Abstract
Adipose tissue has a variety of diverse functions that maintain energy homeostasis. In conditions of excess energy availability, adipose tissue increases its lipid storage and communicates the nutritional abundance to various organs in the body. In conditions of energy depletion, such as fasting, cold exposure, or prolonged exercise, triglycerides stored in adipose tissue are released as free fatty acids to support the shift to catabolic metabolism. These diverse functions of storage, communication, and energy homeostasis are shared between numerous adipose depots including subcutaneous, visceral, brown, beige, intramuscular, marrow, and dermal adipose tissue. As organisms age, the cellular composition of these depots shifts to facilitate increased inflammatory cell infiltration, decreased vasculature, and increased adipocyte quantity and lipid droplet size. The purpose of this review is to give a comprehensive overview of the molecular and cellular changes that occur in various aged adipose depots and discuss their impact on physiology. The molecular signature of aged adipose leads to higher prevalence of metabolic disease in aged populations including type 2 diabetes, cardiovascular disease, Alzheimer’s disease, and certain types of cancer.
Introduction:
The world population demographic is shifting; both lifespan and rates of obesity are increasing. In the US alone, the aged population is expected to double, with models predicting that by 2050 there will be 88.5million individuals above the age of 65 [1]. At the same time, obesity in the aged population is expected to rise past its current level of 42.8% to a rate of 64.3% by 2050 [2]. When combined, aging and obesity are predictive risk factors for type 2 diabetes, Alzheimer’s, cardiovascular disease, and certain types of cancer. More work is needed to understand the age-associated morphological and molecular changes in adipose tissue to combat metabolic disease.
Increased adipose tissue with aging or obesity is aligned with the ‘thrifty phenotype’ under which organisms accumulate fat to survive during food shortage [3]. To respond to environmental shifts in food availability, adipose tissue functions as an endocrine organ: responding to external cues, assessing the local abundance of stored triglycerides, and then communicating the energy availability to the rest of the body. The external cues acting on adipocytes are numerous including insulin, glucagon, and relative abundance of macronutrients such as circulating sugars and lipids. The internal environment of the adipose tissue is also complex with variations in adipocyte size, numerous cell types, and differences in vascular infiltration (Figure 1). Response to external and internal cues is mediated by secreted factors, known as adipokines, that are released from adipocytes and signal to target cells in the liver, pancreas, and hypothalamus [4]. These adipokines regulate satiety, insulin sensitivity, glucose metabolism, and lipid storage. While adipose tissue is necessary for survival in food shortage, there is no selective pressure for over abundance. As organisms accumulate fat with age or obesity, the environmental response is diminished with lower adipokine production and signaling.
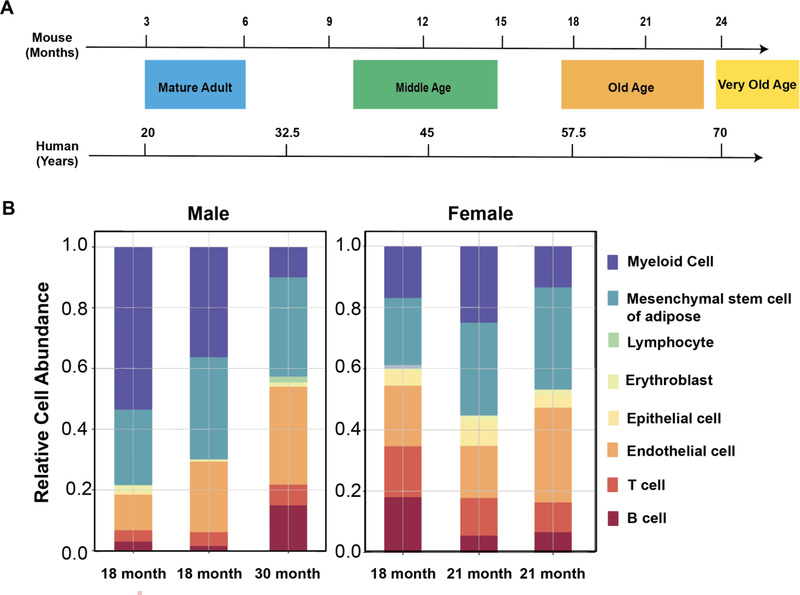
Figure 1: Impact of age range on adipose tissue cellular abundance.
A) Time scale for phases of aging including mature adult being 3–6 months in mice (20–30 years in humans), middle-age of 10–14 months in mice (38–47 years in humans), old age of 18–24 months in mice (56–69 years in humans), and very old of 24 months+ in mice (70 and above in humans). Modified from [8]. B) Single cell RNA-sequencing using microfluidic droplet (droplet) of combined adipose tissue from old age and very old age in male and old age in female C57BL6J mice. Data from Tabula Muris Senis [5].
The age-associated changes in the cellular and molecular composition of adipose are mediated in a depot specific manner. There are several different adipose depots including: visceral white adipose tissue (vWAT), subcutaneous white adipose tissue (sWAT), brown adipose tissue (BAT), beige adipose tissue, inter- and intramuscular adipose tissue, marrow adipose tissue (MAT), and dermal adipose tissue (dWAT). The depots that specialize in storage and communication of stored nutrient availability include the sWAT depots (located in the flank, hip, femoral, gluteal, and abdominal regions) and vWAT depots (located within the peritoneal cavity). With aging these depots have increased infiltration of inflammatory cells, larger lipid droplets, and a higher abundance of senescent cells [5]. Thermogenic adipose tissue depots, including the BAT and beige adipose tissue, have decreased function with age including lower mitochondrial abundance, increased lipid storage, and decreased fuel utilization [6]. The depots of adipose tissue that are buried intramuscularly and in the bone marrow are increased during aging, which shifts body composition leading to decreased muscle mass and bone density. Finally, adipose depots that provide padding and structural support such as the dermal adipose tissue decrease in very old age, with increased fibrosis and decreased adipocyte quantity [7]. These age-associated shifts in the various adipose tissue depots culminate in decreased basal metabolic rate, decreased muscle mass, decreased bone density, worsened insulin responsiveness, increased ectopic deposition of lipids, and subsequent lipotoxicity.
The purpose of this review is to summarize the morphological and molecular changes in aged adipose tissue in a depot specific manner. The initial sections will outline age-associated morphology including adipose tissue expansion, decreased vascularity, increased fibrosis, and immune cell infiltration. The following sections will discuss depot specific changes in these morphological and molecular features. Molecular characterization has largely been performed in mice, and we have denoted scales of aging that follow the described Jackson Laboratories age range for C57BL/6J with mature adult being 3–6 months (20–30 years in humans), middle-age of 10–14 months (38–47 years in humans), old age of 18–24 months (56–69 years in humans), and very old of 24 months+ (70 and above in humans) (Figure 1)[8]. These age groups are defined by biomarkers including transcriptional measurements of senescence, which appear in middle age and are fully expressed in old age, as well as reproductive capacity which is highest in mature adults and decreases with middle age. These age-associated changes are driven by altered tissue morphology, cell infiltration, and molecular signaling and are correlated with the development of diseases including osteoporosis, sarcopenia, type 2 diabetes, and atherosclerosis.
Morphological changes in adipose tissue
Although adipose tissue is primarily composed of adipocytes scaffolded together by a web of vasculature, the stromal vascular fraction (SVF) also includes preadipocytes, endothelial cells, macrophages, and a variety of other immune cells. The quality and relative quantity of these different cell types is important for maintaining proper function. Morphological changes in aging adipose tissue decreases metabolic flexibility leading to insulin resistance, inflammation, and decreased energy expenditure.
Hypertrophy and hyperplasia
Adipocytes expand with age-associated increase in body weight. In mice, total body fat reaches its maximum at middle age and then decreases with advanced age, a trend that is mirrored in humans [9, 10]. The expansion of adipocytes can be categorized as hypertrophic, in which adipocyte size increases, or hyperplastic, in which adipocyte number increases [11]. Both morphologies have been observed in obesity as well as aging. Mean adipocyte size increases between middle and old age, then decreases with advanced age [12]. Although increasing cell size allows adipose tissue to store more lipids and protect other organs from lipotoxic stress, hypertrophy leads to a low surface area to volume ratio. Decreasing this ratio leads to inefficient nutrient transport and poor cell signaling which promotes tissue dysfunction and metabolic disorder. Indeed, hypertrophic growth in white adipose tissue correlates with diabetes in obese humans [13]. Hyperplastic growth, on the other hand, is generally associated with improved insulin sensitivity in human subjects [14]. Hypertrophic growth from increased adiposity in obesity or aging can lead to changes in oxygen availability, vascularity, fibrosis, and immune cell populations [15].
Oxygen availability and vasculature
Severe hypertrophy can increase the adipocyte size beyond the diffusion limit of oxygen as well as reduce access to the blood supply, promoting a hypoxic environment. Immunohistochemical staining with the hypoxia sensor, pimonidazole, showed hypoxia in the white adipose tissue (WAT) of obese (leptin deficient) mice [16]. Hypoxia induces a response mediated by the hypoxia-inducible factors (HIFs) to upregulate angiogenesis and replenish oxygen levels. This response may be dysregulated with obesity, as hypoxic WAT had decreased blood vessel density and lower levels of the angiogenic factor VEGFA [16]. Another potential mechanism of hypoxia with obesity is increased oxygen consumption through activation of uncoupled respiration by free fatty acids [17]. Adipose tissue hypoxia contributes to diabetes etiology, and eliminating components of the hypoxic response, such as HIF-1α or its binding partner ARNT, in adipocytes alleviated diet-induced insulin resistance [17, 18].
