Abstract
Angiostrongylus cantonensis, the main causative agent of human neuroangiostrongyliasis, is a food-borne parasitic zoonosis, particularly in Southeast Asia and Mainland China. Angiostrongylus malaysiensis, a cryptic species, has not been unequivocally identified as a causative agent for human angiostrongyliasis. Here, we investigated a local incidence of human angiostrongyliasis in Kalasin Province, northeastern part of Thailand. Field and laboratory investigations, clinical symptoms, and treatment of the disease are also discussed. Five sera and three cerebrospinal fluid samples were taken from each patient who displayed clinical symptoms of mild or severe headache without neck stiffness after ingesting a local dish containing Pila virescens. With molecular evidence using PCR and DNA sequencing approaches, we confirmed the presence of A. malaysiensis and A. cantonensis DNA in the patient samples. In addition, P. virescens and Pomacea canaliculata collected in the vicinity were also examined for the existence of angistrongylid larvae. The rate of infection in the snail population was 33.3% (18 infection out of 54 examined), with A. cantonensis as the predominant species. Notably, two snails were found to be co-infected with both A. malaysiensis and A. cantonensis. This discovery comes after several years of suspicion that it could be a zoonotic pathogen. Therefore, our findings are important for public health and clinical diagnosis since clinicians are not aware of the zoonotic potential of A. malaysiensis in humans.
Keywords: Angiostrongylus cantonensis, Angiostrongylus malaysiensis, Aquatic snails, Eosinophilic meningitis, Thailand
Abbreviations: ELISA, Enzyme-linked immunosorbent assay; SYBR green qPCR, SYBR green quantitative real-time polymerase chain reaction; Cq, quantification cycle in qPCR; CSF, cerebrospinal fluid; Cytb, cytochrome b; rpm, round per minute
Graphical abstract
Highlights
-
•
A. malaysiensis as a potential zoonotic pathogen of human angiostrongyliasis.
-
•
A. cantonensis and A. malaysiensis coexist in snails where human cases detected.
-
•
Discussions on related clinical manifestations and patient profiles of Angiostrongylus spp. co-infection.
1. Introduction
Human neuroangiostrongyliasis is a food-borne parasitic zoonosis distributed worldwide, particularly in East Asia and Southeast Asia. This includes Thailand, where has been recognized as one of the highest incidence areas of angiostrongyliasis with eosinophilic meningoencephalitis (Wang et al., 2008), particularly in the northeastern region of the country (Punyagupta et al., 1970; Pipitgool et al., 1997; Eamsobhana, 2013). Human Angiostrongylus infection generally causes central nervous system disorders with eosinophilia pleocytosis, i.e. eosinophilic meningitis, meningoencephalitis, and occasionally ocular angiostrongyliasis (Ketsuwan and Pradatsundarasar, 1966; Malhotra et al., 2006; Sinawat et al., 2008; Ansdell and Wattanagoon, 2018). Larval stage of the nematode Angiostrongylus cantonensis, commonly known as rat lungworm is the prime causative agent (Cross, 1979a, Cross, 1979b; Wang et al., 2008). Humans get infected via ingestion of raw molluscan intermediate hosts, harboring the infective third-stage larvae (L3) of the nematode (Eamsobhana et al., 2010a, Eamsobhana et al., 2010b).
Over twenty Angiostrongyulus spp. are described so far, mainly in rodents and other wildlife as definitive hosts, while terrestrial and aquatic snails serve as intermediate hosts (Spratt, 2015; Cowie, 2019; Almeida et al., 2020). Only two species, A. cantonensis and A. costaricensis, are documented as human pathogens, causing neurological and abdominal angiostrongyliasis, respectively (Bhaibulaya, 1991; Kramer et al., 1998). Since the parasitic stages are rarely detected in clinical samples, i.e. blood and cerebrospinal fluid (CSF), immunodiagnostic assays have been developed for diagnosing presumptive infection rather than methods for parasitic worm detection (Kuberski et al., 1979). Consequently, questions remain as to whether humans can be exposed to and potentially be infected by other angiostrongylid species in endemic areas, such as Thailand.
Angiostrongylus malaysiensis formally recognized as the Malaysian strain of A. cantonensis (Bhaibulaya and Cross, 1971), is one of angiostrongylid parasites occurring in Southeast Asia. In terms of morphological and genetic information, A. cantonensis and A. malaysiensis are closely related (Bhaibulaya, 1979; Eamsobhana et al., 2015; Chan et al., 2020), and the two cryptic species can utilize the same definitive and intermediate host species (Bhaibulaya and Techasoponmani, 1972). Additionally, mixed infections of A. cantonensis and A. malaysiensis in freshwater snails (intermediate hosts) and rodents (definitive hosts) have been widely recorded (Lim and Heyneman, 1965; Stafford et al., 1976; Lim et al., 1977; Jakkul et al., 2021). Given the close morphological and genetic relationship between A. cantonensis and A. malaysiensis, coupled with the ability to complete their life-cycle using similar hosts, and having been found coexisting in the same geographical localities, an underestimation of the prevalence of A. malaysiensis in the region could have occurred. Although there has yet to be any clear evidence of whether A. malaysiensis can infect humans, these compelling factors led us to hypothesize that A. malaysiensis has the potential to.
In Thailand, aquatic apple snails (i.e. Pila spp. and Pomacea spp.) have been recognized as one of the important intermediate hosts of Angiostrongylus spp., leading cause of human neuroangiostrongyliasis, occurring through the consumption of uncooked snail meat in a traditional dish called “Koi-hoi” (Punyagupta et al., 1970; Pipitgool et al., 1997; Eamsobhana et al., 2009). Here, we investigate a recent sporadic outbreak of neuroangiostrongyliasis at a local community of Kalasin Province, northeastern Thailand. Our specific objective is to explore the potential co-occurrence of A. cantonensis and A. malaysiensis in human cases and Pila snails collected from an endemic area in the community. Additionally, we also revealed the first incidence of A. cantonensis and A. malaysiensis co-infection in humans from the five patients who had a history of consuming the snail dish Koi-hoi (from Pila).
2. Materials and methods
2.1. Study site
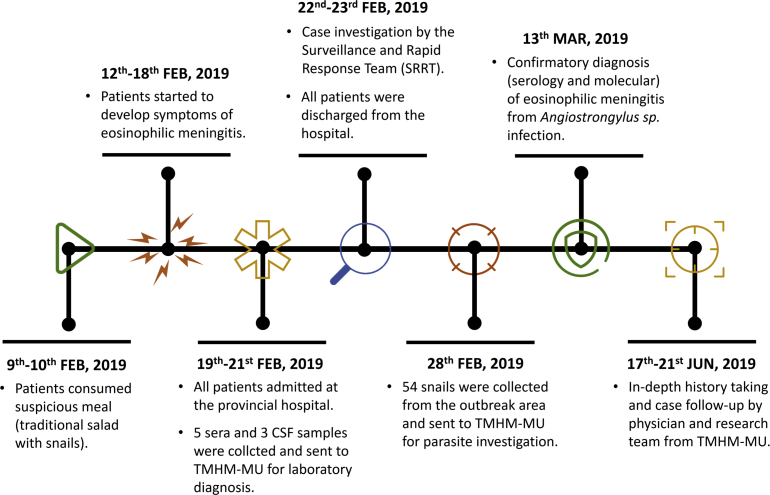
From February to June 2019, field parasitological and human angiostrongyliasis investigations took place at Ban Na Kham Village, Sam Kha Subdistrict, Kuchinaria District, Kalasin Province, Thailand (Latitude: 16.504501, Longitude: 103.937461), (Fig. 1). This study investigated human angiostrongyliasis incidence carried out by parasitologists from the Department of Helminthology, Faculty of Tropical Medicine, Mahidol University (TMHM-MU); staffs from Kuchinarai District Health Office, and the Crown Prince Kuchinarai Hospital, Kalasin Province. Parasitological data, human angiostrongyliasis cases' history, and biological specimens were collected in collaboration with various representatives, including the parasitologists from TMHM-MU, local public health officers, responsible physicians, primary health care units (PHCU), village chiefs, and local health volunteers. A detailed description of the study timeline is summarized in Fig. 2. This study was approved by the Human Ethics Committee of the Faculty of Tropical Medicine, Mahidol University (Ethical Clearance No. MUTM 2019-028-01) and the Faculty of Tropical Medicine – Animal Care and Use Committee, Mahidol University (Certification No. FTM-ACUC 010/2019E).
