Abstract
The objective of this study was to investigate the effects of dietary administration of l-arginine (Arg) or N-carbamylglutamate (NCG) on hepatic energy status and mitochondrial functions in suckling Hu lambs with intrauterine growth retardation (IUGR). Forty-eight newborn Hu lambs of 7 d old were allocated into 4 treatment groups of 12 lambs each, in triplicate with 4 lambs per replicate (2 males and 2 females) as follows: CON (lambs of normal birth weight, 4.25 ± 0.14 kg), IUGR (3.01 ± 0.12 kg), IUGR + 1% Arg (2.99 ± 0.13 kg), or IUGR + 0.1% NCG (3.03 ± 0.11 kg). The experiment lasted for 21 d, until d 28 after birth, and all lambs were fed milk replacer as a basal diet. Compared with IUGR lambs, NCG or Arg administration increased (P < 0.05) the adenosine triphosphate (ATP) level and the activities of complexes I/III/IV, isocitrate dehydrogenase and citrate synthase in the liver. Compared with CON lambs, the relative mRNA levels of adenosine monophosphate-activated protein kinase α1 (AMPKα1), peroxisome proliferator-activated receptor γ coactivator-1α (PGC1α) and transcription factor A (TFAM) were increased (P < 0.05) in the liver of IUGR lambs, but were decreased (P < 0.05) in the liver of NCG- or Arg-treated lambs compared with those in the IUGR lambs. Compared with IUGR lambs, NCG or Arg administration decreased (P < 0.05) the total AMPKα (tAMPKα)-to-phosphorylated AMPKα (pAMPKα) ratio and the protein expression of PGC1α and TFAM. The results suggested that dietary Arg or NCG supplements improved hepatic energy status and mitochondrial function and inhibited the AMPK-PGC1α-TFAM pathway in IUGR suckling lambs.
Keywords: l-Arginine, Liver, Intrauterine growth restriction, Energy status, Mitochondrial function, N-carbamylglutamate
1. Introduction
Intrauterine growth restriction (IUGR) is a serious condition in livestock production, as fetal growth restriction is associated with long-term limitations in health and performance in postnatal life (Eke et al., 2019). IUGR animals experience higher rates of neonatal morbidity/mortality and lower postnatal growth rate and reproductive performance (Hales et al., 1991). Moreover, IUGR has been shown to impair the mitochondrial biogenesis and hepatic oxidative phosphorylation in rats (Park et al., 2003) and piglets (Zhang et al., 2016a, 2017).
Timely nutritional interventions could alleviate the adverse metabolic consequences of IUGR. l-Arginine (Arg), a nutritionally semi-essential amino acid involved in multiple biological functions, has been shown to increase gluconeogenesis and trigger the hormonal regulation of energy metabolism (Tangara et al., 2010; Wu et al., 2009). Dietary Arg or N-carbamylglutamate (NCG) supplementation has been shown to alleviate IUGR-induced hepatic oxidative damage in newborn lambs via activating the nitric oxide (NO) pathway as well as the antioxidative and phase II metabolizing enzymes (Zhang et al., 2020). It is also well-known that Arg can promote mitochondrial biogenesis and functions via the NO signaling pathway (Nisoli et al., 2003), which is one of the pathways involved in the intracellular redox environment modulation (Yin et al., 2014). As an analogue of N-acetylglutamate synthase (NAG), NCG is metabolically stable and can boost the Arg content in plasma and enhance its endogenous synthesis via the activation of intestinal carbamoyl phosphate synthase-1 and pyrroline-5 carboxylate synthase (Wu et al., 2004).
Mitochondria are the main organelle that produces reactive oxygen species (ROS) and adenosine triphosphate (ATP), which exerts an important role in regulating cellular oxidative injury (Zhang et al., 2018a). The function of mitochondria is closely related to the availability of nutrients (Theurey et al., 2016). Hepatic mitochondrial dysfunction and oxidative injury have been reported in IUGR pigs (Zhang et al., 2018b) and heat-stressed broilers (Zhang et al., 2018c). However, little information is available on the role of dietary Arg or NCG administration on hepatic mitochondrial function in IUGR lambs.
The adenosine monophosphate-activated protein kinase (AMPK), peroxisome proliferator-activated receptor γ coactivator-1α (PGC1α) and silent information regulator 1 (SIRT1) are recognized to play vital roles in regulating cellular energy metabolism (Chau et al., 2010). Additionally, PGC1α, the upstream factor of the nuclear respiratory factors 1 and 2 (NRF1/2) and the transcription factor A mitochondrial (TFAM) can increase the biogenesis of mitochondria, and thus can be used as biomarkers to sense nutrient availability (Rodgers et al., 2005). However, the regulatory mechanism regarding hepatic mitochondrial metabolism in IUGR suckling lambs supplemented with NCG or Arg is still unclear.
Therefore, the current study aimed at investigating the effects of dietary NCG or Arg supplementation on hepatic energy status and mitochondrial function and linking these responses to AMPK-PGC1α-TFAM pathway activation in the liver of IUGR suckling Hu lambs.
2. Materials and methods
All experimental protocols were approved by the Animal Care and Use Committee of the Yangzhou University (SXXY, 2015-0054) and were performed following the practical animal protection law and the Guide for the Care and Use of Laboratory Animals formulated by the National Research Council (China).
2.1. Animals and treatments
Suckling Hu lambs were identified as IUGR when the birth weights were ≥1.5 standard deviation (SD) lower than the average. Suckling Hu lambs with birth weights near the mean litter birth weight (within 0.5 SD) were deemed to have normal birth weights (CON) (Zhang et al., 2019a). On d 7 after birth, 12 and 36 newborn Hu lambs weighing 4.25 ± 0.14 kg (CON) and 3.01 ± 0.12 kg (IUGR), respectively, were selected from a cohort of 432 twin lambs at the Jiangyan Experimental Station (Taizhou, Jiangsu, China). Lambs were weaned and separated from their dams at 7 d old and were assigned, based on their initial body weight (BW), to 1 of 4 treatment groups with 12 animals in each group in triplicates with 4 lambs per replicate (2 males and 2 females). The treatment groups were as follows: CON (4.25 ± 0.14 kg), IUGR (3.01 ± 0.12 kg), IUGR + 1% Arg (2.99 ± 0.13 kg), or IUGR + 0.1% NCG (3.03 ± 0.11 kg) (Zhang et al., 2020). Three replications were in each treatment, and 4 lambs were in each replication. The trial lasted for 21 d (until d 28 after birth) during which period lambs in each replicate pen were housed in a 4 m × 1 m indoor pen with free access to clean water and fed on iso-caloric and iso-nitrogenous basal diets of milk replacer (Table 1) to meet nutrient requirements of suckling lambs (NRC, 2007). Nitrogen was equilibrated using l-alanine (Ajinomoto Co., Ltd., Beijing, China). NCG with 97% purity was provided by Sigma–Aldrich Corporation (Louis, Missouri, US) whereas Arg was provided by Ajinomoto Co., Ltd., (Beijing, China). The dose of NCG (0.1%) and Arg (1%) was determined based on previous trials on lambs (Zhang et al., 2018d, 2019b), piglets (Yang et al., 2013) and rats (Cao et al., 2016). The milk replacer feeding rate was adjusted every 10 d at a rate of 2% of the live body weight of each lamb. Before feeding, milk replacer was dissolved in hot water to make a 40 °C solution (containing 16.67% DM), and it was given to every lamb 3 times a day (at 07:00, 13:00 and 19:00). Experienced farm personnel were responsible for feeding the lambs with milk replacer, thus avoiding confounding factors such as daily handling and management. The individual daily intake of milk replacer was calculated as the difference between the offered and refused amounts. The average dry matter intake (ADMI) of milk replacer was also computed daily via multiplying the average daily milk intake by its DM level (%).
