Abstract
Background
Hepatic hemangiomas are the most typical benign mesenchymal lesions of the liver. Most of these lesions are asymptomatic. Giant hepatic hemangiomas (GHH) (>10 cm) are often symptomatic and require surgical intervention. This study aimed to describe the clinical findings, risk factors, diagnostic approach and management of GHH.
Methods
We performed a retrospective analysis of patients with GHH treated at our hospital from January 2008 to December 2018. The medical records of each patient were reviewed to obtain the clinical and surgical data.
Results
Twelve patients with GHH were treated during the study period. 9 were female and 3 were male. The mean age of diagnosis was 48,2 years. The most common presenting symptom was abdominal pain. Eight patients presented an abdominal mass. Indications for surgical resections were rupture (n = 2), Kasabach-Merritt syndrome (n = 1) and abdominal pain (n = 9). Right hepatectomy was done in four patients, left lobectomy in four patients, and enucleation in four patients. Embolization was performed in 4 patients, but due to the persistence of symptoms or bleeding, surgery was indicated. The mean operative time was 3.5 h, and median blood loss was 870 ml. The median hospital stay was 5.3 days. For four patients, we registered postoperative complications causing death in one case. All alive patients were asymptomatic at a median follow-up of 55 months.
Conclusion
Despite limitations and alternative modalities, surgery remains the only effective curative treatment for GHH.
Keywords: Giant hepatic hemangioma, Liver resection /surgery
Highlights
-
•
Hepatic hemangiomas are the most common benign tumors of the liver with the incidence of 0.4–20%.
-
•
Giant hepatic hemangiomas (GHH) are usually responsible for overt clinical symptoms and complications.
-
•
Management of liver hemangiomas ranges from close observation to surgery depending upon the site, size and symptoms.
-
•
Surgical resection is indicated in patients with abdominal complaints or complications, or when diagnosis remains inconclusive.
1. Introduction
Hepatic hemangiomas are the most common benign tumors of the liver, with an incidence of 0.4–20% [1,2]. Most of them are usually small in size, ranging from a few mm to 3 cm in diameter. They are composed of clusters of blood-filled cavities lined by endothelial cells and fed by the hepatic artery [3]. They are most often discovered incidentally in imaging studies. Typical hemangiomas usually do not increase in size over time and therefore are unlikely to cause symptoms. Giant hepatic hemangiomas (GHH) are defined as hemangiomas larger than 5 cm [3,4]. They are usually responsible for overt clinical symptoms and complications consisting, most often, of upper abdominal pain, hemorrhage, biliary compression, or a consumptive coagulopathy that may require prompt surgical intervention or other treatments [5]. Management of liver hemangiomas ranges from close observation to surgery depending upon the site, size, and symptoms.
It is widely accepted that intervention is indicated only for symptomatic hemangiomas. Surgical resection is indicated in patients with abdominal complaints or complications or when the diagnosis remains inconclusive. The ideal surgical treatment for GHH is still controversial; however enucleation is the preferred surgical method [6,7]. Based on the existing literature. In this study, we report our 17-year experience in the clinical management of GHH. the work has been reported in line with the PROCESS criteria [8].
2. Materials and methods
A retrospective review of patients who underwent surgery for GHH in the Department of digestive surgery, Sahloul hospital, Tunisia, was performed between January 2008 and December 2018. The data collection included patient demographics, clinical presentation, tumor characteristics, diagnostic studies, surgical procedures, and outcomes.
3. Results
A total of twelve patients of GHH were treated by surgery over the 10-year of the study, which were radiologically and histologically proven liver hemangioma. The characteristics of twelve patients with giant liver hemangiomas are shown in Table 1.
Table 1.
