Abstract
Introduction
Recreational use of nitrous oxide (N2O) is a growing practice in France and all around the world and is often associated with neurological complications. We report detailed clinical and paraclinical presentations of 12 patients with combined degeneration of the spinal cord and peripheral neuropathies in relation to N2O consumption, possibly favored by lockdowns due to SARS-CoV-2 pandemic.
Results
With variable levels of consumption, the 12 patients presented spinal cord and/or peripheral nerve damage, with mostly motor and ataxic symptoms, motor axonal nerve damage, and medullary T2-weighted hyperintensities on MRI. There was a clear improvement in symptoms after vitamin B12 substitution, although some sequelae remained, particularly sensory.
Discussion
We report detailed clinical, electrophysiological, radiological, and biological consequences of N2O abuse in 12 patients. Our data support the clinical and paraclinical observations reported in the literature. The mechanisms of neurological N2O toxicity are still debated. There is currently no precise recommendation on the therapeutic management. The clinical evolution after vitamin B12 substitution seems sufficient but could depend on early management. Effective messages targeting at risk population, but also the health professionals involved, seem crucial as does a better legal framework for this growing practice.
Keywords: Nitrous oxide, Neuropathy, Myelopathy, Neurological disorder
Introduction
Exposure to nitrous oxide (N2O) is associated with multiple complications, especially neurological ones [1]. The first reported cases mainly concerned situations of occupational or post-operative exposure.
Recreational use of N2O, mainly through freely bought food cartridges, inhaled either directly or via a balloon, has sharply increased since a few years. It was believed to be the second most common psychoactive substance consumed by French students between 2015 and 2017 [2]. Since then, there has been a growing frequency of serious complications linked to this consumption, particularly in France, as indicated in reports by the French addictovigilance network and the French Health Security Agency in June 2020. Surveillance data on psychoactive substances consumed in France identify an important and sustained N2O consumption during the SARS-CoV-2 epidemic, especially during the first lockdown, in relation to a difficulty of access to other substances and a need to consume due to inactivity [3]. Worldwide, N2O abuse is already responsible for several cases of death [1].
In our article, we describe clinically, biologically, radiologically and neurophysiologically 12 cases of spinal cord injury and/or peripheral neuropathies secondary to N2O recreational consumption that occurred between August 2020 and April 2021 and were treated in our Neurology Department at the Avicenne University Hospital. All the data were obtained from the records of patients.
Results
The clinical and paraclinical data from all 12 patients are detailed in Table 1. There were 6 males and 6 females with an average age of 22.2 ± 3.3 years. The mean weekly consumption of N2O was of 3109 ± 2800 g with a mean duration of 12.7 ± 8.3 months between the onset of consumption and the hospitalization. Ten patients were regular smokers (cigarette or Hookah), three were occasional cannabis users, and seven were alcohol users (only one in a daily basis). None of them was occupationally exposed to heavy metals, nitrogen protoxide, or industrial solvents. Four of them were unemployed or out of school, one was a student, and the others worked as animators, cleaning lady or delivery drivers.
Table 1.