Table 1.
Ingredients and nutrient composition of milk replacer (%, dry-matter basis)1.
| Item | Content |
|---|---|
| Ingredients | |
| Whey protein concentrate, 34% CP | 30.00 |
| Milk fat powder, 11% CP | 35.00 |
| Whole milk powder | 20.00 |
| α-Casein | 5.00 |
| Glucose | 6.00 |
| Pre-mixed mixture2 | 4.00 |
| Nutrient content (analyzed)3 | |
| GE, MJ/kg | 19.36 |
| CP, % | 23.77 |
| EE, % | 15.65 |
| Ash, % | 7.64 |
| Lysine, % | 1.53 |
| Methionine, % | 0.41 |
| Threonine, % | 0.87 |
| l-Arginine, % | 0.63 |
| Ca, % | 0.99 |
| TP, % | 0.73 |
GE = gross energy; CP = crude protein; EE = ether extract; Ca = calcium; TP = total phosphorus.
This table is cited from Zhang et al. (2020).
Main contents of the pre-mixed mixture (per kilogram of the pre-mixed mixture): Cu (as CuSO4·5H2O) 600 mg; Mn (MnSO4·H2O) 315 mg; Fe (FeSO4·7H2O) 8,400 mg; Zn (ZnSO4·7H2O) 12,500 mg; Se (as Na2SeO3) 17 mg; vitamin A 55,000 IU; vitamin E 400 IU; vitamin D 5,500 IU; vitamin K 12.5 mg; biotin 2 mg; folacin 7.5 mg; choline 15 mg; riboflavin 100 mg; vitamin B6 175 mg; thiamin 317.5 mg; and vitamin B12 500 mg.
Nutrient levels are all measured values.
2.2. Hepatic sample collection
On d 28 of age (final day of the experiment) and before sacrifice, lambs were anesthetized by intravenous injection of sodium pentobarbital (15 mg/kg). Then, hepatic tissue was extracted immediately and weighed, frozen promptly in liquid nitrogen, and preserved at −80 °C until further analysis.
2.3. Determination of hepatic adenosine diphosphate (ADP), ATP and adenosine monophosphate (AMP) levels
The hepatic ADP, ATP and AMP levels were determined by high-performance liquid chromatography (HPLC) following the method of Wang et al. (2016). The levels of the total adenine nucleotide (TAN) and adenylate energy charges (AEC) were calculated using the following equations (Zhang et al., 2019b):
| TAN = ATP + ADP + AMP; |
| AEC = (ATP + 0.5 ADP)/(ATP + ADP + AMP). |
2.4. Determination of hepatic tricarboxylic acid (TCA) cycle key enzyme activities
The activities of hepatic TCA key enzymes, including isocitrate dehydrogenase, citrate synthase and alpha-oxoglutarate dehydrogenase complex, were determined in hepatic tissue using the commercial ovine enzyme-linked immunosorbent assay (ELISA) kits (Shanghai Yuan ye Biotechnology Co., Shanghai, China) following the manufacturer's instruction. In brief, the standard solution (50 μL) or the supernatant of the liver tissue extract after dilution was added into each well in the plate. Subsequently, horseradish peroxidase (HRP)-conjugated reagent (100 μL) was added and the plates were incubated at 37 °C for 60 min. Thereafter, the plates were rinsed 5 times with a washing solution, followed by the addition of chromogen solutions A (50 μL) and B (50 μL) and then the plates were mixed and incubated again at 37 °C for 15 min. Finally, a stopping solution (50 μL) was added into the plates and the absorbance was read by the ELISA reader at 450 nm (Model 550; Bio-Rad, Hercules, CA, USA) (Zhang et al., 2019, b; Pi et al., 2014).
2.5. Determination of hepatic mitochondrial complex's activities
Mitochondria were isolated from the fresh liver using a tissue mitochondria isolation kit (No. C3606, Beyotime, Nantong, China) following the manufacturer's instruction. Activities of mitochondrial complexes, including nicotinamide-adenine dinucleotide (NADH) ubiquinone reductase (complex I), succinate ubiquinone reductase (complex II), ubiquinol cytochrome c reductase (complex III) and cytochrome c oxidase (complex IV), were detected following the descriptions of Medja et al. (2009) and Hargreaves et al. (2018). All results were normalized to total protein concentration in each sample for inter-sample comparison (Zhang et al., 2018e).
2.6. Assessment of nicotinamide adenine dinucleotide (NAD) and NADH contents in the hepatic homogenate
Hepatic tissue was homogenized using a lysis buffer, followed by 15 min of centrifugation at 15,000 × g and 4 °C. Then, the supernatant was isolated for measuring the levels of NADH and NAD using a NAD/NADH Quantification Kit (No. MAK037, Sigma, MO, USA) (Zhang et al., 2018a).
2.7. Determination of the mitochondrial DNA (mtDNA) copy number
Total DNA in hepatic tissues was separated by a QIAamp DNA Mini Kit (No.51304, QIAGEN, Hilden, Germany), and the mtDNA copy number was quantified with qRT-PCR using the genomic DNA as the loading control (Zhang et al., 2016b). The mitochondrion-coding cytochrome b (cyt b, NCBI: KY662385.1) and the glyceraldehyde-3-phosphate dehydrogenase (GAPDH, XM_012166462.1) genes were used to quantify mtDNA and nuclear DNA, separately. The primer sequences are displayed in Table 2. The PCR reaction was prepared with a final volume of 20 μL and performed with a 3-step PCR amplification program. At the end of amplification, the melting curve was analyzed, and the relative mtDNA copy number was measured based on the mtDNA-to-nuclear DNA ratio.
Table 2.
Primer sequences used in the real-time PCR.
| Gene | Sequence (5′–3′) | GenBank accession number |
|---|---|---|
| AMPKα1 | F:GACTGCTACTCCACAGAGATCG | NM006251 |
| R:TCAGCATCTGAATCACTCCTTT | ||
| AMPKα2 | F:TGCGGAGGGCCATTCA | NM023991 |
| R:TGAGACAGAGGACGACATGCT | ||
| SIRT1 | F:CGAGAGGCGAGGAGGAGAAG | XM_015104377.1 |
| R:ATCGTTCGAGGATCTGTGCC | ||
| SIRT3 | F: GCCCAATGCCACTCACTACT | XM_004019744.3 |
| R: GGGATGCCAGATGCTCTCTC | ||
| PGC1α | F:CACCCACAACTCCTCCTCAT | AY321517.1 |
| R:CCTTCCTTTCCTCGTGTC | ||
| NRF1 | F:CGGAAGAGACAGCAAACACG | AY368269.1 |
| R:GGTTGGGTTTGGAGGGAGAG | ||
| NRF2 | F: CGAGCCGGTGTGAGTAGA | AY369137.1 |
| R: TTCCGTGGCCCAGTGTAAAG | ||
| ESRRA | F: TGCAGATCACCAAGCGAGAG | XM_015103167.1 |
| R: GTCTCACCTGTCTTCCGAGG | ||
| TFAM | F: CGCTCCCCCTTTAGTTTTGC | XM_015104510.1 |
| R: CTGCCAGTCTGCCCTGTAAG | ||
| mtD-loop | F: ACTCGGAGACCCAGACAACT | KY662385.1 |
| R: TGTAGGGGTGTTCAACTGGC | ||
| GAPDH | F:GTCAAGGCAGAGAACGGGAA | XM_012166462.1 |
| R:GGTTCACGCCCATCACAAAC |
AMPK = AMP-activated protein kinase; SIRT = silent information regulator; PGC1α = peroxisome proliferator-activated receptor γ coactivator-1α; NRF = nuclear respiratory factor; ESRRA = estrogen-related receptor alpha; TFAM = transcription factor A, mitochondrial; GAPDH = glyceraldehyde-3-phosphate dehydrogenase.