Adipose tissue hypoxia has also been shown to occur with aging by immunohistochemistry and direct measurements of the interstitial partial pressure of oxygen [19]. Age associated hypertrophic growth leads to reduced oxygen diffusion that is worsened by inadequate compensation from the vasculature. The in vitro angiogenic potential of adipose-derived stem cells declines with donor age, and adipose tissue from mice show age-dependent decreases in VEGF expression and vascular density [20–22]. Despite a lack of angiogenesis, HIF-1α appears to be upregulated in aged adipose tissue, although the instability of the HIF-1α protein can make it a challenge to quantify [23, 24]. HIF-1α can reduce the expression of genes involved in mitochondrial complex IV assembly, which have decreased expression in aged adipose tissue. Downregulation of mitochondrial activity with aging may underly hypertrophic growth: adipocyte-specific knockout of HIF-1a restored expression of several complex IV transcripts and reduced mean adipocyte size in middle-aged mice [23]. Currently, it is not known if increased hypoxia is simply a by-product of the morphological changes associated with aging, or if hypoxic signaling regulates adipose tissue function and the progression of metabolic disorders, as is observed in obesity.
Extracellular matrix remodeling and fibrosis
Changes in vascularity and oxygen availability during adipose tissue expansion are accompanied by a remodeling of the extracellular matrix (ECM) that is associated with fibrosis. HIF-1α is involved in the upregulation of many ECM genes including collagens [16]. Deposition of collagens resulting in fibrosis is observed in obese mice and humans [16, 25]. Aging is also associated with adipose tissue fibrosis, vWAT from old mice showed a seven-fold increase in collagen staining [20]. Fibrosis is a key factor in the metabolic dysfunction associated with hypertrophic growth. Obese mice lacking a key collagen protein (collagen VI) had increased adipocyte size but improved glucose and lipid clearance. Mechanistically, reducing fibrosis may relieve stress on the adipocyte membrane, leading to downregulated inflammation and increased flexibility to form receptor-stabilizing structures like caveolae [26]. Changes in non-structural components of the ECM with aging may also impact the metabolic flexibility of adipose tissue. For example, the matricellular protein periostin was decreased in brown and white fat with age, a change that may contribute to impaired lipid utilization [27].
Immune cells and inflammation
Adipose tissue growth is also associated with quantitative and qualitative changes in resident immune cells [28]. Obesity is characterized by an enrichment of proinflammatory macrophages, particularly in vWAT [29]. Accumulation of adipose tissue macrophages is likely caused by both recruitment of monocytes from the circulation as well as increased proliferation stimulated by the cytokine monocyte chemoattractant protein 1 (MCP-1) [30]. Aging is also associated with accrual of inflammatory markers in adipose tissue that can be produced by either adipocytes or other cells found in the stromovascular fraction (SVF). However, unlike obesity, accumulation of inflammatory markers with age is independent of macrophage abundance [31, 32]. Rather, macrophage polarization is shifted away from the native, anti-inflammatory M2-like profile towards an inflammatory M1-like and “double negative” profile [32]. Hypoxia is one of several factors that may induce macrophage polarization in adipose tissue with obesity or aging, as expression of hypoxia-related genes correlated with inflammatory M1-like but not anti-inflammatory M2-like macrophages [33]. Indeed, culturing SVF cells under hypoxic conditions increased the expression of inflammatory cytokines [33]. Free fatty acids and other circulating factors may also induce polarization through toll-like receptor 4 (TLR4) signaling and downstream NF-kB activation. In obesity, TLR4 can be activated by dietary free fatty acids via the adaptor protein fetuin-A, which is upregulated with high-fat diet [34, 35]. TLR4 signaling is also relevant in aging as its deletion results in decreased transcript levels of inflammatory interleukin 6 (IL-6) and MCP-1 in old mice [36]. However, adipose tissue expression and serum levels of fetuin-A are decreased in old mice, indicating an alternate mechanism of TLR4 activation during aging [36].
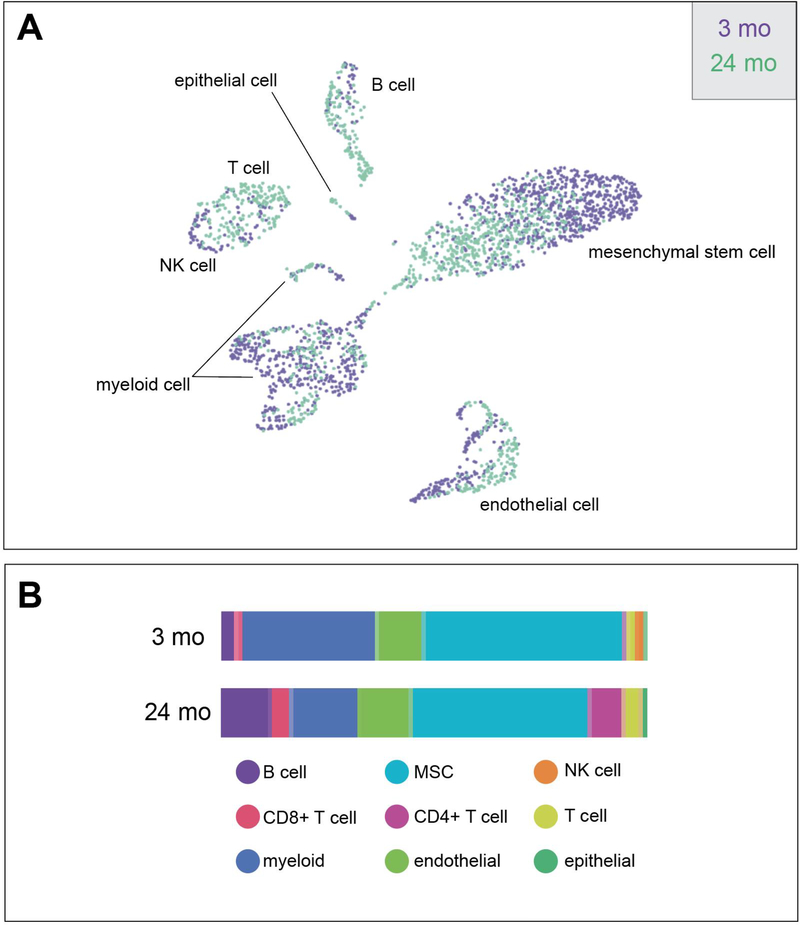
Although the number of macrophages does not increase with age, quantitative changes in other immune cells have been observed. Cluster of differentiation 8 positive (CD8+) and CD4+ T cells were increased in the vWAT of old mice [32, 37]. Interestingly, eliminating CD4+ regulatory T cells, and thus their canonical anti-inflammatory role, improved age-associated insulin resistance, indicating that a balance of immune cells is required for proper adipose tissue function [37]. In female mice, aging is also associated with an expansion of resident adipose tissue B cells that are transcriptionally distinct from age-associated splenic B cells. Accrual of these cells may be a key regulatory event in the immuno-remodeling of adipose tissue with age, as depletion of all B cells prevented the accumulation of regulatory T cells in the visceral WAT of old mice [38] (Figure 2).
Figure 2: Changes in Tissue Composition with Aging from Tabula Muris Senis.
A) uniform manifold approximation and projection (UMAP) and B) bar graph from FACS of gonadal white adipose tissue from 3- and 24-month old C57BL6JN mice. Acronyms: mesenchymal stem cell (MSC), Cluster of differentiation 4/8 (CD4/CD8), natural killer (NK).
The architecture of adipose tissue shifts with growth due to obesity or aging. Remodeling events include hypertrophic adipocytes with decreased access to the vasculature, increased fibrosis, and inflammatory polarization or accumulation of different types of immune cells. A common link between these changes is hypoxia, which is well characterized in obesity and may also play a role with aging. However, there are likely mechanisms of adipose tissue remodeling that are distinct to aging, such as alternate activation of TLR4 signaling.
Mechanisms of adipose remodeling with age may carry over to humans. Average adipocyte size increases with age in WAT biopsies [39]. As was shown in mice, hypoxia-related mitochondrial dysfunction could underly this hypertrophic growth since expression of COX5B decreased with age in human WAT [23]. In regard to hypoxia and vascularity, the in vitro angiogenic capacity of adipose stem cells declined with the age of the owner [40]. Human aging is also associated with systemic, low-grade inflammation, although the extent that adipose tissue contributes is not yet clear [41]. Adipose macrophage content has been shown to positively and significantly correlate with aging, although that relationship may plateau and begin to reverse at middle age [42, 43]. While studies in model organisms allow for separation of phenotypes due to obesity versus aging, this distinction is less clear in human studies, and age-associated changes can be compounded by overnutrition and obesity.
The morphological changes outlined above are general trends associated with adipose tissue growth from obesity or aging. More specific changes manifest depending on the type of adipocyte, which are categorized as white, brown, or beige depending on their unique gene expression and function (Figure 4). Additionally, the impact of aging varies depending on the anatomical location of the adipose tissue depot, which have diverse morphologies and functions. Many of the mouse studies referenced above were performed in only one type of adipose tissue (gonadal WAT) and the conclusions drawn may not be ubiquitously applicable. We will next specify the age-associated changes in different types of fat. We will go over the three major types of adipocyte: white, brown, and beige which will cover the cellular precursors and transdifferentiation between white and beige adipocytes. We will focus our discussion on the locations of various adipose tissue depots including white (distinguished as visceral or subcutaneous), brown, beige, muscular, bone marrow, and dermal (Figure 3). The impact of aging in other types of fat such as perivascular will not be discussed here but have been highlighted in recent reviews [44].
