Abstract
Cerebellar dysfunction is associated with neurological soft signs (NSS), which is a promising endophenotype for schizophrenia spectrum disorders. However, the relationship between cerebellar-cerebral resting-state functional connectivity (rsFC) and NSS is largely unexplored. Moreover, both NSS and cerebellar-cerebral rsFC have been found to be correlated with negative symptoms of schizophrenia. Here, we investigated the correlations between NSS and cerebellar-cerebral rsFC, explored their relationship with negative symptoms in a main dataset, and validated the significant findings in a replication dataset. Both datasets comprised schizophrenia patients and healthy controls. In schizophrenia patients, we found positive correlations between NSS and rsFC of the cerebellum with the inferior frontal gyrus and the precuneus, and negative correlations between NSS and rsFC of the cerebellum with the inferior temporal gyrus. In healthy controls, NSS scores were positively correlated with rsFC of the cerebellum with the superior frontal gyrus and negatively correlated with rsFC between the cerebellum and the middle occipital gyrus. Cerebellar-prefrontal rsFC was also positively correlated with negative symptoms in schizophrenia patients. These findings were validated in the replication dataset. Our results suggest that the uncoupling of rsFC between the cerebellum and the cerebral cortex may underlie the expression of NSS in schizophrenia. NSS-related cerebellar-prefrontal rsFC may be a potential neural pathway for possible neural modulation to alleviate negative symptoms.
Keywords: neurological soft signs, schizophrenia, cerebellar-cerebral connectivity, functional connectivity
Introduction
Neurological soft signs (NSS), originally defined as minor non-localizable neurological impairments, are characterized by deficits in sensory integration, motor coordination, and disinhibition.1,2 Accumulating evidence3–8 suggests that NSS may be one of the promising endophenotypes for schizophrenia spectrum disorders,3,9 which can bridge the gap between heterogeneous phenotypes (clinical symptoms and behaviors) and complex genotypes.3,10,11 With the advent of neuroimaging techniques, investigating the neural underpinnings of NSS may help to ascertain the etiology of schizophrenia.
Resting-state functional magnetic resonance imaging (rsfMRI) is an important tool to understand the intrinsic organization of spontaneous signal fluctuations or coupling among distributed brain networks, which can be measured by resting-state functional connectivity (rsFC).12 Dysfunction of the cerebellum has been proposed to play a role in the pathophysiology of schizophrenia.13 More recently, evidence suggests that the cerebellum is a critical node in multiple functional networks,14,15 and changes in cerebellar-cerebral rsFC in patients with schizophrenia have also been reported.16–18 Although previous studies have suggested that cerebellar-cerebral rsFC in schizophrenia is associated with NSS,19,20 few empirical studies have directly examined such relationship. To the best of our knowledge, only two previous studies have utilized rsfMRI to investigate the relationship between NSS and neural correlates in schizophrenia. Galindo et al21 found that NSS were correlated with altered functional connectivity (FC) in the default mode network in patients with schizophrenia, while Hirjak et al22 found that NSS were correlated with alterations of the frontocerebellar and frontoparietal networks in patients with schizophrenia. However, these two studies used the independent component analysis (ICA), which is a data-driven method and is different from the whole-brain seed-based FC approach. The latter rather than the former method could test a-priori hypothesis involving specific regions of interest (ROIs).
Since NSS are related to sensorimotor dysfunctions1,8 and general cognitive impairment in schizophrenia,23–26 we constructed cerebellar-cerebral rsFC by focusing on the sensorimotor and executive control networks, using cerebellar ROIs which has been found to be involved in the respective functional networks.14,15,27,28 Specifically, substantial evidence suggests that the function of the cerebellum is beyond motor control and coordination29 and is linked to cognitive function.30,31 Moreover, rsFC studies have confirmed the functional heterogeneity of the cerebellum and found that cerebellar-cortical circuits within the anterior lobe and lobule VII are mapped onto the sensorimotor network and those in the posterior lobe are mapped onto the executive control network.14,15,27,28 Utilizing cerebellar ROIs in the sensorimotor and executive control networks may provide opportunities to explore the various rsFC patterns in schizophrenia.
In addition, previous studies have reported that cerebellar-cerebral rsFC is correlated with negative symptoms in schizophrenia.18,32 Importantly, NSS, especially the motor coordination signs, have been found to correlate with negative symptoms in schizophrenia.23–34 Taken together, these previous findings suggest that the cerebellar-cerebral functional circuits may be the shared neural underpinnings between NSS and negative symptoms in schizophrenia.
In this study, we aimed to (1) investigate whether patients with schizophrenia had more NSS deficits compared with healthy controls; (2) examine the relationship between NSS and cerebellar-cerebral rsFC in patients with schizophrenia and healthy controls using a ROI-whole-brain voxel rsFC approach; (3) explore whether the NSS-related cerebellar-cerebral rsFC was correlated with negative symptoms in schizophrenia; and (4) validate the significant findings in an independent dataset. Based on previous findings, we hypothesized that patients with schizophrenia would exhibit more NSS.2 Regarding the NSS-related rsFC, we hypothesized that rsFC between the cerebellum and the frontal cortex would be correlated with NSS in patients with schizophrenia,22 and rsFC between the cerebellum and more generalized cerebral regions would be correlated with NSS in healthy controls.35 We further hypothesized that negative symptoms would be correlated with motor coordination-related rsFC of the cerebellum with the frontal cortex in schizophrenia patients.23,32 Lastly, we hypothesized that the independent dataset would replicate and validate the significant findings.
Methods
Participants
Two independent samples were included in this study: a main dataset of 51 patients with schizophrenia and 50 healthy controls as well as a replication dataset of 34 patients with schizophrenia and 34 healthy controls. The detailed information of recruiting participants can be found in the Supplementary Methods. The diagnosis of schizophrenia was ascertained by qualified psychiatrists using the Structure Clinical Interview for DSM-IV Axis I Disorder.36 Their clinical symptoms were assessed by qualified psychiatrists using the Positive and Negative Syndrome Scale (PANSS)37 and the Scale for Assessment of Negative Symptoms (SANS).38 All patients were in clinical stabilization.