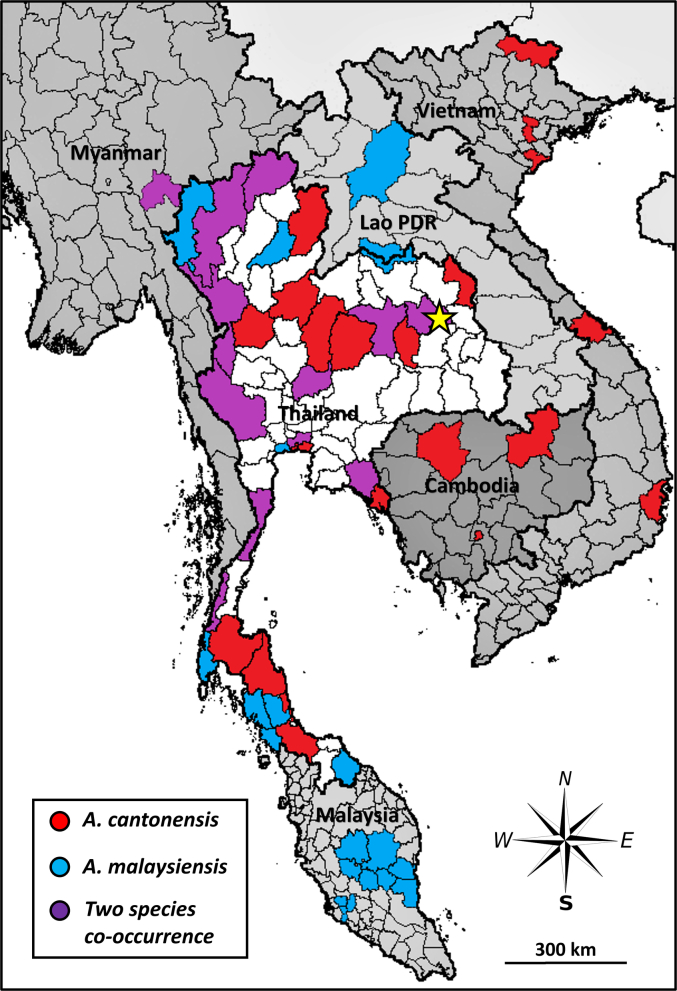
Fig. 1.
Geographic distribution of A. malaysiensis and A. cantonensis in Thailand and neighboring countries. The outbreak area in Kalasin Province, Northeast Thailand, is highlighted in a yellow star. (Data was derived from the following publications: Eamsobhana et al., 2010; Yong et al., 2015; Rodpai et al., 2016; Yong et al., 2016; Vitta et al., 2016a; Vitta et al., 2016b; Dusitsittipon et al., 2017; Eamsobhana et al., 2018; Lv et al., 2018; Chaisiri et al., 2019; Dumidae et al., 2019; Eamsobhana et al., 2019; Valentyne et al., 2020). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
Timeline of the sporadic incidence of human eosinophilic meningitis by Angiostrongylus in a local village of Kuchinarai District, Kalasin Province, Thailand.
2.2. Sera and CSF samples from human angiostrongyliasis cases
Five sera and three cerebrospinal fluid (CSF) from human angiostrongyliasis cases were collected by the Crown Prince Kuchinarai Hospital and sent to the Department of Helminthology, Faculty of Tropical Medicine, Mahidol University for laboratory investigation. These include serological and molecular diagnoses with immunoblotting assay and SYBR green quantitative real-time PCR, respectively (details and procedures are given in the following sections).
2.3. Western blot analysis for human angiostrongyliasis confirmation
Antibodies against Angiostrongylus spp. in the patients' sera were detected using an in-house western blot protocol targeting the 31 kDa antigenic protein (Nuamtanong, 1996; Eamsobhana and Yong, 2009). Crude somatic antigens were prepared from A. cantonensis adult worms harvested from experimentally infected rats, R. norvegicus. The parasites were ground in a mortar and pestle containing distilled water and aluminum powder (Sigma, US) under cold condition (4 ๐C). The parasite suspension was homogenized with an Ultrasonic Processor XL 2020 (Misonix, Farmingdale, US) for 10 min at 1 min intervals, and centrifuged at 13,000 rpm (x 9447 g) for 45 min at 4 ๐C. The supernatant was collected and kept at −80 ๐C until used. The protein concentration was quantified by Coomassie Plus Protein Assay Reagent Kit (Pierce, US). Briefly, membrane strips were incubated with 1 ml of a 1:50 mixture of individual patient serum in PBST (1× phosphate buffer solution +0.05% Tween) containing 0.02% NaN3 overnight. After three washes with PBST, each strip was incubated with anti-human IgG conjugated with horseradish peroxidase (Southern Biotech) at 1:1000 dilution in PBST for 2 h. Three additional washes with PBST were performed. After washing, each strip was arranged on a flat plate and covered with freshly prepared substrate solution (C12H7NCl2O2 in PBS containing H2O2) for 2 min. The reaction was stopped by adding distilled water until the water became clear.
2.4. Investigation of Angiostrongylus larvae from snail intermediate hosts
Aquatic snails were collected by the local health volunteers, as per traditional snail collection methods from natural water sources in the village. The method was similar to that used by local villagers to gather snails for food consumption. GPS coordinates were recorded at the points where snails were collected.
Snails were examined and identified to species level based on morphological characteristics following Brandt (1974) and Ng et al. (2020). Snails were euthanized at 0 ๐C for 20 min and had their shells removed. The foot and mantle parts of each snail were removed and cut into smaller pieces and incubated in artificial tissue digesting solution: 1% HCl-1% Pepsin (1:10,000; equivalent to 10,000 FCC units/mg; VWR International, UK) at 37 ๐C for an hour. The digestive suspension was stirred every 15 min during the digestion period. Tissue debris and suspension were filtered through an aluminum sieve (No. 25, mesh size 700 um) and left to sediment for at least 20 min in a small glass bowl (10 cm diameter). Approximately two-thirds of the supernatant was then decanted, and the remaining sediment was microscopically examined for the presence of L3 Angiostrongylus spp. Third stage Angiostrongylus larvae (L3) were morphologically identified using the following characteristics: the presence of subterminal caudal constriction (pointed tail tip), a pair of cephalic chitinous rods, and body length of 480–520 μm (Lim and Ramachandran, 1979; Moreira et al., 2013). The Angiostrongylus larvae were counted and pooled per snail and preserved in 70% ethanol at −20 °C for subsequent molecular identification. The rate of parasitic infection, mean abundance (mean number of parasites found in all hosts in the population), mean intensity (mean number of parasites found in individual infected hosts), and the range of L3 Angiostrongylus infection in the snails were estimated using Quantitative Parasitology version 3.0 (Reiczigel et al., 2019).