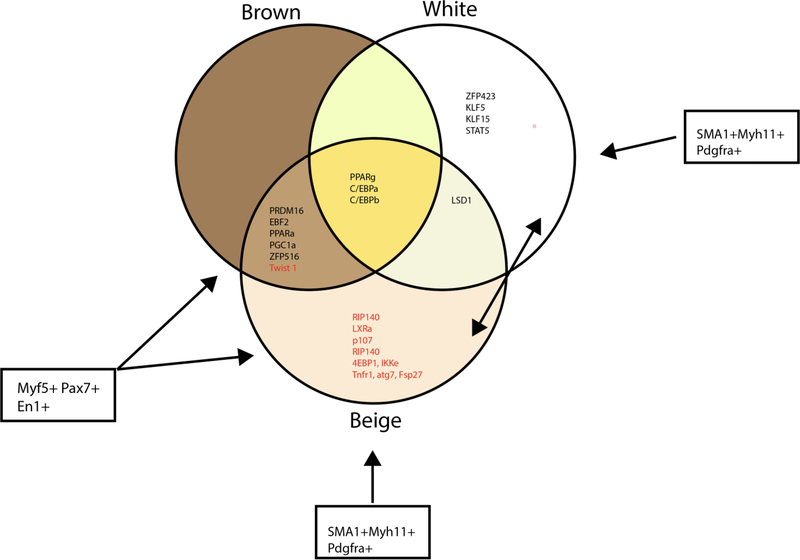
Figure 4: Key Transcriptional Regulators in Brown, White, and Beige Adipose Tissue.
A large body of literature has focused on the characterization of transcriptional regulation in adipocyte differentiation and maintenance. Transcriptional regulators that drive the adipogenic program (black lettering) and inhibitors of adipogenesis (red lettering) have been describes in brown, white, and beige adipocytes. Although there is significant overlap between these depots, unique transcriptional regulators have been identified for white and beige adipocytes. More work is needed to understand how these transcriptional regulators change with aging in a depot specific manner.
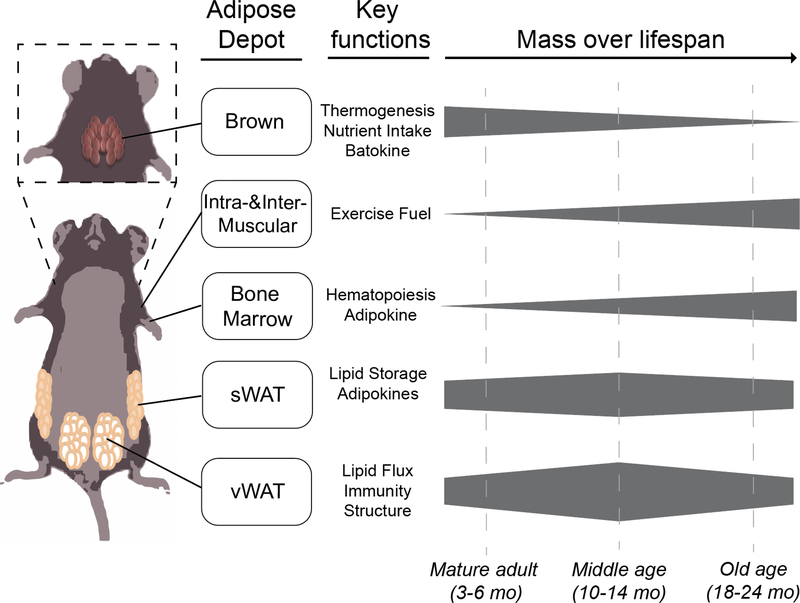
Figure 3: Depot Specific Changes in Aging Adipose Tissue.
The various adipose tissue depots in mice have a range of function from thermogenesis, regulation of glucose homeostasis through adipokine production, and lipid storage. These tissues expand and contract in a depot specific manner between the stages of aging from mature adult, middle age, and old age.
Aging in Adipose Tissue Depots
White Adipose Tissue
White adipose tissue is a highly dynamic organ that functions in lipid storage and release, insulation and padding, and endocrine signaling. Generally, it is distributed in the visceral (vWAT) depot surrounding organs in the peritoneal cavity, or the subcutaneous (sWAT) depot underlying the skin, particularly in the gluteal-femoral region. Although these two depots share fundamental similarities, they have morphological and molecular differences that lead them to serve distinct purposes in physiology and disease (as reviewed in [45]). vWAT contains primarily unilocular adipocytes characterized by a single, large lipid droplet and a high degree of innervation, while sWAT contains a heterogenous population of unilocular and multilocular (multiple small lipid droplets) adipocytes surrounded by a high amount of connective tissue [46]. Both depots have the canonical function of a lipid repository, however vWAT is more susceptible to lipolysis in response to signaling from the nervous system, while sWAT is the preferred depot for long term lipid storage. When the storage capacity of sWAT is reached, lipids are deposited in the visceral depot. Increased visceral adiposity is associated with diabetes, cardiovascular disease, and other metabolic disorders, which could be explained by anatomical location, as a portion of vWAT has direct access to liver via the portal vein [47]. However, characteristics inherent to the two depots may also contribute to their opposing effects on metabolic outcomes. Transplantation of sWAT into the visceral location in mice resulted in improved glucose homeostasis and weight loss, indicating that sWAT may have intrinsically beneficial qualities [48]. With age, the ratio of vWAT to sWAT increases [10]. Underlying this shift are changes in cellular functions, such as impaired adipocyte generation, altered secretion of cytokines and hormones, and dysregulated lipid accumulation and breakdown.
The generation of lipid-storing adipocytes requires tight regulation to maintain the metabolic flexibility of WAT. Adipogenesis involves the conversion of mesenchymal stem cells into committed preadipocytes, which then replicate or differentiate into mature white adipocytes. Differentiation involves an epigenetic transition followed by the cooperative action of the transcription factors PPARγ and C/EBPα to promote adipocyte-specific gene expression, a process that is responsive to a variety of positive and negative regulators [49]. With age, tissue plasticity is impaired. In rats, the ability of preadipocytes to differentiate in culture declines with the donor age, and the expression of C/EBPα is decreased in multiple WAT depots in vivo [50, 51]. In addition to differentiation, the capacity of preadipocytes to self-renew may also be impaired. Stable isotope labeling and turnover modeling indicated that replication of adipocyte progenitors in the SVF was impaired specifically in sWAT of old mice [52].
The decline in adipogenic potential can be attributed to cellular senescence, which is a state of halted cell cycle progression triggered canonically by telomere shortening or prematurely by other stressors [53]. Markers of senescence—such as p16Ink4a (inhibitor of CDK4) expression and senescence-associated B-galactosidase activity—were elevated across WAT depots in very old female mice [54]. Inducing the death of p16Ink4a senescent cells in old mice restored PPARγ and C/EBPα expression and alleviated age-associated fat loss [55]. Premature senescence specifically in WAT may be associated with impaired processing of microRNA (miRNA), a category of nucleic acid that regulates gene expression post-transcriptionally. The protein that processes miRNA—Dicer—was downregulated in vWAT and sWAT but unchanged in the liver with age. Knockout of Dicer in preadipocytes induced a senescent phenotype and impaired their doubling time, although differentiation in this case was not affected [56].
In humans, adipogenesis declines with age in sWAT, as measured by deuterium labeling of nuclei and lipid droplets [57]. Senescent pathways may impair adipogenic capacity, potentially in a cell-type specific manner as was similarly observed in mice. Adipose-derived stems cells from older donors had increased expression of p16Ink4a that was associated with reduced differentiation capacity in culture [58]. Whole sWAT biopsies showed no age-dependent expression of p16 but rather TP53 (tumor protein 53), which functions in an alternate route of the same senescent pathway [39]. Senolytic drugs are a promising intervention to reduce senescent cell burden and boost regenerative potential in adipose tissue [59].
Cell senescence, along with other aging-associated events, impact the endocrine function of adipose tissue. Healthy WAT releases a variety of molecules that maintain systemic energy balance, including leptin and adiponectin. Secretion of these adipokines is impacted with aging, which has been reviewed thoroughly in [60]. Adipose tissue also secretes cytokines and chemokines as part of the immune response.
Resident immune cells are a major source of inflammatory cytokines, but adipocytes and preadipocytes have also been shown to secrete them. Senescence causes preadipocytes to secrete inflammatory molecules such as IL-6 that can promote inflammation in other cells in vitro [61]. A mechanism of inflammation potentially related to senescence is impaired autophagy—the process that clears and recycles accumulated proteins and damaged organelles—resulting in ER stress [62]. Markers of ER stress were elevated in the vWAT of old mice, particularly in stromal cells like preadipocytes. Drugs that inhibit ER stress reduced the age-dependent increase in IL-6 and MCP-1 in preadipocytes and TNFα in macrophages [63]. Overall, the combination of polarized macrophages, accumulated lymphocytes, and stressed preadipocytes and adipocytes contribute to the inflammatory phenotype of WAT during aging.
Inflammation from obesity and aging can inhibit the ability of adipocytes to store lipids. Insulin is a key hormone for promoting lipid storage, and insulin-stimulated lipogenesis was shown to be decreased in adipocytes from sWAT of middle-aged rats [64]. Inflammatory molecules such as TNFα have been shown to disrupt the insulin signaling pathway in adipocytes, their accumulation with aging may contribute to insulin resistance in WAT [65].
The breakdown and release of lipids is also affected by age-associated inflammation. During conditions of energy demand like fasting, catecholamines are sent by the sympathetic nervous system to initiate lipolysis of stored triglycerides in adipocytes. However, in aged vWAT, expression of catecholamine-degrading enzymes is elevated in adipose macrophages, preventing the signal from reaching adipocytes [66]. Altered gene expression in the macrophages is driven by the nod-like receptor pyrin domain-containing 3 (NLRP3) inflammasome, an innate immune sensor that can be activated by a variety of pathogenic or endogenous molecules. Deletion of NLRP3 rescued fasting-induced lipolysis in vWAT in old mice [66]. The exact trigger of the NLRP3 inflammasome in aged WAT is unclear, but potentially an array of factors including circulating fatty acids and ceramides, intracellular organelle dysfunction, and unchecked post-translational acetylation contribute to its activation [67, 68].