This study was conducted in accordance with the Helsinki Declaration and approved by the Ethics Committees of the Institute of Psychology, the Chinese Academy of Sciences (protocol number: H13014, H15027, H16015), the New Territories West Cluster Clinical and Research Ethics Committee (protocol number: NTWC/CREC/1293/14), and the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (UW14-325).
Measures
The abridged version of the Cambridge Neurological Inventory (CNI) was used to assess NSS.8,39 It consists of 25 items divided into the motor coordination (eg, rapid finger tapping and fist-edge-palm), sensory integration (eg, extinction and stereognosis) and disinhibition (eg, go/no-go response and head movement) subscales. Each item was scored on a dichotomized scale with “0” for absence and “1” for presence of NSS by trained experimenters. Higher score indicates more NSS. The intraclass correlation coefficients for the subscales and the total scale were greater than 0.80.39
Participants’ IQ was estimated using the short form (the information, arithmetic, similarities, and digit span subtests) of the Chinese version of the Wechsler Adult Intelligence Scale-Revised.40
MRI Data Acquisition and Preprocessing
The rsfMRI data and high-resolution T1-weighted structural imaging data were acquired using a 3-T MR scanner (see Supplementary Methods for scanning sequences). Details on imaging preprocessing can be found in the Supplementary Methods. In brief, rsfMRI data were preprocessed using the Statistical Parametrical Mapping 12 (SPM 12; https://www.fil.ion.ucl.ac.uk/spm/software/spm12) and the Conn Functional Connectivity Toolbox (CONN).40 Standardized denoising steps were performed using the ART41 and the anatomical component-based noise correction method (aCompCor)42 in CONN (see Supplementary Methods for details). Global signal was not regressed out as it may lead to artefactual negative FC results.43 The mean frame-wise displacement (FD) for each participant was taken as a covariate in the subsequent analysis to minimize the effects of head motion.44
FC Analysis
Previous studies27,28 have found that the cerebellar seeds are able to identify cerebellar-cerebral executive control and sensorimotor networks. Thus, six ROIs for the executive control network27 and four ROIs for the sensorimotor network27,28 were defined with a radius of 6 mm in the bilateral cerebellum to construct cerebellar-cerebral rsFC in the CONN (Supplementary table 1). Second, mean time series of each seed were calculated and correlated with the rest of the whole-brain voxels. Then, the correlation maps for each participant across all ROIs were transformed to Z-maps through Fisher’s r-to-z transformation.
Statistical Analysis
Demographic and Behavioral Data Analysis
Demographic and behavioral data were compared between groups in each dataset using independent sample t-tests or chi-square tests in SPSS (Version 17).
Regression Analysis Between Cerebellar-Cerebral rsFC and NSS
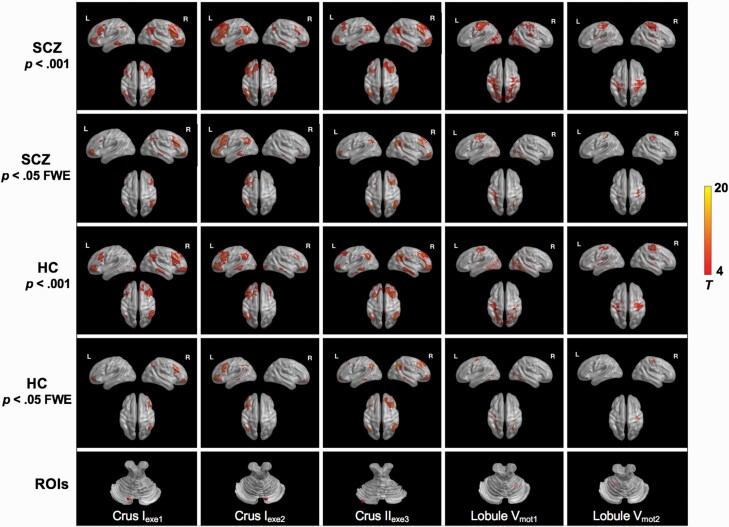
Before regression analysis, the spatial topography of cerebellar-cerebral rsFC in healthy controls and schizophrenia patients was verified by one-sample t-tests in each dataset (see figure 1, Supplementary Results, and Supplementary table 2). The threshold for significance was set at voxel-level family-wise error (FWE) corrected P < .05.
Fig. 1.
Resting-state cerebellar-cerebral functional connectivity in SCZ and HC in the main dataset. The representative cerebellar seeds shown here include: Crus Iexe1, Crus Iexe2, Crus IIexe3, Lobule Vmot1, Lobule Vmot2. SCZ, patients with schizophrenia; HC, healthy controls; ROIs, regions of interest.
To establish the neural correctional patterns between cerebellar-cerebral rsFC and CNI total and subscale scores for each group in the main dataset, Fisher Z-maps were included into a linear regression model comprising CNI total score (or subscale scores) as a regressor for patients with schizophrenia or healthy controls separately in the CONN. Age, gender, and mean FD44 were entered as covariates. Given the correlation between NSS and cognition,26,45 we did not include Intelligence Quotient (IQ) as a covariate. Length of education, duration of illness, average daily antipsychotic dosage, and gray matter volume of the cerebellum were included as covariates separately to control for the potential confounding effects (see Supplementary Material). The clusters were considered significant if they reached a threshold of voxel-level P < .001 with cluster-level FWE corrected P < .005 (in order to correct for multiple comparisons of 10 ROIs).
Correlation Analysis Between NSS-Related rsFC and Clinical Symptoms
The rsFC values of significant clusters were extracted in CONN. Partial correlation analysis was conducted to investigate the relationship between the NSS-related rsFC values and scores on the SANS subscales as well as the composite score in schizophrenia patients in the main dataset using SPSS. Covariates for the partial correlation analysis in the two schizophrenia patient groups included age, gender, mean FD, and antipsychotic dosage. The significance level for the partial correlation analysis was set at P < .05.
Validation Analysis
The significant findings in the main dataset were validated in the replication dataset. In order to test whether the significant correlations between CNI scores and the corresponding cerebral rsFC of the cerebellar functional network in the main dataset were also found in the replication dataset, each CNI score which was significantly correlated with rsFC in the main dataset and the Fisher Z-map from the corresponding cerebellar functional network were adopted into a linear regression model in the replication dataset. A mask of the significant cerebral regions found in the main dataset was generated using the WFU PickAtlas software (http://www.nitrc.org/projects/wfu_pickatlas/) for small volume correction. The threshold was voxel-level P < .005 with cluster-level FWE corrected P < .05.
