Abstract
Impaired emotional processing and cognitive functioning are common in schizophrenia, schizoaffective disorder, and bipolar disorders, causing significant socioemotional disability. While a large body of research demonstrates abnormal cognition/emotion interactions in these disorders, previous studies investigating abnormalities in the emotional scene response using event-related potentials (ERPs) have yielded mixed findings, and few studies compare findings across psychiatric diagnoses. The current study investigates the effects of emotion and repetition on ERPs in a large, well-characterized sample of participants with schizophrenia-bipolar syndromes. Two ERP components that are modulated by emotional content and scene repetition, the early posterior negativity (EPN) and late positive potential (LPP), were recorded in healthy controls and participants with schizophrenia, schizoaffective disorder, bipolar disorder with psychosis, and bipolar disorder without psychosis. Effects of emotion and repetition were compared across groups. Results displayed significant but small effects in schizophrenia and schizoaffective disorder, with diminished EPN amplitudes to neutral and novel scenes, reduced LPP amplitudes to emotional scenes, and attenuated effects of scene repetition. Despite significant findings, small effect sizes indicate that emotional scene processing is predominantly intact in these disorders. Multivariate analyses indicate that these mild ERP abnormalities are related to cognition, psychosocial functioning, and psychosis severity. This relationship suggests that impaired cognition, rather than diagnosis or mood disturbance, may underlie disrupted neural scene processing in schizophrenia-bipolar syndromes.
Keywords: electroencephalography, psychosis spectrum, affective processing
Introduction
Emotional and cognitive impairments, and their interaction, are key features of schizophrenia-bipolar syndromes, jointly causing significant socioemotional deficits.1–5 Emotional scenes elicit a distinct, well-researched electrophysiological response, which is used to examine emotional processing in these syndromes. These studies indicate little to no evidence for abnormal basic emotional scene processing in psychosis or bipolar disorders,6–10 despite clear socioemotional deficits related to emotion perception in other modalities.1,11 However, studies more consistently indicate abnormal cognition/emotion interactions across the schizophrenia-bipolar spectrum.12–18 In the current study, we examine a possible interaction between emotion and cognition by inspecting electrophysiological suppression and enhancement of the emotional scene response with scene repetitions. This approach could be more relevant to social cognition across the schizophrenia-bipolar spectrum by evaluating response suppression and enhancement in emotional responding.
Emotional scene perception evokes distinct event-related potential (ERP) components indexing motivational and attentional processes, notably the late positive potential (LPP) and early posterior negativity (EPN). The LPP, most evident from approximately 400–900 ms at centro-parietal scalp locations, shows enhanced voltage positivity for emotional relative to neutral scenes.19,20 The EPN, occurring around 150–300 ms in bilateral occipitotemporal regions, shows an enhanced voltage negativity to emotional scenes.21,22
Additionally, researchers have demonstrated that repeated exposures to such emotional scenes moderate the amplitude of neural responses.23–26 Various study designs have been employed to examine the interaction between scene novelty and emotional quality. Results indicate that component amplitudes change with stimulus repetition, and the direction and extent of this change often differs with study design. A review summarizing studies of massed and distributed scene repetitions concludes that mid-latency components (EPN, N2, and P2) are decreased with both forms of repetition, while the LPP is decreased with massed repetitions but enhanced with distributed repetitions.27 Decreases and increases in amplitude can be considered response suppression and enhancement, respectively, though these effects and their mechanisms remain to be fully understood. It is suggested that suppression-like and enhancement-like effects could be due to cognitive processes, such as perceptual fluency and episodic memory.27
Despite these modulatory effects of stimulus repetition, the underlying effect of scene emotion on the LPP remains, showing discrimination between neutral and emotional scenes throughout all study designs, despite hundreds of repetitions.23,24,27 The preservation of this emotion effect demonstrates that 2 different brain processes are at work when viewing emotional scenes: cognitively guided neural suppression/enhancement and the (automatic) emotional response. The depth of the literature on these different neural processes in unselected participants and the large sample size of the current patient study allow us to examine how these different processes might be operating in mood and psychosis syndromes.
In the current study, we examined the effect of repeated exposure to emotional scenes on ERPs in a large, well-characterized group of participants with schizophrenia-bipolar syndromes and in healthy individuals. Clinical groups included schizophrenia, schizoaffective disorder, bipolar I disorder with a lifetime history of psychotic features, and bipolar I disorder without psychotic features. This approach allowed us to assess if findings were psychosis specific or shared with other severe psychiatric syndromes. We compared LPP and EPN responses to neutral, pleasant, and unpleasant scenes between distributed presentations and groups. We hypothesized that LPP amplitudes would be enhanced and EPN amplitudes would be suppressed with multiple presentations; however, these repetition effects would be reduced in clinical groups. Furthermore, as abnormalities in behavioral emotion and cognition measures appear to increase from affective to nonaffective psychosis (facial emotion recognition,3 cognition,2 and perceived scene arousal28), we hypothesized that differences from healthy subjects will follow a stair-step, dimensional pattern with significant and large differences between healthy and nonaffective psychosis (schizophrenia) and small or nonsignificant differences between healthy and affective syndromes (bipolar disorders). Qualitatively, we expected dimensional amplitude reductions and reduced within-subject effects with increasingly nonaffective psychosis.