In human sWAT, ex vivo catecholamine-stimulated lipolysis activity also significantly decreased with age. This was attributed to catecholamine degradation in adipocytes rather than in macrophages as was observed in mice [69]. NLRP3 inflammasome activity has been associated with metabolic dysfunction in human WAT, but the extent that it contributes to lipolysis defects with human aging remains to be determined [70].
White adipose tissue plays an essential role in regulating systemic energy homeostasis—it can store excess lipids to prevent lipotoxicity in other organs as well as release them during times of energy demand. Age-associated events including senescence and inflammation disrupt these functions, resulting in aberrant lipid accumulation and metabolic dysfunction. In sWAT, the ability to store lipids declines potentially because: 1) mature adipocytes reach their hypertrophic limit, 2) hyperplastic growth declines due to impaired replication and differentiation of preadipocytes, and 3) insulin signaling is dampened by inflammation. Instead of being stored in sWAT, lipids accumulate in vWAT and ectopically in other organs such as the liver. Inflammation-driven defects in lipolysis, resulting in low turnover of lipids, also contributes to the growth of vWAT with age. Expansion of visceral fat can lead to many of the morphological changes listed in the section above and is associated with poor metabolic outcomes. Despite the pathological consequences of increased visceral and ectopic white fat, balanced accumulation may be an important adaption for longevity due to its role in energy storage and endocrine function, as suggested in [3]. Indeed, a complete loss of fat can result in the same pathologies as over-accrual, such as insulin resistance.
Brown Adipose Tissue
Like white adipose, brown adipose tissue (BAT) contributes to systemic energy homeostasis. Instead of energy storage and release, however, BAT specializes in energy consumption for thermogenesis. Brown adipocytes are primarily derived from progenitor cells expressing the transcription factors Pax7, En1, and Myf5, a lineage that is shared with skeletal muscle precursors [71]. Adipogenesis of brown fat is similar to white fat in requiring involvement of PPARy and C/EBP proteins, but is otherwise distinctly driven by PRDM16, which in combination with C/EBPβ is sufficient to induce the brown fat program [72]. Brown adipocytes are acutely activated through β-adrenergic signaling from highly abundant sympathetic nerve fibers in response to cold or other stimuli [73]. The resulting thermogenic program—mediated by PGC-1α—upregulates mitochondrial biogenesis and expression of the mitochondrial protein UCP1, which uncouples respiration from ATP production to produce heat [74]. Thermogenesis increases the uptake and catabolism of circulating sugars and lipids in brown adipocytes, which has made BAT an attractive target for combating obesity and related metabolic diseases. In addition to thermogenesis, BAT is also capable of secreting factors that act in an autocrine fashion to optimize activity or a paracrine fashion to regulate metabolism in other tissues. Aging is associated with decreased adipogenesis, thermogenesis, and endocrine functions in BAT.
Unlike the marked increase in WAT mass with age, BAT mass stays relatively constant after maturation but begins to decline with very old age in mice [75, 76]. Morphologically, brown adipocytes show hypertrophic growth and increased lipid droplet size with age in both male and female mice [75, 77]. Metabolomics analyses show accumulation of specific types of lipids with age, including long chain mono-unsaturated ceramides and dolichols, while acylcarnitine species decreased [78].
As with WAT, the adipogenic function of BAT may be impaired with aging and is likely regulated by multiple intra- and extra-cellular mechanisms. Intracellularly, the expression of PRDM16 in whole BAT and the ability of preadipocytes to differentiate in vitro declined with age [75]. Accumulation of ceramide lipids, potentially due to inflammation, in BAT with age may also impact adipogenic potential, as treating preadipocytes with ceramide 16:0 reduced expression of UCP1 and PGC-1α in vitro [78]. Multiple extracellular regulators of BAT differentiation are impaired with aging. One of the receptors for the neuropeptide orexin, an established regulator of BAT, was decreased with age, and orexin supplementation alleviated the age-associated adipogenic defect in culture [75].
Thermogenic capacity in BAT is reduced with age in rodents although the extent is sex-specific [79]. Levels of UCP1 are decreased in old mice when kept at non-thermoneutral conditions (any temperature below that of the body, including room temperature) [75, 78]. However, upregulation of UCP1 expression in response to β3-adrenegeric receptor agonism (CL-316,243) was equivalent between young and old mice [80]. Induction of thermogenic gene expression may not be uniform across adipocytes. Single-cell RNA sequencing revealed two populations of adipocytes present in BAT—one highly thermogenic population with elevated mitochondrial content and expression of UCP1, and one lowly thermogenic population with high fatty acid uptake [81]. Cold exposure stimulated substantial conversion of low- to high-thermogenic adipocytes; however, this flexibility was lost in middle-aged mice [81]. This indicates that heterogeneity among brown adipocyte may contribute to the age-associated decline in thermogenesis and should further be explored.
Among the many factors that mediate adaptive thermogenesis, nuclear factor erythroid 2-like 1 (Nfe2l1/Nrf1) has emerged as an important regulator due to its role in increasing proteasome activity to maintain proteostasis [82]. Nrf1 expression is upregulated during cold exposure, and deletion of Nrf1 in brown/beige adipocytes resulted in ER stress, abnormal mitochondrial morphology and function, and impaired thermogenesis [82]. Considering that loss of proteostasis and mitochondrial dysfunction are hallmarks of aging, it would be worthwhile to investigate if the induction of Nrf1 during cold exposure is maintained with increasing age [83].
Impaired intake and utilization of fuel with age may also impact thermogenic function. Activated BAT imports fuel sources derived from other tissues, so age-associated impairments in other organs can impact thermogenesis. Cold exposure results in increased levels of circulating acylcarnitine species that are derived from the liver and taken up by BAT. In old mice, production of hepatic acylcarnitines was impaired, and restoring their levels through carnitine supplementation rescued age-associated cold sensitivity [84]. Once imported, fuels like glucose and lipids are catabolized to replenish the mitochondrial proton gradient, which is dissipated by UCP1 to produce heat. Upregulation of oxidative pathways in response to β3-adrenegeric receptor (CL-316,243) was impaired in BAT of old mice [80]. Mechanistically, this was attributed to a decline in the iron-sulfur-cluster pathway and subsequently decreased production of lipoic acid, which serves as an essential cofactor for several enzymes involved in catabolic pathways, such as pyruvate dehydrogenase [80]. Old mice also fail to mobilize resident lipids in brown adipocytes during cold exposure, although ATGL-mediated lipolysis in BAT may not be required for adaptive thermogenesis [75, 85, 86].
In addition to thermogenesis, BAT also functions as an endocrine organ, producing common adipokines such as adiponectin, as well as unique “batokines” that can include peptides and lipids [87]. During energy demanding conditions like cold exposure or exercise, BAT secretes the lipid 12,13-diHOME which can act as an autocrine or paracrine signal to itself or skeletal muscle to increase fatty acid uptake [88]. The function of many batokines and how they are impacted with age has yet to be fully elucidated.
Loss of BAT quantity and function also occurs with human aging. BAT depots in humans are less distinct than the interscapular depot in rodents, making it a challenge to measure their loss in mass. Rather, BAT is imaged by its uptake of labeled glucose during cold exposure using positron emission tomography-computed tomography (PET/CT). Use of PET/CT shows that presence of BAT declines with age [89, 90]. Aging also has a functional impact on BAT thermogenesis in humans [79]. BAT biopsies collected from deep neck surgery had decreased expression of UCP1 with age [91]. Additionally, preadipocytes from these biopsies that were differentiated in culture showed a reduction in β-adrenergic-stimulated uncoupled respiration with the age of the donor [91]. The endocrine function of BAT may also decline in human aging. Secretion of 12,13-diHOME in response to exercise differed between old and young adults with similar activity levels [92]. Impaired BAT function can lead to decreased thermogenesis and cold sensitivity in older adults. Additionally, because BAT plays a significant role in whole-body glucose and energy homeostasis, loss of BAT with age can contribute to the development of insulin resistance, obesity, and type 2 diabetes [93].
Beige Adipose Tissue
Besides BAT, adaptive thermogenesis is also carried out by beige adipocytes that reside within sWAT. Beige adipocytes are an inducible form of thermogenic adipocytes that develop in response to prolonged cold exposure. Like BAT, beige adipocytes are rich in mitochondria, express UCP1, and differentiation is driven by PRDM16, C/EBP, and PPARγ. However, there are distinct differences in transcriptional regulation and progenitor cell population between brown and beige adipocytes (Figure 4). Lineage tracing analysis revealed beige adipocytes are derived from two mechanisms including transdifferentiation from mature white adipocytes and differentiation from the stem cell pool [94]. Beige adipocytes differentiated from stem cells arise from numerous progenitor cell, the majority are derived from a smooth muscle progenitor cells expressing smooth muscle actin (SMA) and myosin heavy chain 11 (MYH-11) [95] [96], but beige adipocytes from Myf5+ progenitors have also been observed in sWAT (Figure 4) [97].
Cold-induced beiging is decreased with aging [98]. Transcriptional markers of beige adipocytes begin to decrease at 16 weeks of age in the sWAT of C57BL/6J mice. At 6 months of age, transcriptional markers of beige adipocytes are entirely lost and sWAT displays white adipocyte morphology only [98]. Similarly, in humans there are decreases in beige adipose tissue, however, there is some dispute in humans whether identified BAT depots represent brown or beige, with gene expression of biopsy studies showing evidence of both [99–102]. There are multiple mechanisms that regulate this decrease in beige adiposity including increased senescence, decreased differentiation, and decreased transdifferentiation.
