Abstract
Trio-next generation sequencing is useful to identify undiagnosed inherited diseases. We have attended a patient with trigenic ADH5/ALDH2/ADGRV1 pathogenic variants, which caused two distinct diseases, myelodysplastic syndrome and Usher syndrome. Whole genome sequencing of peripheral blood from the patient and his parents were applied to identify disease-causing genes. Sanger sequencing was performed to validate the identified ADH5/ALDH2/ADGRV1 variants. Our results identified disease-associated variants in ADGRV1 (disease inheritance autosomal recessive) and in ADH5 (disease inheritance also autosomal recessive) and a variant in ALDH2 (disease inheritance autosomal dominant). Although the variants identified in ADH5 and ALDH2 have been reported, their co-existence in association with disease-causing variation in a third gene has not. They broaden the spectrum of ADGRV1 in Usher syndrome. Findings on next generation sequencing guided rapid and accurate diagnosis, resulting in patient-tailored therapeutic intervention.
Keywords: ADH5/ALDH/ADGRV1 variants, Myelodysplastic syndrome, Trigenic mutations, Trio-next generation sequencing, Usher syndrome
Highlights
-
•
Trigenic ADH5 / ALDH2 / ADGRV1 variants in myelodysplastic syndrome with Usher syndrome were identified.
-
•
Two novel pathogenic frameshift variants in ADGRV1 in compound heterozygous state with Usher syndrome type II were described.
-
•
Findings on next generation sequencing guided rapid and accurate diagnosis, resulting in patient-tailored therapy.
ADH5/ALDH/ADGRV1 variants, Myelodysplastic syndrome, Trigenic mutations, Trio-next generation sequencing, Usher syndrome.
1. Introduction
Since the advent of next generation sequencing (NGS), digenic or trigenic inheritance has come to the forefront of attention. We have encountered a rare instance of co-existent myelodysplastic syndrome (MDS) and Usher syndrome (USH) diagnosed by NGS of the patient and his unaffected parents (trio-NGS).
MDS is a group of clonal hematopoietic stem cell diseases characterized by cytopenia, dysplasia in at least one major myeloid lineage, ineffective hematopoiesis, and increased risk of acute myeloid leukemia (Cazzola, 2020). Combined deficiency of both cytosolic alcohol dehydrogenase 5 (ADH5) and mitochondrial aldehyde dehydrogenase 2 (ALDH2) triggers the development of MDS in both mouse and human (Dingler et al., 2020). ADH5 is the main enzyme that detoxifies the ubiquitous, highly reactive molecule formaldehyde, which is detrimental to our genome (Sanghani et al., 2000; Staab et al., 2008, 2009; Teng et al., 2001). ALDH2, active mainly in detoxifying acetaldehyde, also takes part in formaldehyde detoxification (Marchitti et al., 2008; Schmidt et al., 1972; Uotila and Koivusalo, 1974; Wang et al., 2002, 2009). DNA crosslink repair by the products of genes mutated in Fanconi anemia, such as BRCA2, provides an essential backstop against the effects of formaldehyde (Dingler et al., 2020). Dingler et al. discovered that endogenous formaldehyde is physiologically the principal substrate for ALDH2 and ADH5; established methods to quantify blood formaldehyde; and demonstrated that combined ADH5 and ALDH2 deficiency leads to formaldehyde accumulation, with in consequence profound hematopoietic disruption and reduced numbers of hematopoietic stem cells (Dingler et al., 2020). The features of MDS with ADH5 or ALDH2 mutations are diverse. They resemble those of Fanconi's anemia, with skin pigmentation, café-au-lait spots, mild mental retardation, short stature, vitiligo, and microcephaly (Auerbach, 2009; Dingler et al., 2020). Oka and colleagues concurrently reported that a functional G>A single-nucleotide polymorphism, rs671, in ALDH2 (exon 12, Glu504Lys) when present in heterozygous or homozygous state causes facial flushing in response to drinking alcohol (frequent in Asians). The rs671 polymorphism protects against alcoholism, but also is associated with an increased risk of cardiovascular disorders and certain types of cancer. Furthermore, in combination with mutations in ADH5 the rs671 polymorphism causes a newly named disorder, AMeD (aplastic anemia, mental retardation, and dwarfism) syndrome, as do other ALDH2 variants (Oka et al., 2020).
USH is an autosomal recessive disease characterized by sensorineural hearing loss and retinitis pigmentosa (RP). Its prevalence ranges from 3.2 - 6.2/10000, with 3.2/100000 for Colombia (Tamayo et al., 1991), 4.4/100000 for the USA (Boughman and Fishman, 1983), and 6.2/100000 for Birmingham, UK (Hope et al., 1997).
