Abstract
Bone regeneration is a crucial part in the treatment of periodontal tissue regeneration, in which new attempts come out along with the development of nanomaterials. Herein, the effect of cerium oxide nanoparticles (CeO2 NPs) on the cell behavior and function of human periodontal ligament stem cells (hPDLSCs) was investigated. Results of CCK-8 and cell cycle tests demonstrated that CeO2 NPs not only had good biocompatibility, but also promoted cell proliferation. Furthermore, the levels of alkaline phosphatase activity, mineralized nodule formation and expressions of osteogenic genes and proteins demonstrated CeO2 NPs could promote osteogenesis differentiation of hPDLSCs. Then we chose electrospinning to fabricate fibrous membranes containing CeO2 NPs. We showed that the composite membranes improved mechanical properties as well as realized release of CeO2 NPs. We then applied the composite membranes to in vivo study in rat cranial defect models. Micro-CT and histopathological evaluations revealed that nanofibrous membranes with CeO2 NPs further accelerated new bone formation. Those exciting results demonstrated that CeO2 NPs and porous membrane contributed to osteogenic ability, and CeO2 NPs contained electrospun membrane may be a promising candidate material for periodontal bone regeneration.
Keywords: CeO2 nanoparticles, Periodontal ligament stem cells, Bone regeneration, Electrospinning, Fibrous membrane
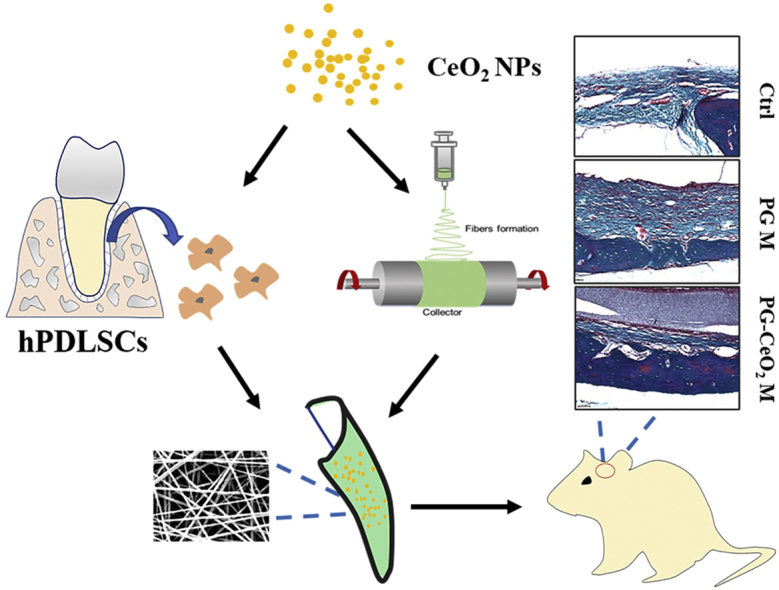
Graphical abstract
Schematic illustration of the in vitro and in vivo study of CeO2 NPs' effects on accelerating bone regeneration.
Highlights
-
•
CeO2 nanoparticles are induced in the therapy of periodontal regeneration as a bio-activator.
-
•
CeO2 nanoparticles have potential promotion on osteogenic differentiation of periodontal stem cells.
-
•
Fibrous membrane containing CeO2 nanoparticles was fabricated by electrospinning with biodegradable materials.
-
•
Incorporation CeO2 nanoparticles with PCL/gelatin enhance bone regeneration in rat critical bone defect.
1. Introduction
Periodontitis, a chronic inflammatory disease, results in not only tissue defects including alveolar bone, gingiva, periodontal ligament and cementum, but also system diseases such as adverse pregnancy outcomes, rheumatoid arthritis, Alzheimer's disease and cancer [[1], [2], [3]]. The ultimate goal of periodontal regenerative treatment is to reconstruct the periodontal complex with both hard and soft tissues, in which the alveolar bone is an indispensable part. In clinical practice, guided tissue regeneration (GTR) and guided bone regeneration (GBR) are two important regeneration approaches to restore functional bone tissues, which can be used alone or in combination with artificial/autogenous bone graft. Most commonly, the GTR/GBR membranes were used to prevent the infiltration of fibrous tissue during periodontal and alveolar bone regeneration [4]. While commercial resorbable barrier membranes have been used for single-step surgical regeneration procedures in clinical, they still have limitations such as high cost, poor mechanical property, improper biodegradation, and unpredictable clinical outcomes especially in horizontal periodontal bone defects [5]. Therefore, the development of artificial biodegradable membranes with low cost, unique structures, proper physicochemical properties and biological ability to achieve more satisfactory tissue regeneration attract increasing attention recently [[6], [7], [8]].
Electrospinning is an effective way to fabricate biodegradable fibrous membranes with porous microstructures. Such fibrous membranes mimic the natural extracellular matrix structure for stem cells to migrate, adhere, proliferate, differentiate. In addition, flexible electrospun fibrous materials made by synthetic high-molecular-weight polymers or natural polymers or the blends of them can endow fiber materials with proper properties including mechanical property and regulatable biodegradation by changing the compositions and adjusting the blending ratios [9]. These membranes can also be easily cut into desired shapes to match tissue defects. Moreover, it is easy to incorporate bioactive agents into [10] fibers during electrospinning or onto the surface [11] of fibers to achieve sustained drug delivery. These features impart the electrospun fibrous membranes with translational promise.
Due to their low cost, high safety, biodegradability, and convenient availability, poly (ε-caprolactone) (PCL) and gelatin have been used as biomaterials for decades [12]. As their complementary characteristics in degradation speed and hydrophobicity, PCL and gelatin are frequently combined in blend electrospinning for biomedical applications [10,[13], [14], [15]]. Nevertheless, osteogenic properties of PCL/gelatin membranes are often limited. To impart the electrospun membranes with tissue regeneration ability, bioactive growth factors (GFs), such as bone morphogenetic protein 2 (BMP-2) [16] and platelet-derived growth factor (PDGF) [17], were incorporated. However, the short protein half-life, possible denaturation as well as high cost have limited the use of bioactive GFs. Compared with bioactive GFs, nanomaterials with tissue regeneration ability possessed some unique advantages, such as high stability and relatively low cost. Therefore, numerous nanomaterials, such as bioactive glass nanoparticles [18], nanohydroxyapatite (nHA) [19], gold nanoparticle (Au NP) [20], graphene oxide (GO) [21] and cerium oxide nanoparticles (CeO2 NPs) [22], have been explored as bioactive agent substitutes for tissue engineering in bone defect healing [23]. Among them, CeO2 NPs have a potential in improving healing process of different tissues which is attributed to their excellent biological properties including anti-oxidation, anti-inflammation, antibacterial activities and angiogenic potential [24]. Therefore, they have been applied in the therapy of drug-induced liver injury [25] and inflammatory bowel disease [26]. CeO2 NPs have also been applied in bone tissue engineering because they can control the growth and differentiation of mesenchymal stem cells (MSCs) [27,28], promote bone regeneration on titanium surface [29] and enhance vascularization [30,31]. Interestingly, Ce exists in healthy bone and accumulates with ages [32]. However, the effects of CeO2 NPs on periodontal ligament stem cells (PDLSCs, cells isolated and expanded from periodontal ligament tissue with “stemness”) [33] have not been explored yet.
In this work, to develop new membranous substitutes with a favorable ability on promoting bone regeneration for GTR and GBR application, we utilized electrospinning to prepare CeO2 NPs loaded membranes. By taking advantage of the osteogenesis potential of CeO2 NPs (as a bio-activator), the CeO2 NPs loaded PCL/gelation composite (PG-CeO2) nanofibrous membranes were prepared. The effects of CeO2 NPs on the osteogenic differentiation of hPDLSCs were first investigated. Furthermore, the PG-CeO2 membranes were implanted into rat skull critical-sized bone defect models to evaluate their in vivo bone regeneration activity. It showed that CeO2 NPs promoted proliferation and osteogenesis differentiation of hPDLSCs. When combined with electrospinning, PG-CeO2 membranes exhibited promoted ability on bone regeneration, which implied they have potential application in alveolar bone regeneration for periodontal tissue engineering.
2. Experimental section
2.1. Materials
Poly (ε-caprolactone) (PCL, 80000 kD) and Gelatin (Gel, 300 g Bloom) were bought from Sigma-Aldrich. Hexafluoroisopropanol (HFP) were bought from Aladdin Reagent Company, acetic acid (HAc) were bought from Sinopharm. Cell Counting Kit-8 (CCK-8) were bought from Donjindo Molecular Technologies.
2.2. Synthesis of CeO2 nanoparticles and fabrication of fibrous membranes
The CeO2 NPs were synthesized according to a previous report [34] as the following process: cerium nitrate (6 mmol) was dissolved in 100 mL of H2O and ethylene glycol solution (1:1) at room temperature. The cerium nitrate solution was heated up to 60 °C, and then 20 mL NH4OH was introduced. CeO2 NPs were obtained after vigorous stirring for 3 h. Then CeO2 NPs were functionalized with carboxyl groups by citric acid treatment for better hydrophilicity. The fibrous membranes were fabricated by an electrospinning technique. PCL and Gelatin were dissolved in HFP, then HAc was added. The composition was listed in Table 1. After adding CeO2 NPs, the solution was stirred and ultrasonically processed for 30 min. The electrospinning parameters were: voltage 16 kV, height 15 cm, flow rate 1 mL/h. The air humidity was 40%–60%. All the samples were collected on aluminum foil, and then lyophilized. After removal of the aluminum foil, the membranes were stored in clean polythene bags for the following experiments.