The decrease in newly differentiated beige adipocytes with aging is largely dependent upon cellular senescence as a driver. Cellular senescence occurs through immune dysregulation and the induction of cell cycle arrest programs such as p53 resulting in a permanent arrest of the cell cycle [103]. In aging, senescent cells increase in number, as observed by transcriptional markers of senescence which are increased in the inguinal white adipose tissue (iWAT, a subset of sWAT) in aged mice. In SMA+ cells specifically, overexpression of senescent programs, measured through SA-ß-galactose staining and expression of tumor suppressor genes, causes a significant decrease in the beiging of iWAT, accompanied by an increase in serum glucose levels and an inability to defend body temperature [98]. In C57BL/6J adult and middle-aged mice, an inhibition of the senescent program through SMA+ specific deletion of Ink4a/Arf using a tamoxifen inducible SMA-CreGFP driver rescued the inability of aged mice to induce beiging of iWAT upon cold exposure [95] [98]. This deletion also decreased serum glucose levels, increased glucose uptake in the iWAT, and allowed mice to defend their body temperature more readily upon exposure to 4°C. Interestingly, inhibiting the cellular senescent pathway through the oncogene p53 restored cold-induced beiging in aged mice through decreasing mitophagy [103]. Preadipocytes isolated from the sWAT of middle age humans have a similar decrease in beige adipocyte differentiation and increase in transcriptional markers of senescence including p21, p16Ink4a, and IGFBP5, suggesting that senescence is a major driver in age-related beige adipocyte decline in humans and mice [98] [104].
The molecular regulation of transdifferentiation between beige to white adipocytes in aging remains largely unclear. Evidence from Duteil et al., suggest that lysine-specific demethylase 1 (Lsd1) is decreased in iWAT as mice age from 6 weeks to 30 weeks accompanied by the loss of beige adipocytes [105]. Furthermore, overexpression of Lsd1 in iWAT of middle-aged C57BL6/N resulted in an increase in beige adipocytes while adipose specific knockout of Lsd1 decreased beiging and increased the beige to white adipocyte transition. Surprisingly, this regulation was determined to be PPARα dependent rather than the well-established adipocyte transcriptional master regulator, PPARγ. ChIP-qPCR revealed that Lsd1 was enriched at the PPARα promoter upon stimulation of beige adipogenesis. PPARα inhibition also ablated Lsd1’s ability to promote beiging of iWAT in middle-aged mice [105]. More work is needed to assess the contribution of beige adipose tissue to total energy expenditure and determine the mechanisms that regulate the differentiation and transdifferentiation with aging.
While studies have determined mechanisms that regulate the decrease in beige adiposity with age, many unanswered questions remain. One intriguing observation is that continued β-adrenergic stimulation via the agonist CL-316,243 over the course of one week can induce beiging in middle-aged mice [106]. The discrepancy of beige responsiveness between cold and β-adrenergic stimulation requires further study. Because β3 adrenergic agonists can induce beiging in aged mice, the loss of cold induction may be dependent upon the ability to sense the cold through thermoreceptors in the skin or through loss of β3 adrenergic receptor activation in cold. Alternatively, there is evidence to support distinct signaling pathways for β3-adrenergic stimulation and cold exposure [107]. Outstanding questions include how transcriptional regulators of brown, beige, and white adipose tissue are altered with aging. A large body of literature has focused on transcriptional regulation with differentiation (Figure 4) with numerous factors identified that either promotor inhibit differentiation. The transcriptional regulation of aging in adipose tissue, however, is just beginning to be explored.
Muscle Localized Adipose Tissue
Adipocytes can form both in and around skeletal muscle, these depots are known as intramuscular and intermuscular adipose tissue. Intramuscular adipose tissue is a population of adipocytes dispersed within skeletal muscle fibers, while intermuscular adipose tissue develops in between and surrounding the skeletal muscle but does not infiltrate the muscle fibers itself. There is also a potential for intramyocellular lipid droplet formation in muscle cells, which is characterized as ectopic lipid storage [3, 108, 109]. We will focus our discussion on the intra- and intermuscular adipocytes which are a distinct population of adipocytes, rather than the intramyocellular lipid droplets which can form in response to insulin resistance, elevated lipolysis, and in trained athletes [108]. Typically, these adipose depots increase with age and begins to occupy more of the muscle tissue leading to decrease function of the muscles and sarcopenia, the age dependent loss of muscle tissue.
Intermuscular adipose tissue increases with age in C57BL/6J mice and positively correlates with sarcopenia. Zhu et al., found that old mice showed significantly increased intermuscular adipose tissue compared to young mice [110]. This increase in intermuscular adipose tissue on the hind limbs was accompanied by a decrease in cross sectional muscle fiber, correlating it with sarcopenia. Aged mice also showed increases in gene expression associated with cellular senescence, protein degradation, and inflammation. Genes associated with differentiation, tissue fibrosis, angiogenesis, and mitochondrial lipid regulation were down regulated in aged mice compared to young [110].
Physiological studies in elderly humans provide a link between intramuscular adipose tissue and a decrease in muscle function. A large, cross-sectional study of men and women (n=3075) associated increased adipose tissue infiltration into muscle (both intramuscular and intermuscular) with poorer lower extremity performance [111]. A study of 109 elderly subjects (mean age of 74 years) found a correlation with increased intramuscular adipose tissue of the thigh and a decrease of leg muscle mobility [112]. Moreover, increased abundance of lipid deposition in the muscle as measured by x-ray absorbitrometry leads to decreased muscle size and quality with decreased strength [113]. These observations were confirmed with muscle biopsies in aged individuals that showed an increase in lipid deposition corresponds with a decrease in the size and number of muscle fibers[114]. This loss in muscle mass is associated with both sarcopenia and frailty. Sarcopenia is defined as lower muscle mass associated with aging while frailty is assessed by the presence of three of the five following criteria including: unintentional weight loss, exhaustion, low physical activity, slow gait speed, and low grip strength [115]. Biopsy studies on frail and non-frail controls showed that increased intramuscular adipocytes caused elevated muscle inflammation through increased IL-6 production [116]. A complete understanding of the various mechanisms that regulate the development of sarcopenia and frailty would lead to better therapeutic intervention for quality of life.
Bone Marrow Adipose Tissue
Bone marrow adipose tissue (MAT) is a fat depot located in the skeletal system that is spatially and temporally regulated, comprising 10–15% of total fat mass [117]. Two distinct subpopulations of MAT are recognized which change in prevalence and histological patterns throughout life. MAT formation begins at the earliest stages of life, forming dense adipocytes know as constitutive MAT (cMAT), which histologically resembles WAT. Throughout life, regulated MAT (rMAT) begin to occupy red bone marrow [117, 118]. The cMAT population is static, while the rMAT population expands with obesity, PPARγ agonist, and aging. MAT is known to regulate physiology and bone integrity, increased MAT abundance with aging decreases bone density.
MAT accumulates in bone as mice age, with adipocytes increasing in both number and size [119, 120]. The increase in MAT is accompanied by a loss of bone volume, bone mineral content, and an increase in trabecular spacing [117]. In humans, increased adipocyte volume and decreased trabecular bone volume are correlated with aged-related osteoporosis [121]. This increase in MAT also has major consequences in regulation of whole body glucose homeostasis, PET/CT studies demonstrating that MAT is a major site of glucose uptake and that MAT glucose utilization is decreased with aging [122].
The age-associated changes in MAT are transcriptionally regulated. As mice age, decreasing levels PGC-1α in skeletal stem cells corresponds with increasing adipogenesis and decreasing osteogenesis. PGC-1α controls the downstream transcriptional co-activator TAZ, promoting osteogenesis while inhibiting PPARγ and subsequent adipogenesis. PGC-1α KO mice have significantly decreases TAZ expression and impairs the osteogenic-adipogenic balance in skeletal stem cells [123]. Other notable changes in gene expression profiles of aging MAT include increased inflammatory and lipogenesis markers, but a decrease in mitochondrial transcripts [119].
The cell populations of MAT are altered with aging. Lineage tracing using a mT/mG reporter mouse crossed with a Zfp423-EGFP reporter revealed a mesenchymal stem cell origin for MAT, specifically CD45- CD31- Sca1+ CD24- cells. Use of the osterix-cre:mT/mG mice found this progenitor pool was distinct from white and brown adipocytes [124]. CD45- CD31- Sca1+ CD24+ also displayed a tri-lineage adipogenic, osteogenic, and hematopoietic potential. In old C57BL/6J mice, the adipogenic potential of CD45- CD31- Sca1+ cells did not change; however, osteogenic progenitor potential was reduced. When the mice were given a 1-day HFD, aged mice significantly increased the purely adipogenic and multipotent progenitors. Committed preadipocytes (marked by expression of Zfp443) were also significantly increased with one day of HFD in middle-aged mice but not young (2 months) mice [125]. Further work is needed to harness the therapeutic potential of the molecular regulation of MAT through transcription and cell population in the prevention or reversal of age-associated diseases including osteoporosis.
Dermal Adipose Tissue
Dermal adipose tissue resides below the reticular dermis. In mice this adipose tissue depot is separated from subcutaneous adipose tissue by muscle known as the panniculus carnosus, but in humans there is no physical separation even though the dermal and subcutaneous adipocytes are morphologically distinct. Dermal adipose tissue abundance is constantly fluctuating and increases with hair follicle growth, obesity, PPARγ agonist, infection, and age [126, 127]. There are also signals that lead to the contraction of dermal adipose tissue including hair regression, lipodystrophy, and fibrosis [127]. The expansion and contraction of dermal adipose tissue thought to be regulated by lipogenesis rather than lipolysis, since drivers of lipolysis, such as treatment with β3-adrenergic receptor agonist CL-316,243, have no impact on dermal adipose thickness. Other variables lead to changes in base line abundance include an observed sexual dimorphism with females having increased dermal adipose tissue abundance compared to males [126]. The abundance of dermal adipose tissue with age is similarly flexible with molecular restructuring resulting in dedifferentiation and an adipocyte-myofibroblast transition.
