Abstract
Heparan sulfate (HS), a highly sulfated linear polysaccharide, is involved in diverse biological functions in various tissues. Although previous studies have suggested a possible contribution of HS to the differentiation of white adipocytes, there has been no direct evidence supporting this. Here, we inhibited the synthesis of HS chains in 3T3-L1 cells using CRISPR–Cas9 technology, resulting in impaired differentiation of adipocytes with attenuated bone morphogenetic protein 4 (BMP4)–fibroblast growth factor 1 (FGF1) signaling pathways. HS reduction resulted in reduced glucose uptake and decreased insulin-dependent intracellular signaling. We then made heterozygous mutant mice for the Ext1 gene, which encodes an enzyme essential for the HS biosynthesis, specifically in the visceral white adipose tissue (Fabp4-Cre+::Ext1flox/WT mice, hereafter called Ext1Δ/WT) to confirm the importance of HS in vivo. The expression levels of transcription factors that control adipocyte differentiation, such as peroxisome proliferator–activated receptor gamma, were reduced in Ext1Δ/WT adipocytes, which contained smaller, unilocular lipid droplets, reduced levels of enzymes involved in lipid synthesis, and altered expression of BMP4–FGF1 signaling molecules. Furthermore, we examined the impact of HS reduction in visceral white adipose tissue on systemic glucose homeostasis. We observed that Ext1Δ/WT mice showed glucose intolerance because of insulin resistance. Our results demonstrate that HS plays a crucial role in the differentiation of white adipocytes through BMP4–FGF1 signaling pathways, thereby contributing to insulin sensitivity and glucose homeostasis.
Keywords: heparan sulfate, adipocyte, adipogenesis, insulin resistance, diabetes, bone morphogenetic protein, fibroblast growth factor
Abbreviations: BMP4, bone morphogenetic protein 4; DMEM, Dulbecco's modified Eagle's medium; Fabp4, fatty acid–binding protein 4; FGF1, fibroblast growth factor 1; HFD, high-fat diet; HS, heparan sulfate; HSBP, HS-binding protein; Irs, insulin receptor substrate; ITT, insulin tolerance test; LD, lipid droplet; ND, normal diet; NIH, National Institutes of Health; PPARγ, peroxisome proliferator–activated receptor gamma; TGF-β, transforming growth factor β; vWAT, visceral WAT; WAT, white adipose tissue
Heparan sulfate (HS) is a linear chain of repeated disaccharide subunits that are highly sulfated on their carbohydrate residues (1). HS covalently attaches to various core proteins and forms HS proteoglycans, which are abundantly distributed on cell surfaces and in the extracellular matrix. Biosynthesis of HS chains is regulated by diverse enzymes involved in chain polymerization and modification. Chain elongation is predominantly catalyzed by an enzymatic complex of Ext1 and Ext2, which sequentially adds 50 to 250 disaccharide units of N-acetylglucosamine and glucuronic acid. Various sulfotransferases promote the sulfation of these HS chains, providing them with a high negative charge density. This promotes the interaction of HS with positively charged biomolecules (HS-binding protein [HSBP]), such as growth factors and cytokines, at cell surfaces. The role of HS as a coreceptor of various signaling molecules enhances ligand–receptor encounters and results in the augmentation of intracellular signaling (2). In particular, the essential role of HS in the binding of fibroblast growth factors (FGFs) to their receptors (FGF receptors) is well documented (3).
Previous studies have revealed that HS is involved in diverse physiological functions, such as developmental processes (4), synaptic organization (5), axonal guidance (6), and angiogenesis (7). Recent reports have shown that HS is associated with various pathological conditions, including autism (8) and osteochondroma (9), attracting attention to HS as a therapeutic target in various disorders. Indeed, palovarotene, a retinoic acid receptor γ selective agonist, has been proven to possess therapeutic potential in a mouse model of osteochondroma caused by HS loss (10) and is currently under clinical trial for treatment of human osteochondroma. In addition, genome-wide association studies have indicated a risk variant of an HS synthesis gene in type 2 diabetes (11, 12, 13). Our previous studies have demonstrated that HS plays important roles in the differentiation and proliferation of pancreatic β-cells and contributes to normal insulin secretion in mice (14). A small clinical study also indicated that loss-of-function mutations in EXT1 or EXT2 impaired insulin secretion (15). Although these studies emphasize the involvement of HS in insulin secretion, its involvement in insulin sensitivity is poorly understood.
White adipose tissue (WAT) is one of the most important tissues that determine insulin sensitivity and control glucose homeostasis. White adipocytes can store excess energy as triglycerides in a unilocular lipid droplet (LD), protecting other organs from lipotoxicity. They also act as endocrine cells, releasing various adipokines to control systemic energy homeostasis (16). Differentiation of preadipocytes to mature white adipocytes dramatically potentiates their functions in glucose uptake, energy storage, and adipokine secretion, leading to the improvement of systemic glucose homeostasis (17). Previous studies have shown a prominent increase in HS during adipocyte differentiation and the involvement of several HSBPs in the differentiation process (18), suggesting that HS in white adipocytes might modulate several signaling pathways, inducing adipogenic differentiation and leading to normal insulin sensitivity and glucose homeostasis. However, the direct involvement of HS in adipocyte function remains to be elucidated.
In the present study, we inhibited the synthesis of HS chains in 3T3-L1 adipocytes, using CRISPR–Cas9 technology, to analyze the importance of HS in adipocyte differentiation and insulin-dependent glucose uptake. Then, we specifically deleted HS in mouse WAT, using the Cre-loxP system, to examine the role of HS in vivo. Our results clearly demonstrate that HS promotes the differentiation of adipocytes and contributes to normal glucose homeostasis.
Results
HS deletion in 3T3-L1 adipocytes impaired their differentiation
First, we investigated the expression of enzymes essential for HS biosynthesis during 3T3-L1 differentiation. A prominent increase in Ext1 expression, in the course of differentiation (Fig. S1A), indicates the importance of Ext1-dependent HS synthesis in 3T3-L1 differentiation. To investigate the detailed roles of HS in 3T3-L1 differentiation, we deleted the Ext1 gene in 3T3-L1 adipocytes using CRISPR–Cas9 technology to obtain Ext1-heterodeleted cells (Ext1+/− cells) (Fig. S1, B–D). Although the differentiation medium induced LD formation in Ext1+/+ 3T3-L1 cells (control cells), no LD formation was observed in Ext1+/− cells (Fig. 1A). RT-PCR analysis showed reduced expression of the differentiation-related transcription factors peroxisome proliferator–activated receptor gamma (Pparγ) and Cebpα (Fig. 1B). The expression of acetyl-CoA carboxylase 1, which encodes a rate-limiting enzyme for adipogenesis (19), was significantly decreased in Ext1+/− cells (Fig. S1E). Furthermore, the expression of fatty acid–binding protein 4 (Fabp4), commonly used as a marker for differentiated adipocytes, was decreased by HS reduction (Fig. 1B). To eliminate the possibility that off-target effects of the CRISPR–Cas9 system affected differentiation (20), we evaluated the effect of HSase on the differentiation and impact of Ext1 overexpression on Ext1+/− cells. Microscopic observation and RT-PCR analysis showed the impairment of differentiation and adipogenesis by HSase treatment (Fig. S2). Because transfection efficiency was 70% 1 day after the electroporation and Ext1 overexpression was transient, we could not completely rescue the phenotype of Ext11+/− cells. However, transient Ext1 overexpression in Ext1+/− cells induced LD formation and significantly increased the expression levels of several genes essential for adipogenic differentiation such as Cebpα and Srebp1 (Fig. S3), indicating that impaired phenotypes of Ext1+/− were not derived from off-target effects. These results demonstrate that HS reduction in 3T3-L1 cells attenuates their adipogenic differentiation.
Figure 1.
