Highlights
-
•
Vegetables and whole grains delivered weekly to households with diabetes risk.
-
•
Children with prediabetes increased liking of whole grains and vegetables.
-
•
Children with prediabetes increased consumption of whole grains but not vegetables.
-
•
Adults improved health outcomes but children did not, despite being study target.
Abbreviations: T2DM, type 2 diabetes mellitus; SES, socioeconomic status; HOMA-IR, homeostatic model assessment for insulin resistance; CSA, community-supported agriculture; HbA1c, Hemoglobin A1c
Keywords: Metabolic disorders, Prediabetes, Endocrinology, Preventive medicine
Abstract
Introduction
The incidence of pediatric prediabetes and type 2 diabetes mellitus (T2DM) is increasing, with those from low socioeconomic status (SES) households at increased risk. Dietary fiber (e.g., whole grains) is shown to improve glucose control and there is need for innovative strategies that address barriers to consumption (e.g., limited availability).
Methods
Food Overcoming our Diabetes Risk (FoodRx) was a pre-post study (N = 47) that provided 16 weeks of home-delivered whole grains, vegetables, and beans/legumes to households of low-income children in [blinded for submission] who had obesity and prediabetes. Child liking and intake (24-hour diet recalls) was evaluated. Anthropometrics and T2DM-related laboratory measurements (e.g. glycated hemoglobin) were measured for children, and for caregivers to evaluate potential spillover effect.
Results
Post-intervention, children increased liking of whole grains, vegetables, and beans/legumes (P < 0.05 for all). Child whole grain intake increased from 1.7 to 2.5 oz-equivalent servings/day (P < 0.001), and the percent of total grain intake that were whole increased from 30% to 44% (P < 0.001). Children’s body mass index, blood pressure, and serum triglyceride levels increased (+14.6 mg/dl, P = 0.04). Caregivers fasting glucose (-7.5 mg/dl; P = 0.03), fasting insulin (-2.5 μIU/ml, P = 0.0009) and homeostatic model assessment for insulin resistance (HOMA-IR) decreased (-0.8, P = 0.01).
Conclusions
Home deliveries of fiber rich foods improved liking and intake among children at risk for T2DM. There was spillover effect on caregivers, who demonstrated improvement in T2DM-related laboratory measurements instead of the children.
1. Introduction
The incidence of prediabetes and type 2 diabetes mellitus (T2DM), an obesity-related chronic disease, among U.S. children and adolescents is growing at an alarming rate, (Mayer-Davis et al., 2017) with those from low socioeconomic status (SES) backgrounds at greatest risk. (Nagarajan et al., 2017) An analysis of National Health and Nutrition Examination Survey (NHANES) 1999–2014 found that the prevalence of prediabetes/diabetes significantly increased from 21.4% to 28.0% among adolescents (12–18 years) from low SES households. (Jackson et al., 2018) Insulin resistance, with the compensatory hyper-secretion of insulin (even during fasting), is a hallmark of prediabetes and an important link to related cardiometabolic risk factors (e.g. central adiposity, elevated blood pressure, and abnormalities with serum lipids such as hypertriglyceridemia). (Li et al., 2009) Lifestyle interventions in children aimed at reducing their risk of obesity-related chronic disease, such as diabetes, examine their improvement with respect to these clinical markers and estimations of their insulin resistance. (Monzavi et al., 2006, Brage et al., 2004)
A fiber-rich diet (e.g., whole grains, vegetables, beans/legumes) is associated with decreased risk of developing diet-related chronic disease such as T2DM. (Weickert and Pfeiffer, 2008) Unfortunately, the majority of U.S. children are not meeting the Dietary Guidelines for Americans (DGAs) recommendations. (Thomson et al., 2019) For example, based on a 2000 calorie diet, it is recommended that children and adults consume 3 oz-equivalents (oz.-eq.) of whole grains/day and that whole grains should account for at least half of total grain intake. (USDHHS and USDA., 2015) An analysis of national data from 2005 to 2012 showed that U.S. adolescents (13–18 years) consumed 0.8 oz.-eq./day of whole grains, and adolescents from low SES households ate fewer whole grains than their higher SES peers. (Tester et al., 2017)
The aforementioned disparities underscore the need to reach children at risk for T2DM from low SES households and address barriers to making and sustaining positive diet behaviors (e.g., a dislike of fiber-rich foods and limited neighborhood access of fiber-rich foods). Research suggests that while children only modestly like whole grains and vegetables, (Burgess-Champoux, 2006, De Wild et al., 2015, Leak et al., 2019) interventions that repeatedly expose children to the target food (s) are shown to improve liking, (Wadhera et al., 2015) and increased liking is associated with increases in intake of the target food. (Appleton et al., 2018) An additional barrier is the limited access to fiber-rich foods in low SES neighborhoods. (Larson et al., 2009) There are increasing examples of novel approaches where vegetables grown with community supported agriculture (CSA) are prescribed by clinicians to patients at risk of diet-sensitive chronic disease, (Joshi et al., 2019, Cavanagh et al., 2017, Bryce et al., 2017) or delivered to homes in low SES neighborhoods. (Hanson et al., 2017) To our knowledge, there has yet to be an intervention where vegetables and whole grains were delivered together to households affected by chronic disease risk.
