Abstract
Background
Between unaffected mental health and diagnosable psychiatric disorders, there is a vast continuum of functioning. The hypothesized link between striatal dopamine signaling and psychosis has guided a prolific body of research. However, it has been understudied in the context of multiple interacting factors, subclinical phenotypes, and pre-postsynaptic dynamics.
Method
This work investigated psychotic-like experiences and D2/3 dopamine postsynaptic receptor availability in the dorsal striatum, quantified by in vivo [11C]-raclopride positron emission tomography, in a sample of 24 healthy male individuals. Additional mediation and moderation effects with childhood trauma and key dopamine-regulating genes were examined.
Results
An inverse relationship between nondisplaceable binding potential and subclinical symptoms was identified. D2/3 receptor availability in the left putamen fully mediated the association between traumatic childhood experiences and odd beliefs, that is, inclinations to see meaning in randomness and unfounded interpretations. Moreover, the effect of early adversity was moderated by a DRD2 functional variant (rs1076560). The results link environmental and neurobiological influences in the striatum to the origination of psychosis spectrum symptomology, consistent with the social defeat and diathesis–stress models.
Conclusions
Adversity exposure may affect the dopamine system as in association with biases in probabilistic reasoning, attributional style, and salience processing. The inverse relationship between D2/3 availability and symptomology may be explained by endogenous dopamine occupying the receptor, postsynaptic compensatory mechanisms, and/or altered receptor sensitivity. This may also reflect a cognitively stabilizing mechanism in non-help-seeking individuals. Future research should comprehensively characterize molecular parameters of dopamine neurotransmission along the psychosis spectrum and according to subtype profiling.
Keywords: dopamine, D2 receptor, PET, striatum, childhood trauma, psychosis spectrum
Introduction
The dopaminergic system is strongly involved in cognition, goal-directed behavior, and the etiology of neuropsychiatric disorders.1,2 The prominent dopamine (DA) hypothesis of schizophrenia postulates the illness is caused by modulations of dopaminergic neurotransmission.3 The role of DA in psychotic disorders is also substantiated by the mechanisms of antipsychotic medication, commonly D2-like receptor (D2, D3, and D4) antagonism.4 As a complementary approach to post mortem studies,5 early molecular imaging intensely investigated D2-like receptor densities and pharmacokinetics, as enabled by the introduction and refinement of radiopharmaceuticals incorporating positron-emitting radionuclides.6 Recent studies in the field highlight the nigrostriatal pathway rather than the mesolimbic pathway as the locus of highest dysregulation of DA signaling,7 with its elevated synthesis and higher stimulated release8,9 being the most consistent findings. Moreover, correlations observed between DA synthesis and positive symptom severity10,11 further corroborate these associations.
In comparison with presynaptic DA signaling, the role of postsynaptic signaling in psychotic symptoms remains less clear.12 Notably, some previous evidence13 and related conceptualizations14,15 accentuate the complex dynamics of pre- and postsynaptic dopaminergic neurotransmission. Whether DA imbalances are late manifestations of schizophrenia or reflective of a more generic mechanism shared by a broader psychosis phenotype remains debated. Some previous positron emission tomography (PET) studies point to heightened striatal DA synthesis capacity in individuals at high risk for psychosis16 and its progressive increase in transitioning from prodromes to psychotic episodes.17 This aligns with the psychosis continuum,18,19 suggesting gradable symptomology along a spectrum between severe and subclinical forms, such as psychotic-like experiences, schizotypal personality, or “at-risk” states.20–22 The existing evidence also suggests that baseline D2-like receptor availability may index inter-individual differences.23 Nevertheless, definitive conclusions have been hindered by the lack of consensus regarding the structure of schizotypy and other psychosis-related phenomena.24 Notably, even among patients with clinical psychosis, the substantial heterogeneity in treatment response and side effects suggests the existence of discrete dopaminergic subtypes.25,26 Therefore, more work is necessary to investigate the coinciding and discriminant aspects of these symptoms in the context of construct validity and trait-state dynamics, as well as to understand the link between neurobiology and the cognitive-perceptual dimensions.
Aberrant salience processing has been proposed to occur downstream in the pathway to psychotic symptoms.27 This may underlie perceived reality distortion as a plausible mechanism linking altered dopaminergic neurotransmission to symptoms.28 The putative causality path also includes interference with the dopaminergic system by early environmental triggers, with a prominent role of childhood adversities and trauma exposure, which may precipitate or exacerbate disorders.29 Attributing salience to threatening environmental stimuli may cause a state of endogenous sensitization at subsequent exposures, together with modulations in the brain neurocircuitry.30 A meta-analysis and a review identified strong associations between schizotypy or schizophrenia and childhood adversity, observing that affected individuals were 2.01–4.15 times31 and 2.72 times32 more likely, respectively, to have a history of childhood trauma than unaffected controls. There is also evidence that genetically determined DA neurotransmission contributes to the propensity for unfounded beliefs and delusion-prone perception.33 The inter-individual differences in D2-like receptor expression and its striatal availability have been linked to functional genetic variation in DRD2 and other DA-regulating genes,34 while the responses to early stressors may be subject to gene × environment interactions.35,36
First, this work investigates the hypothesis of an association between baseline postsynaptic D2/3 receptor availability (D2/3R) within the dorsal striatum, as quantified by PET [11C]-raclopride, and psychotic-like experiences in explicitly healthy individuals. In doing so, we also postulated that subtly orthogonal symptom measures may differ in how they relate to D2/3R availability. Second, we hypothesized that the latter mediates the relationship between childhood trauma exposure and psychotic-like experiences. In an exploratory analysis, we also examined possible interactions between D2/3R availability and early trauma with single nucleotide polymorphisms (SNPs) in key DA-regulating genes.
Methods
Participants
This study was part of a larger project investigating psychotic-like experiences37 approved by the Ethics Committee Zurich (KEK-ZH-No. 2011-0423) and conducted in compliance with the Declaration of Helsinki. We recruited 24 healthy right-handed males of European Caucasian descent between 20 and 40 years of age. All participants gave written informed consent. Present or prior history of mental illness or substance abuse was ruled out with the Mini-International Neuropsychiatric Interview.38 The measurements were performed between 4 and 5 pm in a dimly lit hospital room, where probands were asked to lie down comfortably without falling asleep.
Imaging Data Acquisition
[11C]-Raclopride is a selective competitive D2/D3 antagonist (KD ~ 1–10 nM) from the benzamides family used for in vivo estimation of receptor availability.39 The emission scans were acquired using a PET/CT scanner (Discovery STE and RX, GE Healthcare, Waukesha, WI, USA) operating in 3D mode, with an axial field of view of 15.3 cm. [11C]-Raclopride was radio-synthesized on site according to the Good Manufacturing Practice and administered as a bolus infusion into an antecubital vein. The mean (±SD) injected radioactivity was 180.96 ± 19.10 MBq. The dynamic scan started immediately after tracer application and continued for 60 min (31 frames: 4 × 15 s, 8 × 30 s, 9 × 60 s, 2 × 180 s, 8 × 300 s). Emission data were reconstructed by filtered back projection, applying a 6-mm FWHM filter, with CT-based attenuation correction (120 kV/80 mA). For the coregistration, high-resolution T1-weighted anatomical images (TR, 8.2 ms; TE, 3.8 ms; flip angle, 8°; in-plane resolution, 1 × 1 × 1 mm3; FOV, 160 × 240 mm2; 160 slices) were collected using a 3 T MRI system (Philips Achieva, Philips Medical Systems, Best, Netherlands) equipped with an 8-channel head coil.
Positron Emission Tomography Image Processing
PET image processing was performed using PMOD version 3.5 (PMOD Technologies Ltd., Zurich, Switzerland). Regional time–activity curves were derived and fitted to the simplified reference tissue model40 with the cerebellum as a reference,41 thereby enabling the quantification of receptor kinetics without arterial sampling. Regional nondisplaceable binding potential (BPND) values, reflecting the ratio at the equilibrium of the bound radioligand to the nondisplaceable radioligand,42 were extracted from the bilateral putamen and caudate nuclei, showing the highest and most reliable uptake in response to [11C]-raclopride.43 The region definitions were based on the parcellation algorithm implemented in PMOD (Neuro-Tool) and the individually segmented anatomical MRI in Montreal Neurological Institute stereotactic space. The accuracy of parcellated regions-of-interest (ROIs) was verified and manually adjusted as necessary by a neuroscientist knowledgeable about striatal dissection.
Genotyping
Using the proteinase K method, genomic DNA was isolated from whole blood collected into EDTA tubes. Genotyping was performed with the Illumina Infinium PsychArray (Illumina, San Diego, CA, USA). Six widely studied polymorphic loci were chosen based on previous literature linking PET D2-like receptor availability with genetic variation in DA regulation34: rs1079597, rs6277, rs1076560, rs1799732 (DRD2), rs1800497 (ANKK1), and rs4680 (COMT).