2.5. DNA extraction from CSF specimens and L3 Angiostrongylus larvae from snails
Human CSF samples, adult males of A. cantonensis and A. malaysiensis (from archived specimens at the Department of Helminthology) for use as positive controls in SYBR green quantitative real-time PCR, and Angiostrongylus L3 isolated from the snails were subjected for DNA extractions. As per standard lab procedure, the human CSF samples were centrifuged at 10,000 rpm (x 5590 g) for 30 min at 4 ๐C (Eppendorf, USA) to completely separate sediment from the fluid. The sediment of each sample was subsequently used for DNA extraction. Due to the very small size of the Angiostrongylus L3 larvae (450–500 սm) obtained from snails, using traditional tissue disruption methods (e.g. tissue grinding with plastic pestle) may risk tissue loss, and subsequently yield poor results. To facilitate efficient tissue homogenization and cell disruption, a bead-beating method was used on pooled larvae by employing 20 mg of 0.1 mm silica beads in 200 μl of lysis buffer in Tissue Lyser LT (Qiagen, Germany) at 50 Hz for 30 s (Jakkul et al., 2021). Three rounds of homogenization were performed for each sample, with an instant cooling step on ice before each round to prevent DNA degradation from excess heat. Finally, DNA extraction was processed by the Genomic DNA Mini Kit (Geneaid, Taipei, Taiwan) for DNA. The manufacturer's standard protocol was followed for DNA extraction, and the recovered product was stored at −20 ๐C for subsequent molecular investigation.
2.6. SYBR green quantitative real-time PCR for quantification and identification of Angiostrongylus spp
Genomic DNA isolated from human CSF and Angiostrongylus larvae were amplified targeting the mitochondrial Cytb gene using SYBR green quantitative real-time PCR (qPCR). Two pairs of species-specific primers were applied to discriminate between A. cantonensis and A. malaysiensis, following Jakkul et al. (2021). The primers: AC4_cytb_F: 5′AAT GTT TGT TGA GGC AGA TC 3′ and AC5_cytb_R: 5′ GCT ACA ACA CCC ATA ACC T 3′ were used to amplify A. cantonensis DNA (amplicon size 117 bp), whereas the primers: AM3_cytb_F: 5′ CGA GAT ATT TAT TGA GGC TG 3′ and AM4_cytb_R: 5′GAC AAA ACC CTC ATC AAT AA 3′ were used to amplify A. malaysiensis DNA (amplicon size 141 bp). For positive controls, the gDNA obtained from the adult males were serially diluted (ten-fold dilution ranging from 1 to 1 × 10−4 ng/μl) to construct qPCR standard curves for each species. All qPCR reactions were conducted following the protocol described by Jakkul et al. (2021). The qPCR products were checked band size of the species-specific amplicon on 1.5% agarose gel at 50 V for 40 min. All qPCR amplicons were then subjected to DNA Sanger sequencing with the PCR species-specific primers by Macrogen, an external biotechnology company (Seoul, South Korea). The obtained nucleotide sequences were compared with the other Cytb sequences of A. cantonensis and A. malaysiensis available in Genbank (NCBI databases via standard nucleotide BLAST) for species confirmation.
3. Results
3.1. Patients' profiles and clinical features
The ages of the five Angiostrongylus-infected patients (four males and one female) ranged from 26 to 51 years old. Their chief complaint was headache (100%) without neurological defects (Table 1). All patients were admitted to the hospital after presenting with headache and they indicated ingesting “koi-hoi” (a form of sour and spicy traditional salad dish) with aquatic snail meat. The patients revealed that they had “koi-hoi” for several meals (3–7 times), with more than 10 snails ingested per meal. In terms of clinical laboratory assessment, complete blood count (CBC) showed a high white blood cell count with 18–52% eosinophils. Slight cloudiness with elevated CSF protein was observed in all samples. The CSF profiles showed normal sugar levels (30–53 mg/dL) but increased protein (81.8–226.5 mg/dL) with significantly elevated eosinophil number (35–77%), (see more details in Table 1).
Table 1.
Profiles of infected patients with chief complaints and initially laboratory investigations.
| Genders/Ages |
Chief complaints |
Sera |
CSF |
||||
|---|---|---|---|---|---|---|---|
| WBC count (cell x 103) | Eosinophils (%) | Sugar (mg/dL) | Protein (mg/dL) | WBC count (cell x 103) | Eosinophils (%) | ||
| Male 26 yearsa,b | Severe headache, weakness | 18,120 | 28 | 47.0 | 118.9 | 720 | 52 |
| Male 34 years b | Weakness, loss of appetite | 13,120 | 39 | 53.0 | 116.0 | 660 | 40 |
| Male 49 yearsa,b | Headache with muscle pain and hot sensation in periorbital areas | 21,000 | 52 | 30.0 | 226.5 | 1720 | 77 |
| Male 51 years b | Headache | 14,200 | 35 | 50 | 115.0 | 600 | 35 |
| Female 40 yearsa,b | Headache radiated to neck stiffness, weakness | 11,200 | 18 | 44 | 81.8 | 1440 | 56 |
Patients' CSF specimens were investigated.
Patients' sera were investigated.
3.2. Laboratory investigations on human samples
Confirmatory serological diagnosis performed with immunoblotting showed an angiostrongyliasis-specific reactive band at 31 kDa in all the five infected patients from whom sera had been obtained (Data not shown).
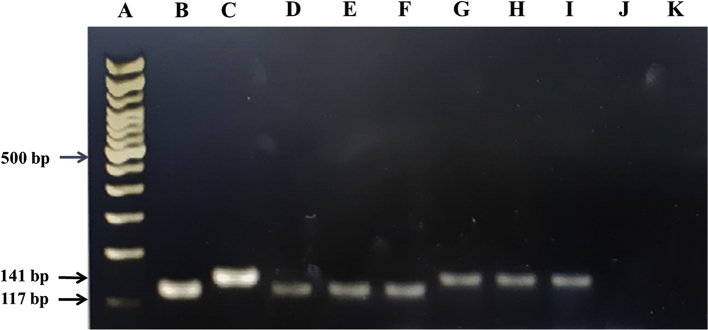
Molecular detection of Angistrongylus was performed by qPCR on three CSF samples. Both A. cantonensis and A. malaysiensis DNA were detected in the CSF samples, as evidenced by the species-specific PCR bands on the gel along with the DNA sequences after Sanger sequencing (Fig. 3, Fig. 4, and Supplementary Fig. 1). These results confirm that both A. cantonensis and A. malaysiensis DNA were present in the same CSF. In addition, qPCR results showed that the DNA concentration of A. cantonensis was relatively higher than A. malaysiensis; a relative ratio of A. cantonensis: A. malaysiensis in CSF-A, CSF-B, and CSF-C were 95.5: 4.5, 70.5: 29.5 and 89.7: 10.3, respectively (Table 2). The presence of non-specific PCR products from the heterogeneous DNA (primer-dimer) also confirmed that amplification occurred without cross-reaction. Primer-dimer usually occurs when a reaction has a low concentration of template, and it was easily separated from the expected product due to their lower melting temperature.
Fig. 3.