Aging in dermal adipose tissue is cyclic with expansion and contraction at various stages of life. As mice age there is increased damage from ultraviolet radiation that increases fibrosis and leads to the final contraction with very old age [7]. Due to its recent discovery and characterization, there are many questions remaining with dermal adipose tissue in relation to aging. Particularly, its functional role and contribution to systemic metabolism; dermal adipose tissue has been shown to have numerous roles in insulation, dermal thickness and integrity, and wound healing. More work is needed to understand how the cyclic nature of dermal adipose tissue is regulated with age, how this depot changes in human aging, and how the function of this tissue changes with aging.
Perspectives
As organisms age, the energy demands of growth and reproduction subside leading to decreased basal metabolic rate. The decrease in energy expenditure with age leads to increased adipose tissue mass in visceral, subcutaneous, marrow, intermuscular, and intramuscular adipose tissue. Age-associated decrease in energy expenditure is exacerbated by decreased thermogenesis in brown and beige adipose tissue. Other depot specific changes are more complex and undergo cyclic expansion and contraction with age such as dermal adipose tissue, which expands with old age and weight gain but decreases with very old age. Age-associated changes are more than just altered hypertrophy or hyperplasia in response to the shift in energy utilization—they represent an entire remodeling that includes immune cell infiltration, decreased vasculature, and increased fibrosis. Cumulatively, these shifts lead to increased age-associated metabolic diseases including type 2 diabetes, certain types of cancer, sarcopenia, osteoporosis, and cardiovascular disease. Developing our understanding of aging adipose tissue will create therapies that improve quality of life in old age.
The complexity of cells in aging adipose tissue has only been appreciated recently with the advent of novel tools including single cell RNA-sequencing. Recently, the Tabula Muris Consortium performed single cell RNA-sequencing on numerous tissues in male and female C57BL/6JN mice aged 1 month, 3 months, 18 months, 21 months, 24 months, and 30 months [5]. Their analysis included BAT, sWAT, and two depots of vWAT- gonadal and mesenteric. Data in this study were collected by two methods: microfluidic droplet (droplet) (Figure 1) and fluorescence-activated cell sorting (FACS) (Figure 2). For the droplet analysis all of the adipose tissues were processed together and were only harvested from 18-month females, 18-month males, 21-month females, and 30-month males. In the FACS sorting the brown, subcutaneous, mesenteric, and gonadal adipose from 3-month, 18-month, and 24-month old mice were processed. The data produced from Tabula Muris Senis will add a deeper understanding to the field, with the ability to interpret inter-organ communication since multiple organs from the same mouse were processed in a controlled environment.
The improvements in imaging technology have led to a renaissance of understanding adipose tissue mass and expansion in real time. The use of positron emission tomography with computed tomography (PET/CT) has allowed us to image the uptake of glucose in brown and beige adipose tissue and determine how fuel utilization decreases with age. Similarly, magnetic resonance imaging (MRI) has allowed us to quantify and measure dWAT and determine its cyclic changes with age. Use of various labels, including osmium tetroxide staining with micro-computed tomography, have led to the characterization of MAT with rosiglitazone treatment, obesity, and aging. New technology is needed to quantify intra- and intermuscular adipose tissue with aging in mice to determine the molecular regulation of this depot. In humans the use of bioimpedance-derived fluid volume combined with dual-energy X-ray absorptiometry (DXA) and improved computational assessment, has been used to determine muscle quality, density, and adiposity with aging [128]. Future work to build the resolution and application of this technology will lead to a deeper understanding of these buried adipose tissue depots and their changes with age.
Further molecular characterization is needed beyond the single cell transcriptomics. Single cell proteomics, metabolomics, and lipidomics has become a possibility within the next decade with the power to unlock key molecular features of senescence and aging that have been previously unknown [129]. The use of single cell assessment of protein and metabolite pools holds the answer to many small depots such as dermal and intramuscular adipose tissue depots. Other advances in technology include the improved resolution of matrix-assisted laser desorption ionization time of flight (MALDI-TOF) when coupled with ion mobility to understand the spatial distribution of cell types. This type of in tissue resolution could decipher the heterogeneity of adipose tissue including the factors that regulate beige adipocyte differentiation in sWAT, and the variations in cMAT and rMAT and how these depots change with aging.
The application of novel technology to aged adipose tissue has the ability to guide our knowledge towards the development of therapeutics to combat age-associated diseases. As we explore the composition, cellular signaling, and environmental response of adipose tissue with aging complexity arises with depot specific variation. Many adipose tissue depots remain unexplored or under-explored in the context of aging including marrow, dermal, and beige adipose tissue. Understanding how these various depots are altered with aging will inform on the aging process and associated changes in metabolic physiology.
ACKNOWLEDGEMENTS
We would like to thank all members of the Simcox lab for reviewing the manuscript and offering edits. We thank Drs Alan Attie and Rozalyn Anderson for their valuable insights and comments.
FUNDING
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health, the Office of The Director, National Institutes of Health (OD) and the National Cancer Institute (NCI) under Award Number K12HD101368. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The work was also supported in part by the NIH/National Institute on Aging through the University of Wisconsin Madison Center for Demography of Health and Aging Pilot Grant (P30AG017266) and startup funds from the University of Wisconsin-Madison School Department of Biochemistry to J.A.S.. Other funds that supported this publication include funds from the Diabetes Research Center at Washington University in St. Louis of the National Institutes of Health under award number P30DK020579.
Acronyms:
- sWAT
subcutaneous white adipose tissue
- vWAT
visceral white adipose tissue
- BAT
brown adipose tissue
- MAT
marrow adipose tissue
- SVF
Stromal vascular fraction
- VEGFA
Vascular endothelial growth factor A
- HIF-1a
Hypoxia-inducible factor 1 alpha
- ARNT
Aryl hydrocarbon receptor nuclear translocator
- ECM
Extracellular matrix
- MCP1
Monocyte chemoattractant protein 1
- TLR4
Toll-like receptor 4
- NF-kB
Nuclear Factor kappa-light-chain-enhancer of activated B cells
- IL-6
Interleukin 6
- CD8+
Cluster of differentiation 8 positive
- CD4+
Cluster of differentiation 4 positive
- PPARγ
Peroxisome proliferator activated receptor gamma
- C/EBP
CCAAT-enhancer-binding protein
- INK4a
Inhibitor of CDK4
- TP53
Tumor protein 53
- TNFa
Tumor necrosis factor alpha
- NLRP3
Nod-like receptor pyrin domain-containing 3
- PAX7
Paired box 7
- EN1
Engrailed homeobox 1
- MYF5
Myogenic factor 5
- PRDM16
PR domain containing 16
- PGC-1a
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- UCP1
Uncoupling protein 1
- ATGL
Adipose triglyceride lipase
- TAZ
transcriptional co-activator with PDZ-binding motif
References
- 1.Fakhouri TH, et al. , Prevalence of obesity among older adults in the United States, 2007–2010. NCHS Data Brief, 2012(106): p. 1–8. [PubMed] [Google Scholar]
- 2.Batsis JA and Zagaria AB, Addressing Obesity in Aging Patients. The Medical clinics of North America, 2018. 102(1): p. 65–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conte M, et al. , The Dual Role of the Pervasive “Fattish” Tissue Remodeling With Age. Frontiers in Endocrinology, 2019. 10(114). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jura M and Kozak LP, Obesity and related consequences to ageing. Age (Dordr), 2016. 38(1): p. 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Almanzar N, et al. , A single-cell transcriptomic atlas characterizes ageing tissues in the mouse. Nature, 2020. 583(7817): p. 590–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zoico E, et al. , Brown and Beige Adipose Tissue and Aging. Frontiers in endocrinology, 2019. 10: p. 368–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kruglikov IL and Scherer PE, Skin aging: are adipocytes the next target? Aging (Albany NY), 2016. 8(7): p. 1457–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flurkey K, Currer JM, and Harrison DE, Chapter 20 - Mouse Models in Aging Research, in The Mouse in Biomedical Research (Second Edition), Fox JG, et al. , Editors. 2007, Academic Press: Burlington. p. 637–672. [Google Scholar]
- 9.Hemmeryckx B, et al. , Age-associated adaptations in murine adipose tissues. Endocrine journal, 2010. 57(10): p. 925–30. [DOI] [PubMed] [Google Scholar]
- 10.Kuk JL, et al. , Age-related changes in total and regional fat distribution. 2009, Elsevier. p. 339–348. [DOI] [PubMed] [Google Scholar]
- 11.Choe SS, et al. , Adipose tissue remodeling: Its role in energy metabolism and metabolic disorders. 2016, Frontiers Media S.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller KN, et al. , Aging and caloric restriction impact adipose tissue, adiponectin, and circulating lipids. Aging Cell, 2017. 16(3): p. 497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muir LA, et al. , Adipose tissue fibrosis, hypertrophy, and hyperplasia: Correlations with diabetes in human obesity. Obesity, 2016. 24(3): p. 597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arner E, et al. , Adipocyte turnover: Relevance to human adipose tissue morphology. Diabetes, 2010. 59(1): p. 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crewe C, An YA, and Scherer PE, The ominous triad of adipose tissue dysfunction: Inflammation, fibrosis, and impaired angiogenesis. 2017, American Society for Clinical Investigation. p. 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halberg N, et al. , Hypoxia-Inducible Factor 1 Induces Fibrosis and Insulin Resistance in White Adipose Tissue. Molecular and Cellular Biology, 2009. 29(16): p. 4467–4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee YS, et al. , Increased adipocyte O2 consumption triggers HIF-1α, causing inflammation and insulin resistance in obesity. Cell, 2014. 157(6): p. 1339–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang C, et al. , Disruption of hypoxia-inducible factor 1 in adipocytes improves insulin sensitivity and decreases adiposity in high-fat diet-fed mice. Diabetes, 2011. 60(10): p. 2484–2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, et al. , Aging is associated with hypoxia and oxidative stress in adipose tissue: implications for adipose function. American Journal of Physiology-Endocrinology and Metabolism, 2011. 301(4): p. E599–E607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donato AJ, et al. , The impact of ageing on adipose structure, function and vasculature in the B6D2F1 mouse: Evidence of significant multisystem dysfunction. Journal of Physiology, 2014. 592(18): p. 4083–4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimoda Y, et al. , Aging differentially alters the expression of angiogenic genes in a tissue-dependent manner. Biochemical and Biophysical Research Communications, 2014. 446(4): p. 1243–1249. [DOI] [PubMed] [Google Scholar]