Methods
Participants
Across 5 Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP 2) sites and 3 Psychosis and Affective Research Domains and Intermediate Phenotypes sites (which were also B-SNIP 2 sites), researchers recruited 429 healthy controls (HC), 272 participants with schizophrenia (SZ), 245 with schizoaffective disorder (SAD), 191 with bipolar I disorder with a lifetime history of psychotic features (BDP), and 73 with bipolar I disorder without a history of psychotic features (BDNP). Trained masters- or doctoral-level clinicians diagnosed participants according to the Structured Clinical Interview for DSM-IV-TR disorders.29 Demographics are provided in table 1, mean scores on clinical scales in supplementary table S1, and medication details in supplementary tables S2–S4.
Table 1.
Demographics
| HC | BDNP | BDP | SAD | SZ | Statistic | P | |
|---|---|---|---|---|---|---|---|
| N | 426 | 73 | 190 | 242 | 268 | F(4,1194) = 12.09*** | <.001 |
| Mean age | 34 | 41 | 37 | 39 | 40 | ||
| Age SD | 12.35 | 12.63 | 11.50 | 11.26 | 11.80 | ||
| Sex (% F) | 58 | 73 | 57 | 55 | 37 | χ 2(4) = 45.41*** | <.001 |
| N from each site | |||||||
| Boston | 90 | 9 | 27 | 27 | 53 | ||
| Chicago | 57 | 0 | 57 | 57 | 51 | ||
| Dallas | 78 | 27 | 42 | 36 | 60 | ||
| Georgia | 97 | 2 | 21 | 49 | 47 | ||
| Hartford | 35 | 35 | 43 | 73 | 57 |
Note. HC, healthy controls; BDNP, bipolar I disorder without psychotic features; BDP, bipolar I disorder with psychotic features; SAD, schizoaffective disorder; SZ, schizophrenia.
Healthy subjects had no history of psychosis, mania, or recurrent depressive episodes, or any first-degree family members with a history of psychotic disorders according to the Family History Research Diagnostic Criteria.30,31 Exclusionary criteria for all participants included current illicit drug use (established by urine toxicology screening), alcohol or substance abuse within 1 month of the study, alcohol or substance dependence within 3 months of the study, an extensive history of past abuse, and any major neurological, cognitive, or major medical disorder affecting the central nervous system. For more information about procedures, see Tamminga et al.32
All subjects provided written informed consent prior to participation after obtaining a complete description of the study procedures. This project was approved by the institutional review board at all participating sites, and procedures were in accordance with the Helsinki Declaration of 2013.
Procedures
Emotional Stimuli
Sixty scenes (exemplars in supplementary figure S1) were obtained through internet searches and consistent with the International Affective Picture System (IAPS).33 Scenes were presented in grayscale with a red focus point in the center and balanced for statistical equivalence (P > .20) in luminance and 90% quality JPEG file size as a rough index of complexity. For additional details on stimulus selection and editing, see Sabatinelli and Frank34 and Frank and Sabatinelli35. Twenty neutral, 20 pleasant, and 20 unpleasant scenes were viewed in pseudo-random order. This 60-picture set was presented in identical order 3 times during data collection, or in 3 blocks, to analyze the effect of scene repetition (supplementary figure S1). Each participant viewed the picture set in the same order and was instructed to passively view scenes with their eyes loosely fixed on the red central fixation point.
Data Collection
Electroencephalography (EEG) data were recorded using a 10–20 system with 64 sensors, including CB1/CB2, mastoids, nose, and forehead ground (QuikCap, Compumedrics Neuroscan). Sensor impedances were kept below 10 kΩ, and data were sampled at 1000 Hz with a bandpass filter of direct current - 100Hz. During data collection, participants viewed an IAPS scene for 1000 ms followed by 3.5 s of black screen. After the EEG recording, participants rated each scene according to experienced arousal and valence using the Self-Assessment Manikin.36 These ratings were examined for valence and group effects using mixed-design ANOVAs with a 3 (valence: neutral, pleasant, and unpleasant) × 5 (group: HC, SZ, SAD, BDP, and BDNP) design. We applied a Holm–Bonferroni Procedure to these ANOVAs to control for family-wise error rate. Alpha values for each test are reported alongside results. Glass’ Delta effect sizes were calculated using healthy comparisons as the control group.
EEG Data Processing
Raw data were inspected for bad sensor recordings and interpolated in BESA (MEGIS Software) with no more than 5% of channels interpolated per subject. Data were sampled at a rate of 1000 Hz, transformed into an average reference, and digitally filtered from 0.1 (12 dB/oct, zero phase) to 100 Hz (48 dB/oct, zero phase) with a notch filter at 60 Hz and width of 2 Hz. Eye blinks, heart rate, and muscle tension artifacts were minimized using the Independent Components Analysis toolbox in EEGLAB37 under Matlab (MathWorks), with a maximum of 5 components removed per subject. Data were downsampled to 500 Hz, and epochs containing an amplitude greater than 120 μV at any sensor were excluded. No less than 10 trials were included in each subject’s ERP waveform per valence/block combination with an average of 19.12 trials included (SD = 1.41). This number did not significantly differ by group (F[4,1126] = 1.77, P = .13).