PCR amplicons of CSF samples from the patients. (A) 100 bp ladder marker, (B) positive control - 0.1 ng gDNA of A. cantonensis, (C) positive control - 0.1 ng gDNA of A. malaysiensis, (D—F) A. cantonensis specific amplicon of CSF-A, CSF-B and CSF-C, (G-I) A. malaysiensis specific amplicon of CSF-A, CSF-B and CSF-C, (J and K) negative controls.
Fig. 4.
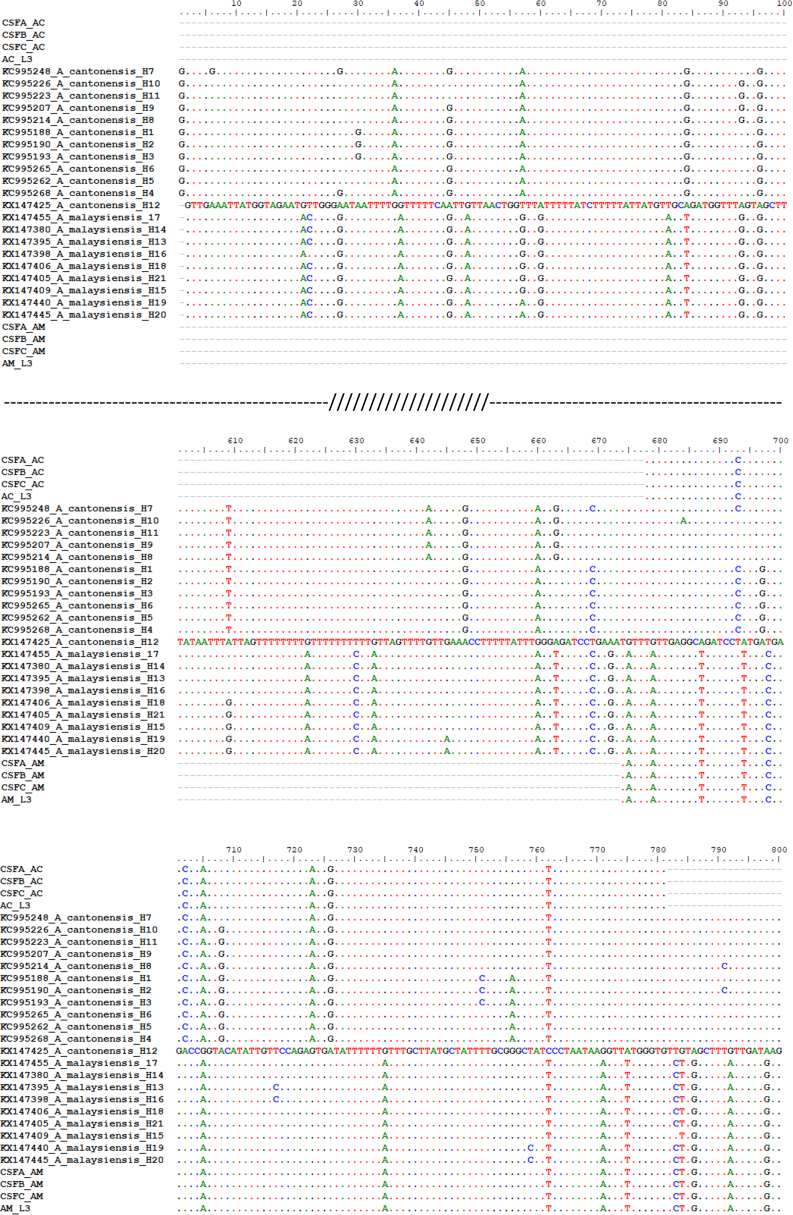
Aligned mitochondrial Cytb sequences of the CSF samples (CSFA_AC, CSFB_AC, CSFC_AC for A. cantonensis, CSFA_AM, CSFB_AM, CSFC_AM for A. malaysiensis), the L3 isolated from snails (AC_L3 for A. cantonensis and AM_L3 for A. malaysiensis) and NCBI Genbank reference sequences of haplotypes existed in Thailand (see Dusitsittipon et al., 2017), showing the fixed nucleotide positions.
Table 2.
The starting quantity (SQ) of the parasites DNA in CSF samples. Relative ratio is proportion between A. cantonensis (Ac) and A. malaysiensis (Am) in a CSF sample.
| Samples | Starting quantity (SQ) in ng/սl |
Relative ratio of SQAc (%) | Relative ratio of SQAm (%) | |
|---|---|---|---|---|
| A. cantonensis (SQAc) | A. malaysiensis (SQAm) | |||
| CSF-A | 3.684 × 10−3 | 1.701 × 10−4 | 95.5 | 4.5 |
| CSF-B | 1.403 × 10−4 | 5.853 × 10−5 | 70.5 | 29.5 |
| CSF-C | 1.105 × 10−2 | 1.267 × 10−3 | 89.7 | 10.3 |
3.3. Therapeutic approach
The onset of the disease was sudden, and the duration of the disease was short. The patients received lumbar puncture (LP) or spinal tap to release intracranial pressure, followed by supportive treatment (Punyagupta, 1979). Of the five patients involved, three received spinal tap, and CSF samples were collected for laboratory confirmation, whereas the other two showed no intense headache. According to the treatment plan and due to medical ethics, the physician did not conduct a spinal tap on the latter two patients. The medication administered was albendazole (200 mg) 2 tablets b.i.d. (after meals in the morning and afternoon) for 10 days with prednisolone (5 mg) 4 tablets t.i.d. (after breakfast, lunch, and dinner) for 2 days. All patients were relieved of their persistent headache after one to two days of treatment, and subsequently discharged from the hospital after a few days.
3.4. Prevalence and species identification of Angiostrongylus L3 from snails
A total of 54 aquatic snails were collected from different natural water bodies located in the study area. Of them, 48 were morphologically identified as Pila virescens (formerly referred to Pila polita as a synonym) (Ng et al., 2020), while six were Pomacea canaliculata.
Microscopic examination revealed that 18 of 54 (33.3%) snails were infected with Angiostrongylus larvae. Angiostrongylus L3 were found only in P. virescens, while no P. canaliculata was infected (Table 3). Molecular identification of the larvae to the species level based on qPCR revealed that 16 snails were infected by A. cantonensis (Cq ranges from 25.01–34.0) and two snails harbored both A. cantonensis (Cq = 25.74 and 29.86) and A. malaysiensis (Cq = 32.74 and 33.16). The findings indicate a higher prevalence of A. cantonensis in the snail population as compared to A. malaysiensis, with prevalence of infection at 33.3% and 3.7% respectively. Additionally, the mitochondrial Ctyb sequences of A. cantonensis and A. malaysiensis derived from the infected-snails were consistent with the same haplotypes of both parasite species found in the patient CSF samples (Fig. 4).
Table 3.
