Abstract
Blastomyces is an endemic fungal pathogen found in regions of North America. It is endemic in the Ohio and Mississippi river valleys, New York, Wisconsin, Colorado, Texas, Kansas, Nebraska, and other regions of the United States. It is common in Canada, mainly Ontario and Manitoba. Here, we report a case of tracheal and pulmonary blastomycosis. Interestingly, this case presented as an unexpected diagnosis as part of a malignancy workup. To our knowledge, this is only the second case of tracheal blastomycosis reported in the literature.
Keywords: Blastomycosis, Pulmonary nodules, Tracheal blastomycosis
1. Introduction
Blastomyces is a dimorphic fungus found in the soil of warm, moist, and wooded areas that causes localized or systemic infections. Blastomyces infection follows the inhalation of conidia or through direct skin inoculation. Blastomycosis is endemic in North America, mostly in the Ohio and Mississippi river valleys, New York, Colorado, Wisconsin, Texas, Kansas, and Nebraska, with an annual incidence rate of 0.3–1.8 cases per 100,000 in the United States [1,2]. Canada has an incidence of 0.133–0.179 cases per 100,000 people, with most cases in provinces around the Great Lakes, Quebec, Ontario, and Manitoba [[3], [4], [5]]. Infection occurs commonly in older persons who participate in outdoor activities [3]. Most infections result in pulmonary illness, but the involvement of bones, genitourinary system, central nervous system, liver, spleen, heart, lymph nodes, and musculoskeletal system can occur [2]. Tracheal blastomycosis is a rare manifestation of the disease that has been previously reported once in the literature. We report a case of tracheal and pulmonary blastomycosis that was discovered following routine lung cancer screening.
2. Case presentation
An 81-year-old male with a past medical history significant for chronic obstructive pulmonary disease, coronary artery disease, hypertension, hypothyroidism, diabetes mellitus, and hyperlipidemia who has had a chronic cough productive of thick yellowish sputum for the past 6–12 months, superimposed upon chronic shortness of breath that had been present for several years. These symptoms were slowly progressive in nature. The patient had an episode of pneumonia 2 years prior to presentation, followed by another episode of pneumonia a month later, in both instances treated with oral antibiotics as an outpatient. At a routine visit on day 0 with the patient's primary care physician, the decision was made to obtain a low dose chest computed tomography scan for routine lung cancer screening, which revealed multiple irregular nodular opacities in the bilateral lower lung zones, right middle lobe, and lingula up to 2 × 3 cm in size, tree-in-bud opacities, and soft tissue attenuation of the luminal surface of the left lateral aspect of the trachea, right lateral aspect of the trachea, and evidence of prior granulomatous disease (Fig. 1A). Due to the patient's rural location and challenges of care coordination with another healthcare system, 3 months elapsed before additional imaging was obtained. The follow-up non-contrast computed tomography scan on day 125 showed continued bi-basilar nodular densities at the lung bases, with a 2.2 cm nodular density appearing more mass-like at this time and mild nodular thickening at the proximal tracheal wall that was similar in appearance as the prior exam. A positron emission tomography-computer tomography was undertaken on day 139, which showed 18F-fluorodeoxyglucose (FDG)-avid thickening of the left side of the upper trachea, a 4.4 cm right middle lobe opacity which was mildly avid, and mildly avid patchy opacities at the left base. The patient was referred for pulmonary consultation and bronchoscopic examination.
Fig. 1.
Computed Tomography (CT) imaging of the tracheal lesion. CT images of the tracheal lesion are shown serially at (A) initial diagnosis, (B) mid-way through therapy with oral itraconazole, and (C) after completion of therapy.
The patient's medications included: metformin, levothyroxine, atorvastatin, lisinopril, tiotropium inhaler, albuterol inhaler, amlodipine, terazosin, budesonide/formoterol inhaler, multi-vitamins, fish oil, soluble fiber, B vitamin complex, aspirin, chlorthalidone, and omeprazole. The patient had no known drug allergies. The patient formerly smoked 1 pack per day for 30 years but had quit 30 years ago. He drank alcohol infrequently. The patient was formerly in the Army and served at Fort Drum in Kentucky and overseas in Germany. He then became a trucker. He pursued hunting and typically camped along the Moose River in northern Vermont, USA.
