Abstract
Myasthenia gravis (MG) is the most common autoimmune disease affecting the neuromuscular junction by specific autoantibodies. The etiology of MG and its heterogeneity in clinical courses are poorly understood, although it was recently shown that gut microbial dysbiosis plays a critical role. Since levels of Calprotectin (CLP) seem to correlate with level of dysbiosis, we hypothesize that CLP may serve as potential disease activity biomarker in MG. Sera from 251 patients with MG and 90 controls were analyzed in an explorative, cross-sectional design. Prospectively, we tested CLP levels in MG patients up to 3 years. Association of CLP levels with socio-demographics, disease activity (quantitative myasthenia gravis (QMG) score, myasthenia gravis-specific Activities of Daily Living scale (MG-ADL)), antibody (Abs) status, history of myasthenic crisis, treatment regime, and history of thymectomy were investigated using univariate analysis. Mean baseline serum levels of CLP were significantly higher in MG patients compared to controls (4.3 μg/ml vs. 2.1 μg/ml; p < 0.0001). Higher levels of CLP were associated with a higher clinical disease severity measured by MGFA classification and QMG score. Nevertheless, the only weak correlation of CLP with clinical outcome parameters needs confirmation in future studies. Currently, there are no validated blood biomarkers for MG. The significantly elevated CLP and mild correlation with parameters of disease activity suggests that CLP holds promise as a biomarker for measurement of individual disease severity.
Keywords: Myasthenia gravis, Calprotectin, Microbial dysbiosis, Biomarker, Disease severity
Highlights
-
•
The etiology of myasthenia gravis (MG) is still poorly understood, although gut microbial dysbiosis plays a critical role.
-
•
Levels of Calprotectin (CLP) seem to correlate with level of gut dysbiosis.
-
•
CLP levels are elevated in MG patients correlating with markers of disease severity.
-
•
CLP might be a promising biomarker for measuring MG disease activity.
1. Introduction
Myasthenia gravis (MG) is an autoimmune disease affecting the neuromuscular junction by specific autoantibodies [1,2]. While the final pathways of the disease and its effectors disturbing the functions of the neuromuscular junction are relatively well known, the etiology of MG and its heterogeneity in clinical course are poorly understood. Importantly, there is an urgent need for a sensitive biomarker in MG that reliably predicts the individual disease course and exacerbation, as well as guiding immune suppressive treatment, especially in the light of emerging and more specific therapy options for MG patients [3].
Both, genetic and environmental factors have been considered crucially involved in the etiology of MG [4]. While the exact factors responsible for predisposition to MG remain elusive, a crucial role for gut microbiota in the pathogenesis of MG has been hypothesized, since MG patients show a high level of microbial dysbiosis [[5], [6], [7]].
Similarly to inflammatory bowels diseases (IBD) [8], the incidence of MG is increasing in newly industrialized countries [9,10], supporting further an association between “westernization of lifestyles”, gut microbiome and MG.
Calprotectin (CLP), a calcium-binding protein of the S100 family, performs various biological functions via interaction with Toll-like receptor 4 [11] on the surface of leukocytes and is manly released by activated neutrophils, monocytes and early differentiation states of macrophages [12]. CLP has been shown to perform various biological functions, especially in triggering signaling pathways involved in inflammatory processes and inhibition of microbial growth [12]. In IBD, levels of CLP not only correlated with the level of microbial dysbiosis [13], but were also significantly increased in patients with active disease [14] and strongly predicted disease relapse and treatment response [15]. CLP was also investigated in other autoimmune diseases, mainly rheumatoid arthritis (RA) [16], where prediction of disease activity and treatment outcome was proven high. Of highlight, CLP leads to the release of pro-inflammatory cytokines [17,18] and induction of auto-reactive CD8+ T cells [18,19], which play a key role in the pathogenesis of MG [20].
Therefore, we want to test the hypothesis that CLP may serve as a potential disease activity biomarker using a cohort of MG patients compared to controls.
2. Methods
2.1. Standard protocol approvals, registrations, and patient consent
The study was approved by the ethics committee of the Charité-Universitätsmedizin Berlin (EA1/281/10). All patients gave written informed consent in accordance with the Declaration of Helsinki in its currently applicable form.
2.2. Study design
This is an explorative cross-sectional and prospective study comparing serum CLP levels of MG patients and controls to assess the potential of CLP to measure disease activity as assessed by MGFA classification system, quantitative myasthenia gravis (QMG) score, and myasthenia gravis-specific Activities of Daily Living scale (MG-ADL).
2.3. Patients and controls
This study was performed at the certified integrated Center for Myasthenia gravis (iMZ) of the Charité-Universitätsmedizin Berlin, Germany. Patients over the age of 18 years with confirmed diagnosis of myasthenia gravis based on the current guidelines of the German Neurological Society [21] were included independent of disease duration and severity. Overall, 251 patients were consecutively screened at the iMZ clinic between March 2016 and May 2020 and were further categorized in subgroups according to age at onset (early-onset MG [EOMG] was defined as onset at ≤50 years of age, late-onset MG was defined as onset >50 years of age [22]) and thymus pathology (thymoma-associated MG [TAMG]). Prospectively, we tested CLP levels in an explorative design in a limited cohort of 58 MG patients over 3 years.
