Abstract
Introduction
The survival of patients with relapsed small cell lung cancer (SCLC) has achieved little progress in the last several decades. ALTER1202 confirmed the efficacy and safety of anlotinib as a third- or further-line option for relapsed SCLC. This study aimed to assess the cost-effectiveness of anlotinib compared with placebo as third- or further-line treatment for advanced SCLC in China.
Methods
A Markov model was developed to simulate the process of advanced SCLC and estimate the incremental cost-effectiveness ratio (ICER) of anlotinib versus placebo. The health outcomes and utilities were derived from the ALTER1202 (NCT03059797) and published sources, respectively. Total costs were calculated from the perspective of Chinese society. One-way and probabilistic sensitivity analyses (PSA) were conducted to explore the model uncertainties.
Results
Anlotinib was estimated to result in an additional 0.12 quality-adjusted life-years (QALYs) at an incremental cost of $2131.32, resulting in an ICER of $17,741.94/QALY. The ICER did not exceed the willingness-to-pay (WTP) threshold of $30,833 per QALY, which was three times the gross domestic product (GDP) per capita of China in 2019. One-way sensitivity analysis showed that the cost of anlotinib exerted the maximum influence on the result of the model, followed by the utility of progression-free survival (PFS) state in the anlotinib group and median overall survival (mOS) in the anlotinib group. In PSA, the probability of anlotinib being cost-effective was 26.6% and 78.5% when the WTP threshold was one and three times the GDP per capita, respectively.
Conclusion
Anlotinib is likely to be a cost-effective option compared with placebo for patients with relapsed SCLC who experience failure of at least two lines of chemotherapy in China.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-021-01889-2.
Keywords: Anlotinib, Cost-effectiveness, SCLC, Targeted therapy, Third-line treatment
Key Summary Points
| In China, lung cancer is the most common carcinoma, and the proportion of SCLC shows an increasing tendency, which leads to considerable economic burden |
| Anlotinib is the only approved third-line therapy for SCLC in China; however, the exorbitant price of the novel therapy is always a key consideration for cancer treatment |
| This study aimed to investigate whether anlotinib was cost-effective in the third- or further-line setting for patients with relapsed SCLC in China |
| The results showed that the ICER of anlotinib versus placebo was $17,741.94 per QALY, which did not exceed the WTP threshold of $30,833 per QALY, so anlotinib is a cost-effective option as third- or further-line treatment for SCLC in China |
| Sensitivity analyses showed that the cost of anlotinib and utility of PFS state in the anlotinib group were the most influential factors in the model, and the probability of anlotinib being cost-effective was 26.6% and 78.5% when the WTP threshold was one and three times the GDP per capita, respectively |
Introduction
According to the latest global cancer statistics from 2020, lung cancer is ranked the second most common malignancy and is still the leading cause of cancer death [1, 2]. Small cell lung cancer (SCLC) accounts for approximately 13–15% of all diagnosed lung cancer [3]. It is characterized by rapid growth, being highly aggressive and quick development of chemo-resistance [4]. The general 5-year survival for SCLC is only about 6% [3, 5], showing a clearly poorer prognosis compared to non-small cell lung cancer (NSCLC) (5-year survival, 24%). Little progress has been made in the medication therapy of SCLC except for the standard treatment with platin/etoposide. No effective treatment is available after disease relapse, so new strategies for treatment of refractory SCLC, especially third- or further-line options, are urgently need.
Targeted therapy has achieved great success in lung cancer, but is mainly reflected in NSCLC. Anlotinib, a novel oral multi-target tyrosine kinase inhibitor (TKI), significantly prolonged both progression-free survival (PFS) (4.1 vs. 0.7 months, P < 0.0001) and overall survival (OS) (7.3 vs. 4.9 months; P = 0.0210) compared with placebo for refractory SCLC as a third- or further-line treatment [6]. It is a promising treatment option for patients with relapsed SCLC who failed ≥ 2 lines of chemotherapy and has been approved by National Medical Products Administration (NMPA) of China in 2019.
