Highlights
-
•
Spontaneous dog cancers closely resemble human cancer.
-
•
Dogs with EGFR associated tumors were immunized with an EGFR/HER2 peptide vaccine.
-
•
EGFR peptide vaccinated dogs developed anti-EGFR/HER2 antibodies.
-
•
Vaccinated dogs have anti-EGFR antibody and T cells infiltrating tumors.
-
•
Vaccinated dogs with osteosarcoma had tumor regression and increased survival.
Keywords: EGFR; Vaccine; Peptide; Canine; Osteosarcoma; Abbreviations: BSA, bovine serum albumin; CTLA-4, cytotoxic T-lymphocyte associated protein 4; DAPI, 4′,6-diamidino-2-phenylindole; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; FBS, fetal bovine serum; GAPDH, glyceraldehyde-3 phosphate dehydrogenase; HER2, human epidermal growth factor receptor 2, HER3, human epidermal growth factor receptor 3; HER4, human epidermal growth factor receptor 4; MFI, mean fluorescence intensity; MHC, major histocompatibility complex; OD, optical density; OSA, osteosarcoma; PBS, phosphate buffered saline; pERK, phosphorylated extracellular signal-regulated kinase; pNPP, p-nitrophenyl phosphate; RT, room temperature
Abstract
Epidermal Growth Factor Receptor (EGFR) is overexpressed on a number of human cancers, and often is indicative of a poor outcome. Treatment of EGFR/HER2 overexpressing cancers includes monoclonal antibody therapy (cetuximab/trastuzumab) either alone or in conjunction with other standard cancer therapies. While monoclonal antibody therapy has been proven to be efficacious in the treatment of EGFR/HER2 overexpressing tumors, drawbacks include the lack of long-lasting immunity and acquired resistance to monoclonal therapy. An alternative approach is to induce a polyclonal anti-EGFR/HER2 tumor antigen response by vaccine therapy. In this phase I/II open-label study, we examined anti-tumor immunity in companion dogs with spontaneous EGFR expressing tumors. Canine cancers represent an outbred population in which the initiation, progression of disease, mutations and growth factors closely resemble that of human cancers. Dogs with EGFR expressing tumors were immunized with a short peptide of the EGFR extracellular domain with sequence homology to HER2. Serial serum analyses demonstrated high titers of EGFR/HER2 binding antibodies with biological activity similar to that of cetuximab and trastuzumab. Canine antibodies bound both canine and human EGFR on tumor cell lines and tumor tissue. CD8 T cells and IgG deposition were evident in tumors from immunized dogs. The antibodies inhibited EGFR intracellular signaling and inhibited tumor growth in vitro. Additionally, we illustrate objective responses in reducing tumors at metastatic sites in host animals. The data support the approach of amplifying anti-tumor immunity that may be relevant in combination with other immune modifying therapies such as checkpoint inhibitors.
Introduction
Immunotherapy, including monoclonal antibodies, has become an integral treatment strategy for human cancers. The monoclonal antibodies, cetuximab and trastuzumab, target malignancies such as colorectal cancer and breast cancer that overexpress the ErbB family members EGFR and HER2, respectively [1,2]. Overexpression of these molecules on tumors is often associated with a more aggressive tumor behavior and carries a worse prognosis [3], [4], [5].
We recently pursued strategies to actively elicit B and T lymphocyte immunity to the ErbB tumor protein family [6]. Advantages of vaccination to stimulate anti-tumor immunity include ease of administration, and induction of durable, tumor-specific immune responses compared to monoclonal antibodies that require repeated administration and do not confer long lasting tumor immunosurveillance. We previously demonstrated that mice immunized with a peptide derived from the extracellular domain of EGFR generated antibodies that specifically bound both EGFR and HER2, promoted tumor cytotoxicity, and inhibited tumor growth both in vitro and in vivo [6].
Herein, we assess the biological activity of EGFR/HER2 peptide-based immunotherapy in dogs with appendicular osteosarcoma and other ErbB associated tumors. Dogs represent a relatively outbred population in which disease arises spontaneously with a diversity of cancers similar to humans [7]. Studies have demonstrated that expression of ErbB family proteins in aggressive canine cancers, including osteosarcoma and hemangiosarcoma, is correlated with shortened survival times [8], [9], [10], [11], [12], [13], [14]. ErbB immunization strategies are an attractive option because of ease of administration, low toxicity, affordability, limited clinic visits, and potential clinical effectiveness. Anti-tumor antibodies also enhance the efficacy of conventional chemotherapies, radiation therapy, and checkpoint inhibitors [15], [16], [17], [18]. Finally, relatively few effective immunotherapeutic options exist for the treatment of highly metastatic malignancies in the dog [19].
In this study, we report the biological activity of antibodies generated in dogs immunized with a conserved peptide from the extracellular domain of EGFR with shared amino acid sequence homology to HER2 and HER3 extracellular domains. Immunization with canine EGFR p527 (cEGFR p527) resulted in the generation of antibodies that bound both canine and human EGFR as well as HER2. These antibodies were capable of inhibiting EGFR signaling, attracted antibodies and CD8 T cells to the in vivo tumor microenvironment and inhibited tumor growth in vitro. We report 12-month survival outcomes in a first group of dogs with appendicular osteosarcoma that received cEGFRp527 vaccination as adjuvant treatment. As a first open-label canine clinical trial, these data demonstrate that immunization with a specific EGFR/HER peptide induces cell-mediated and humoral responses in canine recipients and could be a promising immunotherapeutic strategy for canines with tumors that express ErbB family proteins.
Materials and methods
Peptides and cell lines
Canine EGFR p527–545 (IKCAHYIDGPHCVKTCPAG) was synthesized by GenScript (Piscataway, NJ; > 95% purity). The EGFR+ human epidermoid carcinoma cell line A431 (CRL-1555) and the canine osteosarcoma cell line D17 (CRL-6248) were obtained from American Type Culture Collection (ATCC; Manassas, VA), as was the HER2+/EGFR- human mammary gland carcinoma cell line MDA-MB-453 (HTB-131, ATCC) [20]. Cell lines were authenticated by ATCC for viability, growth, and morphology.