Socio-demographics (age, sex, disease duration), history of myasthenic crisis, antibody status (acetylcholine receptor antibody [AChR-Abs], muscle specific receptor tyrosine kinase antibody [MuSK-Abs], lipoprotein-related protein 4 [LRP4], seronegative), current MG specific medication (cholinesterase inhibitors, glucocorticoids, and long-term immunosuppressant's), history of thymectomy, and comorbidities were collected in a database. Exclusion criteria were age <18, previous history of cancer except thymoma [23], and diagnosis of RA or IBD due to potential influence on CLP levels. 77 age and gender matched voluntary HC were enrolled as a healthy control group, as well as 13 patients with non-inflammatory neurological diseases (NC) recruited from the outpatient clinic for polyneuropathies as a diseased control group. Exclusion criteria for controls were history of autoimmune disorders, neurological diseases other than polyneuropathies, obesity, cardiovascular diseases as well as history of cancer.
2.4. Clinical assessment
Clinical outcome was assessed using the MGFA classification for disease classification [24] and the QMG score for disease severity. Using the MGFA classification, patients were grouped into remission (MGFA 0), ocular (MGFA I) or generalized MG patients (MGFA II-IV) at time of study inclusion and blood sampling. We have not used the MGFA classification by employing the most severely affected muscles of disease history but for current disease severity to define the patient's MGFA class [24].
The QMG score was developed as a tool for assessing disease severity as well as the pattern of deficits based on quantitative testing of sentinel muscle groups [24,25]. Its reliability and validity have been demonstrated in several studies [25,26]. QMG scores were assessed at baseline and follow up to evaluate disease severity. Moreover, patient reported outcome regarding impact on daily living was assessed at both corresponding time points using the MG-ADL [27,28].
2.5. Laboratory testing
Blood samples were collected from patients with MG and controls immediately centrifuged and stored at −80 °C until being analyzed in May 2020. Serum levels of CLP were measured using the fCAL turbo method® on a COBAS 8000 semi-automated analyses (Bühlmann Laboratories AG, Schönenbuch, Switzerland) according to manufacturer's protocol [29,30]. The fCAL turbo method® has been validated for accurate measurement of CLP levels in serum [29].
2.6. Statistical analysis
All statistical analyses were performed using GraphPad Prism (version 8.2.1, GraphPad Software, San Diego, CA, USA) and SPSS (version 25; SPSS Inc., Chicago, USA). Continuous data are presented as means and standard deviation (SD) and categorical variables as absolute frequencies and percentages. Baseline serum levels of CLP between MG patients and HC were compared using the one-way analysis of variance (ANOVA) with corrections for multiple comparisons. Correlation between CLP levels, clinical, and laboratory assessments were examined using nonparametric Spearman correlation analysis. A univariate analysis was performed using Mann-Whitney nonparametric test to analyze the differences between groups, and the Kruskal-Wallis test was used to analyze the differences between three or more groups. To illustrate the predictive performance of CLP in regard to disease severity as measured by QMG and MG-ADL, we calculated a delta for changes in QMG, MG-ADL and CLP levels, performed a correlation using Spearman correlation coefficient as well as a Mann-Whitney test. A p-value<0.05 was considered statistically significant.
2.7. Data availability
Anonymized data will be shared upon reasonable request from a qualified investigator.
3. Results
3.1. Demographics and baseline characteristics of MG patients
Overall, we included 251 patients with MG, 77 HC and 13 NC (Table 1) and for prospective analysis 58 MG patients (Table 2). Mean age was 54.4 years (SD 17.4), 147 (59 %) were female. Median disease duration was 4.0 years (2.0–10.0). Mean age at disease onset was 46.1 years (SD 19.0). 208 (83 %) of MG patients were positive for AChR-Abs, 10 (3 %) for MusK-Abs, 0 for LRP4-Abs (0 %), and 34 (15 %) remained seronegative (SN). Disease severity at time of sampling ranged from MGFA class 0–IIIB (median II), mean QMG was 8.1 (SD 6.5) and mean MG-ADL was 4.9 (SD 3.9). 50 MG patients (20 %) had a history of myasthenic crisis as defined by rapid worsening of muscle weakness and potential airway compromise from ventilatory or bulbar dysfunction [31].
Table 1.
Baseline characteristics and medical history of MG patients and controls.