Clinicoradiological characteristics.
| Patient № | Age (years)/gender | Clinical presentation | location | Size (cm) |
|---|---|---|---|---|
| 1 | 54/F | Right upper quadrant pain and mass, vomiting | Left lobe | 11*9 |
| 2 | 47/F | Right upper quadrant pain and mass | Right lobe | 13*10 |
| 3 | 27/H | Right upper quadrant pain and mass, vomiting | Left lobe | 12*8 |
| 4 | 44/F | Right upper quadrant pain and mass | Right lobe | 17*8 |
| 5 | 36/H | Right upper quadrant pain | Left lobe | 15*13 |
| 6 | 32/F | Right upper quadrant pain and mass | Left lobe | 18*12 |
| 7 | 60/F | Right upper quadrant pain and mass, vomiting | Left lobe | 12*9 |
| 8 | 38/F | Hemorrhage shock | Right lobe | 22*16 |
| 9 | 51/F | Right upper quadrant pain and mass | Right lobe | 13*6 |
| 10 | 69/F | Hemorrhage shock | Left lobe | 19*15 |
| 11 | 66/F | Kasabach-Merritt syndrome | Left lobe | 17*10 |
| 12 | 55/H | Right upper quadrant pain and mass | Right lobe | 14*10 |
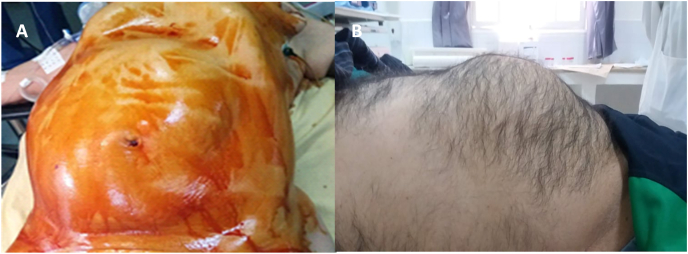
During the study period, about twelve patients were operated for GHH. Of these patients, 9 were female (75%), and 3 were male (25%). The mean age of diagnosis was 48,2 years (range 27–69 years). Three patients had a medical history of diabetes, four patients had hypertension, and one patient had Myocardial Infarction 5 years ago. In addition six patients had a history of surgery: two patients were operated on for acute cholecystitis, one patient for ovarian cyst, one patient for appendicitis, and one patient for breast cancer. The most common presenting symptom was abdominal pain (9/10cases) and vomiting (3/10cases). Eight patients presented an abdominal mass (Fig. 1). Indications for surgical resections were rupture (n = 2), Kasabach-Merritt syndrome (n = 1) and abdominal pain (n = 9).
Fig. 1.
(A,B) preoperative abdominal mass.
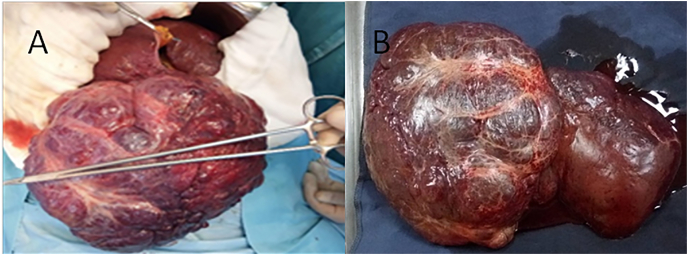
In all the patients, the imaging findings were obtained from the case records. The extent and severity of the disease were evaluated by hepatic ultrasound (US), abdominal spiral CT scan, and/or magnetic resonance imaging (MRI). The hemangiomas were usually multiple (50%) and bilobar (25%) and had a median size of 15.2 cm (range, 11–22 cm) (Fig. 2).
Fig. 2.
GHH of the right liver (A), right hepatectomy carrying out the GHH (B).
The type of liver resection was decided based on the hemangioma's size, location and morphology (Table 2). Right hepatectomy was done in four patients (Fig. 3), left lobectomy in four patients, and enucleation in four patients.
Table 2.