However, the exorbitant price of the novel therapy is always a key consideration for cancer treatment. Hence, we are interested in investigating whether anlotinib is cost-effective in the third- or further-line setting for patients with relapsed SCLC in China.
Methods
Patients
Clinical data were mainly derived from a phase II randomized controlled trial (ALTER 1202, NCT03059797) that compared anlotinib versus placebo in patients with relapsed SCLC who had experienced failure of at least two lines of chemotherapy [6]. Patients were randomly assigned (2:1) to receive anlotinib (n = 82) or placebo (n = 38) 12 mg orally once daily for 2 weeks and then 1 week off until disease progression or development of intolerable toxicity. Patients received subsequent therapy after progression according to the ALTER1202 protocol. Baseline characteristics such as age, gender, Eastern Cooperative Oncology Group (ECOG) performance status, tumor stage, smoking and treatment history were well balanced between the two arms (see Table S1 in the Electronic Supplementary Material).
Model Structure
A Markov model was established using TreeAge Pro 2011 (TreeAge Software, Inc., Williamstown, MA) to simulate the disease process of advanced SCLC, which included three exclusive health states: PFS, progressive disease (PD) and death (Fig. 1). All patients were assumed to initially enter the model in the PFS state and subsequently survived or died. Patients who survived either remained in the PFS state or transferred to the PD state, and those transferred to the PD state either remained or died [7–10]. The Markov cycle was set as 1 month with a time horizon of 5 years, considering that the probability of survival to year 5 after the SCLC diagnosis was only 6% [11] and the median OS of patients with advanced SCLC was < 1 year in ALTER 1202 [6]. Transition probabilities between different health states were calculated based on the equation as follows: P (1 month) = 1 − (0.5)(1/median time to event), derived from the equations: P = 1 − e−R and R = − ln[0.5]/(time to event/number of treatment cycles) (for clinical outcomes, see Table 1) [12, 13]. A 5% discount rate for health utilities and costs was assumed based on the recommendation of the China Guidelines for Pharmacoeconomic Evaluations [14]. This model-based economic study was based on previously conducted studies and did not contain any new studies with human participants or animals performed by any of the authors and so did not require the approval of an independent ethics committee.
Fig. 1.
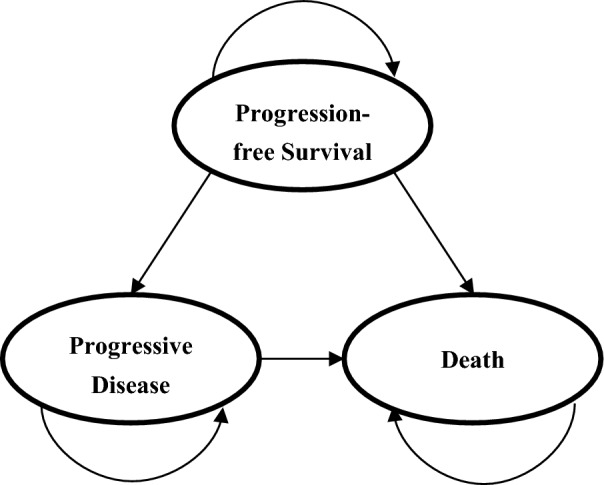
Markov model with three exclusive health states for advanced SCLC
Table 1.