As for the significant correlations between NSS-related rsFC and clinical symptoms found in the main dataset, the correlation between the rsFC of the Crus Iexe1 with the inferior frontal gyrus and the SANS avolition subscale score was carried out in the replication dataset to verify its stability.
Results
Behavioral Results
The demographic, behavioral, and clinical characteristics of the participants are shown in table 1. Patients with schizophrenia had significantly higher CNI subscale and total scale scores than healthy controls.
Table 1.
Demographic, Clinical, and NSS Information of Main and Replication Datasets
| Main Dataset | Replication Dataset | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HC (n = 49) | SZ (n = 50) | t/χ 2 | P | Cohen’s d | HC (n = 34) | SZ (n = 34) | t/χ 2 | P | Cohen’s d | |
| Demographics | ||||||||||
| Age (y) | 27.45 (6.42) | 27.68 (6.27) | t97 = −0.18 | .857 | 0.036 | 43.18 (12.81) | 43.94 (12.17) | t66 = −0.25 | .804 | 0.061 |
| Gender (male, %) | 46.94% | 52.00% | 0.25 | .689 | 35.29% | 33.33% | 0.03 | 1.000 | ||
| Education (y) | 15.92 (2.85) | 12.50 (3.34) | t97 = 5.47** | <.001 | 1.102 | 12.15 (3.46) | 11.48 (2.67) | t66 = 0.88 | .384 | 0.217 |
| Estimated IQ | 128.84 (12.00) | 99.26 (13.96) | t97 = 11.29** | <.001 | 2.272 | 119.65 (11.91) | 105.78 (16.63) | t66 = 3.91** | <.001 | 0.959 |
| CNI | ||||||||||
| Motor coordination | 1.33 (1.28) | 3.04 (1.93) | t97 = −5.20** | <.001 | 1.044 | 1.18 (1.19) | 2.67 (1.88) | t66 = −3.88** | <.001 | 0.947 |
| Sensory integration | 0.37 (0.70) | 1.18 (1.38) | t97 = −3.69** | <.001 | 0.740 | 1.21 (0.98) | 1.79 (1.02) | t66 = −2.38* | .020 | 0.580 |
| Disinhibition | 1.18 (0.69) | 1.90 (1.27) | t97 = −3.66** | <.001 | 0.704 | 0.44 (0.70) | 1.09 (0.98) | t66 = −3.12* | .003 | 0.763 |
| CNI total | 2.86 (1.93) | 6.14 (3.30) | t97 = −6.03** | <.001 | 1.213 | 2.82 (1.93) | 5.55 (2.92) | t66 = −4.52** | <.001 | 1.103 |
| Clinical characteristics | ||||||||||
| Course of illness (y) | 1.18 (1.08) | 19.89 (10.63) | ||||||||
| DUP (y) | 0.82 (1.06) | NA | ||||||||
| PANSS_Positive | 9.72 (3.09) | 11.25 (5.88) | ||||||||
| PANSS_Negative | 11.38 (5.96) | 12.62 (5.34) | ||||||||
| PANSS_General | 20.70 (5.09) | 24.33 (8.32) | ||||||||
| SANS_Affective Flattening | 5.06 (6.37) | 2.68 (3.76) | ||||||||
| SANS_Alogia | 1.78 (2.92) | 1.50 (2.46) | ||||||||
| SANS_Avolition | 1.58 (2.32) | 2.32 (3.07) | ||||||||
| SANS_Anhedonia | 2.72 (4.08) | 2.44 (3.47) | ||||||||
| SANS_Inattention | 2.38 (2.22) | 0.76 (1.52) | ||||||||
| SANS_Composite total | 3.44 (3.97) | 8.94 (10.87) | ||||||||
| CPZ equivalent dose (mg) | 340.83 (174.32) | 224.92 (192.11) | ||||||||
Note: HC, healthy controls; SZ, patients with schizophrenia; CNI, the abridged version of the Cambridge Neurological Inventory; NA, not applicable; PANSS: the Positive and Negative Syndrome Scale; CPZ: chlorpromazine; DUP, duration of untreated psychosis; SANS: the Scale for Assessment of Negative Symptoms; SANS_Composite total, the total score of composite items but excluding attention item.
*P < .05, **P < .001.
Correlations Between Cerebellar-Cerebral rsFC and CNI Scores
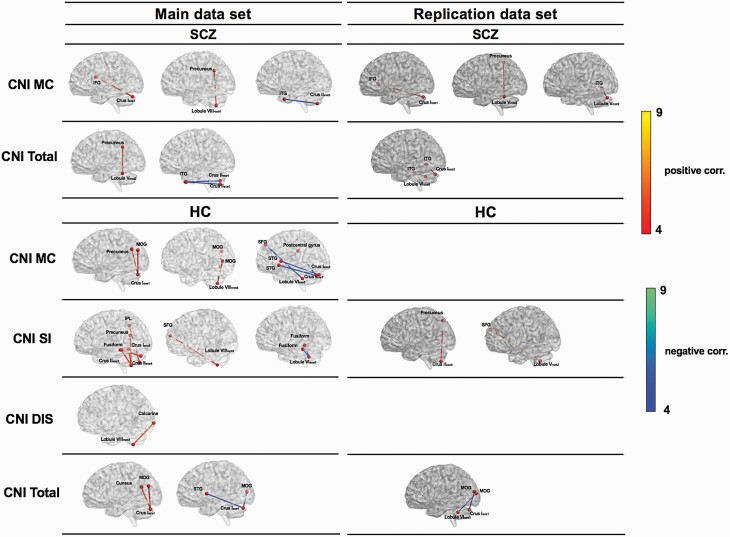
Table 2 and figure 2 show the significant correlations between cerebellar-cerebral rsFC and CNI scores in patients with schizophrenia in the main dataset. The rsFC between the Crus Iexe1 and the inferior frontal gyrus and the rsFC between the lobule VIIImot4 and the precuneus were significantly and positively correlated with the motor coordination subscale score, indicating stronger connectivity with more severe motor coordination deficits. Moreover, the rsFC between the Crus IIexe3 and the inferior temporal gyrus was negatively correlated with the motor coordination subscale score, indicating weaker connectivity with more severe motor coordination deficits. CNI total score was positively correlated with the rsFC between the precuneus and the Lobule Vmot2 and negatively correlated with the rsFC between the inferior temporal gyrus and the Crus IIexe3 as well as the Crus IIexe4. There were no significant correlations between scores on the sensory integration and disinhibition subscales and rsFC.
