Abstract
Background:
Relative to the abundance of publications on dementia and clock drawing there is limited literature operationalizing ‘normal’ clock production.
Objective:
To operationalize subtle behavioral patterns seen in normal digital clock drawing to command and copy conditions.
Methods:
From two research cohorts of cognitively-well participants aged 55+ who completed digital clock drawing to command and copy conditions (n=430), we examined variables operationalizing clock face construction, digit placement, clock hand construction, and a variety of time-based, latency measures. Data are stratified by age, education, handedness, and number anchoring.
Results:
Normative data are provided in supplemental tables. Typical errors reported in clock research with dementia were largely absent. Adults age 55+ produce symmetric clock faces with one stroke, with minimal overshoot and digit misplacement, and hands with expected short to long ratio. Data suggest digitally acquired graphomotor and latency differences based on handedness, age, education, and anchoring.
Discussion:
Data provide useful benchmarks from which to assess digital clock drawing performance in Alzheimer’s Disease and related dementias.
Mesh terms: executive function; attention; memory, short-term; digital technology; cognition; aging; reference standards
MeSH Key Words: dementia, neuropsychological tests, adult, middle aged, laterality
“If I could give only one test to a patient, the test I would choose would be the Clock Drawing Test”
- Edith Kaplan
Introduction
There is considerable research demonstrating how clock drawing test (CDT) patterns can assess cognition for individuals with mild cognitive impairment (MCI) and Alzheimer’s/Vascular Spectrum Dementia (AVSD); [1] see [2–13]. Dementias with dominant subcortical changes such as Parkinson’s disease with dementia (PDD) and small vessel vascular dementia (SVD) produce greater graphomotor impairment and more errors of commissions relative to peers with dementia such as Alzheimer’s disease (AD) [1, 4, 6, 10]. When comparing command versus copy clock construction patterns, individuals with AD often improve from command to copy, while individuals with PDD and SVD do not [2, 4, 6, 7, 14]. Depending on the disease severity, individuals with AD and PDD often draw poor clock face contours and display omissions, commissions, and poor ordering of numbers. Failure to draw two hands, poor hand placement, and superfluous markings are also observed in neurodegenerative samples [15]. These are some reasons why the CDT is an excellent first-line measure to screen for emergent neurodegenerative conditions [16].
Relative to the abundance of publications on dementia and paper and pencil clock drawing normative references [15], there is limited literature describing digitally acquired ‘normal’ clock production. To date, neuropsychology research on clock drawing is largely administered via paper-and-pencil, and scoring is based on accuracy or error production. This approach is inherently limiting for appreciating subtle behavioral patterns in cognitively-well adults who produce few to no errors on examination. Through digital technology advances [17], researchers can now record subtle behavioral features of normal clock drawing construction. For example, researchers report clock drawing latency measurements differ by age groups [18], associate with traditional neuropsychological domains in non-demented older adults [19], provide insight into subtle cognitive changes after surgical procedures [20], present differently for individuals with clinical depression [21], mild cognitive impairment [22], and that the act of anchoring numbers (placing 12, 3, 6, 9 prior to other numbers) informs us about brain connectomics [23].
To date, however, there is only one normative publication on digital clock drawing. Piers and colleagues (2017) worked within a large sample of stroke- and dementia-free Framingham Heart Study participants to provide information on processing speed and higher order problem solving as measured by digitally-acquired total clock drawing time and intercomponent latencies. The publication provides important evidence on latency metrics and pen stroke frequency for six different age groups (age ranges 20–98). Older adult cohorts (primarily age ≥60) took longer (in seconds) to complete clock drawing, had longer latencies between clock drawing components (time between face, hand, numbers), and produced more drawing strokes. Although the team presents an impressive sample size across each age group, the authors recognized that the older adult samples, in particular, may have included individuals with some cognitive impairment. Additionally, the study is largely focused on time-based metrics; graphomotor behaviors addressing clockface, number, and hand production behaviors are not provided for normative comparison.
We designed the current investigation to provide a more comprehensive normative reference of digitally-acquired clock drawing behaviors from well characterized cognitively-well adults age 55 or above. The goal was to operationalize the production of a “normal” appearing clock as measured with digital technology. We specifically chose to examine a relative circumscribed corpus of digitally obtained clock variables related to the three components necessary to ‘draw the face of a clock, put in all the numbers, and set the hands to ten after eleven’ including (1) drawing of the clockface, (2) drawing and placement of numbers inside the clock face, and (3) the accurate placement of the clock hands. The corpus’ digital clock variables were stratified by age, education, handedness, and the use of anchor digits.
Methods
Participants:
Participants provided written informed consent and the investigation as conducted in accordance with the Declaration of Helsinki. Data are from two cohorts: 1) Framingham Heart Study (FHS) 2nd generation participants who had completed neuropsychological protocols and the digital clock drawing test (dCDT); 2) cognitively well participants from Florida, who had completed neuroimaging, neuropsychological protocol, and dCDT via federally funded research investigations.
FHS Cohort: Inclusion- Individuals age 55 plus who had maintained consistent neuropsychological follow-up within the Gen 2 cohort for exams 7, 8, and 9, and had completed exam 9 dCDT with standard neuropsychological metrics and were identified as “cognitively-well” through Latent Class Analysis (LCA) steps: 1) The LCA required inclusion of nine neuropsychological tasks as indicator variables [24]: Wechsler Memory Scale (WMS) Logical Memory immediate free recall, delayed free recall and delayed recognition; Wechsler Adult Intelligence Scale (WAIS) Digit Span Backwards; WAIS Similarities; the Boston Naming Test; Trails Making Test- Parts A and B; Verbal Fluency task for the letters F, A and S; and the ‘animal’ fluency test; 2) Raw performance scores were converted to z-scores based on the grand mean and entered into an LCA that yielded four groups; 3) Individuals with average or higher standardized scores relative to sample were retained for the investigation. Exclusion- Did not meet FHS study criteria requiring review for suspected cognitive impairment.