| Total MG | EOMG | LOMG | TAMG | Controls | HC | NC | |
|---|---|---|---|---|---|---|---|
| Demographics | 251 | 97 (39%) | 154 (61 %) | 30 (12 %) | 90 | 77 (86 %) | 13 (14 %) |
| Sex Female |
147 (59 %) | 77 (69 %) | 69 (45 %) | 19 (68 %) | 52 (58 %) | 46 (60 %) | 6 (38 %) |
| Age at diagnosis (YEARS) | 54.4 ± 1.4 | 36.1 ± 8.4 | 66.2 ± 9.9 | 58.1 ± 12.5 | 51.9 ± 11.8 | 47.2 ± 7.7 | 65.6 ± 10.9 |
| Disease duration (years) | 4.0 (2-0-10.0) | 4.0 (2.0–7.0) | 4.0 (2.0–11.3) | 3.5 (1.3–8.3) | – | – | – |
| History of myasthenic crisis | 50 (20 %) | 21 (22 %) | 27 (18 %) | 11 (39%) | – | – | – |
| MGFA classification at time point of sampling 0 I II III IV V |
38 (15 %) 42 (17 %) 144 (57 %) 27 (11 %) 0 (0 %) 0 (0 %) |
20 (21 %) 13 (13 %) 43 (44 %) 13 (13 %) 0 (0 %) 0 (0 %) |
18 (12 %) 24 (16 %) 80 (52 %) 13 (8 %) 0 (0 %) 0 (0 %) |
0 (0 %) 5 (18 %) 21 (75 %) 1 (4 %) 0 (0 %) 0 (0 %) |
– | – | – |
| QMG- Score | 8.1 ± 6.5 | 8.1 ± 6.3 | 7.9 ± 6.5 | 9.4 ± 6.7 | – | – | – |
| MG-ADL- Score | 4.9 ± 3.9 | 5.0 ± 3.9 | 4.5 ± 3.7 | 4.6 ± 4.2 | – | – | – |
| History of thymectomy | 129 (52 %) | 66 (68 %) | 62 (40 %) | 28 (100 %) | – | – | – |
| MG-specific treatment at baseline Cholinesterase inhibitors monotherapy Corticosteroids Azathioprine MycophenolatE mofetil Methotrexate Rituximab Eculizumab |
56 (16 %) 75 (43 %) 86 (29 %) 28 (11 %) 12 (5 %) 10 (4.0 %) 7 (3 %) |
30 (31 %) 28 (29 %) 22 (23 %) 9 (9 %) 6 (6 %) 6 (6 %) 2 (2 %) |
32 (21 %) 83 (54 %) 48 (31 %) 17 (13 %) 6 (4 %) 3 (3 %) 5 (3 %) |
2 (7 %) 10 (36 %) 12 (43 %) 2 (7 %) 0 (0 %) 4 (14 %) 0 (0 %) |
– | – | – |
Data are mean (SD) and n (%) for the baseline variables and median (IQR) for disease duration. Disease duration is the time from diagnosis until baseline. Abbreviations: EOMG = early onset myasthenia gravis, HC = healthy controls, IQR = interquartile range; LOMG = late onset myasthenia gravis; MG = myasthenia gravis; MGFA = Myasthenia gravis foundation of America classification; MG-ADL = MG-activity of daily life score; NC = non-inflammatory neurological controls, SD−standard deviation, TAMG = thymoma - associated myasthenia gravis, QMG = quantitative myasthenia gravis score.
Table 2.
Demographical and clinical characteristics of MG patients of prospective analysis.
| V1* | V2** | |
|---|---|---|
| Demographics | 58 | 58 |
| Sex Female |
23 (40 %) | 23 (40 %) |
| Age at diagnosis (YEARS) | 57.6 ± 16.4 | 60.5 ± 16.1 |
| Disease duration (years) | 5.0 (1.8–14.0) | 8.0 (2.0–7.0) |
| History of myasthenic crisis | 8 (14 %) | 8 (14 %) |
| MGFA classification at time point of sampling 0 I II III IV V |
6 (10 %) 16 (28 %) 33 (57 %) 2 (4 %) 0 (0 %) 0 (0 %) |
13 (22 %) 11 (19 %) 31 (54 %) 3 (5 %) 0 (0 %) 0 (0 %) |
| QMG- Score | 6.6 ± 4.9 | 7.2 ± 5.7 |
| MG-ADL- Score | 4.2 ± 3.4 | 4.0 ± 3.7 |
| History of thymectomy TYMOMYA THYMITIS WITHOUT PATHOLOGY |
26 (45 %) 6 (22 %) 10 (39 %) 10 (39 %) |
33 (57 %) 8 (24 %) 10 (30 %) 15 (46 %) |
| MG-specific treatment at Sample time Cholinesterase inhibitors monotherapy Corticosteroids Azathioprine MycophenolatE mofetil Methotrexate Rituximab Eculizumab |
7 (12 %) 8 (14 %) 12 (21 %) 1 (2 %) 5 (9 %) 1 (2 %) 0 (0 %) |
10 (17 %) 9 (16 %) 21 (36 %) 8 (14 %) 9 (16 %) 2 (3 %) 0 (2 %) |
Data are mean (SD) and n (%) and median (IQR) for disease duration. Disease duration is the time from diagnosis until baseline. Abbreviations: IQR = interquartile range; MG = myasthenia gravis; MGFA = Myasthenia gravis foundation of America classification; MG-ADL = MG-activity of daily life score; SD−standard deviation, QMG = quantitative myasthenia gravis score, V1 = baseline visit, V2 = follow-up visit after 3 years.
At time of sampling, 129 (52 %) of MG patients had already undergone a thymectomy, 56 MG patients (16 %) used symptomatic monotherapy with cholinesterase inhibitors, while the majority of patients additionally used oral corticosteroids (n = 75; 42 %) and/or steroid sparing immunosuppressive therapy (n = 86; 29 %). In 17 MG patients (7 %), escalation therapies (rituximab; eculizumab) were required.