2.8. Real-time PCR for measuring the mRNA expression
Total RNA in hepatic tissues was separated, quantified, and reversely transcribed to the complementary DNA (cDNA) following a previous report (Pi et al., 2014). The expression of the target gene was measured relative to the GAPDH level by the 2−ΔΔCT approach (Livak and Schmittgen, 2001). The relative mRNA level of each target gene was standardized to that in the CON group. Table 2 displays the sequences and the GenBank accession numbers of primer sets used to amplify target genes.
2.9. Western blotting
Hepatic protein expression was measured as previously reported (Kang et al., 2015). In brief, an equivalent amount of protein was isolated from the hepatic supernatant solutions by using 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), which was then transferred to the blotting membrane to carry out immunoblotting (Wang et al., 2015). The primary antibodies used in this study included total AMPKα (tAMPKα), rabbit phosphorylated AMPKα (pAMPKα or Thr172), SIRT1, PGC1α, NRF1, mouse anti-β-actin (Sigma–Aldrich, Inc.) and TFAM (Cell Signaling Technology Inc., Danvers, MA, USA). All were diluted at a rate of 1:1,000. The secondary antibodies adopted in this study included goat anti-mouse IgG HRP and goat anti-rabbit IgG HRP. Both were diluted at a rate of 1:1,500 (Antgene Biotech). The pAMPKα, tAMPKα, SIRT1, PGC1α, TFAM and NRF1 protein bands were developed by an enhanced chemiluminescence western blotting kit (Amersham), visualized using the Gene Genome Bioimaging System and analyzed through the GeneTools software (Syngene). The phosphorylated AMPKα was standardized according to its expression level, which was the total protein expression of AMPKα.
2.10. Statistical analysis
Results are expressed as means with pooled SEM. The fixed effect of sex was included in the original statistical model but was not significant (P > 0.05); thus, it was eliminated from the final model, in which treatment alone was the fixed effect. Data analysis was performed by one-way analysis of variance (ANOVA) using SPSS 10.0 (SPSS, Chicago, IL, USA). Tukey's post hoc test was applied for multiple comparisons among treatment groups. The significance level was set to P < 0.05. The data are presented as means and SEM.
3. Results
3.1. Hepatic ADP, ATP and AMP contents
The IUGR lambs experienced a decline in ATP, TAN, ADP, AMP and AEC levels with a rise in the AMP-to-ATP ratio in the liver (P < 0.05) compared to the CON lambs (Table 3). Compared to IUGR lambs, lambs that received NCG or Arg supplementation showed an increase (P < 0.05) in the contents of ADP, ATP, AMP and TAN with a decrease (P < 0.05) of the AMP-to-ATP ratio in the liver. Compared to CON lambs, the contents of ADP, ATP, AMP and TAN were lower (P < 0.05) and the AMP-to-ATP ratio was greater (P < 0.05) in the liver in lambs that received NCG or Arg supplementation. The hepatic AEC level was similar (P > 0.05) between IUGR lambs and those that received NCG or Arg. There were no differences (P > 0.05) in the ATP, TAN, ADP, AMP, AEC levels, and AMP-to-ATP ratio in the liver of lambs between NCG and Arg supplementation.
Table 3.
Effects of l-arginine or N-carbamylglutamate (NCG) supplementation on adenylate purines in the liver of intrauterine growth retarded suckling lambs1.
| Item | Groups2 |
SEM | P-value | |||
|---|---|---|---|---|---|---|
| CON | IUGR | IUGR+1%Arg | IUGR+0.1%NCG | |||
| ATP, μg/g wet wt | 108a | 61.4c | 84.1b | 82.0b | 5.95 | 0.007 |
| ADP, μg/g wet wt | 139 | 71.1 | 99.3b | 103b | 9.11 | 0.010 |
| AMP, μg/g wet wt | 183a | 136c | 151b | 159b | 11.24 | 0.013 |
| AMP-to-ATP ratio | 1.69c | 2.24a | 1.83b | 1.92b | 0.171 | 0.005 |
| TAN3, μg/g wet wt | 431a | 269c | 335b | 344b | 14.32 | 0.009 |
| AEC4 | 0.43a | 0.35b | 0.40ab | 0.39ab | 0.012 | 0.021 |
ATP = adenosine triphosphate; ADP = adenosine diphosphate; AMP = adenosine monophosphate; TAN = total adenine nucleotide; AEC = adenylate energy charge.
a,b,c Mean values within a row with different superscript letters were significantly different (P < 0.05).
Mean values with their SEM, n = 12 in each group.
CON: the normal birth weight group given a control diet; IUGR: intrauterine growth retardation group given a control diet; IUGR+1% Arg: intrauterine growth retardation group given a 1% l-arginine-supplemented diet; IUGR+0.1%NCG: intrauterine growth retardation group given a 0.1% N-carbamylglutamate-supplemented diet.
TAN = ATP + ADP + AMP.
AEC = (ATP + 0.5 ADP)/(ATP + ADP + AMP).
3.2. Activities of key enzymes of hepatic TCA cycle
The IUGR lambs showed a decline (P < 0.05) in the hepatic alpha-oxoglutarate dehydrogenase complex, isocitrate dehydrogenase and citrate synthase activities compared with those in the CON lambs (Table 4). Dietary NCG or Arg supplementation increased (P < 0.05) the hepatic alpha-oxoglutarate dehydrogenase complex, isocitrate dehydrogenase, and citrate synthase activities compared to IUGR lambs. Compared to CON lambs, the activities of hepatic alpha-oxoglutarate dehydrogenase complex, isocitrate dehydrogenase, and citrate synthase were lower (P < 0.05) in the liver in lambs that received NCG or Arg supplementation. There were no differences (P > 0.05) in the hepatic alpha-oxoglutarate dehydrogenase complex, isocitrate dehydrogenase and citrate synthase activities in lambs between NCG and Arg supplementation.
Table 4.
Effects of l-arginine or N-carbamylglutamate (NCG) supplementation on TCA cycle key enzyme activities in the liver of intrauterine growth retarded suckling lambs1.
| Item | Groups2 |
SEM | P-value | |||
|---|---|---|---|---|---|---|
| CON | IUGR | IUGR+1%Arg | IUGR+0.1%NCG | |||
| Citrate synthase, mIU/g protein | 295a | 209c | 253b | 251b | 18.25 | 0.008 |
| Isocitrate dehydrogenase, mIU/g protein | 49.6a | 30.2c | 38.5b | 38.1b | 2.79 | 0.009 |
| Alpha-oxoglutarate dehydrogenase complex, μg/g protein | 384a | 266c | 312b | 320b | 21.96 | 0.011 |
a,b,c Mean values within a row with different superscript letters were significantly different (P < 0.05).