Florida Cohort: Individuals age 55 plus with intact instrumental activities of daily living [25], and who had completed a cognitive screening for dementia over the phone (TICS-M; [26]), a face-to-face interview with a clinical neuropsychologist, and completed the dCDT neuropsychological research protocol with tests standardized according to available published normative references [27]. Exclusion criteria: Individuals with MCI (single or multidomain) were excluded from the final sample based on the comprehensive criteria from Jak and colleagues (2009) using age-adjusted normative data [28, 29]; medical illness potentially limiting lifespan; major psychiatric disorder; history of head trauma; documented learning disorder; <6th grade education; substance abuse within the last year; major cardiac disease (i.e. congestive heart failure); English as a second language; major chronic medical illness limiting lifespan.
Digital Clock Drawing Test:
In the present study, we applied the digital technology referenced in Souillard-Mandar et al., (2016)[17]. A trained test administrator administered the dCDT to command using the instructions to “Draw the face of a clock, put in all the numbers, and set the hands to ten after eleven” followed by a copy condition. Drawings were completed using digital pen from Anoto, Inc. and associated smart paper [17]. Although drawings are digitally recorded, the pen acts as a normal ballpoint pen, therefore, in the event of corrupted digital data a traditional hard copy (ink on paper) is available to the examiner. The pen technology captures and measures pen positioning on smart paper 75 times/second. 8.5 × 11inch smart paper folded in half giving participants a drawing area of 8.5 × 5.5inch. An in-house software system (dCDT classification assist tool) classified each pen-stroke with at least 84% accuracy. To ensured appropriate scoring, a trained rater with high reliability (93–99% accuracy) then deconstructed and replayed each clock drawing recordings to review all computer classifications [17].
dCDT Variables of Interest (Tables 1 & 2).
Table 1:
Digital Clock Variables Organized by Clock Element
| Variable Name (units) | Variable Description |
|---|---|
| Graphomotor Variables | |
| cTotal Strokes | Total number of strokes used in drawing all components of this clock |
| Clockface Variables | |
| cCF Total Strokes | Total number of strokes used to draw the clockface |
| dCF Overshooting Distance (mm) | The minimum distance between start of the longest clockface stroke and the end, divided by the total length of the stroke. Gives a normalized measure of how far apart or overlapped the closest points near the end of the stroke are |
| dCF Overshooting Angle (degrees) | Number of degrees between start and end of the longest stroke in first clockface, measured from center of ellipse. Positive: end of stroke goes past the start, Negative: end does not go past the start |
| bCF Drawing Direction | Direction clockface was drawn, either clockwise or counterclockwise |
| dCF Area (CFA) (mm) | aArea of a circle fitted to the drawn clockface |
| dCF Symmetry (mm) | aMeasure of how circular or oblong the clockface is, using horizontal and vertical dimensions |
| Digit Variables | |
| cMissing | Number of digits not appearing on the clock |
| cOutside | Number of digits placed outside the clockface |
| cPerseverations digits | Number of digits that are repeated |
| cConsecutive Digits | Number of digits drawn consecutively, with no items, such as hands or center dot, drawn between them |
| dDistance to Clockface (mm) | aAverage distance from each digit to the nearest point on the clockface |
| dWidth (mm) | Average width of the bounding boxes for all numbers on the clock |
| dHeight (mm) | Average height of the bounding boxes for all numbers on the clock |
| cAnchoring | Placement of 12,3,6,9 prior to other digits |
| dMisplacement (degrees) | aA summation of each digit’s distance from ideal placement |
| Hour and Minute Hand Specific Variables | |
| dLength3 (mm) | Distance from innermost to outermost point on the hand |
| dDistance to Center (mm) | Distance from inner end of each hand to center of the clockface represented as an ellipse |
| dHour and Minute Hand angle (degrees) | Difference between each hand angle and the perfect angle; Negative for the hour or minute hand indicates the hour hand is to the left of the ideal hour hand placement |
| dHour Hand/Minute Perseverations hands | aRepresents the ratio of hour hand length the minute hand length |
| dTime and Latency Variables (secs) | |
| Total Clock Time (TCT) | Total time to draw all clock components |
| CF Total Time | Time to draw the clockface |
| Post Clockface Latency (PCFL) | Delay between finishing the last stroke in the clockface and whatever is drawn next |
| Pre-First Hand Latency (PFHL) | Delay between whatever was drawn before the first clock hand and starting to draw that clock hand |
| Pre-Second Hand Latency (PSHL) | Delay between whatever was drawn before the second clock hand and starting to draw that clock hand |
| Pre-Center Dot Latency (Pre-CDL) | Lag before start of drawing center dot (if there is one) |
| Post-Center Dot Latency (Post-CDL) | Lag after drawing the center dot (if there is one) |
Variable required calculations. See Appendix 1 for details and calculation formulas
Nominal Data
Ordinal Data
Interval/Ratio Data
Table 2:
Digital Clock Variable Calculation Descriptions and Formulae
| Variable Name | Description of Calculation | Formula |
|---|---|---|
| CF Area (CFA) | The vertical and horizontal diameters of the fitted ellipse are halved to form radii. Average the vertical and horizontal radii to generate the radius of the closest fit perfect circle. Then use this radius in the formula for area of a circle | |
| CF Symmetry | Signifies a ratio between the symmetry of the top and bottom halves of the ellipse with the right and left halves of the ellipse. If the ratio is less than 1, the ellipse is vertically oblong. If the ratio is greater than one, the ellipse is horizontally oblong. |
Note: horizontal symmetry is measured as the average distance between corresponding points on either side of the face, and vertical symmetry is measured as the average distance between corresponding points on the top and bottom of the face |
| Distance to Clockface | The average of each digit’s distance to the nearest point on the clockface. |
Note: The distance for a single digit is calculated using the center of the digit’s bounding box, which is a rectangle surrounding the digit’s highest and widest points |
| Misplacement | The degrees of difference from ideal for each digit could be positive or negative depending on whether it is placed clockwise or counterclockwise of its ideal location. To account for this, and just identify how far the variable is from ideal, the absolute value of each digit’s ideal difference was calculated and then summed. | |
| Hour Hand/Minute Hand | Ratio between the lengths of the hour hand and minute hand. When this ratio is less than one, the hour hand is shorter than the minute hand. |
The dCDT provides over 2500 potential variables. Based on our team’s collective experience with neurology, neuropsychology, dementia, and clock research, we selected variables considered relevant for understanding clock face, digit, and hand placement, as well as component latency variables.