Clinical efficacy and the most frequent treatment-related AEs
| Variables | Base case value | |
|---|---|---|
| Anlotinib | Placebo | |
| Clinical efficacy (months, 95% CI) | ||
| Median OS | 7.3 (6.1–10.3)* | 4.9 (2.7–6.0) |
| Median PFS | 4.1 (2.8–4.2)* | 0.7 (0.7–0.8) |
| AEs (grade ≥ 3) (%) | 35.8 | 15.4 |
| Hypertension | 13.6 | 2.6 |
| Anorexia | 1.2 | 0 |
| Fatigue | 1.2 | 0 |
| Hand-foot syndrome | 4.9* | 0 |
| Elevated alanine transaminase | 1.2 | 5.1 |
| Lymphopenia | 2.5* | 0 |
| Hypertriglyceridemia | 3.7* | 0 |
OS overall survival, PFS progression-free survival, AEs adverse events
*P < 0.05
Utility Estimates
The survival time was adjusted by health-related quality of life (QoL) to estimate quality-adjusted life-years (QALYs). Health utilities reflected the QoL in different health states. The primary utility values were 0.804 and 0.321 for PFS and PD state, respectively, which were obtained from the previously published studies [15, 16]. The utility decrements associated with side effects were calculated based on the occurrence of grade ≥ 3 adverse events (AEs) in ALTER 1202 [6]. Thus, the utility of PFS state was 0.791 for the anlotinib group and 0.803 for placebo. The utility of PD state and death was 0.321 and zero, respectively, in both arms (Table 2).
Table 2.
Costs and utility scores
| Variables | Base case value | |
|---|---|---|
| Anlotinib | Placebo | |
| Direct medical costs for PFS state ($/cycle) | ||
| Anlotinib | 830.50 | 0.00 |
| Tests | 270.65 | 270.65 |
| Outpatient fees | 5.07 | 5.07 |
| Grade ≥ 3 AEs | 47.50 | 18.11 |
| Total direct medical costs for PFS state ($/cycle) | 1153.72 | 293.84 |
| Societal costs ($/cycle) | ||
| Travel | 12.13 | 12.13 |
| Loss of salary | 35.95 | 35.95 |
| Total societal costs ($/cycle) | 48.08 | 48.08 |
| Total costs for PFS state ($/cycle) | 1201.80 | 341.92 |
| Total costs for PD state ($/cycle) | 847.00 | 847.00 |
| Utility | ||
| PFS state | 0.791 | 0.803 |
| PD state | 0.321 | 0.321 |
PFS progression-free survival, AEs adverse events, PD progressive disease
Measurement of Costs
Total costs were calculated from the perspective of Chinese society, including two parts: direct medical costs and societal costs. Direct medical costs covered drugs, tests, management of grade ≥ 3 AEs and outpatient fees. Societal costs consisted of travel and time (loss of salary) costs. We referred to China's national drug reimbursement list 2020 to estimate the cost of anlotinib ($44.49/12 mg). Expenses for other drugs and tests were derived from the standards of Changzhou No. 2 People’s Hospital, Nanjing Medical University. We assumed the average societal costs were identical between anlotinib and placebo. Travel and time costs were estimated on the basis of the taxi fare per kilometer ($12.13 per trip) and the average monthly salary ($35.95 per day) [17] in China, respectively [7, 18]. According to ALTER1202, chemotherapy was the most common subsequent therapy after progression in both arms (82.2% vs. 83.3) [6]. Based on the Chinese Society of Clinical Oncology (CSCO) guideline for SCLC and local clinical expert opinions, the subsequent therapy after progression was assumed to be carboplatin + etoposide, and the expenses after PD referred to the previous study [10]. All costs were converted into US dollars ($1 = RMB 6.8976, CNY Central Historical Parity Rate 2020). The estimated costs per cycle are shown in Table 2.