Antibodies
Cetuximab (anti-human EGFR monoclonal antibody) was a gift of Dr. David Rimm (Yale School of Medicine). Rituximab (human anti-CD20 monoclonal antibody) and trastuzumab (anti-human HER-2 monoclonal antibody) were obtained from the Smilow Cancer Center Pharmacy, New Haven, CT. Anti-human EGFR mouse monoclonal antibodies, Ab-10 (clone 111.6; ThermoScientific; Waltham, MA) and Ab-1 (clone 528; Calbiochem; Temecula, CA), were used for the detection of canine EGFR as demonstrated by previous studies [21].
Animals and immunization
Protocols were consistent with accepted guidelines of the NIH for the care and use of animals as well as approved by the Yale University Institutional Animal Care and Use Committee. Canine cancer patients were recruited by MedVet (Norwalk, CT) as well as licensed veterinary practices. All dog owners provided written informed consent for enrollment of their dogs in the study, a total of 93 patients reported herein, including 43 patients with pathology confirmed appendicular OSA and enrolled in the vaccination protocol >12 months (for survival outcomes as defined below). Canine cancer patients were first confirmed to have ErbB associated tumors by tissue pathology (board certified pathologists) and were recruited for the study. OSA patients received standard of care (both amputation and 4–6 rounds of carboplatin; 250–300 mg/m2 IV, q 3 weeks), followed by p527 vaccination beginning a minimum of 3 weeks after the last cycle of chemotherapy. Dogs were not required to be free of metastasis prior to enrollment.
Montanide ISA 51VG (Seppic, Inc.) was utilized for an adjuvant as it has been used in human clinical vaccine trials [22]. The vaccination emulsion was assembled under sterile conditions according to manufacturer's specifications and stored at 4 °C. At day 0, a pre-immune serum sample was obtained, and patients were immunized s.c. with 0.8 mg of cEGFR p527 with 15% (v/v) LymeVax® (Zoetis, Inc.; Parsippany, NJ) emulsified in Montanide ISA 51VG (Seppic, Inc.; Fairfield, NJ) in a total volume of 200 µl in the interscapular region. Twenty-one days later, a second serum sample was obtained, and patients were boosted s.c. in an identical manner. Convalescent serum samples were obtained at day 40–50 after the first vaccination. Healthy (non-tumor bearing) dog sera were obtained with consent through the Clinton Veterinary Hospital (Clinton, CT). Finally, a second cohort of dogs were immunized and boosted in an identical manner with 0.8 mg of cEGFR p527 with CpG ODN 2006 (200 µg/dose; InvivoGen; San Diego, CA) emulsified in Montanide ISA 51VG in a total volume of 200 µl. Selected patients provided follow-up imaging (radiographs) with analysis interpreted by radiologists at intervals indicated by the presiding veterinarian, however, regular imaging was not a requirement of the study.
ELISAs for EGFR and Borrelia burgdorferi antibodies
Anti-EGFR serum antibodies were assessed as previously described [6]. cEGFR p527 peptide (50 µg/μl), was adsorbed to microtiter plates, followed by incubation with serum samples (1:100 dilution). Alkaline phosphatase conjugated-goat anti-dog IgG antibody (Southern Biotech; Birmingham, AL) was added to wells followed by incubation with pNPP substrate. IgG units are defined as (OD immune serum/OD healthy dog serum)/(OD pre-immune serum/OD healthy dog serum). To confirm specificity of the anti-cEGFR response, 1:100 dilutions of serum were pre-incubated overnight with 10 mg/ml cEGFR p527 or irrelevant control peptide murine histone H2b p21 (AQKKDGKKRKRSRKE, AnaSpec, San Jose, CA) prior to detecting EGFR binding by ELISA.
Antibodies to Borrelia burgdorferi were detected as previously described [6,23]. In brief, microtiter plates were adsorbed with B. burgdorferi extract in carbonate coating buffer, followed by canine serum samples (diluted 1:100). Plates were washed, incubated with phosphatase conjugated-goat anti-dog IgG antibody (Southern Biotech, 1:1000 dilution) pNPP added and read at 405 nm.
Flow cytometry
A431, D17 and MDA-MB 453 cells (105 cells/sample) were stained with a 1:100 dilution of canine serum followed by incubation with a 1:100 dilution of anti-dog IgG AlexaFluor 488 (Jackson Immunoresearch; West Grove, PA. Cells were analyzed on a FACSCalibur (BD Biosciences; San Jose, CA) with FlowJo software (Tree Star; Ashland, OR). Controls included cells stained with secondary antibody alone, cetuximab (2 µg/105 cells), Ab-10 (1 µg/105) and trastuzumab (20 μg/105).
Immunofluorescence staining
Tumor tissues were procured from deceased study dogs at the time of necropsy with owner consent and fixed in 10% neutral buffered formalin, paraffin embedded (FFPE). Tissues were deparaffinized, rehydrated and underwent antigen retrieval using Retrieve-All solution (Biolegend; Dedham, MA) according to manufacturer's protocol. Controls included healthy dog serum (1:100 dilution), mouse anti-EGFR mAb (Ab-10 clone 111.6, 1:20 dilution), and isotype control (purified mouse IgG2a, clone MC2a-53, Biolegend). Sections were blocked with 5% goat serum, washed and stained for EGFR using a 1:100 dilution of either preimmune or cEGFR p527 immune serum overnight at 4 °C, followed by 1:50 dilution rabbit anti-mouse IgG Alexa Fluor 488 (Jackson Immunoresearch). Dog IgG deposition was detected using anti-dog IgG Alexa Fluor 488 (1:50 dilution, Jackson Immunoresearch). CD8 T cells were stained in tissues using mouse anti-dog CD8 (MAB6709; R&D Systems, Minneapolis, MN), followed by goat anti-mouse IgG (H + L) Alexa Fluor 488 (A11017; Life Technologies, Eugene, OR) and counterstaining with DAPI (Molecular Probes; Eugene, OR). Stained tissue sections were divided into four equal quadrants, and the mean fluorescent intensity of each quadrant quantified by Image J [24].