Across the FHS and Florida cohorts we stratified dCDT variables by age in ten-year groups starting at age 55, resulting in four groups. Education was grouped into three categories: <13 years, 13–16 years, >16 years. Handedness was defined as right or left-dominant. For ambidextrous individuals, the hand used for clock drawing determined left or right handedness.
Statistical Analyses:
Cohort Invariance: We used measurement invariance (MI) in a structural equation modeling (SEM) framework [30, 31] to determine if dCDT metrics were comparable between the two participant cohorts [32]. Following published recommendations [32, 33] we assessed MI using both χ2 difference tests for the four sequential models, alternative fit indices (i.e., comparative fit index [CFI], root mean square error of approximation [RMSEA], and standardized root mean square residual [SRMR]) with invariance defined as an absolute decline ≥ 0.005 in CFI (ΔCFI), as well as an increase ≥ 0.010 in RMSEA (ΔRMSEA) and/or an increase ≥ 0.005 in SRMR (ΔSRMR). Since we were not interested in deriving structural elements of the model (e.g., creating a scale score for dCDT variables), we did not assess the equivalence of factor variances, covariances, or means (i.e., structural invariance [32].
Normative values for continuous dCDT variables across the combined cohort are expressed as means and standard deviations, medians, and interquartile ranges (IQR), and ranges. Categorical dCDT variables are expressed as frequencies and percentages. Additional normative references with mean, standard deviation, and range are provided based on the following groupings: a) age: 55–65, 66–75, and 76–85; b) education: high school or less (<13), some college to college graduate (13–16), graduate school (>16); c) anchoring; and d) handedness. Data are provided for normative purposes only and to guide future research.
Results
Participant demographics (Figure 1a, b; Tables 3, 4):
Figure 1a.
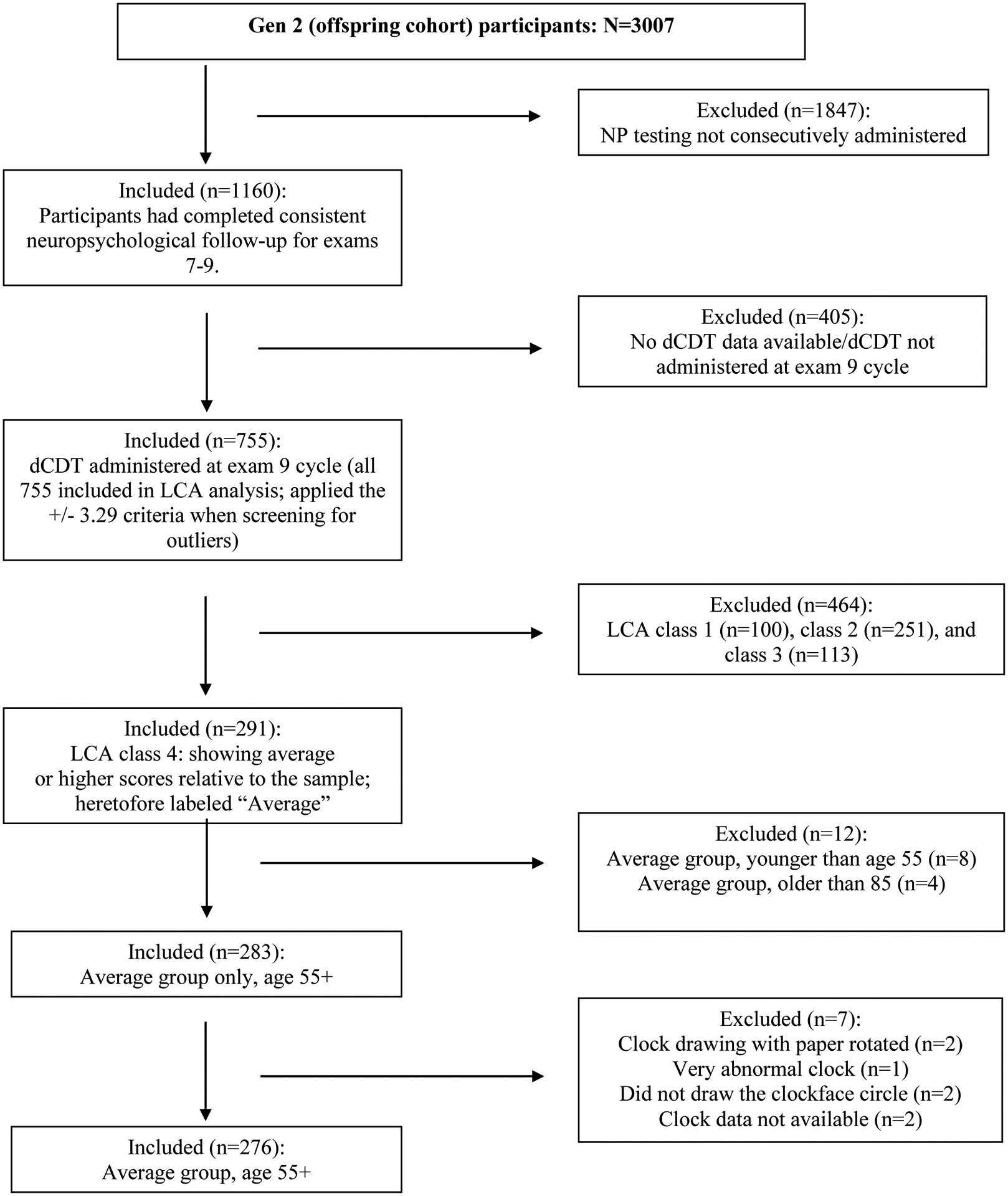
Framingham Corpus Selection
Figure 1b.