Psychometric Measures
Psychotic-Like Experiences
Psychotic-like experiences were measured with the 32-item Exceptional Experiences Questionnaire-Revised (PAGE-R),37,44,45 which uses a five-point frequency Likert scale from 0 (never) to 4 (very often). Unlike many other instruments, the items are not based on clinical symptoms or their attenuated forms, but on reports of healthy individuals seeking counseling for their experiences.45 Notably, the measure captures experiences not only associated with distress, but also those evoking neutral connotations or comfort (e.g., seeing meaning in coincidences). This relatively new instrument was cross-validated with other measures of positive symptomology37 and applied in previous brain imaging studies.46 The analysis used the total score as well as three empirically derived scales for odd beliefs (OB), dissociative anomalous perceptions (DAP), and hallucinatory anomalous perceptions (HAP), with corresponding scale ranges of 0–128, 0–44, 0–28, and 0–36, respectively. Example items are provided in the Supplementary Material.
Schizotypal Personality Traits
For comparisons with an established measure of schizotypy, the 74-item Schizotypal Personality Questionnaire (SPQ)47 was administered, using the total score and three higher-order factors: cognitive-perceptual (positive facet), interpersonal (negative facet), and disorganized.48
Childhood Trauma Exposure
Histories of childhood neglect and maltreatment were measured using the 28-item Childhood Trauma Questionnaire (CTQ).49–51 The analyses employed the total score, which reflects the general severity of exposure, ranging in our sample between 25 and 83, from “none or minimal” through “low-to-moderate” to “severe” (single case).
Statistical Analysis
First, Spearman’s rank correlation coefficients were used to assess the relationships between the D2/3R availability, subclinical symptoms, childhood trauma exposure, and age.52 Second, to expand on the predictive value of BPND from four brain regions on the separate symptom measures (SPQ, PAGE-R), a dominance analysis53,54 was conducted within the linear regression framework using the “dominanceanalysis” R package.55 This approach provides a statistical ranking of the importance of a predictor in the presence of multicollinearity (as observed in our striatal parcellation data), where relying on typical beta coefficients would be inadequate.54,56,57 Specifically, relative importance was derived from R2 estimates for three hierarchical dominance types: complete (most strict, holding across all predictors and all possible regression subset models), conditional (the averaged additional contribution of a given model level is higher than that of another predictor), and general (greater average mean additional contribution across all model levels).53,54 To assess the robustness of results, bootstrapping with 5000 replicates was conducted, first, to determine the proportion of bootstrapped samples reproducing the observed effects between each pair of predictors and, second, to determine the average dominance estimates across all bootstrapped samples. Third, a mediation analysis tested D2/3R availability as a mediator, trauma as an independent variable, and subclinical symptoms as dependent variables following the procedures of Baron and Kenny.58 The model tested our specific hypothesis of D2/3R availability accounting for the relation (and not just the strength of the relation) between predictor and outcome. The significance of indirect effects was examined using bootstrapping with 5000 replicates and 95% bias-corrected confidence intervals (CI), as implemented in the “mediation” R package for causal mediation analysis, following quasi-Bayesian estimation.59 This method decomposes the effects of causally interpretable benchmarks instead of relying on typical regression coefficients using the product method. Specifically, the average causal mediation effect (ACME), average direct effect (ADE), and total effect (TE), that is, their sum, were computed. As causal mediation is based on a strong and directly untestable assumption that the mediator-outcome effect is not confounded (i.e., the sequential ignorability assumption), the main analysis was supplemented by a sensitivity analysis60 using the medsens function, This introduces ρ, a parameter representing the correlation of error terms in the mediator and outcome models, and also compares the coefficient of determination between both models. Accordingly, the robustness of the mediation effect was determined by probing which levels of violation to the assumption would reverse or invalidate the conclusion from the main analysis. The mathematical foundation for the above methods of dominance and mediation/sensitivity analyses is detailed elsewhere.53,54,60,61
Lastly, two regressions under an additive genetic model with normalization and adjustment for age were conducted to investigate possible interactions of individual SNPs with D2/3R availability (explaining subclinical symptoms) and with trauma load (explaining D2/3R availability). These exploratory analyses were limited to the variables from the primary imaging finding. Second-level statistics were calculated using R version 3.6.1 at a P < .05 threshold, with the Benjamini–Hochberg procedure used to control the false discovery rate (FDR).
Results
Descriptive Statistics
Table 1 provides the descriptive statistics, psychometric measures, [11C]-raclopride BPND, and injection activities of the study sample.
Table 1.
Demographic Characteristics and Study Measures
| Characteristic/Measure (N = 24) | M | SD | Min.–Max. |
|---|---|---|---|
| Sex | 24 m | – | – |
| Age (y) | 27.38 | 5.17 | 21–38 |
| Estimated IQ* | 111.54 | 13.96 | 91–143 |
| Education (formal years) | 13.96 | 2.14 | 10–17 |
| Body mass index (BMI) | 23.81 | 2.26 | 18.52–27.78 |
| Psychotic-like experiences | |||
| PAGE-R total score | 16.92 | 16.15 | 0–49 |
| PAGE-R odd beliefs (OB) | 7.25 | 6.09 | 0–18 |
| PAGE-R dissociative anomalous perceptions (DAP) | 1.96 | 2.96 | 0–12 |
| PAGE-R hallucinatory anomalous perceptions (HAP) | 2.92 | 3.11 | 0–9 |
| Schizotypal traits | |||
| SPQ total score | 17.92 | 9.94 | 2–38 |
| SPQ cognitive-perceptual schizotypy (positive) | 8.08 | 6.07 | 0–22 |
| SPQ interpersonal schizotypy (negative) | 5.46 | 4.01 | 1–18 |
| SPQ disorganized schizotypy (disorganized) | 4.38 | 3.62 | 0–13 |
| Childhood trauma exposure | |||
| CTQ total score | 38.83 | 13.24 | 25–83 |
| [11C]-Raclopride injection activity (MBq) | 180.96 | 19.10 | 155.59–222.28 |
| [11C]-Raclopride BPND: | |||
| Putamen left | 2.990 | 0.279 | 2.504–3.740 |
| Putamen right | 2.970 | 0.261 | 2.418–3.604 |
| Caudate left | 2.127 | 0.256 | 1.678–2.746 |
| Caudate right | 2.064 | 0.272 | 1.471–2.799 |
Note: BPND, nondisplaceable binding potential; CTQ, Childhood Trauma Questionnaire; PAGE-R, Exceptional Experiences Questionnaire-Revised; M, mean; SD, standard deviation; SPQ, Schizotypal Personality Questionnaire.
*Premorbid intelligence was estimated by extrapolating IQ values from the Multiple-Choice Vocabulary Intelligence Test.
The specific tracer activity was M = 32.58 GBq/µmol, SD = 11.04. This value was an estimation based on standard values acquired for quality control. Within the setup, the specific activity was not measured directly.
Spearman’s Rank Correlations
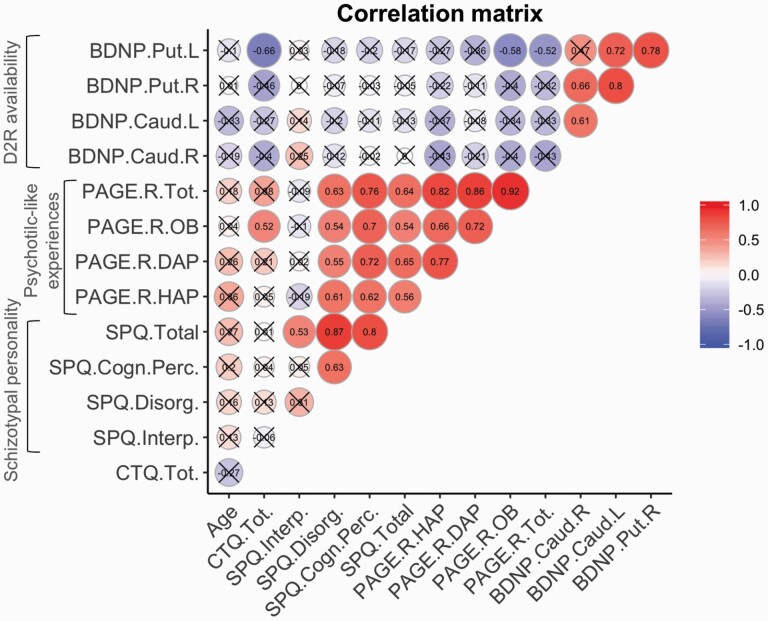
After FDR correction, there were positive significant bivariate correlations between BPND for all four striatal regions, except for the left putamen−right caudate pair. Similarly, all measures of symptomology were positively associated with the exception of the “Disorganized” facet of SPQ (which was correlated solely with SPQ total score). Additionally, we found negative associations between PAGE-R and BPND, which were nominally significant for the left putamen (with PAGE-R total score and OB). Finally, childhood trauma was significantly associated with PAGE-R OB (positively) and BPND in the left putamen (negatively) (figure 1 and figure S1).
Fig. 1.
Correlation matrix. The results were filtered for significance at FDR-corrected P < .05 (uncrossed circles). BDNP, [11C]-raclopride BPND; Caud, caudate; CTQ, Childhood Trauma Questionnaire; PAGE.R, Exceptional Experiences Questionnaire-Revised; Put, putamen; SPQ, Schizotypal Personality Questionnaire.