Three subtypes, I, II and III, are historically distinguished by age of onset, severity, progression of clinical symptoms, and absence or presence of balance disorder (Boughman et al., 1983; Espinos et al., 1998; Hope et al., 1997; Rosenberg et al., 1997; Vernon, 1969). USH type I is most debilitating, with severe congenital hearing loss, vestibular areflexia, and early onset of RP (within the first decade, as a rule). Type II is most frequently encountered, with moderate to severe hearing loss, normal vestibular function, and onset of RP in the second decade. USH type III is characterized by progressive postlingual hearing loss, variable onset of RP, and variable vestibular response (Keats and Corey, 1999; Kimberling et al., 2010; Mathur and Yang, 2015). Vestibular-function phenotype does not, however, map exactly to USH subtype (Wafa et al., 2021). To date, 12 genes have been associated with USH. USH type I genes are MYO7A, USH1C, CDH23, PCDH15, USH1G, and CIB2. USH type II genes are USH2A, ADGRV1 (USH2C), PDZD7 (DFNB57), and WHRN (DFNB31). USH type III genes are CLRN1 and HARS1 (Mathur and Yang, 2015). The roles of 3 are disputed; CIB2, PDZD7, and HARS are thought to be very rare contributors to USH (Booth et al., 2018; Wafa et al., 2021). USH2A is most often identified as mutated in USH type II patients (80%); but ADGRV1 mutation also causes USH type II. ADGRV1 encodes the largest known cell surface protein (6306 amino-acid residues), G protein-coupled receptor 98 or very large G protein coupled receptor 1 (VLGR1), expressed in brain and in cochlear and retinal structures (Foord et al., 2002; Schwartz et al., 2005). VLGR1 is critical for proper hair cell development and maintenance of photoreceptor structures (Liu et al., 2007; Yang et al., 2010).
We initially suspected that our patient had an inherited bone marrow (BM) failure syndrome such as Fanconi's anemia or dyskeratosis congenita. We performed trio-NGS to identify candidate disease genes. This permitted successful diagnosis of 2 distinct hereditary diseases, MDS with ADH5/ALDH2 mutations and USH type II with ADGRV1 mutations. The sequencing data were very helpful in clinical decision making.
2. Materials and methods
2.1. Study participants and clinical evaluations
The use of material and clinical information was approved by the Research Ethics Committee of the Faculty of Medicine, Juntendo University, and was in accordance with the Declaration of Helsinki. Written informed consent for genetic testing was preparatorily obtained from the parents of the patient.
2.2. Audiologic evaluation
Audiologic evaluation included otoscopy, pure-tone threshold testing by air conduction (0.125, 0.25, 0.5, 1, 2, 4, 8 kHz) and bone conduction (0.25, 0.5, 1, 2, 4 kHz), and conventional tympanometry (226 Hz). Distortion-product (DP) otoacoustic emission studies (DPOAE) were conducted using the DP-gram in response to pure tones in the frequency range of 0.5–8 kHz.
2.3. Ophthalmologic evaluation
Ophthalmologic evaluation included ocular examination, electroretinography, and optical coherence tomography. Full-field electroretinograms were recorded using a four-primary ganzfeld stimulator and were obtained using standard protocols detailed by the International Society for Clinical Electrophysiology of Vision (McCulloch et al., 2015). Retinal images were obtained by Spectralis spectral-domain optical coherence tomography (Heidelberg Engineering, Heidelberg, Germany).
2.4. Hematologic evaluation
Complete blood counts and determinations of various biomarker values were performed. To detect morphologic dysplasia and blasts, iliac-crest BM aspiration and biopsy were performed, with chromosome analysis and routine cytopathologic and histopathologic evaluation.
2.5. Genomic DNA preparation
Genomic DNA was isolated from patient and parent whole blood using Maxwell RSC Whole Blood DNA Kit (Promega, Madison, WI).
2.6. Genetic analysis
Indexed genomic DNA libraries were prepared from patient genomic DNA. A whole genome sequencing (WGS) library was established using a MGIEasy DNA Library Prep Kit V1.1 (BGI, Shenzhen, China) according to the manufacturers’ protocols. Sequencing was performed on a MGISEQ-2000 using MGISEQ-2000RS High-throughput Sequencing Set PE100 V3.0 (BGI). After the sequencing step, reads were obtained by Trimmomatic V.0.39 (Bolger et al., 2014; Kohda et al., 2016). Read alignment was performed and aligned to human GRCh/hg19 genome using Burrows-Wheeler Aligner V.0.6.1. (Li and Durbin, 2009). The aligned data were processed with Samtools V.1.11 (Li et al., 2009) and Picard [http://picard.sourceforge.net./]. GATKV.4.1.9.0 (McKenna et al., 2010) was also used for insertion and deletion realignment, quality recalibration, and variant calling. Detected variants were annotated using both Annotate Validation (ANNOVAR) V.11/12/2014 (Wang et al., 2010) and custom Ruby scripts. The variants were assessed in silico for likely effects using PROVEAN protein program V.1.1.3 (Choi and Chan, 2015)), DDIG-in software V.9/13/2020 (Folkman et al., 2015), and NMDEscPredictor (Coban-Akdemir et al., 2018).
2.7. Sanger sequencing
Mutation analyses of ADGRV1 (exons 4 and 86), ADH5 (exon 8) and ALDH2 (exon 12) were performed by PCR and direct sequencing. PCR amplification was performed using PrimeSTAR GXL DNA Polymerase (Takara Bio, Shiga, Japan; primer sequences, Table 1). PCR cycle conditions were 30 cycles of 98 °C for 10sec, 60 °C for 15sec, and 68 °C for 7min. Purified DNA for sequencing was obtained by eluting PCR products from gels using a NucleoSpin Gel and PCR Clean-up (Takara Bio). DNA sequencing was performed by FASMAC (Kanagawa, Japan).