Prevalence (%), mean intensity (MI), mean abundance (MA) and range of Angiostrongylus infection (L3) in aquatic snails collected from the outbreak area in Kalasin Province, Thailand. Data on individual parasite burden is reported in Supplementary Table 1.
| Snail species (n) | No. of infected snail | (%) Prevalence [95% CI] | MI [95% CI] | MA [95% CI] | Range |
|---|---|---|---|---|---|
| Pila virescens (48) | 18 | 37.5 [24.8–52.1] | 14.8 [6.06–33.3] | 5.5 [2.1–13.2] | 1 to >100 |
| Pomacea canaliculata (6) | – | – | – | – | – |
| Total (N = 54) | 18 | 33.3 [22.1–47.2] | 14.8 [5.7–32.4] | 4.9 [1.7–11.7] | 1 to > 100 |
4. Discussion
This study reports incidence of human eosinophilic meningitis caused by Angiostrongylus spp. in a local village in Kuchinarai District, Kalasin Province, Northeastern Thailand. Interestingly, our molecular laboratory investigations revealed evidence of co-occurrence between A. cantonensis and A. malaysiensis in CSF samples of the patients. People in this region like to consume a traditional dish called “koi-hoi” containing improperly cooked snails. The consumption of snails containing infective Angiostrongylus L3 larvae is a likely cause of human angiostrongyliasis (Eamsobhana et al., 2009). Alternatively, infection in humans might also be acquired through drinking water or consuming vegetables contaminated with the parasite (Heyneman and Lim, 1967; Tsai et al., 2004; Howe et al., 2019).
In humans, the common clinical manifestations of neuroangiostrongyliasis are severe headache, nuchal stiffness, nausea, vomiting, and paresthesia involving the head, body, or extremities (Chotmongkol and Sawanyawisuth, 2002; Wang et al., 2008). In our study, three of five patients showed DNA evidence of A. cantonensis and A. malaysiensis co-occurrence. The patients developed only few clinical symptoms, encountering mild to severe headache for a couple of days without nausea, vomiting, or other neurological signs. Specifically, neurological disorders of nuchal stiffness, convulsion, paresthesia, blurred vision, and squint were absent. However, the severity of angiostrongyliasis symptoms can depend on the intensity and location of infection, parasite species or strains, and a person's tolerance level of their immune response (Punyagupta, 1979; Murphy and Johnson, 2013). In Taiwan, the Hualien strain of A. cantonensis showed lower infectivity; delayed fecundity and poor development in rats, and caused milder pathology and lower mortality in mice than the Pingtung strain (Lee et al., 2014). Studies have revealed that the lethal effects seen in hosts caused by A. cantonensis might be reduced due to co-infection with A. malaysiensis via an undefined interaction and pathway. Cross et al. (1979) showed that the lethal effects due to heavy infection of A. cantonesis (10,000 larvae) were reduced in monkeys that were previously exposed to A. malaysiensis, suggesting that A. malaysiensis infection was able to immunize monkeys against the lethal effect of A. cantonensis challenge. Additionally, low infectivity of definitive hosts and mild pathogenic symptoms in non-permissive hosts have been observed in A. malaysiensis infection (Lim et al., 1977). Accordingly, we hypothesize that coinfection between A. cantonensis and A. malaysiensis might lead to a neutral interaction resulting in less severe symptoms, as shown by the three patients that we found with evidence of both A. cantonensis and A. malaysiensis. Nonetheless, further studies are needed to address whether A. malaysiensis do indeed cause milder pathology and reduced mortality in humans. Here, our findings present the first evidence of A. malaysiensis infection in humans, despite many years of suspicion that it could be a zoonotic pathogen.
In our study, a probable diagnosis of human neuroangiostrongyliasis caused by A. cantonensis was based on a combination of a history of ingesting undercooked aquatic snails and headache as the chief complaint. Laboratory findings detected increased eosinophil numbers in peripheral blood, and CSF helped to confirm the diagnosis. Basic CBC showed a high WBC count with eosinophilia in the peripheral blood associated with eosinophilic pleocytosis in all CSF specimens. Our results corroborate earlier reports from the northeastern and central parts of Thailand, showing an average cell count in CSF ranging from 90 to 3244 × 103 cell/μl, eosinophil count ranging from 10% to 98%, and peripheral blood eosinophils ranging from normal to 42% (Tangwanicharoen et al., 2001; Kittimongkolma et al., 2007).
Generally, Angiostrongylus spp. larvae are rarely detected in the blood or CSF samples of patients during microscopic examination (Kuberski et al., 1979). In rare cases, however, Rai et al. (2014) detected larvae in CSF samples. From our qPCR detection assay, we were able to detect Angiostrongylus DNA in CSF samples, although no larva was recovered in the CSF. Our assay also allowed for the simultaneous detection of A. cantonensis and A. malaysiensis in all three CSF specimens. The difference in PCR amplicon size was used to discriminate between the two Angiostrongylus spp. through comparison with positive controls (Fig. 3). The concentration of A. cantonensis with respect to A. malaysiensis in the CSF and snails was also relatively higher based on our qPCR results. Moreover, our result showing complete alignment of mitochondrial Cytb sequences (Fig. 4) also clearly indicates that the haplotypes of A. cantonensis and A. malaysiensis isolated from both patient CFS samples and the infected snails belong to the haplotypes that previously existed in Thailand (Dusitsittipon et al., 2017).
Our result showed the same specific reactive bands at 31 kDa in all five sera in terms of immunoblot diagnosis. This might reflect conserved antigens derived from the crude somatic extract of A. cantonensis adults, containing a large complexity of protein components present in the “31 kDa band” as 2D-electrophoresis demonstrated earlier (Morassutti et al., 2012). Alternatively, this could represent cross-reaction against A. malaysiensis antibodies from the co-infections as the two species are closely related in morphological and genetic aspects (Bhaibulaya, 1979; Eamsobhana et al., 2015; Chan et al., 2020). Further research on developing serological diagnosis to differentiate between the two Angiostrongylus spp., and assessing potential co-infection will vastly aid in understanding the epidemiology and clinical manifestations of neuroangiostrongyliasis, particularly in endemic regions.
The prevalence of A. malaysiensis infection in the snails was lower than that of A. cantonensis. Notably, co-infection of the two Angistrongylus spp. occurred in snails that carried high parasitic abundance (i.e. 65 and > 100 larvae), whereas snails harboring only A. cantonensis had lower parasitic abundance (1–51 larvae) (Supplementary Table 1). We infer that the A. malaysiensis population in the study area might be less successful than A. cantonensis, either due to a suitable and more conducive environmental niche for A. cantonensis or potential competition between the two species. Congruent with our inference, Sawabe and Makiya (1995) discovered that the infectivity and survival capacities of A. malaysiensis larvae were much lower than A. cantonensis in Biomphalaria glabrata snails. Additionally, A. malaysiensis appeared to be more susceptible to environmental extrinsic and host intrinsic factors such as temperature, desiccation, pH, and proteases in the snail body compared to A. cantonensis (Sawabe and Makiya, 1995). These could potentially explain the lower prevalence of A. malaysiensis in the snail intermediate hosts from the study area. Recently, Jakkul et al. (2021) reported an opposite trend from a survey of terrestrial snails in urban public parks of Bangkok, i.e. A. malaysiensis was the dominant species over A. cantonensis. This may due to differences in geographical distribution and environmental niche between the two places (peri-domestic habitat with agricultural practices in Kalasin versus urban setting in Bangkok). In addition, different snail intermediate hosts were investigated in the two studies, i.e. fresh water snails P. virescens (local species) in Kalasin and terrestrial snails Achatina fulica (invasive species) in Bangkok. Interestingly, this raises further research questions on the potential influence of host species (either intermediate or definitive hosts) and the role of invasive species on the distribution of A. malaysiensis and A. cantonensis, in the context of parasite ecology.