The overall aim of the current study was to examine the efficacy of the 16-week Food Overcoming our Diabetes Risk (FoodRx) feasibility study where low SES households that had a child with obesity and prediabetes received home deliveries of whole grains (biweekly), and vegetables and beans/legumes (weekly). Our hypothesis was that children would increase their liking and intake of our primary outcomes of interest (whole grains, vegetables, beans/legumes). Additionally, we explored pre-post changes in anthropometric and T2DM-related laboratory measures in the children, our primary target of the intervention, as well as in their caregivers to examine spillover effect.
2. Material and methods
2.1. Participants
Children (8–17 years) were identified from a patient population being seen in a specialty clinic for pediatric obesity (body mass index [BMI] ≥ 95th percentile). They were eligible if they had laboratory of evidence of prediabetes (fasting glucose 100–125 mg/dL and/or glycated hemoglobin [HbA1c] 5.7–6.4%). (American Diabetes Association, 2010) The most recent available value within12 months was used; for some, it was the elevated laboratory value that had prompted referral to our specialty clinic, and for others it was recent laboratory data gathered from existing patients.
A caregiver with whom the child resided at least five days/week, and who reported being responsible for at least half of the grocery shopping for that household, and could speak either English or Spanish was also recruited to participate. Participation was limited to low SES households (defined by enrollment in public insurance) located in two specific inner-city neighborhoods (defined by zip code) of Oakland and Hayward, California. The study protocol was approved by the Children's Hospital & Research Center Oakland Institutional Research Board.
2.2. Procedures
At the baseline study visit, children and caregivers visited the specialty obesity clinic to provide consent/assent, anthropometric and T2DM-related laboratory measurements, survey data, and a diet recall (child only). Home deliveries were discussed and caregivers received a binder with educational materials and recipes featuring the delivered foods. After the visit, two telephone recalls were collected with the child.
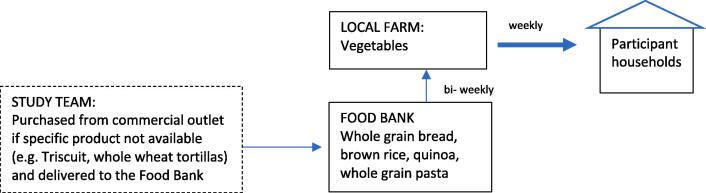
Once baseline data were collected, whole grains, vegetables, and beans/legumes were delivered to participant homes for 16 weeks as a part of a coordinated effort between a local food bank that provided beans/legumes and whole grains, and local CSA farm that provided the vegetables weekly. (Fig. 1) Delivery amounts varied by household size (~ a half-cup serving of vegetables per person/day): 5.5 lb (2–3 people), 8.5 lb (4–5 people), or 10.5 lb (6 or more people). Every other week, deliveries included either two 15-oz cans or 1 lb of dried beans/legumes (lentils, black beans, pinto beans, and kidney beans) and at least 1-oz-eq/day of whole grains per person (brown rice, quinoa, barley, whole grain pasta, whole grain bread, whole grain cereal, whole grain crackers, and whole wheat tortillas). The amount of vegetables, beans/legumes, and whole grains provided were broadly selected in an attempt to double the typical amount of those foods consumed by US adolescents. (Tester et al., 2017, Kimmons et al., 2009)
Fig. 1.
Deliveries to participant households consisted of weekly deliveries of vegetables from a local farm which were accompanied by whole grain foods delivered from the Food Bank to the participating farm.
Participants were also invited to attend monthly cooking classes held by the public health nutrition department in the recruitment zip codes. Classes covered three whole grain recipes included in the binder (fried brown rice, whole grain pasta with vegetables, and quinoa black bean salad). Caregivers were also texted links to: videos of these recipes made by the bilingual instructor who led the classes, other available videos relevant to delivery contents (e.g., how to roast vegetables), and additional information (e.g., tips about storing carrots).
After completion of the intervention, participants visited the research clinic to repeat data collection. Households received a $25 gift card for each clinic visit.
2.3. Measures
2.3.1. Child, caregiver, and household characteristics
At baseline, caregivers provided sociodemographic (age, gender, ethnicity and race) and household-level information: income, number of people in the household, participation in the Supplemental Nutrition Assistance Program (SNAP), and household food security using the USDA 18-item Food Security Module (scored as either food-secure [score 0–2] or food-insecure [score 3–18]). (Bickel et al., n.d.) All survey data was collected using REDCap hosted at [blinded for submission].