Table 1.
Primers for validating ADH5, ALDH2 and ADGRV1 variants by Sanger sequencing.
| Gene | Primer sequence | PCR product size (bp) | |
|---|---|---|---|
| ADGRV1 | Exon 4 | 5′-aatttttcatttggaacttcttaacca-3′ | 690 |
| 5′-ttctttcaatatgctttctcatctcc-3′ | |||
| Exon 86 | 5′-tttgcgctgaaagtgtctgag-3′ | 363 | |
| 5′-ccaagaagcaggagaaactgg-3′ | |||
| ADH5 | Exon 8 | 5′-ctctccatcccctcaaactca-3′ | 479 |
| 5′-ttccagagttctgtcctgggta-3′ | |||
| ALDH2 | Exon 12 | 5′-atacagggggtcctgggagt-3′ | 500 |
| 5′-acagagcagaggctgggtct-3′ | |||
2.8. TA cloning
The purified PCR products were incubated with A-overhang enzyme at 65 °C for 10 minutes to add dATP and were then subcloned into the pMD20-T vector (Mighty TA Cloning Kit, Takara Bio). Inserted sequence was confirmed using the forward primer 5′-CAGGAAACAGCTATGAC-3′ and the reverse primer 5′-GTTTTCCCAGTCACGAC-3′ (FASMAC).
3. Results
3.1. Clinical findings
An 18-year-old man was referred to our hospital for evaluation of chronic idiopathic anemia. He had hearing loss, retinal degeneration, short stature, and mild mental retardation, all since childhood. Unexplained anemia had required occasional transfusions of red blood cells for a decade. His parents and an older brother are well. The parents are not consanguine. At presentation, he had no abnormal physical features, including his skin and hair color, and he was normocephalic. The patient's white blood cell count was 1,500/μL, his hemoglobin 7.9 g/dL, and his platelet count 48,000/μL. No clinical-biochemistry abnormalities were identified. Unfortunately, we had no access to measurement of serum formaldehyde concentrations.
BM biopsy and aspiration specimens yielded normocellular marrow with multilineage dysplasia manifest as micromegaryocytosis and, in neutrophil granulocytes, as pseudo-Pelger-Huët anomaly and hypogranular cytoplasm (Figure 1A). BM chromosome analysis found 3 different populations containing clonal aberrations characterized by unbalanced translocation and duplication. Abnormalities of chromosomes 5, 7, 8, and 20 – del (5q), del (7q), +8, del (20q) – characteristic of MDS, were not detected (Cazzola, 2020).
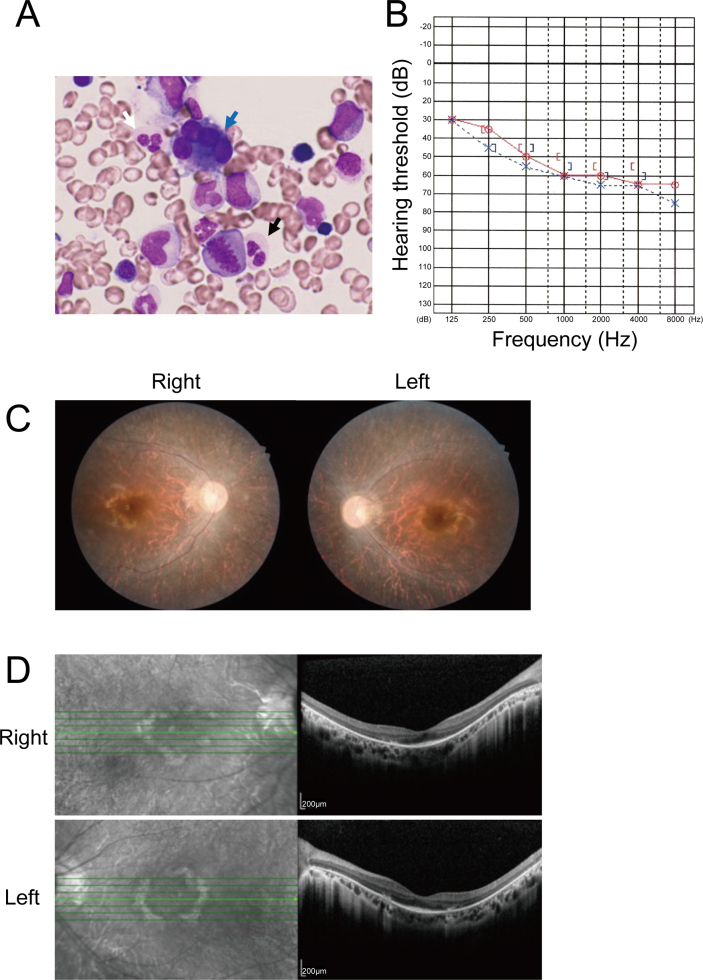
Figure 1.