Nondisplaceable Binding Potential (D2/3R Availability)
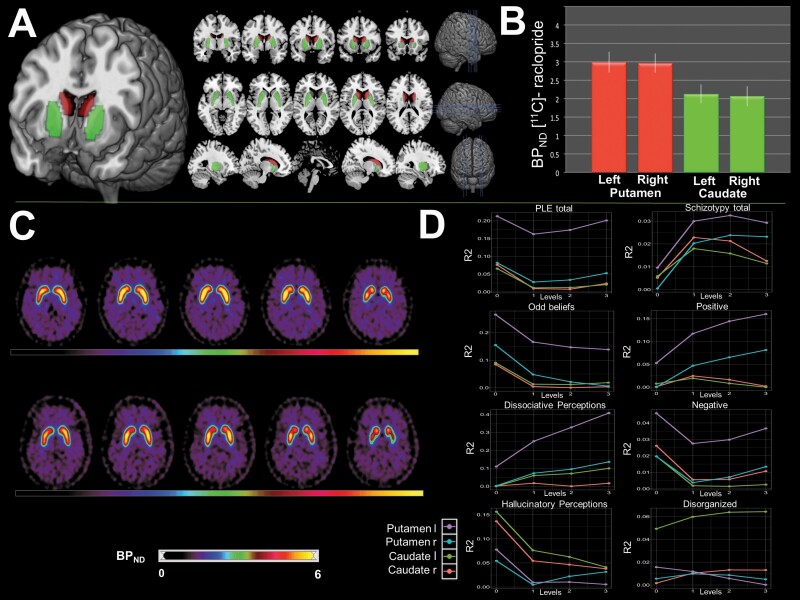
Figure 2 depicts the ROIs, corresponding BPND, and associations with subclinical symptoms. Overall, the dominance analysis indicated a prevailing impact of BPND in the left putamen across most symptom dimensions, with systematically assessed contributions from the remaining regions (Figure 2D). Reproducibility rates (RR) of ≥70% indicate high confidence in an observed effect being present in a population,62 and robust complete dominance was found for the left putamen across all submodels in relation to both PAGE-R total (RR = 74%−79%) and PAGE-R OB (RR = 74%−81%). The proportions of variance (R2) in BPND explained by PAGE-R total and PAGE-R OB were 0.188 (bs.E [bootstrapped estimate] = 0.201, bs.SE [bootstrapped standard error] = 0.121) and 0.179 (bs.E = 0.196, bs.SE = 0.097), respectively. An extended presentation of this analysis is provided in the Supplementary Material.
Fig. 2.
[11C]-Raclopride PET results. (A) Placement of the anatomical regions-of-interest. (B) Mean (± SD) BPND. (C) PET images from two representative subjects (aged 22 and 21) with high and low striatal BPND (upper and lower panels, respectively). (D) Conditional dominance of BPND in relation to psychotic-like experiences ([PLE] PAGE-R, left column) and schizotypy (SPQ, right column). Plots depict the explained variance (R2, y-axis) as a function of predictors in the model from level “0” (a single predictor) to “3” (all four predictors) (x-axis).
Mediation Analysis
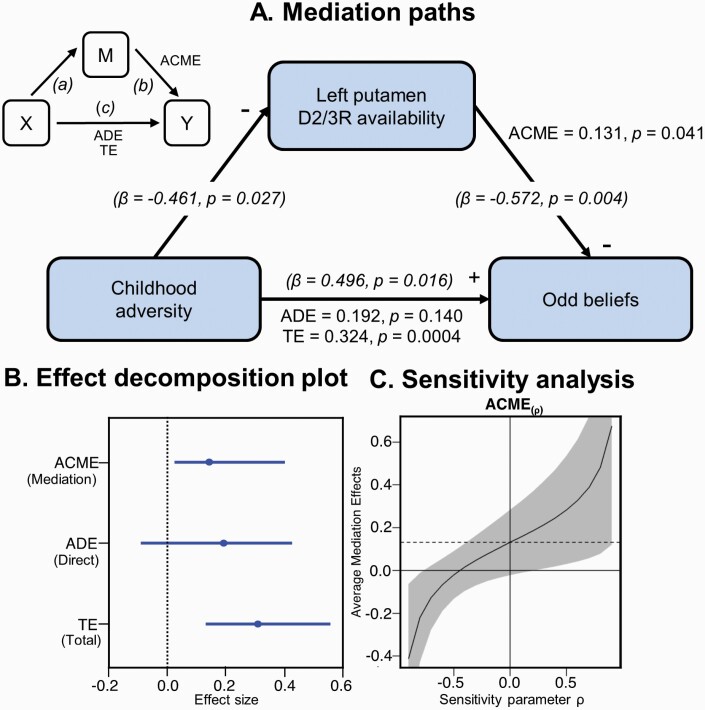
A preliminary analysis using fitted regressions supported the mediational hypothesis in the path-tracing step, showing significant effects (P < .05) of CTQ on PAGE-R OB and both D2/3R availability in the left putamen and D2/3R availability on PAGE-R OB. Given the impact of outliers and high leverage points in mediation analyses,63 data were checked for multivariate outliers using robust Mahalanobis distance (D). One dataset was clearly an outlier (D > 8) and was discarded from subsequent analyses. Bootstrapping revealed a significant effect via the mediator (ACME = 0.131, 95% CI [0.035–0.400], P = .041), a nonsignificant direct (unmediated) effect (ADE = 0.192, 95% CI [−0.082–0.420], P = .14), and a significant total effect (TE = 0.324, 95% CI [0.157–0.530], P = .0004). The proportion of the total effect mediated by D2/3R availability was 41% (95% CI [0.128–1.930], P = .044). As the effect became nonsignificant after the inclusion of the mediator, the results reflected a full mediation according to the classical framework.58Figure 3A depicts these key results. The sensitivity analysis indicated that the ρ value at which ACME reached 0 was approximately −0.45, indicating the robustness of the mediation estimate to violations of the sequential ignorability assumption.64 The potentially omitted total variance from unobserved confounders in the mediator-outcome relationship was estimated as 28% (√0.078).
Fig. 3.
Mediation analysis of childhood adversity, left putamen D2/3R availability, and odd beliefs. (A) Mediation path diagram with decomposition effects: ACME, average causal mediation effects; ADE, average direct effects; TE, total effects. Additional standardized regression coefficients (β) and corresponding P-values for each path are in brackets. (B) Bootstrap confidence intervals for the three effects. (C) Sensitivity analysis plot for ACME. The solid black curve corresponds to the estimated ACME at different increments of ρ (95% CI), and the dashed line marks the point at which sequential ignorability holds at ρ = 0.
Interactions with Dopamine-Regulating Genes
After FDR correction, the interaction between SNP rs1076560 and childhood trauma exposure remained significant in predicting D2/3R availability (estimate = 0.483, Puncorr. = .004, Pcorr. = .048). Specifically, this effect increased with the presence of risk allele A (1 × estimate for CA genotypes, 2 × estimate for AA genotypes).
The Supplementary Material provides further details on the methods and results.
Discussion
Dopamine, Subclinical Symptoms, and Trauma
This study examined the associations among the psychotic-like symptoms, early traumatic experience, and striatal [11C]-raclopride BPND. As the key finding intersecting these measures, postsynaptic D2/3R availability in the left putamen was a (full) mediator of the effect of trauma exposure on having odd beliefs. This aligns with the view that early stressful experiences may impair or sensitize the DA system later in life29 and a finding linking childhood adversity to left basal ganglia function during reward-related processing.65 The laterality aspect may reflect asymmetrical projections of dopaminergic neurons.66 Considerably, the exposure to stressors in animal models exerts well-documented interference with DA neurotransmission in association with glucocorticoid secretion.67–69 These observations support the social defeat hypothesis, implying detrimental environmental influences and a prolonged subordinate position as risk factors for psychotic disorders.70 Such adversities may cause biases in cognitive schemas towards more bizarre and delusion-like interpretations.71 Aberrant DA signaling presumably renders coincidental incidents as salient events, which can eventually become hardwired as DA-dependent psychotic beliefs.27 Notably, the phenomena involved in our mediation model were odd beliefs, manifesting as biases in probabilistic reasoning, atypical attributional style, and altered theory of mind.72 The present findings extend our previous conclusions from large cohorts, which indicated that the pathway connecting childhood trauma with psychotic-like experiences is through subjective stress appraisal.73 Additionally, we found that early trauma was associated with having odd beliefs alone or together with perceptual anomalies.73,74 Collectively, psychosocial adversities, including neglect and abuse, are a plausible vulnerability factor for symptoms even below diagnostic cut-off points, in association with DA neurotransmission.