Heparan sulfate (HS) plays important roles in 3T3-L1 differentiation via modulating BMP4–FGF1 signaling.A, observation of 3T3-L1 cells at 0 and 7 days after inducing differentiation. The white scale bar represents 100 μm. B, relative mRNA expression levels of differentiation markers at 0 and 7 days after inducing differentiation. n = 3–8. The mean mRNA expression level of control 3T3-L1 cells at day 0 was set to 1. Gray: control cells at day 0; red: control cells at day 7; white: Ext1+/− cells at day 0; blue: Ext1+/− cells at day 7. ∗Comparison of gray and red; †comparison of white and blue; #comparison of red and blue. C, relative mRNA expression levels of differentiation-related genes in control 3T3-L1 cells. n = 4–6. Gray and white bars indicate the day after inducing differentiation. The mean mRNA expression level at day 0 was set to 1. D, observation of 3T3-L1 cells at 7 days after inducing differentiation, with or without BMP4–FGF1 treatment. Upper pictures: without BMP4–FGF1 treatment; lower pictures: with BMP4–FGF1 treatment. The white scale bar represents 100 μm. E, relative mRNA expression levels of BMP4–FGF1 signaling and differentiation-related genes. n = 6–9. The mean mRNA expression level of control 3T3-L1 cells without BMP4–FGF1 treatment was set to 1. Gray: control cells without BMP4–FGF1 treatment; red: control cells with BMP4–FGF1 treatment; white: Ext1+/− cells without BMP4–FGF1 treatment; blue: Ext1+/− cells with BMP4–FGF1 treatment. ∗Comparison of gray and red; †Comparison of gray and white; #Comparison of white and blue. ∗,#p < 0.05; ∗∗,##p < 0.01; and ∗∗∗,†††p < 0.005. BMP4, bone morphogenetic protein 4; Bmpr1a, bone morphogenetic protein receptor 1 a; Cebpα, CCAAT/enhancer-binding protein α; Fabp4, fatty acid–binding protein 4; FGF1, fibroblast growth factor 1; Fgfr1, fibroblast growth factor receptor 1; Pparγ, peroxisome proliferator–activated receptor γ; Tgfβr1, TGFβ receptor 1; Tgfβr2, TGFβ receptor 2; Tgfβr3, TGFβ receptor 3.
BMP4–FGF1 signaling–mediated adipogenic differentiation was inhibited by HS deletion
Next, we investigated how HS modulates differentiation of preadipocytes into mature adipocytes. Among diverse HSBPs, there is increasing evidence of the importance of bone morphogenetic protein 4 (BMP4) and FGF1 in adipocyte differentiation. BMP4 is essential for the onset of adipocyte differentiation via the increased expression of PPARγ, a master regulator for adipocyte differentiation (21, 22). FGF1 inhibits FGF2 expression in adipocytes. Since FGF2 is necessary to maintain preadipocytes in an immature state and to suppress adipogenesis, FGF1-induced FGF2 inhibition facilitates differentiation (23). Thus, we examined the involvement of BMP4–FGF1 signaling pathways in HS-dependent differentiation. Stimulation of 3T3-L1 cells with differentiation medium for 7 days in increased expression of BMP receptor 1a and FGF receptor 1 and reduced expression of Fgf2 (Fig. 1C). The expression of receptors for transforming growth factor β (TGF-β), which is an HSBP that modulates adipocyte differentiation (24), was unchanged (Fig. 1C), supporting the importance of BMP4–FGF1 signaling pathways in adipocyte differentiation. In normal 3T3-L1 cells, treatment with BMP4–FGF1 facilitated LD enlargement and a robust increase in the expressions of Pparγ and Cebpα, with decreased expression of Fgf2 (Fig. 1, D and E). The impact of BMP4–FGF1 treatment was limited in Ext1+/− 3T3-L1 cells, although the treatment promoted differentiation through the upregulation of Pparγ and Fabp4 (Fig. 1, D and E). These results suggest that BMP4–FGF1 signaling pathways are involved in HS-dependent adipogenic differentiation.
Glucose uptake by 3T3-L1 cells was attenuated by HS deletion
We examined whether the impaired differentiation, caused by HS deletion, would affect glucose uptake by 3T3-L1 cells. Basal expression levels of insulin receptor substrate (Irs) and Akt, which are important proteins for insulin-dependent glucose uptake, did not differ between control and Ext1+/− cells (Fig. 2A). However, phosphorylation of Irs and Akt by insulin stimulation was significantly impaired in Ext1+/− cells (Fig. 2A). Moreover, insulin-dependent glucose uptake in Ext1+/− cells became half of that measured in control cells (Fig. 2B). These in vitro experiments demonstrate that HS plays important roles in regulating differentiation and contributes to insulin-dependent glucose uptake.
Figure 2.
Insulin-dependent glucose uptake was decreased in Ext1-heterozygous knockout 3T3-L1 (Ext1+/−) cells.A, left, representative images of Western blot for phosphorylated insulin receptor substrate (pIrs), Irs, pAkt, and Akt, with or without insulin stimulation. Middle, protein levels of pIrs corrected by Irs intensity, with or without insulin stimulation. n = 3. Right, the protein levels of pAkt corrected by Akt intensity, with or without insulin stimulation. n = 3. The mean intensity of control 3T3-L1 cells with insulin was set to 1. B, measurement of deoxy-d-glucose uptake in 3T3-L1 cells, with or without insulin stimulation. Gray: control cells, white: Ext1+/− cells. n = 12. ∗p < 0.05 and ∗∗∗p < 0.005.
HS reduction in mouse WAT resulted in insufficient fat accumulation and lower body weight
Next, we investigated the in vivo functions of HS in WATs. A WAT-specific HS-deleted mouse was generated by crossing Ext1flox/flox mice with Fabp4-cre mice, which express Cre recombinase specifically in their WAT. Since homozygous Ext1 deletion in WAT resulted in embryonic lethality, we produced a male mouse with heterozygous Ext1 deletion, specifically in WAT (Fabp4-Cre+::Ext1flox/WT mice, hereafter called Ext1Δ/WT). Ext1flox/WT mice were used as controls. First, we examined the Cre-dependent DNA recombination of the Ext1 gene, Ext1 mRNA expression, and HS levels in visceral (epididymal) WAT (vWAT), because Fabp4 is dominantly expressed in visceral but not subcutaneous WAT (25). Ext1 gene recombination, decreased Ext1 mRNA expression, and HS reduction in Ext1Δ/WT vWAT were confirmed (Fig. S4, A–D). No compensatory increase of other HS synthases was observed in vWAT (Fig. S4B). We also detected Cre recombinase expression in subcutaneous WAT, brown adipose tissue, and monocytes (26) (Fig. 5G). However, Ext1 mRNA was reduced only in vWAT probably because of low expression levels of Cre recombinase in subcutaneous WAT, brown adipose tissue, and monocytes (Fig. S5, B, D, and F). In addition, previous study showed ectopic Fabp4 expression of heart, kidney, and liver in mice (27). However, Ext1 mRNA expressions in these organs were not changed (Fig. S5H). These results demonstrated that we obtained mice with HS reduction, specifically in vWAT.
Body weight and composition did not differ between control and Ext1Δ/WT mice fed with a normal diet (ND) (Fig. 3, A and B and Table 1). Although a high-fat diet (HFD) induced robust weight gain with increased adipose tissue in control mice, HFD-fed Ext1Δ/WT mice exhibited lower body weight with smaller vWAT (Fig. 3, A and B and Table 1), indicating the involvement of HS in adipogenesis of vWAT, in vivo. In addition, higher calorie and fat intakes were observed in Ext1Δ/WT mice. A lower plasma concentration of leptin, which is released from WAT to suppress appetite, might have contributed to increased food intake (Fig. 3, C and D).
Figure 3.
Body weight and amount of visceral adipose tissue were decreased in Ext1-heterozygous deleted mice (Ext1Δ/WT) fed with HFD.A, body weight over time. n = 3–28. B, representative images of fat by μCT. Upper pictures, control mouse and Ext1Δ/WT mouse fed with ND; lower pictures, control mouse and Ext1Δ/WT mouse fed with HFD. Blue: subcutaneous fat; beige: visceral fat. C, left, total calorie intake per day. Right, fat calorie intake per day. n = 5. D, plasma leptin concentration 6 h after starvation; gray: control mice fed with ND; red: Ext1Δ/WT mice fed with ND; blue: control mice fed with HFD; and green: Ext1Δ/WT mice fed with HFD. n = 4–5. ∗Comparison of mice fed ND; #comparison of mice fed HFD. ∗ and #p < 0.05, ∗∗p < 0.01, and ###p < 0.005. μCT, micro–computed tomography; HFD, high-fat diet; ND, normal diet.