Cost-Effectiveness and Sensitivity Analysis
The primary outcomes of our model included total cost and QALYs. Then the incremental cost-effectiveness ratio (ICER) of anlotinib versus placebo was calculated and compared with a willingness-to-pay (WTP) threshold. If the ICER was less than or equal to the threshold, then anlotinib was considered to be an economical strategy compared to placebo; otherwise, anlotinib would not be considered an economical choice. To test the uncertainty of the model, one-way and probability sensitivity analyses (PSA) were performed. In one-way sensitivity analysis, all variables varied across a plausible range and were obtained from 95% confidence intervals (95% CI) or by assuming a variance of 20% [9, 19]. PSA was performed using a Monte Carlo simulation of 1000 iterations, with all parameters simultaneously varied with a specific pattern of distribution. Based on the recommendations of the ISPOR-SMDM Modeling Good Research Practice Working Group, health utility and probability parameters were set to beta analysis; cost and medical resource utilization parameters were set to gamma distribution [20, 21]. Results of one-way and PSA analyses were presented as a tornado diagram and cost-effectiveness acceptability curve (CEAC), respectively. The WTP threshold was set at three times the gross domestic product (GDP) per capita of China in 2019 ($30,833) [14, 22].
Results
Base Case Outcomes
From the perspective of Chinese society, the total cost was $6907.77 for the anlotinib group and $4776.45 for the placebo group, respectively. Treatment with anlotinib was estimated to provide an incremental 0.12 QALYs compared with placebo group. Thus, the ICER was $17,741.94 per QALY, which did not exceed our established threshold of $30,833/QALY (Table 3). Based on the results above, we consider anlotinib to be a cost-effective option as the third- or further-line treatment of relapsed SCLC.
Table 3.
Cost-effectiveness analysis
| Variables | Anlotinib | Placebo |
|---|---|---|
| Cost ($) | ||
| PFS state | 4232.79 | 278.21 |
| PD state | 2674.97 | 4498.24 |
| Total costs | 6907.77 | 4776.45 |
| Incremental costs | 2131.32 | – |
| Effectiveness (QALYs) | ||
| PFS state | 0.23 | 0.05 |
| PD state | 0.08 | 0.14 |
| Total effectiveness | 0.32 | 0.20 |
| Incremental effectiveness | 0.12 | – |
| ICER ($/QALY) | 17741.94 | |
PFS progression-free survival, PD progressive disease, QALYs quality-adjusted life-years, ICER incremental cost-effectiveness ratio
Sensitivity Analysis
In one-way sensitivity analysis, we varied the mOS and mPFS based on the 95% CI and other variables across a range of ± 20%. The results are shown in a tornado diagram (Fig. 2). The cost of anlotinib was the most influential factor in our study. When the cost of anlotinib ranged from $498.3 to $1162.7, the ICER increased from $8002.19/QALY to $27,481.40/QALY. In addition, the cost-effectiveness analysis was also sensitive to the utility of PFS state for the anlotinib group (uPFS1) and median OS of the anlotinib group (mOS1). When uPFS1 ranged from 0.633 to 0.949, the ICER decreased from $28897.21/QALY to $12800.52/QALY. When mOS1 ranged from 6.1 to 10.3 months, the ICER increased from $11,509.80/QALY to $22,884.27/QALY. Furthermore, median OS of placebo group (mOS2), cost of PD state, median PFS of anlotinib group (mPFS1), utility of PD state and utility of PFS state for placebo group (uPFS2) also influenced the ICER. One-way sensitivity analysis was used to explore the more competitive price of anlotinib, the results of which suggested that when the monthly cost of anlotinib was not > $575.70, the ICER was less than one time the GDP per capita, where anlotinib was worthwhile for third- or further-line treatment of SCLC. After running 1000 iterations of Monte Carlo simulation, the mean costs in anlotinib group were $6840.62 ± 1315.30 with 0.32 ± 0.05 QALYs. PSA suggested that the probability of anlotinib being cost-effective versus the placebo was 78.5% at a WTP of $30,833 per QALY (Fig. 3). In addition, the probability of anlotinib being cost-effective was 26.6% and 61.9% at a WTP threshold of one and two times the GDP per capita, respectively.
Fig. 2.