Associations with Postsynaptic D2/3R Availability
While the positive link between childhood trauma load and psychosis spectrum is corroborated by previous evidence,73,75,76 the negative coupling of D2/3R availability with trauma and symptoms is seemingly at odds with the theory of DA hyperactivity, at least in its over-simplistic version. We observed a low D2/3R availability for high psychotic-like experiences across all four striatal regions, with the strongest effects in the left putamen and somewhat weaker effects in the right caudate. An early study using [123I]IBZM (benzamide) single-photon emission computed tomography (SPECT) reported the same negative relationship between psychoticism and D2/3R availability in the basal ganglia.77 This finding was interpreted as compensatory down-regulation of postsynaptic receptor activity, through decreased receptor number or sensitivity. However, psychoticism is the least clear-cut of Eysenck’s personality dimensions, linking rather weakly related mental functions of overinclusive thinking, nonconformism, and impulsive tendencies.78 Two subsequent [11C]-raclopride PET studies independently confirmed the negative relationship between striatal D2/3R availability and detachment (i.e., social avoidance and withdrawal) partially defines the negative facet of schizotypy.79,80 In contrast, a positive relationship was previously identified between the “Disorganized” facet of schizotypy and right striatal D2/3R availability, as measured by the [123I]IBZM SPECT.81 The aforementioned compensative postsynaptic mechanisms may account for our results, commensurate with the proposition that these may be responses that normalize the primary insult of elevated dopamine synthesis.82 Indeed, an elegant within-subject study with healthy individuals found an inverse correlation between the striatal endogenous DA synthesis rate and D2/3R binding, as measured with l-[β-11C]DOPA and [11C]-raclopride radiotracers, respectively.13 This effect may involve the phasic and tonic components of subcortical DA release, one representing direct neuron firing, with the other setting the background level of DA stimulation and regulating responsivity to DA for autoreceptor and postsynaptic sites.83
Another explanation of our findings is putative competition between the injected radioligand and endogenous DA at D2/3R sites. Accordingly, the lower binding may reflect a higher receptor occupancy masking actual DA activity. Together with different sensitivities of radioligands to endogenous DA, this mechanism might have contributed to the discrepancy between the PET results using spiperone (butyrophenone derivative) (e.g., [11C]-methylspiperone) and benzamide family tracers (e.g., [11C]-raclopride), which tend to show higher receptor densities and no elevation or more inconsistent results,84 respectively. Our choice of [11C]-raclopride was dictated by its high selectivity and being the standard ligand for D2/3R imaging. However, D2 agonists (e.g., endogenous DA) and antagonists (e.g., raclopride, antipsychotics) also differ in the way they bind between two affinity states, i.e., D2Low and D2High, with the latter being involved in activating the second-messenger cascade. Based on findings from animal models, psychotic symptoms may be linked to a higher proportion of D2 receptors configured in the high-affinity state.85 Such an effect was preliminarily demonstrated for the putamen, in the absence of total baseline receptor differences between schizophrenia patients and controls.86 Given that 8%–21% of striatal D2 receptors may be occupied at baseline by endogenous DA, which preferentially competes for binding at D2High sites, while [11C]-raclopride seems non-preferential,87 the functionally significant affinity state might have been partially obscured by this mechanism in our results.
If the psychosis continuum holds true, our findings contradict numerous previous results within the clinical spectrum linking higher D2-like receptor availability to psychotic disorders.88–90 Still, other studies have detected no difference between drug-naïve schizophrenia patients and healthy controls,91–94 reporting positive correlations with unspecific symptoms86 or no correlations,92,94 but also negative correlations with positive symptoms.95 A recent high-quality meta-analysis of striatal DA function in schizophrenia highlights augmented DA synthesis and release capacity as the most robust pathological characteristics.96 However, the same meta-analysis based on 34 studies involving 485 schizophrenia cases and 485 controls indicated no standardized mean difference, but significant heterogeneity across studies assessing D2/D3 receptor availabilities, DA transporter availabilities, and synaptic DA levels was found. This work concludes that these three parameters may be dependent on patient subgroups,96 consistent with previous observations.97 Notably, the proposed inverted-U–shaped function between DA availability and D2 receptor status may reflect balanced versus unbalanced cognition performance.98 Within this possible variability spectrum, our findings indicate the higher incidence and degree of positive symptoms in the presence of lower postsynaptic D2/3R availability. As a novel proposed interpretation, postsynaptic downregulation may reflect a cognitively stabilizing mechanism, specific to overtly healthy individuals with psychotic-like experiences. Interestingly, cognitive flexibility and stability were shown to be mediated by D2 receptor signalling.99,100 Paradoxically, some of these experiences, particularly odd beliefs, are not necessarily burdensome, but may be valued positively and enhance well-being.72 They may constitute responses to a load of other symptoms and distressful experiences, possibly linked to excessive dopaminergic tone. Indeed, a cognition mode similar to odd beliefs was found to decrease stress under perceptual ambiguity101 and reinstate a sense of agency under a lack of control.102
Further considerations relate to spatial striatal subdivisions. Higher [11C]-raclopride BPND values in the putamen relative to the caudate are consistent with the D2 receptor distribution in the human brain.43,103,104 The spot in the left putamen is consonant with case reports of psychosis triggered by an infarction in this area,105 but also with an intriguing resolution of psychotic symptoms after a local hemorrhage.106 It suggests that an organic disruption in this region may induce a dramatic, possibly causative change in symptomology. Furthermore, the loss of left putamen volume was identified as a correlate of delusions107 and a risk stratifier and predictor of symptom development in individuals at-risk for psychosis.108 Other studies have reported abnormalities in the putamen in association with schizotypy or psychosis without laterality effects. For example, aberrant signal circuitry in the putamen was correlated with delusions and dependent on the salience network.109 Intriguingly, bilateral putamen enlargement was associated with schizotypal personality disorder, which has been interpreted as a mechanism mitigating the development of overt psychosis.110 These contradictory volumetric findings may be resolved by integrating the status of D2-like receptors, such as the availability of D2High versus total binding sites or levels of endogenous DA.111,112 Notably, the striatum is strategically positioned within the cortico-striato-thalamic re-entrant circuit. Therefore, any structural or functional change in this region per se may cause atypical corticostriatal signaling, subserving cognition, emotion, and behavior and their (in-)coherent functioning.113,114 Our findings can also be interpreted within the role of striatal DA neuron populations for coding reward prediction error,115 aberrations of which can result in the allocation of attention, which may further drive associative learning and contribute to the formation of delusional beliefs.116
Gene × Environment Interaction
This research also reveals some insights into a polymorphism in DRD2 (rs1076560; C>A). This intronic SNP is involved in regulating pre-postsynaptic DA signaling balance117 and has been linked to a range of schizophrenia-related endophenotypes in a meta-analysis.118 The possible mechanism underlying our result is a coupling of A alleles with a reduced short (D2S) to long variant (D2L) expression ratio. Both D2S and D2L autoreceptors117 can differentially modulate the GABA-dependent inhibition of DA release.119 Our finding aligns with the diathesis-stress model120 and observed interactions with either rs1076560 or DA risk allele load, for anxiety state and trait,121 parenting style,122 and childhood trauma.123 SNP rs1076560 was also found to interact with the binding of [123I]IBZM in the putamen to affect the degree of schizotypy.124 In consideration of cortico-thalamic input, D2 receptor density, and neuroplasticity, both genetic and environmental mechanisms may interact locally in the putamen in a way that merits further investigation.
Differentiated Phenotyping
More differentiated phenotyping may better capture symptoms in relation to neurobiology. Despite high positive correlations between SPQ and PAGE-R, only the latter was significantly associated with PET parameters and trauma. Schizotypal traits and psychotic-like experiences are among the phenotypic indicators of schizotypy, conceived as a latent proneness to psychosis or attenuated symptoms.125 Both reflect a trait-state specificity, with either more time-invariant or transient features.126 The awareness of the measured phenotype, conceptual clarity, and a future consensus78 are important, because diverse assessments might have contributed to mixed findings.127 Such an observation was noted for the mentioned association between detachment measured by the Karolinska Scales of Personality and D2/3R binding, which did not generalize to other measures of detachment, despite their positive correlations.79 Additionally, the sensitivity of [11C]-raclopride to cognitive states may result in different binding in dependency of the expectation to PET scanning.128 Importantly, psychotic-like experiences represent a societal issue, with prevalence rates between 5% and 27%.19,129 There is evidence for a rather epi-phenomenological nature of these signs; for 80% and 20% of individuals, they remit and become persistent, respectively, with 7% surpassing the threshold for a clinically relevant psychotic disorder.130
Cognitive Level and Transition to Clinical Manifestation
Beyond neurobiological implications, the cognition and clinical ramifications also merit discussion. Odd beliefs refer to a mental scaffolding for appraising the world, conferring cognitive-perceptual schemas defying factual knowledge or a conventional understanding of reality.131,132 They involve both representational content and assumed veracity,133 remaining self-evident for the holder despite being unauthenticated. These may include beliefs in meaningful coincidences, supernatural phenomena, or bizarre irrational thoughts. Existing research literature recognizes the role of cognitive biases for peculiar belief formation and maintenance.134 This may involve biases in jumping to conclusions (i.e., assigning meta-cognitive evidence to observations and experiences),135 hypersalience of evidence–hypothesis matches,136 or intolerance of ambiguity.137 In experimental settings, delusional ideation has been associated with endorsing interpretations despite disconfirmatory evidence and demonstrated in at least one nonclinical population.138 In agreement with our study noting the effect of early stress exposure, one prevalent view situates belief formation at the interface between cognitive and affective information processing.139,140 While the cognitive and learning principles are probably involved in the described mechanisms, compared with more modular systems (such as memory or attention), beliefs refer to much more distributed cognitive processes. Therefore, no complete account of beliefs exists,141 and more research is needed. On the functional level and in consideration of our study sample dealing with anomalous experiences, top-down meaning-making mechanisms seem a very plausible driver of belief formation seeking to reconcile uncertainty, pre-existing beliefs, and self-identity.