ERP Analysis
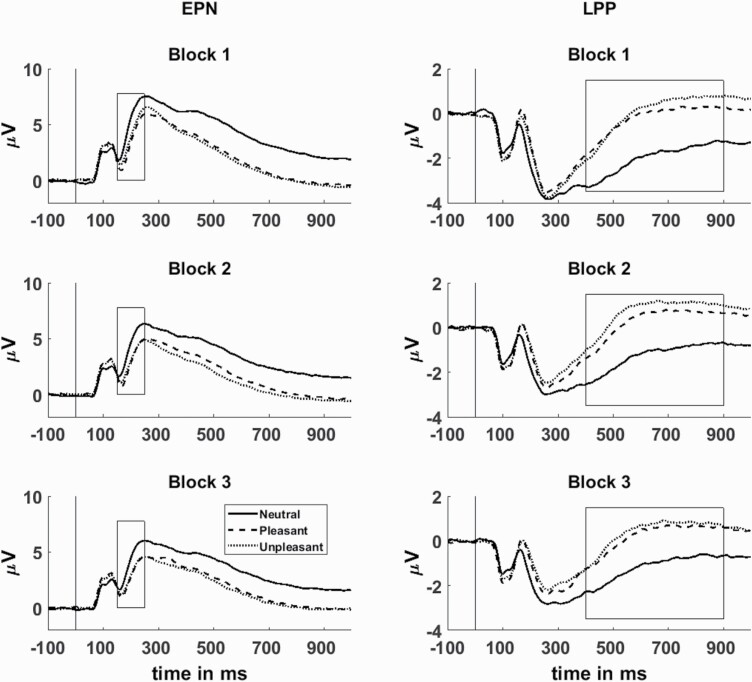
As in our previous publication,10 we chose 6 occipitotemporal sensors (P7, PO7, CB1, P8, PO8, and CB2) averaged over 150–200 ms to quantify the EPN (figure 1; supplementary figure S2). For the LPP, data from 9 centroparietal sensors (FC1, FC2, FCz, C1, C2, Cz, CPz, CP1, and CP2) were averaged over 400–900 ms (figure 1; supplementary figure S2). Data were adjusted for age using the methods described in Dukart et al.38 Mixed-design ANOVAs with a 3 (valence: neutral, pleasant, and unpleasant) × 3 (block: first, second, and third) × 5 (group: HC, SZ, SAD, BDP, and BDNP) design were conducted for each ERP and a Holm–Bonferroni Procedure was applied. Alpha values are reported alongside results. Follow-up Tukey tests were conducted as appropriate and Glass’ Delta effect sizes were calculated using healthy participants as the control group.
Fig. 1.
Grand-average event-related potentials of the occipitoparietal early posterior negativity (EPN; left) and centroparietal late positive potential (LPP; right). Analyzed time ranges and are shown surrounded by a box (EPN: 150–250 ms, LPP: 400–900 ms). Blocks 1, 2, and 3 are shown descending. Neutral scene response is depicted with a solid line, pleasant with a dashed line, and unpleasant with a dotted line.
Canonical Correlation for Relationship With Clinical Measures
To assess bidirectional relationships between emotional ERP measures, clinical symptoms, functioning, and cognition, we performed a canonical correlation analysis (CCA) across all groups. CCA is a data-driven multivariate approach that identifies the relationship between 2 sets of variables by maximizing correlations between “predictor” and “criterion” variable sets.39 CCA is particularly useful when there are high intercorrelations within variable sets and the relationship between variable sets is nondirectional/biorthogonal.39 Results of a CCA are correlated pairs of latent variates. Each pair is independent and composed of weighted sums of the predictor variables that maximally correlate with the weighted sums of the criterion variables. Interpretation of what the latent variates represent and how they are related to each other can be determined by the weighted sums or loadings of individual measures on the latent structure, much like principal components analysis.10,40–43 In the present study, the first set included emotional ERP measures (for each block of neutral, pleasant, and unpleasant; total of 18 variables) and the second set included clinician-rated behavioral and cognitive measures (positive and negative psychosis symptoms, mania, depression, anxiety, psychosocial functioning, and cognition; details on scales are in supplementary table S1). These dimensional measures of clinically relevant features are more statistically powerful than categorical distinctions (ie, active mania vs euthymia).
Results
EPN Amplitude
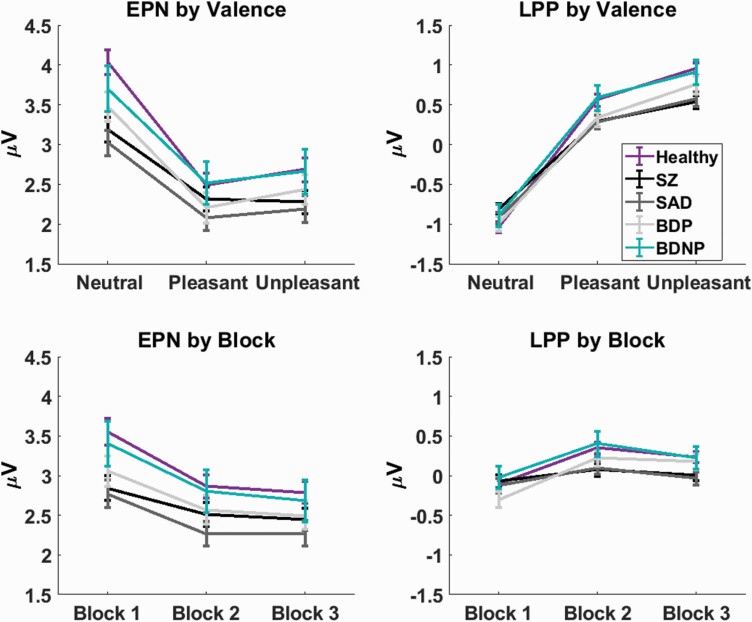
At the EPN, there was not a significant main effect of group (F[4,1194] = 2.69, P = .03, α = .0125) or a 3-way interaction (F[16,4776] =1.51, P = .09, α = .0125). Group by valence (F[8,2388] = 11.02, P < .001, α = .025) and group by block interactions (F[8,2388] = 3.50, P =.001, α = .017) were significant (figure 2). As expected, the EPN had enhanced negativity for emotional scenes (P < .001) and decreases in amplitude with each consecutive block (P < .001), indicating enhanced early processing of emotional scenes and response suppression with decreased stimulus novelty.