Tornado diagram of one-way sensitivity analysis to identify influential factors. The influential factors are listed in descending order of value. The dashed line intersecting the bars represents the ICER of $17,741.94 per QALY from the base case results. PFS progression-free survival, OS overall survival, PD progressive disease, AEs adverse events, ICER incremental cost-effectiveness ratio, QALY quality-adjusted life-year
Fig. 3.
Cost-effectiveness acceptability curves. The curves summarize the results of probabilistic sensitivity analysis by estimating probabilities of different treatments being considered optimal strategies at varying WTP thresholds. QALY: quality-adjusted life-year
Discussion
In China, lung cancer is the most commonly diagnosed cancer with 815,563 new cases per year [23], and SCLC is a highly lethal type of lung cancer that has seen few therapeutic advances. When SCLC relapses, there are relatively few effective options for treatment, especially in patients with at least two lines of chemotherapy. Although immunotherapy has been an effective option in various cancer types in recent years, outcomes for SCLC have been modest [24]. A phase 1/2 trial (CheckMate 032) indicated that the median PFS was 1.4 months (95% CI 1.3–1.6) and the median OS was 5.6 months (95% CI 3.1–6.8) for SCLC treated with nivolumab in the third-line setting [25]. ALTER1202 showed anlotinib, a novel multi-target TKI, had significant efficacy versus placebo as third- or further-line treatment for relapsed SCLC, which was a great breakthrough in molecular targeted therapy for SCLC. Our study was the first to evaluate the health and economic outcomes of anlotinib for patients with SCLC. According to our analysis, the ICER was $17,741.94/QALY, which did not exceed our established threshold ($30,833 per QALY). Based on this, we consider anlotinib to be a cost-effective strategy compared with placebo for patients with SCLC who have been treated with at least two lines of chemotherapy in China.
Studies on cost-effectiveness of therapy for SCLC are limited. Smare et al. reported that the ICER was $111,054/QALY for nivolumab versus IV topotecan and $73,110/QALY versus oral topotecan, and nivolumab was considered cost-effective for third-line treatment of SCLC in the USA [26]. However, nivolumab has not received approval for the indication of SCLC in China, and whether it is a worthwhile choice still needs to be explored. Currently, anlotinib is the only approved third-line therapy for SCLC in China. A previous study by Huang [27] found anlotinib is not a cost-effective regimen as the third-line and later treatment for NSCLC. According to that study, anlotinib yielded an additional 0.08 QALY at a cost of $8960.04, resulting in an ICER of $112,000.5 per QALY, which surpassed the WTP threshold (three times the GDP per capita in 2018) in China. One-way sensitivity analysis indicated the utility of PFS state and the cost of anlotinib were the most influential factors, which was similar to the result of our study. Therefore, we speculated that anlotinib was cost-effective in SCLC but not in NSCLC, which might mainly be attributed to the price of anlotinib, which had a significant reduction (36.99%) in early 2021 in China. We found that the ICER would be $32,137.88/QALY for SCLC and beyond the threshold of three times the GDP per capita before China’s national drug price negotiation.
Currently, there is no consensus on the ICER threshold for cost-effectiveness analysis. Different countries have their own standards, and even in the same country, several different threshold values exist simultaneously [28–31]. According to some estimates, each life-year is valued at around three times the annual earnings [32]. The World Health Organization (WHO) recommends that: if the ICER is less than one time the GDP per capita, then the incremental cost is worthwhile; if the ICER is between one and three times the GDP per capita, then the incremental cost is acceptable; and if the ICER is higher than three times the GDP per capita, then the incremental cost is not worthwhile. Three times the GDP per capita for each QALY is the most common WTP threshold adopted in health technology assessment in China [31]. Our study showed that the ICER of anlotinib versus placebo for SCLC was $17,741.94, which lay between one and three times the GDP per capita, so the incremental cost incurred by anlotinib was considered acceptable. Additionally, if the criterion of one times the GDP per capita suggested by Ochalek et al. [31] and Zhao et al. [33] was adopted, the probability of anlotinib being cost-effective was about 26.6%. Because of the impact of the SARS-CoV-2 outbreak on the economic system, the GDP per capita in 2019 rather than 2020 was used in this study.