Mean values with their SEM, n = 12 in each group.
CON: the normal birth weight group given a control diet; IUGR: intrauterine growth retardation group given a control diet; IUGR+1% Arg: intrauterine growth retardation group given a 1% l-arginine-supplemented diet; IUGR+0.1%NCG: intrauterine growth retardation group given a 0.1% N-carbamylglutamate-supplemented diet.
3.3. Hepatic mitochondrial complex's activities
The activities of hepatic mitochondrial complexes I/III/IV were lower (P < 0.05) in IUGR lambs than those in the CON group (Table 5). The NCG- or Arg-supplemented lambs had greater and lower (P < 0.05) activities of hepatic mitochondrial complexes I/IV compared with those of the IUGR and CON lambs, respectively. Comparing NCG or Arg supplementation with the CON lambs, no difference (P > 0.05) was found in the activity of hepatic mitochondrial complex III. Hepatic mitochondrial complex II activity was similar (P > 0.05) among treatment groups. No differences (P > 0.05) were found in the activities of hepatic mitochondrial complexes I/III/IV in lambs between NCG and Arg supplementation.
Table 5.
Effect of l-arginine or N-carbamylglutamate (NCG) supplementation on mitochondrial electron transport chain enzymes (I, II, III and IV) in the liver of intrauterine growth retarded suckling lambs (nmol/mg protein per min)1.
| Item | Groups2 |
SEM | P-value | |||
|---|---|---|---|---|---|---|
| CON | IUGR | IUGR+1%Arg | IUGR+0.1%NCG | |||
| Complex I | 316a | 240c | 274b | 278b | 13.89 | 0.009 |
| Complex II | 342 | 337 | 340 | 342 | 9.75 | 0.201 |
| Complex III | 603a | 518b | 594a | 602a | 11.25 | 0.009 |
| Complex IV | 309a | 220c | 258b | 260b | 9.38 | 0.006 |
a,b,c Mean values within a row with different superscript letters were significantly different (P < 0.05).
Mean values with their SEM, n = 12 in each group.
CON: the normal birth weight group given a control diet; IUGR: intrauterine growth retardation group given a control diet; IUGR+1% Arg: intrauterine growth retardation group given a 1% l-arginine-supplemented diet; IUGR+0.1%NCG: intrauterine growth retardation group given a 0.1% N-carbamylglutamate-supplemented diet.
3.4. Hepatic NADH and NAD contents
The NAD level and NAD-to-NADH ratio were increased (P < 0.05) in the liver of IUGR lambs compared to those of the CON lambs (Table 6). The NAD level and NAD-to-NADH ratio were reduced (P < 0.05) in the liver of IUGR lambs treated with NCG or Arg compared to those in IUGR lambs. Compared to CON lambs, the hepatic NAD level and NAD-to-NADH ratio were greater (P < 0.05) in lambs that received NCG or Arg supplementation. The hepatic NADH level was similar (P > 0.05) among treatment groups. No differences (P > 0.05) were found in the NAD level and NAD-to-NADH ratio in the liver of lambs between NCG and Arg supplementation.
Table 6.
Effect of l-arginine or N-carbamylglutamate (NCG) supplementation on NAD and NADH level in the liver of intrauterine growth retarded suckling lambs1.
| Item | Groups2 |
SEM | P-value | |||
|---|---|---|---|---|---|---|
| CON | IUGR | IUGR+1%Arg | IUGR+0.1%NCG | |||
| NAD, nmol/mg protein | 2.07c | 5.89a | 3.86b | 3.79b | 0.312 | 0.006 |
| NADH, nmol/mg protein | 1.37 | 1.41 | 1.35 | 1.39 | 0.061 | 0.119 |
| NAD-to-NADH ratio | 1.51c | 4.18a | 2.86b | 2.74b | 0.089 | 0.007 |
NAD = nicotinamide adenine dinucleotide; NADH = nicotinamide adenine dinucleotide hydride.
a,b,c Mean values within a row with different superscript letters were significantly different (P < 0.05).
Mean values with their SEM, n = 12 in each group.
CON: the normal birth weight group given a control diet; IUGR: intrauterine growth retardation group given a control diet; IUGR+1% Arg: intrauterine growth retardation group given a 1% l-arginine-supplemented diet; IUGR+0.1%NCG: intrauterine growth retardation group given a 0.1% N-carbamylglutamate-supplemented diet.
3.5. Gene expression
The relative mtDNA copy number and the mRNA expression of AMPKα1, AMPKα2, SIRT1, SIRT3, PGC1α, NRF1 and TFAM were higher (P < 0.05) in the liver of IUGR lambs compared to those of the CON ones (Table 7). The relative mtDNA copy number and the mRNA expression of AMPKα2, SIRT1, SIRT3, PGC1α, and NRF1 were lower and greater (P < 0.05) in the liver of IUGR lambs treated with NCG or Arg in comparison to those of the IUGR and CON lambs, respectively. Comparing NCG or Arg supplementation with the CON lambs, no differences (P > 0.05) were found in the hepatic mRNA expression of AMPKα1 and TFAM. There were no differences (P > 0.05) in the relative mtDNA copy number and mRNA expression of the above-mentioned genes in the liver of lambs between NCG and Arg supplementation.
Table 7.
Effect of l-arginine or N-carbamylglutamate (NCG) supplementation on gene expression related to energy metabolism and mitochondrial biogenesis in the liver of intrauterine growth retarded suckling lambs1.
| Item | Groups2 |
SEM | P-value | |||
|---|---|---|---|---|---|---|
| CON | IUGR | IUGR+1%Arg | IUGR+0.1%NCG | |||
| Relative mtDNA copy number | 1.00c | 2.23a | 1.52b | 1.59b | 0.101 | 0.006 |
| AMPKα1 | 1.00b | 1.89a | 1.13b | 1.09b | 0.082 | 0.009 |
| AMPKα2 | 1.00c | 1.97a | 1.45b | 1.51b | 0.088 | 0.004 |
| SIRT1 | 1.00c | 2.18a | 1.66b | 1.64b | 0.112 | 0.012 |
| SIRT3 | 1.00c | 3.09a | 2.17b | 2.11b | 0.131 | 0.007 |
| PGC1α | 1.00c | 3.54a | 2.31b | 2.29b | 0.079 | 0.015 |
| NRF1 | 1.00c | 3.04a | 1.99b | 2.03b | 0.062 | 0.021 |
| NRF2 | 1.00 | 0.95 | 0.99 | 1.02 | 0.101 | 0.209 |
| ESRRA | 1.00 | 1.07 | 0.96 | 1.05 | 0.068 | 0.117 |
| TFAM | 1.00b | 1.74a | 1.09b | 1.11b | 0.059 | 0.022 |
AMPK = AMP-activated protein kinase; SIRT = silent information regulator; PGC1α = peroxisome proliferator-activated receptor γ coactivator-1α; NRF = nuclear respiratory factor; ESRRA = estrogen-related receptor alpha; TFAM = transcription factor A, mitochondrial.
a,b,c Mean values within a row with different superscript letters were significantly different (P < 0.05).
Mean values with their SEM, n = 12 in each group.
CON: the normal birth weight group given a control diet; IUGR: intrauterine growth retardation group given a control diet; IUGR+1% Arg: intrauterine growth retardation group given a 1% l-arginine-supplemented diet; IUGR+0.1%NCG: intrauterine growth retardation group given a 0.1% N-carbamylglutamate-supplemented diet.