Clinical findings in the patient. (A) Hematopathologic findings, MDS. Blue, white, and black arrows respectively indicate micromegakaryocytes and, in neutrophils, pseudo-Pelger-Huet anomaly and hypogranular cytoplasm (May-Grünwald-Giemsa, original magnification, 1000 x). (B) Audiogram image. Audiogram image, red circles (right ear) and blue crosses (left ear) indicate air-conduction hearing, and square brackets (red, right ear; blue, left ear) indicate bone-conduction hearing. The audiogram shows moderate hearing loss. (C) Fundoscopy images. Pigmentary deposits and attenuation of retinal vessels are shown. (D) Optical coherence tomograms, both eyes, demonstrate binocular thinning and atrophy of outer retinal segments.
The diagnosis of MDS with multilineage dysplasia was assigned (2016 WHO criteria), with a Revised International Prognostic Scoring System (IPSS-R) score of 6.5 (very high risk of malignancy). Median survival per IPSS-R was predicted as 0.8 years with BM transplantation (BMT) urgently required. Allogeneic BMT was successful, with complete remission for 1 year to date and without severe acute graft-versus-host disease. BM examination after remission showed no morphologic markers of MDS, and donor chimerism was over 99.9%. His hematologic indices showed no abnormalities, and he is not dependent on blood transfusion.
External auditory canals and tympanic membranes were intact on otoscopy. Pure tone audiometry showed moderate to severe sensorineural hearing loss with gradient worsening at increasing frequencies (Figure 1B). DPOAE assessment found bilateral cochlear hearing impairment at all frequencies tested. Tympanometry found no middle-ear dysfunction. Findings typical of RP were identified on fundoscopy, including pigmentary deposits resembling bone spicules and attenuation of retinal vessels (Figure 1C). Electroretinography could not be completed due to claustrophobia. Optical coherence tomograms demonstrated binocular thinning and atrophy of outer retinal segments (Figure 1D).
3.2. Genetic findings
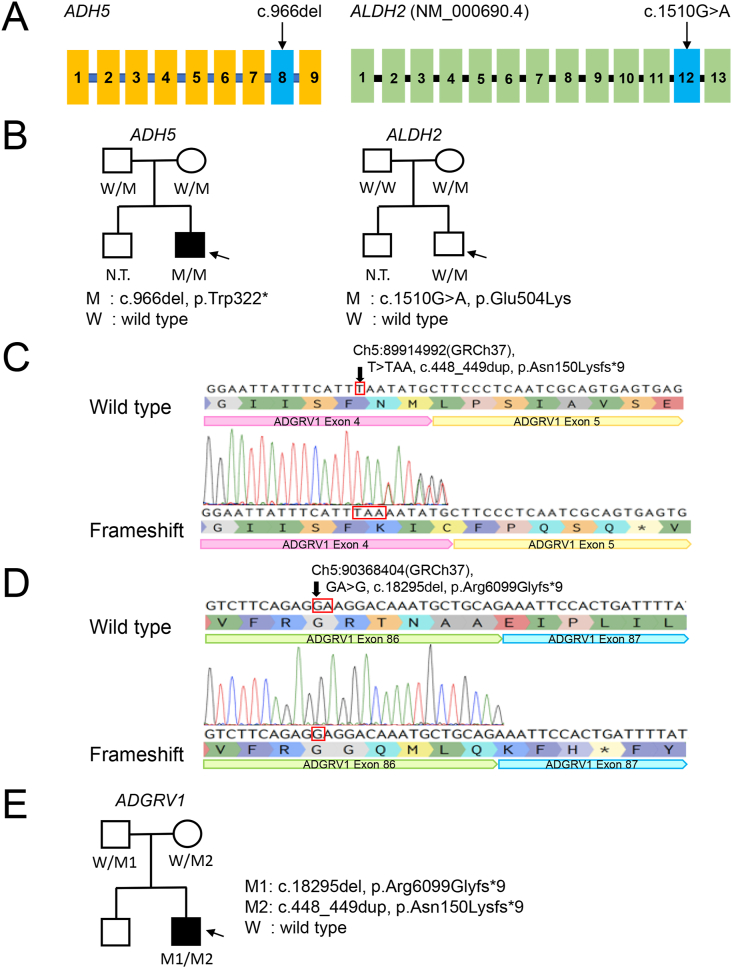
Trio WGS revealed homozygosity for a variant (c.966delG) in ADH5 (NM_000671.4), predicted to yield a frameshift with a premature stop codon (p.Trp322∗) causing functional deficiency of formaldehyde detoxification. The patient also was heterozygous for a maternally inherited variant (c.1510G>A) in ALDH2 (NM_000690.4), predicted to yield an amino-acid residue substitution (p.Glu504Lys) contributing to functional deficiency of formaldehyde detoxification (Figure 2A and B). Both these variants are reported (Oka et al., 2020). These variants were confirmed by Sanger sequencing using highly specific primers to detect only ADH5 rather than variation in a pseudogene. We also analyzed 22 genes associated with Fanconi syndrome, because dysfunction of these can cause genome instability and give rise to a phenocopy of ADH5/ALDH2 disease (Auerbach, 2009). No pathogenic variants were detected. The variant c.966delG in ADH5 was uniformly predicted to be deleterious (PROVEAN protein)/disease-causing (DDIG-in) and to result in nonsense-mediated mRNA decay or truncated protein production (NMDEscPredictor); scores and probabilities are listed in Table 2.
