.33–6.54) | 0.008 |
| Diabetes | ||||
| No | Reference | Reference | ||
| Yes | - | 0.561 | - | 0.789 |
| Cardiovascular Diseases | ||||
| No | Reference | Reference | ||
| Yes | - | 0.577 | - | 0.633 |
| Smoking | ||||
| No | Reference | Reference | ||
| Yes | - | 0.881 | - | 0.847 |
| Surgery Type | ||||
| Partial nephrectomy | Reference | Reference | ||
| Radical nephrectomy | - | 0.183 | 4.65 (1.51–14.28) | 0.007 |
| Laterality | ||||
| Left | Reference | Reference | ||
| Right | - | 0.889 | - | 0.828 |
| AJCC stage | ||||
| I | Reference | Reference | ||
| II | - | 0.290 | 1.13 (0.11–6.88) | 0.583 |
| III | - | 0.203 | 2.10 (0.68–6.46) | 0.196 |
| IV | - | 0.077 | 11.53 (4.62–28.75) | <0.001 |
| T-stage | ||||
| T1 | Reference | Reference | ||
| T2 | - | 0.794 | - | 0.033 |
| T3 | - | 0.297 | - | 0.491 |
| T4 | - | 0.162 | - | 0.310 |
| N-stage | ||||
| N0 | Reference | Reference | ||
| N1 | - | 0.056 | - | 0.254 |
| M-stage | ||||
| M0 | Reference | Reference | ||
| M1 | 8.39 (3.87–18.19) | <0.001 | - | 0.338 |
| Fuhrman Grade | ||||
| I | Reference | Reference | ||
| II | - | 0.891 | - | 0.878 |
| III | - | 0.936 | - | 0.870 |
| IV | - | 0.029 | - | 0.189 |
| ALB | ||||
| Reference | Reference | |||
| 0.155 | - | 0.125 | ||
| NLR | ||||
| Reference | Reference | |||
| 0.709 | - | 0.359 | ||
| PLR | ||||
| Reference | Reference | |||
| 0.233 | - | 0.874 | ||
| SIRI | ||||
| Grade 1 | Reference | Reference | ||
| Grade 2 | 1.91 (0.87–4.21) | 0.109 | 1.27 (0.78–5.04) | 0.513 |
| Grade 3 | 4.93 (2.21–11.00) | <0.001 | 6.29 (2.28–17.39) | <0.001 |
Abbreviations: OS, overall survival; CSS, cancer-specific survival; CI, confidence interval; BMI, body mass index; AJCC, American Joint Committee on Cancer; SIRI, systemic inflammation response index.
Figure 4.
The nomogram predicting 1-year and 3-year OS and CSS rate of RCC patients the training cohort. (A) OS nomogram; (B) CSS nomogram.
Abbreviations: OS, overall survival; CSS, cancer-specific survival; SIRI, systemic inflammation response index.
Figure 5.
Calibration plot of the nomogram for predicting 1-year and 3-year OS and CSS in training cohort. (A) 1-year OS; (B) 1-year CSS; (C) 3-year OS; (D) 3-year CSS.
Abbreviations: OS, overall survival; CSS, cancer-specific survival.
Discussion
Regarding the predictive indicators of RCC prognosis, the traditional concept is that the stage of the tumor is closely related to the prognosis, and a higher stage often indicates shorter survival.18 Many literatures have reported that tumor diameter, lymph node metastasis status, tumor invasion and other conditions are closely related to RCC prognosis.19 Inflammatory and immune markers in routine blood have also been used to study the correlation with cancer occurrence, progression and outcome.20 Lymphocytes and monocytes in the blood, which are closely related to immunity, have been reported to correlate with the prognosis of a variety of tumors in the body.21 Peripheral blood indicators have more of a preoperative predictive role in malignancies and are useful in guiding treatment options such as the extent of surgery and the choice of postoperative radiotherapy.22
In recent years, there has been a strong interest in the prognostic value of peripheral blood biomarkers in RCC patients, such as PLR and NLR can be used as prognostic predictors of RCC.23,24 A variety of inflammatory and immune cells have been confirmed to participate in the whole process of malignant tumor progression, and the body’s immune response to tumor cells is an important factor in the prognosis of malignant tumors. As an important component of the tumor microenvironment, lymphocytes, which are produced by lymphoid organs, participated in tumor cell genesis and development.25 The presence of peritumor lymphocyte infiltration in patients with RCC is an important independent prognostic marker.26 Lymphocytes are important performers in the body’s immune function, which exert cytotoxic effects directly or secret interleukins and other cytokines to mediate immune responses to inhibit tumor growth and distant metastasis and exert immune surveillance.27 Lymphocytes can play a role in eliminating tumor cells and inhibiting their proliferation and growth through CD4 and CD8 lymphocytes and natural killer cells.28 Since lymphocytes are both an indicator of inflammation and a marker that can determine the prognosis of patients with malignant neoplasms, the relationship between inflammatory response and malignancy is closely linked.