3.2. CLP levels are higher in MG
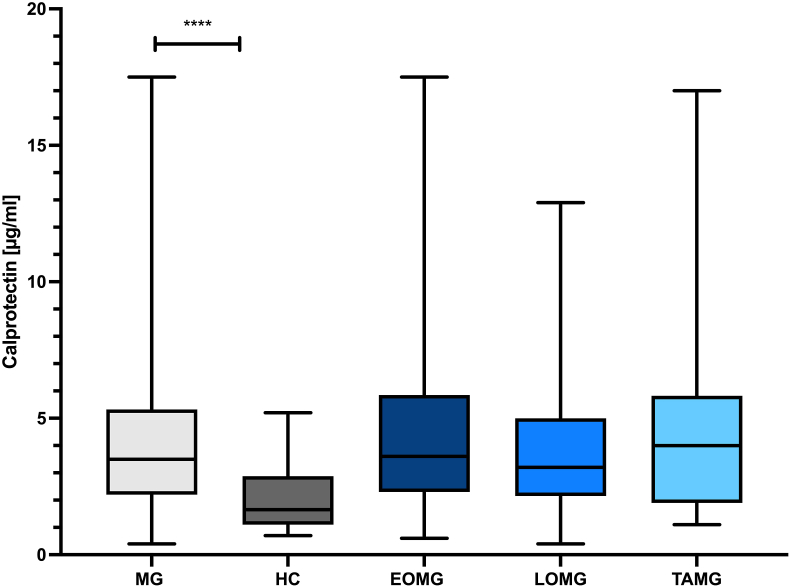
Baseline serum CLP levels were significantly higher in MG patients with a mean of 4.3 μg/ml (SD 3.0, 95 % CI 3.8–4.6) compared to HC (mean 2.1 μg/ml (SD 1.1, 95 % CI 1.2–2.2) and NC (mean 2.0 μg/ml (SD 1.2, 95 % CI 1.6–2.3); p < 0.0001; Fig. 1), with an area under the receiver operating curve (AUC) of 0.77 (95 % confidence interval (CI) 0.70–0.83; p < 0.0001). With a cut-off-value of 1.55 μg/ml CLP discriminated MG patients from controls with a sensitivity of 90.4 % and a specificity of 45.1 %.
Fig. 1.
Calprotectin levels are elevated in MG patients compared to controls. The box plot bar represents the mean baseline serum calprotectin (CLP) level with SD in all myasthenia gravis (MG) patients (n = 251) and subgroups compared to healthy controls (HC) (n = 77), n = number of patients with evaluable data. Mean CLP levels were significantly increased in all MG patients compared to HC regardless of MG-subtype (p < 0.001). Abbreviations: EOMG = early onset myasthenia gravis, LOMG = late onset myasthenia gravis; MG = myasthenia gravis; TAMG = thymoma - associated myasthenia gravis.
There was a trend of highest levels in TAMG patients (4.9 μg/ml (SD 3.7, 95 % CI 3.4–6.2)), but not reaching statistical significance (p = 0.218, Kruskal-Wallis test).
Serum CLP levels in MG patients and controls were neither correlated to age (r = −0.04, p = 0.459) nor associated to gender (p = 0.9246; Mann-Whitney test).
Mean CLP levels in AChR-Abs positive patients (n = 208) were 4.2 μg/ml (SD 3.0), in MuSK-Abs positive patients (n = 10) 4.6 μg/ml (SD 4.1), and in seronegative MG patients (n = 34) 4.9 μg/ml (SD 2.9) showing no significant in between group differences (p = 0.21, Kruskal-Wallis test). Moreover, in AChR-Abs positive and MuSK-Abs positive MG patients, CLP levels did not correlate with Abs level (r = 0.03, p = 0.677 for AChR-Abs; r = 0.04, p = 0.55 for MuSK-Abs) (Spearman correlation coefficient). There was no significant difference in CLP levels in patients with (n = 51) and without (n = 200) a history of myasthenic crisis (n = 51) (p = 0.213, Mann-Whitney test).
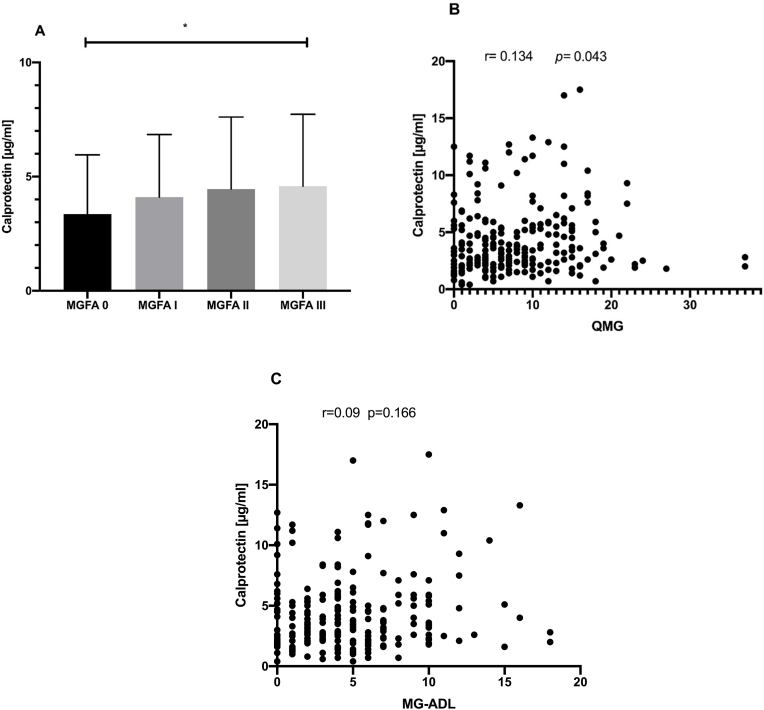
3.3. Baseline CLP levels correlates with disease severity
Clinical severity measured by MGFA classification ranging from remission, I– III at time point of sampling were compared regarding CLP levels and revealed significant higher CLP levels in patients with a generalized compared with pure ocular MG or patients in remission (p = 0.0435, Kruskal-Wallis test, Fig. 2A). QMG score at baseline correlated weakly, but significantly with serum CLP levels (r = 0.134, p = 0.043, Spearman correlation coefficient, Fig. 2B). However, there was no significant correlation with the patient outcome parameter MG-ADL (r = 0.09, p = 0.1664, Spearman correlation coefficient, Fig. 2C).