Table 1.
Quantification of fat and muscle mass by micro–computed tomography
| Genotype | Visceral fat (g) | Subcutaneous fat (g) | Muscle (g) | Body weight (g) |
|---|---|---|---|---|
| Control (ND) | 0.11 ± 0.01 | 2.04 ± 0.03 | 15.28 ± 0.56 | 24.49 ± 0.45 |
| Ext1Δ/WT (ND) | 0.08 ± 0.01 | 1.93 ± 0.01 | 14.21 ± 0.38 | 24.95 ± 0.34 |
| Control (HFD) | 1.12 ± 0.01 | 2.57 ± 0.15 | 20.77 ± 0.33 | 31.65 ± 0.49 |
| Ext1Δ/WT (HFD) | 0.45 ± 0.10a | 2.24 ± 0.18 | 20.20 ± 0.39 | 29.65 ± 0.45b |
Eight-week-old mice were used in this experiment. Comparison of mice fed HFD. n = 4–6.
p < 0.005.
p < 0.01.
Impaired differentiation and adipogenesis in Ext1Δ/WT adipocytes
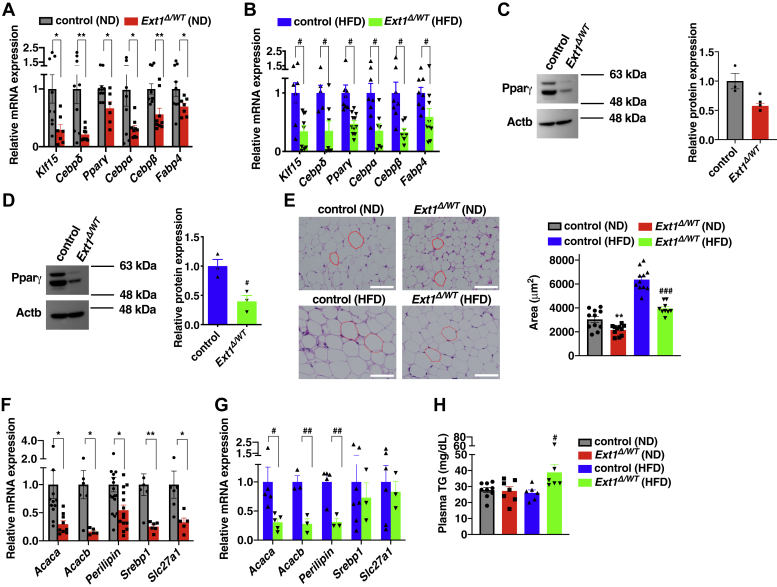
We investigated the impact of HS reduction on vWAT differentiation. The expression levels of transcriptional factors, including Klf15 and Cebpα, and the differentiation marker Fabp4 were decreased in Ext1Δ/WT vWAT (Fig. 4, A and B). We also investigated the level of protein expression of the master differentiation regulator, Pparγ, and confirmed that this was reduced in Ext1Δ/WT vWAT (Fig. 4, C and D). H&E staining showed that the visceral white adipocytes of Ext1Δ/WT mice were smaller than that of control mice, regardless of diet (Fig. 4E). Several factors of adipogenesis, such as acetyl-CoA carboxylase 1, acetyl-CoA carboxylase 2, and perilipin (19), were reduced in Ext1Δ/WT vWAT (Fig. 4, F and G). These results indicate that HS reduction impairs adipocyte differentiation and results in the formation of small adipocytes with lower adipogenesis and lipid storage. The lower capacity of lipid storage in visceral white adipocytes of Ext1Δ/WT mice might contribute to the higher plasma triglyceride levels found in Ext1Δ/WT mice fed with HFD (Fig. 4H). In addition, we did not observe the alteration of subcutaneous white adipocytes and brown adipocytes between two groups (Fig. S6).
Figure 4.
The differentiation of visceral white adipocytes was attenuated in Ext1Δ/WTmice of epididymal white adipose tissue.A and B, relative mRNA expression levels of differentiation markers in vWAT. n = 5–11. C and D, left, representative images of Western blot in Pparγ. Right, relative protein level of Pparγ in vWAT. n = 3. The mean Pparγ expression level in control vWAT was set to 1. E, left, observation of vWAT by H&E staining. The white scale bar represents 50 μm. Right, the averaged area of adipocytes in upper pictures. n = 9–11. At least 100 areas were measured, per mouse. F and G, relative mRNA expression levels of adipogenic genes in vWAT. n = 3–18. In A, B, F, and G, the mean mRNA expression level of control vWAT was set to 1. H, plasma triglyceride concentration 6 h after starvation. n = 6–9. Gray: control mice fed with ND; red: Ext1Δ/WT mice fed with ND; blue: control mice fed with HFD; and green: Ext1Δ/WT mice fed with HFD. ∗Comparison of mice fed ND; #Comparison of mice fed HFD. ∗ and #p < 0.05; ∗∗ and ##p < 0.01. Acaca, acetyl-CoA carboxylase alpha; Acacb, acetyl-CoA carboxylase beta; Cebpδ, CCAAT/enhancer-binding protein δ; Cebpβ, CCAAT/enhancer-binding protein β; HFD, high-fat diet; Klf15, krüppel-like factor 15; ND, normal diet; Pparγ, peroxisome proliferator–activated receptor gamma; Slc27a1, long-chain fatty acid transport protein 1; Srebp1, sterol regulatory element–binding protein 1; vWAT, visceral white adipose tissue.
We further examined the effect of HS deletion on BMP4–FGF1 signaling pathways in vWAT. RT-PCR analysis showed decreased mRNA expression of the ligands and their receptors and a significant increase in Fgf2 expression in Ext1Δ/WT vWAT (Fig. 5), indicating that HS inhibition affected BMP4–FGF1 signaling pathways and led to attenuated adipogenic differentiation.
Figure 5.
Expressions of BMP4–FGF1 signaling molecules were altered in vWAT of Ext1Δ/WTmice.A and C, relative mRNA expression levels of BMP4–FGF1 pathway–related genes in vWAT. n = 5–18. The mean mRNA expression level of control vWAT was set to 1. B and D, left, representative images of Western blot in Fgfr1. Right, relative protein level of Fgfr1 in vWAT. n = 3. The mean Fgfr1 expression level of control WAT was set to 1. We intentionally showed the same Western blot images of Actb for Figures 4C and 5B because we used same adipose tissues for Western blotting in these experiments. We also showed the same Actb images for Figures 4D and 5D because we used same adipose tissue homogenates to examine the expression levels of Pparγ (Fig. 4D) and Fgfr1 (Fig. 5D). Gray: vWAT isolated from control mice fed with ND; red: vWAT isolated from Ext1Δ/WT mice fed with NDl; blue: vWAT isolated from control mice fed with HFD; and green: vWAT isolated from Ext1Δ/WT mice fed with HFD. ∗Comparison of mice fed with ND; #Comparison of mice fed with HFD. ∗ and #p < 0.05; ##p < 0.01. BMP4, bone morphogenetic protein 4; FGF1, fibroblast growth factor 1; Fgfr1, fibroblast growth factor receptor 1; HFD, high-fat diet; ND, normal diet; Pparγ, peroxisome proliferator–activated receptor gamma; vWAT, visceral white adipose tissue.
Glucose intolerance because of insulin resistance in Ext1Δ/WT mice
We examined the involvement of HS in glucose homeostasis. The mRNA expression levels of glucose transporter 4, insulin receptor, and Irs1, which encode essential proteins for insulin-dependent glucose uptake, were reduced in Ext1Δ/WT vWAT (Fig. 6, A and B). Upon glucose challenge, Ext1Δ/WT mice fed with ND showed significantly higher glucose levels (Fig. 6C). When stressed by HFD, aggravated glucose intolerance was observed in Ext1Δ/WT mice. Although plasma insulin levels were not decreased in Ext1Δ/WT mice compared with control mice (Fig. 6D), the hypoglycemic action of insulin was impaired in Ext1Δ/WT mice, according to the insulin tolerance test (ITT; Fig. 6E). These results revealed that Ext1Δ/WT mice had glucose intolerance because of insulin resistance, with lower expression levels of insulin-signaling molecules in white adipocytes.