EGFR and HER2 signaling detection and inhibition by western blotting
Epidermal growth factor receptor (rhEGF, R&D Systems) stimulation of A431 and D17 cells in the presence of preimmune or immune sera (1:25 dilution) and subsequent western blotting was performed as previously described [25]. Cetuximab or Ab-1 (both at 10 µg/ml) served as positive controls for A431 and D17 cells, respectively. PD98059 (1 μg/ml, Sigma) was a positive control for inhibiting ERK phosphorylation in MDA-MB-453 cells. Antibodies used were against pEGFR (Y1068), pERK (Thr202/Tyr204) and GAPDH (Cell Signaling Technology, Danvers, MA). Densitometry was performed on scanned images of films using Image J.
Tumor cell proliferation assays
The ability of immune sera to inhibit the growth of the EGFR overexpressing A431 or D17 cell lines were measured using a 3H-thymidine incorporation assay as previously described using 4000 A431 cells/well, 1000 D17 cells/well and 4 × 104 MDA-MB-453 cells/well [6]. Control antibodies included cetuximab (10 µg/ml), rituximab (anti-CD20; 10 µg/ml), Ab-1 (10 µg/ml), mouse IgG2a (BioLegend; Dedham, MA) and trastuzumab (21 μg/ml). Percent cell growth inhibition: [(Pre-immune serum CPM – Immune serum CPM)/pre-immune serum CPM] x 100.
Statistics
Survival data from this study was independently reviewed and analyzed by the Yale Center for Analytical Sciences. Results are expressed as means ± SEM or N (%). The group comparison was performed using the Mann-Whitney test (Prism, GraphPad Software). Survival analysis was conducted using the Kaplan Meier method to estimate 1-year survival rate, median survival time and mean survival time, while incorporating censored data. Point estimates and 95% confidence intervals were reported. This analysis was conducted using SAS 9.4 (SAS Institute Inc., Cary, NC, USA). The statistical significance was set as p < 0.05, two-sided.
Results
Rationale, selection, and formulation of canine EGFR peptide (cEGFR p527) vaccination
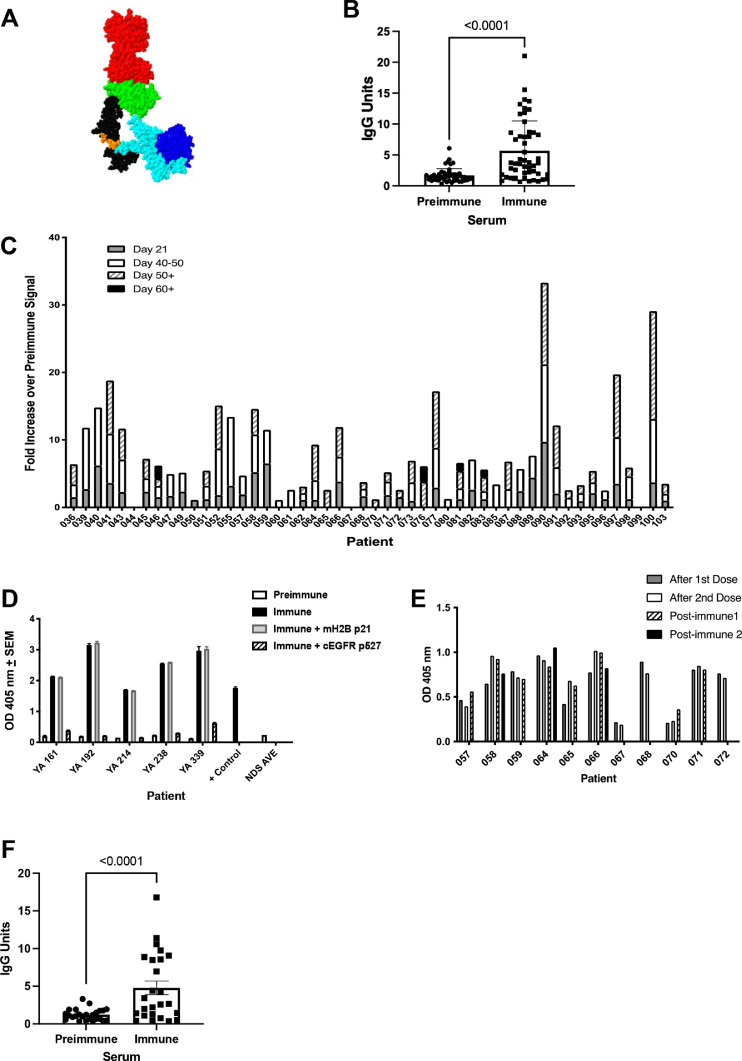
In the prior studies, we examined immunogenic cryptic self-peptides representing the membrane proximal, extracellular domain IV of EGFR (Fig. 1A) in the proximity of the known cetuximab-binding region [6]. Strategically, we investigated immunogenic peptides within homologous domains shared between EGFR, HER2, and HER3, given the expression of heterodimeric rearrangements of these ErbB family proteins on tumor cells [26]. The crystal structure of the human EGFR extracellular domain co-crystalized with the Fab fragment of cetuximab is illustrated in Fig. 1A [27]. Cetuximab binding to domain III is shown, while the p527 region, a site examined in the present study, is illustrated in domain IV. The p527 region was chosen, in part, for its amino acid sequence identity with surface exposed extracellular regions on HER2 and HER3 (Supplementary Table 1) and the near identical homology to the same region on human and murine EGFR (Supplementary Table 2), with only a single amino acid difference at the amino terminus.
Fig. 1.