3.6. Protein expression
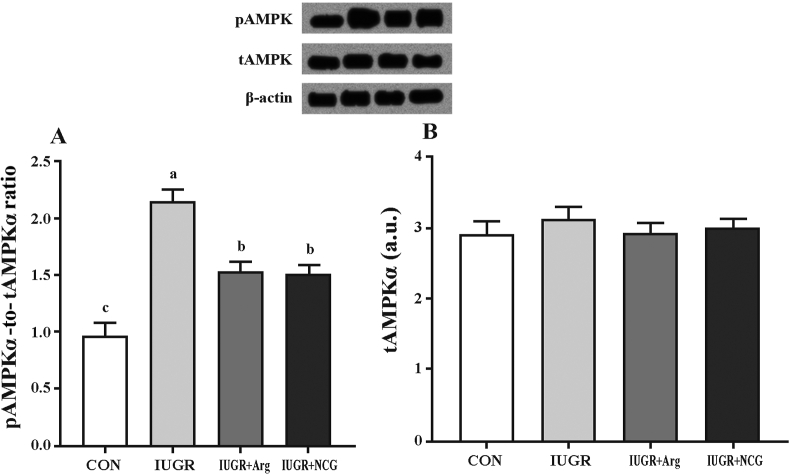
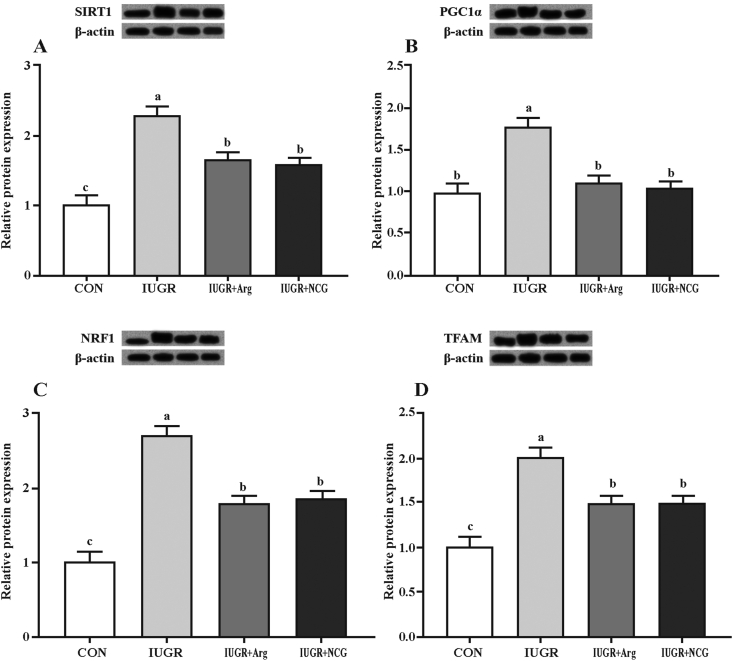
The pAMPKα-to-tAMPKα ratio and the protein expression of PGC1α, SIRT1, NRF1 and TFAM were up-regulated in IUGR lambs compared to those of the CON ones (P < 0.05) (Fig. 1A and Fig. 2). Compared to IUGR and CON lambs, NCG- or Arg-supplemented lambs had lower and greater (P < 0.05) pAMPKα-to-tAMPKα ratio and protein expression of SIRT1, NRF1 and TFAM in the liver, respectively. Comparing NCG or Arg supplementation with the CON lambs, no difference (P > 0.05) was found in the PGC1α protein expression. There were no differences (P > 0.05) in hepatic PGC1α, SIRT1, NRF1, TFAM, and tAMPKα protein levels between Arg and NCG treatments (Fig. 1B).
Fig. 1.
Effects of l-arginine or N-carbamylglutamate (NCG) supplementation on the phosphorylated AMPKα (pAMPKα)-to-total AMPKα (tAMPKα) ratio (A) and the protein abundance of tAMPKα (B) in the liver of IUGR suckling lambs. The bands shown are the representative western blot images of pAMPKα, tAMPKα and β-actin. Beta-actin was from the same blot as the proteins of interest. Data were analyzed as repeated measures with treatments. Mean values in columns without a common letter differ (P < 0.05). Values are means (n = 12 per group), with their standard errors represented by vertical bars. a.u. = arbitrary units; AMPK, AMP-activated protein kinase. CON: the normal birth weight group given a control diet; IUGR: intrauterine growth retardation group given a control diet; IUGR+1% Arg: intrauterine growth retardation group given a 1% l-arginine-supplemented diet; IUGR+0.1%NCG: intrauterine growth retardation group given a 0.1% N-carbamylglutamate-supplemented diet.
Fig. 2.
Effects of l-arginine or N-carbamylglutamate on the protein abundances of silent information regulator 1 (SIRT1) (A), PPARγ coactivator-1α (PGC1α) (B), nuclear respiratory factor 1 (NRF1) (C), and transcription factor A (TFAM) (D) in the liver of IUGR suckling lambs. The bands shown are the representative Western blot images of sirtuin 1 (SIRT1), PGC1α, NRF1, TFAM and β-actin. Beta-actin was from the same blot as the proteins of interest. Data were analyzed as repeated measures with treatments. Mean values in columns without a common letter differ (P < 0.05). Values are means (n = 12 per group), with their standard errors represented by vertical bars. CON: the normal birth weight group given a control diet; IUGR: intrauterine growth retardation group given a control diet; IUGR+1% Arg: intrauterine growth retardation group given a 1% l-arginine-supplemented diet; IUGR+0.1%NCG: intrauterine growth retardation group given a 0.1% N-carbamylglutamate-supplemented diet.
4. Discussion
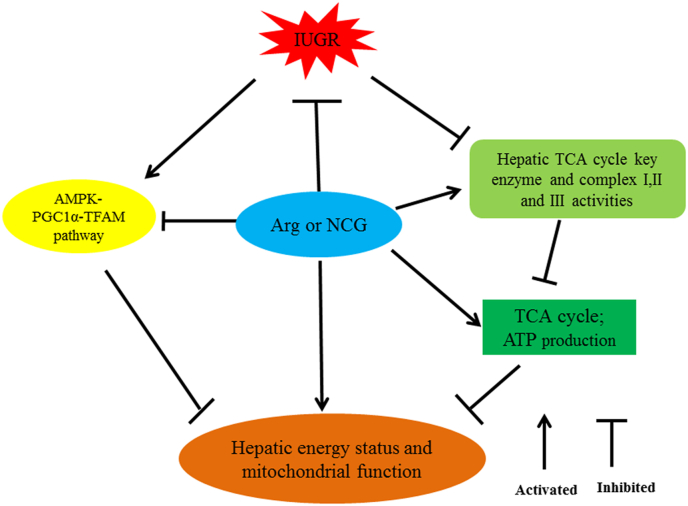
The liver exerts a vital role in the regulation of whole-body energy metabolism. ATP represents a major energy source in most cell functions, and the level of AEC represents the high-energy phosphate content in the cells (Hardie and Hawley, 2001; Kang et al., 2015). The AMP-to-ATP ratio can be used as a biomarker for intracellular energy status (Hardie and Hawley, 2001). Relative to the individual nucleotide scale, energy charge within the adenyl pool may be potentially used as a favorable approach for measuring the energy status in tissues (Hou et al., 2011). In this study, dietary supplementation of NCG or Arg increased the hepatic ATP and AEC levels but decreased the AMP-to-ATP ratio, which indicated that NCG or Arg enhanced the ATP production to increase the IUGR-mediated energy supply. Our results are consistent with those of Zhang et al. (2019b) who reported that the ATP content in the intestine of IUGR suckling lambs was increased by dietary supplementation of NCG or Arg. These results imply that dietary NCG or Arg administration could mitigate IUGR-induced hepatic mitochondrial dysfunctions through modulating the adenine nucleotide pool (Fig. 3).