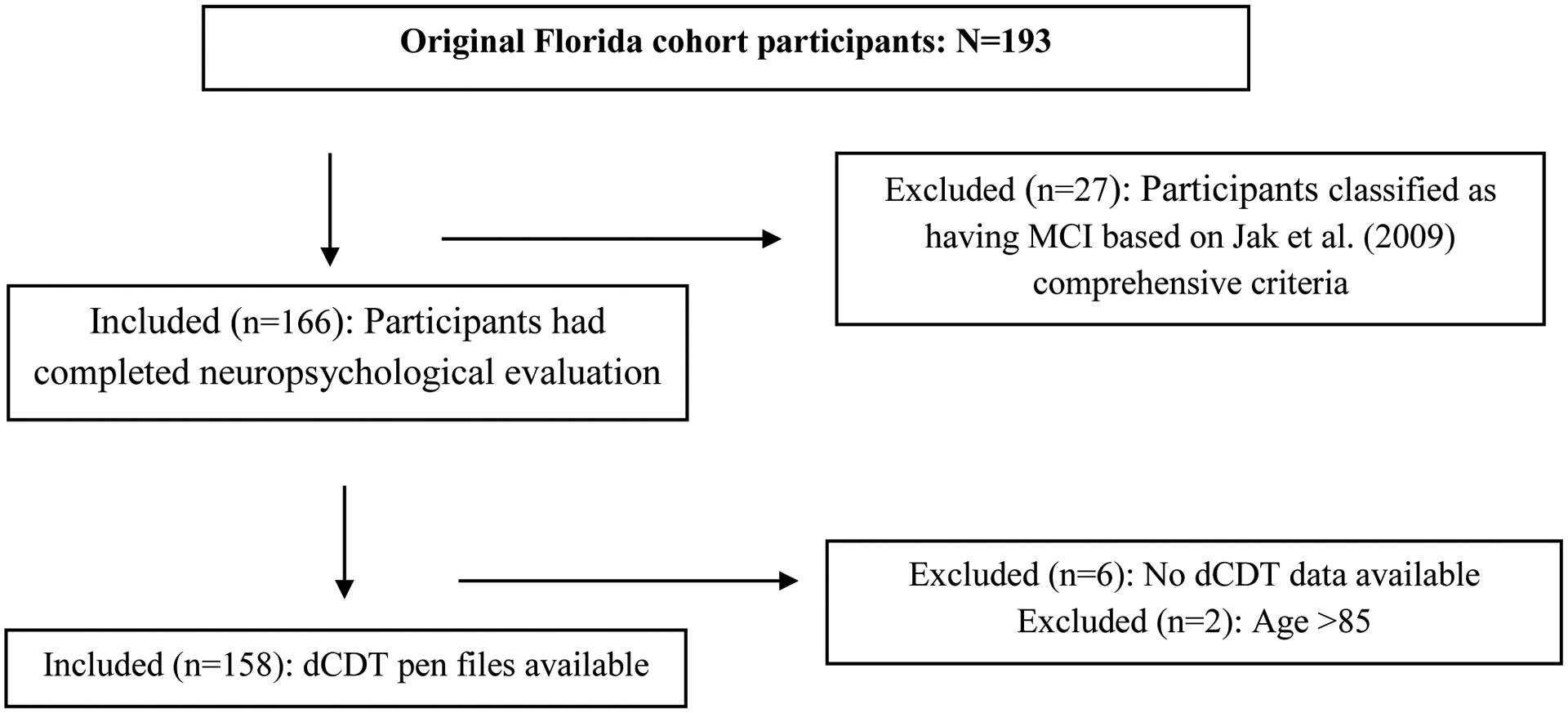
Florida Cohort Selection
Table 3:
Demographics and Raw Neuropsychological Test Scores by Cohort
| Test | Mean (SD) [Min, Max] | |
|---|---|---|
| FHS Cohort (n=272) | Florida Cohort (n=158) | |
| Age | 67.85 (6.46) [55, 84] | 68.30 (5.49) [55, 83] |
| Education | 16.75 (3.34) [8, 21] | 16.31 (2.61) [9, 24] |
| WRAT | 51.52 (3.82) [38, 57] | 52.77 (3.20) [41, 57] |
| WTAR | -- | 43.63 (5.77) [27, 50] |
| MMSE2 | 29.34 (0.94) [25, 30] | 28.80 (1.07) [25, 30] |
| WAIS/WAIS-III Digit Span Forward | 6.90 (1.24) [4, 9] | 6.98 (1.24) [5, 9] |
| WAIS/WAIS-III Digit Span Backward | 5.08 (1.23) [3, 8] | 5.40 (1.23) [2, 8] |
| TMT-A | 29.85 (9.30) [13, 71] | 32.40 (9.34) [16, 64] |
| TMT-B | 74.51 (34.19) [30, 274] | 74.40 (24.54) [31, 190] |
| COWA Letter Fluency | 44.25 (11.38) [16, 78] | 43.51 (11.87) [11, 75] |
| COWA Semantic Fluency | 20.50 (4.72) [7, 35] | 22.49 (4.42) [13,37] |
| BNT | 29.01 (1.16) [25, 30] | 57.40 (2.47) [48, 60] |
Note. Mini Mental State Examination: MMSE; Wechsler Test of Adult Reading: WTAR; Word Reading Association Test: WRAT; Trail Making Test Part A & B: TMT-A & TMT-B; Controlled Oral Word Association: COWA; Boston Naming Test: BNT. For the FHS cohort BNT even-item form (0–30) was administered, whereas for the Florida cohort the score represents full BNT scores (0–60).
WRAT: FHS (n=272), Florida cohort (n=110); WTAR: Florida cohort (n=47).