Detailed demographics, clinical, and paraclinical characteristics of the patients
| Case | Age (y) Sex (M/F) |
N2O Consumption (g/week)* / Duration (weeks) |
Other consumption | Motor dysfunction | Sensory deficit | Ataxia | Areflexia | Other clinical features | MRS on first assessment (0–6)** | Posterior cord lesion on MRI | MRI enhancement | ENMG | Lab findings: B12 (pmol/l) HCY (µmol/l) MMA (µmol/l) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
21 M |
240 / 12 | h |
+ (UL and LL, distal, predominantly in TA) |
+ (UL and LL, distal) |
+ | + (Achilles) | Lh | 1 |
+ (C2–C7) |
+ | Axonal motor length-dependent polyneuropathy of the LL with distal predominance |
217: nl 32: ↑ 1.5: ↑ |
| 2 |
24 F |
4200 / 72 |
a t c |
+ (LL, distal, predominantly in TA) |
+ (LL) |
+ | + (diffuse) | Brief psychotic disorder | 3 |
+ (C2–C5) |
− | Severe axonal length-dependent polyneuropathy of LL with sensory predominance |
134: ↓ 178.5: ↑ 0.6: ↑ |
| 3 |
23 M |
2800 / 72 |
a t c |
− |
+ (T2 level) |
+ | + (diffuse) | Lh, VSD | 4 |
+ (cervico-dorso-lumbar) |
− | Severe motor axonal neuropathy of LL |
158: N 76.8: ↑ 2.1: ↑ |
| 4 |
21 F |
8700 / 96 |
a t |
+ (LL and right UL) |
+ (T4 level) |
+ |
+ (LL) |
Lh, paresthesias of UL and LL, VSD | 3 |
+ (C2–C4) |
− | Pure motor impairment of the two common fibulars |
1142: ↑ 7.6 (after supplementation): ↑ 1.2: ↑ |
| 5 |
21 M |
> 2800 / unk |
h a |
− |
+ (LL) |
+ | + (Achilles) | − | 4 | − | − | Advanced motor sensory axonal neuropathy of LL |
103: ↓ unk unk |
| 6 |
25 F |
20.000 / 1 |
a t |
+ (LL, distal) |
+ (C5 level) |
+ |
+ (diffuse) |
VSD | 4 |
+ (C2–C5) |
+ (medullary and meningeal) |
Pure motor axonal polyneuropathy length dependent |
1208: ↑ 44.9: ↑ 1.4: ↑ |
| 7 |
20 F |
33.600 / 40 | − |
+ (LL, distal) |
+ (T10 level) |
+ |
+ (diffuse except bicipital) |
Paresthesia of LL, Lh, VSD, visual disturbance, paranoia |
4 | − | − | Motor and sensory neuropathy |
165.7: N 94.2: ↑ 17.3: ↑ |
| 8 |
19 M |
5800 / 48 |
a t c |
+ (Psoas) |
+ (T12 level) |
+ | − | Pyramidal reflexes, VSD | 4 |
+ (cervico-thoracic) |
− | N |
151: N 70.1: ↑ 3.8: ↑ |
| 9 |
28 F |
480 / 48 | T |
+ (UL and LL, and axial) |
+ (LL) |
+ | + (diffuse) | VSD, disorientation | 3 | + | − | N |
63.6: ↓ 130.2: ↑ 1.54: ↑ |
| 10 |
27 M |
1680 / 3 | − | − |
+ (UL and LL, distal) |
+ |
+ (LL) |
− | 2 | − | − | Severe axonal and demyelinating neuropathy of LL |
> 2000 (after supplementation): ↑ 154.3: ↑ 13.5: ↑ |
| 11 |
17 H |
720 / 24 | h |
+ (Psoas) |
− | + |
+ (diffuse) |
− | 3 | − | − | Severe axonal length-dependent neuropathy with sensory and motor predominance in UL and LL, respectively |
91: ↓ 117: ↑ 32: ↑ |
| 12 |
20 F |
3360 / 24 |
a t |
+ (UL and LL, distal) |
+ (UL and LL, distal) |
+ | − | − | 2 |
+ (C2–C7) |
− |
Motor axonal polyneuropathy length dependent |
111: ↓ 21.3: ↑ 6.68: ↑ |
y years, M Masculine, F Feminine, a Alcohol, h Hookah, c Cannabis, t Tobacco, ENMG Electroneuromyogram, B12 Vitamin B12 (normal values: 145–570 pmol/L), HCY homocysteine (normal values < 15 µmol/L), MMA Methylmalonic acid (normal values < 0.4 µmol/L), N Normal, unk unknown, Lh Lhermitte sign, TA tibialis anterior, LL lower limbs, UL upper limbs, VSD vesico-sphincterian disorders, + present, − absent,
*Consumption in g per week (1 cartridge = 10 ml = 8 g; 1 canister = 100 cartridges)
**Modified Rankin scale from 0 (normal) to 6 (death)
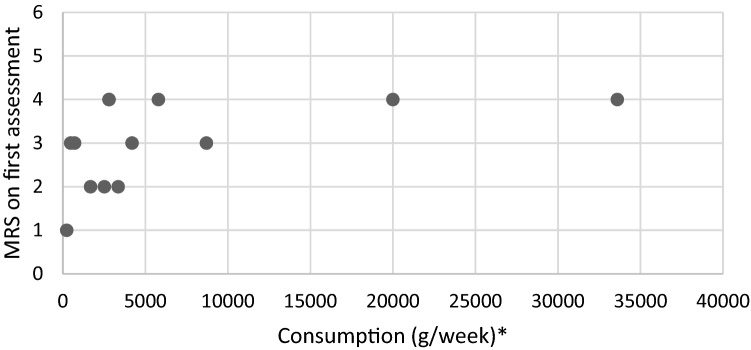
All of the patients presented impaired vibratory sensation or proprioceptive ataxia of lower limbs (12/12) and frequent motor deficits (9/12) predominantly in the lower limbs. Seven patients had spinal cord injury symptoms (pyramidal reflexes, Lhermitte sign, vesico-sphincterian disorders, sensitive spine level). Other neurologic manifestations included dysexecutive syndrome (1/12) and visual disturbance (1/12). Two patients experienced paranoia, one of them (patient 2) requiring a psychiatric hospitalization for a first and transitory psychotic disorder, responsible for a delay in B12 supplementation. As shown in Fig. 1, every patient with an important N2O consumption (> 5000 g/week) had a Rankin scale ≥ 3. However, the small number of included patients did not allow us to highlight a statistically significant association.