Fig. 2.
Event-related potential amplitudes arranged by measure and effect. Early posterior negativity (EPN) amplitudes are to the left, late positive potential (LPP) to the right, valence effects at the top, and block effects at the bottom. Healthy control is in purple, schizophrenia (SZ) in black, schizoaffective disorder (SAD) in dark gray, bipolar I disorder with psychotic features (BDP) in light gray, and bipolar I disorder without psychotic features (BDNP) in teal. Line plots are shown with 1 SE.
EPN: Group × Valence Interaction
Neutral scene responses differentiated groups (figure 2; P < .001), with HC > SZ (P = .001, Glass’ Δ = .27) and SAD (P < .001, Glass’ Δ = .32), indicating reduced processing of neutral scenes in schizophrenia and schizoaffective disorder. All groups displayed significant main effects of valence (all P < .001); however, only HC and BDP displayed significantly higher pleasant than unpleasant response amplitudes (both P < .001), suggesting that SZ and SAD could have reduced sensitivity to differences in arousal between pleasant and unpleasant scene categories or a diminished emotional response.
EPN: Group × Block Interaction
Block 1 showed a significant difference between groups (P = .004; figure 2). Tukey tests showed that HC > SZ (P = .02, Glass’ Δ = .20) and SAD (P = .01, Glass’ Δ = .22), indicating reduced responses to novel stimuli. All groups displayed significant main effects of block with block 1 > blocks 2 and 3 (all P < .001), demonstrating intact response suppression of the EPN among psychiatric groups.
LPP Amplitude
There was not a significant main effect of group (F[4,1194] = 1.22, P = .30, α = .025) or 3-way interaction (F[16,4776] = 1.16, P = .29, α = .017), but there was a significant group by valence interaction (F[8,2388] = 11.71, P < .001, α = .017) and group by block interaction (F[8,2388] = 7.43, P < .001, α = .0125; figure 2). Within-subject effects were significant and in expected directions, with increased amplitudes to emotional scenes (P < .001) and an increase in amplitude from block 1 to 2 (P < .001), followed by a decrease from block 2 to 3 (P < .001). These effects indicate enhanced elaborative processing of emotional scenes and initial response sensitization followed by suppression.
LPP: Group × Valence Interaction
Pleasant and unpleasant scene responses significantly differentiated groups (P = .04, .001; figure 2). For pleasant responses, uncorrected t-tests indicated that HC > SZ and SAD (both P = .02, Glass’ Δ: SZ = .17, SAD = .17). For unpleasant responses, Tukey tests showed that HC > SZ (P = .003, Glass’ Δ = .26) and SAD (P = .01, Glass’ Δ = .24). All groups showed significant effects of valence, with unpleasant responses > pleasant > neutral (all P < .001). In sum, results suggest that SZ and SAD have reduced LPPs for pleasant and unpleasant scenes, but their neural discrimination of emotional from neutral scenes is intact.
LPP: Block × Group Interaction
All groups displayed a significant effect of block, following a quadratic-like function with the peak amplitude at block 2 (figure 2). However, the difference between blocks 1 and 2 differed across groups, with SZ < HC/BDP/BDNP (all P < .05, Glass’ Δ = .38) and SAD < HC/BDP (both P < .01, Glass’ Δ = .28), suggesting that response enhancement at block 2 was attenuated in these groups.
Relationship With Behavioral Measures
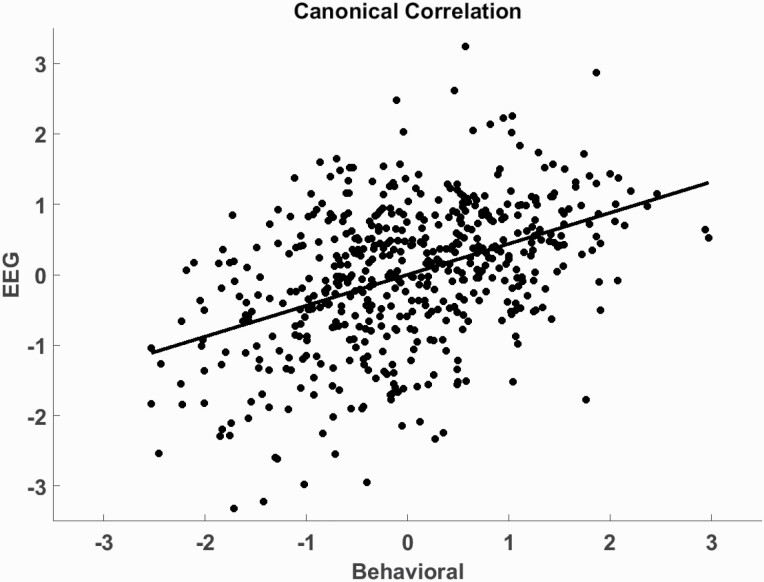
One CCA variate pair was significant (r = .44, F[180,4505.36] = 1.38, P = .001, eigenvalue = .24, Wilks Statistic = .62). Individual scores on the resulting latent variables and a regression line are shown in figure 3. Loadings indicated that low cognition (loading = −.84), low premorbid IQ (loading = −.70), low social (loading = .53) and global functioning (loading = .46), and high psychosis symptoms (loadings = .50, .46, .33 for positive, negative, and general, respectively) are related to lower LPP amplitudes to repeated emotional scenes (loadings = −.46, −.41, −.33, −.31 for unpleasant 3, unpleasant 2, pleasant 3, and pleasant 2, respectively) and lower EPN amplitude to novel neutral scenes (loading = −.31). The overall correlation between resulting latent variables was .44 (P = .001; figure 3).