Immunized canine patients generate anti-cEGFR p527 antibodies. Representative individual serum samples from dogs were tested by ELISA for binding to the cEGFR p527 peptide. IgG Units are defined in the Materials and Methods. A. EGFR-cetuximab crystal structure (PDB ID 1YY9). The cetuximab Fab fragment is at the top (red). The human EGFR extracellular domain I (blue), domain II (cyan), domain III (green) and membrane proximal domain IV (black) are shown with the p527 peptide sequence (orange) highlighted. B. Individual IgG responses to cEGFR p527 before and after immunization. C. Cumulative IgG response in individual dogs (represented as fold increase over preimmune response). D. p527 Representative sera (1:100 dilutions) were pre-incubated overnight with cEGFR p527 or control irrelevant peptide mH2B p21 to confirm specificity of the antibody binding. E. IgG response to Borrelia burgdorferi antigen in dogs immunized with cEGFR p527. F. Individual IgG responses to immunization peptide emulsified in Montanide ISA51 VG with CpG before and after immunization (+/- SEM) (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
cEGFR p527 immunization elicits anti-cEGFR peptide antibodies
Ninety-three dogs were enrolled based on specific pathologic identification of tumor types associated with ErbB family tumor expression. Male and female breeds of all ages (2 −13 years) were enrolled, exhibiting different ErbB family tumor types with osteosarcoma (OSA) and hemangiosarcoma (HSA) occurring with the highest frequency in our study (Supplemental Tables 3 and 4). In dogs that received myelosuppressive chemotherapy, vaccination was not performed until >3weeks after the final cycle.
Overall, we observe a highly significant increase in anti-EGFR antibodies upon vaccination and boost with p527 peptide (p < 0.001; Fig. 1B). The overall range of response is between 4 and 30 fold to greater relative to pre-immune responses in recipients (Fig. 1C). Antibody development appeared independent of breed, gender, or tumor type (Supplementary Table 3) and independent of chemotherapy administered to dogs in this study. While most experienced a significant increase in anti-EGFR antibodies with the second (booster) vaccination, others experienced a significant increase in antibody response with only a single dose compared to pre-immune serum responses (Fig. 1C). The antibodies generated by EGFR p527 immunization were specific for EGFR, as the antibody signal decreased when the immune sera were pre-incubated with EGFR p527 yet was not altered when incubated with an irrelevant peptide (mH2B) (Fig. 1D).
Canine LymeVax® was included as a small component of the vaccine formulation to help determine if recipients may be immune compromised and assessed together with anti-EGFR titers in individual dogs. As shown in Fig. 1E, most dogs tested had anti-Borrelia IgG, indicating an intact immune response to vaccination. However, two patients, 067 and 070 exhibited weak anti-Borrelia IgG immunity (Fig. 1E) and also did not produce anti-cEGFR p527 antibodies (Fig. 1C), suggesting that these two were immune compromised.
Finally, we have also examined anti-EGFR antibody responses in 35 dogs immunized with cEGFR p527 emulsified in Montanide ISA 51 VG with CpG (Fig. 1F). As indicated, CpG also serves as an effective toll-like receptor agonist adjuvant in generating strong anti-cEGFR p527 antibodies in a manner identical to those observed in the other formulation. There were no other changes in anti-EGFR biological activity between the formulations as defined below.
No immediate hypersensitivity reactions were noted in any dogs, though approximately 14–30% of dogs (depending on the whether the vaccine was administered at MedVet or by participating veterinarians) developed inflammation and a sterile abscess between 10 and 28 days after vaccination at the site of immunization. Warm compresses were applied to these sites, and most of the abscesses resolved over a span of two weeks without any additional intervention.
cEGFR p527 immune sera bind live cell-associated canine EGFR and human EGFR and HER2
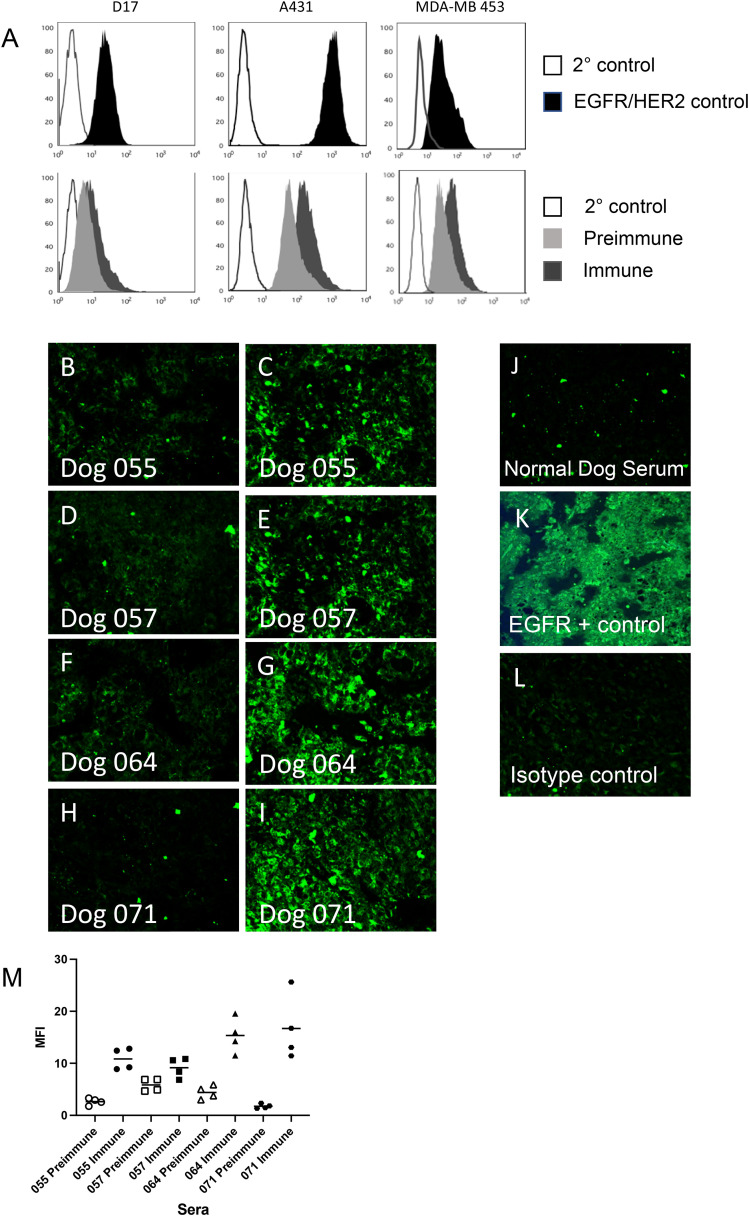
The antibodies elicited by immunization also bound intact EGFR on both human and canine tumor cell lines (Fig. 2A; representative serum analysis on lower panels). Canine osteosarcoma D17 and human A431 cells both express EGFR (Fig. 2A, top panels) as illustrated by anti-EGFR positive control binding. cEGFR p527 immune serum bound to EGFR on the surface of the D17 cells to higher degree than preimmune serum (Fig. 2A, lower panel). Similarly, binding was demonstrated to the human EGFR overexpressing cell line A431. Overall, cEGFR p527 immunization elicits antibodies binding both canine and human EGFR expressed on cell membranes, a critical observation to support the ability to bind tumor cells in vivo. Finally, immune canine serum also stains cell populations of MDA-MB-453, a cell line positive for HER2 but not observed to express EGFR (Fig. 2A, bottom right panel). The upper MDA-MB 453 panel illustrates binding by trastuzumab as a control.