On exam, the patient was afebrile, with unremarkable vital signs. His oxygen saturation was 94% in room air. He was in no acute distress, had no oral lesions, no parotid swelling or tenderness, and no cervical lymphadenopathy. His lungs were clear bilaterally on lung exam. His cardiac exam was normal, and the rest of his exam was unremarkable. There was no rash nor skin lesions. Routine laboratory studies revealed a white blood cell count of 8.5 10*3/microliter (Ref range 4.5–11.0), serum creatinine of 1.41 mg/dL (Ref range 0.5–1.5), and a mild elevation in erythrocyte sedimentation rate and c-reactive protein.
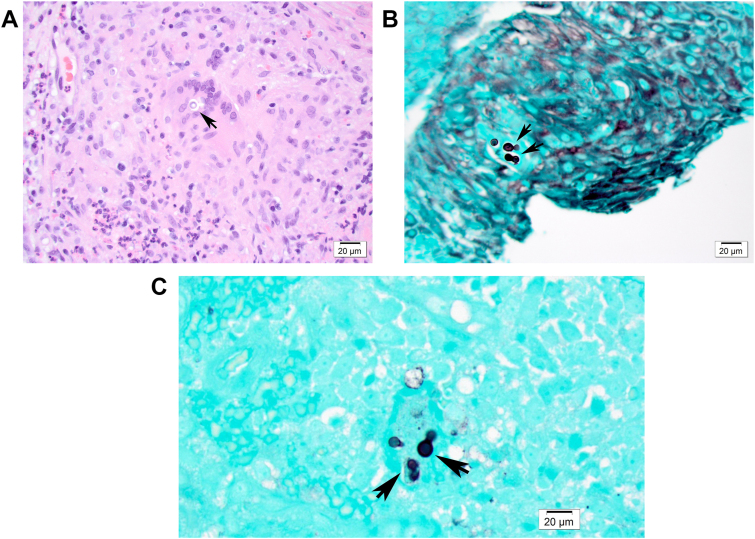
Upon pulmonary consultation, there was concern noted for the tracheal lesion seen on imaging studies. A bronchoscopy was performed on day 191, which revealed a friable fungating mass on the left anterolateral aspect of the proximal trachea, with initial cytopathology worrisome for malignancy. Cultures of the tracheal lesion were not obtained during bronchoscopy as there was no suspicion for an infectious etiology at the time. However, further pathologic evaluation revealed multiple hyperplastic squamous epithelium fragments with acute and chronic inflammation, including eosinophils and multinucleated giant cells in the submucosa. Round, broad-based budding fungi were visible on hematoxylin and eosin, periodic acid-Schiff, and Grocott's methenamine silver-stained sections (Fig. 2). These yeast forms had thick walls and multiple nuclei up to 12 μm in diameter. These histologic findings were diagnostic for Blastomyces infection.
Fig. 2.
Histopathological analysis of tracheal tissue. A. Hematoxylin and eosin stain of tracheal tissue demonstrates a large round thick-walled yeast cell. B and C., Grocott's methenamine silver stain section showing a round broad-based budding yeast.
On day 222, the patient was started on oral itraconazole 200 mg three times a day for three days, as a loading dose, then continued twice a day. Omeprazole was discontinued, and the dose of atorvastatin was reduced to minimize the possibility of drug-drug interactions. The patient tolerated the itraconazol well, and serum levels were confirmed to be in the therapeutic range (1.73 mcg/mL, reference range 1– 4mcg/mL). Follow-up computed tomography scan at 6 months (day 383) showed that the upper trachea's nodular thickening was substantially reduced (Fig. 1B). The bi-basilar opacities were smaller and more linear in appearance, super-imposed upon a background suggestive of bi-basilar scarring. The patient had overall improvement of cough/sputum production, but had some level of shortness of breath and modest cough/sputum production.
Another computed tomography scan was obtained near the end of therapy on day 492, which revealed interval decrease in size and heterogeneity of the opacity in the inferior right middle lobe with persistent linear and nodular scarring. Persistent airspace and nodular opacities in the lingula and bilateral lower lobes were markedly improved. There was scattered mucus plugging most conspicuously in the left lower lobe. Some persistent nodular thickening of the trachea just below the thoracic inlet remained (Fig. 1 C). Because of the concern for continued aspiration, omeprazole was re-started, guaifenesin was continued, and nasal steroids were initiated. Approximately 9 months after completion of itraconazole therapy, a follow-up visit on day 782 revealed no signs or symptoms suggestive of active pulmonary infection.