Figure 2.
Genetic findings in the patient. (A) Location of mutations in ADH5 and ALDH2. (B) Family pedigree, patient. Symbols for clinically unaffected individuals are empty (circle, female; square, male). The symbol, a square, for the patient is filled. M1 and M2 indicate the mutant alleles of ADH5, whereas W indicates a wild-type allele. (C and D) Sanger sequencing results, ADGRV1 variants in the patient. In C, a chromatogram is abnormal; the arrow indicates the duplicated nucleotide within ADGRV1, i.e., c.448_449dup. In D, achromatogram again is abnormal; the arrow indicates the site at which a nucleotide within ADGRV1 has been deleted, i.e., c.18295del. (E) ADGRV1 variants were identified in a non-consanguine family. Symbols for clinically unaffected individuals are empty (circle, female; square, male). The symbol, a square, for the patient is filled. M1 and M2 indicate the mutant alleles of ADGRV1, whereas W indicates a wild-type allele.
Table 2.
Characteristics of the mutations found in the patient and his parents.
| Chromosome | Position (GRCh37) | Gene | CCDS ID | cDNA mutation | Protein change | rsID | PROVEAN | DDIG-in | GnomAD % | TOPMed % |
|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 89914992 | ADGRV1 | 47246.1 | c.448_449dup | p.Asn150Lysfs∗9 | - | - | disease | No data | No data |
| 5 | 90368404 | ADGRV1 | 47246.1 | c.18295del | p.Arg6099Glyfs∗9 | - | Deleterious | disease | No data | No data |
| 4 | 99993857 | ADH5 | 47111.1 | c.966del | p.Trp322∗ | rs748162259 | Deleterious | disease | 0.05 | 0.0004 |
| 12 | 112241766 | ALDH2 | 9155.1 | c.1510G>A | p.Glu504Lys | rs671 | Deleterious | - | 25.7 | 0.9 |
The patient also was compound heterozygous for biallelic variants in ADGRV1 (NM_032119.4) (Figure 2C and D). One variant, within exon 4, is c.448_449dup (p.Asn150Lysfs∗9) (Figure 2C). The other, within exon 86, is c.18295del (p.Arg6099Glyfs∗9) (Figure 2D). The variants were confirmed by Sanger sequencing (Figures 2C and D), with that in exon 86 confirmed by subcloning (Figure 2D). Each variant in ADGRV1 was inherited from a different parent (Figure 2E). The variants were uniformly predicted to be deleterious (PROVEAN)/disease-causing (DDIG-in) and to result in nonsense-mediated mRNA decay or truncated protein production (NMDEscPredictor); scores and probabilities are provided in Table 2. None of the variants is recorded in the gnomAD V2.1.1 (Karczewski et al., 2020) database or the NCBI database. We consider them novel.
4. Discussion
Many mechanisms protect the genome from formaldehyde. Endogenous formaldehyde clearance is especially important for normal hematopoiesis (Dingler et al., 2020; Oka et al., 2020). Our patient was homozygous for a variant in ADH5 and heterozygous/wild-type for a variant in ALDH2, whereas DNA crosslink repair genes were intact.
Our patient required BMT to cure MDS. Before BMT, high-dose chemotherapy was needed to eradicate dysplastic cells. Cytotoxic agents are contraindicated in patients with MDS associated with Fanconi's anemia or dyskeratosis congenita because of deficiencies in DNA repair. Trio NGS identified that our patient had neither, permitting a usual pre-transplant chemotherapy regimen. Diagnosis by trio-NGS thus was valuable in clinical decision-making.
ADGRV1 variants account for a minority of instances of USH type II, with small insertions, point mutations, deletions, and splicing alterations reported (Wei et al., 2018; Weston et al., 2004). VLGR1 is a major component of hair cell stereocilia ankle links, which are critical to normal development of auditory hair bundles (McGee et al., 2006). Our patient had moderate to severe hearing loss and progressive RP without vestibular dysfunction, clinically typical in USH type II. Born to non-consanguine parents, our patient harboured in trans the ADGRV1 variants c.448_449dup and c.18295del, each derived from a different parent. These findings permitted assignment of the diagnosis of USH type II.
In summary, our results identified trigenic ADH5/ALDH2/ADGRV1 mutations in association with MDS and USH type II, an unprecedented combination of disorders. Furthermore, we report 2 novel pathogenic frameshift variants in ADGRV1 associated in compound heterozygous state with USH type II in a non-consanguineous family. Our work contributes to expanding the perspective not only of MDS with ADH5/ALDH2 mutations, but also of USH with ADGRV1 pathogenic variants.
Declarations
Author contribution statement
Shintaro Kinoshita, Jun Ando, Yoshiki Furukawa, Yoko Azusawa: Analyzed and interpreted the data; Wrote the paper.
Miki Ando: Conceived and designed the experiments; Wrote the paper.
Midori Ishii: Performed the experiments; Analyzed and interpreted the data.
Osamu Tomita, Shuichi Shirane, Norio Komatsu: Contributed reagents, materials, analysis tools or data.