Fig. 2.
Serum from cEGFR p527 peptide immunized dogs bind EGFR/HER2 on both canine and human cells and osteosarcoma tumor tissue. A. Representative serum from immunized dogs were analyzed by flow cytometry for binding to EGFR-bearing canine OSA D17 or human A431 cells, or to HER2-bearing (EGFR negative) human MDA-MB 453 cells. Upper panels (left to right) illustrate secondary control antibody staining (dark lines) or positive control antibody staining (filled peaks) of D17 cells (Ab-10), A432 (cetuximab), and MDA-MB 453 (trastuzumab). Lower panels illustrate representative staining of D17, A431, or MDA-MB 453 cells with secondary control antibody (dark line), preimmune canine sera (light gray peaks) or day 40–50 cEGFR p527 post immune sera (black peaks). B-L. FFPE canine osteosarcoma tissue samples were stained using either preimmune or immune sera from cEGFR p527 immunized dogs. Panels B, D, F and H are each stained with an individual preimmune serum. Panels C, E, G and I are each stained with the corresponding individual immune serum. Panels J, K and L represent staining with healthy dog sera, positive EGFR control monoclonal antibody (Ab-10) or isotype control IgG antibody, respectively. M. Mean fluorescent intensity (MFI) of preimmune and immune sera stained tissues from panels B-I as analyzed by Image J. Magnification is 40x. (For comparison of staining differences between panels B-L, reader is referred to the web version of this article.)
These results were further confirmed by the staining of canine osteosarcoma tissue using cEGFR p527 immune and preimmune sera. Canine osteosarcomas have been reported to express varying levels of ErbB family proteins (EGFR/HER2) and/or mRNA [8,12,28]. Post-operative OSA bone tumor tissues were obtained from local patients and analyzed by immunofluorescence for binding by pre- and post-immune canine serum samples (Fig. 2B,I). Immune sera from cEGFR p527 immunized dogs bound to osteosarcoma tissue (Fig. 2C,E,G,I – each panel a separate serum) whereas there was little tissue binding seen with the corresponding preimmune tissue (Fig. 2B,D,F,H). These results were confirmed as the mean fluorescent intensity (MFI) of the immune sera-stained tissue was greater than those of preimmune stained sera (Fig. 2M). Healthy dog serum (non-tumor bearing) (Fig. 2J) or isotype control secondary antibody (Fig. 2L) fail to significantly bind OSA tissue sections. In contrast, positive anti-EGFR monoclonal antibody (clone Ab-10) control stained OSA tissues with a more diffuse overall pattern (Fig. 2K).
cEGFR p527 immunization mediates CD8 infiltration and IGG deposition into tumors
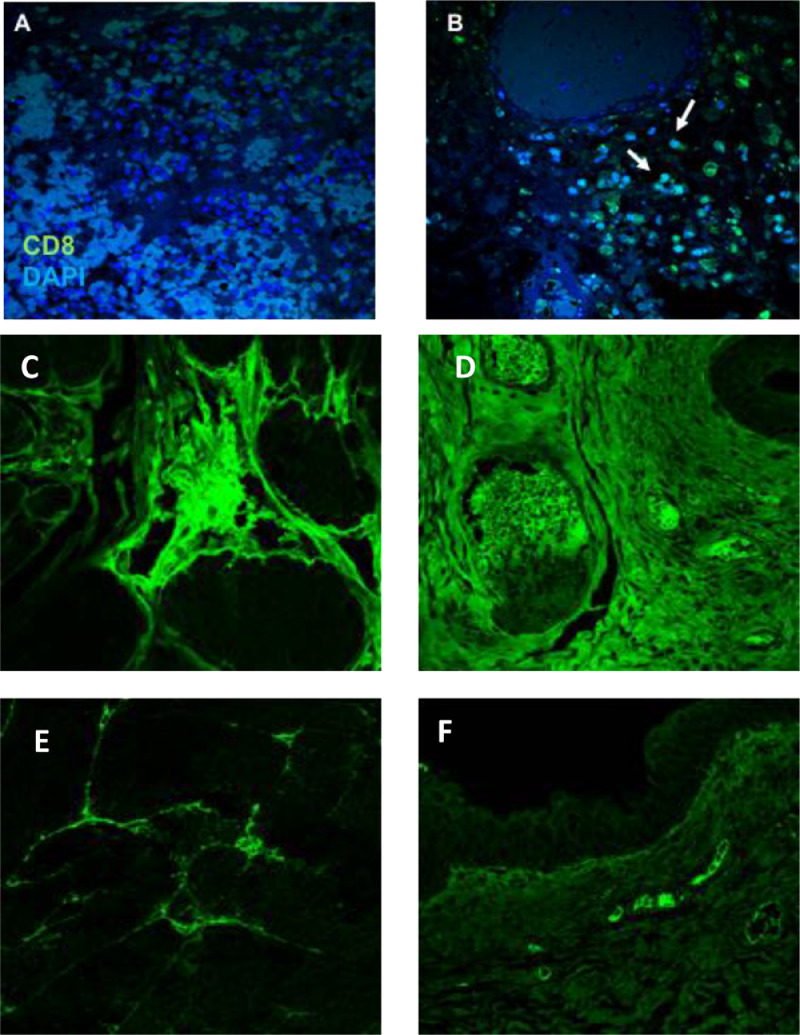
Tumor-specific immune responses were also assessed for the infiltration of CD8 T cells into the tumor site [29,30]. Fresh bladder tissue carcinoma was obtained from a cEGFR p527 immune dog with urothelial carcinoma. The tissue was analyzed for infiltrating antibody and T cells into the bladder tumor versus normal, non-cancerous bladder tissue from a second healthy donor (Fig. 3). DAPI single stained tissue is illustrated in panel A (blue cells), while CD8 T cells were detected in tissue from a cEGFR p527 immunized dog (green cells; Fig. 3B). The bladder tumor from the cEGFR p527 immune patient also stained positive for dog IgG (Fig. 3C and D), while bladder tissue from a non-immunized dog was negative for IgG deposition (Fig. 3E and F). These observations show that cEGFR p553 immunization can promote antibody deposition and CD8 T cell infiltration into the tumor microenvironment.