MMSE: n=324; MoCA: n=112. MoCA scores were converted to MMSE scores [41]
Table 4:
Demographics for the Combined Cohorts
| Variable | N=430 | Range |
|---|---|---|
| Age, mean (SD) | 68.01 (6.12) | [55, 84] |
| Gender: Female N (%) | 249 (57.91 %) | - |
| Race: White N (%) | 423 (98.37%) | - |
| Education, mean (SD) | 16.59 (3.09) | [8, 24] |
| Handedness: righthanded N (%) | 381 (88.60 %) | - |
FHS Cohort: From 3,007 individuals within the Gen 2 cohort, we identified 755 with full neuropsychological protocols and dCDT at exam 9. LCA analyses identified 291 high-performing individuals with average or higher neuropsychology scores. None of the individuals had FHS review classification for MCI or dementia. After exclusion for corrupt or missing digital files, the final sample included 272 individuals.
Florida Cohort: From a total of 193 participants who completed dCDT as part of the neuropsychological protocol, 27 were excluded due to meeting criteria for MCI, and 6 had missing pen files. The final Florida sample included 158 individuals.
The final total sample includes 436 participants between ages 55 and 92, with an education ranging from 8–24 years, split across sexes (57.57% female), Caucasian (98.39%), and self-reported dominant right-handed (88.76%). There were no significant differences between cohort age or education (p’s> .13). Tables 3, 4
Cohort Invariance analysis:
MI analyses showed adequate support for combining cohorts for both conditions (Supplemental Table 1). dCDT variables are summarized by domain (general strokes, clock face, digit placement, hands) below. For the misplacement sum variable, we excluded individuals with missing digits (n=5). For average digit misplacement, we included all individuals but divided by the number of digits.
Supplemental Tables 2–19 provide descriptive data for dCDT variables of interest.
Supplemental Figures 1–10 show distributions of the raw digital clock drawing variables by cohort and condition.
Total Pen Strokes:
Participants took on average 24.92 (±5.22) pen strokes in the command and 24.02 (±2.57) pen-strokes in the copy condition. Age, education, anchoring, handedness: There were no discernable differences in number of pen strokes.
Clockface (CF):
Command: Participants predominantly produced the CF circle using a single pen stroke (median=1.00, mean=1.12 pen strokes) with minimal overshoot distance (median=0.00, mean=0.00), and an overshooting angle of 11.32 degrees (median= 8.79, mean=11.32). Fourteen of 430 (3%) participants drew an incomplete CF. On average, CFs were slightly more elongated horizontally (CF Symmetry; median= 1.20, mean= 1.32) and CF area had a wide distribution. Copy: Participants continued to predominantly produce the CF circle using a single pen stroke (median=1.00, mean=1.07 pen strokes) with minimal overshoot distance (median= 0.00, mean= 0.00), and moderate overshooting angle (median=11.39, mean=13.87). Nine of 430 participants drew an incomplete CF. CFs were slightly more elongated horizontally (CF Symmetry; median= 1.24, mean= 1.35), and CF area was generally smaller in the copy relative to the command condition. Age, education, anchoring, and handedness: There were no discernable differences in CF production based on these demographics. The majority of right-handers produced the CF in counterclockwise direction (94.23%) whereas less than half of left-handers (45.83%) did so.
Digits:
Command: On average, digits were placed 6.69mm away from the CF (median= 6.64, mean= 6.69), with small deviation from ideal angle (median= 60.01 degrees, mean= 70.53 degrees). In the command, 5 of 430 participants (1.16%) omitted one or more of the twelve digits, and 33 of 430 (7.67%) placed one to twelve digits outside the CF. Copy: Digits were placed, on average, 5.61mm away from the CF (median= 5.43mm, mean= 5.61mm), with deviation from ideal angle (median= 60.92 degrees, mean= 65.82 degrees). In the copy condition, five participants (1.16%) omitted only one digit, and 10 participants (2.32%) placed one to three digits outside the CF circle. No participant displayed perseverated digits for command or copy. Age, education, anchoring, and handedness: There were no discernable age and handedness differences. Less misplacement was observed in more educated groups for command (<13 years: 77.88 degrees, 13–16 years: 74.54 degrees, and >16 years: 63.27 degrees) and copy (<13 years: 73.82 degrees, 13–16 years: 65.82 degrees, >16 years: 63.90 degrees). Non-anchorers displayed higher digit misplacement relative to anchorers in the command (non-anchorers: 76.78 degrees; anchorers: 64.56 degrees) and copy (non-anchorers: 66.37 degrees; anchorers: 65.06 degrees). More participants anchored for command (51.16%) than copy (42.09%). Anchoring frequency was higher for younger older adults in our cohort (ages 55–64 = 54.35%; ages 75–84 = 44.44%) and for individuals with a college education (>50%) relative to high school or fewer years of formal schooling (34.09%). There were no anchoring patterns by handedness.
Clock Hands (CH):
Command: Across the age samples, the lowest/innermost points of the hour hand and minute hands were drawn above the center of the CF. There was minimal difference in CH from the ideal angle, with an expected shorter hour to minute hand (hour to minute hand ratio= 0.76, median= 0.68). Copy: Across the age groups, the lowest/innermost points of the hands were drawn closer to the center of the CF, with an expected shorter hour to minute hand (mean ratio= 0.63, median= 0.61). There were no unistrokes, i.e., no participant connected the numbers “11” and “10” by drawing a single line. Age, education, anchoring, and handedness: There were no discernable differences.
Latencies:
Command: Participants took on average 35.02 (±12.52) seconds to complete the drawing with a post-CF latency of 1.51 (±1.81) seconds, a pre-first hand latency of 2.54 (±2.36) seconds, and a pre-center dot latency of 1.98 (±2.12) seconds. A subset of participants (24.65%) did not use a center dot. Pre-second hand latency (1.57 seconds) was on average shorter than pre-first hand latency (2.54 seconds). Copy: Participants took on average 27.40 (±8.93) seconds to complete the drawing, with a post-CF latency of 1.33 (±1.13) seconds, a pre-first hand latency of 1.15 (±0.95) seconds, and a pre-center dot latency of 1.21 (±0.87) seconds. A subset of participants (30.00%) did not use a center dot. Pre-second hand latency (1.06 seconds) was on average slightly shorter than pre-first hand latency (1.15 seconds). There were shorter copy condition times relative to command condition for TCT, CF Total Time, PCFL, PFHL, and PSHL. Age, education, anchoring, and handedness: Latency variables increased across the age and education cohorts. Data shows that anchorers took longer to draw the command clock (~1.5 seconds longer) relative to non-anchorers. Left-handed participants took longer to draw clocks (~2 seconds longer) for command and copy conditions relative to right-handers.