Fig. 2.
Baseline CLP and clinical disease severity. A: Column bar graph of association of CLP level with clinical severity measured by Myasthenia gravis foundation of America classification (MGFA) using Kruskal-Wallis test. B: Correlation analysis of quantitative myasthenia gravis (QMG) score with serum level of CLP using Spearman correlation coefficient. C: Correlation analysis of myasthenia gravis activity of daily life (MG-ADL) score with serum level of CLP using Spearman correlation coefficient.
3.4. Relationship between CLP levels and treatment regime
Patients receiving only symptomatic monotherapy showed the highest levels of CLP at baseline (n = 75; 42 %; 4.1 μg/ml (SD 2.8)), whereas patients treated with eculizumab (n = 7 (3 %); 2.1 μg/ml (SD 0.4)) had the lowest, although not reaching statistical significance (p = 0.072; Kruskal-Wallis test). MG patients with a history of thymectomy (at least >2 years) had similar mean baseline CLP levels compared to patients without history of thymectomy (4.3 μg/ml (SD 3.3) vs. 4.2 μg/ml (SD 2.7); Mann-Whitney test).
3.5. CLP not predictive with individual disease severity activity
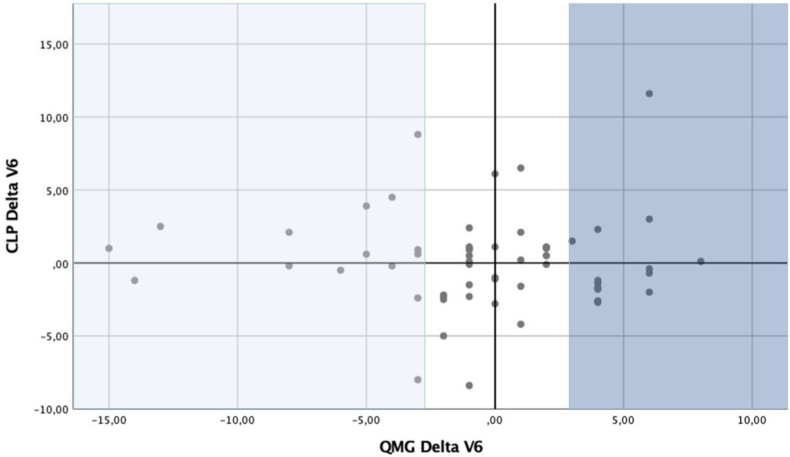
To investigate the predictive performance of CLP regarding to individual changes in clinical (QMG) as well as patient reported outcome parameters (MG-ADL), we calculated a delta for each time point as well as a delta for individual change in CLP level and performed a Mann-Whitney test. We did not observe significant delta changes as defined by ≥ 2 points for QMG and ≥3 for MG-ADL in correlation with delta changes of CLP using Spearman correlation coefficient (r = 0.241 for QMG, r = 0.495 for MG-ADL). There was only a tendency for correlation in regard to changes over time for QMG score in total MG population at baseline (n = 251) and year 3 (n = 58) (p = 0.410) (Fig. 3).
Fig. 3.
Correlation of QMG score with CLP levels over time. Using grouped scattered plot we examined correlation in individual change (delta score) between clinical outcome parameter QMG score and CLP over two time points (baseline (n = 251 patients; year 3 (n = 58 patients). A significant change in delta QMG was defined as improvement ≥2 points presented as a positive delta (dark blue); significant change in CLP level as a negative delta (light blue).
4. Discussion
This explorative cross-sectional and prospective study revealed that serum CLP levels of MG patients were significantly higher in comparison to controls. Moreover, baseline CLP levels correlated weakly, but positively with clinical disease activity as measured by QMG score and MGFA classification. Nevertheless, the only weak correlation of CLP with clinical outcome parameters needs confirmation in future studies.
It should be emphasized, that baseline CLP levels were elevated in all MG patients regardless of Abs status (AChR-Abs, anti-MuSK-Abs, SN), which might be helpful in suspected MG cases, since about 15 % of the MG patients remain SN [1] and we have not found elevated CLP levels in the control group. Nevertheless, CLP is a sensitive but unspecific marker of inflammation. Its potential value therefore does not lie in the ability to discriminate different autoimmune diseases, but rather to reflect different degrees of disease activity, as proposed in other conditions like IBD [14] and RA [16,32], where CLP has become a routinely measured biomarker for disease severity. In addition, CLP has the potential to be a marker of microbial dysbiosis [13], which has been proposed to play a critical pathophysiological role in MG [7,33,34].
CLP leads to induction of auto-reactive CD8+ T cells, IL-17 [35] and other pro-inflammatory cytokines like IL-1β. The imbalance of inflammatory cytokines are involved in the pathogenesis of MG and play a central role in the development of inflammation at the neuromuscular junction [20,35]. IL-1β was reported to be a key cytokine which promotes Th17 cell generation, which is crucially involved in pathogenesis of MG [36]. In an experimental autoimmune MG mouse model, IL-17- knock out mice were developing fewer myasthenic symptoms and less pathogenic AChR-specific Abs [35]. Furthermore, increased IL-17 levels have been observed in MG patients [36], and an increased frequency of IL-17- producing CD4+ T cells has been demonstrated in particular for TAMG [37], which might explain the trend towards higher mean CLP level in TAMG patients in our cohort.