Yoshihito Kishita, Yukiko Yatsuka, Hidetaka Eguchi, Yasushi Okazaki: Performed the experiments.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Written informed consent was obtained from the patient for publication of the case report and accompanying images. We thank A.S. Knisely for critical reading of the manuscript. This work was carried out in part at the Intractable Disease Research Center, Juntendo University Graduate School of Medicine.
References
- Auerbach A.D. Fanconi anemia and its diagnosis. Mutat. Res. 2009;668(1-2):4–10. doi: 10.1016/j.mrfmmm.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth K.T., Kahrizi K., Babanejad M., Daghagh H., Bademci G., Arzhangi S., Zareabdollahi D., Duman D., El-Amraoui A., Tekin M., Najmabadi H., Azaiez H., Smith R.J. Variants in CIB2 cause DFNB48 and not USH1J. Clin. Genet. 2018;93(4):812–821. doi: 10.1111/cge.13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boughman J.A., Fishman G.A. A genetic analysis of retinitis pigmentosa. Br. J. Ophthalmol. 1983;67(7):449–454. doi: 10.1136/bjo.67.7.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boughman J.A., Vernon M., Shaver K.A. Usher syndrome: definition and estimate of prevalence from two high-risk populations. J. Chron. Dis. 1983;36(8):595–603. doi: 10.1016/0021-9681(83)90147-9. [DOI] [PubMed] [Google Scholar]
- Cazzola M. Myelodysplastic syndromes. N. Engl. J. Med. 2020;383(14):1358–1374. doi: 10.1056/NEJMra1904794. [DOI] [PubMed] [Google Scholar]
- Choi Y., Chan A.P. PROVEAN web server: a tool to predict the functional effect of amino acid substitutions and indels. Bioinformatics. 2015;31(16):2745–2747. doi: 10.1093/bioinformatics/btv195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coban-Akdemir Z., White J.J., Song X., Jhangiani S.N., Fatih J.M., Gambin T., Bayram Y., Chinn I.K., Karaca E., Punetha J., Poli C., Baylor-Hopkins Center for Mendelian G., Boerwinkle E., Shaw C.A., Orange J.S., Gibbs R.A., Lappalainen T., Lupski J.R., Carvalho C.M.B. Identifying genes whose mutant transcripts cause dominant disease traits by potential gain-of-function alleles. Am. J. Hum. Genet. 2018;103(2):171–187. doi: 10.1016/j.ajhg.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingler F.A., Wang M., Mu A., Millington C.L., Oberbeck N., Watcham S., Pontel L.B., Kamimae-Lanning A.N., Langevin F., Nadler C., Cordell R.L., Monks P.S., Yu R., Wilson N.K., Hira A., Yoshida K., Mori M., Okamoto Y., Okuno Y., Muramatsu H., Shiraishi Y., Kobayashi M., Moriguchi T., Osumi T., Kato M., Miyano S., Ito E., Kojima S., Yabe H., Yabe M., Matsuo K., Ogawa S., Gottgens B., Hodskinson M.R.G., Takata M., Patel K.J. Two aldehyde clearance systems are essential to prevent lethal formaldehyde accumulation in mice and humans. Mol. Cell. 2020 doi: 10.1016/j.molcel.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinos C., Millan J.M., Beneyto M., Najera C. Epidemiology of usher syndrome in Valencia and Spain. Community Genet. 1998;1(4):223–228. doi: 10.1159/000016167. [DOI] [PubMed] [Google Scholar]
- Folkman L., Yang Y., Li Z., Stantic B., Sattar A., Mort M., Cooper D.N., Liu Y., Zhou Y. DDIG-in: detecting disease-causing genetic variations due to frameshifting indels and nonsense mutations employing sequence and structural properties at nucleotide and protein levels. Bioinformatics. 2015;31(10):1599–1606. doi: 10.1093/bioinformatics/btu862. [DOI] [PubMed] [Google Scholar]
- Foord S.M., Jupe S., Holbrook J. Bioinformatics and type II G-protein-coupled receptors. Biochem. Soc. Trans. 2002;30(4):473–479. doi: 10.1042/bst0300473. [DOI] [PubMed] [Google Scholar]
- Hope C.I., Bundey S., Proops D., Fielder A.R. Usher syndrome in the city of Birmingham--prevalence and clinical classification. Br. J. Ophthalmol. 1997;81(1):46–53. doi: 10.1136/bjo.81.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karczewski K.J., Francioli L.C., Tiao G., Cummings B.B., Alfoldi J., Wang Q., Collins R.L., Laricchia K.M., Ganna A., Birnbaum D.P., Gauthier L.D., Brand H., Solomonson M., Watts N.A., Rhodes D., Singer-Berk M., England E.M., Seaby E.G., Kosmicki J.A., Walters R.K., Tashman K., Farjoun Y., Banks E., Poterba T., Wang A., Seed C., Whiffin N., Chong J.X., Samocha K.E., Pierce-Hoffman E., Zappala Z., O'Donnell-Luria A.H., Minikel E.V., Weisburd B., Lek M., Ware J.S., Vittal C., Armean I.M., Bergelson L., Cibulskis K., Connolly K.M., Covarrubias M., Donnelly S., Ferriera S., Gabriel S., Gentry J., Gupta N., Jeandet T., Kaplan D., Llanwarne C., Munshi R., Novod S., Petrillo N., Roazen D., Ruano-Rubio V., Saltzman A., Schleicher M., Soto J., Tibbetts K., Tolonen C., Wade G., Talkowski M.E., Genome Aggregation Database C., Neale B.M., Daly M.J., MacArthur D.G. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581(7809):434–443. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keats B.J., Corey D.P. The Usher syndromes. Am. J. Med. Genet. 1999;89(3):158–166. [PubMed] [Google Scholar]