2.3.2. Child whole grain, vegetable, beans/legume liking
Children rated their liking of 8 whole grains, 17 vegetables, and 4 beans/legumes that were included in the deliveries using a 4-point scale (never tried, do not like, like a little, and like a lot), which is used in fruit and vegetable studies and shown to be effective with children as young as fourth grade. (Domel et al., 1993)
2.3.3. Child diet intake
Diet intake was measured using three 24-hour diet recalls (pre-post intervention), which were entered into the Nutrition Data System for Research (NDSR 2015), a diet analysis program developed by the University of Minnesota-Twin Cities (UMN) Nutrition Coordinating Center (NCC). A diet recall was conducted in person at both study visits, with food models to aid with portion size estimations. Each in-person recall was followed by two unannounced telephone recalls within two weeks, and NDSR food amounts booklets were given to assist portion estimation. Efforts were made to collect two weekday and one weekend recall. Children 8–11 years were the primary respondent with caregiver assistance, and children 12+ years were independent respondents. Study staff were trained by a researcher (Dr. Leak, co-author) certified by the UMN NCC in using NDSR, and that researcher also led data processing (e.g., data cleaning, identification of outliers). In addition to the primary measures (whole and refined grains, vegetables and beans/legumes), total energy (kcal/day) and total fiber (grams/day) were assessed.
2.3.4. Child and caregiver anthropometric and blood pressure measurements
At study visits, weight was measured using a digital Tanita scale, height using a calibrated stadiometer, and waist circumference (at iliac crest to the nearest 0.1 cm) using Gulick tape. Weight and height were used to calculate BMI as well as age- and sex- specific BMI percentiles and z-scores using standard methods. (Vidmar et al., 2013) The ratio of the waist circumference to height (WHtR; <0.5 is considered normal for children and adults of both genders) was used to assess central adiposity. (Maffeis et al., 2008) Lastly, blood pressure (diastolic [DBP] and systolic [SBP] blood pressure) was measured according to standard protocol. (Flynn and Falkner, 2017) Pediatric age-, sex-, and height- specific blood pressure percentiles were calculated. (Sorensen and Bruun, 2018)
2.3.5. Child and caregiver laboratory measurements
The number of elapsed days between the date of the HbA1c used to determine eligibility and the pre-intervention visit was noted for analysis. At both study visits, fasting (8 + hours) biomarkers of hyperglycemia and insulin resistance (i.e., HbA1c, fasting glucose, fasting insulin, and Homeostatic Model Assessment of Insulin Resistance [HOMA-IR], an index of insulin resistance calculated with glucose and insulin (Antuna-Puente et al., 2011) and lipids (total cholesterol [T chol], low-density lipoprotein cholesterol [LDL-C], high-density lipoprotein cholesterol [HDL-C], non-HDL cholesterol, triglycerides [TG]) were measured. Fasting insulin and HOMA-IR were collected for all participants, but omitted from analysis in the caregivers who were taking insulin for their T2DM.
2.4. Data analysis
Descriptive analysis of child, caregiver, and household characteristics were conducted and percentages, means and standard deviations were reported. Paired t-test was used to compare pre- to post-intervention means, reporting p value, and using alpha 0.05 as the level of significance. McNemar’s test was used to evaluate the binary/dichotomized outcomes (e.g., dichotomized stages of change), reporting the p value of the Chi-squared test. Data analysis was conducted using Stata 15.
3. Results
3.1. Characteristics
Children and a caregiver were enrolled in the study between January and June 2017 until a total of 60 children were recruited. This target number was commensurate to available resources. Between May – October 2017, 47 pairs of children and caregivers returned for data collection post-intervention, yielding a follow-up rate of 78%.
Children were 12.9 ± 2.4 years, and the majority were male (53%) and Hispanic (61.7%). The mean age for caregivers was 43 ± 9.8 years, and the majority were female (96%) with a large percentage not employed outside of the home (49%). Households had on average 5.8 ± 2.5 people, with 3.3 ± 1.9 adults and 2.5 ± 1.3 children. The majority of households had an annual household income ≤ $30,000 (59%), received SNAP benefits (52.5%), and were food-insecure (55%). Child, caregiver, and household characteristics are described in Table 1. Participants who enrolled but did not complete follow-up did not vary from the final study sample with respect to all characteristics noted in Table 1.
Table 1.
Baseline Child, Caregiver, and Household Characteristics (N = 47).
| Children N = 47 | Age, mean years (SD) | 12.9 (2.4) |
| Gender, n (%) Male Female |
25 (53%) 22 (47%) |
|
| Race/ethnicity, n (%) Non-Hispanic White Non-Hispanic Black Hispanic Asian Mixed/other |
0 (0%) 12 (26%) 31(66%) 1 (2%) 3 (6%) |
|
| Cargivers N = 47 | Age, mean years (SD) | 43.0 (9.8) |
| Gender, n (%) Female Male |
45 (96%) 2 (4%) |
|
| Race/ethnicity, n (%) Non-Hispanic White Non-Hispanic Black Hispanic Asian Mixed/other |
1 (2%) 11 (24%) 34 (72%) 1 (2%) 0 (0%) |
|
| Employment Status, n (%) Not employed outside home Employed part-time Employed full-time |
23 (49%) 15 (32%) 9 (19%) |
|
| Households N = 47 | Number of adults, mean (SD) | 3.3 (1.9) |
| Number of children, mean (SD) | 2.5 (1.3) | |
| Household income, n (%) $0 – $15,000 $15,001-$30,000 $30,001-$45,000 $45,001- $60,000 Decline |
11(24%) 16 (35%) 12 (26%) 2 (4%) 6 (11%) |
|
| Household use SNAP, n (%) Currently In the past Never |
24 (52%) 13 (28%) 9 (20%) |
|
| Household Food Insecurity, n (%) Yes No |
26 (55%) 21 (45%) |
|
3.2. Changes in child whole Grain, Vegetable, Beans/legume liking
There were eight whole grains that were included in the deliveries; the average percent that had been “ever tried” increased from 65% to 81% (P < 0.0001) pre-post intervention. The average percent of whole grains (out of eight) that were “liked” increased from 59% to 70% (P = 0.0005). Regarding the 17 vegetables included in the deliveries, the number of vegetables that children had “ever tried” increased from 66 to 77% (P < 0.01). Also, the average percent of these vegetables (out of 17) that were “liked” increased from 52 to 59% (P = 0.01). Lastly, among the 4 beans/legumes included in the deliveries, the average percent children had “ever tried” increased from 75 to 89%, and the percent that were “liked” increased from 60 to 76% (P < 0.05 for both).