One-way sensitivity analysis suggested uPFS1 was the second most influential factor in our study. Specific utility values of different health states for SCLC patients have not been established, and the clinical trial ALTER 1202 did not collect adequate information of QoL. Utility values for NSCLC were often applied in previous cost-effectiveness analyses for SCLC [10, 34, 35] because most symptoms between the two subtypes of lung cancer are similar. Nafees et al. [19] explored utilities for metastatic NSCLC through a time trade-off interview of oncologists from different countries including China. Thus, we used the Chinese utility values afforded by Nafees [15, 16] and subtracted the utility decrements induced by grade ≥ 3 AEs [10, 16].
Several limitations of our study should be pointed out. First, the clinical data were not based on patient-level data in clinical practice, but mainly derived from a phase II trial (ALTER1202) with a relatively small sample size. Second, one-way analysis showed the utilities exerted great influence on the results of the model. However, because the critical data were not available in the phase II trial, we collected utility values from previously published literature, which may not be consistent with the real world. Third, both clinical outcomes and costs in this study were derived from Chinese patients, and this may affect the generalizability of our research to other countries.
Conclusion
The inspiring efficacy of anlotinib for third- or later-line treatment of SCLC marks great progress in this recalcitrant disease. Most remarkably, this scheme was found to be cost-effective in our study. The results demonstrated that treatment with anlotinib may improve health outcomes of patients with relapsed SCLC and allow more efficient use of financial resources from the perspective of Chinese society.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Y. Lin for her contributions to part of the costs data collection.
Funding
This study, including the journal’s Rapid Service Fee, was supported by the Medical Pharmacy Fund of Jiangsu Pharmaceutical Association (no. Q202031; A202022) and Changzhou Sci & Tech Program (no. CJ20209013; CE20195048).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Z. Sun and G. Liu conceived the research idea and supervised the work. Z. Sun, J. Gong and Q. Wan designed the study. J. Gong and Q. Wan conducted statistical analysis and interpretation. J. Shang and X. Qian extracted information. D. Su validated the model. J. Gong drafted the original manuscript, Z. Sun and G. Liu reviewed and edited the manuscript.
Disclosures
J. Gong, Q. Wan, J. Shang, X. Qian, D. Su, Z. Sun and G. Liu have nothing to disclose.
Compliance with Ethics Guidelines
This study is based on previously conducted studies and dose not contain any new studies with human participants or animals performed by any of the authors and so does not require the approval of an independent ethics committee.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
Contributor Information
Zhiqiang Sun, Email: jungler@sina.cn.