- Kimberling W.J., Hildebrand M.S., Shearer A.E., Jensen M.L., Halder J.A., Trzupek K., Cohn E.S., Weleber R.G., Stone E.M., Smith R.J. Frequency of Usher syndrome in two pediatric populations: implications for genetic screening of deaf and hard of hearing children. Genet. Med. 2010;12(8):512–516. doi: 10.1097/GIM.0b013e3181e5afb8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohda M., Tokuzawa Y., Kishita Y., Nyuzuki H., Moriyama Y., Mizuno Y., Hirata T., Yatsuka Y., Yamashita-Sugahara Y., Nakachi Y., Kato H., Okuda A., Tamaru S., Borna N.N., Banshoya K., Aigaki T., Sato-Miyata Y., Ohnuma K., Suzuki T., Nagao A., Maehata H., Matsuda F., Higasa K., Nagasaki M., Yasuda J., Yamamoto M., Fushimi T., Shimura M., Kaiho-Ichimoto K., Harashima H., Yamazaki T., Mori M., Murayama K., Ohtake A., Okazaki Y. A comprehensive genomic analysis reveals the genetic landscape of mitochondrial respiratory chain complex deficiencies. PLoS Genet. 2016;12(1) doi: 10.1371/journal.pgen.1005679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., Marth G., Abecasis G., Durbin R., Genome Project Data Processing, S. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25(16):2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Bulgakov O.V., Darrow K.N., Pawlyk B., Adamian M., Liberman M.C., Li T. Usherin is required for maintenance of retinal photoreceptors and normal development of cochlear hair cells. Proc. Natl. Acad. Sci. U. S. A. 2007;104(11):4413–4418. doi: 10.1073/pnas.0610950104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchitti S.A., Brocker C., Stagos D., Vasiliou V. Non-P450 aldehyde oxidizing enzymes: the aldehyde dehydrogenase superfamily. Expet Opin. Drug Metabol. Toxicol. 2008;4(6):697–720. doi: 10.1517/17425250802102627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur P., Yang J. Usher syndrome: hearing loss, retinal degeneration and associated abnormalities. Biochim. Biophys. Acta. 2015;1852(3):406–420. doi: 10.1016/j.bbadis.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch D.L., Marmor M.F., Brigell M.G., Hamilton R., Holder G.E., Tzekov R., Bach M. ISCEV Standard for full-field clinical electroretinography (2015 update) Documenta ophthalmologica. Adv. Ophthalmol. 2015;130(1):1–12. doi: 10.1007/s10633-014-9473-7. [DOI] [PubMed] [Google Scholar]
- McGee J., Goodyear R.J., McMillan D.R., Stauffer E.A., Holt J.R., Locke K.G., Birch D.G., Legan P.K., White P.C., Walsh E.J., Richardson G.P. The very large G-protein-coupled receptor VLGR1: a component of the ankle link complex required for the normal development of auditory hair bundles. J. Neurosci. : Off. J. Soc. Neurosci. 2006;26(24):6543–6553. doi: 10.1523/JNEUROSCI.0693-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna A., Hanna M., Banks E., Sivachenko A., Cibulskis K., Kernytsky A., Garimella K., Altshuler D., Gabriel S., Daly M., DePristo M.A. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20(9):1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka Y., Hamada M., Nakazawa Y., Muramatsu H., Okuno Y., Higasa K., Shimada M., Takeshima H., Hanada K., Hirano T., Kawakita T., Sakaguchi H., Ichimura T., Ozono S., Yuge K., Watanabe Y., Kotani Y., Yamane M., Kasugai Y., Tanaka M., Suganami T., Nakada S., Mitsutake N., Hara Y., Kato K., Mizuno S., Miyake N., Kawai Y., Tokunaga K., Nagasaki M., Kito S., Isoyama K., Onodera M., Kaneko H., Matsumoto N., Matsuda F., Matsuo K., Takahashi Y., Mashimo T., Kojima S., Ogi T. Digenic mutations in ALDH2 and ADH5 impair formaldehyde clearance and cause a multisystem disorder, AMeD syndrome. Sci. Adv. 2020;6(51) doi: 10.1126/sciadv.abd7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg T., Haim M., Hauch A.M., Parving A. The prevalence of Usher syndrome and other retinal dystrophy-hearing impairment associations. Clin. Genet. 1997;51(5):314–321. doi: 10.1111/j.1399-0004.1997.tb02480.x. [DOI] [PubMed] [Google Scholar]