3.3. Changes in child intake of grains, vegetables, and beans/legumes
All children had at least two reliable diet recalls. The percentage with three reliable recalls was 36/47 (76%) and 45/47 (96%) at baseline and follow-up, respectively. Changes in grain, vegetable, and bean/legume intake are presented in Table 2. Pre-intervention total energy intake was 1490 ± 500 kcal/day and post-intervention was 1456 ± 550 (p = 0.67). Total fiber was 14.4 +/-5.8 g/day at pre-intervention and 15.8 +/-7.7 g (P = 0.23) at post-intervention.
Table 2.
Changes in Diet Intake of Fiber-rich Foods Among Children (N = 47).
| Baseline (N = 47) |
Post- Intervention(N = 47) |
Paired t-test |
|
|---|---|---|---|
| Mean ± SD | Mean ± SD | P value | |
| Grains (ounce equivalents/day) | |||
| Total grains | 5.8 ± 2.5 | 6.1 ± 2.5 | 0.54 |
| Refined grains | 4.2 ± 2.2 | 3.6 ± 2.1 | 0.11 |
| Whole grains | 1.7 ± 1.0 | 2.5 ± 1.5 | 0.0002* |
| Vegetables (servings/day) | |||
| Total vegetables | 2.3 ± 1.7 | 2.4 ± 1.5 | 0.81 |
| Dark green vegetables | 0.2 ± 0.3 | 0.3 ± 0.6 | 0.11 |
| Yellow vegetables | 0.2 ± 0.3 | 0.2 ± 0.3 | 0.90 |
| White potatoes | 0.1 ± 0.4 | 0.1 ± 0.3 | 0.61 |
| Starchy vegetables | 0.1 ± 0.2 | 0.1 ± 0.2 | 0.65 |
| Other vegetables | 0.7 ± 0.7 | 0.7 ± 0.6 | 0.86 |
| Beans/Legumes (servings/day) | |||
| Beans/legumes | 0.3 ± 0.4 | 0.4 ± 0.5 | 0.30 |
indicates statistical significance at p < 0.05.
Whole grain consumption increased from 1.7 to 2.5 oz.-eq./day (+0.9, SD 1.5, P < 0.001). The proportion of total grains that were whole grains increased 30% to 44% (+13%, SD 24%, P = 0.0005). There were no changes in total grains (6.1 ± 2.5 oz.-eq./d versus 5.9 ± 2.5 oz.-eq./d, P = 0.54) or refined grains (3.6 ± 2.1 versus 4.2 ± 2.2 oz.-eq./d, P = 0.11). Total vegetable intake was 2.3 ± 1.7 servings/day at baseline and remained unchanged (2.4 ± 1.5; P = 0.81). Though there were trends towards increased dark green vegetables (0.2 ± 0.3 to 0.3 ± 0.6 servings, P = 0.11) and increased bean/legume intake (0.3 ± 0.4 to 0.4 ± 0.5 servings, P = 0.30), these were not statistically significant.
3.4. Changes in child anthropometric, blood Pressure, and laboratory measures
3.4.1. Anthropometrics and blood pressure
Weight increased (+2.9 ± 4.0 kg) and also height (+1.3 ± 1.3 cm), translating to a BMI increase of + 0.5 mg/kg2 (P < 0.05), though the change in BMI z-score (+0.01) was not significant (Table 3). SBP increased by 3 ± 1.1 mmHg, and DBP increased by 3.3 ± 8.3 mmHg, though the change in respective age-, sex-, and height- specific percentiles for both SBP and DBP were not significant.
Table 3.