5. Conclusions
A. cantonensis and A. malaysiensis are closely related, sharing similarities in morphological characteristics, genetic information, and life cycle. Despite these similarities, the evidence for zoonotic infection by A. malaysiensis has not yet been clearly revealed. Although the parasite infection rate in the snails was relatively low for A. malaysiensis compared with A. cantonensis, the importance of zoonotic A. malaysiensis infection in humans should not be overlooked. The mechanism underlying the occurrence observed is unknown and more investigations in both intermediate and definitive hosts are needed. In particular, the paucity of information on clinical manifestations in patients infected with A. malaysiensis warrants additional data. This study provides the first evidence of A. malaysiensis in humans and supports the potential of coinfection with both A. cantonensis and A. malaysiensis. This breaking evidence follows many years of speculation that A. malaysiensis could be a zoonotic pathogen.
The following are the supplementary data related to this article
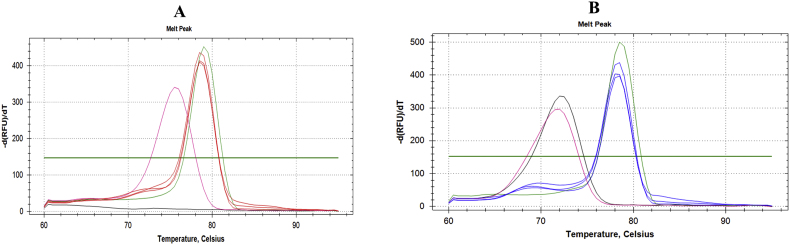
Appendix Fig. 1: The qPCR melting curves analysis of A. cantonensis (A) and A. malaysiensis (B) assays. In A. cantonensis assay (A): CSF samples = red, A. cantonensis positive control = green, A. malaysiensis positive control = pink, and negative control = black. In A. malaysiensis assay (B), CSF samples = blue, A. malaysiensis positive control = green, A. cantonensis positive control = pink, and negative control = black. For A. malaysiensis, non-specific amplification occurred, as indicated by the negative control (black line) being detected at the same temperature as the non-target samples.
Appendix Table 1: Angiostrongylus larval examination in aquatic snails collected from the study site in Kuchinarai District, Kalasin Province, Thailand based on morphological and molecular identifications.
Data statement
Other data that support the findings of this study are available from the corresponding author upon reasonable request.
Declaration of Competing Interest
All authors declare that we have no conflicts of interest regarding the content in this article.
Acknowledgements
The authors acknowledge the internal research grant of the Faculty of Tropical Medicine, Mahidol University, for financial support to conduct a field survey in Kalasin Province. We would like to deliver special appreciations also to the staffs from the local administrations: Kuchinarai District Health Office, Kalasin Provincial Health Office, and Crown Prince Kuchinarai Hospital, Kalasin Province, as well as local health volunteers, villagers, and importantly anonymous angiostrongyliasis patients for their kind cooperation and facilitation during the fieldwork conducted at their properties. Grateful thanks also to Mr. Aran Manalang (the University of Hawaii at Manoa, Hawaii, US – internship) for his help in field investigation and laboratory examination.
References
- Almeida L.R., Souza J.G.R., Santos H.A., Torres E.J.L., Vilela R.V., Cruz O.M.S., Rodrigues L., Pereira C.A.J., Maldonado A., Lima W.S. Angiostrongylus minasensis n. sp.: new species found parasitizing coatis (Nasua nasua) in an urban protected area in Brazil. Rev. Bras. Parasitol. Vet. 2020;29 doi: 10.1590/S1984-29612019103. [DOI] [PubMed] [Google Scholar]
- Ansdell V., Wattanagoon Y. Angiostrongylus cantonensis in travelers: clinical manifestations, diagnosis, and treatment. Curr. Opin. Infect. Dis. 2018;31:399–408. doi: 10.1097/QCO.0000000000000481. [DOI] [PubMed] [Google Scholar]
- Bhaibulaya M. Morphology and taxonomy of major Angiostrongylus species of eastern Asia and Australia. In: Cross J.H., editor. Studies on Angiostrongyliasis in Eastern Asia and Australia. U.S. Naval Medical Research Unit No. 2. Taipei, Taiwan. 1979. pp. 4–13. [Google Scholar]
- Bhaibulaya M. Snail borne parasitic zoonoses: Angiostrongyliasis. Southeast Asian J. Trop. Med. Public Health. 1991;22:189–193. [PubMed] [Google Scholar]
- Bhaibulaya M., Cross J.H. Angiostongylus malaysiensis (Nematoda: Metastrongylidae), a new species of rat lung-worm from Malaysia. Southeast Asian J. Trop. Med. Public Health. 1971;2:527–533. [PubMed] [Google Scholar]
- Bhaibulaya M., Techasoponmani V. Mixed infections of Angiostrongylus spp. in rats. Southeast Asian J. Trop. Med. Public Health. 1972;3:451–455. [PubMed] [Google Scholar]
- Brandt R.A.M. The non-aquatic molluscan of Thailand. Archiv. Molluskenkunde. 1974;105:1–423. [Google Scholar]
- Chaisiri K., Dusitsittipon S., Panitvong N., Ketboonlue T., Nuamtanong S., Thaenkham U., Morand S., Dekumyoy P. Distribution of the newly invasive New Guinea flatworm Platydemus Manokwari (Platyhelminthes: Geoplanidae) in Thailand and its potential role as a paratenic host carrying Angiostrongylus malaysiensis larvae. J. Helminthol. 2019;93:711–719. doi: 10.1017/S0022149X18000834. [DOI] [PubMed] [Google Scholar]
- Chan A.H.E., Chaisiri K., Dusitsittipon S., Jakkul W., Charoennitiwat V., Komalamisra C., Thaenkham U. Mitochondrial ribosomal genes as novel genetic markers for discrimination of closely related species in the Angiostrongylus cantonensis lineage. Acta Trop. 2020;211:105645. doi: 10.1016/j.actatropica.2020.105645. [DOI] [PubMed] [Google Scholar]
- Chotmongkol V., Sawanyawisuth K. Clinical manifestations and outcome of patients with severe eosinophilic meningoencephalitis presumably caused by Angiostrongylus cantonensis. Southeast Asian J. Trop. Med. Public Health. 2002;33:231–234. [PubMed] [Google Scholar]
- Cowie R.H. Annotated catalogue of species of Angiostrongylus and the related genera Gallegostrongylus, Rodentocaulus and Stefanskostrongylus (Nematoda: Metastrongyloidea, Angiostrongylidae) J. Helminthol. 2019;93:389–423. doi: 10.1017/S0022149X19000270. [DOI] [PubMed] [Google Scholar]
- Cross J.H. 1979. Studies on angiostrongyliasis in Eastern Asia and Australia. U.S. Naval Medical Research Unit No. 2. Taipei, Taiwan; p. 164. [Google Scholar]
- Cross J.H. Experimental studies of Angiostrongylus species and strains in monkeys and laboratory animals. In: Cross J.H., editor. Studies on Angiostrongyliasis in Eastern Asia and Australia. U.S. Naval Medical Research Unit No. 2. Taipei, Taiwan. 1979. pp. 118–137. [Google Scholar]