Figure 6.
Ext1Δ/WTmice showed impaired glucose tolerance because of insulin resistance.A and B, relative mRNA expression levels of glucose uptake–related genes in vWAT. n = 6–18. The mean expression level of control vWAT was set to 1. C, blood glucose levels in mice after intraperitoneal glucose injection. n = 8–27. D, plasma insulin concentrations after intraperitoneal injection of glucose. n = 4–9. E, blood glucose levels in mice after intraperitoneal insulin injection: n = 8–13. Gray: control mice fed with ND; red: Ext1Δ/WT mice fed with ND; blue: control mice fed with HFD; and green: Ext1Δ/WT mice fed with HFD. ∗Comparison of mice fed with ND; #Comparison of mice fed with HFD. ∗ and #p < 0.05; ∗∗∗p < 0.005. Glut4, glucose transporter 4; HFD, high-fat diet; Ir, insulin receptor; Irs1, insulin receptor substrate 1; ND, normal diet; vWAT, visceral white adipose tissue.
Discussion
In this study, we investigated the role of HS in 3T3-L1 adipocytes and mouse vWAT. Our experiments using 3T3-L1 cells demonstrate that HS is required for their differentiation via the induction of adipogenic transcriptional factors and contributes to normal insulin-dependent glucose uptake. In vivo experiments reveal that HS in vWAT induces differentiation and plays an important role in normal insulin sensitivity. It is noteworthy that Ext1Δ/WT mice fed with ND develop glucose intolerance because of insulin resistance in the absence of the detrimental effects of HFD on metabolic organs, emphasizing the critical importance of HS in vWAT for glucose homeostasis. A possible contribution of BMP4–FGF1 signaling pathways to HS-dependent adipocyte differentiation is also indicated.
We analyzed a male mouse with heterozygous Ext1 deletion, specifically in WAT because of lethality of homozygous Ext1 deletion in WAT. Systemic Ext1 homozygous deficiency led to embryonic lethality because of failure of gastrulation, lack of organized mesoderm, and extraembryonic tissues at embryonic day 8.5 (E8.5) (4), demonstrating that HS is essential for embryonic development. In the present study, we could confirm that Fabp4-Cre+::Ext1flox/flox mice were alive at E16.5 (Fig. S7). Previous study has shown that Fabp4 is also expressed in the heart, kidney, and liver (27), and Cre expression is detected in cardiomyocytes and hepatocytes of Fabp4-Cre+ mice (28). Indeed, we confirmed that the Cre expression in the heart, kidney, and liver of Fabp4-Cre+ mice were detected at E16.5 (Fig. S7). In addition, Wang et al. (29) reported that vWAT developed postnatally. These results might indicate that Fabp4-Cre+::Ext1flox/flox mice might die of cardiac, hepatic, and/or renal dysfunction from E16.5 to P0, although further research is required to determine the responsible organ for embryonic lethality of Fabp4-Cre+::Ext1flox/flox mouse.
Ext1 mRNA was decreased in vWAT but not in subcutaneous WAT. Although both WATs have a large capacity to store excessive energy in the form of triglycerides, they have different developmental origins and distinct characteristics (30). On the one hand, transplantation of subcutaneous WAT can ameliorate metabolic dysregulation (31). On the other hand, an excess of vWAT is associated with insulin resistance, and removal of vWAT during bariatric surgery improves insulin sensitivity (32). Our study also showed the strong contribution of vWAT to systemic glucose homeostasis. These evidences indicate the contradictory impact of these WATs on energy homeostasis. Since the importance of HS in subcutaneous WAT was not evaluated in this study, further studies should examine its role in differentiation and energy homeostasis.
Ext1Δ/WT mice showed glucose intolerance because of insulin resistance and demonstrates the strong contribution of vWAT to systemic glucose homeostasis. One of the limitations of our study was that we could not evaluate the importance of HS in subcutaneous WAT. Further studies are required to examine its role in differentiation and energy homeostasis.
Glucose intolerance because of insulin resistance, the metabolic phenotype induced by HS deletion in vWAT, is usually found in obese subjects with metabolic syndrome. However, Ext1Δ/WT mice fed with HFD had a lean phenotype, with decreased visceral fat mass and increased food intake. These characteristics are also observed in other diabetic animal models. Attenuated adipocyte differentiation because of the disruption of transcription factors can result in decreased energy storage, smaller fat mass, and glucose intolerance (17, 33). Lipoatrophic diabetes, caused by abnormal lipid homeostasis, results in less lipid storage in the adipose tissues and voracious appetite, with lower leptin secretion, higher blood triglyceride levels, and insulin resistance (33, 34). In the present study, we confirmed no ectopic accumulation of fat in liver and muscles (Fig. S8, A–C). In addition, we examined home cage locomotor activity test to assess whether increased activity of Ext1Δ/WT mice resulted in elevated energy expenditure. However, locomotor activity was not changed between two groups (Fig. S8D), indicating that energy expenditure might not be altered. Therefore, it can be deduced that HS deletion impairs adipocyte differentiation, leading to insufficient capacity for energy storage and a spillover of excessive glucose and triglyceride into the blood, without inducing obesity.
The importance of BMP4 and FGF1 in adipocyte differentiation and insulin sensitivity has been clearly demonstrated by various studies (21, 22, 23, 35). In our in vitro study, BMP4–FGF1-induced adipogenic differentiation was inhibited by HS deletion in 3T3-L1 cells. Moreover, our in vivo study revealed that a significant reduction in Bmp4, Fgf1, and their receptors was induced by HS deletion in Ext1Δ/WT mice. These data indicate substantial interaction of HS with BMP4–FGF1 signaling pathways in the differentiation of adipocytes and insulin sensitivity. However, we could not rule out the possibility that other HS-dependent signaling pathways also affected metabolic phenotypes. Treatment of Ext1+/− 3T3L1 cells with exogenous BMP4–FGF1 had a limited effect on adipogenic differentiation. Previous studies have shown that BMP4 or FGF1 deficiency in mice induces various phenotypes, such as adipocyte enlargement or macrophage infiltration, although these studies were concerned with the impact of whole-body homozygous deletion of the ligands (35, 36). Several reports have shown the inhibitory effect of TGF-β, one of the HSBPs, on adipocyte differentiation (24). Although we examined the involvement of TGF-β pathways by evaluating the expression levels of three TGF-β receptors, HS deletion in 3T3-L1 cells, and vWAT did not alter their expression (Fig. S9, A–C). Previous reports have shown that an HS proteoglycan betaglycan (TGF-β receptor III) is involved in TGF-β–dependent adipogenic differentiation (37). TGF-β could bind to the core protein betaglycan as well as to HS chains. Indeed, TGF-β stimulation induced phosphorylation of Smad3 in 3T3-L1 cells regardless of HS amount. (Fig. S9D). Thus, the interaction between TGF-β and core protein betaglycan, but not HS chains, might be sufficient to transduce their signals; however, further studies are required to examine the involvement of HS in TGF-β signaling pathways in adipocytes. Wnt ligands also have a significant impact on the differentiation of various tissues (38). In WAT, the canonical pathway inhibits adipogenesis, and the noncanonical pathway induces it (39, 40). HS 6-O-sulfation, which is accelerated by HS-6-sulfotransferase and removed by glucosamine-6-sulfatases (Sulf1 and Sulf2), determines the preference for canonical or noncanonical pathways. In Xenopus, Sulf1 overexpression inhibits the canonical pathway and enhances the noncanonical pathway (41). In muscle cells, deletion of Sulf1 and Sulf2 promotes the noncanonical pathway (42). Since Ext1 deletion results in the attenuation of both signaling pathways, further research focusing on HS-6-sulfotransferase, Sulf1, and Sulf2 will clarify the importance of fine tuning the HS chains in adipogenic differentiation. The identified binding ligands of HS include growth factors, extracellular matrix components, cell–cell adhesion molecules, lipoproteins, cytokines, chemokines, and coagulation factors (2). Therefore, it is conceivable that several HS-dependent signaling pathways cooperatively regulate adipocyte differentiation. Further studies are required to investigate the detailed molecular mechanisms of HS-dependent adipocyte differentiation.