Fig. 1.
Relation between nitrous oxide consumption and modified Rankin scale on first assessment. MRS modified Rankin scale. *Consumption in gram per week (1 cartridge = 10 ml = 8 g; 1 canister = 100 cartridges)
Electroneuromyograms showed a neuropathy in 10/12 patients, with typical axonal and motor damage, predominantly in the lower limbs. One presented a demyelinating neuropathy, another initially presented a normal electroneuromyography, but two months later (after supplementation) displayed a pure motor impairment of the lower limbs. Axonal loss was predominant on peroneal nerves with no apparent relationship with N2O consumption. Among the 3 patients with an absence of motor response on peroneal nerves, 2 had a consumption lower than the median.
MRI of the spinal cord showed extensive T2-weighted hyperintensities in 9/12 patients, with a stereotypical involvement of posterior cords, and cervical predominance. One of the patients with normal spinal cord MRI had the highest N2O consumption. Two patients (patients 1 and 6) presented gadolinium enhancement of the posterior cervical cord, one of which was also meningeal (patient 6). MRI control at 2 and 4 weeks, in patients 1 and 6, respectively, showed a stability of the T2-weighted hyperintensities but an improvement in the medullary and meningeal enhancement. Cerebrospinal fluid analyses performed in those two subjects and another patient showed no inflammatory, no oligoclonal bands, and no viral abnormalities. Metabolic, infectious (e.g., HIV, syphilis), and inflammatory tests were performed and were all negative except for a folate deficiency found in three subjects, and a selenium, zinc, and iron deficiency in one patient. As the clinical presentations were all acute or subacute, no genetic testing was performed. In addition, no patient reported a family history of neurological disorders and they all started to clinically improve after N2O weaning and vitamin supplementation.
All our patients benefited from intramuscular (10/12) or oral (2/12) B12 supplementation, but one did not wish to continue treatment (patient 2). Symptoms stabilized in all of them consecutive to N2O withdrawal and B12 vitamin substitution. Long-term follow-up was mostly difficult, with poor adherence to care and patients were frequently lost to follow-up. Eight out of twelve patients have been re-evaluated at a mean time of 7.6 ± 6.9 weeks after supplementation. These patients notably improved with a Rankin score variation of 1.7 ± 1.0, but all of them displayed persistent motor or sensory symptoms at last visit.
Discussion
Here, we described 12 cases of patients developing neurological complications with spinal cord and peripheral nerve involvement secondary to N2O abuse during the SARS-CoV-2 pandemic. Importantly, their clinical and paraclinical conditions improved after B12 supplementation.
Since the summer of 2020, the resurgence of such neurological complications in our Parisian suburb of Seine-Saint Denis Department suggests that the SARS-CoV-2 pandemic with its successive lockdowns may have favored an increased consumption of N2O. Indeed, at least 7 of our 12 patients started their consumption in 2020 or 2021, mostly exceeding the framework of a simply social and "festive" consumption.
