Abstract
The spectrum of human pathogens and the infectious diseases they cause is continuously changing through evolution, selection and changes in the way human populations interact with their environment and each other. New human pathogens often emerge or re-emerge from an animal reservoir, emphasizing the central role that non-human reservoirs play in human infectious diseases. The 1918 pandemic of influenza virus A/H1N1 and the 2020 pandemic of coronavirus disease 2019 (COVID-19) are the most dramatic examples of this in recent human history. Pathogens can also re-emerge with new characteristics, such as multidrug resistance, or in different places, such as Ebola virus in West Africa in 2013 and Zika virus in Brazil in 2015, to cause new epidemics. Most human pathogens have a history of evolution in which they first emerge and cause epidemics, become unstably adapted, re-emerge periodically and then – without intervention – eventually become endemic, with the potential for future outbreaks.
Keywords: Drivers of emergence, emerging infections, hotspots for emergence, MRCP, species jump, zoonosis
Key points.
-
•
Infectious diseases are continuously emerging
-
•
Most known human pathogens, including severe acute respiratory syndrome coronavirus 2, are zoonoses, and most that are not have zoonotic origins
-
•
Globalization and human invasiveness create more opportunities for emergence
-
•
Global surveillance and research consortia and novel technologies will allow for more frequent and more rapid detection of novel pathogens
-
•
A more proactive and global approach to mitigation of emergence events is required
Introduction
In the 1970s, with antibiotics and vaccines at hand and the eradication of smallpox within reach, there was general optimism that infectious diseases would soon be a thing of the past. In 1972 the Nobel laureate Macfarlane Burnet concluded that: ‘If … we retain a basic optimism and assume no major catastrophes occur and that any wars are kept at the ‘brush fire’ level, the most likely forecast about the future of infectious disease is that it will be very dull.’ The HIV pandemic crushed this optimism, and infectious diseases were put back on the global health agenda, of which the 1992 publication Emerging Infections: Microbial Threats to Health in the United States has been a landmark (see Further Reading).
Since then, the emergence of antimicrobial resistance among many different pathogens, including against last-resort antibiotics, the continuous emergence of (mostly) viruses with potential for human-to-human or pandemic spread – severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the disease it causes, coronavirus disease 2019 (COVD-19), being the most recent and most dramatic example – and the intentional release of pathogens as terrorist weapons continuously reminds us that infectious diseases are actually far from dull.
Definitions
‘Emerging infectious diseases’ are defined as ‘those whose incidence in humans has increased within the past two decades or threatens to increase in the near future’. Emergence can be from the spread of new agents (including known agents with a new resistance mechanism), from recognition of an infection that has been present but undetected in the population, or from the realization that an established disease has an infectious origin. Emergence can also be used to describe the reappearance (or re-emergence) of a known infection after a decline in incidence (see Further Reading).
Zoonotic emergence
Pathogen
There are >1400 known human pathogens, most (60%) of which are transmitted to humans zoonotically and depend on an animal reservoir for survival. An additional smaller proportion (5–10%) is environmentally transmitted, and the remainder consists of pathogens that can be maintained by an exclusively human-to-human transmission cycle. Among emerging infections, the proportion of zoonotic infections is even higher (73%), indicating that the human–animal interface presents a risk for emergence.1 In addition, almost all (now) established strictly human pathogens have zoonotic origins: these pathogens have moved from animals into humans and fully adapted to them during many millennia of human and pathogen evolution.1 , 2
Human
Because most human pathogens rely on an animal or environmental reservoir, the interactions between human populations and their surrounding ecosystem determine the local pathogen spectrum, and the interpopulation interactions determine the spread of these pathogens. Historically, there have been several profound and distinct transitions in human environmental and interpopulation interactions that have radically changed the spectrum and causes of infectious disease in human populations (Table 1 ). Today, we are living through the fourth great historical transition. The invasiveness of human activity into all geographical areas of the world, the globalization of economic activities and culture, the speed and accessibility of distant contact, the spread and intensification of urbanization, and our increasing reliance on either intricate or massive technology, are reshaping the relations between humans and microbes.3
Table 1.