CLP was elevated in MG patients with high disease activity as scored with MGFA and QMG, which can effectively reflect the severity of the disease. This important finding relates to studies of CLP in IBD and RA, where CLP is routinely used as a disease activity and treatment response marker, especially for biologicals [15,38]. Although not statistically significant, this fact is further supported by the finding of lowest CLP levels in patients receiving eculizumab, providing additional evidence of the strong therapeutic efficiency of complement inhibition [39].
Socio-demographic parameters, history of myasthenic crisis, as well as Abs-status and levels had no effect on CLP levels, although several longitudinal studies in RA patients observed higher CLP levels in patients being positive for rheumatoid factor [16]. In addition, patients with a history of thymectomy >2 years showed no relevant difference regarding CLP level, which might be due to the lower classification regarding MGFA- and QMG-score at time of sampling in comparison to time of diagnosis.
In the longitudinal analysis, the individual disease activity did not show a significant correlation in regard to delta changes. However, mean changes in primary outcome parameters QMG and MG-ADL were not significantly differing over the observed follow up time in our cohort, which might be mainly due to the low number of therapy naïve patients. In addition, the precise cut-off value for significant delta changes of QMG and MG-ADL score remains unclear and need to be explored in larger, longitudinal studies.
There are several limitations to our study. Although we included a rather high number of MG-patients in the cross-sectional design, our findings are limited to the rather small sample size in subgroups as well as the low number of included patients for the follow up assessment, which was due to the explorative design of our study. Future conformational and larger prospective studies are strongly needed to further examine the potential utility of CLP as a disease activity biomarker in MG.,. In addition, the main proportion of included patients was not therapy naïve as being heterogeneous in regards to disease duration and clinical severity. Nevertheless, this diversity reflects the typical demography of a specialized MG clinic. The control population was rather small, although in line with studies examining CLP in other autoimmune diseases.
5. Conclusion
In conclusion, this explorative cross-sectional and prospective study demonstrates that CLP levels were significantly higher in MG compared to controls. Additionally, we provide evidence, that CLP might reflect disease severity. There is an unmet need of a validated, non-invasive biomarker to assess disease activity and potentially guiding treatment. Further multicentric, longitudinal investigations are strongly needed to determine the potential utility of CLP as a biomarker for better care of patients with MG.
Study funding
His study was supported by the NeuroCure Clinical Research funding (Grant/Award Number: Exc 257).
Author contributions
Frauke Stascheit, MD: Design and conceptualized study, analyzed and interpreted the data, drafted the manuscript for intellectual content; the author takes full responsibility to the integrity of the data analyzed, Benjamin Hotter, MD: Analyzed and Interpreted the data, revised the manuscript for intellectual content; Sarah Hoffman, MD: Major role in acquisition of data; interpreted the data; revised the manuscript for intellectual content; Siegfried Kohler, MD: Major role in acquisition of data; interpreted the data; revised the manuscript for intellectual content; Sophie Lehnerer, MD: interpreted the data, revised the manuscript for intellectual content; Andreas Sputtek, MD: Laboratory analysis, revised the manuscript for intellectual content; Andreas Meisel, MD: Design and conceptualized study, interpretetd the data, revised the manuscript for intellectual content, funding acquisition.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests. Andreas Meisel reports financial support was provided by Charite University Hospital Berlin NeuroCure Clinical Research Center. F. Stascheit reports no competing financial interests or personal relationships. B. Hotter reports no competing financial interests or personal relationships. S. Hoffmann received speaker honoraria from Alexion. S. Kohler reports speaker's honoraria from Novartis and Biomarin. S. Lehnerer reports speaker's honoraria and honoraria for attendance at advisory boards from Alexion Pharmaceuticals. A. Sputtek reports no competing financial interests or personal relationships. A. Meisel received speaker honoraria from Alexion, GRIFOLS and Hormosan. He received honoraria from Alexion, MorphoSys and Vitaccess for consulting services and financial research support from Octapharma and Alexion. Andreas Meisel is chairman of the medical advisory board of the German Myasthenia Gravis Society.
Acknowledgements
We thank our co-workers of the NeuroCure Clinical Research Center Claudia Heibutzki and Dike Remstedt for patient management of the MG outpatient department, Arun Prakash-Singh for management of serum samples, and M. Heinold, S. Märschenz and S. Lischewski for administration support.