The PPARγ agonists pioglitazone and rosiglitazone are widely used to ameliorate insulin resistance in patients with type II diabetes. Although these agonists affect energy homeostasis via pleiotropic action on various tissues, several reports have revealed that their induction of adipocyte differentiation plays a role in lowering blood glucose (43, 44). This raises the idea that promotion of adipocyte differentiation would be an effective therapy against insulin resistance. Recent preclinical studies have shown that BMP4 gene therapy, using adeno-associated virus 8, enhances insulin sensitivity (45), and that exogenous FGF1 administration sensitizes the tissue to insulin (46). Our study has shown the significant contribution of HS to adipocyte differentiation through BMP4–FGF1 signaling pathways and insulin sensitivity. In addition, our previous study revealed the involvement of HS in pancreatic β-cell differentiation and insulin secretion (14). Taken together, HS could be a promising target for drug development to improve insulin resistance via BMP4–FGF1-dependent adipogenic differentiation, and also to increase insulin secretion from β-cells. Therefore, it may be worthwhile to examine the therapeutic potential of increasing HS on metabolic organs and develop novel drugs that increase HS itself and/or HS-dependent signaling pathways.
In conclusion, we generated Ext1-heterozygous knockout 3T3-L1 cells (Ext1+/− cells) and visceral white adipocyte–specific heterozygous Ext1-deleted mice (Ext1Δ/WT mice) to investigate the importance of HS in white adipocytes. Our results demonstrate that HS plays a crucial role in the differentiation of white adipocytes, thereby contributing to normal insulin sensitivity and glucose homeostasis. Overall, these findings could improve our understanding of diabetes and lead to the development of novel therapies for diabetes by targeting HS.
Experimental procedures
Cell culture and adipocyte differentiation
The 3T3-L1 cells (American Type Culture Collection) were maintained in Dulbecco's modified Eagle's medium (DMEM; Wako Pure Chemical Industries, Ltd) supplemented with 10% bovine calf serum (GE Healthcare) and 5% CO2 at 37 °C. At 2 days after confluence (day 0), differentiation was induced by exchanging the previous medium for DMEM containing 10% fetal bovine serum (GE Healthcare), 1 μg/ml insulin (Wako), 0.25 μM dexamethasone (Nacalai Tesque, Inc), and 0.5 mM 3-isobutyl-1-methylxanthine (Nacalai), for 48 h. At day 2, the culture medium was changed with DMEM containing 10% fetal bovine serum and 1 μg/ml insulin. At days 4 and 6, the culture medium was changed with DMEM containing 10% fetal bovine serum (47). At day 7, we observed LDs using an inverted microscope (IX-71; Olympus Life Science).
Heterozygous deletion of the Ext1 gene from 3T3-L1 cells (Ext1+/− cells)
The CRISPR–Cas9 system was introduced to generate Ext1-heterozygous deleted 3T3-L1 (Ext1+/−) cells. The oligonucleotides corresponding to Ext1 exon 1, containing the protospacer adjacent motif sequence, were inserted into the BbsI restriction site of the pX459 v2.0 vector (plasmid number #62988; Addgene). The guide RNA sequences used in this study were designated using CRISPR direct (48) and are shown in Table S1. Transfection was performed using Lipofectamine 2000 (Thermo Fisher Scientific) and Opti-MEM (Thermo Fisher Scientific) according to the manufacturer's protocol. Briefly, 2.5 × 105 cells per well were seeded in a 6-well plate before transfection and incubated overnight with 250 μl of a mixture of Lipofectamine 2000 and Opti-MEM. After that, we selected the puromycin-resistant stable transformants for further culture. After the dideoxy sequencing of isolated genomic DNA from the transformants to confirm the genome editing, Ext1+/− cells were established.
Quantitative RT-PCR
RNA isolation, RT, and real-time PCR were performed, as described previously (49). Gene expression levels were normalized against beta-actin expression. The primers used in this study are shown in Table S2.
Immunocytochemistry
3T3-L1 cells seeded in a 6-well plate were washed with PBS and fixed with methanol (Wako)/ethanol (Wako) at −20 °C for 15 min. After blocking with PBS containing 0.5% bovine serum albumin (Nacalai) and 0.05% sodium azide (Wako), 3T3-L1 cells were incubated with primary antibody at 4 °C overnight. After washing, cells were incubated with fluorescent dye–conjugated secondary antibody for 1 h at room temperature. The antibodies used in this study are shown in Table S3. Nuclei were stained with Hoechst 33258 (Dojindo). Images were captured using a confocal microscope C2si (Nikon).
Heparinase treatment of 3T3-L1 cells
To pharmacologically remove HS from 3T3-L1 cells, we treated 3T3-L1 cells by heparinase III (HSase, 40 mU/ml; New England Biolabs), which degrades HS (50), as described previously with slight modification (51). HSase treatment was performed at day 0, day 2, day 4, and day 6.
BMP4 and FGF1 stimulation
3T3-L1 cells were incubated with BMP4 (40 ng/ml; Wako) for 2 days (from day −2 to day 0). After that, we induced differentiation as described previously. FGF1 (10 ng/ml; Miltenyi Biotec) was added to the differentiation medium.
Transient Ext1 overexpression
3T3-L1 cells were electroporated as follows. First, we mixed 1.0 × 106 3T3-L1 cells and 20 μg of pCI-neo mammalian expression vector (Promega) with or without mouse Ext1 complementary DNA in 200 μl of Opti-MEM (Thermo Fisher Scientific). After these complexes were transferred to an electroporation cuvette, electroporation was performed using CUY21EDIT II (Bex Co, Ltd) according to the manufacturer's protocol. After that, we seeded the cells at 24-well plate and induced differentiation as described previously.
Insulin and TGF-β stimulation
At 7 days after differentiation, 3T3-L1 cells were washed with Tris-buffered saline and stimulated in a culture medium containing insulin (50 nM) and TGF-β (1 ng/ml; Peprotech), respectively, for 30 min. After that, proteins were extracted using radioimmunoprecipitation assay buffer (50 mM Tris–HCl buffer, pH 7.6, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% SDS) containing sodium orthovanadate (Wako).
Western blotting
Isolated adipose tissues were lysed with radioimmunoprecipitation assay buffer. The levels of proteins were measured by bicinchoninic acid assay, as described previously (52). Aliquots containing 10 μg protein were separated on 8% or 12% SDS-polyacrylamide gels, and the proteins were then transferred to polyvinylidene difluoride membranes, which were blocked with 5% nonfat dry milk solution for 1 h. The membranes were incubated with primary antibodies at 4 °C overnight and then incubated with secondary antibodies at room temperature for 1 h. The antibodies used in this study are shown in Table S3. Immunoreactive bands were detected using an EzWestLumi plus (ATTO), and bands were recorded using the Ez-capture MG chemiluminescence imaging system (ATTO). The intensity levels were calculated using ImageJ (National Institutes of Health [NIH]) (53).
Glucose uptake by the 3T3-L1 adipocytes
We used 3T3-L1 cells at 7 days after differentiation and incubated them in serum-free DMEM medium 2 h before the assay. The assay was performed as described previously (54, 55). Briefly, the cells were washed several times with no-glucose DMEM (Nacalai) and treated with 1 μM insulin for 10 min at 37 °C. Then, the cells were incubated at 37 °C in no-glucose DMEM with 0.5 μCi of [3H]-2-deoxy-d-glucose (PerkinElmer) and 5 mM of unlabeled 2-deoxy-d-glucose (Nacalai) for 10 min. After that, the cells were washed with ice-cold perfusion in PBS containing 1 mM CaCl2 and MgCl2 and lysed with 100 mM NaOH. Finally, the lysates were mixed with 2 ml of liquid scintillator cocktail (Ultima Gold XR; PerkinElmer), and the radioactivity was measured with an LSC-1000 (Hitachi Aloka Medical). Protein concentrations were measured using a Coomassie Protein Assay Kit (Thermo Fisher Scientific).