Psychotic-like experiences are associated with a heightened risk of developing a psychotic disorder in the future.19,142 At the same time, some positive (but not negative) features of schizotypy have been linked to psychological well-being.143 Numerous researchers also point to personal144 and environmental145 resources moderating or mitigating symptom deterioration. Without further empirical data, it is difficult to determine whether similar DA-related downregulation mechanisms rendering deviant experiences more tolerant may operate in clinical psychoses. We speculate this may be the case for certain subgroups according to some latent factors. In addition to D2-like receptor availability, corticostriatal inhibitory and excitatory influences, which seem to co-vary with positive symptomology,146 may be the biological factors underlying such clinical differentiation. Notably, phenomena such as supersensitivity psychosis indicate the dynamic and compensatory nature of D2-like receptors, which are currently not fully understood.147 The transition to clinical manifestation also touches upon the fundamental question of how psychotic-like experiences are positioned within developmental psychopathology. While substantial evidence supports behavioral, cognitive, and neurobiological overlaps across the psychosis spectrum,125,148 recent findings suggest that schizotypy and even more psychotic-like experiences, may share less of a genetic basis with schizophrenia than previously thought.149 Additionally, some brain morphological findings, such as a tendency to have a thicker prefrontal cortex, may reflect putative protective mechanisms.150 In this context, our data complement these intriguing findings, focusing on a direct differential molecular effect of DA neurotransmission.
Study Limitations
These findings are based on male participants, precluding examination of the link between ovarian hormone cycles and DA151, the known sex-dependent variability in striatal DA release,152 and D2/3R binding characteristics.153 Moreover, the retrospective measures used may be susceptible to memory bias. Nevertheless, self-reported abuse and trauma tend to be stable across time, independent of current symptomology, and may be reliably collected from healthy individuals and patients.154 The magnitude of symptoms spanned low to medium values of the measures utilized, suggesting we did not explore more pronounced symptomology. However, this somewhat limited range is as expected because we screened for explicitly healthy individuals and stronger symptoms might correspond to clinical portions of the spectrum. The results are presented in terms of statistical significance, which may not fully overlap with clinical relevance, understood as the impact on clinical practice.155 Nevertheless, differences between participants with high and low D2/3R availability in the putamen in our sample reached nearly 40%, which seems to be a considerable level. While the sample size is within the range typical for PET studies, given the costs and exposure to radioactivity, this remains a limitation. Furthermore, the genetic component of this work requires replication in a larger sample and should be considered exploratory. Lastly, we could not quantify the relative contribution of D3 receptor binding. However, the expression of D3 receptors and their binding in the dorsal striatum are low.156 Future studies may combine PET tracers acting pre- and postsynaptically, targeting the affinity states of D2 receptors (e.g., with long-lived [18F]-agonist ligands), and involve the radiolabeled DA precursor, depletion of endogenous DA, or pharmacological/task-related challenges. Despite these limitations, this study is a rare effort integrating in vivo measurements of DA with other multilevel factors to elucidate the symptoms at the healthy end of the psychosis spectrum.
Conclusions
These results present an updated proposition on psychotic-like symptom origination. Accordingly, childhood adversity may modulate DA signaling in the putamen, with effects on cognitive-perceptual aberrance linked to a biological predisposition. Although emerging below clinical thresholds, these processes may inform our knowledge of the disease phenotype, adaptive/protective factors, and the scientific understanding of unfounded beliefs.
Supplementary Material
Acknowledgments
We thank Drs Thomas Wyss and Lui Unterrassner for helping in the preparatory phase of this study.
This work was supported by the Donald C. Cooper-Fonds and the Zurich Program for Sustainable Development of Mental Health Services.
Conflict of Interest Statement
Dr Walitza has received royalties from Thieme Hogrefe, Kohlhammer, Springer, and Beltz and lecture honoraria from Opopharma in the last 5 years. Her research has been supported by the Swiss National Science Foundation (SNSF), several EU FP7s, HSM High Specialized Medicine of the Canton Zurich, Switzerland, Bfarm Germany, the Hartmann Müller Foundation, the Olga Mayenfisch Foundation, and the Gertrud Thalmann Foundation. Dr Falkai has been an honorary speaker for Janssen-Cilag, AstraZeneca, Eli Lilly, Bristol-Myers-Squibb, Lundbeck, Pfizer, Bayer Vital, SmithKline Beecham, Wyeth, and Essex. During the last 5 years, but not currently, he was a member of the advisory boards of Janssen-Cilag, AstraZeneca, Eli Lilly, and Lundbeck. All the other authors declare no biomedical financial interests or potential conflicts of interest.
References
- 1.Grace AA. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci. 2016;17(8):524–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63(1):182–217. [DOI] [PubMed] [Google Scholar]
- 3.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophr Bull. 2009;35(3):549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lally J, MacCabe JH. Antipsychotic medication in schizophrenia: a review. Br Med Bull. 2015;114(1):169–179. [DOI] [PubMed] [Google Scholar]
- 5.Seeman P. Dopamine receptor sequences. Therapeutic levels of neuroleptics occupy D2 receptors, clozapine occupies D4. Neuropsychopharmacology. 1992;7(4):261–284. [PubMed] [Google Scholar]
- 6.Kanthan M, Cumming P, Hooker JM, Vasdev N.. Classics in neuroimaging: imaging the dopaminergic pathway With PET. ACS Chem Neurosci. 2017;8:1817–1819. [DOI] [PubMed] [Google Scholar]
- 7.McCutcheon RA, Abi-Dargham A, Howes OD. Schizophrenia, dopamine and the striatum: From biology to symptoms. Trends Neurosci. 2019;42(3):205–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fusar-Poli P, Meyer-Lindenberg A. Striatal presynaptic dopamine in schizophrenia, part II: meta-analysis of [(18)F/(11)C]-DOPA PET studies. Schizophr Bull. 2013;39(1):33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howes OD, Kambeitz J, Kim E, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69(8):776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howes OD, Montgomery AJ, Asselin MC, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66(1):13–20. [DOI] [PubMed] [Google Scholar]
- 11.Hietala J, Syvälahti E, Vilkman H, et al. Depressive symptoms and presynaptic dopamine function in neuroleptic-naive schizophrenia. Schizophr Res. 1999;35(1):41–50. [DOI] [PubMed] [Google Scholar]
- 12.Kuepper R, Skinbjerg M, Abi-Dargham A. The dopamine dysfunction in schizophrenia revisited: new insights into topography and course. In: Gross G, Geyer MA, eds. Current Antipsychotics. Heidelberg: Springer; 2012:1–26. [DOI] [PubMed] [Google Scholar]
- 13.Ito H, Kodaka F, Takahashi H, et al. Relation between presynaptic and postsynaptic dopaminergic functions measured by positron emission tomography: implication of dopaminergic tone. J Neurosci. 2011;31(21):7886–7890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slifstein M, Abi-Dargham A. Is it pre- or postsynaptic? Imaging striatal dopamine excess in schizophrenia. Biol Psychiatry. 2018;83(8):635–637. [DOI] [PubMed] [Google Scholar]
- 15.De Mei C, Ramos M, Iitaka C, Borrelli E. Getting specialized: presynaptic and postsynaptic dopamine D2 receptors. Curr Opin Pharmacol. 2009;9(1):53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egerton A, Chaddock CA, Winton-Brown TT, et al. Presynaptic striatal dopamine dysfunction in people at ultra-high risk for psychosis: findings in a second cohort. Biol Psychiatry. 2013;74(2):106–112. [DOI] [PubMed] [Google Scholar]
- 17.Howes O, Bose S, Turkheimer F, et al. Progressive increase in striatal dopamine synthesis capacity as patients develop psychosis: a PET study. Mol Psychiatry. 2011;16(9):885–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeRosse P, Karlsgodt KH. Examining the psychosis continuum. Curr Behav Neurosci Rep. 2015;2(2):80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39(2):179–195. [DOI] [PubMed] [Google Scholar]
- 20.Cannon TD. The current state of the clinical high risk for psychosis research paradigm. Biol Psychiatry. 2020;88(4):284–286. [DOI] [PubMed] [Google Scholar]
- 21.Mennigen E, Bearden CE. Psychosis risk and development: what do we know from population-based studies? Biol Psychiatry. 2020;88(4):315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor JH, Calkins ME, Gur RE. Markers of psychosis risk in the general population. Biol Psychiatry. 2020;88(4):337–348. [DOI] [PubMed] [Google Scholar]