- Sanghani P.C., Stone C.L., Ray B.D., Pindel E.V., Hurley T.D., Bosron W.F. Kinetic mechanism of human glutathione-dependent formaldehyde dehydrogenase. Biochemistry. 2000;39(35):10720–10729. doi: 10.1021/bi9929711. [DOI] [PubMed] [Google Scholar]
- Schmidt R.P., Mock R.E., Shiner D.S. Lactic dehydrogenase in lung tissue and plasma of rhesus monkeys. Lab. Anim. Sci. 1972;22(5):728–730. [PubMed] [Google Scholar]
- Schwartz S.B., Aleman T.S., Cideciyan A.V., Windsor E.A., Sumaroka A., Roman A.J., Rane T., Smilko E.E., Bennett J., Stone E.M., Kimberling W.J., Liu X.Z., Jacobson S.G. Disease expression in Usher syndrome caused by VLGR1 gene mutation (USH2C) and comparison with USH2A phenotype. Invest. Ophthalmol. Vis. Sci. 2005;46(2):734–743. doi: 10.1167/iovs.04-1136. [DOI] [PubMed] [Google Scholar]
- Staab C.A., Alander J., Brandt M., Lengqvist J., Morgenstern R., Grafstrom R.C., Hoog J.O. Reduction of S-nitrosoglutathione by alcohol dehydrogenase 3 is facilitated by substrate alcohols via direct cofactor recycling and leads to GSH-controlled formation of glutathione transferase inhibitors. Biochem. J. 2008;413(3):493–504. doi: 10.1042/BJ20071666. [DOI] [PubMed] [Google Scholar]
- Staab C.A., Alander J., Morgenstern R., Grafstrom R.C., Hoog J.O. The Janus face of alcohol dehydrogenase 3. Chem. Biol. Interact. 2009;178(1-3):29–35. doi: 10.1016/j.cbi.2008.10.050. [DOI] [PubMed] [Google Scholar]
- Tamayo M.L., Bernal J.E., Tamayo G.E., Frias J.L., Alvira G., Vergara O., Rodriguez V., Uribe J.I., Silva J.C. Usher syndrome: results of a screening program in Colombia. Clin. Genet. 1991;40(4):304–311. doi: 10.1111/j.1399-0004.1991.tb03100.x. [DOI] [PubMed] [Google Scholar]
- Teng S., Beard K., Pourahmad J., Moridani M., Easson E., Poon R., O'Brien P.J. The formaldehyde metabolic detoxification enzyme systems and molecular cytotoxic mechanism in isolated rat hepatocytes. Chem. Biol. Interact. 2001;130–132(1-3):285–296. doi: 10.1016/s0009-2797(00)00272-6. [DOI] [PubMed] [Google Scholar]
- Uotila L., Koivusalo M. Formaldehyde dehydrogenase from human liver. Purification, properties, and evidence for the formation of glutathione thiol esters by the enzyme. J. Biol. Chem. 1974;249(23):7653–7663. [PubMed] [Google Scholar]
- Vernon M. Sociological and psychological factors associated with hearing loss. J. Speech Hear. Res. 1969;12(3):541–563. doi: 10.1044/jshr.1203.541. [DOI] [PubMed] [Google Scholar]
- Wafa T.T., Faridi R., King K.A., Zalewski C., Yousaf R., Schultz J.M., Morell R.J., Muskett J., Turriff A., Tsilou E., Griffith A.J., Friedman T.B., Zein W.M., Brewer C.C. Vestibular phenotype-genotype correlation in a cohort of 90 patients with Usher syndrome. Clin. Genet. 2021;99(2):226–235. doi: 10.1111/cge.13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Li M., Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38(16):e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.F., Han C.L., Yin S.J. Substrate specificity of human and yeast aldehyde dehydrogenases. Chem. Biol. Interact. 2009;178(1-3):36–39. doi: 10.1016/j.cbi.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Wang R.S., Nakajima T., Kawamoto T., Honma T. Effects of aldehyde dehydrogenase-2 genetic polymorphisms on metabolism of structurally different aldehydes in human liver. Drug Metabol. Dispos.: the biological fate of chemicals. 2002;30(1):69–73. doi: 10.1124/dmd.30.1.69. [DOI] [PubMed] [Google Scholar]
- Wei C., Yang L., Cheng J., Imani S., Fu S., Lv H., Li Y., Chen R., Leung E.L., Fu J. A novel homozygous variant of GPR98 causes usher syndrome type IIC in a consanguineous Chinese family by next generation sequencing. BMC Med. Genet. 2018;19(1):99. doi: 10.1186/s12881-018-0602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston M.D., Luijendijk M.W., Humphrey K.D., Moller C., Kimberling W.J. Mutations in the VLGR1 gene implicate G-protein signaling in the pathogenesis of Usher syndrome type II. Am. J. Hum. Genet. 2004;74(2):357–366. doi: 10.1086/381685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Liu X., Zhao Y., Adamian M., Pawlyk B., Sun X., McMillan D.R., Liberman M.C., Li T. Ablation of whirlin long isoform disrupts the USH2 protein complex and causes vision and hearing loss. PLoS Genet. 2010;6(5) doi: 10.1371/journal.pgen.1000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.