Mice
All mice used in this study were handled in accordance with the Principles for the use of Research Animals of Tohoku University (2019MdA-327-03 and 2019MdLMO-203-02). All mice were kept under a 12-h light/dark cycle (8:00 AM/8:00 PM) in a humidity- and temperature-controlled room, and water and food were provided ad libitum. Labo MR Stock (3.29 kcal/g, crude fat: 3.9%; NOSAN Yokohama) and Quick FAT (3.96 kcal/g, crude fat: 13%; CLEA Japan, Inc) were used as ND and HFD, respectively. The creation of the loxP-modified Ext1 allele (Ext1 flox) (6) and Fabp4-Cre transgenic mice (The Jackson Laboratory) has been previously described (56). In this experiment, 8- to 12-week-old male mice were used, unless otherwise noted.
Immunohistochemistry
The WAT was quickly removed after perfusion of PBS and 4% paraformaldehyde (Wako). After isolation of WAT, WAT was further fixed in 10% formalin neutral buffer solution (Wako). After embedding in paraffin and making sections of 8 μm thicknesses, sections were blocked with 10% normal goat serum (Nichirei Biosciences, Inc) for 15 min at room temperature. Then, sections were incubated with primary antibodies overnight at 4 °C. After washing with PBS, sections were incubated with secondary antibody for 2 h at room temperature. The antibodies used in this study are shown in Table S3. Finally, sections were mounted with Vectashield Hardset with 4′,6-diamidino-2-phenylindole (Vector Laboratories). Images were captured using a confocal microscope C2si (Nikon).
Monocytes separation
Blood samples were collected from abdominal aorta with heparin sodium treatment (Mochida Pharmaceutical Co, Ltd). After that, monocytes were separated using lymphocyte separation solution (Nacalai) with modifications (57).
Micro-computed tomography analysis of adipose tissue
Body fat composition was analyzed as described previously, with some modifications (58). In brief, mice were sacrificed and scanned between the proximal end of the first vertebra and the distal end of the tibia, using the Latheta LCT-200 experimental animal X-ray CT system (micro–computed tomography; Hitachi). Tail, feet, and head were excluded because they contain negligible amounts of fat. The voxel size was 96 × 96 μm, and Latheta software was used to characterize the volume and mass of adipose tissues.
Glucose tolerance test and ITT
Glucose (Wako; 2 g/kg body weight) and insulin (0.75 U/kg body weight) were injected intraperitoneally. Both tests were performed as described previously (14). Blood samples were taken from the tail. The blood glucose concentrations were determined with an ACCUCHEK comfort blood glucose analyzer (Roche Ltd).
Measurement of the concentration of HS in WAT
Isolated adipose tissues were incubated with 0.5 M Tris–HCl (pH 7.5) containing actinase E (Kaken Pharmaceutical Co, Ltd; 2 mg/ml) at 55 °C overnight. After that, the levels of HS were determined using the HS ELISA kit (Seikagaku Biobusiness Corporation).
Measurement of plasma insulin and leptin
The concentrations of insulin and leptin were determined by ELISA (insulin; Morinaga, leptin; Shibayagi). Blood samples were taken from the tail vein. For leptin measurement, mice were fasted for 6 h before the experiment.
Measurement of adipocyte size by H&E staining
After perfusion and fixation, H&E staining was performed as described previously (59). A BZ-9000 Fluorescence Microscope (Keyence) was used for taking the images, and ImageJ software (NIH) was used for morphometric analysis. The areas of white adipocytes were calculated using ImageJ (53).
Oil red O staining
The liver, tibialis anterior muscle, and gastrocnemius muscle were quickly removed after perfusion with PBS and then was fixed in 4% paraformaldehyde (Wako). After isolation of these tissues, they were fixed in 10% formalin neutral buffer solution (Wako). Oil red O staining was performed as described previously with some modifications (60). A BZ-9000 Fluorescence Microscope was used for observation, and the Hybrid Cell Count image analysis program (Keyence) was used for morphometric analysis.
Home cage locomotor activity
Home cage locomotor activity was assessed as described previously (61). Mice were transferred to individual home cage and habituated for 24 h prior to the recording of locomotor activity. Locomotor activity was measured using an activity-monitoring system with infrared-beam apparatus (SUPERMEX; Muromachi Kikai Co Ltd). Data were collected and analyzed using CompACT AMS software (Muromachi Kikai Co Ltd).
Statistical analysis
Experiments were analyzed using GraphPad Prism 8 (GraphPad Software) for statistical analysis. Student's t tests were utilized, except for body weights, glucose tolerance test, plasma insulin levels, and ITT, which were analyzed using two-way ANOVA. A p value <0.05 was regarded as significant. Data are presented as means ± SEM.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Supporting information
This article contains supporting information.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
We acknowledge the technical support of the Biomedical Research Core of Tohoku University Graduate School of Medicine and the Biomedical Research unit of Tohoku University Hospital.
Author contributions
T. M., F. I., Y. Y., K. Y., and T. Y. conceptualization; T. M., M. M., and T. Y. methodology; T. M. validation; T. M. formal analysis; T. M., A. S., and Y. M. investigation; T. M., M. M., A. S., R. O., and Y. M. data curation; T. M., K. Y., and T. Y. writing–original draft; Y. Y. and T. Y. writing–review and editing; T. M. visualization; F. I. supervision; T. M., R. O., and T. Y. funding acquisition.
Funding and additional information
This work was supported by Japan Society for the Promotion of Science KAKENHI grant number JP20J11179 (to T. M.); Nishinomiya Basic Research Fund (to T. M.), Japan; Anzai Diabetes Memorial Research Fund, Japan (to T. Y.); and National Institutes of Health grant R01 AR055670 (to Y. Y.), USA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Edited by Qi-Qun Tang
Supporting information
References
- 1.Bourdon M.A., Krusius T., Campbell S., Schwartz N.B., Ruoslahti E. Identification and synthesis of a recognition signal for the attachment of glycosaminoglycans to proteins. Proc. Natl. Acad. Sci. U. S. A. 1987;84:3194–3198. doi: 10.1073/pnas.84.10.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esko J.D., Lindahl U. Molecular diversity of heparan sulfate. J. Clin. Invest. 2001;108:169–173. doi: 10.1172/JCI13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yayon A., Klagsbrun M., Esko J.D., Leder P., Ornitz D.M. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991;64:841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- 4.Lin X., Wei G., Shi Z., Dryer L., Esko J.D., Wells D.E., Matzuk M.M. Disruption of gastrulation and heparan sulfate biosynthesis in EXT1-deficient mice. Dev. Biol. 2000;224:299–311. doi: 10.1006/dbio.2000.9798. [DOI] [PubMed] [Google Scholar]
- 5.Zhang P., Lu H., Peixoto R.T., Pines M.K., Ge Y., Oku S., Siddiqui T.J., Xie Y., Wu W., Archer-Hartmann S., Yoshida K., Tanaka K.F., Aricescu A.R., Azadi P., Gordon M.D. Heparan sulfate organizes neuronal synapses through neurexin partnerships. Cell. 2018;174:1450–1464.e1423. doi: 10.1016/j.cell.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inatani M., Irie F., Plump A.S., Tessier-Lavigne M., Yamaguchi Y. Mammalian brain morphogenesis and midline axon guidance require heparan sulfate. Science. 2003;302:1044–1046. doi: 10.1126/science.1090497. [DOI] [PubMed] [Google Scholar]