Similarly, monocytes have been reported that they can affect the progression of RCC and are associated with RCC patient poor prognosis.29 Further in the mechanism of monocytes affecting RCC, it was found that monocytes in the tumor microenvironment are stimulated by inflammatory factors to differentiate into tumor-associated macrophages, which can be activated into different subtypes that, in the presence of different subtypes, not only inhibit the growth of tumor cells but also promote their proliferation and metastasis.30 Monocyte count can reflect the tumor microenvironment infiltrating macrophages, and also directly supply energy to cancer cells, promote angiogenesis in cancer foci, and facilitate tumor evasion from immune surveillance and subsequently promote tumor progression.31
LMR is an indicator that integrates lymphocytes and monocytes and provides a more comprehensive picture of the chronic inflammatory state of the body.32 Its diagnostic and prognostic value has been demonstrated in a variety of tumors. The results of a meta-analysis showed that low LMR levels were significantly associated with shorter overall survival, progression-free survival, and tumor-specific survival in non-hematologic malignancies and hematologic malignancies.33
The development of tumor is the outcome of the interaction between the body and the tumor, and the prognosis of tumor patients is not only related to the qualities of the tumor itself, but also to the immune status and nutrition of the patients.34 Hemoglobin, as an important parameter of complete blood count, reflects to some extent the degree of anemia and the nutritional status of the patient’s body. It has been suggested that hemoglobin is closely related to the prognosis of colorectal cancer, gastric cancer, endometrial cancer and many other cancers.35–37
A combination of multiple inflammatory cells has been shown to correlate with the prognosis of various cancers. Based on NLR, PLR and LMR, Ferro et al38 developed prostatic systemic inflammatory markers (PSIM) and found that PSIM was the only independent prognostic variable affecting the probability of adverse pathology in prostate cancer. In addition, the usefulness of inflammation-based SIRI prognostic scores for patients with RCC has been reported. By investigating 161 patients with metastatic RCC who underwent cytoreductive nephrectomy, Gu et al16 found that high SIRI was associated with poorer OS and was an independent prognostic predictor of OS, with aggressive tumor behavior significantly associated. Fukuda et al17 retrospectively analyzed data from 161 patients who underwent cytoreductive nephrectomy for metastatic RCC underwent cytoreductive nephrectomy in 152 patients and found that SIRI predicted survival in patients with RCC. In our study, we performed a multicenter clinical study and found that SIRI better predicted OS and CSS in RCC patients than LMR and hemoglobin, and that SIRI was an independent risk factor for OS and CSS in RCC patients. In addition, we constructed prognostic nomograms for OS and CSS based on SIRI, which could better predict survival of RCC patients.
In speculating the reasons for the poor prognosis of RCC patients in the high SIRI group, we found that patients in the high SIRI group included more cases of AJCC III/IV stage, T3/T4 stage, M1 stage and Fuhrman III/IV grade, which may contribute to the poorer prognosis of patients in the grade 3 group.
This study also has several limitations. First, we did not assess the patient’s quality of life, energy level or postoperative nutritional status. Second, we did not include other treatments in the study, which could also have an impact on the prognosis. Finally, although this is a multicenter study, it was still a retrospective study which requires an expanded sample for prospective study.
Conclusion
In general, we created a biomarker that better reflects systemic inflammation in RCC patients, SIRI. In addition, we found that SIRI was an independent prognostic factor for OS and CSS in RCC patients undergoing laparoscopic nephrectomy and SIRI-based prognostic nomograms were good predictors of survival in RCC patients.
Funding Statement
This study was supported by the Scientific Research Foundation of Graduate School of Southeast University (YBPY2173), Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX21_0156), the National Natural Science Foundation of China (81672551), Jiangsu Provincial Key Research and Development Program (BE2019751), Innovative Team of Jiangsu Provincial (2017XKJQW07), and The National Key Research and Development Program of China (SQ2017YFSF090096).
Data Sharing Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The methodology of this study was ethically approved by the Ethics Committees and Institutional Review Boards of all participating institutions (SHSY-IEC-BG/02.04/04.0-81602469 and ZDKYSB077).