Fig. 3.
Canonical correlation between emotional electroencephalography (EEG) measures and clinician-rated behavioral measures. The dots depict individual subject scores on the 2 latent variables (“Behavioral” and “EEG”) and the regression line shows the correlation between variables (r = .44).
Self-Report
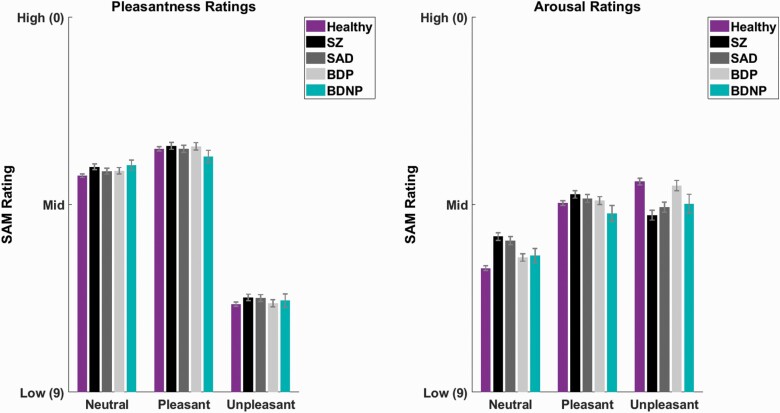
Analysis of self-reported scene ratings (figure 4) did not yield significant main effects of group (pleasantness: F[4,1161] = 2.12, P = .08, α = .017, arousal: F[4,1161] = .87, P = .48, α = .05). Pleasantness ratings did not show a group by valence interaction (F[8,2322] = .78, P = .60, α = .05). However, arousal ratings displayed a significant group by valence interaction (F[8,2322] = 117.50, P < .001, α = .0125).
Fig. 4.
Average pleasantness (left) and arousal ratings (right) on the Self-Assessment Manikin for each group and scene category. Ratings range from 0 to 9 where 9 = low pleasantness/arousal and 0 = high pleasantness/arousal. Healthy control is in purple, schizophrenia (SZ) in black, schizoaffective disorder (SAD) in dark gray, bipolar I disorder with psychotic features (BDP) in light gray, and bipolar I disorder without psychotic features (BDNP) in teal. Neutral, pleasant, and unpleasant categories are shown along the x-axis. Bars are shown with 1 SE.
Arousal: Group × Valence Interaction
Neutral and unpleasant scenes (both P = .001) but not pleasant scenes (P = .09), displayed group differences in arousal (figure 4). For neutral scenes, Tukey tests indicated that SZ/SAD < HC/BDP (all P < .02; Glass’ Δ: SZ = .65, SAD = .56), with lower scores indicating higher arousal. For unpleasant scenes, tests showed that SZ/SAD > HC/BDP (all P < .03; Glass’ Δ: SZ = .49, SAD = .37), with higher scores indicating lower arousal. Results indicate higher arousal to neutral scenes and lower arousal to unpleasant scenes in schizophrenia-like syndromes (figure 4).
Main Effect of Valence
As expected, the main effects of valence were significant for both pleasantness and arousal, with pleasantness showing that pleasant < neutral < unpleasant (all P < .001; lower ratings indicate more perceived pleasantness) and arousal showing that unpleasant and pleasant < neutral (both P < .001; lower ratings indicate more perceived arousal).
Discussion
This study investigated neural responses to scene stimuli in schizophrenia, schizoaffective disorder and bipolar disorder with and without psychosis. The EPN and LPP were used to transdiagnostically examine the effects of emotional content and scene repetition. Results indicated that both emotional processing and response suppression/enhancement are abnormal in schizophrenia and schizoaffective disorder. Furthermore, clinical profiles characterized by cognitive deficit, low functioning, and active psychosis symptoms were most associated with reduced LPPs to repeated emotional scenes and reduced EPNs to novel neutral scenes.
Self-Report
Subjects with SZ and SAD reported higher arousal to neutral scenes and lower arousal to unpleasant scenes, but there were no group differences in perceived pleasantness. Findings support an abnormal subjective experience of emotion in schizophrenia-like psychosis, as demonstrated in previous research with large samples.28,44 The theory of intact hedonic responses in psychosis perhaps explains the lack of significant differences in pleasantness ratings.45 However, recent work suggests that pleasantness perception in nonaffective psychosis may be characterized by higher emotional coactivation.46 Future transdiagnostic work should consider using a bivariate model of pleasantness to investigate emotional coactivation.