Table 1.
The composition of electrospinning solution for membrane fabrication.
| HFP (mL) | PCL (g) | Gel (g) | HAc (μL) | CeO2 NPs (g) | |
|---|---|---|---|---|---|
| PCL/Gel | 15 | 1.05 | 1.05 | 20 | 0 |
| PCL/Gel/CeO2 | 15 | 1.05 | 1.05 | 20 | 0.105 |
2.3. Characterization of the CeO2 NPs and CeO2 loaded fibrous membranes
X-ray powder diffraction (XRD) measurements of CeO2 NPs were carried out in a D8-ADVANCE (Bruker AXS Inc, Madison, WI, USA). The size and distribution were studied by transmission electron microscopy (TEM, Tecnai G2 Spirit Biotwin, FEI Company, USA) and dynamic light scattering (DLS, Nano-ZS, Malvern Instrument). X-ray photoelectron spectroscopy (XPS) was performed using a PHI 5000 VersaProbe (Ulvac-Phi, Japan). Fibers with or without CeO2 were observed by TEM as well. The fibers with or without CeO2 NPs were coated by platinum and then the micromorphology was observed by scanning electron microscopy (SEM, S–3400 N II,Hitachi, Tokyo, Japan), equipped with a detector for energy dispersive spectroscopy (EDS). Those SEM images were used for fiber diameters measurement by the ImageJ software. Elemental analysis of cerium ions in fibrous membranes was conducted on an inductive coupled plasma optical emission spectrometer (ICP-OES, OPTIMA530DV, PerkinElmer). Mechanical properties detection was carried out at room temperature by using a universal testing machine (Instron 3365, USA). Samples were cut into rectangles (4 × 1 cm) and their thickness was measured by a micrometer caliper. The constant cross-head speed was set as 10 mm/min and stress-strain curves were recorded. Thermo-gravity analysis (TGA) was used to evaluate the variation of thermal stability of membranes in the presence or absence of CeO2 NPs (TGA Simultaneous Thermal Analyzer, Mettler-Toledo, Switzerland). The in vitro degradation of fibers was detected by SEM for surface morphology change and degradation rates were calculated by weight loss in physiological solution on days 0, 1, 3, 7 and 14. Membrane samples were first weighted (W0) and subsequently incubated in 5 mL PBS in EP tubes at 37 °C. Weights of every sample were recorded at predetermined time points (Wt) after drying. The remained mass rates were calculated as %.
2.4. In vitro antioxidant study of CeO2 NPs in membranes
The SOD-like activities of CeO2 NPs in fibrous membranes were assessed by monitoring the change of •O2− by using nitro blue tetrazolium (NBT) as a probe [35]. Briefly, NBT (2 mM), EDTA (0.1 M) and riboflavin (1.2 mM) were mixed into Tris-HCl buffer (0.1 M, pH 7.4). After mixing for 5 min at 37 °C, PG-CeO2 membranes (1 mg/mL) were added and irradiated with a LED lamp for 110 s. The absorption spectra of the mixed solution were then obtained by using a microplate reader. The CAT-like activities of CeO2 NPs were assessed by measuring the amount of generated oxygen with a dissolved oxygen meter (SevenExcellence Multiparameter, Mettler Toledo Co., Ltd). After adding 3 mg PG-CeO2 membrane into 3 mL H2O2 (0.5 M) solution, the amount of generated oxygen (mg/L) was detected and recorded every 30 s.
2.5. hPDLSCs culture and viability assay
Human PDL stem cells were obtained and identified as our previous work [36]. In short, premolars from young patients (aged < 16 y) were obtain from volunteers for orthodontic reasons with consent forms, and PDL tissue blocks were isolated from the middle third of the root surface and cultured in complete medium. Then, P0 cell suspension was seeded at the concentration of 500 cells/mL in 96-well plates for single cell-derived colonies selection. Those individual colonies were passaged and expanded for identification and following experiments. Cells of passage 3–5 were used. The cell viability and proliferation were detected by CCK-8. Briefly, the cells were cultured with CeO2 NPs at different concentrations for 48 h. 10 μL of CCK-8 reagent was added into 100 μL medium each well for incubation, then the optical density (OD) was measured by a SpectraMax M3 microplate reader (Molecular Devices, CA, USA) at a wavelength of 450 nm every 30 min until the OD was 1.0–2.0. OD values of cell proliferation on days 1, 3, 5, and 7 were measured in the same way. For cell cycle detection, cells were stained by propidium iodide (PI, 50 μg/mL) which contained RNase (100 μg/mL), and then incubated for 30 min at 37 °C for flow cytometry (BD Biosciences, Mountain View, CA). The populations of the G1, G2 and S were quantified by the FlowJo software. Cells’ debris and clumps were excluded.
2.6. TEM imaging of cells
To investigate intracellular CeO2 localization, hPDLSCs were cultured with CeO2 NPs for 24 h. Then the samples were washed by PBS for three times and fixed overnight in 2.5% glutaraldehyde. After that, the cells were post-fixed with 1% osmium tetroxide (Merck, Germany) for 2 h and dehydrated using graded ethanol series: 50, 75, 95 and 100% twice for 10 min and finally embedded in an epoxy resin for ultrathin sectioning. The sectioned samples were used for TEM imaging at an accelerating voltage of 120 kV.
2.7. Osteogenic differentiation
For cell osteogenic induction, the culture medium was changed by osteogenic differentiation medium (OS, growth medium supplemented with 0.1 μM dexamethasone, 10 mM β-glycerophosphate, and 50 μM ascorbic acid). Alkaline phosphatase (ALP) activity and Alizarin Red Staining (ARS) were performed to evaluate the osteogenic potential. ALP activity assay and NBT/BCIP ALP staining kits (Beyotime Biotechnology, China) were used to analyze the ALP activity according to the manufacturer's instructions on day 7. For ARS staining, 10% ARS solution (Sigma-Aldrich) was used to staining cells on day 21 for 5 min and washed by water. Then red mineral deposition that stained by ARS quantitation was applied by measuring the absorbance at 562 nm after mineral nodes were desorbed by 10% (w/v) cetylpyridinium chloride (CPC, Sigma-Aldrich).
2.8. Real-time quantitative PCR
To evaluate the effect of CeO2 NP on hPDLSCs’ differentiation at different concentrations, in vitro co-culture of the cell and CeO2 NP for 7 days was performed. Then, the expression of osteogenic-related genes was examined, including Runt-related transcription factor 2 (RUNX2), bone morphogenetic protein (BMP-2), osteocalcin (OCN), and osteopontin (OPN). All primer sequences were summarized in Table 2. Total cellular RNA was isolated by TRIzol (Invitrogen, Karlsruhe, Germany), then further reverse transcribed to cDNA using PrimeScript™ II 1st Strand cDNA Synthesis Kit for real-time polymerase chain reaction (RT-PCR, Takara, Japan). qRT-PCR was carried out using Universal SYBR Green Supermix (Bio-Rad, USA). Each reaction was performed in a final volume of 10 μL containing 1 μL cDNA, and 0.5 mM of each primer, 5 μL SYBR Green PCR Master Mix, and ddH2O. The relative level of target gene expression was calculated using the comparative 2-△△Ct. All the samples were run in triplicate and normalized to GAPDH.
Table 2.
Primer sequences.
| Gene | Forward primer sequence (5′–3′) | Reverse primer sequence (5′–3′) |
|---|---|---|
| RUNX2 | GGAGTGGACGAGGCAAGAGTTT | AGCTTCTGTCTGTGCCTTCTGG |
| BMP-2 | TAGTGACTTTTGGCCACGACG | GCTTCCGCTGTTTGTGTTTG |
| OCN | GGCAGCGAGGTAGTGAAGAG | GATGTGGTCAGCCAACTCGT |
| OPN | GATGGCCTTGTATGCACCATTC | GCAGACCTGACATCCAGTACC |
| GAPDH | CGCTCTCTGCTCCTCCTGTT | CCATGGTGTCTGAGCGATGT |
2.9. Western blot
Total cellular protein was extracted from cultured cell populations using the RIPA buffer (Thermo Scientific, Rockford, IL, USA) supplemented with protease inhibitor cocktail, following the manufacturer's specifications. Protein concentration was measured by the Bradford method (Bio-Red Laboratories, Benicia, CA, USA) with bovine serum albumin (BSA) as a standard. Protein samples were loaded at 10 μg protein per well on 4%–20% sodium, dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyacrylamide fluoride (PVDF) membranes. The membranes were blocked with 3% BSA in PBST (PBS with 0.1% Tween). Primary antibodies of ALP, BMP-2, OPN, and OCN (Abcam, Cambridge, MA, USA) were applied in 1:500 dilution. Immune complexes were detected with anti-rabbit secondary antibody (Abcam, Cambridge, MA, USA) and chemiluminescence reagents. The amount of protein expression was compared after normalization with β-actin.