- 22.Efimenko A, et al. , Angiogenic properties of aged adipose derived mesenchymal stem cells after hypoxic conditioning. J Transl Med, 2011. 9: p. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soro-Arnaiz I, et al. , Role of Mitochondrial Complex IV in Age-Dependent Obesity. Cell Rep, 2016. 16(11): p. 2991–3002. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Rodriguez A, et al. , Essential role of protein tyrosine phosphatase 1B in obesity-induced inflammation and peripheral insulin resistance during aging. Aging Cell, 2012. 11(2): p. 284–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Divoux A, et al. , Fibrosis in human adipose tissue: Composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes, 2010. 59(11): p. 2817–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan T, et al. , Metabolic Dysregulation and Adipose Tissue Fibrosis: Role of Collagen VI. Molecular and Cellular Biology, 2009. 29(6): p. 1575–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graja A, et al. , Loss of periostin occurs in aging adipose tissue of mice and its genetic ablation impairs adipose tissue lipid metabolism. Aging Cell, 2018. 17(5): p. 12810–12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trim W, Turner JE, and Thompson D, Parallels in immunometabolic adipose tissue dysfunction with ageing and obesity. 2018, Frontiers Media S.A. p. 169–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weisberg SP, et al. , Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest, 2003. 112(12): p. 1796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amano SU, et al. , Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metabolism, 2014. 19(1): p. 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu D, et al. , Aging Up-Regulates Expression of Inflammatory Mediators in Mouse Adipose Tissue. The Journal of Immunology, 2007. 179(7): p. 4829–4839. [DOI] [PubMed] [Google Scholar]
- 32.Lumeng CN, et al. , Aging Is Associated with an Increase in T Cells and Inflammatory Macrophages in Visceral Adipose Tissue. The Journal of Immunology, 2011. 187(12): p. 6208–6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujisaka S, et al. , Adipose tissue hypoxia induces inflammatory M1 polarity of macrophages in an HIF-1alpha-dependent and HIF-1alpha-independent manner in obese mice. Diabetologia, 2013. 56(6): p. 1403–12. [DOI] [PubMed] [Google Scholar]
- 34.Shi H, et al. , TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest, 2006. 116(11): p. 3015–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pal D, et al. , Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nature Medicine, 2012. 18(8): p. 1279–1285. [DOI] [PubMed] [Google Scholar]
- 36.Ghosh AK, et al. , Toll-like receptor 4 (TLR4) deficient mice are protected from adipose tissue inflammation in aging. Aging, 2017. 9(9): p. 1971–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bapat SP, et al. , Depletion of fat-resident T reg cells prevents age-associated insulin resistance. Nature, 2015. 528(7580): p. 137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Camell CD, et al. , Aging Induces an Nlrp3 Inflammasome-Dependent Expansion of Adipose B Cells That Impairs Metabolic Homeostasis. Cell Metab, 2019. 30(6): p. 1024–1039 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gustafson B, Nerstedt A, and Smith U, Reduced subcutaneous adipogenesis in human hypertrophic obesity is linked to senescent precursor cells. Nat Commun, 2019. 10(1): p. 2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madonna R, et al. , Age-dependent impairment of number and angiogenic potential of adipose tissue-derived progenitor cells. Eur J Clin Invest, 2011. 41(2): p. 126–33. [DOI] [PubMed] [Google Scholar]
- 41.Krabbe KS, Pedersen M, and Bruunsgaard H, Inflammatory mediators in the elderly. Exp Gerontol, 2004. 39(5): p. 687–99. [DOI] [PubMed] [Google Scholar]
- 42.Harman-Boehm I, et al. , Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J Clin Endocrinol Metab, 2007. 92(6): p. 2240–7. [DOI] [PubMed] [Google Scholar]
- 43.Ortega Martinez de Victoria E, et al. , Macrophage content in subcutaneous adipose tissue: associations with adiposity, age, inflammatory markers, and whole-body insulin action in healthy Pima Indians. Diabetes, 2009. 58(2): p. 385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Queiroz M and Sena CM, Perivascular adipose tissue in age-related vascular disease. Ageing Res Rev, 2020. 59: p. 101040. [DOI] [PubMed] [Google Scholar]
- 45.Lee MJ, Wu Y, and Fried SK, Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med, 2013. 34(1): p. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berry DC, et al. , The developmental origins of adipose tissue. Development, 2013. 140(19): p. 3939–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Item F and Konrad D, Visceral fat and metabolic inflammation: the portal theory revisited. Obesity Reviews, 2012. 13(SUPPL.2): p. 30–39. [DOI] [PubMed] [Google Scholar]
- 48.Tran TT, et al. , Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab, 2008. 7(5): p. 410–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cristancho AG and Lazar MA, Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol, 2011. 12(11): p. 722–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kirkland JL, Hollenberg CH, and Gillon WS, Age, anatomic site, and the replication and differentiation of adipocyte precursors. American Journal of Physiology - Cell Physiology, 1990. 258(2 27–2). [DOI] [PubMed] [Google Scholar]
- 51.Karagiannides I, et al. , Altered expression of C/EBP family members results in decreased adipogenesis with aging. Am J Physiol Regul Integr Comp Physiol, 2001. 280(6): p. R1772–80. [DOI] [PubMed] [Google Scholar]
- 52.Kim SM, et al. , Loss of white adipose hyperplastic potential is associated with enhanced susceptibility to insulin resistance. Cell Metabolism, 2014. 20(6): p. 1049–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu Z, et al. , The role of adipose tissue senescence in obesity- and ageing-related metabolic disorders. Clin Sci (Lond), 2020. 134(2): p. 315–330. [DOI] [PubMed] [Google Scholar]
- 54.Stout MB, et al. , Growth hormone action predicts age-related white adipose tissue dysfunction and senescent cell burden in mice. Aging, 2014. 6(7): p. 575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu M, et al. , Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife, 2015. 4: p. e12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mori MA, et al. , Role of microRNA processing in adipose tissue in stress defense and longevity. Cell Metabolism, 2012. 16(3): p. 336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guillermier C, et al. , Imaging mass spectrometry demonstrates age-related decline in human adipose plasticity. JCI Insight, 2017. 2(5): p. e90349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alt EU, et al. , Aging alters tissue resident mesenchymal stem cell properties. Stem Cell Res, 2012. 8(2): p. 215–25. [DOI] [PubMed] [Google Scholar]
- 59.Hickson LJ, et al. , Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine, 2019. 47: p. 446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mancuso P and Bouchard B, The Impact of Aging on Adipose Function and Adipokine Synthesis. Front Endocrinol (Lausanne), 2019. 10: p. 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu M, et al. , JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci U S A, 2015. 112(46): p. E6301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ghosh AK, et al. , Impaired autophagy activity is linked to elevated ER-stress and inflammation in aging adipose tissue. Aging (Albany NY), 2016. 8(10): p. 2525–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ghosh AK, et al. , Elevated Endoplasmic Reticulum Stress Response Contributes to Adipose Tissue Inflammation in Aging. J Gerontol A Biol Sci Med Sci, 2015. 70(11): p. 1320–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sertie RA, et al. , Metabolic adaptations in the adipose tissue that underlie the body fat mass gain in middle-aged rats. Age (Dordr), 2015. 37(5): p. 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hotamisligil GS, et al. , IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science, 1996. 271(5249): p. 665–8. [DOI] [PubMed] [Google Scholar]
- 66.Camell CD, et al. , Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature, 2017. 550(7674): p. 119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Youm YH, et al. , Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab, 2013. 18(4): p. 519–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.He M, et al. , An Acetylation Switch of the NLRP3 Inflammasome Regulates Aging-Associated Chronic Inflammation and Insulin Resistance. Cell Metab, 2020. 31(3): p. 580–591 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gao H, et al. , Age-Induced Reduction in Human Lipolysis: A Potential Role for Adipocyte Noradrenaline Degradation. Cell Metab, 2020. [DOI] [PubMed] [Google Scholar]
- 70.Rheinheimer J, et al. , Current role of the NLRP3 inflammasome on obesity and insulin resistance: A systematic review. Metabolism, 2017. 74: p. 1–9. [DOI] [PubMed] [Google Scholar]
- 71.Inagaki T, Sakai J, and Kajimura S, Transcriptional and epigenetic control of brown and beige adipose cell fate and function. Nat Rev Mol Cell Biol, 2016. 17(8): p. 480–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kajimura S, et al. , Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature, 2009. 460(7259): p. 1154–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cannon B and Nedergaard J, Brown Adipose Tissue: Function and Physiological Significance. 2004, American Physiological Society. p. 277–359. [DOI] [PubMed] [Google Scholar]