Event-related potentials
In the current study, both SZ and SAD displayed EPN response reductions to neutral and novel stimuli. The multivariate correlation showed that these reductions were related to high cognitive deficit, low functioning, and high psychosis symptoms. Results suggest that early emotional scene processing is disrupted for individuals with schizophrenia-like psychoses. The particularly reduced EPN amplitude in response to neutral scenes could reflect inappropriate activation of fear circuitry in response to neutral stimuli, as found in face processing studies.47 This could indicate a generalized hyperactivation of emotional circuitry in emotionally ambiguous situations.
Furthermore, SZ and SAD EPNs also did not show significant differences between pleasant and unpleasant scenes, consistent with the reduced arousal ratings of unpleasant scenes in these groups. Results could reflect reduced sensitivity to unpleasant stimuli or a generally diminished emotional response. BDNP also did not display a significant EPN difference between pleasant and unpleasant scenes, likely due to the smaller sample size (table 1).
SZ and SAD were further characterized by reduced LPP amplitudes to emotional scenes and weakened LPP enhancement at block 2. While emotional LPP amplitude was reduced in schizophrenia-like psychoses, we note that within-subject effects of emotion were still intact. Findings in schizophrenia, therefore, indicate mildly reduced processing of emotional content and attenuated response modulation by repeated exposures. While the exact cause of response suppression and enhancement in this paradigm is not clear, abnormalities could be due to cognitive deficits, such as deficient habituation, memory, or perceptual fluency. The relationship between LPPs to repeated emotional stimuli and cognition is further supported by results of the CCA, where current global cognition and premorbid IQ had the strongest loadings on the latent behavioral variate.
Given the modest effect sizes, it is perhaps not surprising that similar studies with smaller samples have found inconsistent ERP results. Additionally, patterns of significance at the early component and effect sizes in the SAD group indicate that inclusion of this group in schizophrenia samples could boost the likelihood of significant findings in emotional processing studies, emphasizing the importance of transdiagnostic research.
Limitations
Participants were taking a wide range of psychoactive medications. Medications showed few associations with variables, and additional effects of interacting medications cannot be ruled out. However, we note that the treatment of acute psychosis with antipsychotic medication does not appear to alter emotion perception.48 Lithium treatment had associations with all EPN variables (supplementary table S5), with higher response amplitudes in those receiving lithium than those who did not. Observed effects were still present in bipolar disorder alone (all P < .05), so they do not appear to be an effect of diagnosis. While associations did not survive Bonferroni correction and the current cross-sectional study was not designed to probe medication effects, lithium treatment studies should perhaps consider its effects on early visual and emotional processing.
Additionally, participants had a range of illness lengths and varying histories of medication use. While these factors could affect behavioral and neural measures, this diverse sample is reasonably representative of community populations with schizophrenia-bipolar syndromes. Most participants were clinically stable and not experiencing severe mood or psychosis symptoms at the time of testing, limiting the range of symptomology in this sample. Studies of acutely ill patients and first-episode psychosis could usefully explore relationships between ERPs and acute symptomology. To the degree that emotional deficits are more persistent in schizophrenia and state related in bipolar disorder, such studies add knowledge regarding differences in state-related variability across the schizobipolar continuum.
Future Directions
A critical direction of psychiatry is emphasizing biological relevance across diagnostic boundaries. To this end, the B-SNIP consortium has identified 3 biological subtypes of psychosis, “Biotypes,” during the first iteration of the B-SNIP project,49 which display larger between-group biological differences than DSM diagnoses.43,50–52 Biotypes have unique clinical profiles, with one type displaying severe deficits in cognition and social functioning,53 suggesting that this type (biotype 1) could have unique emotional processing abnormalities. Another research group has identified subtypes of psychosis with intact and impaired emotional experience,54 which could reflect biotypes or an additional level of behavioral differences. Once biotypes of participants in the current study are established, we will examine emotional scene processing and other emotional measures (specifically emotion recognition3) in the context of broader dense phenotyping.
Conclusions
This study demonstrated that schizophrenia and schizoaffective disorder share disruptions in the neural processing of repeated emotional scenes. Disruptions were highly related to cognitive deficits rather than mood symptoms or diagnosis, so future work should elaborate on the connection between emotion and cognition. Observed differences were small, indicating that the emotional scene response is largely intact and not optimal for studying socioemotional dysfunction in schizophrenia-bipolar syndromes. Future transdiagnostic work is needed to examine processes with higher relevance to social cognition, such as face processing and affective prosody, and their relevance to behavioral outcomes.
Funding
Funding provided by the National Institute of Mental Health (NIMH): MH096942, MH078113, MH096900, MH103366, MH096913, MH077851, MH096957, MH077945, MH103368.