2.10. Cell morphology on fibrous membranes
Fibrous membranes with CeO2 NPs were immersed in 75% ethanol for 20 min in sterile culture dishes, then washed in ddH2O for three times, rinsed from PBS with 10% penicillin/streptomycin 3 times, and soaked in DMEM overnight before cell seeding. Cells cultured on the membranes for 1, 3, 5, and 7 days. The samples were washed thrice with PBS and fixed with 2.5% glutaraldehyde overnight at 4 °C. Next, those samples were progressively dehydrated with 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90% and absolute ethanol for 10 min, respectively. Then the samples were air-dried for SEM observation of cell morphology.
2.11. In vivo experimental design
The membranes were cut into discs with a diameter of 5 mm. The sterilization of samples was performed with UV-light irradiation and 75% ethanol treatment, then washed and immersed by DMEM with 10% penicillin/streptomycin for 24–48 h. The rat cranial defect model was used to evaluate bone regeneration in this study. Thirty Sprague-Dawley rats (male, 7–8 weeks old) were divided into three groups: control group (defects without repair), PG membrane group, and PG-CeO2 membrane group. After anesthetized by pentobarbital sodium, the rats were positioned in a stereotaxic frame and immobilized during surgery. The hairs over the skull were shaved, then a surgical incision along the midline from the nasofrontal to the occipital region was made for dorsal calvarium exposure. A saline-cooled stainless-steel trephine (5 mm outer diameter) was used to remove full-thickness bones to create defects on both left and right sides of the dorsal calvarium for membrane implantation. After the implantation for 4 weeks and 8 weeks, 5 rats in each group were sacrificed, and their calvarias were collected and fixed in 10% neutral buffered formalin for Micro-CT (VivaCT 80, Scanco Medical, Switzerland) analysis. The parameters for scanning were set as source voltage at 70 kV, source current at 114 μA and the scanning slice thickness was 18 μm. Then decalcified for histological evaluation. Three-dimensional (3D) pictures and bone volume (BV)/tissue volume (TV) ratios were reconstructed and analyzed. After that, those samples were decalcified in EDTA-Na2, then embedded in paraffin for sectioning. Haematoxylin-eosin (HE) and Masson's trichrome staining were performed.
2.12. Data analysis
Data were expressed as the mean ± standard deviation (SD). At least three duplicate samples were tested in all experiments.
2.13. Ethics approval and consent to participate
The animal experiments were carried out with the approval of the Ethics Committee and guidelines of the Animal Care Committee of Nanjing Medical University (IACUC-1912027). Teeth were taken after informed consent of the patients. The manuscript does not contain clinical studies or patient data.
3. Results
3.1. Characterization of CeO2 NPs and cell biocompatibility evaluation
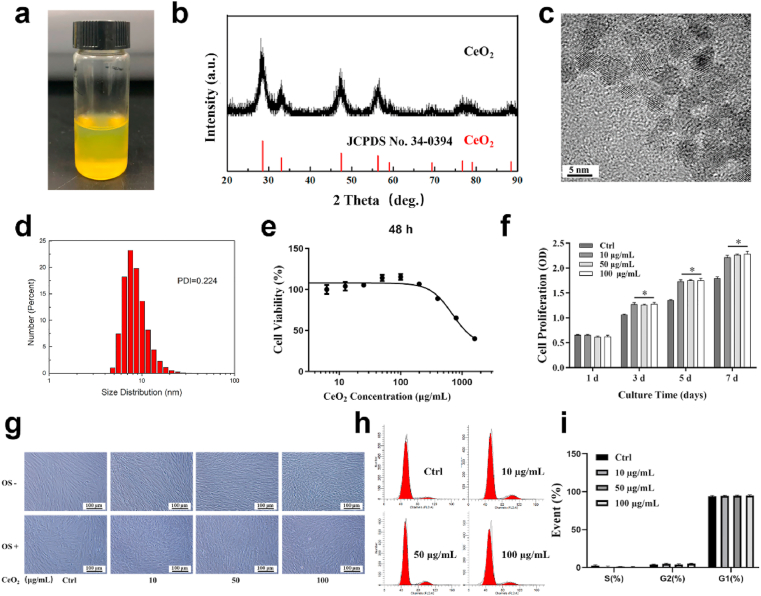
As prepared CeO2 NPs aqueous solution (Fig. 1a) appeared as an orange colloidal solution with evenly dispersible CeO2 NPs, XRD pattern of CeO2 NP sample (Fig. 1b) showed diffraction peaks matched the standard diffraction pattern of CeO2 NPs without impure peaks, confirming the single cubic phase of crystalline CeO2 NPs. As shown in TEM (Fig. 1c) and DLS (Fig. 1d) images, CeO2 NPs were well dispersed, and their average particle size was 8.1 ± 4.6 nm. The long-term stability of CeO2 NPs was investigated by DLS. After storing for more than 6 months, CeO2 NPs are still well dispersed in water, demonstrating that the long-term stability of CeO2 NPs was quite good (Fig. S1). Since the ratio of Ce3+ to Ce4+ on their surfaces is an important property of CeO2 NPs, XPS spectrum was collected to quantify the ratio of the Ce species. As shown in Fig. S2, both the Ce3+ and Ce4+ existed on CeO2 NPS’ surface, and the Ce3+ fraction was 29.87%. As shown in Fig. 1e–f, concentrations of CeO2 NPs up to 100 μg/mL had no significant effect on cell viability. CeO2 NPs seemed to promote cell proliferation when co-cultured for more than 3 days. And also, after incubating CeO2 NPs with hPDLSCs for 48 h, cells had little morphological change (Fig. 1g), and negligible cell cycle change (Fig. 1h–i), more than 90% of cells were at the G1 phase. These results indicated that the CeO2 NPs had good biocompatibility. Based on the above results, we chose four concentrations of CeO2 NPs (0, 10, 50, and 100 μg/mL) to investigate the effects of CeO2 NPs on the osteogenic differentiation of hPDLSCs.
Fig. 1.
Characterization and biocompatibility of CeO2 NPs. (a) Digital photo, (b) XRD pattern, (c) TEM image and (d) size distribution histogram of CeO2 NPs. (e) Viability and (f) proliferation of hPDLSCs when treated with CeO2 NPs at different concentrations. (g) Light microscope images of hPDLSCs when culture with or without CeO2 NPs under normal (OS-) and osteogenic induction medium (OS+). (h, i) Cell cycle analysis of hPDLSCs treated with CeO2 NPs for 3 days.
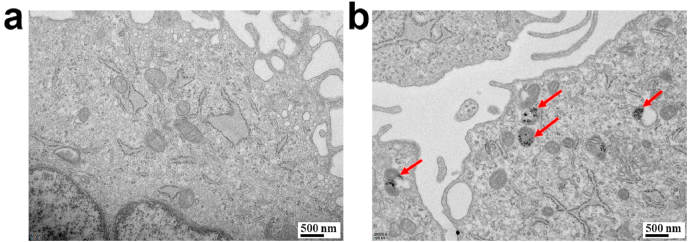
3.2. Internalization and localization of CeO2 NPs in hPDLSCs
Ultrastructural analysis was performed to detect the localization of CeO2 NPs within the cells. Typical TEM images (Fig. 2) of cells after incubation with 10 μg/mL CeO2 NPs for 24 h showed that the cells incubated with CeO2 NPs contain black dots corresponding to clusters of aggregated particles, whereas these dots were not observed in the cells incubated without NPs. CeO2 NPs were uptake by hPDLSCs, and endosomal compartments containing nanoparticles were seen inside the cells.
Fig. 2.