References
- 1.Gilhus N.E., Gravis Myasthenia. N. Engl. J. Med. 2016;375:2570–2581. doi: 10.1056/NEJMra1602678. [DOI] [PubMed] [Google Scholar]
- 2.Gilhus N.E., Verschuuren J.J. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol. 2015;14:1023–1036. doi: 10.1016/S1474-4422(15)00145-3. [DOI] [PubMed] [Google Scholar]
- 3.Tannemaat M.R., Verschuuren J. Emerging therapies for autoimmune myasthenia gravis: towards treatment without corticosteroids. Neuromuscul. Disord. : NMD. 2020;30:111–119. doi: 10.1016/j.nmd.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Gilhus N.E., Tzartos S., Evoli A., Palace J., Burns T.M., Verschuuren J. Myasthenia gravis. Nat Rev Dis Primers. 2019;5:30. doi: 10.1038/s41572-019-0079-y. [DOI] [PubMed] [Google Scholar]
- 5.Qiu D., Xia Z., Jiao X., Deng J., Zhang L., Li J. Altered gut microbiota in myasthenia gravis. Front. Microbiol. 2018;9:2627. doi: 10.3389/fmicb.2018.02627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moris G., Arboleya S., Mancabelli L., Milani C., Ventura M., de Los Reyes-Gavilán C.G. Fecal microbiota profile in a group of myasthenia gravis patients. Sci. Rep. 2018;8:14384. doi: 10.1038/s41598-018-32700-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng P., Li Y., Wu J., Zhang H., Huang Y., Tan X. Perturbed microbial ecology in myasthenia gravis: evidence from the gut microbiome and fecal metabolome. Adv. Sci. (Weinheim, Baden-Wurttemberg, Germany) 2019;6:1901441. doi: 10.1002/advs.201901441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng S.C., Shi H.Y., Hamidi N., Underwood F.E., Tang W., Benchimol E.I. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet (London, England) 2018;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 9.Zieda A., Ravina K., Glazere I., Pelcere L., Naudina M.S., Liepina L. A nationwide epidemiological study of myasthenia gravis in Latvia. Eur. J. Neurol. 2018;25:519–526. doi: 10.1111/ene.13535. [DOI] [PubMed] [Google Scholar]
- 10.Ogawa Y., Nakagawa M., Yoshida T., Yazaki M., Sekijima Y., Hashimoto T. Survey of epidemiology, clinical picture and current treatments for elderly-onset (≥ 65 years) patients with myasthenia gravis in Nagano Prefecture, Japan. Neurol. Clinic. Neurosci. 2017;5:107–112. [Google Scholar]
- 11.Ehrchen J.M., Sunderkötter C., Foell D., Vogl T., Roth J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J. Leukoc. Biol. 2009;86:557–566. doi: 10.1189/jlb.1008647. [DOI] [PubMed] [Google Scholar]
- 12.Shabani F., Farasat A., Mahdavi M., Gheibi N. Calprotectin (S100A8/S100A9): a key protein between inflammation and cancer. Inflamm. Res. 2018;67:801–812. doi: 10.1007/s00011-018-1173-4. [DOI] [PubMed] [Google Scholar]
- 13.Halfvarson J., Brislawn C.J., Lamendella R., Vázquez-Baeza Y., Walters W.A., Bramer L.M. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat. Microbiol. 2017;2:17004. doi: 10.1038/nmicrobiol.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suárez Ferrer C., Abadía Barno M., Martín Arranz E., Jochems A., García Ramírez L., Poza Cordón J. The use of serum calprotectin as a biomarker for inflammatory activity in inflammatory bowel disease. Rev. Esp. Enferm. Dig. : Org. Off. Soc. Espanola de Patol. Digestiva. 2019;111:744–749. doi: 10.17235/reed.2019.5797/2018. [DOI] [PubMed] [Google Scholar]
- 15.Kalla R., Kennedy N.A., Ventham N.T., Boyapati R.K., Adams A.T., Nimmo E.R. Serum calprotectin: a novel diagnostic and prognostic marker in inflammatory bowel diseases. Am. J. Gastroenterol. 2016;111:1796–1805. doi: 10.1038/ajg.2016.342. [DOI] [PubMed] [Google Scholar]
- 16.Abildtrup M., Kingsley G.H., Scott D.L. Calprotectin as a biomarker for rheumatoid arthritis: a systematic review. J. Rheumatol. 2015;42:760–770. doi: 10.3899/jrheum.140628. [DOI] [PubMed] [Google Scholar]
- 17.Sunahori K., Yamamura M., Yamana J., Takasugi K., Kawashima M., Yamamoto H. The S100A8/A9 heterodimer amplifies proinflammatory cytokine production by macrophages via activation of nuclear factor kappa B and p38 mitogen-activated protein kinase in rheumatoid arthritis. Arthritis Res. Ther. 2006;8:R69. doi: 10.1186/ar1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loser K., Vogl T., Voskort M., Lueken A., Kupas V., Nacken W. The Toll-like receptor 4 ligands Mrp8 and Mrp14 are crucial in the development of autoreactive CD8+ T cells. Nat. Med. 2010;16:713–717. doi: 10.1038/nm.2150. [DOI] [PubMed] [Google Scholar]
- 19.Loser K., Vogl T., Voskort M., Lueken A., Kupas V., Nacken W. The Toll-like receptor 4 ligands Mrp8 and Mrp14 are crucial in the development of autoreactive CD8+ T cells. Nat. Med. 2010;16:713–717. doi: 10.1038/nm.2150. [DOI] [PubMed] [Google Scholar]