3. Discussion
Blastomycosis is an uncommon disease that has both pulmonary and extra-pulmonary manifestations; upper airway involvement and especially tracheal involvement (either isolated or in the presence of pulmonary infection) is even rarer.
A literature search revealed only one other published case of tracheal blastomycosis infection, involving a 17-year-old female in the 1980s [6]. While tracheal infections are infrequently reported, there have been many cases of oropharyngeal and laryngeal infections that have a more robust literature base around them. The proximity and functionally similar mucosa and circulation of tracheal and laryngeal anatomy allows us to use these cases as a useful comparison. A longitudinal study of blastomycosis in Mississippi showed that 2 out of 326 (0.6%) recorded cases of blastomycosis had laryngeal involvement and another study by the Mayo Clinic saw laryngeal involvement in 5/102 (4.9%) [7].
Contributing to the difficult diagnosis of this condition is the frequent lack of clinical suspicion of blastomycosis based on overall incidence. This is partially due to lack of mandatory reporting in most states except Arkansas, Louisiana, Michigan, Wisconsin, and Mississippi, resulting in underestimated disease prevalence and incidence [1,3,[8], [9], [10]]. Classically, this disease has been reported as endemic in the southcentral, southeastern, and midwestern regions of the United States. However, an increasing number of cases reported does not fall within this geographical area, with some even outside of North America [1,[11], [12], [13]]. The U.S. Centers for Disease Control and Prevention indicates that the fungus’ endemic area spans the entire eastern half of the continental United States, save the southern tip of Florida and some coastal regions of the northeast [9]. Cases have been reported in the northeast United States, including New York and Vermont [10,14,15]. Cases in Vermont have typically been found in the northwestern region of the state, where there is a higher concentration of residents [16]. While clearly present in a much larger distribution than originally thought, it is difficult to precisely define the endemic area of this fungal disease.
Pulmonary manifestations are the most common form of blastomycosis due to the inhalation of conidia as the most common route of initial pathogenesis [17]. Blastomycosis is primarily a lower respiratory infection. Presenting symptoms of blastomycosis are often mild or absent in isolated upper airway disease or overshadowed by coexisting pulmonary symptoms, likely causing cases to be undercounted. Often, these mild blastomycosis infections are subclinical and self-limiting [19]. It is important to note that the constellation of symptoms associated with pulmonary blastomycosis is not unique compared to signs and symptoms of other lung pathologies, and as a result, diagnostic delays are not uncommon. Patients may be asymptomatic or may present with acute pneumonia and respiratory distress. Alternatively, the presentation may include night sweats, hemoptysis, weight loss and show significant radiographic changes that may be mistaken for tuberculosis, or malignancy, as was the case in our patient. Symptoms can progress to impending or fulminant respiratory failure and even death [4,8,13]. Common radiographic findings include pulmonary nodules, as well as lobar consolidation or solitary or multiple abscesses [7]. Tracheal and laryngeal lesions that co-occur with a pulmonary infection may be easily overlooked if the diagnostic imaging employed is nonspecific, as with a chest x-ray or ultrasound, especially in milder cases.
Extrapulmonary manifestations are rare but may include nearly every organ, including bones, genitourinary system, central nervous system, liver, spleen, heart, lymph nodes, and muscles [2]. Skin lesions are well documented in the literature. Involvement of the larynx can occur as a result of the spread of quiescent pulmonary disease via a lymphohematogenous route or as a result of fungus-containing sputum traversing the larynx and causing infection by direct inoculation [18]. In the other case report of tracheal blastomycosis, it was speculated that a similar route may be present for tracheal infection. However, it is not well understood why tracheal involvement is so much rarer than laryngeal involvement.