(a) TEM image of hPDLSCs. (b) hPDLSCs treated with CeO2 NPs for 24 h (red arrows: CeO2 NPs). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3. In vitro effects of CeO2 NPs on osteogenic differentiation
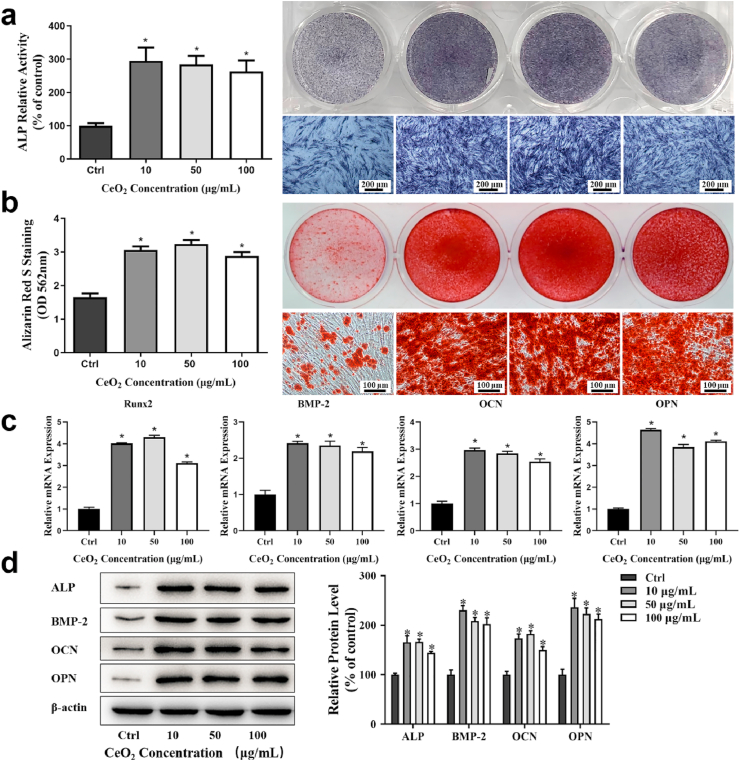
The impact of CeO2 NPs on osteogenic differentiation of hPDLSCs was evaluated by ALP activity and staining, RT-PCR, and Western Blot. hPDLSCs were cultured with CeO2 NPs in OS medium. ALP activity was tested by ALP activity assay and ALP staining. On day 7, ALP activity levels of hPDLSCs were increased when incubating with CeO2 NPs at different concentrations (Fig. 3a). The effects of CeO2 NPs on the formation of the mineralized nodules were tested by ARS on day 21, by which calcium nodule can be stained red, and histogram image of CPC quantification on the left (Fig. 3b). The gene expressions of bone-related mRNA (RUNX2, BMP-2, OCN and OPN) (Fig. 3c), and protein expression levels (ALP, BMP-2, OCN and OPN) (Fig. 3d) were also detected. Those results had a consistent trend in our study, in which the osteogenic markers of hPDLSCs were all up-regulated at all concentrations of CeO2 NPs, while with no statistical significance. Taken together, these results showed that CeO2 NPs significantly promoted hPDLSCs osteogenic differentiation even at the lowest concentration of 10 μg/mL.
Fig. 3.
Osteogenic effects of CeO2 NPs on hPDLSCs after co-cultured at concentration of 0, 10, 50, and 100 μg/mL (a) ALP relative activity (left) and corresponding stained images (right) on day 7. (b) ARS assay of mineralization, OD values at 562 nm (left) and corresponding stained images (right) on day 21. (c) Osteogenic related gene expressions on day 7. (d) Osteogenic related protein expressions on day 14.
3.4. Characterization of fibrous membranes incorporated with CeO2 NPs
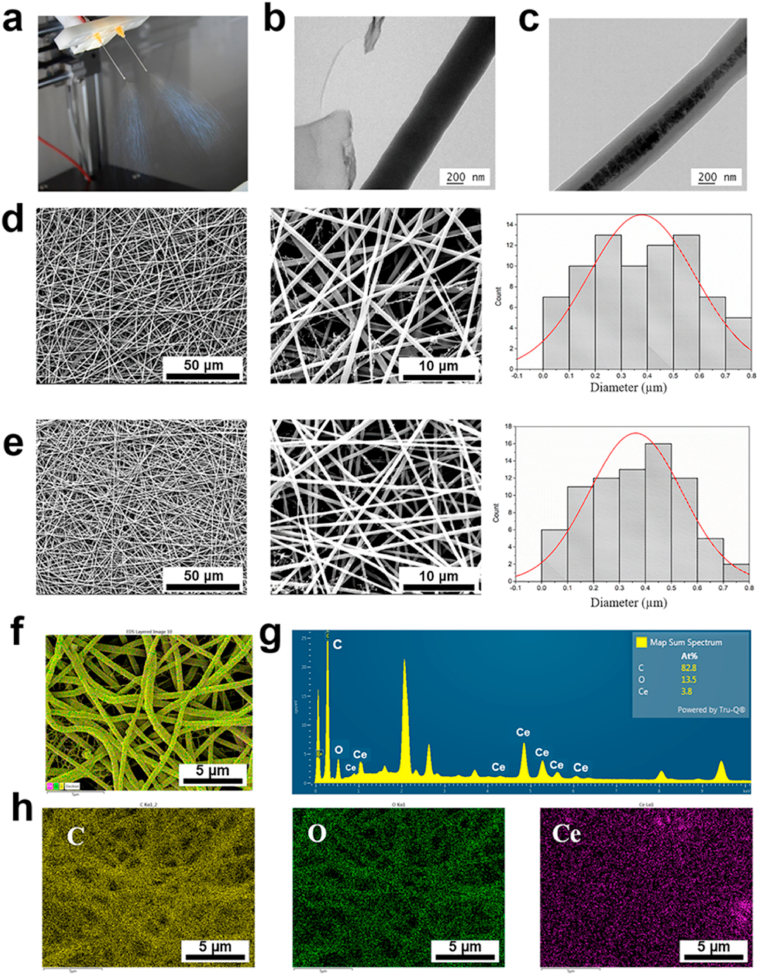
For utilizing CeO2 NPs in periodontal regeneration in a proper way, electrospinning was selected for the NPs encapsulation and local delivery. Fig. 4a showed the process of electrospinning to fabricate membranes. When the original fiber jet was stable, it was swapped to the fiber formation mode and the samples were collected as non-woven membranes. TEM and SEM images of fibers without or with CeO2 NPs were presented in Fig. 4b and d, 4c and 4e, respectively. The fiber with CeO2 NPs had black particles inside, indicating the successful encapsulation of CeO2 NPs (Fig. 4c). SEM images showed both kinds of fibers were macroscopically smooth without any gross defects. The average diameter of blank fibers and fibers with CeO2 was 378 ± 204 nm and 355 ± 181 nm, respectively. There was no statistical significance between the two types of fibers. To confirm CeO2 NPs were incorporated in fibrous membranes, EDS mapping was presented in Fig. 4f–h, which showed Ce, O, and C elements were in the fiber, and the atomic percent of Ce was 3.8%. And also, the result of inductive coupled plasma optical emission spectrometer (ICP) showed weight ratio of Ce in the composite was 3.84%, which means the proportion of CeO2 was 4.72%. Those results demonstrated CeO2 NPs have been successfully incorporated inside the fibers.
Fig. 4.
(a) Photograph of electrospinning. Representative TEM images of (b) PG fiber and (c) PG-CeO2 fiber. Representative SEM images with histograms of fiber diameters of PG membranes (d) and PG-CeO2 fibrous membranes (e). EDS elemental mapping for the merged image of all elements (f), spectrum (g) and elements C in yellow, O in green, Ce in purple (h). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
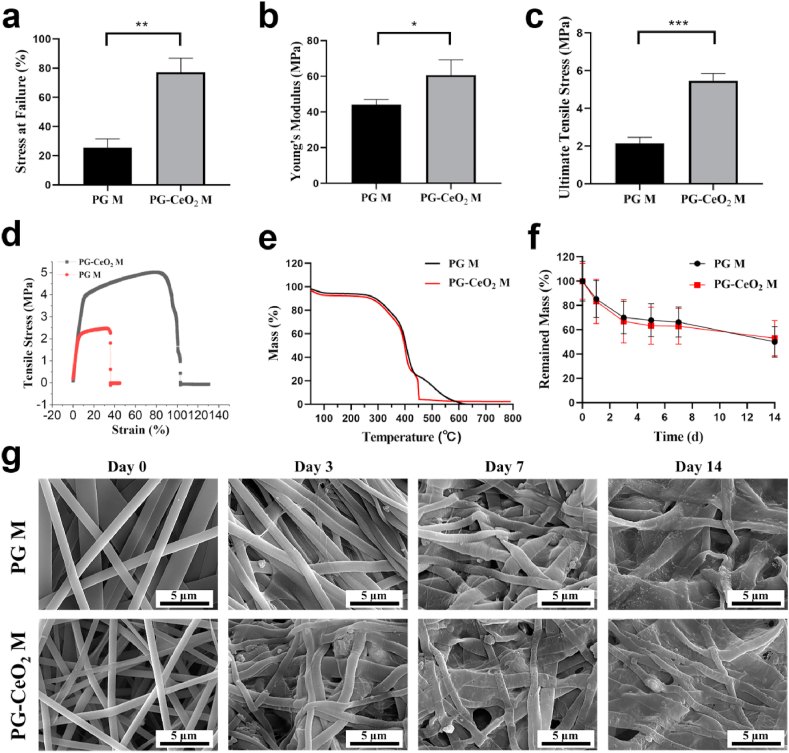
Fig. 5a–c showed the mechanical properties of membranes, the ultimate tensile strength data of PG and PG-CeO2 were 2.15 ± 0.32 MPa and 5.46 ± 0.40 MPa. The stress at failure data were 25.6 ± 6.02 and 77.09 ± 9.71, and the data of Young's modulus were 44.00 ± 3.00 MPa and 60.64 ± 8.67 MPa, respectively. All three parameters showed the mechanical properties of membrane with CeO2 NPs were remarkably enhanced. In addition, the representative tensile stress-strain curves of membranes were showed in Fig. 5d, indicating that both of the membranes were elastic. TGA technique was used to evaluate the thermal stability of membranes. As shown in Fig. 5e, the initial degradation temperature of PG-CeO2 membrane was slightly lower than PG membrane, but the mass loss had a consistent trend because the main composite of both membranes was PCL and gelatin. Membranes degraded gradually in physiological solution, which was recorded by weight measurements and SEM. As shown in Fig. 5f, there was a sharp drop of weight in the first week of both groups. Compared with PG membrane, the weight loss was more prominent in PG-CeO2 membrane group, which may be attributed to the incorporation of CeO2 NPs. While at the second week, weight loss slowed down. The reason may be attributed to the release of CeO2 NPs that speed up the degradation of fibers by providing an increase in surface area of fibers at the first week. The same trend was observed in SEM images. Fibers had significant morphological changes during the immersion, and fibers became rough and fused. Compared with the PG membrane, micropore appeared in fibers of PG-CeO2 membranes on day 7 and became more obvious on day 14, which might be attributed to the CeO2 release from fibers. To further verify it, EDS mappings of PG-CeO2 membranes on days 3, 7 and 14 in the degradation test were collected as well. As shown in Fig. S3, the atomic ratio of Ce was decreasing along with the extension of soak time in PBS, from 3.8% before immersion, to 0.6%, 0.5% and 0.3% on days 3, 7 and 14, respectively. Those results verified incorporation of CeO2 can affect degradable speed and fiber morphology to a certain extent, and also proved the release of CeO2 from membrane along with degradation.