Table 2.
Associations of CNI Subscale and Total Scores With Cerebellar-Cerebral Functional Connectivity in the Main Dataset
| MNI Coordinates | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cerebellar Seeds | Direction of Association | Regions | P (FWE-Corr) | Cluster Size | x | y | z | T |
| A. Patients with schizophrenia | ||||||||
| CNI MC | ||||||||
| Crus Iexe1 | Positive | Inferior frontal gyrus | .001 | 123 | 56 | 8 | 18 | 5.54 |
| Crus IIexe3 | Negative | Inferior temporal gyrus | .001 | 126 | −44 | 6 | −36 | 4.47 |
| Lobule VIIImot4 | Positive | Precuneus | <.001 | 195 | −4 | −54 | 34 | 5.64 |
| CNI SI | No significant results | |||||||
| CNI DIS | No significant results | |||||||
| CNI total | ||||||||
| Crus IIexe3 | Negative | Inferior temporal gyrus | .003 | 106 | −46 | 8 | −40 | 4.66 |
| Crus IIexe4 | Negative | Inferior temporal gyrus | .003 | 110 | −46 | 10 | −40 | 5.13 |
| Lobule Vmot2 | Positive | Precuneus | .001 | 121 | −8 | −50 | 38 | 5.21 |
| B. Healthy controls | ||||||||
| CNI MC | ||||||||
| Crus Iexe1 | Positive | Middle occipital gyrus | .001 | 130 | −46 | −78 | 28 | 5.4 |
| Positive | Precuneus | .001 | 128 | −12 | −64 | 30 | 4.51 | |
| Negative | Superior temporal gyrus | <.001 | 339 | −62 | −2 | −2 | 5.82 | |
| Negative | Postcentral gyrus | .004 | 101 | 60 | −10 | 24 | 5.33 | |
| Crus Iexe2 | Negative | Superior temporal gyrus | .0046 | 98 | −64 | −8 | 8 | 4.7 |
| Lobule VIexe5 | Negative | Medial superior frontal gyrus | .0046 | 95 | 10 | 54 | 40 | 5.3 |
| Lobule VIIImot4 | Positive | Middle occipital gyrus | <.001 | 180 | −22 | −78 | 12 | 4.77 |
| Positive | Middle occipital gyrus | <.001 | 129 | 28 | −68 | 18 | 4.47 | |
| CNI SI | ||||||||
| Crus Iexe2 | Positive | Fusiform gyrus | .001 | 121 | −26 | −42 | −12 | 5.15 |
| Crus IIexe3 | Positive | Precuneus | .004 | 100 | 24 | −50 | 18 | 5.34 |
| Positive | Fusiform gyrus | .003 | 101 | −26 | −44 | −12 | 5.05 | |
| Positive | Fusiform gyrus | .001 | 125 | 30 | −44 | −14 | 4.83 | |
| Crus IIexe4 | Positive | Inferior parietal lobule | <.001 | 141 | 30 | −46 | 40 | 5.03 |
| Lobule VIexe5 | Negative | Fusiform gyrus | <.001 | 299 | −34 | −38 | −16 | 5.49 |
| Negative | Fusiform gyrus | <.001 | 139 | 30 | −42 | −20 | 4.98 | |
| Lobule VIIImot3 | Positive | Superior frontal gyrus | .002 | 100 | 30 | 60 | −2 | 4.5 |
| CNI DIS | ||||||||
| Lobule VIIImot3 | Positive | Calcarine sulcus | <.001 | 132 | −6 | −100 | −8 | 5.84 |
| CNI total | ||||||||
| Crus Iexe1 | Positive | Cuneus | <.001 | 304 | −20 | −58 | 22 | 5.77 |
| Positive | Middle occipital gyrus | .001 | 118 | −44 | −74 | 24 | 5.25 | |
| Negative | Middle occipital gyrus | .002 | 109 | 38 | −86 | 8 | 5.11 | |
| Negative | Superior temporal gyrus | <.001 | 170 | −60 | 4 | 4 | 4.55 | |
Note: CNI, the abridged version of the Cambridge Neurological Inventory; MC, motor coordination subscale; SI, sensory integration subscale; DIS, disinhibition subscale. The brain regions in bold were validated in the replication dataset. The threshold was voxel-level P < .001 and cluster-level FWE correction P < .005.
Fig. 2.
Associations of CNI subscale and total scores with cerebellar-cerebral resting-state functional connectivity in the main (P < .001 with cluster-level FWE corrected P < .005) and the replication (P < .005 with cluster-level FWE corrected P < .05) datasets. CNI, the abridged version of the Cambridge Neurological Inventory; MC, motor coordination subscale; SI, sensory integration subscale; DIS, disinhibition subscale; IFG, inferior frontal gyrus; ITG, inferior temporal gyrus; MOG, middle occipital gyrus; SFG, superior frontal gyrus; STG, superior temporal gyrus; IPL, inferior parietal lobule.
Table 2 and figure 2 show the significant correlations between cerebellar-cerebral rsFC and CNI scores in healthy controls in the main dataset. Scores on the motor coordination subscale were positively correlated with the rsFC between the Crus Iexe1 and the middle occipital gyrus as well as the precuneus. Scores on the sensory integration subscale were positively correlated with the rsFC of the fusiform gyrus with the Crus Iexe2 as well as the Crus IIexe3, and the rsFC of the superior frontal gyrus with the Lobule VIIImot3. Moreover, there were significant positive correlations between CNI total score, motor coordination and disinhibition subscale scores and the rsFC of the occipital cortex with the Crus Iexe1, the Lobule VIIImot4, and the Lobule VIIImot3, respectively. Most rsFC results remained significant after including the covariates (see Supplementary Tables).