Fig. 3.
Possible mechanism of Arg or NCG on the regulation of the hepatic energy status and mitochondrial function in IUGR suckling Hu lambs. Arg = l-arginine; NCG = N-carbamylglutamate; IUGR = intrauterine growth retardation; TCA = tricarboxylic acid cycle; ATP = adenosine triphosphate; AMPK = adenosine monophosphate-activated protein kinase; PGC1α = peroxisome proliferator-activated receptor γ coactivator-1α; TFAM = transcription factor A, mitochondrial.
The TCA cycle may be used as a critical approach to produce energy in the liver (Kang et al., 2015) via the TCA cycle key enzymes such as isocitrate dehydrogenase, citrate synthase and α-ketoglutarate dehydrogenase complex. The citrate synthase can catalyze the first step in this cycle through the attachment of acetate molecules onto oxaloacetate (Wiegand and Remington, 1986). Isocitrate dehydrogenase is also capable of catalyzing oxidative decarboxylation for isocitrate, which can thereby generate a-ketoglutarate and CO2 (Corpas et al., 1999). The a-ketoglutarate dehydrogenase complex, the complex constituted by different enzymes, can convert α-ketoglutarate into succinyl-CoA (Corpas et al., 1999). According to our results, dietary supplements of NCG or Arg to IUGR lambs could alleviate the reduced critical enzyme activities during the TCA cycle; this is because NCG or Arg is converted to aspartate, which is further transformed into the TCA cycle intermediates (like oxaloacetate) (Sivakumar et al., 2008). Specifically, the increased oxaloacetate content can enhance citrate synthase activity (Sivakumar et al., 2008). Thus, our results indicate that dietary NCG or Arg supplementation could improve the hepatic energy status by enhancing the activities of the TCA cycle key enzymes. Also, Arg participates in the urea cycle, and the product, fumaric acid, makes a connection between the urea cycle and the TCA cycle to increase oxaloacetate. According to Schanbacher et al. (1981), the serum isocitrate dehydrogenase level was elevated in liver injury in a cattle model. Consistent with the results of liver ATP content of this study, the NCG- or Arg-treated IUGR lambs had higher liver isocitrate dehydrogenase, citrate synthase and α-oxoglutarate dehydrogenase complex activities, which might trigger the TCA cycle to produce more ATP, thus alleviating IUGR-derived metabolic stress.
AMPK has a vital role in the cellular adaptation to nutritional deprivation in vitro, together with physiopathological stresses in vivo (Amaral et al., 2016). Also, AMPK prevents cellular ATP deficiency and mediates cellular responses to metabolic stress, and can be activated at a greater AMP: ATP ratio (Hardie et al., 1998). Accordingly, activated AMPK can prevent excess ATP consumption and elevate ATP generation in response to the limited available energy (Bolster et al., 2002). Among the 7 SIRT subtypes (1 to 7), SIRT1 was the most extensively investigated; it triggers fatty acid oxidation-related gene expression like carnitine palmitoyltransferase 1 (mCPT-1) (Lagouge et al., 2006). Also, the transcription coactivator PGC1α can regulate mitochondrial biogenesis and functions (D'errico et al., 2011) and genes of various biological reactions such as adaptive thermogenesis, mitochondrial biogenesis and glucose/fatty acid metabolism (Liang and Ward, 2006). Additionally, AMPK can also boost SIRT1 activity to meet energy demand and simultaneously regulate the deacetylation and activation of PGC1α; an essential cell response to the increased mitochondrial metabolism (Fernandez-Marcos and Auwerx, 2011). In the current study, IUGR lambs were found to have elevated expressions of AMPKα2, AMPKα1, PGC1α, and SIRT1 at both mRNA and protein levels with an elevated level of AMPKα phosphorylation. In contrast, dietary NCG or Arg supplementation to our IUGR lambs downregulated the AMPKα2, AMPKα1, PGC1α, and SIRT1 expression in the liver at mRNA and protein levels and reduced the phosphorylation level of AMPKα. Thus, dietary NCG or Arg supplementation inhibited the liver AMPK signal transduction pathway in response to IUGR, which conformed to the reduced AMP-to-ATP ratio and the increased liver ATP level. Possibly, the reduced liver AMP-to-ATP ratio together with the increased liver ATP level might inhibit the AMPK-SIRT1-PGC1α signal transduction pathway in IUGR lambs treated with NCG or Arg.
Mitochondria play a vital role in regulating oxidative stress, and any alteration in them is reported to contribute to cellular adaptation to IUGR in terms of metabolism (Zhang et al., 2017). Activating the AMPK can switch on the ATP-generating catabolic pathway through boosting the mitochondria level (Chen et al., 2013). In this study, IUGR decreased the hepatic mitochondrial complexes I, III and IV activities whereas it up-regulated the PGC1α expression, the downstream AMPK and NRF1 and TFAM expression (the targets of PGC1α). Also, IUGR promoted efficient electron transport in mitochondria based on the fact that low-potential mitochondria maintained low levels of ATP production, oxygen consumption and ROS production (Bisht and Dada, 2017). In this study, IUGR lambs treated with dietary NCG or Arg supplements efficiently restored the hepatic mitochondrial complex activities, the expression of mitochondrial regulators (PGC1α, NRF1 and TFAM) and the mtDNA copy number; dietary Arg or NCG supplementation restored the PGC1α expression, a key factor that coactivated NRF1. The NRF1 can modulate TFAM, while the latter can, in turn, control the transcription and duplication of mitochondrial DNA (Hock and Kralli, 2009). These findings suggested that dietary Arg or NCG supplementation counteracted the increase in NRF1 and TFAM expression levels caused by IUGR.
Sirtuins represent the NAD-dependent protein deacetylases family. SIRT1 is the first gene identified of this family and plays a leading role in energy homeostasis as well as being able to activate the downstream AMPK through modulating the NAD content, the SIRT1 substrate (Canto et al., 2009). According to our results, the mRNA and protein abundance of SIRT1 was up-regulated, but the complex I activity was decreased, and the NAD-to-NADH ratio was elevated in IUGR lambs. After dietary Arg or NCG supplementation, the mRNA and protein abundance of SIRT1 and NAD-to-NADH ratio were down-regulated. Additionally, SIRT1 has the functions of binding, deacetylating, and activating PGC1α, which can thereby promote mitochondrial biogenesis (Rodgers et al., 2005). On the other hand, SIRT3, another SIRT family member, was up-regulated responding to IUGR, and SIRT3 knockdown reduced the AMPK phosphorylation and PGC1α expression, suggesting that SIRT3 has a vital role in regulating energy metabolism (Palacios et al., 2009). These findings further demonstrated that IUGR efficiently promoted mitochondrial biogenesis, thus adapting to metabolic stresses.