Fig. 5.
(a) Strain at failure, (b) Young's modulus, (c) ultimate tensile stress (n = 3), and (d) representative tensile stress-strain curves of membranes. (e) TGA curves. (f) Degradation profiles and (g) SEM images of fibers on days 0, 3, 7, and 14 during the degradation in PBS.
To investigate whether the process of electrospinning affects the ROS-scavenging activity of CeO2 NPs, in vitro antioxidant capacity of CeO2 NPs that incorporated in membranes had been examined. As shown in Fig. S4, ROS can be eliminated by adding PG-CeO2 membranes, which proved CeO2 NPs in electrospun membranes still have SOD-like (Fig. S4a) and catalase-like (Fig. S4b) activities.
3.5. Cell behavior of hPDLSCs co-culture with membranes
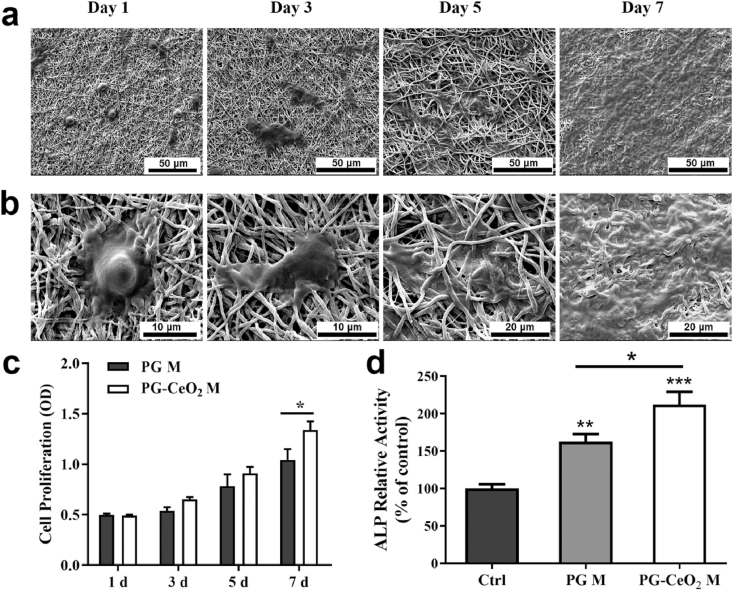
The morphology and distribution of hPDLSCs onto PG-CeO2 membranes after culturing for 1, 3, 5, and 7 days (Fig. 6a), and partial enlarged SEM images were presented in Fig. 6b. The results demonstrated that cells were captured by fibers after co-cultured for 1 day. Then with the extending of culture time, cells spread out on the surface and infiltrated inside gradually. A week later, the surface of the membrane was almost covered by hPDLSCs. We also detected cell proliferation on membranes. Cells seeded on PG-CeO2 membranes obviously promoted cell growth in comparison with blank PG membranes on day 7 (Fig. 6c), while without significant statistical significance on the previous days (day 1, 3, and 5). And the ALP activity of hPDLSCs was increased when cultured with blank PG membranes, and further improved by the addition of CeO2 NPs (Fig. 6d).
Fig. 6.
Representative SEM images of cells on the membranes after seeded for 1, 3, 5, and 7 days (a and b). The images show the adherence and morphology of the cells. (c) Cell proliferation on the membranes after seeded for 1, 3, 5, and 7 days. (d) ALP activity of hPDLSCs on membranes on day 7. (n = 3), *P < 0.05, **P < 0.01, ***P < 0.001.
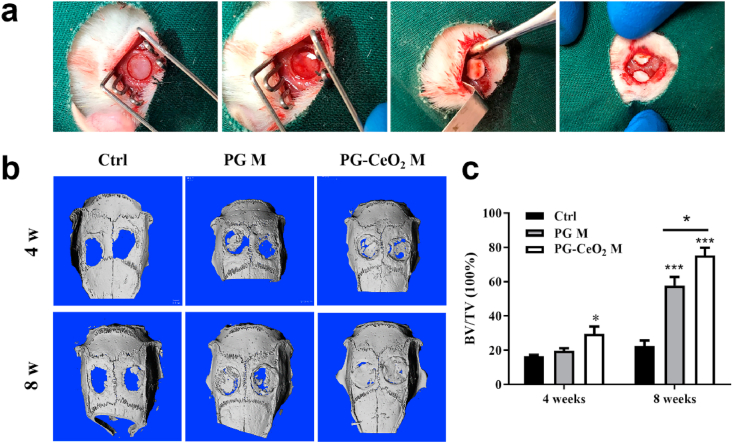
3.6. Micro-CT analysis
The surgical procedures of materials implantation were presented in Fig. 7a. After 4 weeks and 8 weeks’ implantation, the samples of animal calvaria were extracted and assessed by Micro-CT. At the time point of 4 weeks, limited new bone regeneration was observed in the control group (Fig. 7b). In contrast, PG membranes can provide mechanical and space support for cell growth, proliferation and differentiation, and CeO2 NPs can promote the regenerative process. As time went to 8 weeks, each group had more new bone regenerated, the defect area was almost reconstructed in the group of PG-CeO2 membrane. These results were further quantitatively analyzed by calculating the BV/TV (Fig. 7c), which were consistent with the Micro-CT results. The PG-CeO2 membrane group had the highest BV/TV ratio and had significantly statistical differences at week 8.
Fig. 7.
(a) Photographs of the surgical procedure during membranes implantation. (b) Micro-CT evaluation and (c) quantitative analysis of bone growth within harvested samples. BV/TV: ratio of bone volume and tissue volume bone. (n = 5), *P < 0.05, **P < 0.01, ***P < 0.001.
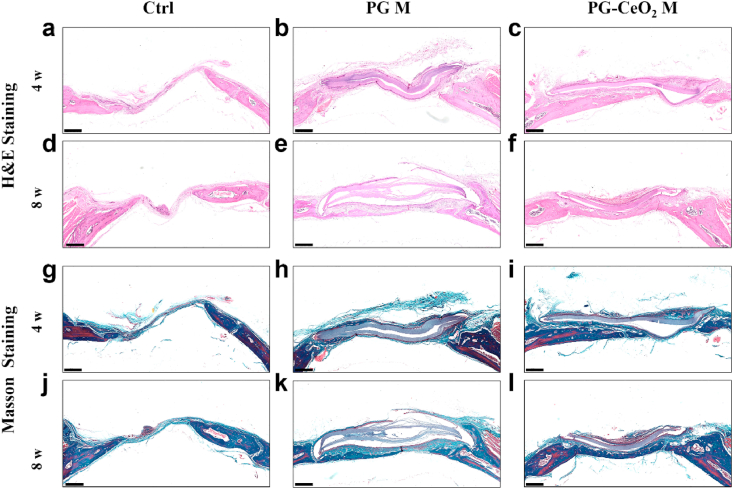
3.7. Histological section results
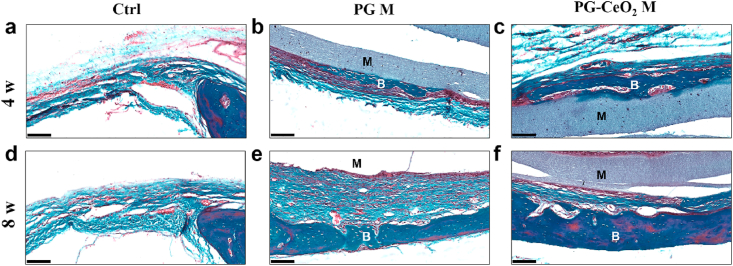
HE and Masson staining of histological sections were shown in Fig. 8. When implanted in vivo for 4 weeks, in the control group (Fig. 8a, d, g, j), there was only connective tissue regenerated with little bone formation, while in implant groups (PG M and PG-CeO2 M), new bone restored the defect along the fibrous membranes. The maximum amount of bone regenerated was the PG-CeO2 membrane group (Fig. 8c, f, i, l), better than the PG membrane group (Fig. 8b, e, h, k), reflected in much more mature and thicker new-formed bone in both 4 weeks and 8 weeks. Because the Masson staining images can clearly distinguish bone and fibrous tissues clearly, microphotographs of Masson staining were locally enlarged (Fig. 9) to further analyze the structure of defect parts. In the control group of both 4 weeks and 8 weeks, defects were filled with fibrous tissue and very limited nascent bone was observed. In the PG membrane group, new bone was formed on the surface of membrane at week 4 (Fig. 9b), and the bone mass became thicker at week 8 (Fig. 9e). In the PG-CeO2 membrane group, consistent with PG membrane group, nascent bone was formed along the membrane at week 4 (Fig. 9c), and accumulated to almost fully fill the defect at week 8 (Fig. 9f).