- 7.van Wijk X.M., van Kuppevelt T.H. Heparan sulfate in angiogenesis: A target for therapy. Angiogenesis. 2014;17:443–462. doi: 10.1007/s10456-013-9401-6. [DOI] [PubMed] [Google Scholar]
- 8.Irie F., Badie-Mahdavi H., Yamaguchi Y. Autism-like socio-communicative deficits and stereotypies in mice lacking heparan sulfate. Proc. Natl. Acad. Sci. U. S. A. 2012;109:5052–5056. doi: 10.1073/pnas.1117881109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsumoto Y., Matsumoto K., Irie F., Fukushi J., Stallcup W.B., Yamaguchi Y. Conditional ablation of the heparan sulfate-synthesizing enzyme Ext1 leads to dysregulation of bone morphogenic protein signaling and severe skeletal defects. J. Biol. Chem. 2010;285:19227–19234. doi: 10.1074/jbc.M110.105338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inubushi T., Lemire I., Irie F., Yamaguchi Y. Palovarotene inhibits osteochondroma formation in a mouse model of multiple hereditary exostoses. J. Bone Miner. Res. 2018;33:658–666. doi: 10.1002/jbmr.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sladek R., Rocheleau G., Rung J., Dina C., Shen L., Serre D., Boutin P., Vincent D., Belisle A., Hadjadj S., Balkau B., Heude B., Charpentier G., Hudson T.J., Montpetit A. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 12.Moore A.F., Jablonski K.A., McAteer J.B., Saxena R., Pollin T.I., Franks P.W., Hanson R.L., Shuldiner A.R., Knowler W.C., Altshuler D., Florez J.C., Diabetes Prevention Program Research G. Extension of type 2 diabetes genome-wide association scan results in the diabetes prevention program. Diabetes. 2008;57:2503–2510. doi: 10.2337/db08-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang Y.C., Liu P.H., Yu Y.H., Kuo S.S., Chang T.J., Jiang Y.D., Nong J.Y., Hwang J.J., Chuang L.M. Validation of type 2 diabetes risk variants identified by genome-wide association studies in Han Chinese population: A replication study and meta-analysis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0095045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuzawa T., Yoshikawa T., Iida T., Karpati A., Kitano H., Harada R., Nakamura T., Sugawara A., Yamaguchi Y., Yanai K. Heparan sulfate in pancreatic beta-cells contributes to normal glucose homeostasis by regulating insulin secretion. Biochem. Biophys. Res. Commun. 2018;499:688–695. doi: 10.1016/j.bbrc.2018.03.213. [DOI] [PubMed] [Google Scholar]
- 15.Bernelot Moens S.J., Mooij H.L., Hassing H.C., Kruit J.K., Witjes J.J., van de Sande M.A., Nederveen A.J., Xu D., Dallinga-Thie G.M., Esko J.D., Stroes E.S., Nieuwdorp M. Carriers of loss-of-function mutations in EXT display impaired pancreatic beta-cell reserve due to smaller pancreas volume. PLoS One. 2014;9 doi: 10.1371/journal.pone.0115662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith U., Kahn B.B. Adipose tissue regulates insulin sensitivity: Role of adipogenesis, de novo lipogenesis and novel lipids. J. Intern. Med. 2016;280:465–475. doi: 10.1111/joim.12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarjeant K., Stephens J.M. Adipogenesis. Cold Spring Harb. Perspect. Biol. 2012;4:a008417. doi: 10.1101/cshperspect.a008417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilsie L.C., Chanchani S., Navaratna D., Orlando R.A. Cell surface heparan sulfate proteoglycans contribute to intracellular lipid accumulation in adipocytes. Lipids Health Dis. 2005;4:2. doi: 10.1186/1476-511X-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clarke A.J., Riffelmacher T., Braas D., Cornall R.J., Simon A.K. B1a B cells require autophagy for metabolic homeostasis and self-renewal. J. Exp. Med. 2018;215:399–413. doi: 10.1084/jem.20170771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X.H., Tee L.Y., Wang X.G., Huang Q.S., Yang S.H. Off-target effects in CRISPR/Cas9-mediated genome engineering. Mol. Ther. Nucleic Acids. 2015;4:e264. doi: 10.1038/mtna.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammarstedt A., Hedjazifar S., Jenndahl L., Gogg S., Grunberg J., Gustafson B., Klimcakova E., Stich V., Langin D., Laakso M., Smith U. WISP2 regulates preadipocyte commitment and PPARgamma activation by BMP4. Proc. Natl. Acad. Sci. U. S. A. 2013;110:2563–2568. doi: 10.1073/pnas.1211255110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suenaga M., Kurosawa N., Asano H., Kanamori Y., Umemoto T., Yoshida H., Murakami M., Kawachi H., Matsui T., Funaba M. Bmp4 expressed in preadipocytes is required for the onset of adipocyte differentiation. Cytokine. 2013;64:138–145. doi: 10.1016/j.cyto.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Hutley L.J., Newell F.S., Kim Y.H., Luo X., Widberg C.H., Shurety W., Prins J.B., Whitehead J.P. A putative role for endogenous FGF-2 in FGF-1 mediated differentiation of human preadipocytes. Mol. Cell Endocrinol. 2011;339:165–171. doi: 10.1016/j.mce.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 24.Choy L., Skillington J., Derynck R. Roles of autocrine TGF-beta receptor and Smad signaling in adipocyte differentiation. J. Cell Biol. 2000;149:667–682. doi: 10.1083/jcb.149.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garin-Shkolnik T., Rudich A., Hotamisligil G.S., Rubinstein M. FABP4 attenuates PPARgamma and adipogenesis and is inversely correlated with PPARgamma in adipose tissues. Diabetes. 2014;63:900–911. doi: 10.2337/db13-0436. [DOI] [PubMed] [Google Scholar]
- 26.Jeffery E., Berry R., Church C.D., Yu S., Shook B.A., Horsley V., Rosen E.D., Rodeheffer M.S. Characterization of Cre recombinase models for the study of adipose tissue. Adipocyte. 2014;3:206–211. doi: 10.4161/adip.29674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yue F., Cheng Y., Breschi A., Vierstra J., Wu W., Ryba T., Sandstrom R., Ma Z., Davis C., Pope B.D., Shen Y., Pervouchine D.D., Djebali S., Thurman R.E., Kaul R. A comparative encyclopedia of DNA elements in the mouse genome. Nature. 2014;515:355–364. doi: 10.1038/nature13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mullican S.E., Tomaru T., Gaddis C.A., Peed L.C., Sundaram A., Lazar M.A. A novel adipose-specific gene deletion model demonstrates potential pitfalls of existing methods. Mol. Endocrinol. 2013;27:127–134. doi: 10.1210/me.2012-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Q.A., Tao C., Gupta R.K., Scherer P.E. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med. 2013;19:1338–1344. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chau Y.Y., Bandiera R., Serrels A., Martinez-Estrada O.M., Qing W., Lee M., Slight J., Thornburn A., Berry R., McHaffie S., Stimson R.H., Walker B.R., Chapuli R.M., Schedl A., Hastie N. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat. Cell Biol. 2014;16:367–375. doi: 10.1038/ncb2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foster M.T., Softic S., Caldwell J., Kohli R., de Kloet A.D., Seeley R.J. Subcutaneous adipose tissue transplantation in diet-induced obese mice attenuates metabolic dysregulation while removal exacerbates it. Physiol. Rep. 2013;1 doi: 10.1002/phy2.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foster M.T., Shi H., Seeley R.J., Woods S.C. Removal of intra-abdominal visceral adipose tissue improves glucose tolerance in rats: Role of hepatic triglyceride storage. Physiol. Behav. 2011;104:845–854. doi: 10.1016/j.physbeh.2011.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seip M., Trygstad O. Generalized lipodystrophy, congenital and acquired (lipoatrophy) Acta Paediatr. Suppl. 1996;413:2–28. doi: 10.1111/j.1651-2227.1996.tb14262.x. [DOI] [PubMed] [Google Scholar]