Discussion
This study used digital technology to operationalize normal clock drawing for adults age 55 plus. The investigation included data from two cohorts selected based on cognitive testing indicating at least average or higher cognitive functions. We applied confirmatory factor analysis to test measurement invariance between the clock drawing performance across the two cohorts and determined feasibility for combining the normative groups. We report on the contributions of age, educational attainment, handedness, and anchoring behavior on various latencies and graphomotor elements of clock drawing.
Within our sample, dCDT to command and copy conditions involved on average 25 pen-strokes with a relatively symmetrical clock face often completed with a single pen movement in a counterclockwise direction, and with minimal CF overshoot. Consistent paper and pencil normative references[6, 15], the command relative to copy condition showed larger CF area, larger digit height and width, longer hands, and larger distance between innermost hand placement relative to ideal center. Digital technology also revealed that number/ digit misplacement was minimal, on average, with six degrees of misplacement per digit. This level of misplacement was maintained across both conditions (maximum misplacement possible per digit is 180 degrees). Post-hoc frequencies for hour and minute hand misplacement shows slightly higher percentage of participants with hand angle misplacement biased towards the twelve (command: 50% vs. copy: 59%), with the minute hand biased towards the spatial location of the number one (command: 52% vs. copy: 65%). The difference in the CF area between the command and copy conditions is consistent with the ratio of command to copy changes for digit height and width, hour hand length, and angles.
Also consistent with normative expectations [6, 15], cognitively-well adults also produced minimal behaviors typically performed by patients with cognitive disorders such as dementia (e.g., perseverations, omissions). Less than 2% of the sample had missing digits (only five individuals missed one digit or did not put in any digits), no participant had perseverated digits, less than 2% had perseverated hands, although some individuals did scratch out hand placement as a self-correction (command: 13.72%; copy: 7.44%). There were no instances where a participant drew a single line connecting the numbers “ten” and “eleven”, a behavior often referred to as a unistroke. A measurable number of participants omit the center dot (approximately 25% per condition) and the significance of this remains to be determined. Collectively, these findings remain consistent with the concept that individuals who are cognitively-well produce minimal perseverative errors or omissions, and when they do make errors, they self-correct [4, 6, 14].
These collective drawing behaviors took on average approximately half of a minute in each condition. Descriptive statistics suggest participants take longer to draw in the command relative to copy condition, with latencies longest for pre-first hand latency and time to set the center dot. Longer drawing and latency times within the command condition appear to be consistent with previous research; command drawing time appears to be more cognitively demanding and accurate hand placement, in particular, at least partially reflects working memory and inhibitory functions [19].
Time to completion was longer across age groups. Total completion time incrementally increased up to 10 seconds from youngest to oldest age group for both conditions. This is consistent with Piers and colleagues (2017) reporting that older age groups have longer total completion time and decision-making latencies on dCDT [18]. Age differences are also seen in the CF area with smaller clocks in the command for older adults and larger clock area in the copy condition. These collective results are consistent with normal age-related expectations of processing speed [34] and suggest that clock drawing nuances are a viable metric for studying aging-related changes of visuoconstruction and attention [35].
Contrary to age, the total years of education appear to differentiate peers on graphomotor output and planning. Anchoring was seen in 50% of the individuals with a college education or higher, but only 34% for individuals with high school or fewer years of formal schooling. In addition, participants who used anchoring showed fewer degrees of digit misplacement and their hands were closer to center than non-anchors. We speculate the trade-off in anchoring behavior may be reflected in the slight time increase in the overall drawing of the command condition (~1.5 seconds) relative to non-anchorers. Prior research shows anchorers outperform non-anchorers on measures of executive function and learning/memory tests, and show more modular brain network involving the ventral (‘what’) visuospatial processing stream as measured by connectomics [23]. It is unknown, however, if anchoring behavior may also reflect a compensatory strategy of those with brain disease. Future research should examine anchoring in low and high educational groups and in neurodegenerative profiles.
Clock drawing performance differed based on self-reported handedness. The most striking difference is the direction of CF construction, with the vast majority of the right-handers producing a CF in counterclockwise direction (94.23%); whereas less than half of left-handers (45.83%) did so. This percentage matches published neuropsychological observations that approximately 50% of left-handers draw clockwise or from right to left [15]. A review of means and standard deviations suggests left-handed participants may take longer to draw clocks (~2 seconds longer) in both conditions. Handedness groups did not differ in missing digits, digits drawn outside the CF, digit perseveration, and anchoring behavior.
The current research study is not without limitations. There is clear lack of ethnic diversity and educational range. Future research needs to expand normative references to ethnoracially, geospatially, and more educationally diverse samples. There is possibility the sample within the FHS included individuals with subjective cognitive impairment, unidentified MCI, or unknown neurological illness. We also a priori selected individuals who had completed annual neuropsychological evaluations and the dCDT on exam 9. Annual evaluations may have inadvertently introduced a practice effect such that the individuals’ cognitive performance was slightly higher than typical at exam 9. Although our percentage of left-handers included in this study are within the range of population estimates [36, 37], our rates are based on self-report relative to dominant writing hand, and likely include participants who were ambidextrous in other physical domains. Future research on handedness and clock drawing may prove beneficial, particularly for left-handed older adults with neurodegenerative disorders [37, 38]. In addition, data regarding employment status and occupation; known contributors to cognitive performance, were not included in the present study and should be considered in future investigations. Given the known contributions of cardiovascular disease risk on cognitive functioning and since we excluded participants with major medical comorbidities, such as cardiac disease, our findings may not generalize to those individuals. Finally, while our MI analyses supported combining the FHS and Florida cohorts, the findings should be interpreted with caution. Future studies should assess MI using an exploratory structural equation modeling procedure. We also encourage future research to examine pen pressure, since it may provide diagnostic classification between healthy individuals and those with amnestic MCI, as well as those with mild dementia due to AD[39]. Given the various digital platforms used for clock drawing in the literature, future research should ascertain construct validity of pen pressure variables across devices such as tablet administration tools.