Cerebellar-Cerebral rsFC and Clinical Features of Schizophrenia
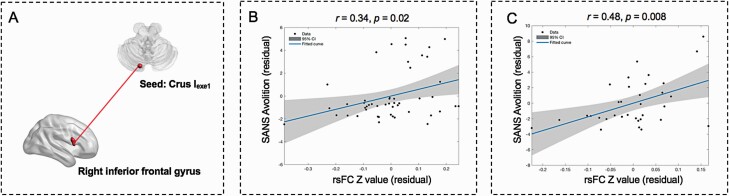
For schizophrenia patients in the main dataset, the rsFC between the Crus Iexe1 and the inferior frontal gyrus, which showed positive correlation with the CNI motor coordination subscale score in the FC analysis, was positively correlated with the SANS avolition subscale score (r = 0.34, P = .02; figure 3). Such correlation remained significant after including daily antipsychotic dosage, duration of untreated psychosis, illness duration, and gray matter volume of the cerebellum as covariates (see Supplementary Results). However, correlational results did not survive multiple comparison correction (P < .002). Exploratory analysis did not find any other significant correlations between rsFC and clinical symptoms.
Fig. 3.
Relationship between CNI-related cerebellar-prefrontal rsFC and negative symptoms. (A) rsFC between the inferior frontal gyrus and the Crus Iexe1. (B) Scatterplot of the significant correlation between two unstandardized residuals for reflecting relationship between SANS avolition subscale score and rsFC of the Crus Iexe1 with the inferior frontal gyrus in the main dataset. (C) Scatterplot of the significant correlation between two unstandardized residuals for reflecting relationship between SANS avolition subscale score and rsFC of the Crus Iexe1 with the inferior frontal gyrus in the replication dataset. CNI, the abridged version of the Cambridge Neurological Inventory; rsFC, resting-state functional connectivity; SANS, the Scale for Assessment of Negative Symptoms.
Validation Results in the Replication Dataset
The replication dataset confirmed that schizophrenia patients had higher CNI scores than healthy controls (table 1). Significant cerebellar-cerebral rsFCs in the executive control network and the sensorimotor network were also found (Supplementary table 3).
Confirming the patterns in the main dataset, patients with schizophrenia in the replication dataset exhibited the same correlations between scores on the motor coordination subscale and the rsFC of the corresponding cerebellar network and cerebral regions (Supplementary table 4 and figure 2). As for CNI total scores, patients with schizophrenia exhibited a negative correlation with the rsFC of the inferior temporal gyrus and the cerebellar executive control network. Healthy controls exhibited a significant positive correlation between the sensory integration subscale score and the rsFC between the superior frontal gyrus and the cerebellar sensorimotor network, and a significant negative correlation between the CNI total score and the rsFC of the middle occipital gyrus with the cerebellar executive control network. Most of the results remained significant after including length of education, duration of illness, and gray matter volume of the cerebellum as covariates (see Supplementary Tables). However, only the rsFC between the inferior frontal gyrus and the Crus Iexe1 was found to be significant after including daily antipsychotic dosage as a covariate.
Lastly, the same significant positive correlation between scores on the SANS avolition subscale and rsFC (r = 0.48, P = .008; figure 3) was found. Such correlation remained significant after including daily antipsychotic dosage and gray matter volume of the cerebellum as covariates (r = 0.51, P = .009) and became marginally significant after including daily antipsychotic dosage and illness duration as covariates (r = 0.49, P = .05).
Discussion
The current study aimed at investigating cerebellar-cerebral rsFC underlying NSS in schizophrenia patients and healthy controls. Our study has two main findings: (1) cerebellar-cerebral rsFC involving the executive control and motor networks were correlated with NSS differently in patients with schizophrenia and healthy controls; and (2) cerebellar-prefrontal rsFC was significantly and positively correlated with motor coordination deficits and negative symptoms in schizophrenia patients.
The correlations between NSS and rsFC of the cerebellum with the inferior frontal gyrus, the inferior temporal gyrus, and the precuneus in schizophrenia patients are generally in line with previously morphological,46,47 task-based fMRI,48–50 and rsfMRI studies,22 providing evidence to support the role of cerebello-thalamo-cortical circuit (CTCC) dysfunction51,52 in the pathophysiology of schizophrenia. Specifically, our findings indicate that increased rsFC of the Crus Iexe1 with the inferior frontal gyrus (a part of the prefrontal cortex) and the lobule VIIImot4 with the precuneus and the decreased rsFC of the Crus IIexe3 with the inferior temporal gyrus are correlated with more motor coordination deficits in schizophrenia patients. These findings extend previous structural and fMRI findings. Previous morphological studies have revealed that the cortical thickness of the prefrontal cortex, the inferior temporal gyrus, and the precuneus were negatively correlated with NSS score in patients with schizophrenia.46,53 Moreover, a task-based fMRI study also found that patients with schizophrenia showed altered prefrontal cortex connectivity during a fist-edge-palm imaging task.48
On the other hand, the inferior frontal gyrus plays a critical role in movement observation, execution of visually guided movements and action monitoring.54–56 Therefore, the uncoupling of brain activities between the inferior frontal gyrus and the cerebellum, in which the inferior frontal gyrus perceives movement information, exerts top-down control and sends signal to the cerebellum, could lead to a failure in visuomotor transformation. The precuneus is known for somatosensory control and movement coordination.57,58 The aberrant cerebellar-cerebral rsFC between the sensorimotor network and the precuneus may lead to impaired processing of basic sensorimotor information and contribute to failure in movement control and coordination. The inferior temporal gyrus has been implicated in auditory processing59 and visual recognition.60 The decreased connectivity between the inferior temporal gyrus and the cerebellum may indicate insufficient communication between the cerebral and cerebellar regions, suggesting abnormal processing for basic auditory and visual stimuli.