Pre- Post Changes in Anthropometrics and Fasting Laboratory Measures for Children and Caregivers (N = 47).
| Children (N = 47) |
Caregivers (N = 47) |
Caregivers with T2DM (N = 14) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Δ | (ΔSD) | P | Pre | Δ | (ΔSD) | P | Pre | Δ | (ΔSD) | P | |
| BMI (kg/m2) | 34.4 | +0.53 | (1.65) | 0.03 | 34.6 | +0.5 | (4.4) | 0.42 | 34.7 | −0.2 | (1.1) | 0.68 |
| BMI Z | 2.34 | +0.01 | (0.19) | 0.66 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| SBP (mmHg) | 110.8 | +3.0 | (1.1) | 0.009 | 119.5 | +1.6 | (14.5) | 0.44 | 121 | +1.2 | (13.7) | 0.75 |
| SBP, %ile | 58.5 | +9.6 | (25.8) | 0.09 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| DBP (mmHg) | 62.3 | +3.3 | (8.3) | 0.01 | 73.5 | +1.5 | (10.6) | 0.34 | 73.6 | +1.6 | (8.8) | 0.52 |
| DBP, %ile | 49.8 | +11.0 | (26.4) | 0.06 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| WHtR (cm) | 0.66 | +0.01 | (0.09) | 0.16 | 0.69 | −0.03 | (0.12) | 0.11 | 0.72 | −0.004 | (0.03) | 0.64 |
| Glucose (mg/dl) | 90.6 | +0.13 | (5.4) | 0.87 | 117.3 | −7.7 | (23.1) | 0.03 | 171 | −20.1 | (38.2) | 0.07 |
| Insulin (μIU/ml) | 31.2 | −1.3 | (13.5) | 0.51 | 17.5 | −2.6 | (5.9) | 0.009a | 17.8 | −1.1 | (4.9) | 0.53a |
| HOMA-IR | 7.0 | −0.3 | (3.4) | 0.56 | 4.8 | −0.8 | (2.0) | 0.01a | 7.4 | −1.1 | (3.2) | 0.38a |
| HbA1c, % | 5.62 | +0.06 | (0.24) | 0.08 | 6.7 | −0.2 | (0.9) | 0.17 | 9.0 | −0.7 | (1.4) | 0.07 |
| Tchol (mg/dl) | 158.5 | +2.0 | (16.4) | 0.39 | 183.6 | −4.3 | (30.6) | 0.35 | 201 | −16.9 | (38.4) | 0.12 |
| HDL-C (mg/dl) | 43.2 | +3.0 | (15.3) | 0.19 | 51.2 | −0.7 | (6.3) | 0.47 | 46 | −1.6 | (4.4) | 0.20 |
| LDL-C (mg/dl) | 90.7 | +0.4 | (17.6) | 0.88 | 107.2 | −1.8 | (26.1) | 0.65 | 122 | −12 | (34) | 0.20 |
| Non-HDL (mg/dl) | 115.3 | −0.9 | (21.3) | 0.77 | 132.4 | −3.6 | (30.0) | 0.42 | 154 | −15 | (37.9) | 0.15 |
| TG (mg/dl) | 117.1 | +14.6 | (46.6) | 0.04 | 120.0 | +5.8 | (35.8) | 0.27 | 141 | +2.7 | (24.3) | 0.68 |
Insulin and HOMA-IR not evaluated in the 6 caregivers who were taking insulin for their T2DM.
3.4.2. Laboratory measures
Mean HbA1c that had been used to determine study eligibility was 5.84% (SD 0.22). By the initial study visit, which was after a median of 170 days later (inter-quartile range/IQR 93–298 days), mean HbA1c was 5.62% (SD 0.20), significantly lower than the eligibility HbA1c, P < 0.001. HbA1c at the post-intervention visit (median interval 126 days, IQR 120–140 days), was 5.69% (0.31), suggesting a non-significant pre- post trend towards greater HbA1c (+0.1%, P = 0.08).
Fasting glucose, fasting insulin and HOMA-IR were unchanged between the two study visits. Though there was a non-significant + 3 mg/dl (SD 15) increase in HDL (P = 0.19), both LDL and non-HDL cholesterol remained nearly the same. There was a statistically significant 12% increase of 14.6 mg/dl in serum triglycerides.
3.5. Changes in caregiver anthropometric, blood pressure, and laboratory measures
3.5.1. Anthropometrics and blood pressure
There were no significant anthropometric changes for the caregivers.
3.5.2. Laboratory measures
Nineteen caregivers (40%) had evidence of prediabetes on the basis of their laboratory testing. Fourteen (29%) had either a known diagnosis and/or laboratory evidence of T2DM at baseline, and six were taking insulin. Three had a new diagnosis of diabetes as a result of study participation.
There was a significant reduction in fasting glucose (-7.7 mg/dl, P = 0.03) among caregivers, overall. Among all caregivers except those taking insulin (N = 41), there was also a significant reduction in fasting insulin (-2.6 μIU/ml, P = 0.0009) and in HOMA-IR (-0.8, P = 0.01). There were no lipid profile markers that changed by more than 5% compared to baseline.
The subgroup of caregivers with T2DM (N = 14) was also evaluated. Though they did not change their weight status (mean BMI was 34.7 kg/cm2 at baseline), changes in fasting glucose (-20.1 mg/dl) and HbA1c (-0.7%) were of greater magnitude than for the overall group, though not significant in this small number of individuals (P = 0.07 for both). For context, a change of at least 0.5% in HbA1c is considered clinically significant. (Little and Rohlfing, 2013) Furthermore, there were roughly 10% decreases in LDL-cholesterol (-12 mg/dl) and non-HDL cholesterol (-15 mg/dl), though these changes were not significant (P > 0.05 for both).