Fig. 3.
cEGFR p527 immunization mediates CD8 infiltration and antibody deposition into canine tumors. Postmortem bladder cancer tissue from a cEGFR p527 immune dog and tissue from healthy, non-cancerous bladder was examined for infiltration of CD8 T cells and endogenous antibody. A. CD8 isotype control. B. CD8 T cell staining (green). Arrows illustrate presence of CD8+ cells within the tumor tissue. C. & D. Antibody deposition in canine bladder tumor tissue from a cEGFR p527 immunized dog (two different fields). E. & F. Canine IgG deposition in normal canine bladder tissue (two different fields). Magnification is 40x (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
Anti-cEGFR p527 antibodies inhibit cell signaling
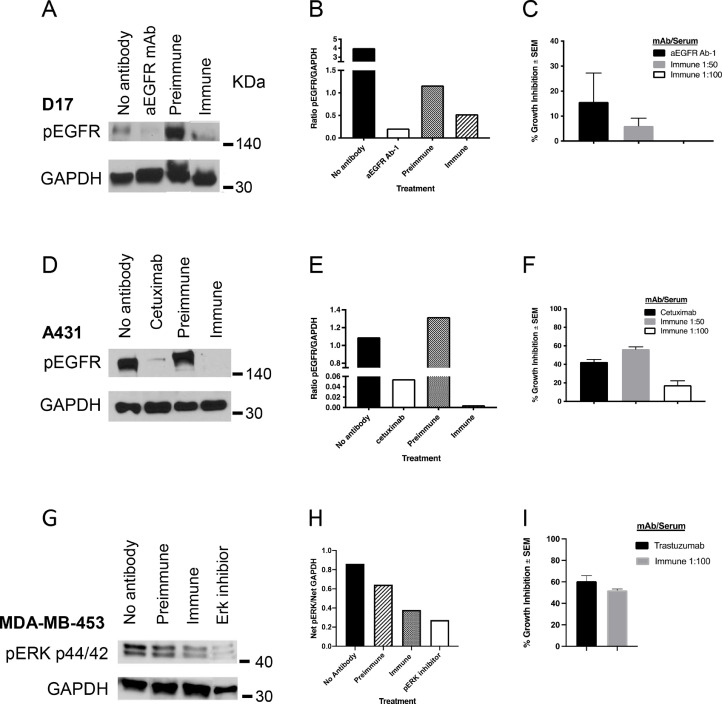
We next examined whether antibodies were also capable of inhibiting EGF- and HER2-mediated intracellular signaling. We assayed the levels of EGFR phosphorylation (pEGFR) in canine D17, human A431, and HER2 signaling in MDA-MB-453 human tumor cell lines was assessed by analysis of pERK (Fig. 4). As illustrated, the levels of phospho-EGFR were reduced upon EGF stimulation in those D17 cells that had been pre-incubated with immune serum as compared to pre-immune serum (Fig. 4A,B). In a similar manner, immune serum decreases EGFR signaling in human A431 cells (Fig. 4D,E). MDA-MB-453 cells are HER2 bearing cells, lacking expression of EGFR. Fig. 4G,H illustrate the ability of cEGFR p527 antisera to interfere with HER2-mediated signaling pathways.
Fig. 4.
Anti-cEGFR p527 antibodies block EGFR and HER2 signaling and in vitro growth. cEGFR p527 immune serum blocks the signaling of EGF induced EGFR phosphorylation and HER2 signaling as assessed by pERK immunoblot. GAPDH was used as a protein loading control. For growth inhibition, cells were incubated with 1:50 or 1:100 dilutions of immune dog serum. Cetuximab served as the positive control for A431 cells, Ab-1 was the positive control for D17 cells and trastuzumab was the positive control for MDA-MB-453 cells. Cell proliferation was measured by 3H-thymidine incorporation and growth inhibition relative to preimmune serum, calculated as described in the Materials and Methods. A. Inhibition of EGF signaling in D17 cells. B. Ratio of pEGFR/GAPDH in D17 cells as determined by densitometry of immunoblots. C. Inhibition of D17 growth. D. Inhibition of EGF signaling in A431 cells. E. Ratio of pEGFR/GAPDH in A431 cells as determined by densitometry of immunoblots. F. Inhibition of A431 growth. G. Inhibition of HER2 signaling in MDA-MB-453 cells. H. Ratio of pERK/GAPDH in MDA-MB-453 cells by densitometry of immunoblots. I. Inhibition of MDA-MB-453 growth.
cEGFR p527 antiserum inhibits tumor cell line growth
In vitro tumor cell growth inhibition is a standard of analysis in assessing the biological efficacy of anti-tumor antibodies. Tumor cell line growth (D17, A431 and MDA-MB-453) was inhibited in the presence of cEGFR p527 immune serum (Fig. 4C, F and I). A431 cell growth was inhibited by approximately 56% in the presence of p527 canine immune serum (1:50 dilution) as compared to cetuximab (approximately 40%) (Fig. 4F). EGFR p527 immune serum inhibited MDA-MB-453 cell growth by approximately 52% (Fig. 4I). Data are normalized to the signal of pre-immune canine patient serum in identical wells.