Transitions in human environmental and interpopulation interactions through time
| Transition, time | Major change |
|---|---|
| Prehistoric transition, millions of years ago | From tree-dwelling to savannah, hunter-gatherer |
| Historic transitions | |
| • First (local), 5000–10,000 years ago | Settlements, crop and livestock domestication |
| • Second (continental), 1000–3000 years ago | Intracontinental military and commercial contacts |
| • Third (intercontinental), from 1500 ce | European exploration and imperialism |
| • Fourth (global), today | Globalization, urbanization, climate change |
The species jump
The jump between species that initiates a first human infection by a new agent is often brought about by a novel or unusual physical contact between potential pathogen and human. Such contacts usually occur because of cultural, social, behavioural or technological change on the part of humans that affects the human–animal interface. The potential for subsequent spread of this ‘new’ infectious disease depends on many different factors, including environmental or social factors. These changes and factors (Table 2 ) are the drivers of emergence (see Further Reading).
Table 2.
Biological, social and environmental drivers of emergence of infectious disease
|
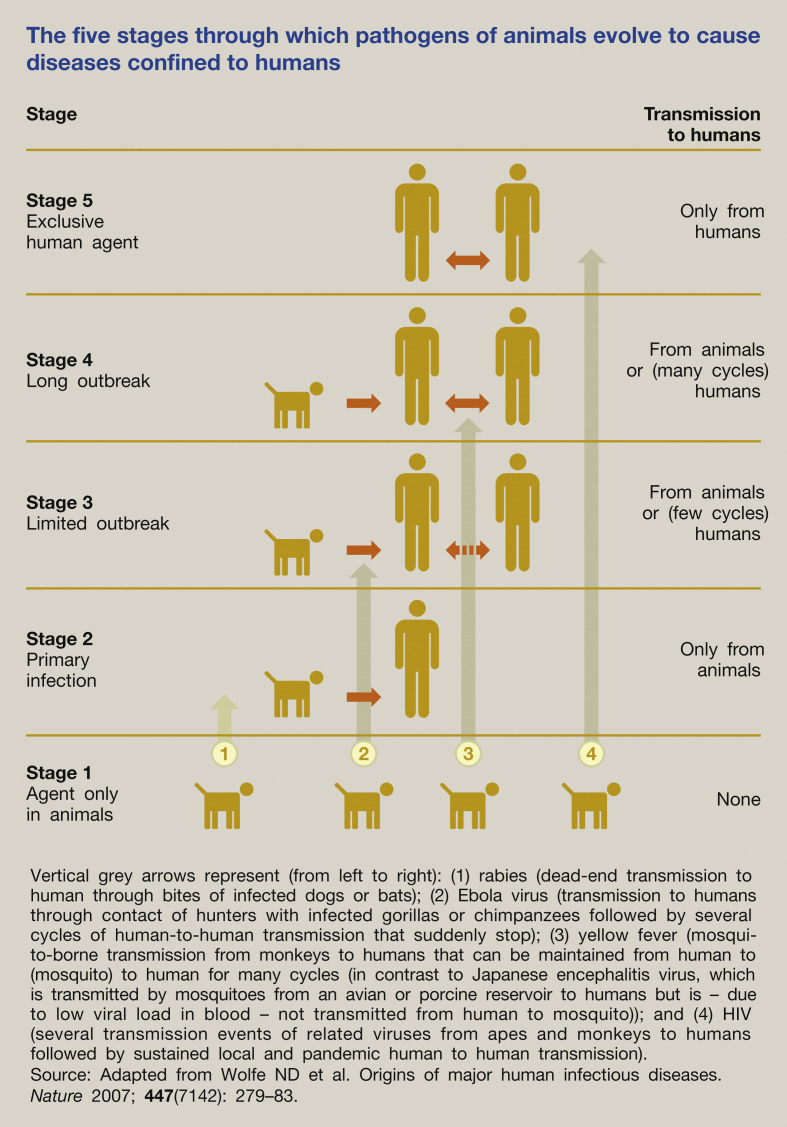
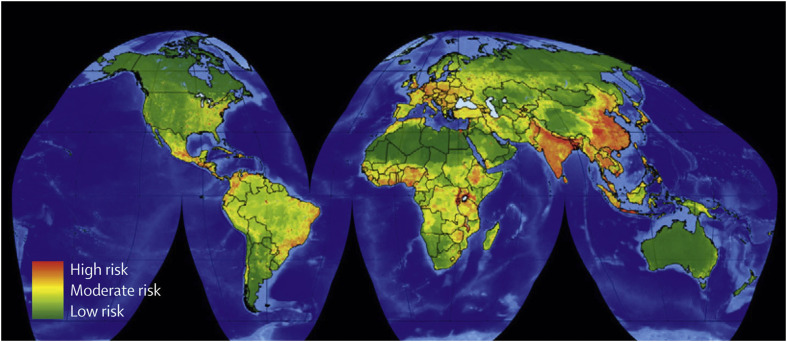
Rather than being a single event, biologically the species jump is often more a transition process involving several stages (Figure 1 ). The pathogen has to overcome various biological barriers (interspecies, intrahuman, interhuman) to move from one stage to the next, to be able finally to cause sustained human-to-human transmission. Based on data from 1940 onwards, the hotspots for emergence of infectious diseases were mapped for zoonotic infections from wildlife and domestic animals, and for drug-resistant and vector-borne organisms. Figure 2 shows that these hotspots are primarily located in South and South-East Asia, South and Central America and Sub-Saharan Africa.4
Figure 1.
The five stages through which pathogens of animals evolve to cause diseases confined to humans.
Figure 2.
Global hotspots for emerging diseases originating in wildlife. Source: From Morse SS, Mazet JA, Woolhouse M, et al. Prediction and prevention of the next pandemic zoonosis. Lancet 2012; 380: 1956–65. With permission from Elsevier.