3.5.3. Engagement with the intervention
At follow-up, 45/47 (96%) of caregivers reported that they still had the binder given to them at the start of the study. A majority (28/47, or 60%) reported that they had prepared included recipes; the most commonly endorsed primary reason for not preparing recipes was thinking that their ‘family would not like the recipes.’ The cooking classes were sparsely attended; only 4 individuals attended a single class. The most commonly endorsed barrier was ‘I did not have time to attend any classes’ (35%). Text messages were sent to all 47 participants, and 45 (96%) reported that they received them. Nearly half (21/47, or 45%) reported that ‘they or someone in their household watched’ any of the online videos texted to them, and 14/47 (30%) of caregivers reported that ‘they or anyone in the household prepared a food based on something learned from a video’ texted to them.
4. Discussion
There is a need for innovative strategies to address inadequate consumption of fiber-rich foods, particularly in populations vulnerable to diet-sensitive chronic disease such as T2DM. Identifying households through the child (rather than the adult) employs a “two for one” approach identifying individuals who often share dietary habits and takes advantage of the higher motivation caregivers often have regarding their child’s health. Though recruitment was oriented around children with evidence of prediabetes, a majority (72%) of the caregivers had prediabetes or T2DM themselves.
Though they were not the primary target of the study, the caregivers demonstrated a spillover effect of the intervention, with positive changes with respect to their T2DM-related laboratory measurements. As a group these caregivers improved their fasting glucose and had lower fasting insulin and HOMA-IR. Though the decrease in HbA1c in the caregivers with T2DM was not significant, the magnitude was clinically meaningful, (Little and Rohlfing, 2013) and comparable to that reported in a produce food prescription program in low-income adults with diabetes. (Bryce et al., 2017) Though not significant, total LDL-cholesterol and non-HDL were lower by roughly 10% in the caregivers with T2DM; consumption of fiber-rich foods is known to reduce serum lipids, (Glore et al., 1994) which is particularly important in people with T2DM given the excess risk of cardiovascular disease. (Association and Management, 2004)
Whole grain consumption increased in the children and nearly reached the recommended USDA target. However, while weekly exposure led to an increase in the number of vegetables and beans/legumes that these children had ever tried and the number that they reported ‘liking’, there was ultimately no observed change in consumption. A cooking skills intervention with children (9–12 years) also demonstrated an increase in the number of vegetables that children had tried (with a paradoxical decrease in their liking) but similarly no change in total vegetable intake. (Overcash et al., 2019) It may be that the strategies required to stimulate increased consumption are different for vegetables than whole grain consumption. Some grains such as pasta are generally familiar to children, and though they require substituting a familiar food with a whole grain version, there is less need for novel food preparation skills. Notably, while “more is better” is true for vegetable consumption, it not true for whole grains; refined grains must be reduced. (USDHHS and USDA., 2015)
Though there is evidence that children with a higher level of whole grain consumption have lower fasting insulin, (Damsgaard et al., 2017) increasing whole grains did not correspond to an improvement in fasting insulin levels or HOMA-IR in this sample. Baseline HbA1c was already in fact significantly lower than the HbA1c used to determine eligibility, pointing to interval improvement in HbA1c that preceded study enrollment (possibly due to rapid lifestyle change after diagnosis of prediabetes by their pediatrician). Furthermore, some cardiometabolic risk factor parameters (e.g. serum triglycerides) worsened post-intervention. It is well-known that carbohydrate load (from both whole and refined grains) contributes to hypertriglyceridemia, (Farquhar et al., 1966) and future studies should explore the balance of refined and whole grains closely, assuring sufficient power to assess for small changes in total and refined grains as well.
Overall limitations of this study include its small sample size, the lack of a control group, and the lack of liking and diet data from caregivers. Because this sample was a treatment-seeking population recruited from a clinic, it is possible that social desirability bias impacted self-reported data. The short duration limits comment on the long-term efficacy of the intervention. Given that the child was the target participant, and due to limited resources, we did not collect diet recall data from caregivers; it is possible that the caregivers improved more than the children because they consumed a larger share of the vegetables provided. It is also possible that the caregivers displaced a greater proportion of their refined grains, leading to greater net benefit from increased whole grain consumption. There are many reasons that access and exposure to nutrient-dense foods is limited. However, children’s dietary preferences often differ from those of their parents, and it is plausible that an intervention increasing access to healthy foods in the home will have differential impact on family members, highlighting the value of measurement of dietary intake in multiple members of a household. Further experimental study after this feasibility study is warranted, with a larger sample size, control comparison, and inclusion of diet recall data from caregivers and/or other household members.
5. Conclusions
In summary, this feasibility study showed that home-delivered vegetables, beans/legumes, and whole grains were liked by children and resulted in increases in whole grain intake. However, while the sample was identified and enrolled because of evidence of prediabetes in the child, it was the caregivers who demonstrated improvement in T2DM-related laboratory measurements. More research is needed to more fully explore the potential of this intervention modality to improve T2DM risk in low SES households.