- 34.Miller S.G., De Vos P., Guerre-Millo M., Wong K., Hermann T., Staels B., Briggs M.R., Auwerx J. The adipocyte specific transcription factor C/EBPalpha modulates human ob gene expression. Proc. Natl. Acad. Sci. U. S. A. 1996;93:5507–5511. doi: 10.1073/pnas.93.11.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jonker J.W., Suh J.M., Atkins A.R., Ahmadian M., Li P., Whyte J., He M., Juguilon H., Yin Y.Q., Phillips C.T., Yu R.T., Olefsky J.M., Henry R.R., Downes M., Evans R.M. A PPARgamma-FGF1 axis is required for adaptive adipose remodelling and metabolic homeostasis. Nature. 2012;485:391–394. doi: 10.1038/nature10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qian S.W., Tang Y., Li X., Liu Y., Zhang Y.Y., Huang H.Y., Xue R.D., Yu H.Y., Guo L., Gao H.D., Liu Y., Sun X., Li Y.M., Jia W.P., Tang Q.Q. BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis. Proc. Natl. Acad. Sci. U. S. A. 2013;110:E798–E807. doi: 10.1073/pnas.1215236110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee M.J., Pickering R.T., Shibad V., Wu Y., Karastergiou K., Jager M., Layne M.D., Fried S.K. Impaired glucocorticoid suppression of TGFbeta signaling in human omental adipose tissues limits adipogenesis and may promote fibrosis. Diabetes. 2019;68:587–597. doi: 10.2337/db18-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Boer J., Wang H.J., Van Blitterswijk C. Effects of Wnt signaling on proliferation and differentiation of human mesenchymal stem cells. Tissue Eng. 2004;10:393–401. doi: 10.1089/107632704323061753. [DOI] [PubMed] [Google Scholar]
- 39.Ross S.E., Hemati N., Longo K.A., Bennett C.N., Lucas P.C., Erickson R.L., MacDougald O.A. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 40.Kanazawa A., Tsukada S., Kamiyama M., Yanagimoto T., Nakajima M., Maeda S. Wnt5b partially inhibits canonical Wnt/beta-catenin signaling pathway and promotes adipogenesis in 3T3-L1 preadipocytes. Biochem. Biophys. Res. Commun. 2005;330:505–510. doi: 10.1016/j.bbrc.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 41.Fellgett S.W., Maguire R.J., Pownall M.E. Sulf1 has ligand-dependent effects on canonical and non-canonical Wnt signalling. J. Cell Sci. 2015;128:1408–1421. doi: 10.1242/jcs.164467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tran T.H., Shi X., Zaia J., Ai X. Heparan sulfate 6-O-endosulfatases (Sulfs) coordinate the Wnt signaling pathways to regulate myoblast fusion during skeletal muscle regeneration. J. Biol. Chem. 2012;287:32651–32664. doi: 10.1074/jbc.M112.353243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hallakou S., Doare L., Foufelle F., Kergoat M., Guerre-Millo M., Berthault M.F., Dugail I., Morin J., Auwerx J., Ferre P. Pioglitazone induces in vivo adipocyte differentiation in the obese Zucker fa/fa rat. Diabetes. 1997;46:1393–1399. doi: 10.2337/diab.46.9.1393. [DOI] [PubMed] [Google Scholar]
- 44.Martinez L., Berenguer M., Bruce M.C., Le Marchand-Brustel Y., Govers R. Rosiglitazone increases cell surface GLUT4 levels in 3T3-L1 adipocytes through an enhancement of endosomal recycling. Biochem. Pharmacol. 2010;79:1300–1309. doi: 10.1016/j.bcp.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 45.Hoffmann J.M., Grunberg J.R., Church C., Elias I., Palsdottir V., Jansson J.O., Bosch F., Hammarstedt A., Hedjazifar S., Smith U. BMP4 gene therapy in mature mice reduces BAT activation but protects from obesity by browning subcutaneous adipose tissue. Cell Rep. 2017;20:1038–1049. doi: 10.1016/j.celrep.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 46.Suh J.M., Jonker J.W., Ahmadian M., Goetz R., Lackey D., Osborn O., Huang Z., Liu W., Yoshihara E., van Dijk T.H., Havinga R., Fan W., Yin Y.Q., Yu R.T., Liddle C. Endocrinization of FGF1 produces a neomorphic and potent insulin sensitizer. Nature. 2014;513:436–439. doi: 10.1038/nature13540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frost S.C., Lane M.D. Evidence for the involvement of vicinal sulfhydryl groups in insulin-activated hexose transport by 3T3-L1 adipocytes. J. Biol. Chem. 1985;260:2646–2652. [PubMed] [Google Scholar]
- 48.Naito Y., Hino K., Bono H., Ui-Tei K. CRISPRdirect: Software for designing CRISPR/Cas guide RNA with reduced off-target sites. Bioinformatics. 2015;31:1120–1123. doi: 10.1093/bioinformatics/btu743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iida T., Yoshikawa T., Matsuzawa T., Naganuma F., Nakamura T., Miura Y., Mohsen A.S., Harada R., Iwata R., Yanai K. Histamine H3 receptor in primary mouse microglia inhibits chemotaxis, phagocytosis, and cytokine secretion. Glia. 2015;63:1213–1225. doi: 10.1002/glia.22812. [DOI] [PubMed] [Google Scholar]
- 50.Greenall S.A., Adams T.E., Johns T.G. Incomplete target neutralization by the anti-cancer antibody rilotumumab. MAbs. 2016;8:246–252. doi: 10.1080/19420862.2015.1122149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yokoyama M., Matsuzawa T., Yoshikawa T., Nunomiya A., Yamaguchi Y., Yanai K. Heparan sulfate controls skeletal muscle differentiation and motor functions. Biochim. Biophys. Acta Gen. Subj. 2020;1864:129707. doi: 10.1016/j.bbagen.2020.129707. [DOI] [PubMed] [Google Scholar]
- 52.Smith P.K., Krohn R.I., Hermanson G.T., Mallia A.K., Gartner F.H., Provenzano M.D., Fujimoto E.K., Goeke N.M., Olson B.J., Klenk D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 53.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harmon A.W., Paul D.S., Patel Y.M. MEK inhibitors impair insulin-stimulated glucose uptake in 3T3-L1 adipocytes. Am. J. Physiol. Endocrinol. Metab. 2004;287:E758–E766. doi: 10.1152/ajpendo.00581.2003. [DOI] [PubMed] [Google Scholar]
- 55.Takahashi M., Takahashi Y., Takahashi K., Zolotaryov F.N., Hong K.S., Kitazawa R., Iida K., Okimura Y., Kaji H., Kitazawa S., Kasuga M., Chihara K. Chemerin enhances insulin signaling and potentiates insulin-stimulated glucose uptake in 3T3-L1 adipocytes. FEBS Lett. 2008;582:573–578. doi: 10.1016/j.febslet.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 56.He W., Barak Y., Hevener A., Olson P., Liao D., Le J., Nelson M., Ong E., Olefsky J.M., Evans R.M. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc. Natl. Acad. Sci. U. S. A. 2003;100:15712–15717. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Satoh M., Ishikawa Y., Itoh T., Minami Y., Takahashi Y., Nakamura M. The expression of TNF-alpha converting enzyme at the site of ruptured plaques in patients with acute myocardial infarction. Eur. J. Clin. Invest. 2008;38:97–105. doi: 10.1111/j.1365-2362.2007.01912.x. [DOI] [PubMed] [Google Scholar]
- 58.Lubura M., Hesse D., Neumann N., Scherneck S., Wiedmer P., Schurmann A. Non-invasive quantification of white and brown adipose tissues and liver fat content by computed tomography in mice. PLoS One. 2012;7 doi: 10.1371/journal.pone.0037026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Serafini S., Santos M.M., Aoun Tannuri A.C., Zerbini M.C.N., de Mendonca Coelho M.C., de Oliveira Goncalves J., Tannuri U. Is hematoxylin-eosin staining in rectal mucosal and submucosal biopsies still useful for the diagnosis of Hirschsprung disease? Diagn. Pathol. 2017;12:84. doi: 10.1186/s13000-017-0673-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mehlem A., Hagberg C.E., Muhl L., Eriksson U., Falkevall A. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat. Protoc. 2013;8:1149–1154. doi: 10.1038/nprot.2013.055. [DOI] [PubMed] [Google Scholar]
- 61.Yamada Y., Yoshikawa T., Naganuma F., Kikkawa T., Osumi N., Yanai K. Chronic brain histamine depletion in adult mice induced depression-like behaviours and impaired sleep-wake cycle. Neuropharmacology. 2020;175:108179. doi: 10.1016/j.neuropharm.2020.108179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.