Various international consortia and large research programmes have been established in an attempt to predict and prevent, or prepare for and mitigate, these novel emergence events. Technical advances enable us to detect, characterize and trace these agents much more rapidly than ever before. Experience shows that we have not been able to appropriately predict, anticipate or tackle emergences of unknown pathogens such as Middle East respiratory syndrome coronavirus (MERS-CoV), known pathogens such as Ebola and Zika virus, or even the annual reappearance of H7N9 influenza in China. A more proactive approach, for example using platform technologies to develop libraries of vaccines and monoclonal agents that can be mass produced when needed, seemed therefore warranted. On the other hand, the emergence of SARS-CoV-2 was predicted from 2005 onward from sequences of coronaviruses in bats in caves in Yunnan, China, closely related to SARS-CoV-1 and able to bind known human receptors.5 But even in the context of a rapidly emerging pandemic, many countries were unable or unwilling to invest resources to prevent entry and spread of the virus in early 2020.
A pandemic is an epidemic that has spread across a large region, for example multiple continents or globally. Globalization and increased air travel are enablers of rapid pandemic spread. In recent history, most zoonotic pandemics were caused by influenza viruses. Because of their rapid transmission and ability to transmit from human to human before the host develops symptoms, if at all (pre-symptomatic or asymptomatic transmission), they are very difficult, if not impossible, to mitigate and contain. From 1900 onward we have seen very severe (1918 H1N1), moderate (1957 H2N2, 1968 H3N2) and mild (2009 H1N1pdm09) pandemics of influenza.
It was thought that coronaviruses like SARS-CoV-1 and MERS-CoV posed a smaller threat of becoming pandemic because of their inability for pre- and asymptomatic transmission. The reports in early 2020 that a substantial proportion of SARS-CoV-2-infected patients did not yet display symptoms or showed no symptoms at all meant almost automatically that SARS-CoV-2 would cause a very different epidemic from SARS-CoV-1 and MERS-CoV, an event that most infectious disease researchers will remember as clearly as the 11 September 2001 attacks on the New York City World Trade Centre.