CRediT authorship contribution statement
June M. Tester: Conceptualization, Methodology, Formal analysis, Resources, Writing– original draft, Funding acquisition. Tashara M. Leak: Conceptualization, Methodology.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by NICHD 1 K23 HD075852 and the Thomas J. Long Foundation. The authors would like to acknowledge the Food as Medicine initiative for making this work possible. Food as Medicine is a collaboration that includes UCSF Benioff Children’s Hospital Oakland, The Alameda County Food Bank, The Alameda County Public Health Department, Dig Deep Farms and the Alameda County “All In” Campaign.
References
- Mayer-Davis E.J., Lawrence J.M., Dabelea D., Divers J., Isom S., Dolan L., Imperatore G., Linder B., Marcovina S., Pettitt D.J., Pihoker C., Saydah S., Wagenknecht L. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. N Engl J Med. 2017;376(15):1419–1429. doi: 10.1056/NEJMoa1610187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan S., Khokhar A., Holmes D.S., Chandwani S. Family Consumer Behaviors, Adolescent Prediabetes and Diabetes in the National Health and Nutrition Examination Survey (2007–2010) J Am Coll Nutr. 2017;36(7):520–527. doi: 10.1080/07315724.2017.1327828. [DOI] [PubMed] [Google Scholar]
- Jackson S.L., Yang E.C., Zhang Z. Income disparities and cardiovascular risk factors among adolescents. Pediatrics. 2018;142(5):e20181089. doi: 10.1542/peds.2018-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Ford E.S., Zhao G., Mokdad A.H. Prevalence of pre-diabetes and its association with clustering of cardiometabolic risk factors and hyperinsulinemia among U.S. adolescents: National health and nutrition examination survey 2005–2006. Diabetes Care. 2009;32(2):342–347. doi: 10.2337/dc08-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzavi R., Dreimane D., Geffner M.E. Improvement in risk factors for metabolic syndrome and insulin resistance in overweight youth who are treated with lifestyle intervention. Pediatrics. 2006;117(6) doi: 10.1542/peds.2005-1532. [DOI] [PubMed] [Google Scholar]
- Brage S, WEDDERKOPP, NIELS EKELUND U, FRANKS PW, WAREHAM NJ, ANDERSEN LB, FROBERG K. Features of the Metabolic Syndrome Are Associated With Objectively Measured Physical Activity and Fitness in Danish Children. Diabetes Care. 2004;27(9):2141-2148. [DOI] [PubMed]
- Weickert M.O., Pfeiffer A.F.H. Metabolic Effects of Dietary Fiber Consumption and Prevention of Diabetes. J Nutr. 2008;138(3):439–442. doi: 10.1093/jn/138.3.439. [DOI] [PubMed] [Google Scholar]
- Thomson J.L., Tussing-Humphreys L.M., Goodman M.H., Landry A.S. Diet quality in a nationally representative sample of American children by sociodemographic characteristics. Am J Clin Nutr. 2019;109(1):127–138. doi: 10.1093/ajcn/nqy284. [DOI] [PubMed] [Google Scholar]
- USDHHS and USDA. 2015-2020 Dietary Guidelines for Americans.; 2015.
- Tester J.M., Leung C.W., Leak T.M., Laraia B.A. Recent Uptrend in Whole-Grain Intake Is Absent for Low-Income Adolescents, National Health and Nutrition Examination Survey, 2005–2012. Prev Chronic Dis. 2017;14(E55) doi: 10.5888/pcd14.160540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess-Champoux T.L. Healthy Whole Grain Choices for Children and Parents: A School-based Pilot Intervention. Published online. 2006 doi: 10.1017/S1368980007001346. [DOI] [PubMed] [Google Scholar]
- De Wild V.W.T., De Graaf C., Boshuizen H.C., Jager G. Influence of choice on vegetable intake in children: Anin-home study. Appetite. 2015;91:1–6. doi: 10.1016/j.appet.2015.03.025. [DOI] [PubMed] [Google Scholar]
- Leak Tashara M., Setiono Felicia, Gangrade Navika, Mudrak Erika. Youth willingness to purchase whole grain snack packs from New York city corner stores participating in a healthy retail program. Int J Environ Res Public Health. 2019;16(18):3233. doi: 10.3390/ijerph16183233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhera D., Capaldi Phillips E.D., Wilkie L.M., Boggess M.M. Perceived recollection of frequent exposure to foods in childhood is associated with adulthood liking. Appetite. 2015;89:22–32. doi: 10.1016/j.appet.2015.01.011. [DOI] [PubMed] [Google Scholar]
- Appleton K.M., Hemingway A., Rajska J., Hartwell H. Repeated exposure and conditioning strategies for increasing vegetable liking and intake: Systematic review and meta-analyses of the published literature. Am J Clin Nutr. 2018;108(4):842–856. doi: 10.1093/ajcn/nqy143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson Nicole I., Story Mary T., Nelson Melissa C. Neighborhood environments: disparities in access to healthy foods in the US. Am J Prev Med. 2009;36(1):74–81.e10. doi: 10.1016/j.amepre.2008.09.025. [DOI] [PubMed] [Google Scholar]