Tumor regression in immunized dogs
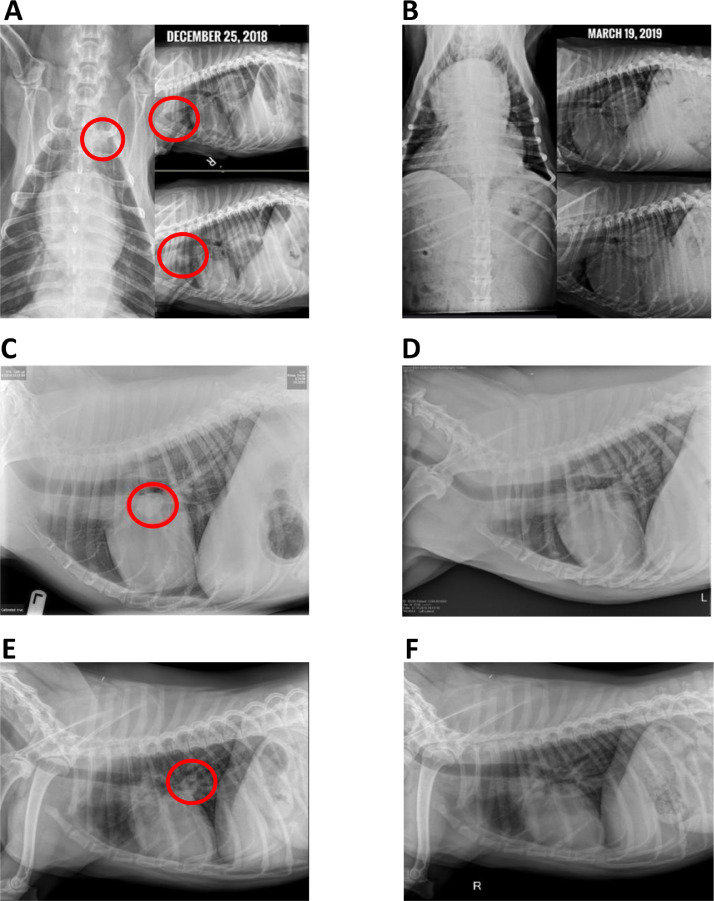
Thoracic radiographs were obtained for some OSA-bearing dogs. Fig. 5 shows radiographs of dogs that presented with macroscopic pulmonary nodules prior to immunization and responses post-immunization, as indicated. All were metastasis-free prior to limb amputation and chemotherapy. The cEGFR p527 immunization was initiated 3 weeks post chemotherapy, at the same time metastases were detected. As illustrated in Fig. 5, all three dogs had visible lung opacities consistent with metastases prior to immunization (Fig. 5A,C,E). The three dogs were positive for anti-EGFR antibodies, as indicated by ELISA and/or flow cytometry following immunizations. The time between serial imaging varied from approximately 3 months (Fig. 5A/B), 11 months (Fig. 5C,D), and 3 months (panel Fig. 5E,F). In each case, previously detectable metastases had resolved at the time of follow-up radiographs. Although these are open-label phase I studies, the data suggest that the cEGFR p527 immunity, potentially in combination with chemotherapy, can promote tumor regression in vivo.
Fig. 5.
Regression of lung metastases in OSA patients immunized with cEGFR p527. Chest X-ray images of three separate dogs diagnosed with OSA lung metastases at the time of immunization with cEGFR p527 vaccine (panels A, C, E) and post immunization (panels B, D, F). Images A&B and E&F: approximately 3 months between imaging. Images C&D: approximately 11 months between images. Red circles represent presence of OSA metastases as defined by radiology reports (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
Twelve-month survival time of OSA dogs immunized with cEGFR p527
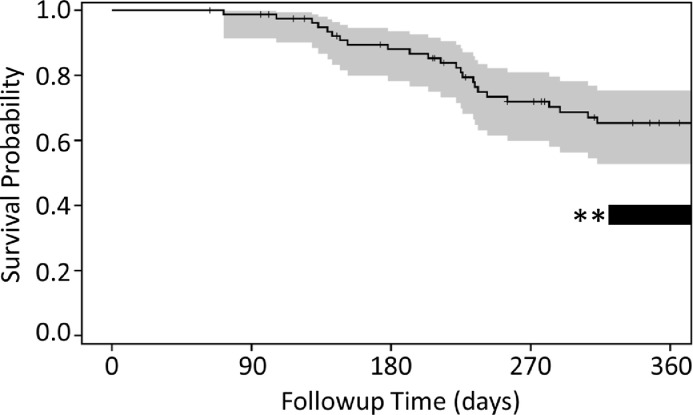
Finally, we examined OSA patients for survival as a secondary endpoint of the phase 1 investigation, with the primary endpoint assessing immunity and safety. In brief, the 12-month survival rate in the population receiving immunization after standard of care (amputation and chemotherapy, N = 43) is 65%, with 95% confidence interval of 53 to 75%, as indicated in Fig. 6. The median survival time is 478 days (95% CI; 418–617 days) and the mean survival time is 496 days (95% CI; 426–565 days). This compares to previous reports indicating approximately 35–40% of dogs treated for OSA with standard-of-care therapy survived 12 months (Fig. 6; dark bar with asterisk) and median overall survival of approximately 307 days [31,32]. While our open label study was not intended to include blinded placebo controls, the data indicate statistically significantly improved survival benefit from cEGFR p527 immunization compared to historical standard of care, amputation and chemotherapy.