Fig. 8.
HE and Masson's trichrome staining of rat cranial defects after implanted with PCL/gelatin membranes without or with CeO2 NPs for 4 weeks and 8 weeks. Scale bars: 500 μm.
Fig. 9.
Masson's trichrome staining of rat cranial defects at higher magnification. M: membrane, B: bone. Scale bars: 100 μm.
4. Discussion
As is well known, in the therapy of oral diseases, bone regeneration is quite essential for the reconstruction of teeth supporting tissue and providing space for dental implants. Many strategies [37,38] about promoting the osteogenic differentiation of stem cells toward enhanced bone regeneration have been carried out in recent years, such as the usage of NPs [20,39] and the modification of bone tissue engineering scaffolds [[40], [41], [42]]. Here, we introduced CeO2 NPs that are promising materials in various biological applications [43] as a bioactive factor to promote bone regeneration, a crucial part of periodontal tissue regeneration. We further combined CeO2 NPs with electrospun membranes for repairing rat cranial defects in vivo.
Cell biocompatibility of nanomaterials was a requirement in their potential biological application [44]. Our results shown in Fig. 1 indicated that CeO2 NPs had good biocompatibility, and even up-regulated cell proliferation at low concentrations, which was consistent with the effects on the BMSCs viability [29] and primary mouse embryonic fibroblasts proliferation [45]. Stem cells are usually sensitive to the cellular uptake of NPs, because the NPs inside the cytoplasm might disturb cell fate and their ability in tissue regeneration [46]. In our study, CeO2 NPs were up-taken by hPDLSCs and located inside vesicular membrane (Fig. 2b). Compared with blank hPDLSCs shown in Fig. 2a, CeO2 NPs did not provoke apparent cell structural damage. However, this result was inconsistent with a previous study showing that CeO2 NPs aggregated and retained inside the cytoplasm of cardiac progenitor cells [47]. The difference could be due to the particle size and incubation time of NPs with cells. As we know, the senescence of in vitro culture over passaging and inflammation stimuli of stem cells lead to the ascent of ROS levels and oxidative injury [48]. Interestingly, intracellular CeO2 NPs might protect stem cells’ physiological function from such oxidative injury by scavenging excessive ROS and thus reducing the ROS-related damage of intracellular components including DNA fragmentation, lipid rupture and protein degradation [49]. This could be a well explanation of the observed good cellular biocompatibility of CeO2 NPs.
Moreover, interactions between nanomaterials and stem cells affect the self-renewal and differentiation behaviors of stem cells [50,51]. To our delight, the CeO2 NPs enhanced osteogenic differentiation-associated markers of hPDLSCs, such as ALP activity at the early stage and mineralized nodules at the later stage (Fig. 3). This is consistent with the previous studies that CeO2 NPs could promote proliferation of stem cells, and played a positive role in new bone regeneration when combined with mesoporous bio-glass scaffolds [52] and gelatin-alginate scaffolds [53]. Ceria nanoparticles have been reported to promote hypertrophic differentiation of BMSCs via DHX15 activation on bone regeneration enhancement [54]. Nevertheless, it should be noted that more work is needed to explore how the CeO2 NPs promote osteogenic differentiation of hPDLSCs in the future.
Electrospinning was used as a fibrous membrane preparation technology to load CeO2 NPs for evaluation of in vivo osteogenic effect. Biodegradable PCL and gelatin were utilized as a matrix to fabricate PG-CeO2 electrospun membranes (Fig. 4c). PCL has suitable ensile property and biocompatibility, and it has been approved for biomedical applications by the U.S. Food and Drug Administration (FDA). However, PCL is hydrophobic and lacks bioactive sites for cell adhesion. Gelatin was therefore used to blend to enhance cell capture on scaffolds by increasing hydrophilicity [10,55]. The characterization of fibrous membranes incorporated with CeO2 NPs (Fig. 4, Fig. 5), which is important for further regenerative studies. As CeO2 NPs used in our work were small, there was no obvious morphological change of fibers loaded with NPs, while the mechanical properties had been improved in PG-CeO2, as well as in PLLA/gelatin composite membrane doped with CeNP [56] and MgO [57]. The anti-oxidative activities of CeO2 are essential in its biomedical application. In this work, CeO2 NPs that were loaded in the electrospun membrane have ROS-scavenging activity (Fig. S4), which also had been proved in a previous study [58].
Fig. 6a and b showed that hPDLSCs were captured, adhered and proliferated on the PG-CeO2 membranes. Compared with cells seeded on blank PG membranes, cells on PG-CeO2 membranes had a faster proliferation rate (Fig. 6c) on day 7. The enhanced proliferation was probably because of the degradation of gelatin induced release of CeO2 NPs, which promoted cell proliferation as the same trend in Fig. 1f. ALP activity shown in Fig. 6d, in agreement with Fig. 3a about the effect of CeO2 NPs and a previous study [13] about non-woven PG membranes, demonstrates that PG-CeO2 membranes could be used as a potential bone tissue engineering scaffold on promoting osteogenic differentiation of stem cells.
In our in vivo study of animal models of critical-sized cranial defect, the rate and quality of new bone formation agreed with the above in vitro results (Fig. 7b and c). The implantation of membranes could provide mechanical support for stem cells facilitating cell migration to the central part of the defect (Fig. 8, Fig. 9). On the other hand, biophysical signals of biomaterials and force application had proved important in stem cell fate regulation [59], stem cell fate can be influenced by cell geometry through plasma membrane order modulation [60], disordered structure of fibers with an arbitrary angle direction might stimulate osteo-differentiation of hPDLSCs [36]. CeO2 NPs further increased the osteogenic differentiation ability of stem cells that homing to the membrane that implanted in defect parts.
5. Conclusions
In summary, we explored the effects of CeO2 NPs on the behavior and function of hPDLSCs. The in vitro data proved that CeO2 NPs promoted hPDLSCs osteogenesis differentiation. The in vivo study demonstrated PG-CeO2 membranes accelerated bone regeneration comparing with blank PG membranes, which further certified the possible roles of CeO2 NPs in new bone formation. We believe CeO2 NPs have great potential in the treatment of periodontal disease and electrospinning could be a suitable way to integrate CeO2 NPs and other nanomaterials in GTR/GBR for convenient clinical practices.
Declaration of competing interest
The authors declare that they have no competing interests.
CRediT authorship contribution statement
Shuangshuang Ren: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Funding acquisition. Yi Zhou: Investigation, Methodology, Formal analysis, Writing – original draft. Kai Zheng: Investigation, Data curation, Writing – original draft. Xuanwen Xu: Validation, Data curation. Jie Yang: Validation, Data curation. Xiaoyu Wang: Validation, Formal analysis, Data curation, Writing – original draft. Leiying Miao: Conceptualization, Project administration. Hui Wei: Conceptualization, Project administration, Writing – review & editing, Supervision. Yan Xu: Conceptualization, Funding acquisition, Project administration, Writing – review & editing, Supervision.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 82001049, 81771074), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD, 2018–87), the Natural Science Foundation of Jiangsu Province (BK. 20200665). The authors are grateful to Professor Yong Hu (Nanjing University) for his critical support on electrospinning technology and valuable discussion of the project.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2021.05.037.
Contributor Information
Leiying Miao, Email: miaoleiying80@163.com.
Hui Wei, Email: weihui@nju.edu.cn.