5. Conclusion
Dietary supplementation of NCG or Arg improved hepatic energy status and mitochondrial function and inhibited the AMPK-PGC1α-TFAM pathway in IUGR suckling Hu lambs. The above findings can potentially recommend NCG or Ag as a dietary treatment for IUGR-induced hepatic mitochondrial dysfunctions. Importantly, our findings can offer valuable clues to enhance our understanding of the elevated hepatic ATP production and mitochondrial biogenesis in Arg- or NCG-treated IUGR lambs, and further contribute to developing a novel nutritional strategy to mitigate the postnatal energy deficiency in IUGR infants.
Author contributions
Hao Zhang and Hongrong Wang designed the research; Hao Zhang, Xiaoyun Liu and Shengnan Ren conducted the research; Mengzhi Wang and Hao Zhang analyzed the data; Hao Zhang and Mabrouk Elsabagh wrote the paper; Hao Zhang and Hongrong Wang had primary responsibility for the final content. All authors read and approved the final manuscript.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
The research was supported by the fund for the National Natural Science Foundation of China (31902180), the Research Project of Natural Science Foundation of Jiangsu Province (BK20170488), the China Postdoctoral Science Foundation (2017M610358), the Science and Technology Innovation Project of Yangzhou University (2019CXJ152), the Top Talents Award Plan of Yangzhou University (2020), and the Cyanine Project of Yangzhou University (2020).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Amaral M.E., Ribeiro R.A., Vanzela E.C., Barbosa-Sampaio H.C. Reduced AMPKalpha2 protein expression restores glucose-induced insulin secretion in islets from calorie-restricted rats. Int J Exp Pathol. 2016;97:50–55. doi: 10.1111/iep.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisht S., Dada R. Oxidative stress: major executioner in disease pathology, role in sperm DNA damage and preventive strategies. Front Biosci (Schol Ed) 2017;9:420–447. doi: 10.2741/s495. [DOI] [PubMed] [Google Scholar]
- Bolster D.R., Crozier S.J., Kimball S.R., Jefferson L.S. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002;277:23977–23980. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- Chen K., Kobayashi S., Xu X., Viollet B., Liang Q. AMP activated protein kinase is indispensable for myocardial adaptation to caloric restriction in mice. PloS One. 2013;8 doi: 10.1371/journal.pone.0059682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C., Gerhart-Hines Z., Feige J.N., Lagouge M., Noriega L., Milne J.C. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpas F.J., Barroso J.B., Sandalio L.M., Palma J.M., Lupiánez J.A., del Rıo L.A. Peroxisomal NADP-dependent isocitrate dehydrogenase. Characterization and activity regulation during natural senescence. Plant Physiol. 1999;121:921–928. doi: 10.1104/pp.121.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W., Xiao L., Liu G., Fang T., Wu X., Jia G. Dietary arginine and Ncarbamylglutamate supplementation enhances the antioxidant statuses of the liver and plasma against oxidative stress in rats. Food Funct. 2016;7:2303–2311. doi: 10.1039/c5fo01194a. [DOI] [PubMed] [Google Scholar]
- Chau M.D., Gao J., Yang Q., Wu Z., Gromada J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK–SIRT1–PGC-1α pathway. Proc Natl Acad Sci Unit States Am. 2010;107:12553–12558. doi: 10.1073/pnas.1006962107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’errico I., Lo Sasso G., Salvatore L., Murzilli S., Martelli N., Cristofaro M. Bax is necessary for PGC1α pro-apoptotic effect in colorectal cancer cells. Cell Cycle. 2011;10:2937–2945. doi: 10.4161/cc.10.17.16791. [DOI] [PubMed] [Google Scholar]
- Eke A., Everett A., Northington F., Vaidya D., McClarin L., Graham E. Identification of intrauterine growth restriction (IUGR) via biomarkers in cord blood and neonatal serum. Am J Obstet Gynecol. 2019;220:S132. [Google Scholar]
- Fernandez-Marcos P.J., Auwerx J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. ASCN. 2011;93:884S–890S. doi: 10.3945/ajcn.110.001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie D.G., Carling D., Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell. Annu Rev Biochem. 1998;67:821–855. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- Hales C.N., Barker D.J., Clark P.M., Cox L.J., Fall C., Osmond C. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ. 1991;303:1019–1022. doi: 10.1136/bmj.303.6809.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves I., Mody N., Land J., Heales S. Blood mononuclear cell mitochondrial respiratory chain complex IV activity is decreased in multiple sclerosis patients: effects of β-interferon treatment. J Clin Med. 2018;7:36. doi: 10.3390/jcm7020036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie D.G., Hawley S.A. AMP-activated protein kinase: the energy charge hypothesis revisited. Bioessays. 2001;23:1112–1119. doi: 10.1002/bies.10009. [DOI] [PubMed] [Google Scholar]
- Hou Y., Yao K., Wang L., Ding B., Fu D., Liu Y. Effects of α-ketoglutarate on energy status in the intestinal mucosa of weaned piglets chronically challenged with lipopolysaccharide. Br J Nutr. 2011;106:357–363. doi: 10.1017/S0007114511000249. [DOI] [PubMed] [Google Scholar]
- Hock M.B., Kralli A. Transcriptional control of mitochondrial biogenesis and function. Annu Rev Physiol. 2009;71:177–203. doi: 10.1146/annurev.physiol.010908.163119. [DOI] [PubMed] [Google Scholar]
- Kang P., Liu Y., Zhu H., Li S., Shi H., Chen F. The effect of aspartate on the energy metabolism in the liver of weanling pigs challenged with lipopolysaccharide. Eur J Nutr. 2015;54:581–588. doi: 10.1007/s00394-014-0739-3. [DOI] [PubMed] [Google Scholar]
- Liang H., Ward W.F. PGC-1α: a key regulator of energy metabolism. Adv Physiol Educ. 2006;30:145–151. doi: 10.1152/advan.00052.2006. [DOI] [PubMed] [Google Scholar]
- Lagouge M., Argmann C., Gerhart-Hines Z., Meziane H., Lerin C., Daussin F. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Medja F., Allouche S., Frachon P., Jardel C., Malgat M., Mousson de Camaret B. Development and implementation of standardized respiratory chain spectrophotometric assays for clinical diagnosis. Mitochondrion. 2009;9:331–339. doi: 10.1016/j.mito.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Nisoli E., Clementi E., Paolucci C., Cozzi V., Tonello C., Sciorati C. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299:896–899. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- NRC . Natl. Acad. Press; Washington, DC: 2007. Nutrient requirements of small ruminants: sheep, goats, cervids and new world camelids. [Google Scholar]
- Palacios O.M., Carmona J.J., Michan S., Chen K.Y., Manabe Y., Ward J.L., 3rd Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging (Albany NY) 2009;1:771–783. doi: 10.18632/aging.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi D., Liu Y., Shi H., Li S., Odle J., Lin X. Dietary supplementation of aspartate enhances intestinal integrity and energy status in weanling piglets after lipopolysaccharide challenge. J Nutr Biochem. 2014;25:456–462. doi: 10.1016/j.jnutbio.2013.12.006. [DOI] [PubMed] [Google Scholar]