The clinical presentations of our patients are comparable to that of cases previously described in the literature [1, 4, 5]. Peripheral nerve damage seems to be at least as frequent as spinal cord damage, with essentially motor, sensory, and ataxic symptoms. The peripheral neuropathy is responsible for a dramatic impairment of autonomy as is the case in our patients, three of them having a modified Rankin score of 4. Although not frequent, we also report the development of psychiatric disorders.
Electroneuromyographic studies described in previous reports showed a high prevalence of motor axonal damages as is the case with our patients, but sometimes results were more heterogeneous, mainly highlighting demyelinating polyneuropathies [4, 6].
T2-weighted hyperintensities on spinal cord MRI are usually stereotyped, essentially posterior, symmetrical, often extensive, and predominantly cervical, with a reported prevalence of 68% to 78% depending on the studies [1, 4]. None of our four patients with brain MRI had any abnormalities; these have rarely been described and appear to be aspecific. Surprisingly, 2/12 patients showed spinal cord contrast enhancement which has only been rarely described [7]. To our knowledge, no cases of meningeal contrast enhancement have been previously reported. Although these patients presented a typical clinico-radiological disorder with clear improvement after N2O withdrawal and B12 vitamin substitution, the hypothesis of an underlying or associated inflammatory disorder remains possible. In such cases, prolonged clinical and radiological follow-up appears essential.
The most sensitive biological markers of these N2O-related disorders in the literature seem to be an increase in methylmalonic acid and homocysteine levels [4] Vitamin B12 levels are often below normal limits, although the deficit might be, at least initially, only functional. The assays performed in our patients are consistent with these observations. A control, carried out in 2 of our patients, one month after supplementation showed a normalization of methylmalonic acid and homocysteine levels.
There is no consensus on the mode, dose, and minimum duration of vitamin B12 supplementation. Although essentially intramuscular, some authors propose an oral supplementation by analogy with the management of pernicious anemia where high daily oral doses (1000–2000 µg/d) could bring similar results [8]. Interestingly, a myeloprotective efficacy of methionine has been demonstrated in certain animal models of vitamin B12 deficiency [9] and although no controlled studies have been carried out in humans, some authors propose this molecule as a complement to vitamin substitution.
Among the cases reported in the literature, follow-up data, when available, show that the clinical course is mostly favorable, with partial if not complete recovery [1]. This is similar to supplemented cases of combined marrow sclerosis due to vitamin B12 deficiency for which long-term sequelae remain in only 6% of patients [10]. However, some authors point to a higher rate of sequelae [11, 12]. Early vitamin B12 supplementation and N2O withdrawal seem to play a determining role in this evolution [10, 12].
A threshold for toxic consumption seems difficult to determine, because of the wide variety of consumption patterns and modes, and because it is likely that this threshold varies between subjects depending on different factors [13]. However, a probability model has recently been proposed, illustrating a dose–response relationship between the number of cartridges consumed per session and the occurrence of paresthesia [14].
Several pathophysiological hypotheses have been proposed to explain the occurrence of N2O complications and involve the inactivation of vitamin B12 by oxidation of the cobalt atom it contains. Two pathways resulting from the active forms of this vitamin in the human body (AdoB12 and MetB12) were implicated to explain those complications: Either from an imbalance of fatty acids and by extension of myelin synthesis or via a decrease in the production of S-adenosyl methionine, a universal CH3 donor, which would play an important role in the synthesis of myelin binding protein and myelin lipids. More recently, it has been proposed that myelopathy lesions could result from a disturbance in the cytokine balance between TNF alpha and IL6/IL10, with S-adenosyl methionine appearing to be involved in their production, and their myelotoxic or myeloprotective roles having, respectively, been demonstrated [15].
Although our study is limited by its retrospective feature, we reported numerous cases of neurological N2O-induced complications. Further prospective studies are needed notably to determine which treatment regimen is preferable and to assess the long-term improvement by clinical examination, biological tests, ENMG, and medullary MRI.
Regarding the increasing frequency of N2O abuse and its potential neurological sequelae, the importance of effective public health messages targeting at-risk populations and of information for the health professionals who have to deal with them seems crucial, as should be a better legal framework for this practice.
Acknowledgements
The authors thank Sébastien Valverde for English editing
Funding
This study was not supported by any funding.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
Code availability
Not applicable.
Declarations
Conflicts of interest
The authors declare that they have no competing interests.