The unique presenting symptom in an isolated laryngeal infection is hoarseness, and others include mild cough, shortness of breath, and dysphagia [20]. A tracheal lesion, on the other hand, will not involve the vocal cords and are far enough from the oropharynx to avoid dysphagia. This makes symptomatic detection possible only incidentally or when lesions have progressed to occlude the airway and impede aeration of the lung, as was found in the case presented by Kaufmann et al. [6]. In many of the cases of isolated laryngeal infection, the patient identified a mild cough in the weeks prior to the development of laryngeal symptoms that was mild enough that they did not seek medical care. As a lesion progresses and becomes more massive, a patient can develop inspiratory stridor as the airway is occluded.
The cases published on laryngeal blastomycosis infection show it can frequently be initially mistaken for other more common etiologies including squamous cell carcinoma (SCC), sarcoidosis, syphilis, other fungal infections, and polyposis [21]. The case of tracheal infection we present here follows a similar course. Our patient's tracheal lesion was found incidentally on a CT and a biopsy without culture was taken due to a high suspicion for cancer. Both SCC and blastomycosis closely resemble each other in gross and histopathologic findings [20]. If tracheal involvement is suspected, a biopsy and tissue examination with appropriate staining is essential for diagnosis [7,22]. The paucity of confirmed cases may be due to the invasive nature of the biopsy in the context of non life threatening symptoms, similar gross histological findings, and the relative prevalence of SCC. This may prompt treatment for SCC without specific tests, like the histological stains described below, to rule out a fungal infection. Once biopsies are obtained, diagnosis of blastomycosis can be made through histopathology and/or culture. Hematoxylin and eosin stains can confirm inflammation and granulomatous changes, but periodic acid- Schiff or Grocott's methenamine silver stain are best used to show fungal elements [1]. Other methods to assist in the diagnosis of blastomycosis may include serology, enzyme immunoassay, or nucleic acid testing from both serum and bronchoalveolar lavage, as well as sputum or bronchoalveolar lavage cultures. However, a biopsy is required to confirm the cause of the specific tracheal lesion [1,23]. Itraconazole is the preferred drug for mild to moderate, non-central nervous system manifestations of blastomycosis, given its lower toxicity profile, although drug levels still need to be monitored. For more severe infections, liposomal amphotericin B is given until significant clinical improvement is seen. Patients will typically require six to twelve months of treatment [1] In our case, the patient responded clinically and radiographically to 9 months of oral itraconazole therapy.
Though not commonly reported, our case represents an unusual manifestation of this fungal infection. To our knowledge, this is only the second case of tracheal blastomycosis reported in the literature, this time in an adult with exposure in the northernmost part of the northeast United States, leading to both tracheal and pulmonary disease. A high degree of clinical suspicion is required to recognize this endemic mycosis.
Declaration of competing interest
None to report.
Acknowledgements
We thank Dr. Nora Ratcliffe for assistance with the pathology findings and Dr. Frank Brennan for assistance with the radiology images.
References
- 1.Castillo C.G., Kauffman C.A., Miceli M.H. Blastomycosis. Infect Dis Clin North Am. 2016;30:247–264. doi: 10.1016/j.idc.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Saccente M., Woods G.L. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 2010;23:367–381. doi: 10.1128/CMR.00056-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown E.M., McTaggart L.R., Dunn D., Pszczolko E., Tsui K.G., Morris S.K. Epidemiology and geographic distribution of blastomycosis, histoplasmosis, and coccidioidomycosis, Ontario, Canada, 1990–2015. Emerg Infect Dis. 2018;24:1257–1266. doi: 10.3201/eid2407.172063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Litvinov I.V., St-Germain G., Pelletier R., Paradis M., Sheppard D.C. Endemic human blastomycosis in Quebec, Canada, 1988-2011. Epidemiol Infect. 2013;141:1143–1147. doi: 10.1017/S0950268812001860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morris S.K., Brophy J., Richardson S.E., Summerbell R., Parkin P.C., Jamieson F. Blastomycosis in Ontario, 1994–2003. Emerg Infect Dis. 2006;12:274–279. doi: 10.3201/eid1202.050849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaufman J. Tracheal blastomycosis. Chest. 1988;93:424–425. doi: 10.1378/chest.93.2.424. [DOI] [PubMed] [Google Scholar]