Operative details and postoperative outcomes.
| Patient № |
Transcathater aretrial embolization | Surgery | Intra operative blood loss (ml) | Post operative stay (days) | Post operative complications | Follow up (years) | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | Left lobectomy | 750 | 5 | uneventful | 6 | asymptomatic | |
| 2 | Right hepatic artery embolization | Right hepatectomy | 1100 | 5 | uneventful | 9 | asymptomatic |
| 3 | enucleation | 500 | 4 | uneventful | 7 | asymptomatic | |
| 4 | Right hepatectomy | 700 | 6 | uneventful | 5 | asymptomatic | |
| 5 | Left hepatic artry embolization | Left hepatectomy | 600 | 8 | Abdominal hematoma | 8 | asymptomatic |
| 6 | enucleation | 400 | 4 | uneventful | 2 | asymptomatic | |
| 7 | enucleation | 300 | 8 | Abdominal biloma | 1 | asymptomatic | |
| 8 | Right hepatic artery embolization | Right hepatectomy | 1800 | 12 | Abdominal hematoma | 10 | asymptomatic |
| 9 | Right hepatectomy | 900 | 5 | uneventful | 3 | asymptomatic | |
| 10 | Left hepatic artery embolization | Left Lobectomy | 2200 | 2 | pulmonary embolism causing death | 0 | dead |
| 11 | Left lobectomy | 850 | 9 | uneventful | 2 | asymptomatic | |
| 12 | enucleation | 350 | 4 | uneventful | 2 | asymptomatic |
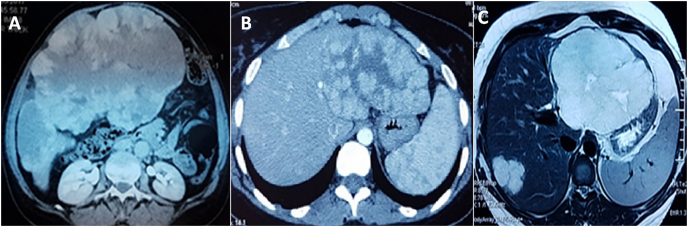
Fig. 3.
Abdominal CT scan revealed GHH of the right lobe (A), Abdominal CT scan revealed GHH of the left lobe (B), Hepatic MRI with T2 sequence reveals GHH of the left lobe associated to another hemangioma localized in segment VII (C).
Embolization was performed in 4 patients. In two cases for ruptured GHH but due to persistence of bleeding, surgery was indicated. In the two other cases, embolization was performed for symptomatic GHH causing abdominal pain. Follow-up arteriography showed complete occlusion of the embolized vessel. Six months later, the symptoms were only partly relieved, and on repeat CT, the tumor size had decreased. Due to persistent symptoms, the patient underwent surgery.
Enucleation was performed by dissecting the tumor from the surrounding hepatic parenchyma along the plane of the tumor capsule. Then, hepatic resection was carried out by removing the hepatic parenchyma containing the hemangioma. The mean operative time was 3.5 h (range, 2–5 h) and median blood loss was 870 ml. The median hospital stay was 5.3 days (range, 4–12 days).
For four patients, we registered postoperative complications causing death in one case. In two cases, it was an abdominal hematoma spontaneously resolved in 4 weeks for the first case and evacuated by radiologic percutaneous drainage in the second case. In one case, it was abdominal biloma spontaneously resolved in 4 weeks, and in another case, the patient presented a pulmonary embolism causing death.
After discharge, all patients were followed up by physical examination, laboratory values (blood count and liver function), and liver ultrasonography at 6 months intervals during the first year and yearlyafter that. In addition, MRI or computed tomography was performed annually. All alive patients were asymptomatic at a median follow-up of 55 months.
4. Discussion
Hemangiomas are composed of multiple, large vessels lined by a single layer of endothelial cells within a thin fibrous stroma. Liver hemangiomas usually occur in the fifth and sixth decades of life and are more common in women [2,8]. Although the exact etiology is still unclear, some authors suggest a genetic predisposition. Exposure to high levels of estrogen and progesterone in pregnancy and oral contraceptives are reasons for the increased incidence in women. In addition, Studies have demonstrated that estrogen stimulates endothelial cell proliferation and organization into capillary-like structures [2].
When the size of the liver hemangioma exceeds 5 cm, it is termed as “giant” hemangioma [9]. Although mosthepatic hemangiomas remain asymptomatic, increasing size or intra-tumoral thrombosis, hemorrhage or infarction can cause pain in the right upper quadrant due to the stretching of the liver capsule.