- Dumidae A., Janthu P., Subkrasae C., Dekumyoy P., Thanwisai A., Vitta A. Genetic characterization of Angiostrongylus larvae and their intermediate host, Achatina fulica, in Thailand. PLoS One. 2019;14 doi: 10.1371/journal.pone.0223257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusitsittipon S., Criscione C.D., Morand S., Komalamisra C., Thaenkham U. Cryptic lineage diversity in the zoonotic pathogen Angiostrongylus cantonensis. Mol. Phylogenet. Evol. 2017;107:404–414. doi: 10.1016/j.ympev.2016.12.002. [DOI] [PubMed] [Google Scholar]
- Eamsobhana P. Angiostrongyliasis in Thailand: epidemiology and laboratory investigations. Hawaii J. Med. Public Health. 2013;72:28–32. [PMC free article] [PubMed] [Google Scholar]
- Eamsobhana P., Yong H.S. Immunological diagnosis of human angiostrongyliasis due to Angiostrongylus cantonensis (Nematoda: Angiostrongylidae) Int. J. Infect. Dis. 2009;13:425–431. doi: 10.1016/j.ijid.2008.09.021. [DOI] [PubMed] [Google Scholar]
- Eamsobhana P., Yoolek A., Punthuprapasa P., Yong H.S. Thai koi-hoi snail dish and angiostrongyliasis due to Angiostrongylus cantonensis: effects of food flavoring and alcoholic drink on the third-stage larvae in infected snail meat. Foodborne Pathog. Dis. 2009;6:401–405. doi: 10.1089/fpd.2008.0191. [DOI] [PubMed] [Google Scholar]
- Eamsobhana P., Lim P.E., Solano G., Zhang H., Gan X., Yong H.S. Molecular differentiation of Angiostrongylus taxa (Nematoda: Angiostrongylidae) by cytochrome c oxidase subunit I (COI) gene sequences. Acta Trop. 2010;116:152–156. doi: 10.1016/j.actatropica.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Eamsobhana P., Yoolek A., Yong H.S. Effect of Thai koi-hoi food flavoring on the viability and infectivity of the third-stage larvae of Angiostrongylus cantonensis (Nematoda: Angiostrongylidae) Acta Trop. 2010;113:245–247. doi: 10.1016/j.actatropica.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Eamsobhana P., Lim P., Yong H. Phylogenetics and systematics of Angiostrongylus lungworms and related taxa (Nematoda: Metastrongyloidea) inferred from the nuclear small subunit (SSU) ribosomal DNA sequences. J. Helminthol. 2015;89:317–325. doi: 10.1017/S0022149X14000108. [DOI] [PubMed] [Google Scholar]
- Eamsobhana P., Yong H.S., Song S.L., Prasartvit A., Boonyong S., Tungtrongchitr A. Cytochrome c oxidase subunit I haplotype reveals high genetic diversity of Angiostrongylus malaysiensis (Nematoda: Angiostrongylidae) J. Helminthol. 2018;92:254–259. doi: 10.1017/S0022149X17000244. [DOI] [PubMed] [Google Scholar]
- Eamsobhana P., Yong H.S., Song S.L., Gan X.X., Prasartvit A., Tungtrongchitr A. Molecular phylogeography and genetic diversity of Angiostrongylus cantonensis and A. malaysiensis (Nematoda: Angiostrongylidae) based on 66-kDa protein gene. Parasitol. Int. 2019;68:24–30. doi: 10.1016/j.parint.2018.09.006. [DOI] [PubMed] [Google Scholar]
- Heyneman D., Lim B.L. Angiostrongylus cantonensis: proof of direct transmission with its epidemiological implications. Science. 1967;158:1057–1058. doi: 10.1126/science.158.3804.1057. [DOI] [PubMed] [Google Scholar]
- Howe K., Kaluna L., Lozano A., Torres Fischer B., Tagami Y., McHugh R., Jarvi S. Water transmission potential of Angiostrongylus cantonensis: larval viability and effectiveness of rainwater catchment sediment filters. PLoS One. 2019;14 doi: 10.1371/journal.pone.0209813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakkul W., Chaisiri K., Saralamba N., Limpanont Y., Dusitsittipon S., Charoennitiwat V., Chan A.H.E., Thaenkham U. Newly developed SYBR green-based quantitative real-time PCRs revealed coinfection evidence of Angiostrongylus cantonensis and A. malaysiensis in Achatina fulica existing in Bangkok metropolitan, Thailand. Food Waterborne Parasitol. 2021;23 doi: 10.1016/j.fawpar.2021.e00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketsuwan P., Pradatsundarasar A. Second case of ocular angiostrongyliasis in Thailand. Am. J. Trop. Med. Hyg. 1966;15:50–51. doi: 10.4269/ajtmh.1966.15.50. [DOI] [PubMed] [Google Scholar]
- Kittimongkolma S., Intapan P.M., Laemviteevanich K., Kanpittaya J., Sawanyawisuth K., Maleewong W. 2007. Eosinophilic meningitis associated with angiostrongyliasis: clinical features, laboratory investigations and specific diagnostic IgG and IgG subclass antibodies in cerebrospinal fluid. Southeast Asian J. Trop. Med. Public Health. 2007 Jan;38(1):24–31. [PubMed] [Google Scholar]
- Kramer M.H., Greer G.J., Quiñonez J.F., Padilla N.R., Hernández B., Arana B.A., Lorenzana R., Morera P., Hightower A.W., Eberhard M.L., Herwaldt B.L. First reported outbreak of abdominal angiostrongyliasis. Clin. Infect. Dis. 1998;26:365–372. doi: 10.1086/516325. [DOI] [PubMed] [Google Scholar]
- Kuberski T., Bart R.D., Briley J.M., Rosen L. Recovery of Angiostrongylus cantonensis from cerebrospinal fluid of a child with eosinophilic meningitis. J. Clin. Microbiol. 1979;9:629–631. doi: 10.1128/jcm.9.5.629-631.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.D., Chung L.Y., Wang L.C., Lin R.J., Wang J.J., Tu H.P., Wu Z.D., Yen C.M. Sequence analysis in partial genes of five isolates of Angiostrongylus cantonensis from Taiwan and biological comparison in infectivity and pathogenicity between two strains. Acta Trop. 2014;133:26–34. doi: 10.1016/j.actatropica.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Lim L.B., Heyneman D. Host-parasite studies on Angiostrongylus cantonensis (Nematoda: Metastrongylidae) in Malaysian rodents: natural infection of rodents and molluscs in urban and rural areas of Central Malaya. Ann. Trop. Med. Parasitol. 1965;59:425–433. doi: 10.1080/00034983.1965.11686328. [DOI] [PubMed] [Google Scholar]
- Lim L.B., Ramachandran C.P. Ecological studies on Angiostrongylus malaysiensis (Nematoda: Metastrongylidae) in Malaysis. In: Cross J.H., editor. Studies on Angiostrongyliasis in Eastern Asia and Australia. U.S. Naval Medical Research Unit No. 2. Taipei, Taiwan. 1979. pp. 26–48. [Google Scholar]
- Lim L.B., Fong Y.L., Krishnansamy M. Studies of Angiostrongylus malaysiensis (Nematoda, Metastrongylidae) in peninsular Malaysia: natural infection in freshwater snails and rodents in rice fields and infectivity experiments. Southeast Asian J. Trop. Med. Public Health. 1977;8:27–35. [PubMed] [Google Scholar]
- Lv S., Guo Y.H., Nguyen H.M., Sinuon M., Sayasone S., Lo N.C., Zhou X.N., Andrews J.R. Invasive Pomacea snails as important intermediate hosts of Angiostrongylus cantonensis in Laos, Cambodia and Vietnam: implications for outbreaks of eosinophilic meningitis. Acta Trop. 2018;183:32–35. doi: 10.1016/j.actatropica.2018.03.021. [DOI] [PubMed] [Google Scholar]