- 7.Clarke A., Skelton J., Fraser R.S. Fungal tracheobronchitis. Report of 9 cases and review of the literature. Medicine (Baltimore) 1991;70:1–14. doi: 10.1097/00005792-199101000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Cates D.J., Rosen C.A., Yassin M.H., Smith L.J. Primary laryngeal blastomycosis: diagnostic challenges and advances in a rare cause of laryngitis. Laryngoscope. 2019;129:2531–2533. doi: 10.1002/lary.27593. [DOI] [PubMed] [Google Scholar]
- 9.Blastomycosis | fungal diseases | CDC. 2020. https://www.cdc.gov/fungal/diseases/blastomycosis/index.html
- 10.Bl P., Wa A., Tj V. Blastomycosis diagnosed in a nonhyperendemic area. WMJ. 2014;113:11–18. quiz 19. [PubMed] [Google Scholar]
- 11.Benedict K., Thompson G.R., Deresinski S., Chiller T. Mycotic infections acquired outside areas of known endemicity, United States. Emerg Infect Dis. 2015;21:1935–1941. doi: 10.3201/eid2111.141950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azar M.M., Assi R., Relich R.F., Schmitt B.H., Norris S., Wheat L.J. Blastomycosis in Indiana: clinical and epidemiologic patterns of disease gleaned from a multicenter retrospective study. Chest. 2015;148:1276–1284. doi: 10.1378/chest.15-0289. [DOI] [PubMed] [Google Scholar]
- 13.Bradsher R.W. The endemic mimic: blastomycosis an illness often misdiagnosed. Trans Am Clin Climatol Assoc. 2014;125:188–203. [PMC free article] [PubMed] [Google Scholar]
- 14.Kiatsimkul P. Increasing incidence of blastomycosis infection in Vermont. Open Forum Infect Dis. 2017;4:S84–S85. doi: 10.1093/ofid/ofx163.032. [DOI] [Google Scholar]
- 15.McDonald R. <em>Notes from the field</em>: blastomycosis cases occurring outside of regions with known endemicity — New York, 2007–2017. MMWR Morb Mortal Wkly Rep. 2018;67 doi: 10.15585/mmwr.mm6738a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seitz A.E., Younes N., Steiner C.A., Prevots D.R. Incidence and trends of blastomycosis-associated hospitalizations in the United States. PLOS ONE. 2014;9 doi: 10.1371/journal.pone.0105466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rucci J., Eisinger G., Miranda-Gomez G., Nguyen J. Blastomycosis of the head and neck. Am J Otolaryngol. 2014;35:390–395. doi: 10.1016/j.amjoto.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 18.Hanson J.M., Spector G., El-Mofty S.K. Laryngeal blastomycosis: a commonly missed diagnosis. Report of two cases and review of the literature. Ann Otol Rhinol Laryngol. 2000;109:281–286. doi: 10.1177/000348940010900309. [DOI] [PubMed] [Google Scholar]
- 19.Vaaler A.K., Bradsher R.W., Davies S.F. Evidence of subclinical blastomycosis in forestry workers in northern Minnesota and northern Wisconsin. Am J Med. 1990;89:470–476. doi: 10.1016/0002-9343(90)90378-q. [DOI] [PubMed] [Google Scholar]
- 20.Dumich P.S., Neel H.B. Blastomycosis of the larynx. Laryngoscope. 1983;93:1266–1270. doi: 10.1002/lary.1983.93.10.1266. [DOI] [PubMed] [Google Scholar]
- 21.Mikaelian A.J., Varkey B., Grossman T.W., Blatnik D.S. Blastomycosis of the head and neck. Otolaryngol Head Neck Surg. 1989;101:489–495. doi: 10.1177/019459988910100415. [DOI] [PubMed] [Google Scholar]
- 22.Yazıcıoğlu Mo in O., Karakurt Z., Aksoy F., Güng r G., Partal M., Adıgüzel N. Bronchoscopy as an indicator of tracheobronchial fungal infection in non-neutropenic intensive-care unit patients. Clin Microbiol Infect. 2013;19:E136–E141. doi: 10.1111/1469-0691.12112. [DOI] [PubMed] [Google Scholar]
- 23.An J., Yang H.-P., Hu C.-P., Cao L.-M., Zhou Y.-F., Xiao Q.-M. Multinodule abnormalities of the tracheobronchus: bronchoscopy findings and clinical diagnosis. Clin Respir J. 2017;11:440–447. doi: 10.1111/crj.12356. [DOI] [PubMed] [Google Scholar]