- 23.Caravaggio F, Fervaha G, Chung JK, et al. Exploring personality traits related to dopamine D2/3 receptor availability in striatal subregions of humans. Eur Neuropsychopharmacol. 2016;26(4):644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwapil TR, Barrantes-Vidal N. Schizotypy: looking back and moving forward. Schizophr Bull. 2015;41 (Suppl 2):S366–S373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howes OD, Kapur S. A neurobiological hypothesis for the classification of schizophrenia: type A (hyperdopaminergic) and type B (normodopaminergic). Br J Psychiatry. 2014;205(1):1–3. [DOI] [PubMed] [Google Scholar]
- 26.Farooq S, Agid O, Foussias G, Remington G.. Using treatment response to subtype schizophrenia: proposal for a new paradigm in classification. Schizophr Bull. 2013;39(6):1169–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160(1):13–23. [DOI] [PubMed] [Google Scholar]
- 28.Winton-Brown TT, Fusar-Poli P, Ungless MA, Howes OD. Dopaminergic basis of salience dysregulation in psychosis. Trends Neurosci. 2014;37(2):85–94. [DOI] [PubMed] [Google Scholar]
- 29.Egerton A, Valmaggia LR, Howes OD, et al. Adversity in childhood linked to elevated striatal dopamine function in adulthood. Schizophr Res. 2016;176(2-3):171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laruelle M. The role of endogenous sensitization in the pathophysiology of schizophrenia: implications from recent brain imaging studies. Brain Res Brain Res Rev. 2000;31(2-3):371–384. [DOI] [PubMed] [Google Scholar]
- 31.Velikonja T, Fisher HL, Mason O, Johnson S. Childhood trauma and schizotypy: a systematic literature review. Psychol Med. 2015;45(5):947–963. [DOI] [PubMed] [Google Scholar]
- 32.Varese F, Smeets F, Drukker M, et al. Childhood adversities increase the risk of psychosis: a meta-analysis of patient-control, prospective- and cross-sectional cohort studies. Schizophr Bull. 2012;38(4):661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmack K, Rössler H, Sekutowicz M, et al. Linking unfounded beliefs to genetic dopamine availability. Front Hum Neurosci. 2015;9:521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gluskin BS, Mickey BJ. Genetic variation and dopamine D2 receptor availability: a systematic review and meta-analysis of human in vivo molecular imaging studies. Transl Psychiatry. 2016;6:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caspi A, Moffitt TE. Gene–environment interactions in psychiatry: joining forces with neuroscience. Nat Rev Neurosci. 2006;7(7):583–590. [DOI] [PubMed] [Google Scholar]
- 36.Howes OD, McCutcheon R, Owen MJ, Murray RM. The role of genes, stress, and dopamine in the development of schizophrenia. Biol Psychiatry. 2017;81(1):9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Unterrassner L, Wyss TA, Wotruba D, Ajdacic-Gross V, Haker H, Rössler W. Psychotic-like experiences at the healthy end of the psychosis continuum. Front Psychol. 2017;8:775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(20):22–33. [PubMed] [Google Scholar]
- 39.Hall H, Köhler C, Gawell L, Farde L, Sedvall G. Raclopride, a new selective ligand for the dopamine-D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12(5):559–568. [DOI] [PubMed] [Google Scholar]
- 40.Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4(3 Pt 1):153–158. [DOI] [PubMed] [Google Scholar]
- 41.Suhara T, Sudo Y, Okauchi T, et al. Extrastriatal dopamine D2 receptor density and affinity in the human brain measured by 3D PET. Int J Neuropsychopharmacol. 1999;2(2):73–82. [DOI] [PubMed] [Google Scholar]
- 42.Innis RB, Cunningham VJ, Delforge J, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27(9):1533–1539. [DOI] [PubMed] [Google Scholar]
- 43.Graff-Guerrero A, Willeit M, Ginovart N, et al. Brain region binding of the D2/3 agonist [11C]-(+)-PHNO and the D2/3 antagonist [11C]raclopride in healthy humans. Hum Brain Mapp. 2008;29(4):400–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Landolt K, Wittwer A, Wyss T, et al. Help-seeking in people with exceptional experiences: results from a general population sample. Front Public Health. 2014;2:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fach W, Atmanspacher H, Landolt K, Wyss T, Rössler W. A comparative study of exceptional experiences of clients seeking advice and of subjects in an ordinary population. Front Psychol. 2013;4:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rössler J, Rössler W, Seifritz E, et al. Dopamine-induced dysconnectivity between salience network and auditory cortex in subjects with psychotic-like experiences: a randomized double-blind placebo-controlled study. Schizophr Bull. 2020;46(3):732–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raine A. The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr Bull. 1991;17(4):555–564. [DOI] [PubMed] [Google Scholar]
- 48.Raine A, Reynolds C, Lencz T, Scerbo A, Triphon N, Kim D. Cognitive-perceptual, interpersonal, and disorganized features of schizotypal personality. Schizophr Bull. 1994;20(1):191–201. [DOI] [PubMed] [Google Scholar]
- 49.Bernstein DP, Stein JA, Newcomb MD, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27(2):169–190. [DOI] [PubMed] [Google Scholar]
- 50.Klinitzke G, Romppel M, Häuser W, Brähler E, Glaesmer H. The German Version of the Childhood Trauma Questionnaire (CTQ): psychometric characteristics in a representative sample of the general population. Psychother Psychosom Med Psychol. 2012;62(2):47–51. [DOI] [PubMed] [Google Scholar]
- 51.Bernstein DP, Ahluvalia T, Pogge D, Handelsman L. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. J Am Acad Child Adolesc Psychiatry. 1997;36(3):340–348. [DOI] [PubMed] [Google Scholar]
- 52.Dang LC, Samanez-Larkin GR, Castrellon JJ, Perkins SF, Cowan RL, Zald DH. Associations between dopamine D2 receptor availability and BMI depend on age. Neuroimage. 2016;138:176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Azen R, Budescu DV. The dominance analysis approach for comparing predictors in multiple regression. Psychol Methods. 2003;8(2):129–148. [DOI] [PubMed] [Google Scholar]
- 54.Budescu DV. Dominance analysis: a new approach to the problem of relative importance of predictors in multiple regression. Psychol Bull. 1993;114(3):542. [Google Scholar]
- 55.Bustos N, Coutinho S.. dominanceanalysis: Dominance Analysis. R package version 1.3.0.; 2010. [Google Scholar]
- 56.Johnson JW, LeBreton JM. History and use of relative importance indices in organizational research. Organ Res Methods 2004;7(3):238–257. [Google Scholar]
- 57.Nimon KF, Oswald FL. Understanding the results of multiple linear regression: Beyond standardized regression coefficients. Organ Res Methods 2013;16(4):650–674. [Google Scholar]
- 58.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. [DOI] [PubMed] [Google Scholar]
- 59.Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. Mediation: R package for causal mediation analysis. J Stat Softw. 2014;59(5):1–38.26917999 [Google Scholar]
- 60.Imai K, Keele L, Yamamoto T. Identification, inference and sensitivity analysis for causal mediation effects. Statistical science. 2010;25(1):51–71. [Google Scholar]
- 61.Hicks R, Tingley D. Causal mediation analysis. Stata J. 2011;11(4):605–619. [Google Scholar]
- 62.Azen R. Using dominance analysis to estimate predictor importance in multiple regression. In: Petscher Y, Schatschneider C, Compton DL, eds. Applied Quantitative Analysis in Education and the Social Sciences. New York, NY:Routledge; 2013:34–64. [Google Scholar]
- 63.Zu J, Yuan KH. Local influence and robust procedures for mediation analysis. Multivariate Behav Res. 2010;45(1):1–44. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Z, Zheng C, Kim C, Van Poucke S, Lin S, Lan P. Causal mediation analysis in the context of clinical research. Ann Transl Med. 2016;4(21):425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dillon DG, Holmes AJ, Birk JL, Brooks N, Lyons-Ruth K, Pizzagalli DA. Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biol Psychiatry. 2009;66(3):206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carlson JN, Fitzgerald LW, Keller RW Jr, Glick SD. Lateralized changes in prefrontal cortical dopamine activity induced by controllable and uncontrollable stress in the rat. Brain Res. 1993;630(1-2):178–187. [DOI] [PubMed] [Google Scholar]
- 67.Walker E, Mittal V, Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu Rev Clin Psychol. 2008;4:189–216. [DOI] [PubMed] [Google Scholar]
- 68.Anstrom KK, Miczek KA, Budygin EA. Increased phasic dopamine signaling in the mesolimbic pathway during social defeat in rats. Neuroscience. 2009;161(1):3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem. 1989;52(5):1655–1658. [DOI] [PubMed] [Google Scholar]