Supplementary Material
References
- 1.Green MF, Horan WP, Lee J. Social cognition in schizophrenia. Nat Rev Neurosci. 2015;16(10):620–631. [DOI] [PubMed] [Google Scholar]
- 2.Hill SK, Reilly JL, Keefe RS, et al. . Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. Am J Psychiatry. 2013;170(11):1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruocco AC, Reilly JL, Rubin LH, et al. . Emotion recognition deficits in schizophrenia-spectrum disorders and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia network on intermediate phenotypes (B-SNIP) study. Schizophr Res. 2014;158(1–3):105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becerril K, Barch D. Influence of emotional processing on working memory in schizophrenia. Schizophr Bull. 2011;37(5):1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schenkel LS, Passarotti AM, Sweeney JA, Pavuluri MN. Negative emotion impairs working memory in pediatric patients with bipolar disorder type I. Psychol Med. 2012;42(12):2567–2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horan WP, Foti D, Hajcak G, Wynn JK, Green MF. Intact motivated attention in schizophrenia: evidence from event-related potentials. Schizophr Res. 2012;135(1-3):95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horan WP, Wynn JK, Kring AM, Simons RF, Green MF. Electrophysiological correlates of emotional responding in schizophrenia. J Abnorm Psychol. 2010;119(1):18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rockstroh B, Junghöfer M, Elbert T, Buodo G, Miller GA. Electromagnetic brain activity evoked by affective stimuli in schizophrenia. Psychophysiology. 2006;43(5):431–439. [DOI] [PubMed] [Google Scholar]
- 9.Weber K, Miller GA, Schupp HT, et al. . Early life stress and psychiatric disorder modulate cortical responses to affective stimuli. Psychophysiology. 2009;46(6):1234–1243. [DOI] [PubMed] [Google Scholar]
- 10.Trotti RL, Parker DA, Sabatinelli D, et al. . Electrophysiological correlates of emotional scene processing in bipolar disorder. J Psychiatr Res. 2020;120:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green MJ, Cahill CM, Malhi GS. The cognitive and neurophysiological basis of emotion dysregulation in bipolar disorder. J Affect Disord. 2007;103(1-3):29–42. [DOI] [PubMed] [Google Scholar]
- 12.Strauss GP, Llerena K, Gold JM. Attentional disengagement from emotional stimuli in schizophrenia. Schizophr Res. 2011;131(1–3):219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullivan SK, Strauss GP. Electrophysiological evidence for detrimental impact of a reappraisal emotion regulation strategy on subsequent cognitive control in schizophrenia. J Abnorm Psychol. 2017;126(5):679–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strauss GP, Kappenman ES, Culbreth AJ, et al. . Emotion regulation abnormalities in schizophrenia: directed attention strategies fail to decrease the neurophysiological response to unpleasant stimuli. J Abnorm Psychol. 2015;124(2):288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strauss GP, Kappenman ES, Culbreth AJ, Catalano LT, Lee BG, Gold JM. Emotion regulation abnormalities in schizophrenia: cognitive change strategies fail to decrease the neural response to unpleasant stimuli. Schizophr Bull. 2013;39(4):872–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ursu S, Kring AM, Gard MG, et al. . Prefrontal cortical deficits and impaired cognition-emotion interactions in schizophrenia. Am J Psychiatry. 2011;168(3):276–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diaz MT, He G, Gadde S, et al. . The influence of emotional distraction on verbal working memory: an fMRI investigation comparing individuals with schizophrenia and healthy adults. J Psychiatr Res. 2011;45(9):1184–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Passarotti AM, Sweeney JA, Pavuluri MN. Emotion processing influences working memory circuits in pediatric bipolar disorder and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49(10):1064–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biol Psychol. 2000;52(2):95–111. [DOI] [PubMed] [Google Scholar]
- 20.Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37(2):257–261. [PubMed] [Google Scholar]
- 21.Junghöfer M, Bradley MM, Elbert TR, Lang PJ. Fleeting images: a new look at early emotion discrimination. Psychophysiology. 2001;38(2):175–178. [PubMed] [Google Scholar]
- 22.Schupp HT, Junghöfer M, Weike AI, Hamm AO. The selective processing of briefly presented affective pictures: an ERP analysis. Psychophysiology. 2004;41(3):441–449. [DOI] [PubMed] [Google Scholar]
- 23.Codispoti M, Ferrari V, Bradley MM. Repetitive picture processing: autonomic and cortical correlates. Brain Res. 2006;1068(1):213–220. [DOI] [PubMed] [Google Scholar]
- 24.Codispoti M, Ferrari V, Bradley MM. Repetition and event-related potentials: distinguishing early and late processes in affective picture perception. J Cogn Neurosci. 2007;19(4):577–586. [DOI] [PubMed] [Google Scholar]
- 25.Ferrari V, Bradley MM, Codispoti M, Karlsson M, Lang PJ. Repetition and brain potentials when recognizing natural scenes: task and emotion differences. Soc Cogn Affect Neurosci. 2013;8(8):847–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrari V, Bradley MM, Codispoti M, Lang PJ. Massed and distributed repetition of natural scenes: brain potentials and oscillatory activity. Psychophysiology. 2015;52(7):865–872. [DOI] [PubMed] [Google Scholar]
- 27.Ferrari V, Codispoti M, Bradley MM. Repetition and ERPs during emotional scene processing: a selective review. Int J Psychophysiol. 2017;111:170–177. [DOI] [PubMed] [Google Scholar]
- 28.Aminoff SR, Jensen J, Lagerberg TV, Andreassen OA, Melle I. Decreased self-reported arousal in schizophrenia during aversive picture viewing compared to bipolar disorder and healthy controls. Psychiatry Res. 2011;185(3):309–314. [DOI] [PubMed] [Google Scholar]