Yan Xu, Email: yanxu@njmu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015;15(1):30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kinane D.F., Stathopoulou P.G., Papapanou P.N. Periodontal diseases. Nat. Rev. Dis. Primers. 2017;3 doi: 10.1038/nrdp.2017.38. [DOI] [PubMed] [Google Scholar]
- 3.Dominy S.S., Lynch C., Ermini F., Benedyk M., Marczyk A., Konradi A., Nguyen M., Haditsch U., Raha D., Griffin C., Holsinger L.J., Arastu-Kapur S., Kaba S., Lee A., Ryder M.I., Potempa B., Mydel P., Hellvard A., Adamowicz K., Hasturk H., Walker G.D., Reynolds E.C., Faull R.L.M., Curtis M.A., Dragunow M., Potempa J. Porphyromonas gingivalis in Alzheimer's disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019;5(1) doi: 10.1126/sciadv.aau3333. eaau3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villar C.C., Cochran D.L. Regeneration of periodontal tissues: guided tissue regeneration, Dent. Clin. North. Am. 2010;54(1):73–92. doi: 10.1016/j.cden.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Bottino M.C., Thomas V., Schmidt G., Vohra Y.K., Chu T.M., Kowolik M.J., Janowski G.M. Recent advances in the development of GTR/GBR membranes for periodontal regeneration--a materials perspective. Dent. Mater. 2012;28(7):703–721. doi: 10.1016/j.dental.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 6.Liang Y., Luan X., Liu X. Recent advances in periodontal regeneration: a biomaterial perspective. Bioact. Mater. 2020;5(2):297–308. doi: 10.1016/j.bioactmat.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang J., Liu R., Chen S., Liu Q., Cai H., Lin Y., Chen Z., Chen Z. Tuning the immune reaction to manipulate the cell-mediated degradation of a collagen barrier membrane. Acta Biomater. 2020;109:95–108. doi: 10.1016/j.actbio.2020.03.038. [DOI] [PubMed] [Google Scholar]
- 8.Qian Y., Zhou X., Zhang F., Diekwisch T.G.H., Luan X., Yang J. Triple PLGA/PCL scaffold modification including silver impregnation, collagen coating, and electrospinning significantly improve biocompatibility, antimicrobial, and osteogenic properties for orofacial tissue regeneration. ACS Appl. Mater. Interfaces. 2019;11(41):37381–37396. doi: 10.1021/acsami.9b07053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xue J., Wu T., Dai Y., Xia Y. Electrospinning and electrospun nanofibers: methods, materials, and applications. Chem. Rev. 2019;119(8):5298–5415. doi: 10.1021/acs.chemrev.8b00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y., Cui W., Zhao X., Wen S., Sun Y., Han J., Zhang H. Bone remodeling-inspired dual delivery electrospun nanofibers for promoting bone regeneration. Nanoscale. 2018;11(1):60–71. doi: 10.1039/c8nr07329e. [DOI] [PubMed] [Google Scholar]
- 11.Hu Y., Dan W., Xiong S., Kang Y., Dhinakar A., Wu J., Gu Z. Development of collagen/polydopamine complexed matrix as mechanically enhanced and highly biocompatible semi-natural tissue engineering scaffold. Acta Biomater. 2017;47:135–148. doi: 10.1016/j.actbio.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 12.Malikmammadov E., Tanir T.E., Kiziltay A., Hasirci V., Hasirci N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018;29(7–9):863–893. doi: 10.1080/09205063.2017.1394711. [DOI] [PubMed] [Google Scholar]
- 13.Gong M., Chi C., Ye J., Liao M., Xie W., Wu C., Shi R., Zhang L. Icariin-loaded electrospun PCL/gelatin nanofiber membrane as potential artificial periosteum. Colloids Surf. B. 2018;170:201–209. doi: 10.1016/j.colsurfb.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Xue J., He M., Liu H., Niu Y., Crawford A., Coates P.D., Chen D., Shi R., Zhang L. Drug loaded homogeneous electrospun PCL/gelatin hybrid nanofiber structures for anti-infective tissue regeneration membranes. Biomaterials. 2014;35(34):9395–9405. doi: 10.1016/j.biomaterials.2014.07.060. [DOI] [PubMed] [Google Scholar]
- 15.Shi R., Geng H., Gong M., Ye J., Wu C., Hu X., Zhang L. Long-acting and broad-spectrum antimicrobial electrospun poly (ε-caprolactone)/gelatin micro/nanofibers for wound dressing. J. Colloid Interface Sci. 2018;509:275–284. doi: 10.1016/j.jcis.2017.08.092. [DOI] [PubMed] [Google Scholar]
- 16.Seong Y.J., Song E.H., Park C., Lee H., Kang I.G., Kim H.E., Jeong S.H. Porous calcium phosphate-collagen composite microspheres for effective growth factor delivery and bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020;109 doi: 10.1016/j.msec.2019.110480. [DOI] [PubMed] [Google Scholar]
- 17.Lee J., Lee S., Ahmad T., Madhurakkat Perikamana S.K., Lee J., Kim E.M., Shin H. Human adipose-derived stem cell spheroids incorporating platelet-derived growth factor (PDGF) and bio-minerals for vascularized bone tissue engineering. Biomaterials. 2020;255 doi: 10.1016/j.biomaterials.2020.120192. [DOI] [PubMed] [Google Scholar]
- 18.Zheng K., Sui B., Ilyas K., Boccaccini A.R. Porous bioactive glass micro- and nanospheres with controlled morphology: developments, properties and emerging biomedical applications. Mater. Horiz. 2021;8(2):300–335. doi: 10.1039/d0mh01498b. [DOI] [PubMed] [Google Scholar]
- 19.Lowe B., Hardy J.G., Walsh L.J. Optimizing nanohydroxyapatite nanocomposites for bone tissue engineering. ACS Omega. 2020;5(1):1–9. doi: 10.1021/acsomega.9b02917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y., Kong N., Zhang Y., Yang W., Yan F. Size-dependent effects of gold nanoparticles on osteogenic differentiation of human periodontal ligament progenitor cells. Theranostics. 2017;7(5):1214–1224. doi: 10.7150/thno.17252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou C., Liu S., Li J., Guo K., Yuan Q., Zhong A., Yang J., Wang J., Sun J., Wang Z. Collagen functionalized with graphene oxide enhanced biomimetic mineralization and in situ bone defect repair. ACS Appl. Mater. Interfaces. 2018;10(50):44080–44091. doi: 10.1021/acsami.8b17636. [DOI] [PubMed] [Google Scholar]
- 22.Seal S., Jeyaranjan A., Neal C.J., Kumar U., Sakthivel T.S., Sayle D.C. Engineered defects in cerium oxides: tuning chemical reactivity for biomedical, environmental, & energy applications. Nanoscale. 2020;12(13):6879–6899. doi: 10.1039/d0nr01203c. [DOI] [PubMed] [Google Scholar]
- 23.Ho-Shui-Ling A., Bolander J., Rustom L.E., Johnson A.W., Luyten F.P., Picart C. Bone regeneration strategies: engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials. 2018;180:143–162. doi: 10.1016/j.biomaterials.2018.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kargozar S., Baino F., Hoseini S.J., Hamzehlou S., Darroudi M., Verdi J., Hasanzadeh L., Kim H.W., Mozafari M. Biomedical applications of nanoceria: new roles for an old player. Nanomedicine. 2018;13(23):3051–3069. doi: 10.2217/nnm-2018-0189. [DOI] [PubMed] [Google Scholar]
- 25.Li F.Y., Qiu Y.P., Xia F., Sun H., Liao H.W., Xie A., Lee J., Lin P.H., Wei M., Shao Y.F., Yang B., Weng Q.J., Ling D.S. Dual detoxification and inflammatory regulation by ceria nanozymes for drug-induced liver injury therapy. Nano Today. 2020;35 [Google Scholar]
- 26.Zhao S., Li Y.X., Liu Q.Y., Li S.R., Cheng Y., Cheng C.Q., Sun Z.Y., Du Y., Butch C.J., Wei H. An orally administered CeO2@Montmorillonite nanozyme targets inflammation for inflammatory bowel disease therapy. Adv. Funct. Mater. 2020;30(45) [Google Scholar]
- 27.Naganuma T., Traversa E. The effect of cerium valence states at cerium oxide nanoparticle surfaces on cell proliferation. Biomaterials. 2014;35(15):4441–4453. doi: 10.1016/j.biomaterials.2014.01.074. [DOI] [PubMed] [Google Scholar]
- 28.Karakoti A.S., Tsigkou O., Yue S., Lee P.D., Stevens M.M., Jones J.R., Seal S. Rare earth oxides as nanoadditives in 3-D nanocomposite scaffolds for bone regeneration. J. Mater. Chem. 2010;20(40):8912–8919. [Google Scholar]
- 29.Li J., Wen J., Li B., Li W., Qiao W., Shen J., Jin W., Jiang X., Yeung K.W.K., Chu P.K. Valence state manipulation of cerium oxide nanoparticles on a titanium surface for modulating cell fate and bone formation. Adv. Sci. 2018;5(2) doi: 10.1002/advs.201700678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiang J., Li J., He J., Tang X., Dou C., Cao Z., Yu B., Zhao C., Kang F., Yang L., Dong S., Yang X. Cerium oxide nanoparticle modified scaffold interface enhances vascularization of bone grafts by activating calcium channel of mesenchymal stem cells. ACS Appl. Mater. Interfaces. 2016;8(7):4489–4499. doi: 10.1021/acsami.6b00158. [DOI] [PubMed] [Google Scholar]