- 70.Selten JP, van der Ven E, Rutten BP, Cantor-Graae E. The social defeat hypothesis of schizophrenia: an update. Schizophr Bull. 2013;39(6):1180–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Howes OD, Murray RM. Schizophrenia: an integrated sociodevelopmental-cognitive model. Lancet. 2014;383(9929):1677–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Unterrassner L, Wyss TA, Wotruba D, Haker H, Rössler W. The intricate relationship between psychotic-like experiences and associated subclinical symptoms in healthy individuals. Front Psychol. 2017;8:1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rössler W, Ajdacic-Gross V, Rodgers S, Haker H, Müller M. Childhood trauma as a risk factor for the onset of subclinical psychotic experiences: exploring the mediating effect of stress sensitivity in a cross-sectional epidemiological community study. Schizophr Res. 2016;172(1−3):46–53. [DOI] [PubMed] [Google Scholar]
- 74.Rössler W, Hengartner MP, Ajdacic-Gross V, Haker H, Angst J. Impact of childhood adversity on the onset and course of subclinical psychosis symptoms–results from a 30-year prospective community study. Schizophr Res. 2014;153(1−3):189–195. [DOI] [PubMed] [Google Scholar]
- 75.Misiak B, Krefft M, Bielawski T, Moustafa AA, Sąsiadek MM, Frydecka D. Toward a unified theory of childhood trauma and psychosis: a comprehensive review of epidemiological, clinical, neuropsychological and biological findings. Neurosci Biobehav Rev. 2017;75:393–406. [DOI] [PubMed] [Google Scholar]
- 76.Read J, van Os J, Morrison AP, Ross CA. Childhood trauma, psychosis and schizophrenia: a literature review with theoretical and clinical implications. Acta Psychiatr Scand. 2005;112(5):330–350. [DOI] [PubMed] [Google Scholar]
- 77.Gray NS, Pickering AD, Gray JA. Psychoticism and dopamine D2 binding in the basal ganglia using single photon emission tomography. Personal Indiv Differ. 1994;17(3):431–434. [Google Scholar]
- 78.Grant P, Green MJ, Mason OJ. Models of Schizotypy: The importance of conceptual clarity. Schizophr Bull. 2018;44(Suppl 2):S556–S563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Breier A, Kestler L, Adler C, et al. Dopamine D2 receptor density and personal detachment in healthy subjects. Am J Psychiatry. 1998;155(10):1440–1442. [DOI] [PubMed] [Google Scholar]
- 80.Farde L, Gustavsson JP, Jönsson E. D2 dopamine receptors and personality traits. Nature. 1997;385(6617):590. [DOI] [PubMed] [Google Scholar]
- 81.Chen KC, Lee IH, Yeh TL, et al. Schizotypy trait and striatal dopamine receptors in healthy volunteers. Psychiatry Res. 2012;201(3):218–221. [DOI] [PubMed] [Google Scholar]
- 82.Frankle WG, Narendran R. Distinguishing schizophrenia subtypes: can dopamine imaging improve the signal-to-noise ratio? Biol Psychiatry. 2020;87(3):197–199. [DOI] [PubMed] [Google Scholar]
- 83.Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41(1):1–24. [DOI] [PubMed] [Google Scholar]
- 84.Seeman P, Kapur S. Schizophrenia: more dopamine, more D2 receptors. Proc Natl Acad Sci U.S.A. 2000;97(14):7673–7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Seeman P. All roads to schizophrenia lead to dopamine supersensitivity and elevated dopamine D2(high) receptors. CNS Neurosci Ther. 2011;17(2):118–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kubota M, Nagashima T, Takano H, et al. Affinity states of striatal dopamine D2 receptors in antipsychotic-free patients with schizophrenia. Int J Neuropsychopharmacol. 2017;20(11):928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Caravaggio F, Iwata Y, Kim J, et al. What proportion of striatal D2 receptors are occupied by endogenous dopamine at baseline? A meta-analysis with implications for understanding antipsychotic occupancy. Neuropharmacology. 2020;163:107591. [DOI] [PubMed] [Google Scholar]
- 88.Pogarell O, Koch W, Karch S, et al. Dopaminergic neurotransmission in patients with schizophrenia in relation to positive and negative symptoms. Pharmacopsychiatry. 2012;45(Suppl 1):S36–S41. [DOI] [PubMed] [Google Scholar]
- 89.Corripio I, Pérez V, Catafau AM, Mena E, Carrió I, Alvarez E. Striatal D2 receptor binding as a marker of prognosis and outcome in untreated first-episode psychosis. Neuroimage. 2006;29(2):662–666. [DOI] [PubMed] [Google Scholar]
- 90.Tune LE, Wong DF, Pearlson G, et al. Dopamine D2 receptor density estimates in schizophrenia: a positron emission tomography study with 11C-N-methylspiperone. Psychiatry Res. 1993;49(3):219–237. [DOI] [PubMed] [Google Scholar]
- 91.Nordstrom A-L, Farde L, Eriksson L, Halldin C. No elevated D2 dopamine receptors in neuroleptic-naive schizophrenic patients revealed by positron emission tomography and [11C] N-methylspiperone. Psychiatry Res: Neuroimag. 1995;61(2):67–83. [DOI] [PubMed] [Google Scholar]
- 92.Talvik M, Nordström AL, Okubo Y, et al. Dopamine D2 receptor binding in drug-naïve patients with schizophrenia examined with raclopride-C11 and positron emission tomography. Psychiatry Res. 2006;148(2−3):165–173. [DOI] [PubMed] [Google Scholar]
- 93.Nakajima S, Caravaggio F, Mamo DC, et al. Dopamine D2/3 receptor availability in the striatum of antipsychotic-free older patients with schizophrenia-A [¹¹C]-raclopride PET study. Schizophr Res. 2015;164(1−3):263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Graff-Guerrero A, Mizrahi R, Agid O, et al. The dopamine D2 receptors in high-affinity state and D3 receptors in schizophrenia: a clinical [11C]-(+)-PHNO PET study. Neuropsychopharmacology. 2009;34(4):1078–1086. [DOI] [PubMed] [Google Scholar]
- 95.Schmitt GJ, Meisenzahl EM, Frodl T, et al. Increase of striatal dopamine transmission in first episode drug-naive schizophrenic patients as demonstrated by [(123)I]IBZM SPECT. Psychiatry Res. 2009;173(3):183–189. [DOI] [PubMed] [Google Scholar]
- 96.Brugger SP, Angelescu I, Abi-Dargham A, Mizrahi R, Shahrezaei V, Howes OD. Heterogeneity of striatal dopamine function in schizophrenia: meta-analysis of variance. Biol Psychiatry. 2020;87(3):215–224. [DOI] [PubMed] [Google Scholar]
- 97.Hietala J, Syvälahti E, Vuorio K, et al. Striatal D2 dopamine receptor characteristics in neuroleptic-naive schizophrenic patients studied with positron emission tomography. Arch Gen Psychiatry. 1994;51(2):116–123. [DOI] [PubMed] [Google Scholar]
- 98.Papenberg G, Karalija N, Salami A, et al. Balance between transmitter availability and dopamine D2 receptors in prefrontal cortex influences memory functioning. Cereb Cortex. 2020;30(3):989–1000. [DOI] [PubMed] [Google Scholar]
- 99.van Holstein M, Aarts E, van der Schaaf ME, et al. Human cognitive flexibility depends on dopamine D2 receptor signaling. Psychopharmacology (Berl.) 2011;218(3):567–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Persson J, Stenfors C. Superior cognitive goal maintenance in carriers of genetic markers linked to reduced striatal D2 receptor density (C957T and DRD2/ANKK1-TaqIA). PLoS One. 2018;13(8):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Beitman BD. Brains seek patterns in coincidences. Psychiatric Ann. 2009;39(5):255–264. [Google Scholar]
- 102.Whitson JA, Galinsky AD. Lacking control increases illusory pattern perception. Science. 2008;322(5898):115–117. [DOI] [PubMed] [Google Scholar]
- 103.Papenberg G, Jonasson L, Karalija N, et al. Mapping the landscape of human dopamine D2/3 receptors with [11 C] raclopride. Brain Struct. Funct. 2019;224(8):2871–2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hall H, Sedvall G, Magnusson O, Kopp J, Halldin C, Farde L. Distribution of D1- and D2-dopamine receptors, and dopamine and its metabolites in the human brain. Neuropsychopharmacology. 1994;11(4):245–256. [DOI] [PubMed] [Google Scholar]
- 105.Farid F, Mahadun P. Schizophrenia-like psychosis following left putamen infarct: a case report. J Med Case Rep. 2009;3(1):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nagara T, Ohara H, Yano K. Disappearance of hallucinations and delusions following left putaminal hemorrhage in a case of schizophrenia. Seishin Shinkeigaku Zasshi. 1996;98:498. [Google Scholar]
- 107.Huang X, Pu W, Li X, et al. Decreased left putamen and thalamus volume correlates with delusions in first-episode schizophrenia patients. Front Psychiatry. 2017;8:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hong SB, Lee TY, Kwak YB, Kim SN, Kwon JS. Baseline putamen volume as a predictor of positive symptom reduction in patients at clinical high risk for psychosis: a preliminary study. Schizophr Res. 2015;169(1−3):178–185. [DOI] [PubMed] [Google Scholar]
- 109.Raij TT, Mäntylä T, Kieseppä T, Suvisaari J. Aberrant functioning of the putamen links delusions, antipsychotic drug dose, and compromised connectivity in first episode psychosis–Preliminary fMRI findings. Psychiatry Res. 2015;233(2):201–211. [DOI] [PubMed] [Google Scholar]
- 110.Chemerinski E, Byne W, Kolaitis JC, et al. Larger putamen size in antipsychotic-naïve individuals with schizotypal personality disorder. Schizophr Res. 2013;143(1):158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Woodward ND, Zald DH, Ding Z, et al. Cerebral morphology and dopamine D2/D3 receptor distribution in humans: a combined [18F]fallypride and voxel-based morphometry study. Neuroimage. 2009;46(1):31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Caravaggio F, Ku Chung J, Plitman E, et al. The relationship between subcortical brain volume and striatal dopamine D2/3 receptor availability in healthy humans assessed with [11 C]-raclopride and [11 C]-(+)-PHNO PET. Hum Brain Mapp. 2017;38(11):5519–5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Looi JC, Walterfang M. Striatal morphology as a biomarker in neurodegenerative disease. Mol Psychiatry. 2013;18(4):417–424. [DOI] [PubMed] [Google Scholar]