- Joshi K, Smith S, Bolen SD, Osborne A, Benko M, Trapl ES. Implementing a Produce Prescription Program for Hypertensive Patients in Safety Net Clinics. Health Promot Pract. Published online 2018:1524839917754090. [DOI] [PubMed]
- Cavanagh Michelle, Jurkowski Janine, Bozlak Christine, Hastings Julia, Klein Amy. Veggie Rx: an outcome evaluation of a healthy food incentive programme. Public Health Nutr. 2017;20(14):2636–2641. doi: 10.1017/S1368980016002081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryce Richard, Guajardo Claudia, Ilarraza Deliana, Milgrom Nicki, Pike Denise, Savoie Kathryn, Valbuena Felix, Miller-Matero Lisa R. Participation in a farmers’ market fruit and vegetable prescription program at a federally qualified health center improves hemoglobin A1C in low income uncontrolled diabetics. Prev Med Reports. 2017;7:176–179. doi: 10.1016/j.pmedr.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson Karla L., Kolodinsky Jane, Wang Weiwei, Morgan Emily H., Pitts Stephanie B. Jilcott, Ammerman Alice S., Sitaker Marilyn, Seguin Rebecca A. Adults and children in low-income households that participate in cost-offset community supported agriculture have high fruit and vegetable consumption. Nutrients. 2017;9(7):726. doi: 10.3390/nu9070726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2010;33(Supplement 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmons J., Gillespie C., Seymour J., Serdula M., Blanck H.M. Fruit and vegetable intake among adolescents and adults in the United States: Percentage meeting individualized recommendations. MedGenMed Medscape Gen Med. 2009;11(1) [PMC free article] [PubMed] [Google Scholar]
- Bickel G, Nord M, Price C, Hamilton W, Cook J. USDA, Food and Nutrition Service, Guide to measuring household food security.
- Domel S.B., Baranowski T., Davis H., Leonard S.B., Riley P., Baranowski J. Measuring fruit and vegetable preferences among 4th- and 5th-grade students. Prev Med (Baltim). 1993;22(6):866–879. doi: 10.1006/pmed.1993.1078. [DOI] [PubMed] [Google Scholar]
- Vidmar S.I., Cole T.J., Pan H. Standardizing anthropometric measures in children and adolescents with functions for egen: Update. Stata J. 2013;13(2):366–378. doi: 10.1177/1536867x1301300211. [DOI] [Google Scholar]
- Maffeis Claudio, Banzato Claudia, Talamini Giorgio. Waist-to-Height Ratio, a Useful Index to Identify High Metabolic Risk in Overweight Children. J Pediatr. 2008;152(2):207–213.e2. doi: 10.1016/j.jpeds.2007.09.021. [DOI] [PubMed] [Google Scholar]
- Flynn J.T., Falkner B.E. New clinical practice guideline for the management of high blood pressure in children and adolescents. Hypertension. 2017;70(4):683–686. doi: 10.1161/HYPERTENSIONAHA.117.10050. [DOI] [PubMed] [Google Scholar]
- Sorensen J, Bruun S. Determinations of Blood Pressure Percentiles in Normal-Weight Children using STATA 15.0. Published online 2018.
- Antuna-Puente B., Disse E., Rabasa-Lhoret R., Laville M., Capeau J., Bastard J.P. How can we measure insulin sensitivity/resistance? Diabetes Metab. 2011;37(3):179–188. doi: 10.1016/j.diabet.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Little R.R., Rohlfing C.L. The long and winding road to optimal HbA1c measurement. Clin Chim Acta. 2013;418:63–71. doi: 10.1016/j.cca.2012.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glore Stephen R, Van Treeck Dianne, Knehans Allen W, Guild Marinell. Soluble fiber and serum lipids: A literature review. J Am Diet Assoc. 1994;94(4):425–436. doi: 10.1016/0002-8223(94)90099-x. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. Dyslipidemia Management in Adults with Diabetes. Diabetes Care. 2004;27(SUPPL. 1). doi:10.2337/diacare.27.2007.s68. [DOI] [PubMed]
- Overcash F.M., Vickers Z., Ritter A.E. An in-home intervention of parent- implemented strategies to increase child vegetable intake : results from a non- randomized cluster-allocated community trial. Published online. 2019:1–13. doi: 10.1186/s12889-019-7079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsgaard Camilla T, Biltoft-Jensen Anja, Tetens Inge, Michaelsen Kim F, Lind Mads V, Astrup Arne, Landberg Rikard. Whole-grain intake, reflected by dietary records and biomarkers, is inversely associated with circulating insulin and other cardiometabolic markers in 8- to 11-year-old children. J Nutr. 2017;147(5):816–824. doi: 10.3945/jn.116.244624. [DOI] [PubMed] [Google Scholar]
- Farquhar J.W., Frank A., Gross R.C., Reaven G.M. Glucose, insulin, and triglyceride responses to high and low carbohydrate diets in man. J Clin Invest. 1966;45(10):1648–1656. doi: 10.1172/JCI105472. [DOI] [PMC free article] [PubMed] [Google Scholar]