Despite limitations, the present report provides comprehensive normative data for objectively-quantified clock drawing latencies and graphomotor features using available digital technology. Digital clock drawing is commercially available to researchers and clinicians, and can be administered either via digital pen technology with associated smart paper[17], or via tablet technologies[40], and so our data can be compared to those other platforms to improve evidence based clinical care approaches. The participants were well characterized and involved in established investigations with the capacity for ruling out cognitive impairment or more severe diseases. Our analyses were limited to descriptive statistics to minimize false positive errors. The reported patterns provide insight into subtle behavioral differences across the middle to older ages, years of education, and handedness. Although we do not include a clinical sample, the current normative references will provide a comparison point for researchers and clinicians needing a normative comparison for clinical samples. Data will also hopefully guide investigations addressing digitally acquired clock drawing profiles in AD and related dementias.
Supplementary Material
Acknowledgements:
We wish to acknowledge and thank the many participants who participated in this investigation, the coordinators (particularly Katie Rodriguez, BS., Ebony Blaize, B.S., and Donna Weber, B.A.) who worked diligently for data recruitment and data entry.
Funding:
Research was supported through the National Institute of Health (R01AG055337, all authors; AG008122, AG016495, AG033040, AG054156, AG049810, AG062109, RA; NIBIB R21EB027344-01, PR; R01NS082386, CP; CD; R01NR014810, CP), as well as N01-HC-25195, RA; HHSN268201500001I, RA; NSF 13-543, CP, DP, RD; IIS 1750192, PR, and Pfizer, RA.
Footnotes
This manuscript is dedicated to Edith Kaplan (1924–2009)
Conflict: Authors have no conflict to disclose.
References
- [1].Emrani S, Lamar M, Price CC, Wasserman V, Matusz E, Au R, Swenson R, Nagele R, Heilman KM, Libon DJ (2020) Alzheimer’s/Vascular Spectrum Dementia: Classification in Addition to Diagnosis. J Alzheimers Dis 73, 63–71. [DOI] [PubMed] [Google Scholar]
- [2].Ahmed S, Brennan L, Eppig J, Price CC, Lamar M, Delano-Wood L, Bangen KJ, Edmonds EC, Clark L, Nation DA, Jak A, Au R, Swenson R, Bondi MW, Libon DJ (2016) Visuoconstructional Impairment in Subtypes of Mild Cognitive Impairment. Appl Neuropsychol Adult 23, 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cahn-Weiner DA, Williams K, Grace J, Tremont G, Westervelt H, Stern RA (2003) Discrimination of dementia with lewy bodies from Alzheimer disease and Parkinson disease using the clock drawing test. Cogn Behav Neurol 16, 85–92. [DOI] [PubMed] [Google Scholar]
- [4].Cosentino S, Jefferson A, Chute DL, Kaplan E, Libon DJ (2004) Clock drawing errors in dementia: neuropsychological and neuroanatomical considerations. Cogn Behav Neurol 17, 74–84. [DOI] [PubMed] [Google Scholar]
- [5].Herrmann N, Kidron D, Shulman KI, Kaplan E, Binns M, Leach L, Freedman M (1998) Clock tests in depression, Alzheimer’s disease, and elderly controls. Int J Psychiatry Med 28, 437–447. [DOI] [PubMed] [Google Scholar]
- [6].Libon DJ, Malamut BL, Swenson MR, Cloud BS (1996) Further analysis of clock drawings among demented and non-demented subjects. Archives of Clinical Neuropsychology 11, 193–211. [PubMed] [Google Scholar]
- [7].Price CC, Cunningham H, Coronado N, Freedland A, Cosentino S, Penney DL, Penisi A, Bowers D, Okun MS, Libon DJ (2011) Clock drawing in the Montreal Cognitive Assessment: recommendations for dementia assessment. Dement Geriatr Cogn Disord 31, 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rouleau I, Salmon DP, Butters N, Kennedy C, McGuire K (1992) Quantitative and qualitative analyses of clock drawings in Alzheimer’s and Huntington’s disease. Brain Cogn 18, 70–87. [DOI] [PubMed] [Google Scholar]
- [9].Royall DR, Palmer RF, Chiodo LK, Polk MJ, Markides KS, Hazuda H (2008) Clock-drawing potentially mediates the effect of depression on mortality: replication in three cohorts. Int J Geriatr Psychiatry 23, 821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Royall DR, Cordes JA, Polk M (1998) CLOX: an executive clock drawing task. J Neurol Neurosurg Psychiatry 64, 588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Schillerstrom JE, Sawyer Baker P, Allman RM, Rungruang B, Zamrini E, Royall DR (2007) Clock drawing phenotypes in community-dwelling African Americans and Caucasians: results from the University of Alabama at Birmingham study of aging. Neuroepidemiology 28, 175–180. [DOI] [PubMed] [Google Scholar]
- [12].Shulman KI (2000) Clock-drawing: is it the ideal cognitive screening test? Int J Geriatr Psychiatry 15, 548–561. [DOI] [PubMed] [Google Scholar]
- [13].Tuokko H, Hadjistavropoulos T, Miller JA, Beattie BL (1992) The Clock Test: a sensitive measure to differentiate normal elderly from those with Alzheimer disease. J Am Geriatr Soc 40, 579–584. [DOI] [PubMed] [Google Scholar]
- [14].Libon DJ, Swenson RA, Barnoski EJ, Sands LP (1993) Clock drawing as an assessment tool for dementia. Arch Clin Neuropsychol 8, 405–415. [PubMed] [Google Scholar]
- [15].Freedman M, Leach L, Kaplan E, Winocur G, Shulman KI, Delis DC (1994) Clock Drawing: A Neuropsychological Analysis, Oxford University Press, New York, NY. [Google Scholar]
- [16].Hazan E, Frankenburg F, Brenkel M, Shulman K (2018) The test of time: a history of clock drawing. Int J Geriatr Psychiatry 33, e22–e30. [DOI] [PubMed] [Google Scholar]