- 114.Rössler J, Unterassner L, Wyss T, et al. Schizotypal traits are linked to dopamine-induced striato-cortical decoupling: a randomized double-blind placebo-controlled study. Schizophr Bull. 2019;45(3):680–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Oyama K, Hernádi I, Iijima T, Tsutsui K. Reward prediction error coding in dorsal striatal neurons. J Neurosci. 2010;30(34):11447–11457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Griffiths O, Langdon R, Le Pelley ME, Coltheart M. Delusions and prediction error: re-examining the behavioural evidence for disrupted error signalling in delusion formation. Cogn Neuropsychiatry. 2014;19(5):439–467. [DOI] [PubMed] [Google Scholar]
- 117.Usiello A, Baik JH, Rougé-Pont F, et al. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 2000;408(6809):199–203. [DOI] [PubMed] [Google Scholar]
- 118.Luykx JJ, Broersen JL, de Leeuw M. The DRD2 rs1076560 polymorphism and schizophrenia-related intermediate phenotypes: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2017;74(Pt A):214–224. [DOI] [PubMed] [Google Scholar]
- 119.Zhang Y, Bertolino A, Fazio L, et al. Polymorphisms in human dopamine D2 receptor gene affect gene expression, splicing, and neuronal activity during working memory. Proc Natl Acad Sci USA. 2007;104(51):20552–20557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Walker EF, Diforio D. Schizophrenia: a neural diathesis-stress model. Psychol Rev. 1997;104(4):667–685. [DOI] [PubMed] [Google Scholar]
- 121.Suchanecka A, Chmielowiec J, Chmielowiec K, et al. Dopamine receptor DRD2 gene rs1076560, personality traits and anxiety in the polysubstance use disorder. Brain Sciences 2020;10(5):262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Si S, Su Y, Zhang S, Zhang J. Genetic susceptibility to parenting style: DRD2 and COMT influence creativity. Neuroimage. 2020;213:116681. [DOI] [PubMed] [Google Scholar]
- 123.Hoffmann C, Van Rheenen TE, Mancuso SG, et al. Exploring the moderating effects of dopaminergic polymorphisms and childhood adversity on brain morphology in schizophrenia-spectrum disorders. Psychiatry Res Neuroimaging. 2018;281:61–68. [DOI] [PubMed] [Google Scholar]
- 124.Taurisano P, Romano R, Mancini M, et al. Prefronto-striatal physiology is associated with schizotypy and is modulated by a functional variant of DRD2. Front Behav Neurosci. 2014;8:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nelson MT, Seal ML, Pantelis C, Phillips LJ. Evidence of a dimensional relationship between schizotypy and schizophrenia: a systematic review. Neurosci Biobehav Rev. 2013;37(3):317–327. [DOI] [PubMed] [Google Scholar]
- 126.Rössler W, Hengartner MP, Ajdacic-Gross V, Haker H, Angst J. Deconstructing sub-clinical psychosis into latent-state and trait variables over a 30-year time span. Schizophr Res. 2013;150(1):197–204. [DOI] [PubMed] [Google Scholar]
- 127.Lee KW, Chan KW, Chang WC, Lee EHM, Hui CLM, Chen EYH. A systematic review on definitions and assessments of psychotic-like experiences. Early Interv Psychiatry. 2016;10(1):3–16. [DOI] [PubMed] [Google Scholar]
- 128.Yoder KK, Kareken DA, Morris ED. What were they thinking? Cognitive states may influence [11C]raclopride binding potential in the striatum. Neurosci Lett. 2008;430(1):38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bourgin J, Tebeka S, Mallet J, Mazer N, Dubertret C, Le Strat Y. Prevalence and correlates of psychotic-like experiences in the general population. Schizophr Res. 2020;215:371–377. [DOI] [PubMed] [Google Scholar]
- 130.Linscott RJ, van Os J. An updated and conservative systematic review and meta-analysis of epidemiological evidence on psychotic experiences in children and adults: on the pathway from proneness to persistence to dimensional expression across mental disorders. Psychol Med. 2013;43(6):1133–1149. [DOI] [PubMed] [Google Scholar]
- 131.Brotherton R, French CC. Belief in conspiracy theories and susceptibility to the conjunction fallacy. Appl Cogn Psychol. 2014;28(2):238–248. [Google Scholar]
- 132.Boden MT, Berenbaum H, Topper M. Intuition, affect, and peculiar beliefs. Pers Individ Dif. 2012;52(7):845–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stephens GL, Graham G. Reconceiving delusion. Int Rev Psychiatry. 2004;16(3):236–241. [DOI] [PubMed] [Google Scholar]
- 134.Broyd A, Balzan RP, Woodward TS, Allen P. Dopamine, cognitive biases and assessment of certainty: a neurocognitive model of delusions. Clin Psychol Rev. 2017;54:96–106. [DOI] [PubMed] [Google Scholar]
- 135.Dudley R, Taylor P, Wickham S, Hutton P. Psychosis, delusions and the “Jumping to Conclusions” reasoning bias: a systematic review and meta-analysis. Schizophr Bull. 2016;42(3):652–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Balzan R, Delfabbro P, Galletly C, Woodward T. Confirmation biases across the psychosis continuum: the contribution of hypersalient evidence-hypothesis matches. Br J Clin Psychol. 2013;52(1):53–69. [DOI] [PubMed] [Google Scholar]
- 137.McKay R, Langdon R, Coltheart M. Need for closure, jumping to conclusions, and decisiveness in delusion-prone individuals. J Nerv Ment Dis. 2006;194(6):422–426. [DOI] [PubMed] [Google Scholar]
- 138.Woodward TS, Buchy L, Moritz S, Liotti M. A bias against disconfirmatory evidence is associated with delusion proneness in a nonclinical sample. Schizophr Bull. 2007;33(4):1023–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fiedler K, Bless H.. The formation of beliefs at the interface of affective and cognitive processes. In: Frijda NH, Manstead AS, Bem S, eds. Emotions and Beliefs: How Feelings Influence Thought. Cambridge:Cambridge University Press. : 2000;144–170. [Google Scholar]
- 140.Sharot T, Garrett N. Forming beliefs: why valence matters. Trends Cogn Sci. 2016;20(1):25–33. [DOI] [PubMed] [Google Scholar]
- 141.Connors MH, Halligan PW. A cognitive account of belief: a tentative road map. Front Psychol. 2014;5:1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hanssen M, Bak M, Bijl R, Vollebergh W, van Os J. The incidence and outcome of subclinical psychotic experiences in the general population. Br J Clin Psychol. 2005;44(Pt 2):181–191. [DOI] [PubMed] [Google Scholar]
- 143.Fumero A, Marrero RJ, Fonseca-Pedrero E. Well-being in schizotypy: the effect of subclinical psychotic experiences. Psicothema. 2018;30(2):177–182. [DOI] [PubMed] [Google Scholar]
- 144.Mohr C, Claridge G. Schizotypy—do not worry, it is not all worrisome. Schizophr Bull. 2015;41(Suppl 2):S436– S443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kuranova A, Booij SH, de Jonge P, et al. Don’t worry, be happy: protective factors to buffer against distress associated with psychotic experiences. Schizophr Res. 2020;223:79–86. [DOI] [PubMed] [Google Scholar]
- 146.Limongi R, Mackinley M, Dempster K, Khan AR, Gati JS, Palaniyappan L. Frontal–striatal connectivity and positive symptoms of schizophrenia: implications for the mechanistic basis of prefrontal rTMS. Eur Arch Psychiatry Clin Neurosci. 2020;271:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yin J, Barr AM, Ramos-Miguel A, Procyshyn RM. Antipsychotic induced dopamine supersensitivity psychosis: a comprehensive review. Curr Neuropharmacol. 2017;15(1):174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ettinger U, Meyhöfer I, Steffens M, Wagner M, Koutsouleris N. Genetics, cognition, and neurobiology of schizotypal personality: a review of the overlap with schizophrenia. Front Psychiatry. 2014;5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nenadić I, Meller T, Schmitt S, et al. Polygenic risk for schizophrenia and schizotypal traits in non-clinical subjects. Psychol Med. 2020;1–11. [DOI] [PubMed] [Google Scholar]
- 150.Kühn S, Schubert F, Gallinat J. Higher prefrontal cortical thickness in high schizotypal personality trait. J Psychiatr Res. 2012;46(7):960–965. [DOI] [PubMed] [Google Scholar]
- 151.Yoest KE, Quigley JA, Becker JB. Rapid effects of ovarian hormones in dorsal striatum and nucleus accumbens. Horm Behav. 2018;104:119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Munro CA, McCaul ME, Wong DF, et al. Sex differences in striatal dopamine release in healthy adults. Biol Psychiatry. 2006;59(10):966–974. [DOI] [PubMed] [Google Scholar]
- 153.Pohjalainen T, Rinne JO, Någren K, Syvälahti E, Hietala J. Sex differences in the striatal dopamine D2 receptor binding characteristics in vivo. Am J Psychiatry. 1998;155(6):768–773. [DOI] [PubMed] [Google Scholar]
- 154.Fisher HL, Craig TK, Fearon P, et al. Reliability and comparability of psychosis patients’ retrospective reports of childhood abuse. Schizophr Bull. 2011;37(3):546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ranganathan P, Pramesh CS, Buyse M. Common pitfalls in statistical analysis: clinical versus statistical significance. Perspect Clin Res. 2015;6(3):169–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Seeman P, Wilson A, Gmeiner P, Kapur S. Dopamine D2 and D3 receptors in human putamen, caudate nucleus, and globus pallidus. Synapse. 2006;60(3):205–211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.