- 31.Das S., Singh S., Dowding J.M., Oommen S., Kumar A., Sayle T.X.T., Saraf S., Patra C.R., Vlahakis N.E., Sayle D.C., Self W.T., Seal S. The induction of angiogenesis by cerium oxide nanoparticles through the modulation of oxygen in intracellular environments. Biomaterials. 2012;33(31):7746–7755. doi: 10.1016/j.biomaterials.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zaichick S., Zaichick V., Karandashev V., Nosenko S. Accumulation of rare earth elements in human bone within the lifespan. Metall. 2011;3(2):186–194. doi: 10.1039/c0mt00069h. [DOI] [PubMed] [Google Scholar]
- 33.Bartold P.M., Gronthos S. Standardization of criteria defining periodontal ligament stem cells. J. Dent. Res. 2017;96(5):487–490. doi: 10.1177/0022034517697653. [DOI] [PubMed] [Google Scholar]
- 34.Cheng H., Lin S., Muhammad F., Lin Y.W., Wei H. Rationally modulate the oxidase-like activity of nanoceria for self-regulated bioassays. ACS Sens. 2016;1:1336–1343. [Google Scholar]
- 35.Liu Y., Zhang Y., Liu Q., Wang Q., Lin A., Luo J., Du Y., Lin Y.-W., Wei H. In vitro measurement of superoxide dismutase-like nanozyme activity: a comparative study. Analyst. 2021;146(6):1872–1879. doi: 10.1039/d0an02164d. [DOI] [PubMed] [Google Scholar]
- 36.Ren S., Yao Y., Zhang H., Fan R., Yu Y., Yang J., Zhang R., Liu C., Sun W., Miao L. Aligned fibers fabricated by near-field electrospinning influence the orientation and differentiation of hPDLSCs for periodontal regeneration. J. Biomed. Nanotechnol. 2017;13(12):1725–1734. doi: 10.1166/jbn.2017.2451. [DOI] [PubMed] [Google Scholar]
- 37.Jun L., Gang C., Hai X., Ke H., Jianfei S., Mei L., Feimin Z., Ning G. Pre-vascularization in fibrin Gel/PLGA microsphere scaffolds designed for bone regeneration. NPG Asia Mater. 2018;10:827–839. [Google Scholar]
- 38.Sculean A., Nikolidakis D., Nikou G., Ivanovic A., Chapple I.L., Stavropoulos A. Biomaterials for promoting periodontal regeneration in human intrabony defects: a systematic review. Periodontol. 2000. 2015;68(1):182–216. doi: 10.1111/prd.12086. [DOI] [PubMed] [Google Scholar]
- 39.Shao D., Lu M., Xu D., Zheng X., Pan Y., Song Y., Xu J., Li M., Zhang M., Li J., Chi G., Chen L., Yang B. Carbon dots for tracking and promoting the osteogenic differentiation of mesenchymal stem cells. Biomater. Sci. 2017;5(9):1820–1827. doi: 10.1039/c7bm00358g. [DOI] [PubMed] [Google Scholar]
- 40.Bose S., Sarkar N. Natural medicinal compounds in bone tissue engineering. Trends Biotechnol. 2020;38(4):404–417. doi: 10.1016/j.tibtech.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xia Y., Fan X., Yang H., Li L., He C., Cheng C., Haag R. ZnO/Nanocarbons-Modified fibrous scaffolds for stem cell-based osteogenic differentiation. Small. 2020;16(38) doi: 10.1002/smll.202003010. [DOI] [PubMed] [Google Scholar]
- 42.Alizadeh M., Li Y., Vahid A., Ataee A., Wen C. High strength porous PLA gyroid scaffolds manufactured via fused deposition modeling for tissue-engineering applications. Smart Mater. Med. 2021;2:15–25. [Google Scholar]
- 43.Xu C., Qu X. Cerium oxide nanoparticle: a remarkably versatile rare earth nanomaterial for biological applications. NPG Asia Mater. 2014;6(3):102–108. [Google Scholar]
- 44.Muhammad Q., Jang Y., Kang S.H., Moon J., Kim W.J., Park H. Modulation of immune responses with nanoparticles and reduction of their immunotoxicity. Biomater. Sci. 2020;8(6):1490–1501. doi: 10.1039/c9bm01643k. [DOI] [PubMed] [Google Scholar]
- 45.Popov A.L., Popova N.R., Selezneva, Akkizov A.Y., Ivanov V.K. Cerium oxide nanoparticles stimulate proliferation of primary mouse embryonic fibroblasts in vitro. Mater. Sci. Eng. C Mater. Biol. Appl. 2016;68:406–413. doi: 10.1016/j.msec.2016.05.103. [DOI] [PubMed] [Google Scholar]
- 46.Celardo I., Pedersen J.Z., Traversa E., Ghibelli L. Pharmacological potential of cerium oxide nanoparticles. Nanoscale. 2011;3(4):1411–1420. doi: 10.1039/c0nr00875c. [DOI] [PubMed] [Google Scholar]
- 47.Pagliari F., Mandoli C., Forte G., Magnani E., Pagliari S., Nardone G., Licoccia S., Minieri M., Di Nardo P., Traversa E. Cerium oxide nanoparticles protect cardiac progenitor cells from oxidative stress. ACS Nano. 2012;6(5):3767–3775. doi: 10.1021/nn2048069. [DOI] [PubMed] [Google Scholar]
- 48.Ma L., Hu J., Cao Y., Xie Y., Wang H., Fan Z., Zhang C., Wang J., Wu C.T., Wang S. Maintained properties of aged dental pulp stem cells for superior periodontal tissue regeneration. Aging Dis. 2019;10(4):793–806. doi: 10.14336/AD.2018.0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mahapatra C., Singh R.K., Lee J.H., Jung J., Hyun J.K., Kim H.W. Nano-shape varied cerium oxide nanomaterials rescue human dental stem cells from oxidative insult through intracellular or extracellular actions. Acta Biomater. 2017;50:142–153. doi: 10.1016/j.actbio.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 50.Li J.J., Kawazoe N., Chen G. Gold nanoparticles with different charge and moiety induce differential cell response on mesenchymal stem cell osteogenesis. Biomaterials. 2015;54:226–236. doi: 10.1016/j.biomaterials.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 51.Qiu J., Li D., Mou X., Li J., Guo W., Wang S., Yu X., Ma B., Zhang S., Tang W., Sang Y., Gil P.R., Liu H. Effects of graphene quantum dots on the self-renewal and differentiation of mesenchymal stem cells. Adv. Healthc. Mater. 2016;5(6):702–710. doi: 10.1002/adhm.201500770. [DOI] [PubMed] [Google Scholar]
- 52.Lu B., Zhu D.Y., Yin J.H., Xu H., Zhang C.Q., Ke Q.F., Gao Y.S., Guo Y.P. Incorporation of cerium oxide in hollow mesoporous bioglass scaffolds for enhanced bone regeneration by activating the ERK signaling pathway. Biofabrication. 2019;11(2) doi: 10.1088/1758-5090/ab0676. [DOI] [PubMed] [Google Scholar]
- 53.Purohit S.D., Singh H., Bhaskar R., Yadav I., Chou C.F., Gupta M.K., Mishra N.C. Gelatin-alginate-cerium oxide nanocomposite scaffold for bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020;116 doi: 10.1016/j.msec.2020.111111. [DOI] [PubMed] [Google Scholar]
- 54.Li J., Kang F., Gong X., Bai Y., Dai J., Zhao C., Dou C., Cao Z., Liang M., Dong R., Jiang H., Yang X., Dong S. Ceria nanoparticles enhance endochondral ossification-based critical-sized bone defect regeneration by promoting the hypertrophic differentiation of BMSCs via DHX15 activation. Faseb. J. 2019;33(5):6378–6389. doi: 10.1096/fj.201802187R. [DOI] [PubMed] [Google Scholar]
- 55.Yin G., Zhao D., Ren Y., Zhang L., Zhou Z., Li Q. A convenient process to fabricate gelatin modified porous PLLA materials with high hydrophilicity and strength. Biomater. Sci. 2016;4(2):310–318. doi: 10.1039/c5bm00414d. [DOI] [PubMed] [Google Scholar]
- 56.Xu Z., Xu Y., Basuthakur P., Patra C.R., Ramakrishna S., Liu Y., Thomas V., Nanda H.S. Fibro-porous PLLA/gelatin composite membrane doped with cerium oxide nanoparticles as bioactive scaffolds for future angiogenesis. J. Mater. Chem. B. 2020;8:9110–9120. doi: 10.1039/d0tb01715a. [DOI] [PubMed] [Google Scholar]
- 57.Liu X., He X., Jin D., Wu S., Wang H., Yin M., Aldalbahi A., El-Newehy M., Mo X., Wu J. A biodegradable multifunctional nanofibrous membrane for periodontal tissue regeneration. Acta Biomater. 2020;108:207–222. doi: 10.1016/j.actbio.2020.03.044. [DOI] [PubMed] [Google Scholar]
- 58.Rather H.A., Thakore R., Singh R., Jhala D., Singh S., Vasita R. Antioxidative study of Cerium Oxide nanoparticle functionalised PCL-Gelatin electrospun fibers for wound healing application. Bioact. Mater. 2018;3(2):201–211. doi: 10.1016/j.bioactmat.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee J.H., Kim D.H., Lee H.H., Kim H.W. Role of nuclear mechanosensitivity in determining cellular responses to forces and biomaterials. Biomaterials. 2019;197:60–71. doi: 10.1016/j.biomaterials.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 60.von Erlach T.C., Bertazzo S., Wozniak M.A., Horejs C.M., Maynard S.A., Attwood S., Robinson B.K., Autefage H., Kallepitis C., Del Río Hernández A., Chen C.S., Goldoni S., Stevens M.M. Cell-geometry-dependent changes in plasma membrane order direct stem cell signalling and fate. Nat. Mater. 2018;17(3):237–242. doi: 10.1038/s41563-017-0014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.