Furthermore, we found that several cerebellar-cerebral rsFCs were also significantly correlated with NSS in healthy controls. A previous study using rsfMRI has reported that NSS were positively correlated with activity of the cerebellar lobule VI61 in the healthy population. Our results are consistent with these previous findings and suggest that more cortical regions including the occipital, temporal, and frontal cortices are involved in the correlations between cerebellar-cerebral rsFC and NSS. For example, rsFC between the Crus Iexe1 and the postcentral gyrus (the primary somatosensory cortex, S1) is negatively correlated with motor coordination deficits. The postcentral gyrus is responsible for attention, proposing somatic sensations and spatial working memory.62 Previous brain structural studies in the healthy population have also found that motor coordination deficits are correlated with morphological changes of the postcentral gyrus.63,64
Another important finding of this study is the significant positive correlation between negative symptoms and cerebellar-prefrontal rsFC, which was also positively correlated with motor coordination deficits in patients with schizophrenia (figure 3). Motor coordination deficits have been found to be correlated with negative symptoms of schizophrenia.23,33 Moreover, a diffusion-tensor imaging study found that white matter volume of the inferior frontal gyrus was negatively correlated with negative symptoms in schizophrenia.65 Another study has found that schizophrenia patients with persistent negative symptoms had smaller gray matter volumes of inferior frontal gyrus compared with patients with nonpersistent negative symptoms.66 These structural MRI studies suggest that the inferior frontal gyrus may play an important role in negative symptoms of schizophrenia. Furthermore, modulation of the connectivity between the cerebellum and the prefrontal cortex using transcranial magnetic stimulation (TMS) has been proposed as a possible intervention to alleviate negative symptoms of schizophrenia.32 Moreover, the application of transcranial direct current stimulation (tDCS) to the prefrontal and cerebellar cortex has been found to improve NSS in patients with bipolar disorder.67 Our finding suggests that cerebellar-prefrontal rsFC may be the common neural pathway of NSS and negative symptoms, and a potential therapeutic target for noninvasive brain stimulation such as TMS and tDCS to improve NSS and negative symptoms in schizophrenia.
The main strength of this study is that a replication dataset has been used to test the reproducibility and reliability of the results found in the main dataset. Furthermore, the majority of the findings remained significant after including covariates such as length of education, duration of illness, and gray matter volume of the cerebellum, although the change in results after including daily antipsychotic dosage suggests that antipsychotic medications may influence brain activity.
This study also has several limitations. First, our patients with schizophrenia were medicated and previous studies have demonstrated that exposure to antipsychotics may affect motor function and brain activity in schizophrenia.68,69 Second, the demographics and clinical features of the two datasets were not matched, and our findings in the control groups of the two datasets differed. However, our main outcomes, such as motor coordination scores, SANS avolition subscale scores, and cerebellar-prefrontal rsFC were not significantly different between two patients’ groups, suggesting that the different illness duration between the two datasets would unlikely affect our findings. Furthermore, a previous study of 738 schizophrenia patients showed that NSS deficits barely changed across the lifespan.4 Third, we chose ROIs in the cerebellum to represent specific networks. Nevertheless, previous evidence suggest that other methods to construct rsFC network are also valid and useful.35 For instance, the established cerebellar network parcellation14 which covers comprehensive networks could be used to explore cerebellar-cerebral rsFC among other networks in schizophrenia in future studies. Finally, other sensorimotor abnormalities in schizophrenia such as catatonia or parkinsonism could also be examined in future research. Previous studies have shown that these abnormalities (NSS, catatonia, and parkinsonism) are related to negative symptoms,70–72 cognition,73,74 and outcome70,71,75 in schizophrenia. Further studies on the neural mechanisms underlying these sensorimotor abnormalities can advance our insights on the psychopathology and development of new treatment for schizophrenia.
In conclusion, we demonstrated altered coupling between NSS and cerebellar-cerebral rsFC in patients with schizophrenia and healthy controls. Furthermore, we found that cerebellar-prefrontal rsFC was positively correlated with both motor coordination deficits and negative symptoms in schizophrenia. Our findings indicate that cerebellar-prefrontal rsFC may be the shared neural mechanism between NSS and negative symptoms in schizophrenia. Altered coupling of NSS-related cerebellar-cerebral functional networks may be potential biomarkers for schizophrenia spectrum disorders.
Funding
This study was supported by a grant from the National Key Research and Development Programme (2016YFC0906402), Beijing Municipal Science & Technology Commission Grant (Z161100000216138), CAS Key Laboratory of Mental Health, Institute of Psychology to Raymond Chan. E.F.C.C. was supported by the Philip K.H. Wong Foundation.
Supplementary Material
Acknowledgments
All authors report no conflict of interests.
References
- 1.Heinrichs DW, Buchanan RW. Significance and meaning of neurological signs in schizophrenia. Am J Psychiatry. 1988;145(1):11–18. [DOI] [PubMed] [Google Scholar]
- 2.Chan RC, Xu T, Heinrichs RW, Yu Y, Wang Y. Neurological soft signs in schizophrenia: a meta-analysis. Schizophr Bull. 2010;36(6):1089–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160(4):636–645. [DOI] [PubMed] [Google Scholar]
- 4.Chan RC, Xie W, Geng FL, et al. Clinical utility and lifespan profiling of neurological soft signs in schizophrenia spectrum disorders. Schizophr Bull. 2016;42(3):560–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu T, Wang Y, Li Z, et al. Heritability and familiality of neurological soft signs: evidence from healthy twins, patients with schizophrenia and non-psychotic first-degree relatives. Psychol Med. 2016;46(1):117–123. [DOI] [PubMed] [Google Scholar]
- 6.Sanders RD, Joo YH, Almasy L, et al. Are neurologic examination abnormalities heritable? A preliminary study. Schizophr Res. 2006;86(1–3):172–180. [DOI] [PubMed] [Google Scholar]
- 7.Chan RC, Xu T, Heinrichs RW, Yu Y, Gong QY. Neurological soft signs in non-psychotic first-degree relatives of patients with schizophrenia: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2010;34(6):889–896. [DOI] [PubMed] [Google Scholar]