Ethical approval
The current study obtained the authorization of the Local Ethics Committee of Avicenne Hospital (CLEA-2021-200).
Consent to participate
Written consents were obtained from all participants.
Consent for publication
Written consents were obtained from all participants.
Footnotes
Raphael Vollhardt and Julie Mazoyer have equally contributed to this work.
References
- 1.Garakani G, Jaffe RJ, Salva D, et al. Neurologic, psychiatric, and other medical manifestations of nitrous oxide abuse: a systematic review of the case literature. Am J Addict. 2016 doi: 10.1111/ajad.12372. [DOI] [PubMed] [Google Scholar]
- 2.Perino J, Letinier L, Mathieu C, et al. Consommation de substances psychoactives : un état des lieux au sein des étudiants de la cohorte i-Share. Ther Recreat J. 2018;73:575. doi: 10.1016/j.therap.2018.09.019. [DOI] [Google Scholar]
- 3.Lapeyre-Mestre M, Boucher A, Daveluy A, et al. Addictovigilance contribution during COVID-19 epidemic and lockdown in France. Therapie. 2020;75:343–354. doi: 10.1016/j.therap.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oussalah A, Julien M, Levy J, et al. Global burden related to nitrous oxide exposure in medical and recreational settings: a systematic review and individual patient data meta-analysis. J Clin Med. 2019 doi: 10.3390/jcm8040551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng D, Ba F, Bi G, et al. The sharp rise of neurological disorders associated with recreational nitrous oxide use in China: a single-center experience and a brief review of Chinese literature. J Neurol. 2020;267:422–429. doi: 10.1007/s00415-019-09600-w. [DOI] [PubMed] [Google Scholar]
- 6.Tani J, Weng H-Y, Chen H-J, et al. Elucidating unique axonal dysfunction between nitrous oxide abuse and vitamin B12 deficiency. Front Neurol. 2019;10:704. doi: 10.3389/fneur.2019.00704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ernst LD, Brock K, Barraza LH, et al. Longitudinally extensive nitrous oxide myelopathy with novel radiographic features. JAMA Neurol. 2015;72:1370–1371. doi: 10.1001/jamaneurol.2015.2141. [DOI] [PubMed] [Google Scholar]
- 8.Stabler SP. Clinical practice. Vitamin B12 deficiency. N Engl J Med. 2013;368:149–160. doi: 10.1056/NEJMcp1113996. [DOI] [PubMed] [Google Scholar]
- 9.Scott JM, Dinn JJ, Wilson P, Weir DG. Pathogenesis of subacute combined degeneration: a result of methyl group deficiency. Lancet. 1981;2:334–337. doi: 10.1016/s0140-6736(81)90649-8. [DOI] [PubMed] [Google Scholar]
- 10.Healton EB, Savage DG, Brust JC, et al. Neurologic aspects of cobalamin deficiency. Medicine (Baltimore) 1991;70:229–245. doi: 10.1097/00005792-199107000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Thompson AG, Leite MI, Lunn MP, Bennett DLH. Whippits, nitrous oxide and the dangers of legal highs. Pract Neurol. 2015;15:207–209. doi: 10.1136/practneurol-2014-001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vasconcelos OM, Poehm EH, McCarter RJ, et al. Potential outcome factors in subacute combined degeneration: review of observational studies. J Gen Intern Med. 2006;21:1063–1068. doi: 10.1111/j.1525-1497.2006.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lacassie HJ, Nazar C, Yonish B, et al. Reversible nitrous oxide myelopathy and a polymorphism in the gene encoding 5,10-methylenetetrahydrofolate reductase. Br J Anaesth. 2006;96:222–225. doi: 10.1093/bja/aei300. [DOI] [PubMed] [Google Scholar]
- 14.Winstock AR, Ferris JA. Nitrous oxide causes peripheral neuropathy in a dose dependent manner among recreational users. J Psychopharmacol. 2020;34:229–236. doi: 10.1177/0269881119882532. [DOI] [PubMed] [Google Scholar]
- 15.Hathout L, El-Saden S. Nitrous oxide-induced B12 deficiency myelopathy: perspectives on the clinical biochemistry of vitamin B12. J Neurol Sci. 2011;301:1–8. doi: 10.1016/j.jns.2010.10.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Not applicable.