- [17].Souillard-Mandar W, Davis R, Rudin C, Au R, Libon DJ, Swenson R, Price CC, Lamar M, Penney DL (2016) Learning Classification Models of Cognitive Conditions from Subtle Behaviors in the Digital Clock Drawing Test. Mach Learn 102, 393–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Piers RJ, Devlin KN, Ning B, Liu Y, Wasserman B, Massaro JM, Lamar M, Price CC, Swenson R, Davis R, Penney DL, Au R, Libon DJ (2017) Age and Graphomotor Decision Making Assessed with the Digital Clock Drawing Test: The Framingham Heart Study. J Alzheimers Dis 60, 1611–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dion C, Arias F, Amini S, Davis R, Penney D, Libon DJ, Price CC (2020) Cognitive Correlates of Digital Clock Drawing Metrics in Older Adults with and without Mild Cognitive Impairment. J Alzheimers Dis 75, 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hizel LP, Warner ED, Wiggins ME, Tanner JJ, Parvataneni H, Davis R, Penney DL, Libon DJ, Tighe P, Garvan CW, Price CC (2019) Clock Drawing Performance Slows for Older Adults After Total Knee Replacement Surgery. Anesth Analg 129, 212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cohen J, Penney DL, Davis R, Libon DJ, Swenson RA, Ajilore O, Kumar A, Lamar M (2014) Digital Clock Drawing: differentiating “thinking” versus “doing” in younger and older adults with depression. J Int Neuropsychol Soc 20, 920–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Binaco R, Calzaretto N, Epifano J, McGuire S, Umer M, Emrani S, Wasserman V, Libon DJ, Polikar R (2020) Machine Learning Analysis of Digital Clock Drawing Test Performance for Differential Classification of Mild Cognitive Impairment Subtypes Versus Alzheimer’s Disease. J Int Neuropsychol Soc 26, 690–700. [DOI] [PubMed] [Google Scholar]
- [23].Lamar M, Ajilore O, Leow A, Charlton R, Cohen J, GadElkarim J, Yang S, Zhang A, Davis R, Penney D, Libon DJ, Kumar A (2016) Cognitive and connectome properties detectable through individual differences in graphomotor organization. Neuropsychologia 85, 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lezak MD, Howieson DB, Loring DW (2004) Neuropsychological Assessment, Oxford University Press, Inc., New York. [Google Scholar]
- [25].Lawton MP, Brody EM (1969) Assessment of older people: self-maintaining and instrumental activities of daily living. The Gerontologist 9, 179–186. [PubMed] [Google Scholar]
- [26].Cook SE, Marsiske M, McCoy KJ (2009) The use of the Modified Telephone Interview for Cognitive Status (TICS-M) in the detection of amnestic mild cognitive impairment. J Geriatr Psychiatry Neurol 22, 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Strauss E, Sherman EMS, Spreen O (2006) A compendium of neuropsychological tests : administration, norms, and commentary, Oxford University Press, Oxford; New York. [Google Scholar]
- [28].Clark LR, Delano-Wood L, Libon DJ, McDonald CR, Nation DA, Bangen KJ, Jak AJ, Au R, Salmon DP, Bondi MW (2013) Are empirically-derived subtypes of mild cognitive impairment consistent with conventional subtypes? J Int Neuropsychol Soc 19, 635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jak AJ, Bondi MW, Delano-Wood L, Wierenga C, Corey-Bloom J, Salmon DP, Delis DC (2009) Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriatr Psychiatry 17, 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Millsap RE (1997) Invariance in measurement and prediction: Their relationship in the single-factor case. Psychological Methods 2. [Google Scholar]
- [31].Borsboom D (2006) The attack of the psychometricians. Psychometrika 71, 425–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Putnick DL, Bornstein MH (2016) Measurement Invariance Conventions and Reporting: The State of the Art and Future Directions for Psychological Research. Dev Rev 41, 71–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chen FF (2007) Sensitivity of goodness of fit indexes to lack of measurement invariance. Structural Equation Modeling: A Multidisciplinary Journal 14, 464–504. [Google Scholar]
- [34].Salthouse TA (1992) Influence of processing speed on adult age differences in working memory. Acta Psychol (Amst) 79, 155–170. [DOI] [PubMed] [Google Scholar]
- [35].Shelton PA, Bowers D, Heilman KM (1990) Peripersonal and vertical neglect. Brain 113 (Pt 1), 191–205. [DOI] [PubMed] [Google Scholar]
- [36].Annette M (1985) Left, Right, Hand, and Brain: The Right Shift Theory, Erlbaum. [Google Scholar]
- [37].Satz P (1972) Pathological left-handedness: an explanatory model. Cortex 8, 121–135. [DOI] [PubMed] [Google Scholar]
- [38].Hugdahl K, Zaucha K, Satz P, Mitrushina M, Miller EN (1996) Left-handedness and age: comparing writing/ drawing and other manual activities. Laterality 1, 177–183. [DOI] [PubMed] [Google Scholar]
- [39].Muller S, Herde L, Preische O, Zeller A, Heymann P, Robens S, Elbing U, Laske C (2019) Diagnostic value of digital clock drawing test in comparison with CERAD neuropsychological battery total score for discrimination of patients in the early course of Alzheimer’s disease from healthy individuals. Sci Rep 9, 3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Muller S, Preische O, Heymann P, Elbing U, Laske C (2017) Diagnostic Value of a Tablet-Based Drawing Task for Discrimination of Patients in the Early Course of Alzheimer’s Disease from Healthy Individuals. J Alzheimers Dis 55, 1463–1469. [DOI] [PubMed] [Google Scholar]
- [41].Bergeron D, Flynn K, Verret L, Poulin S, Bouchard RW, Bocti C, Fulop T, Lacombe G, Gauthier S, Nasreddine Z, Laforce RJ (2017) Multicenter Validation of an MMSE-MoCA Conversion Table. J Am Geriatr Soc 65, 1067–1072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