- 8.Chen EY, Shapleske J, Luque R, et al. The Cambridge Neurological Inventory: a clinical instrument for assessment of soft neurological signs in psychiatric patients. Psychiatry Res. 1995;56(2):183–204. [DOI] [PubMed] [Google Scholar]
- 9.Chan RC, Gottesman II. Neurological soft signs as candidate endophenotypes for schizophrenia: a shooting star or a Northern star? Neurosci Biobehav Rev. 2008;32(5):957–971. [DOI] [PubMed] [Google Scholar]
- 10.Insel TR, Cuthbert BN. Endophenotypes: bridging genomic complexity and disorder heterogeneity. Biol Psychiatry. 2009;66(11):988–989. [DOI] [PubMed] [Google Scholar]
- 11.Gottesman II, Shields J. Genetic theorizing and schizophrenia. Br J Psychiatry. 1973;122(566):15–30. [DOI] [PubMed] [Google Scholar]
- 12.Buckner RL, Krienen FM, Yeo BT. Opportunities and limitations of intrinsic functional connectivity MRI. Nat Neurosci. 2013;16(7):832–837. [DOI] [PubMed] [Google Scholar]
- 13.Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biol Psychiatry. 2008;64(2):81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(5):2322–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Habas C, Kamdar N, Nguyen D, et al. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci. 2009;29(26):8586–8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collin G, Hulshoff Pol HE, Haijma SV, Cahn W, Kahn RS, van den Heuvel MP. Impaired cerebellar functional connectivity in schizophrenia patients and their healthy siblings. Front Psychiatry. 2011;2:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo W, Liu F, Chen J, et al. Resting-state cerebellar-cerebral networks are differently affected in first-episode, drug-naive schizophrenia patients and unaffected siblings. Sci Rep. 2015;5:17275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo W, Liu F, Zhang Z, et al. Increased cerebellar functional connectivity with the default-mode network in unaffected siblings of schizophrenia patients at rest. Schizophr Bull. 2015;41(6):1317–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Picard H, Amado I, Mouchet-Mages S, Olié JP, Krebs MO. The role of the cerebellum in schizophrenia: an update of clinical, cognitive, and functional evidences. Schizophr Bull. 2008;34(1):155–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shinn AK, Baker JT, Lewandowski KE, Öngür D, Cohen BM. Aberrant cerebellar connectivity in motor and association networks in schizophrenia. Front Hum Neurosci. 2015;9:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galindo L, Bergé D, Murray GK, et al. Default mode network aberrant connectivity associated with neurological soft signs in schizophrenia patients and unaffected relatives. Front Psychiatry. 2017;8:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirjak D, Rashidi M, Fritze S, et al. Patterns of co-altered brain structure and function underlying neurological soft signs in schizophrenia spectrum disorders. Hum Brain Mapp. 2019;40(17):5029–5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan RCK, Geng FL, Lui SSY, et al. Course of neurological soft signs in first-episode schizophrenia: relationship with negative symptoms and cognitive performances. Sci Rep. 2015;5:11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan RCK, Dai S, Lui SSY, et al. Re-visiting the nature and relationships between neurological signs and neurocognitive functions in first-episode schizophrenia: an invariance model across time. Sci Rep. 2015;5:11850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cardno AG, Gottesman II. Twin studies of schizophrenia: from bow-and-arrow concordances to star wars Mx and functional genomics. Am J Med Genet. 2000;97(1):12–17. [PubMed] [Google Scholar]
- 26.Cuesta MJ, Moreno-Izco L, Ribeiro M, et al. Motor abnormalities and cognitive impairment in first-episode psychosis patients, their unaffected siblings and healthy controls. Schizophr Res. 2018;200:50–55. [DOI] [PubMed] [Google Scholar]
- 27.Krienen FM, Buckner RL. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex. 2009;19(10):2485–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen-Berg H. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb Cortex. 2010;20(4):953–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evarts EV, Thach WT. Motor mechanisms of the CNS: cerebrocerebellar interrelations. Annu Rev Physiol. 1969;31:451–498. [DOI] [PubMed] [Google Scholar]
- 30.Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage. 2012;59(2):1560–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sokolov AA, Miall RC, Ivry RB. The cerebellum: adaptive prediction for movement and cognition. Trends Cogn Sci. 2017;21(5):313–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brady RO Jr, Gonsalvez I, Lee I, et al. Cerebellar-prefrontal network connectivity and negative symptoms in schizophrenia. Am J Psychiatry. 2019;176(7):512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prikryl R, Ceskova E, Tronerova S, et al. Dynamics of neurological soft signs and its relationship to clinical course in patients with first-episode schizophrenia. Psychiatry Res. 2012;200(2–3):67–72. [DOI] [PubMed] [Google Scholar]
- 34.Chen EY, Hui CL, Chan RC, et al. A 3-year prospective study of neurological soft signs in first-episode schizophrenia. Schizophr Res. 2005;75(1):45–54. [DOI] [PubMed] [Google Scholar]
- 35.Kim DJ, Moussa-Tooks AB, Bolbecker AR, et al. Cerebellar-cortical dysconnectivity in resting-state associated with sensorimotor tasks in schizophrenia. Hum Brain Mapp. 2020;41(11):3119–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frist M, Gibbons M, Sptizer R, Williams J.. User’s guide for the Structured Clinical Interview for DSM-IV Axis I Disorders—research version. New York: Biometrics Research, New York State Psychiatric Institute, 1996. [Google Scholar]
- 37.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 38.Andreasen NC. Negative symptoms in schizophrenia. Definition and reliability. Arch Gen Psychiatry. 1982;39(7):784–788. [DOI] [PubMed] [Google Scholar]
- 39.Chan RC, Wang Y, Wang L, et al. Neurological soft signs and their relationships to neurocognitive functions: a re-visit with the structural equation modeling design. PLoS One. 2009;4(12):e8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–141. [DOI] [PubMed] [Google Scholar]
- 41.Fischer AS, Camacho MC, Ho TC, Whitfield-Gabrieli S, Gotlib IH. Neural markers of resilience in adolescent females at familial risk for major depressive disorder. JAMA Psychiatry. 2018;75(5):493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37(1):90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nalci A, Rao BD, Liu TT. Global signal regression acts as a temporal downweighting process in resting-state fMRI. Neuroimage. 2017;152:602–618. [DOI] [PubMed] [Google Scholar]