- Malhotra S., Mehta D.K., Arora R., Chauhan D., Ray S., Jain M. Ocular Angiostrongyliasis in a child – first case report from India. J. Trop. Pediatr. 2006;52:223–225. doi: 10.1093/tropej/fmi092. [DOI] [PubMed] [Google Scholar]
- Morassutti A.L., Levert K., Perelygin A., da Silva A.J., Wilkins P., Graeff-Teixeira C. The 31-kDa antigen of Angiostrongylus cantonensis comprises distinct antigenic glycoproteins. Vector Borne Zoonotic Dis. 2012;12:961–968. doi: 10.1089/vbz.2011.0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira V.L., Giese E.G., Melo F.T., Simões R.O., Thiengo S.C., Maldonado A., Jr., Santos J.N. Endemic angiostrongyliasis in the Brazilian Amazon: natural parasitism of Angiostrongylus cantonensis in Rattus rattus and R. norvegicus, and sympatric giant African land snails, Achatina fulica. Acta Trop. 2013;125:90–97. doi: 10.1016/j.actatropica.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Murphy G.S., Johnson S. Clinical aspects of eosinophilic meningitis and meningoencephalitis caused by Angiostrongylus cantonensis, the rat lungworm. Hawaii J. Med. Public Health. 2013;72:35–40. [PMC free article] [PubMed] [Google Scholar]
- Ng T.H., Annate S., Jeratthitikul E., Sutcharit C., Limpanont Y., Panha S. Disappearing apple snails (Caenogastropoda: Ampullariidae) of Thailand: a comprehensive update of their taxonomic status and distribution. J. Molluscan Stud. 2020;86:290–305. [Google Scholar]
- Nuamtanong S. The evaluation of the 29 and 31 kDa antigens in female Angiostrongylus cantonensis for serodiagnosis of human angiostrongyliasis. Southeast Asian J. Trop. Med. Public Health. 1996;27:291–296. [PubMed] [Google Scholar]
- Pipitgool V., Sithithaworn P., Pongmuttasaya P., Hinz E. Angiostrongylus infections in rats and snails in Northeast Thailand. Southeast Asian J. Trop. Med. Public Health. 1997;28:190–193. [PubMed] [Google Scholar]
- Punyagupta S. Angiostrongyliasis: Clinical features and human pathology. In: Cross J.H., editor. Studies on Angiostrongyliasis in Eastern Asia and Australia. U.S. Naval Medical Research Unit No. 2. Taipei, Taiwan. 1979. pp. 138–150. [Google Scholar]
- Punyagupta S., Bunnag T., Juttijudata P., Rosen L. Eosinophilic meningitis in Thailand: epidemiologic studies of 484 typical cases and the etiologic role of Angiostrongylus cantonensis. Am. J. Trop. Med. Hyg. 1970;19:950–958. [PubMed] [Google Scholar]
- Rai S., Madi D., Pai S., Baliga S. Unusual larva in the CSF and unique MRI findings in a case of eosinophilic meningitis. J. Clin. Imaging Sci. 2014;4:76. doi: 10.4103/2156-7514.148303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiczigel J., Marozzi M., Fábián I., Rózsa L. Biostatistics for parasitologists - a primer to quantitative parasitology. Trends Parasitol. 2019;35:277–281. doi: 10.1016/j.pt.2019.01.003. [DOI] [PubMed] [Google Scholar]
- Rodpai R., Intapan P.M., Thanchomnang T., Sanpool O., Sadaow L., Laymanivong S., Aung W.P., Phosuk I., Laummaunwai P., Maleewong W. Angiostrongylus cantonensis and A. malaysiensis broadly overlap in Thailand, Lao PDR, Cambodia and Myanmar: a molecular survey of larvae in land snails. PLoS One. 2016;11 doi: 10.1371/journal.pone.0161128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawabe K., Makiya K. Comparative infectivity and survival of first-stage larvae of Angiostrongylus cantonensis and Angiostrongylus malaysiensis. J. Parasitol. 1995;81:228–233. [PubMed] [Google Scholar]
- Sinawat S., Sanguansak T., Angkawinjiwong T., Ratanapakorn T., Intapan P.M., Sinawat S., Yospaiboon Y. Ocular angiostrongyliasis: clinical study of three cases. Eye. 2008;22:1446–1448. doi: 10.1038/eye.2008.135. [DOI] [PubMed] [Google Scholar]
- Spratt D.M. Species of Angiostrongylus (Nematoda: Metastrongyloidea) in wildlife: a review. Int. J. Parasitol. Parasites Wildl. 2015;4:178–189. doi: 10.1016/j.ijppaw.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford E.E., Purnomo T.S., Carney W.P. Angiostrongylus malaysiensis in Indonesia. Southeast Asian J. Trop. Med. Public Health. 1976;7:490–491. [PubMed] [Google Scholar]
- Tangwanicharoen T., Viriyavejakul P., Punpoowong B., Wilairatana P., Kaewkungwal J., Pongponratn E., Riganti M. Cerebrospinal fluid analysis in eosinophilic meningoencephalitis. Southeast Asian J. Trop. Med. Public Health. 2001;32:751–759. [PubMed] [Google Scholar]
- Tsai H.C., Lee S.S., Huang C.K., Yen C.M., Chen E.R., Liu Y.C. Outbreak of eosinophilic meningitis associated with drinking raw vegetable juice in southern Taiwan. Am. J. Trop. Med. Hyg. 2004;71:222–226. [PubMed] [Google Scholar]
- Valentyne H., Spratt D.M., Aghazadeh M., Jones M.K., Šlapeta J. The mitochondrial genome of Angiostrongylus mackerrasae is distinct from a. cantonensis and A. malaysiensis. Parasitol. 2020;147:681–688. doi: 10.1017/S0031182020000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitta A., Polsut W., Fukruksa C., Yimthin T., Thanwisai A., Dekumyoy P. Levels of infection with the lungworm Angiostrongylus cantonensis in terrestrial snails from Thailand, with Cryptozona siamensis as a new intermediate host. J. Helminthol. 2016;90:737–741. doi: 10.1017/S0022149X15001042. [DOI] [PubMed] [Google Scholar]
- Vitta A., Srisongcram N., Thiproaj J., Wongma A., Polsut W., Fukruksa C., Yimthin T., Mangkit B., Thanwisai A., Dekumyoy P. Phylogeny of Angiostrongylus cantonensis in Thailand based on cytochrome c oxidase subunit I gene sequence. Southeast Asian J. Trop. Med. Public Health. 2016;47:377–386. [PubMed] [Google Scholar]
- Wang Q.P., Lai D.H., Zhu X.Q., Chen X.G., Lun Z.R. Human angiostrongyliasis. Lancet Infect. Dis. 2008;8:621–630. doi: 10.1016/S1473-3099(08)70229-9. [DOI] [PubMed] [Google Scholar]
- Yong H.S., Eamsobhana P., Song S.L., Prasartvit A., Lim P.E. Molecular phylogeography of Angiostrongylus cantonensis (Nematoda: Angiostrongylidae) and genetic relationships with congeners using cytochrome b gene marker. Acta Trop. 2015;148:66–71. doi: 10.1016/j.actatropica.2015.04.020. [DOI] [PubMed] [Google Scholar]
- Yong H.S., Song S.L., Eamsobhana P., Lim P.E. Complete mitochondrial genome of Angiostrongylus malaysiensis lungworm and molecular phylogeny of Metastrongyloid nematodes. Acta Trop. 2016;161:33–40. doi: 10.1016/j.actatropica.2016.05.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix Table 1: Angiostrongylus larval examination in aquatic snails collected from the study site in Kuchinarai District, Kalasin Province, Thailand based on morphological and molecular identifications.