Due to the mass effect, large hemangiomas can cause compression of the adjacent structures and produce symptoms such as obstructive jaundice and gastric outlet obstruction [10,11]. In addition, it is possible for intra-tumoral hemorrhage, spontaneous tumor rupture, disseminated intravascular consumptive coagulopathy (Kasabach-Merritt syndrome) to occur in patients with giant hepatic hemangiomas [10,12,13].
Hemangiomas show specific features on radiological imaging. Conventional ultrasound (US) is usually the first used diagnostic exam that reveals hepatic hemangioma (HH) as a hyperechoic homogenous nodule, with well-defined margins and posterior acoustic enhancement [14]. Most extensive lesions can appear inhomogeneous, with mixed echogenicity (hypo- and hyperechoic) because of possible necrosis, hemorrhage, or fibrosis. Lesions that have such echo patterns are defined as atypical HH. On Doppler US, most HH shows minimal or no Doppler signals [15]. During follow-up, stable findings are a very reliable sign for benign disease. On contrast-enhanced CT, typical HH appears as a hypodense, well-defined lesion, which shows peripheral nodular enhancement with progressive centripetal homogeneous filling after contrast injection giving a “nodular peripheral puddling” appearance [3].
Most of HH are small, asymptomatic, and do not require any treatment. However, there is a small number of cases with rapid tumor growth or change in the character of a hemangioma or complications, which prompt for appropriate therapy [3].
The management of giant hepatic hemangioma is controversial. In patients with giant liver hemangioma, observation is justified in the absence of symptoms. Several treatment strategies are available for symptomatic cases: nonsurgical therapy, surgical resection or enucleation [16].
Surgical resection is indicated in patients with abdominal complaints, complications when the diagnosis remains inconclusive, rupture, and Kasabach-Merritt syndrome. In addition Iwatsuki et al. suggested that large hemangiomas may be complicated by rupture or hemorrhage and should be resected [17]. Nevertheless, in another study, Schnelldorfer reported that clinical observation is preferred in most patients, and operative treatment should be reserved for patients with severe symptoms or disease-associated complications [18].
HH can be removed by two main techniques, namely, resection and enucleation. Comparative studies between the two techniques have reported that enucleation is associated with lower morbidity and mortality [19,20,20,21], and this technique is recommended as the surgical procedure of choice for the treatment of HH [22]. Likewise, a meta-analysis conducted in 2017 concluded that enucleation could preserve more normal hepatic parenchyma, decrease the rate of postoperative complications, and should be the preferred surgical procedure for suitable lesions [23].
With the improvement in interventional radiology and super-selective catheterization techniques, transcatheter arterial embolization (TAE) has become another option for HH treatment. Compared with surgery, TAE was less risky and could effectively reduce the tumor, facilitating the surgical resection [1,24].
Radiofrequency ablation (RFA) using both percutaneous and laparoscopic methods has successfully improved abdominal pain in small numbers with symptomatic HH [25]. In addition, hepatic irradiation has been reported to produce complete regression of hepatic hemangioma with minimal morbidity [26]. .
Liver transplantation is described as a rescue treatment in children with hepatic vascular malformations leading to hemodynamic insufficiency and when conventional therapy is unsuccessful [27]. This study has some limitations for its small sample size and retrospective nature.
5. Conclusion
Our experience with these patients serves to reinforce previously published reports suggesting the feasibility and safety of resection and enucleation for giant HH. In fact, after adequate patient selection, surgical treatment of hepatic hemangiomas is a very effective therapeutic choice with no mortality and significant morbidity.
Ethical approval
The study was approved by Ethics Committee of Sahloul Hospital approval references: U2341.
Sources of funding
This study has not received any funding.
Study concept or design – MBM, HA.
Data collection – HA, WF, RG.
Data interpretation – MBM, MAS, EH.
Literature review – AKM, MAS, EH,ABA.
Drafting of the paper – HA, LG, MAS.
Editing of the paper – AR, WF,LG.
Trial registry number
-
1.
Name of the registry: ClinicalTrials.gov
-
2.
Unique Identifying number or registration ID: NCT04709718
-
3.
Hyperlink to the registration (must be publicly accessible): https://clinicaltrials.gov/ct2/show/NCT04709718
Guarantor
Waad Farhat.