- 29.First MB, Spitzer RL, Gibbon M, Williams JB.. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P). New York, NY: Biometrics Research, 2002. [Google Scholar]
- 30.Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria. Reliability and validity. Arch Gen Psychiatry. 1977;34(10):1229–1235. [DOI] [PubMed] [Google Scholar]
- 31.Endicott J.Family History: Research Diagnostic Criteria (FH-RDC). New York, NY: New York State Psychiatric Institute, 1978. [Google Scholar]
- 32.Tamminga CA, Ivleva EI, Keshavan MS, et al. . Clinical phenotypes of psychosis in the bipolar-schizophrenia network on intermediate phenotypes (B-SNIP). Am J Psychiatry. 2013;170(11):1263–1274. [DOI] [PubMed] [Google Scholar]
- 33.Lang PJ, Bradley MM, Cuthbert BN.. International Affective Picture System (IAPS): Technical Manual and Affective Ratings. NIMH Center for the Study of Emotion and Attention. Gainesville, FL: 1997. https://www2.unifesp.br/dpsicobio/adap/instructions.pdf. Accessed October 6, 2017. [Google Scholar]
- 34.Sabatinelli D, Frank DW. Assessing the primacy of human amygdala-inferotemporal emotional scene discrimination with rapid whole-brain fMRI. Neuroscience. 2019;406:212–224. [DOI] [PubMed] [Google Scholar]
- 35.Frank DW, Sabatinelli D. Hemodynamic and electrocortical reactivity to specific scene contents in emotional perception. Psychophysiology. 2019;56(6):e13340. [DOI] [PubMed] [Google Scholar]
- 36.Bradley MM, Lang PJ. Measuring emotion: the self-assessment manikin and the semantic differential. J Behav Ther Exp Psychiatry. 1994;25(1):49–59. [DOI] [PubMed] [Google Scholar]
- 37.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. [DOI] [PubMed] [Google Scholar]
- 38.Dukart J, Schroeter ML, Mueller K; Alzheimer’s Disease Neuroimaging Initiative . Age correction in dementia–matching to a healthy brain. PLoS One. 2011;6(7):e22193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lambert Z, Wildt A, Durand R. Redundancy analysis: an alternative to canonical correlation and multivariate multiple regression in exploring interset associations. Psychol Bull 104:282. Psychol Bull. 1988;104(2):282. [Google Scholar]
- 40.Rodrigue AL, McDowell JE, Tandon N, et al. . Multivariate relationships between cognition and brain anatomy across the psychosis spectrum. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(12):992–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parker DA, Hamm JP, McDowell JE, et al. . Auditory steady-state EEG response across the schizo-bipolar spectrum. Schizophr Res. 2019;209:218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parker DA, Trotti RL, McDowell JE, et al. . Auditory paired-stimuli responses across the psychosis and bipolar spectrum and their relationship to clinical features. Biomarkers Neuropsychiatry. 2020;3(April):100014. doi: 10.1016/j.bionps.2020.100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas O, Parker DA, Trotti RL, et al. . Intrinsic neural activity differences in psychosis biotypes: findings from the bipolar-schizophrenia network on intermediate phenotypes (B-SNIP) consortium. Biomarkers Neuropsychiatry. 2019;1(October):100002. doi: 10.1016/j.bionps.2019.100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Llerena K, Strauss GP, Cohen AS. Looking at the other side of the coin: a meta-analysis of self-reported emotional arousal in people with schizophrenia. Schizophr Res. 2012;142(1–3):65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strauss GP, Cohen AS. The schizophrenia spectrum anhedonia paradox. World Psychiatry. 2018;17(2):221–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strauss GP, Frost KH, Lee BG, Gold JM. The positivity offset theory of anhedonia in schizophrenia. Clin Psychol Sci. 2017;5(2):226–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hall J, Whalley HC, McKirdy JW, et al. . Overactivation of fear systems to neutral faces in schizophrenia. Biol Psychiatry. 2008;64(1):70–73. [DOI] [PubMed] [Google Scholar]
- 48.Herbener ES, Hill SK, Marvin RW, Sweeney JA. Effects of antipsychotic treatment on emotion perception deficits in first-episode schizophrenia. Am J Psychiatry. 2005;162(9):1746–1748. [DOI] [PubMed] [Google Scholar]
- 49.Clementz BA, Sweeney JA, Hamm JP, et al. . Identification of distinct psychosis biotypes using brain-based biomarkers. Am J Psychiatry. 2016;173(4):373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hudgens-Haney ME, Ethridge LE, Knight JB, et al. . Intrinsic neural activity differences among psychotic illnesses. Psychophysiology. 2017;54(8):1223–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ivleva EI, Clementz BA, Dutcher AM, et al. . Brain structure biomarkers in the psychosis biotypes: findings from the bipolar-schizophrenia network for intermediate phenotypes. Biol Psychiatry. 2017;82(1):26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meda SA, Clementz BA, Sweeney JA, et al. . Examining functional resting-state connectivity in psychosis and its subgroups in the bipolar-schizophrenia network on intermediate phenotypes cohort. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1(6):488–497. [DOI] [PubMed] [Google Scholar]
- 53.Clementz BA, Trotti RL, Pearlson GD, et al. . Testing psychosis phenotypes from bipolar-schizophrenia network for intermediate phenotypes for clinical application: biotype characteristics and targets. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5(8):808–818. [DOI] [PubMed] [Google Scholar]
- 54.Strauss GP, Allen DN, Miski P, Buchanan RW, Kirkpatrick B, Carpenter WT Jr. Differential patterns of premorbid social and academic deterioration in deficit and nondeficit schizophrenia. Schizophr Res. 2012;135(1–3):134–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.