- 44.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herold CJ, Duval CZ, Lässer MM, Schröder J. Neurological soft signs (NSS) and cognitive impairment in chronic schizophrenia. Schizophr Res Cogn. 2019;16:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kong L, Herold CJ, Lässer MM, et al. Association of cortical thickness and neurological soft signs in patients with chronic schizophrenia and healthy controls. Neuropsychobiology. 2015;71(4):225–233. [DOI] [PubMed] [Google Scholar]
- 47.Hirjak D, Wolf RC, Kubera KM, Stieltjes B, Maier-Hein KH, Thomann PA. Neurological soft signs in recent-onset schizophrenia: focus on the cerebellum. Prog Neuropsychopharmacol Biol Psychiatry. 2015;60:18–25. [DOI] [PubMed] [Google Scholar]
- 48.Chan RC, Huang J, Zhao Q, et al. Prefrontal cortex connectivity dysfunction in performing the Fist-Edge-Palm task in patients with first-episode schizophrenia and non-psychotic first-degree relatives. Neuroimage Clin. 2015;9:411–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zemankova P, Lungu O, Huttlova J, et al. Neuronal substrate and effective connectivity of abnormal movement sequencing in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2016;67:1–9. [DOI] [PubMed] [Google Scholar]
- 50.Moussa-Tooks AB, Kim DJ, Bartolomeo LA, et al. Impaired effective connectivity during a cerebellar-mediated sensorimotor synchronization task in schizophrenia. Schizophr Bull. 2019;45(3):531–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Friston KJ. Schizophrenia and the disconnection hypothesis. Acta Psychiatr Scand Suppl. 1999;395:68–79. [DOI] [PubMed] [Google Scholar]
- 52.Dong D, Wang Y, Chang X, Luo C, Yao D. Dysfunction of large-scale brain networks in schizophrenia: a meta-analysis of resting-state functional connectivity. Schizophr Bull. 2018;44(1):168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hirjak D, Wolf RC, Stieltjes B, et al. Cortical signature of neurological soft signs in recent onset schizophrenia. Brain Topogr. 2014;27(2):296–306. [DOI] [PubMed] [Google Scholar]
- 54.Gehring WJ, Knight RT. Prefrontal-cingulate interactions in action monitoring. Nat Neurosci. 2000;3(5):516–520. [DOI] [PubMed] [Google Scholar]
- 55.Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. [DOI] [PubMed] [Google Scholar]
- 56.Woolley DG, Wenderoth N, Heuninckx S, Zhang X, Callaert D, Swinnen SP. Visual guidance modulates hemispheric asymmetries during an interlimb coordination task. Neuroimage. 2010;50(4):1566–1577. [DOI] [PubMed] [Google Scholar]
- 57.Christensen LO, Johannsen P, Sinkjaer T, Petersen N, Pyndt HS, Nielsen JB. Cerebral activation during bicycle movements in man. Exp Brain Res. 2000;135(1):66–72. [DOI] [PubMed] [Google Scholar]
- 58.Wenderoth N, Debaere F, Sunaert S, Swinnen SP. The role of anterior cingulate cortex and precuneus in the coordination of motor behaviour. Eur J Neurosci. 2005;22(1):235–246. [DOI] [PubMed] [Google Scholar]
- 59.Chen YH, Edgar JC, Huang M, et al. Frontal and superior temporal auditory processing abnormalities in schizophrenia. Neuroimage Clin. 2013;2:695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Isik L, Singer J, Madsen JR, Kanwisher N, Kreiman G. What is changing when: decoding visual information in movies from human intracranial recordings. Neuroimage. 2018;180(Pt A):147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hirjak D, Thomann PA, Kubera KM, Stieltjes B, Wolf RC. Cerebellar contributions to neurological soft signs in healthy young adults. Eur Arch Psychiatry Clin Neurosci. 2016;266(1):35–41. [DOI] [PubMed] [Google Scholar]
- 62.Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49(1–2):1–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang X, Herold CJ, Kong L, Schroeder J. Associations between brain structural networks and neurological soft signs in healthy adults. Psychiatry Res Neuroimaging. 2019;293:110989. [DOI] [PubMed] [Google Scholar]
- 64.Hirjak D, Wolf RC, Kubera KM, Stieltjes B, Thomann PA. Multiparametric mapping of neurological soft signs in healthy adults. Brain Struct Funct. 2016;221(3):1209–1221. [DOI] [PubMed] [Google Scholar]
- 65.Gu C, Zhang Y, Wei F, Cheng Y, Cao Y, Hou H. Magnetic resonance imaging DTI-FT study on schizophrenic patients with typical negative first symptoms. Exp Ther Med. 2016;12(3):1450–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Benoit A, Bodnar M, Malla AK, Joober R, Lepage M. The structural neural substrates of persistent negative symptoms in first-episode of non-affective psychosis: a voxel-based morphometry study. Front Psychiatry. 2012;3:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Minichino A, Bersani FS, Bernabei L, et al. Prefronto-cerebellar transcranial direct current stimulation improves visuospatial memory, executive functions, and neurological soft signs in patients with euthymic bipolar disorder. Neuropsychiatr Dis Treat. 2015;11:2265–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Whitty PF, Owoeye O, Waddington JL. Neurological signs and involuntary movements in schizophrenia: intrinsic to and informative on systems pathobiology. Schizophr Bull. 2009;35(2):415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peralta V, Cuesta MJ. The effect of antipsychotic medication on neuromotor abnormalities in neuroleptic-naive nonaffective psychotic patients: a naturalistic study with haloperidol, risperidone, or olanzapine. Prim Care Companion J Clin Psychiatry 2010;12(2):PCC.09m00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peralta V, Cuesta MJ. Neuromotor abnormalities in neuroleptic-naive psychotic patients: antecedents, clinical correlates, and prediction of treatment response. Compr Psychiatry. 2011;52(2):139–145. [DOI] [PubMed] [Google Scholar]
- 71.Docx L, Morrens M, Bervoets C, et al. Parsing the components of the psychomotor syndrome in schizophrenia. Acta Psychiatr Scand. 2012;126(4):256–265. [DOI] [PubMed] [Google Scholar]
- 72.Peralta V, de Jalón EG, Campos MS, Cuesta MJ. Covariation between motor signs and negative symptoms in drug-naive subjects with schizophrenia-spectrum disorders before and after antipsychotic treatment. Schizophr Res. 2018;200:85–91. [DOI] [PubMed] [Google Scholar]
- 73.Sachdev P, Hume F, Toohey P, Doutney C. Negative symptoms, cognitive dysfunction, tardive akathisia and tardive dyskinesia. Acta Psychiatr Scand. 1996;93(6):451–459. [DOI] [PubMed] [Google Scholar]
- 74.Dean DJ, Woodward N, Walther S, McHugo M, Armstrong K, Heckers S. Cognitive motor impairments and brain structure in schizophrenia spectrum disorder patients with a history of catatonia. Schizophr Res. 2020;222:335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Os J, Fahy TA, Jones P, et al. Psychopathological syndromes in the functional psychoses: associations with course and outcome. Psychol Med. 1996;26(1):161–176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.