Consent
Written informed consent was obtained from the patient.
Provenance and peer review
Not commissioned, externally peer reviewed.
Declaration of competing interest
The authors declare that they have no conflict of interest.
References
- 1.Zhou J.X., Huang J.W., Wu H., Zeng Y. Successful liver resection in a giant hemangioma with intestinal obstruction after embolization. World J. Gastroenterol.: WJG. 2013;19:2974. doi: 10.3748/wjg.v19.i19.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caseiro Alves F., Brito J., Araujo A.E., Belo-Soares P., Rodrigues H., Cipriano A., Sousa D., Mathieu D. Liver haemangioma: common and uncommon findings and how to improve the differential diagnosis. Eur. Radiol. 2007;17:1544–1554. doi: 10.1007/s00330-006-0503-z. [DOI] [PubMed] [Google Scholar]
- 3.Bajenaru N., Balaban V., Săvulescu F., Campeanu I., Patrascu T. Hepatic hemangioma-review. J. Med. Life. 2015;8:4. [PMC free article] [PubMed] [Google Scholar]
- 4.Mungovan J.A., Cronan J.J., Vacarro J. Hepatic cavernous hemangiomas: lack of enlargement over time. Radiology. 1994;191:111–113. doi: 10.1148/radiology.191.1.8134554. [DOI] [PubMed] [Google Scholar]
- 5.Choi B.Y., Nguyen M.H. The diagnosis and management of benign hepatic tumors. J. Clin. Gastroenterol. 2005;39:401–412. doi: 10.1097/01.mcg.0000159226.63037.a2. [DOI] [PubMed] [Google Scholar]
- 6.Deutsch G.S., Yeh K.A., Bates W.B., III, Tannehill W.B. Embolization for management of hepatic hemangiomas. Am. Surg. 2001;67:159. [PubMed] [Google Scholar]
- 7.Duxbury M.S., Garden O.J. Giant haemangioma of the liver: observation or resection? Dig. Surg. 2010;27:7–11. doi: 10.1159/000268108. [DOI] [PubMed] [Google Scholar]
- 8.Agha R.A., Sohrabi C., Mathew G., Franchi T., Kerwan A., O'Neill N for the Process Group The PROCESS 2020 guideline: Updating consensus preferred reporting of CasE series in surgery (PROCESS) guidelines. Int. J. Surg. 2020;84:231–235. doi: 10.1016/j.ijsu.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Yoon S.S., Charny C.K., Fong Y., Jarnagin W.R., Schwartz L.H., Blumgart L.H., DeMatteo R.P. Diagnosis, management, and outcomes of 115 patients with hepatic hemangioma. J. Am. Coll. Surg. 2003;197:392–402. doi: 10.1016/S1072-7515(03)00420-4. [DOI] [PubMed] [Google Scholar]
- 10.ChandraBose K., Ramanujam A., Muthu Y. Enucleation of a giant hemangioma of liver: Old school revisited. Case Rep. Surg. 2015:2015. doi: 10.1155/2015/234767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adhikari D.R., Thakur V., Telavane P.P., Kulkarni R., Singh R., Joshi R.M. Hypergiant hepatic hemangiomas: case series. Indian J. Surg. 2015;77:40–42. doi: 10.1007/s12262-014-1104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srivastava D., Gandhi D., Seith A., Pande G., Sahni P. Transcatheter arterial embolization in the treatment of symptomatic cavernous hemangiomas of the liver: a prospective study. Abdom. Imag. 2001;26:510–514. doi: 10.1007/s00261-001-0007-x. [DOI] [PubMed] [Google Scholar]