- 74.Uldry M, et al. , Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab, 2006. 3(5): p. 333–41. [DOI] [PubMed] [Google Scholar]
- 75.Sellayah D and Sikder D, Orexin restores aging-related brown adipose tissue dysfunction in male mice. Endocrinology, 2014. 155(2): p. 485–501. [DOI] [PubMed] [Google Scholar]
- 76.Liu Z, et al. , The Dysfunctional MDM2-p53 Axis in Adipocytes Contributes to Aging-Related Metabolic Complications by Induction of Lipodystrophy. Diabetes, 2018. 67(11): p. 2397–2409. [DOI] [PubMed] [Google Scholar]
- 77.Goncalves LF, et al. , Ageing is associated with brown adipose tissue remodelling and loss of white fat browning in female C57BL/6 mice. Int J Exp Pathol, 2017. 98(2): p. 100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gohlke S, et al. , Identification of functional lipid metabolism biomarkers of brown adipose tissue aging. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Florez-Duquet M and McDonald RB, Cold-induced thermoregulation and biological aging. Physiol Rev, 1998. 78(2): p. 339–58. [DOI] [PubMed] [Google Scholar]
- 80.Tajima K, et al. , Mitochondrial lipoylation integrates age-associated decline in brown fat thermogenesis. Nat Metab, 2019. 1(9): p. 886–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Song A, et al. , Low- and high-thermogenic brown adipocyte subpopulations coexist in murine adipose tissue. J Clin Invest, 2020. 130(1): p. 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bartelt A, et al. , Brown adipose tissue thermogenic adaptation requires Nrf1-mediated proteasomal activity. Nat Med, 2018. 24(3): p. 292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lopez-Otin C, et al. , The hallmarks of aging. Cell, 2013. 153(6): p. 1194–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Simcox J, et al. , Global Analysis of Plasma Lipids Identifies Liver-Derived Acylcarnitines as a Fuel Source for Brown Fat Thermogenesis. Cell Metab, 2017. 26(3): p. 509–522 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shin H, et al. , Lipolysis in Brown Adipocytes Is Not Essential for Cold-Induced Thermogenesis in Mice. Cell Metab, 2017. 26(5): p. 764–777 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schreiber R, et al. , Cold-Induced Thermogenesis Depends on ATGL-Mediated Lipolysis in Cardiac Muscle, but Not Brown Adipose Tissue. Cell Metab, 2017. 26(5): p. 753–763 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Villarroya J, Cereijo R, and Villarroya F, An endocrine role for brown adipose tissue? Am J Physiol Endocrinol Metab, 2013. 305(5): p. E567–72. [DOI] [PubMed] [Google Scholar]
- 88.Lynes MD, et al. , The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nat Med, 2017. 23(5): p. 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cypess AM, et al. , Identification and importance of brown adipose tissue in adult humans. N Engl J Med, 2009. 360(15): p. 1509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pfannenberg C, et al. , Impact of age on the relationships of brown adipose tissue with sex and adiposity in humans. Diabetes, 2010. 59(7): p. 1789–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nascimento EBM, et al. , Genetic Markers of Brown Adipose Tissue Identity and In Vitro Brown Adipose Tissue Activity in Humans. Obesity (Silver Spring), 2018. 26(1): p. 135–140. [DOI] [PubMed] [Google Scholar]
- 92.Stanford KI, et al. , 12,13-diHOME: An Exercise-Induced Lipokine that Increases Skeletal Muscle Fatty Acid Uptake. Cell Metab, 2018. 27(6): p. 1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chondronikola M, et al. , Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes, 2014. 63(12): p. 4089–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cinti S, Adipocyte differentiation and transdifferentiation: Plasticity of the adipose organ. Journal of endocrinological investigation, 2002. 25: p. 823–35. [DOI] [PubMed] [Google Scholar]
- 95.Berry DC, Jiang Y, and Graff JM, Mouse strains to study cold-inducible beige progenitors and beige adipocyte formation and function. Nature Communications, 2016. 7(1): p. 10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Long Jonathan Z., et al. , A Smooth Muscle-Like Origin for Beige Adipocytes. Cell Metabolism, 2014. 19(5): p. 810–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sanchez-Gurmaches J and Guertin DA, Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat Commun, 2014. 5: p. 4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Berry DC, et al. , Cellular Aging Contributes to Failure of Cold-Induced Beige Adipocyte Formation in Old Mice and Humans. Cell Metabolism, 2017. 25(1): p. 166–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cypess AM, et al. , Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med, 2013. 19(5): p. 635–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jespersen NZ, et al. , A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab, 2013. 17(5): p. 798–805. [DOI] [PubMed] [Google Scholar]
- 101.Shinoda K, et al. , Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat Med, 2015. 21(4): p. 389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu J, et al. , Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell, 2012. 150(2): p. 366–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fu W, et al. , Transient p53 inhibition sensitizes aged white adipose tissue for beige adipocyte recruitment by blocking mitophagy. Faseb j, 2019. 33(1): p. 844–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Khanh VC, et al. , Aging impairs beige adipocyte differentiation of mesenchymal stem cells via the reduced expression of Sirtuin 1. Biochemical and Biophysical Research Communications, 2018. 500(3): p. 682–690. [DOI] [PubMed] [Google Scholar]
- 105.Duteil D, et al. , Lsd1 prevents age-programed loss of beige adipocytes. Proceedings of the National Academy of Sciences, 2017. 114(20): p. 5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rogers NH, et al. , Aging leads to a programmed loss of brown adipocytes in murine subcutaneous white adipose tissue. Aging cell, 2012. 11(6): p. 1074–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jiang Y, Berry DC, and Graff JM, Distinct cellular and molecular mechanisms for β3 adrenergic receptor-induced beige adipocyte formation. eLife, 2017. 6: p. e30329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li X, et al. , Skeletal Muscle Lipid Droplets and the Athlete’s Paradox. Cells, 2019. 8(3): p. 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Correa-de-Araujo R, et al. , The Need for Standardized Assessment of Muscle Quality in Skeletal Muscle Function Deficit and Other Aging-Related Muscle Dysfunctions: A Symposium Report. Frontiers in physiology, 2017. 8: p. 87–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhu S, et al. , Aging- and obesity-related peri-muscular adipose tissue accelerates muscle atrophy. PLoS One, 2019. 14(8): p. e0221366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Visser M, et al. , Leg Muscle Mass and Composition in Relation to Lower Extremity Performance in Men and Women Aged 70 to 79: The Health, Aging and Body Composition Study. Journal of the American Geriatrics Society, 2002. 50(5): p. 897–904. [DOI] [PubMed] [Google Scholar]
- 112.Marcus RL, et al. , Intramuscular Adipose Tissue, Sarcopenia, and Mobility Function in Older Individuals. Journal of Aging Research, 2012. 2012: p. 629637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Koster A, et al. , Does the amount of fat mass predict age-related loss of lean mass, muscle strength, and muscle quality in older adults? J Gerontol A Biol Sci Med Sci, 2011. 66(8): p. 888–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fragala MS, et al. , Muscle quality index improves with resistance exercise training in older adults. Exp Gerontol, 2014. 53: p. 1–6. [DOI] [PubMed] [Google Scholar]
- 115.Fried LP, et al. , Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci, 2001. 56(3): p. M146–56. [DOI] [PubMed] [Google Scholar]
- 116.Addison O, et al. , Intramuscular fat and inflammation differ in older adults: the impact of frailty and inactivity. J Nutr Health Aging, 2014. 18(5): p. 532–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Scheller EL, et al. , Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nat Commun, 2015. 6: p. 7808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kricun ME, Red-yellow marrow conversion: Its effect on the location of some solitary bone lesions. Skeletal Radiology, 1985. 14(1): p. 10–19. [DOI] [PubMed] [Google Scholar]
- 119.Liu L-F, et al. , Characterization of age-related gene expression profiling in bone marrow and epididymal adipocytes. BMC genomics, 2011. 12: p. 212–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu L-F, et al. , Age-related modulation of the effects of obesity on gene expression profiles of mouse bone marrow and epididymal adipocytes. PloS one, 2013. 8(8): p. e72367–e72367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Justesen J, et al. , Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology, 2001. 2(3): p. 165–171. [DOI] [PubMed] [Google Scholar]
- 122.Suchacki KJ, et al. , Bone marrow adipose tissue is a unique adipose subtype with distinct roles in glucose homeostasis. Nature Communications, 2020. 11(1): p. 3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yu B, et al. , PGC-1α Controls Skeletal Stem Cell Fate and Bone-Fat Balance in Osteoporosis and Skeletal Aging by Inducing TAZ. Cell Stem Cell, 2018. 23(2): p. 193–209.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen J, et al. , Osx-Cre targets multiple cell types besides osteoblast lineage in postnatal mice. PLoS One, 2014. 9(1): p. e85161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ambrosi TH, et al. , Adipocyte Accumulation in the Bone Marrow during Obesity and Aging Impairs Stem Cell-Based Hematopoietic and Bone Regeneration. Cell Stem Cell, 2017. 20(6): p. 771–784.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kasza I, et al. , Thermogenic profiling using magnetic resonance imaging of dermal and other adipose tissues. JCI Insight, 2016. 1(13): p. e87146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang Z, et al. , Dermal adipose tissue has high plasticity and undergoes reversible dedifferentiation in mice. J Clin Invest, 2019. 129(12): p. 5327–5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kuchnia AJ, et al. , Combination of DXA and BIS body composition measurements is highly correlated with physical function-an approach to improve muscle mass assessment. Arch Osteoporos, 2018. 13(1): p. 97. [DOI] [PubMed] [Google Scholar]
- 129.Marx V, A dream of single-cell proteomics. Nature Methods, 2019. 16(9): p. 809–812. [DOI] [PubMed] [Google Scholar]