Guangjun Liu, Email: liugj66@163.com.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;68:1–41. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Sun K, Zheng R, Zeng H, Zhang S, Xia C, et al. Cancer incidence and mortality in china, 2014. Chin J Cancer Res. 2018;30:1–12. doi: 10.21147/j.issn.1000-9604.2018.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stinchcombe TE, Gore EM. Limited-stage small cell lung cancer: current chemoradiotherapy treatment paradigms. Oncologist. 2010;15:187–195. doi: 10.1634/theoncologist.2009-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kraywinkel K, Barnes B. Epidemiology of small cell lung cancer in germany. Onkologe. 2017;23:334–339. doi: 10.1007/s00761-017-0218-6. [DOI] [Google Scholar]
- 5.Demedts IK, Vermaelen KY, van Meerbeeck JP. Treatment of extensive-stage small cell lung carcinoma: current status and future prospects. Eur Respir J. 2010;35:202–215. doi: 10.1183/09031936.00105009. [DOI] [PubMed] [Google Scholar]
- 6.Cheng Y, Wang Q, Li K, Shi J, Liu Y, Wu L, et al. Anlotinib vs placebo as third- or further-line treatment for patients with small cell lung cancer: a randomised, double-blind, placebo-controlled phase 2 study. Br J Cancer. 2021 doi: 10.1038/s41416-021-01356-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou K, Wen F, Zhang P, Zhou J, Zheng H, Sun L, et al. Cost-effectiveness analysis of sensitive relapsed small-cell lung cancer based on jcog0605 trial. Clin Transl Oncol. 2018;20:768–774. doi: 10.1007/s12094-017-1787-y. [DOI] [PubMed] [Google Scholar]
- 8.Liu Q, Luo X, Peng L, Yi L, Tan C. Nivolumab versus docetaxel for previously treated advanced non-small cell lung cancer in china: a cost-effectiveness analysis. Clin Drug Invest. 2019;40:129–137. doi: 10.1007/s40261-019-00869-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han J, Tian K, Yang J, Gong Y. Durvalumab vs placebo consolidation therapy after chemoradiotherapy in stage iii non-small-cell lung cancer: an updated pacific trial-based cost-effectiveness analysis. Lung Cancer. 2020;146:42–49. doi: 10.1016/j.lungcan.2020.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Li LY, Wang H, Chen X, Li WQ, Cui JW. First-line atezolizumab plus chemotherapy in treatment of extensive small cell lung cancer: a cost-effectiveness analysis from china. Chin Med J. 2019;132:2790–2794. doi: 10.1097/CM9.0000000000000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cancer.NetTM. Lung cancer-small cell: Statistics. https://www.Cancer.Net/cancer-types/lung-cancer-small-cell/statistics. Accessed 12 Mar 2021.
- 12.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13:322–338. doi: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- 13.Purmonen T, Martikainen JA, Soini EJO, Kataja V, Vuorinen R-L, Kellokumpu-Lehtinen P-L. Economic evaluation of sunitinib malate in second-line treatment of metastatic renal cell carcinoma in finland. Clin Ther. 2008;30:382–392. doi: 10.1016/j.clinthera.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Liu GE, Hu SL, Wu JH, Wu J, Dong CH, Li HC. China guidelines for pharmacoeconomic evaluations (chinese-english version) Beinjing: China Market Press; 2020. [Google Scholar]
- 15.Nafees B, Stafford M, Gavriel S, Bhalla S, Watkins J. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes. 2008;6:1–84. doi: 10.1186/1477-7525-6-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nafees B, Lloyd AJ, Dewilde S, Rajan N, Lorenzo M. Health state utilities in non-small cell lung cancer: an international study. Asia Pac J Clin Oncol. 2017;13:E195–E203. doi: 10.1111/ajco.12477. [DOI] [PubMed] [Google Scholar]
- 17.National Bureau of Statistics of China. China statistical yearbook (2020). http://www.Stats.Gov.Cn/tjsj/ndsj/2020/indexch.Htm. Accessed 15 July 2021.
- 18.Chen HD, Zhou J, Wen F, Zhang PF, Zhou KX, Zheng HR, et al. Cost-effectiveness analysis of apatinib treatment for chemotherapy-refractory advanced gastric cancer. J Cancer Res Clin Oncol. 2017;143:361–368. doi: 10.1007/s00432-016-2296-z. [DOI] [PubMed] [Google Scholar]
- 19.Wan X, Zhang Y, Tan C, Zeng X, Peng L. First-line nivolumab plus ipilimumab vs sunitinib for metastatic renal cell carcinoma a cost-effectiveness analysis. JAMA Oncol. 2019;5:491–496. doi: 10.1001/jamaoncol.2018.7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Briggs AH, Weinstein MC, Fenwick EAL, Karnon J, Sculpher MJ, Paltiel AD, et al. Model parameter estimation and uncertainty analysis: A report of the ispor-smdm modeling good research practices task force working group-6. Med Decis Making. 2012;32:722–732. doi: 10.1177/0272989X12458348. [DOI] [PubMed] [Google Scholar]
- 21.Richard Edlin CM, Hulme C, Hall P, Wright J. Cost effectiveness modelling for health technology assessment a practical course. Cham: Adis; 2015. [Google Scholar]
- 22.Murray CJL, Evans DB, Acharya A, Baltussen R. Development of who guidelines on generalized cost-effectiveness analysis. Health Econ. 2000;9:235–251. doi: 10.1002/(SICI)1099-1050(200004)9:3<235::AID-HEC502>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 23.International agency for research on cancer. Estimated age-standardized incidence rates (world) in 2020, lung, both sexes, all ages. https://gco.Iarc.Fr/today/online-analysis-map. Accessed 26 Apr 2021.