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest for this work.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 2.Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67(5):913–924. doi: 10.1016/j.eururo.2015.01.005 [DOI] [PubMed] [Google Scholar]
- 3.Margulis V, Sagalowsky AI. Penile cancer: management of regional lymphatic drainage. Urol Clin North Am. 2010;37(3):411–419. doi: 10.1016/j.ucl.2010.04.009 [DOI] [PubMed] [Google Scholar]
- 4.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 5.Tannir NM, Pal SK, Atkins MB. Second-line treatment landscape for renal cell carcinoma: a comprehensive review. Oncologist. 2018;23(5):540–555. doi: 10.1634/theoncologist.2017-0534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mao W, Wang K, Zhang H, et al. Sarcopenia as a poor prognostic indicator for renal cell carcinoma patients undergoing nephrectomy in China: a multicenter study. Clin Transl Med. 2021;11(1):e270. doi: 10.1002/ctm2.270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yong C, Stewart GD, Frezza C. Oncometabolites in renal cancer. Nat Rev Nephrol. 2020;16(3):156–172. doi: 10.1038/s41581-019-0210-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bianchi M, Sun M, Jeldres C, et al. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann Oncol. 2012;23(4):973–980. doi: 10.1093/annonc/mdr362 [DOI] [PubMed] [Google Scholar]
- 9.Ji F, Liang Y, Fu SJ, et al. A novel and accurate predictor of survival for patients with hepatocellular carcinoma after surgical resection: the neutrophil to lymphocyte ratio (NLR) combined with the aspartate aminotransferase/platelet count ratio index (APRI). BMC Cancer. 2016;16:137. doi: 10.1186/s12885-016-2189-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–503. doi: 10.1016/S1470-2045(14)70263-3 [DOI] [PubMed] [Google Scholar]
- 11.Gu L, Ma X, Li H, et al. Prognostic value of preoperative inflammatory response biomarkers in patients with sarcomatoid renal cell carcinoma and the establishment of a nomogram. Sci Rep. 2016;6:23846. doi: 10.1038/srep23846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dupre A, Malik HZ. Inflammation and cancer: what a surgical oncologist should know. Eur J Surg Oncol. 2018;44(5):566–570. doi: 10.1016/j.ejso.2018.02.209 [DOI] [PubMed] [Google Scholar]
- 13.Demirdal T, Sen P. The significance of neutrophil-lymphocyte ratio, platelet-lymphocyte ratio and lymphocyte-monocyte ratio in predicting peripheral arterial disease, peripheral neuropathy, osteomyelitis and amputation in diabetic foot infection. Diabetes Res Clin Pract. 2018;144:118–125. doi: 10.1016/j.diabres.2018.08.009 [DOI] [PubMed] [Google Scholar]
- 14.Motzer RJ, Bacik J, Schwartz LH, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004;22(3):454–463. doi: 10.1200/JCO.2004.06.132 [DOI] [PubMed] [Google Scholar]
- 15.McMillan DC. The systemic inflammation-based Glasgow prognostic score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39(5):534–540. doi: 10.1016/j.ctrv.2012.08.003 [DOI] [PubMed] [Google Scholar]
- 16.Gu L, Ma X, Wang L, et al. Prognostic value of a systemic inflammatory response index in metastatic renal cell carcinoma and construction of a predictive model. Oncotarget. 2017;8(32):52094–52103. doi: 10.18632/oncotarget.10626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukuda H, Takagi T, Kondo T, Shimizu S, Tanabe K. Predictive value of inflammation-based prognostic scores in patients with metastatic renal cell carcinoma treated with cytoreductive nephrectomy. Oncotarget. 2018;9(18):14296–14305. doi: 10.18632/oncotarget.24507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ficarra V, Novara G, Iafrate M, et al. Proposal for reclassification of the TNM staging system in patients with locally advanced (pT3-4) renal cell carcinoma according to the cancer-related outcome. Eur Urol. 2007;51(3):722–9; discussion 9–31. doi: 10.1016/j.eururo.2006.07.010 [DOI] [PubMed] [Google Scholar]
- 19.Lam JS, Breda A, Belldegrun AS, Figlin RA. Evolving principles of surgical management and prognostic factors for outcome in renal cell carcinoma. J Clin Oncol. 2006;24(35):5565–5575. doi: 10.1200/JCO.2006.08.1794 [DOI] [PubMed] [Google Scholar]
- 20.Watson J, Round A, Hamilton W. Raised inflammatory markers. BMJ. 2012;344:e454. doi: 10.1136/bmj.e454 [DOI] [PubMed] [Google Scholar]