- 20.Uzawa A., Kawaguchi N., Himuro K., Kanai T., Kuwabara S. Serum cytokine and chemokine profiles in patients with myasthenia gravis. Clin. Exp. Immunol. 2014;176:232–237. doi: 10.1111/cei.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melzer N., Ruck T., Fuhr P., Gold R., Hohlfeld R., Marx A. Clinical features, pathogenesis, and treatment of myasthenia gravis: a supplement to the Guidelines of the German Neurological Society. J. Neurol. 2016;263:1473–1494. doi: 10.1007/s00415-016-8045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berrih-Aknin S., Frenkian-Cuvelier M., Eymard B. Diagnostic and clinical classification of autoimmune myasthenia gravis. J. Autoimmun. 2014;48–49:143–148. doi: 10.1016/j.jaut.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Markowitz J., Carson W.E., 3rd Review of S100A9 biology and its role in cancer. Biochim. Biophys. Acta. 2013;1835:100–109. doi: 10.1016/j.bbcan.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaretzki A., 3rd, Barohn R.J., Ernstoff R.M., Kaminski H.J., Keesey J.C., Penn A.S. Myasthenia gravis: recommendations for clinical research standards. Task force of the medical scientific advisory board of the myasthenia gravis foundation of America. Neurology. 2000;55:16–23. doi: 10.1212/wnl.55.1.16. [DOI] [PubMed] [Google Scholar]
- 25.Bedlack R.S., Simel D.L., Bosworth H., Samsa G., Tucker-Lipscomb B., Sanders D.B. Quantitative myasthenia gravis score: assessment of responsiveness and longitudinal validity. Neurology. 2005;64:1968–1970. doi: 10.1212/01.WNL.0000163988.28892.79. [DOI] [PubMed] [Google Scholar]
- 26.Barnett C., Katzberg H., Nabavi M., Bril V. The quantitative myasthenia gravis score: comparison with clinical, electrophysiological, and laboratory markers. J. Clin. Neuromuscul. Dis. 2012;13:201–205. doi: 10.1097/CND.0b013e31824619d5. [DOI] [PubMed] [Google Scholar]
- 27.Wolfe G.I., Herbelin L., Nations S.P., Foster B., Bryan W.W., Barohn R.J. Myasthenia gravis activities of daily living profile. Neurology. 1999;52:1487–1489. doi: 10.1212/wnl.52.7.1487. [DOI] [PubMed] [Google Scholar]
- 28.Utsugisawa K., Suzuki S., Nagane Y., Masuda M., Murai H., Imai T. Health-related quality-of-life and treatment targets in myasthenia gravis. Muscle Nerve. 2014;50:493–500. doi: 10.1002/mus.24213. [DOI] [PubMed] [Google Scholar]
- 29.Åsberg A., Løfblad L., Felic A., Hov G.G. Measuring calprotectin in plasma and blood with a fully automated turbidimetric assay. Scand. J. Clin. Lab. Investig. 2019;79:50–57. doi: 10.1080/00365513.2018.1550810. [DOI] [PubMed] [Google Scholar]
- 30.Šumová B., Cerezo L.A., Szczuková L., Nekvindová L., Uher M., Hulejová H. Circulating S100 proteins effectively discriminate SLE patients from healthy controls: a cross-sectional study. Rheumatol. Int. 2019;39:469–478. doi: 10.1007/s00296-018-4190-2. [DOI] [PubMed] [Google Scholar]
- 31.Narayanaswami P., Sanders D.B., Wolfe G., Benatar M., Cea G., Evoli A. International consensus guidance for management of myasthenia gravis. Neurology. 2021;96:114. doi: 10.1212/WNL.0000000000011124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi I.Y., Gerlag D.M., Herenius M.J., Thurlings R.M., Wijbrandts C.A., Foell D. MRP8/14 serum levels as a strong predictor of response to biological treatments in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2015;74:499. doi: 10.1136/annrheumdis-2013-203923. [DOI] [PubMed] [Google Scholar]
- 33.Qiu D., Xia Z., Jiao X., Deng J., Zhang L., Li J. Altered gut microbiota in myasthenia gravis. Front. Microbiol. 2018;9:2627. doi: 10.3389/fmicb.2018.02627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan X., Huang Y., Chai T., Zhao X., Li Y., Wu J. Differential gut microbiota and fecal metabolites related with the clinical subtypes of myasthenia gravis. Front. Microbiol. 2020;11:564579. doi: 10.3389/fmicb.2020.564579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schaffert H., Pelz A., Saxena A., Losen M., Meisel A., Thiel A. IL-17-producing CD4(+) T cells contribute to the loss of B-cell tolerance in experimental autoimmune myasthenia gravis. Eur. J. Immunol. 2015;45:1339–1347. doi: 10.1002/eji.201445064. [DOI] [PubMed] [Google Scholar]
- 36.Roche J.C., Capablo J.L., Larrad L., Gervas-Arruga J., Ara J.R., Sánchez A. Increased serum interleukin-17 levels in patients with myasthenia gravis. Muscle Nerve. 2011;44:278–280. doi: 10.1002/mus.22070. [DOI] [PubMed] [Google Scholar]
- 37.Wang Z., Wang W., Chen Y., Wei D. T helper type 17 cells expand in patients with myasthenia-associated thymoma. Scand. J. Immunol. 2012;76:54–61. doi: 10.1111/j.1365-3083.2012.02703.x. [DOI] [PubMed] [Google Scholar]
- 38.Nordal H.H., Brokstad K.A., Solheim M., Halse A.K., Kvien T.K., Hammer H.B. Calprotectin (S100A8/A9) has the strongest association with ultrasound-detected synovitis and predicts response to biologic treatment: results from a longitudinal study of patients with established rheumatoid arthritis. Arthritis Res. Ther. 2017;19:3. doi: 10.1186/s13075-016-1201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mantegazza R., Vanoli F., Frangiamore R., Cavalcante P. Complement inhibition for the treatment of myasthenia gravis. ImmunoTargets Ther. 2020;9:317–331. doi: 10.2147/ITT.S261414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared upon reasonable request from a qualified investigator.