- 24.Armstrong SA, Liu SV. Immune checkpoint inhibitors in small cell lung cancer: a partially realized potential. Adv Therapy. 2019;36:1826–1832. doi: 10.1007/s12325-019-01008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ready N, Farago AF, Braud FD, Atmaca A, Hellmann MD, Schneider JG, et al. Third-line nivolumab monotherapy in recurrent sclc 032: Checkmate. J Thora Oncol. 2019;14:237–244. doi: 10.1016/j.jtho.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smare C, Dave K, Juarez-Garcia A, Schoenherr N, Abraham P, Penrod JR, et al. A landmark response analysis to determine cost-effectiveness of third-line nivolumab monotherapy for small cell lung cancer. Value Health. 2019;22:S481–S481. doi: 10.1016/j.jval.2019.09.431. [DOI] [Google Scholar]
- 27.Huang J. Cost-effectiveness analysis of anlotinib as a third-line or further treatment in advanced non-small lung cancer. Value Health. 2020;23:S48. [Google Scholar]
- 28.Laupacis A. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ. 1992;146:473. [PMC free article] [PubMed] [Google Scholar]
- 29.Charlton V, Rid A. Innovation as a value in healthcare priority-setting: the UK experience. Soc Just Res. 2019;32:208–238. doi: 10.1007/s11211-019-00333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vanness DJ, Lomas J, Ahn H. A health opportunity cost threshold for cost-effectiveness analysis in the united states. Ann Internal Med. 2020 doi: 10.7326/M20-1392. [DOI] [PubMed] [Google Scholar]
- 31.Ochalek J, Wang H, Gu Y, Lomas J, Cutler H, Jin C. Informing a cost-effectiveness threshold for health technology assessment in china: a marginal productivity approach. Pharmacoeconomics. 2020;38:1319–1331. doi: 10.1007/s40273-020-00954-y. [DOI] [PubMed] [Google Scholar]
- 32.Sachs JD. Direct loss of well-being to the individual. In: Sachs JD, editor. Macroeconomics and Health: Investing in Health for Economic Development, pp 30–33. Switzerland: Geneva;2001.
- 33.Zhao FL, Yue M, Yang H, Wang T, Wu JH, Li SCJMC. Willingness to pay per quality-adjusted life year: is one threshold enough for decision-making? Medical Care. 2011;49:267–272. doi: 10.1097/MLR.0b013e31820192cd. [DOI] [PubMed] [Google Scholar]
- 34.Zhang LF, Hang YF, Liu MB, Li N, Cai HF. First-line durvalumab plus platinum-etoposide versus platinum-etoposide for extensive-stage small-cell lung cancer: a cost-effectiveness analysis. Front Oncol. 2020;10:2717. doi: 10.3389/fonc.2020.602185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou KX, Zhou J, Huang JX, Zhang N, Bai LL, Yang Y, et al. Cost-effectiveness analysis of atezolizumab plus chemotherapy in the first line treatment of extensive-stage small-cell lung cancer. Lung Cancer. 2019;130:1–4. doi: 10.1016/j.lungcan.2019.01.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article/as supplementary information files.