- 21.Murray PJ. Immune regulation by monocytes. Semin Immunol. 2018;35:12–18. doi: 10.1016/j.smim.2017.12.005 [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Florez LJ, Gomez-Alvarez G, Frunza AM, Barneo-Serra L, Martinez-Alonso C, Fresno-Forcelledo MF. Predictive markers of response to neoadjuvant therapy in rectal cancer. J Surg Res. 2015;194(1):120–126. doi: 10.1016/j.jss.2014.10.005 [DOI] [PubMed] [Google Scholar]
- 23.Huszno J, Kolosza Z, Mrochem-Kwarciak J, Rutkowski T, Skladowski K. The role of neutrophil-lymphocyte ratio, platelet-lymphocyte ratio, and platelets in the prognosis of metastatic renal cell carcinoma. Oncology. 2019;97(1):7–17. doi: 10.1159/000498943 [DOI] [PubMed] [Google Scholar]
- 24.Chrom P, Stec R, Bodnar L, Szczylik C. Incorporating neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in place of neutrophil count and platelet count improves prognostic accuracy of the international metastatic renal cell carcinoma database consortium model. Cancer Res Treat. 2018;50(1):103–110. doi: 10.4143/crt.2017.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruddle NH. Lymphatic vessels and tertiary lymphoid organs. J Clin Invest. 2014;124(3):953–959. doi: 10.1172/JCI71611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kauffman EC, Liu H, Schwartz MJ, Scherr DS. Toll-like receptor 7 agonist therapy with imidazoquinoline enhances cancer cell death and increases lymphocytic infiltration and proinflammatory cytokine production in established tumors of a renal cell carcinoma mouse model. J Oncol. 2012;2012:103298. doi: 10.1155/2012/103298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bauer J, Becker AJ, Elyaman W, et al. Innate and adaptive immunity in human epilepsies. Epilepsia. 2017;58(Suppl 3):57–68. doi: 10.1111/epi.13784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raphael I, Nalawade S, Eagar TN, Forsthuber TG. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine. 2015;74(1):5–17. doi: 10.1016/j.cyto.2014.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donskov F, Hokland M, Marcussen N, Torp Madsen HH, von der Maase H. Monocytes and neutrophils as ‘bad guys’ for the outcome of interleukin-2 with and without histamine in metastatic renal cell carcinoma–results from a randomised Phase II trial. Br J Cancer. 2006;94(2):218–226. doi: 10.1038/sj.bjc.6602937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66(1):1–9. doi: 10.1016/j.critrevonc.2007.07.004 [DOI] [PubMed] [Google Scholar]
- 31.Yang M, McKay D, Pollard JW, Lewis CE. Diverse functions of macrophages in different tumor microenvironments. Cancer Res. 2018;78(19):5492–5503. doi: 10.1158/0008-5472.CAN-18-1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goto W, Kashiwagi S, Asano Y, et al. Predictive value of lymphocyte-to-monocyte ratio in the preoperative setting for progression of patients with breast cancer. BMC Cancer. 2018;18(1):1137. doi: 10.1186/s12885-018-5051-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gu LY, Li HZ, Chen LY, et al. Prognostic role of lymphocyte to monocyte ratio for patients with cancer: evidence from a systematic review and meta-analysis. Oncotarget. 2016;7(22):31926–31942. doi: 10.18632/oncotarget.7876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McMillan DC, Watson WS, O’Gorman P, Preston T, Scott HR, McArdle CS. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer. 2001;39(2):210–213. doi: 10.1207/S15327914nc392_8 [DOI] [PubMed] [Google Scholar]
- 35.Wallace K, Li H, Brazeal JG, et al. Platelet and hemoglobin count at diagnosis are associated with survival in African American and Caucasian patients with colorectal cancer. Cancer Epidemiol. 2020;67:101746. doi: 10.1016/j.canep.2020.101746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang XZ, Yang YC, Chen Y, et al. Preoperative anemia or low hemoglobin predicts poor prognosis in gastric cancer patients: a meta-analysis. Dis Markers. 2019;2019:7606128. doi: 10.1155/2019/7606128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Obon-Santacana M, Freisling H, Peeters PH, et al. Acrylamide and glycidamide hemoglobin adduct levels and endometrial cancer risk: a nested case-control study in nonsmoking postmenopausal women from the EPIC cohort. Int J Cancer. 2016;138(5):1129–1138. doi: 10.1002/ijc.29853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferro M, Musi G, Matei DV, et al. Assessment of PSIM (prostatic systemic inflammatory markers) score in predicting pathologic features at robotic radical prostatectomy in patients with low-risk prostate cancer who met the inclusion criteria for active surveillance. Diagnostics (Basel). 2021;11(2):355. [DOI] [PMC free article] [PubMed] [Google Scholar]