- Park K.S., Kim S.K., Kim M.S. Fetal and early postnatal protein malnutrition cause long-term changes in rat liver and muscle mitochondria. J Nutr. 2003;133:3085–3090. doi: 10.1093/jn/133.10.3085. [DOI] [PubMed] [Google Scholar]
- Rodgers J.T., Lerin C., Haas W., Gygi S.P., Spiegelman B.M., Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Sivakumar R., Babu P.V.A., Shyamaladevi C.S. Protective effect of aspartate and glutamate on cardiac mitochondrial function during myocardial infarction in experimental rats. Chem Biol Interact. 2008;176:227–233. doi: 10.1016/j.cbi.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Schanbacher F.L., Willett L.B., Moorehead P.D. Ornithine decarboxylase, serum isocitrate dehydrogenase and clinical chemistry changes during thioacetamide-induced hepatotoxicity in a calf. J Anim Sci. 1981;53:1658–1670. doi: 10.2527/jas1982.5361658x. [DOI] [PubMed] [Google Scholar]
- Tangara M., Chen W., Xu J., Huang F.R., Peng J. Effects of in ovo feeding of carbohydrates and arginine on hatchability, body weight, energy metabolism and perinatal growth in duck embryos and neonates. Brit Poult Sci. 2010;51:602–608. doi: 10.1080/00071668.2010.520303. [DOI] [PubMed] [Google Scholar]
- Theurey P., Tubbs E., Vial G., Jacquemetton J., Bendridi N., Chauvin M.A. Mitochondria-associated endoplasmic reticulum membranes allow adaptation of mitochondrial metabolism to glucose availability in the liver. J Mol Cell Biol. 2016;8:129–143. doi: 10.1093/jmcb/mjw004. [DOI] [PubMed] [Google Scholar]
- Wang X., Liu Y., Li S., Pi D., Zhu H., Hou Y. Asparagine attenuates intestinal injury, improves energy status and inhibits AMP-activated protein kinase signalling pathways in weaned piglets challenged with Escherichia coli lipopolysaccharide. Br J Nutr. 2015;114:553–565. doi: 10.1017/S0007114515001877. [DOI] [PubMed] [Google Scholar]
- Wang L., Yi D., Hou Y., Ding B., Li K., Li B. Dietary supplementation with α-ketoglutarate activates mTOR signaling and enhances energy status in skeletal muscle of lipopolysaccharide-challenged piglets. J Nutr. 2016;146:1514–1520. doi: 10.3945/jn.116.236000. [DOI] [PubMed] [Google Scholar]
- Wiegand G., Remington S.J. Citrate synthase: structure, control, and mechanism. Annu Rev Biophys Biophys Chem. 1986;15:97–117. doi: 10.1146/annurev.bb.15.060186.000525. [DOI] [PubMed] [Google Scholar]
- Wu G., Bazer F.W., Davis T.A., Kim S.W., Li P., Rhoads J.M. Arginine metabolism and nutrition in growth, health and disease. Amino Acids. 2009;37:153–168. doi: 10.1007/s00726-008-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Knabe D.A., Kim S.W. Arginine nutrition in neonatal pigs. J Nutr. 2004;134:2783S–2790S. doi: 10.1093/jn/134.10.2783S. [DOI] [PubMed] [Google Scholar]
- Yang H.S., Fu D.Z., Kong X.F., Wang W.C., Yang X.J., Nyachoti C.M. Dietary supplementation with Ncarbamylglutamate increases the expression of intestinal amino acid transporters in weaned Huanjiang mini-pig piglets. J Anim Sci. 2013;91:2740–2748. doi: 10.2527/jas.2012-5795. [DOI] [PubMed] [Google Scholar]
- Yin J., Ren W.K., Duan J.L., Wu L., Chen S., Li T.J. Dietary arginine supplementation enhances intestinal expression of SLC7A7 and SLC7A1 and ameliorates growth depression in mycotoxin-challenged pigs. Amino Acids. 2014;46:883–892. doi: 10.1007/s00726-013-1643-5. [DOI] [PubMed] [Google Scholar]
- Zhang H., Jin Y., Wang M., Loor Juan J., Wang H. N-Carbamylglutamate and l-arginine supplementation improve hepatic antioxidant status in intrauterine growth-retarded suckling lambs. RSC Adv. 2020;10:11173–11181. doi: 10.1039/c9ra09316h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G.M., Zhang T.T., Jin Y.H., Liu J.L., Guo Y.X., Fan Y.X. Effect of caloric restriction and subsequent re-alimentation on oxidative stress in the liver of Hu sheep ram lambs. Anim Feed Sci Technol. 2018;237:68–77. [Google Scholar]
- Zhang H., Su W., Ying Z., Chen Y., Zhou L., Li Y. N-acetylcysteine attenuates intrauterine growth retardation-induced hepatic damage in suckling piglets by improving glutathione synthesis and cellular homeostasis. Eur J Nutr. 2018;57:327–338. doi: 10.1007/s00394-016-1322-x. [DOI] [PubMed] [Google Scholar]
- Zhang J., Bai K.W., He J., Niu Y., Lu Y., Zhang L. Curcumin attenuates hepatic mitochondrial dysfunction through the maintenance of thiol pool, inhibition of mtDNA damage, and stimulation of the mitochondrial thioredoxin system in heat-stressed broilers. J Anim Sci. 2018;96:867–879. doi: 10.1093/jas/sky009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Zhao F., Peng A., Dong L., Wang M., Yu L. Effects of dietary l-arginine and N-Carbamylglutamate supplementation on intestinal integrity, immune function, and oxidative status in intrauterine-growth-retarded suckling lambs. J Agric Food Chem. 2018;66:4145–4154. doi: 10.1021/acs.jafc.8b00726. [DOI] [PubMed] [Google Scholar]
- Zhang H., Li Y., Chen Y., Zhang L., Wang T. N-Acetylcysteine protects against intrauterine growth retardation-induced intestinal injury via restoring redox status and mitochondrial function in neonatal piglets. Eur J Nutr. 2018;58:3335–3347. doi: 10.1007/s00394-018-1878-8. [DOI] [PubMed] [Google Scholar]
- Zhang H., Peng A., Yu Y., Guo S., Wang M., Coleman D.N. N-carbamylglutamate and l-arginine promote intestinal absorption of amino acids by regulating the mTOR signaling pathway and amino acid and peptide transporters in suckling lambs with intrauterine growth restriction. J Nutr. 2019;149:923–932. doi: 10.1093/jn/nxz016. [DOI] [PubMed] [Google Scholar]
- Zhang H., Peng A., Guo S., Wang M., Loor J.J., Wang H. Dietary N-carbamylglutamate and l-arginine supplementation improve intestinal energy status in intrauterine-growth-retarded suckling lambs. Food Funct. 2019;10:1903–1914. doi: 10.1039/c8fo01618f. [DOI] [PubMed] [Google Scholar]
- Zhang H., Li Y., Hou X., Zhang L., Wang T. Medium-chain TAG improve energy metabolism and mitochondrial biogenesis in the liver of intra-uterine growth-retarded and normal-birth-weight weanling piglets. Br J Nutr. 2016;115:1521–1530. doi: 10.1017/S0007114516000404. [DOI] [PubMed] [Google Scholar]
- Zhang G.M., Deng M.T., Zhang Y.L., Fan Y.X., Wan Y.J., Nie H.T. Effect of PGC-1alpha overexpression or silencing on mitochondrial apoptosis of goat luteinized granulosa cells. J Bioenerg Biomembr. 2016;48:493–507. doi: 10.1007/s10863-016-9684-6. [DOI] [PubMed] [Google Scholar]
- Zhang H., Li Y., Su W., Ying Z., Zhou L., Zhang L. Resveratrol attenuates mitochondrial dysfunction in the liver of intrauterine growth retarded suckling piglets by improving mitochondrial biogenesis and redox status. Mol Nutr Food Res. 2017;61:1600653. doi: 10.1002/mnfr.201600653. [DOI] [PubMed] [Google Scholar]