Non-zoonotic emergence
The emergence of novel zoonotic pathogens is appealing to the imagination and draws plenty of popular and scientific media attention, but does not necessarily represent the only threat from infectious diseases. There is a ‘slow pandemic’ of antimicrobial drug resistance among bacteria and other pathogens. Drug resistance is a threat to the successful treatment of not only HIV, malaria and tuberculosis, but also hospital- and community-acquired infections caused by ‘normal’ Gram-positive and Gram-negative bacteria; these have been predicted to have a major economic impact if not addressed. This has been recognized as a global public health emergency, requiring a global effort to raise awareness, implement regulation and develop novel antibiotics or alternative approaches to prevention and treatment.
Failure of vaccination programmes because of bad press or religious conviction in developed countries can within years cause re-emergence of highly infectious viruses, such as those causing measles or rubella, as has happened in the USA and Europe. Global food production and distribution processes can give rise to widely disseminated foodborne infections that are hard to tackle, and to rapid spread of bacteria with novel resistance mechanisms. Finally, in the Asia-Pacific region, although avian influenza viruses attract most international attention, hand, foot and mouth disease, caused by a fluid spectrum of non-polio enteroviruses, is associated with hundreds of thousands of hospitalizations of children aged <5 every year; this includes with neurological and cardiopulmonary complications and a mortality of around 0.1%, showing that humans can also be a source of emerging infections (Table 3 ).
Table 3.
Selection of important emerging infectious diseases since 2003
| 2020 | SARS-CoV-2 |
| 2016 | Plasmid-mediated colistin resistance in Enterobacteriaceae (mcr-1) |
| 2015 | Zika virus |
| 2013 | Ebola virus in West Africa |
| Influenza virus A/H7N9 | |
| Candida auris invasive hospital-acquired infections | |
| 2012 | MERS-CoV |
| 2011 | Escherichia coli O104:H4 |
| 2010 | New Delhi metalloprotease-associated carbapenem resistance in Enterobacterales |
| Huaiyangshan virus, associated with severe fever and thrombocytopenia syndrome | |
| 2009 | Influenza virus A/H1N1pdm09 |
| 2008 | Artemisinin-resistant Plasmodium falciparum |
| Plasmodium knowlesi | |
| Lujo virus | |
| 2006 | Extensively drug-resistant Mycobacterium tuberculosis |
| 2005 | Human retroviruses HTLV-3 and HTLV-4 |
| 2004 | Re-emergence of influenza virus A/H5N1 |
| 2003 | SARS-CoV |
The role of the doctor
In daily medical practice, it is important that healthcare workers, especially intensive care and infectious disease physicians, are aware of emergence events and countries where processes of emergence and species-jumping are occurring (e.g. by subscribing to ProMED, FluTrackers, the World Health Organization influenza update, International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) or others). It is crucial that each patient's history should include a travel history, which involves more than asking for the countries the patient has visited. In the end, despite sophisticated surveillance programmes, it is usually an astute clinician who, after having seen or heard one or two extraordinary patient histories, makes the connection and sees the first signs of an event of emergence.
Key references
- 1.Taylor L.H., Latham S.M., Woolhouse M.E. Risk factors for human disease emergence. Philos Trans R Soc Lond B Biol Sci. 2001;356:983–989. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss R.A. The Leeuwenhoek Lecture 2001. Animal origins of human infectious disease. Philos Trans R Soc Lond B Biol Sci. 2001;356:957–977. doi: 10.1098/rstb.2001.0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMichael A.J. Environmental and social influences on emerging infectious diseases: past, present and future. Philos Trans R Soc Lond B Biol Sci. 2004;359:1049–1058. doi: 10.1098/rstb.2004.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones K.E., Patel N.G., Levy M.A., et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu B., Zeng L.-P., Yang X.-L., et al. Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further reading
- 6.Lederberg J., Shope R.E., Oaks S.C., Institute of Medicine (US) Committee on Emerging Microbial Threats to Health . National Academy Press; Washington, DC: 1992. Emerging infections: microbial threats to health in the United States. [PubMed] [Google Scholar]