- 13.Aslan A., zu Vilsendorf A.M., Kleine M., Bredt M., Bektas H. Adult Kasabach-Merritt syndrome due to hepatic giant hemangioma. Case Rep. Gastroenterol. 2009;3:306–312. doi: 10.1159/000242420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maruyama M., Isokawa O., Hoshiyama K., Hoshiyama A., Hoshiyama M., Hoshiyama Y. Diagnosis and management of giant hepatic hemangioma: the usefulness of contrast-enhanced ultrasonography. Bangladesh Liver J. 2013:2013. doi: 10.1155/2013/802180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim K.W., Kim T.K., Han J.K., Kim A.Y., Lee H.J., Park S.H., Kim Y.H., Choi B.I. Hepatic hemangiomas: spectrum of US appearances on gray-scale, power Doppler, and contrast-enhanced US. Korean J. Radiol. 2000;1:191–197. doi: 10.3348/kjr.2000.1.4.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim T.K., Han J.K., Kim A.Y., Park S.J., Choi B.I. Signal from hepatic hemangiomas on power Doppler US: real or artefactual? Ultrasound Med. Biol. 1999;25:1055–1061. doi: 10.1016/s0301-5629(99)00058-7. [DOI] [PubMed] [Google Scholar]
- 17.Hoekstra L.T., Bieze M., Erdogan D., Roelofs J.J., Beuers U.H., Gulik T.M.v. Management of giant liver hemangiomas: an update. Expet Rev. Gastroenterol. Hepatol. 2013;7:263–268. doi: 10.1586/egh.13.10. [DOI] [PubMed] [Google Scholar]
- 18.Iwatsuki S., Todo S., Starzl T.E. Excisional therapy for benign hepatic lesions. Surg. Gynecol. Obstet. 1990;171:240. [PMC free article] [PubMed] [Google Scholar]
- 19.Schnelldorfer T., Ware A.L., Smoot R., Schleck C.D., Harmsen W.S., Nagorney D.M. Management of giant hemangioma of the liver: resection versus observation. J. Am. Coll. Surg. 2010;211:724–730. doi: 10.1016/j.jamcollsurg.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Kuo P.C., Lewis W., Jenkins R.L. Treatment of giant hemangiomas of the liver by enucleation. J. Am. Coll. Surg. 1994;178:49–53. [PubMed] [Google Scholar]
- 21.Özden İ., Emre A., Alper A., Tunacı M., Acarlı K., Bilge O., Tekant Y., Arıoğgul O. Long-term results of surgery for liver hemangiomas. Arch. Surg. 2000;135:978–981. doi: 10.1001/archsurg.135.8.978. [DOI] [PubMed] [Google Scholar]
- 22.Hamaloglu E., Altun H., Ozdemir A., Ozenc A. Giant liver hemangioma: therapy by enucleation or liver resection. World J. Surg. 2005;29:890. doi: 10.1007/s00268-005-7661-z. [DOI] [PubMed] [Google Scholar]
- 23.Singh R.K., Kapoor S., Sahni P., Chattopadhyay T.K. Giant haemangioma of the liver: is enucleation better than resection? Ann. R. Coll. Surg. Engl. 2007;89:490–493. doi: 10.1308/003588407X202038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y., Wei X., Wang K., Shan Q., Dai H., Xie H., Zhou L., Xu X., Zheng S. Enucleation versus anatomic resection for giant hepatic hemangioma: a meta-analysis. Gastrointest. Tumors. 2016;3:153–162. doi: 10.1159/000455846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lupinacci R.M., Szejnfedld D., Farah J.F.d.M. Spontaneous rupture of a giant hepatic hemangioma. Sequential treatment with preoperative transcatheter arterial embolization and conservative hepatectomy. Il Giorn. Chir. 2011;32:469–472. [PubMed] [Google Scholar]
- 26.Gao J., Fan R.-F., Yang J.-Y., Cui Y., Ji J.-S., Ma K.-S., Li X.-L., Zhang L., Xu C.-L., Kong X.-L. Radiofrequency ablation for hepatic hemangiomas: a consensus from a Chinese panel of experts. World J. Gastroenterol. 2017;23:7077. doi: 10.3748/wjg.v23.i39.7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toro A., Mahfouz A.-E., Ardiri A., Malaguarnera M., Malaguarnera G., Loria F., Bertino G., Di Carlo I. What is changing in indications and treatment of hepatic hemangiomas. Review, Ann. Hepatol.: Off. J. Mexican Assoc. Hepatol. 2014;13 [PubMed] [Google Scholar]