Fig. 6.
Survival statistics in cEGFR p527 immunized osteosarcoma patients. The plot illustrates 360-day survival among 43 patients with osteosarcoma receiving amputation, 4–6 cycles of carboplatin, followed by cEGFR p527 immunization. Twelve-month survival was observed at 65%, with 95% confidence interval of 53 to 75% (gray). The black bar with asterisks indicates prior studies reporting approximate 35–40% twelve-month survival in osteosarcoma patients similarly treated with standard of care (amputation and carboplatin) as examined in a similar manner herein.
Discussion
Cancer immunotherapy has grown rapidly over the years, utilizing monoclonal antibodies and, more recently, checkpoint inhibitor therapies [1,2]. However, an appreciable number of patients becoming resistant to monoclonal antibody therapy[33], in part due to the rearrangement of ErbB heterodimers and mutational events in tumor cells. Vaccination to tumor proteins can induce long-lasting immunity, with easier administration compared to multiple infusions of monoclonal antibodies.
Many non-mutated self-peptides, including those of tumor proteins can be viewed as immunogenic, termed ‘cryptic self-peptides’ and amplify the pathology of autoimmune diseases and tumor immune pathology via epitope spreading [34], [35], [36], [37], [38]. Additionally, self-proteins, generated by mutational events of DNA or by frameshift in the RNA coding regions in dividing tumor cells are ‘neoantigens’ that break immune tolerance [39,40]. In this study, we have identified an ErbB peptide (cEGFR p527) to which normal immune tolerance has never been established, allowing formerly quiescent B and T lymphocytes to be primed and activated. The primary endpoint of the study is to confirm anti-tumor immunity and safety elicited by EGFR vaccination in spontaneous canine cancers.
Immunotherapy for canine OSA has been of interest for many years. Indeed, a recent prior study examined the immunity exhibited by a Listeria expressing a human chimeric HER2 fusion protein in canine osteosarcoma [28]. Canine osteosarcoma has been reported to express HER2 as well as varying levels of EGFR mRNA and protein [8,12,28]. Similarly, a HER2 chimeric peptide vaccination therapy (emulsified in Montanide ISA 720VG) has demonstrated successful outcomes and efficacy with safety in human Phase I clinical trials of tumor bearing patients [41,42].
This study is based on our prior work illustrating anti-tumor immunity in transplantable ErbB murine cancer models [6]. Moreover, the ErbB domains utilized are surface exposed regions based on the crystal structures of these proteins. Overall, cEGFR p527 immunization was well tolerated, with the only minor adverse event being that of sterile abscess inflammation at the site of injection in approximately 14–30% of patients, the majority of which were self-resolving. We observed no off target tissue adverse events or autoimmunity, as indicated by postmortem pathological analysis (brain, heart, liver, lung, muscle spleen, thymus, pancreas, connective tissue) from 5 dogs immunized in our program (Yale University Department of Comparative Medicine and Pathology; data not shown). Advantages of this approach is in immunity generated to multiple ErbB tumor proteins, EGFR and HER2, often expressed on the same tumor.
A prior study illustrated the binding properties of both cetuximab and trastuzumab to canine EGFR and canine HER2 emphasized the role of humoral immunity in canine cancer therapy [43]. Signaling through EGFR amplifies VEGF expression on tumor cells, a key pathway in tumor resistance to EGFR tyrosine kinase inhibitors [44]. It is clinically relevant that cEGFR p527 immune dog sera block EGFR and HER2 signaling pathways.
Antibody responses arise irrespective of breed, ranging from 4 to 30 fold increased, with differences likely related to diverse MHC backgrounds [45,46]. We do not yet understand how titers of antibody or T cell responses may correlate specifically to clinical outcomes. Twelve-month survival among OSA patients is not significantly correlated with levels of anti-p527 antibody response (data not shown). In a limited number of tissues acquired at postmortem, antibodies and CD8 cells were found to infiltrate the tumor microenvironment. We believe that collaborative binding of both EGFR and HER2 is critical in immunity homing to the tumor microenvironment. Analysis of 43 osteosarcoma patients first treated with amputation and carboplatin, followed with cEGFR p527 immunization revealed a significantly increased 12-month survival. However, there is biological rationale for cEGFR p527 vaccination prior to initiation of chemotherapy in OSA. Significant anti-EGFR IgG immune responses often arise by 3 weeks, accompanied by the development of plasma B cells and memory B cell populations. Terminal stage B cell populations and circulating anti-tumor antibodies are durable throughout myelosuppressive therapies and will continue to provide anti-tumor immune surveillance during cycles of chemotherapy typically provided in OSA. The present study establishes immunotherapy as an important adjunct to surgery and chemotherapy.
Perhaps the most promising data is the observations of regression of established OSA pulmonary metastases, often responsible for OSA mortality [47,48], over the course of cEGFR p527 immunization. This approach is a key step towards canine immunotherapy, and eventually paves the road toward human cancer vaccines. These interesting clinical findings of vaccination efficacy await confirmation through expanded prospective, randomized, placebo-controlled clinical trials. Future studies will further assess the features of individual tumors, including levels of ErbB expression that may be important in the clinical outcomes of cEGFR p527 immunization.
Conclusion
This is the first report of a canine ErbB peptide vaccine that elicits antibodies capable of binding both human and canine EGFR/HER2, inhibiting tumor growth and EGFR signaling in vitro and triggers homing of antibodies and CD8+ T cells to solid tumors in vivo. Antibodies to EGFR and/or HER2 synergize or increase sensitivity to radiation therapy or adjuvant chemotherapy [15], [16], [17]. These findings suggest that ErbB p527 immunization is a viable therapeutic strategy for the treatment of EGFR overexpressing osteosarcomas in the dog, and potentially other canine tumors, either alone or as adjunct to other forms of therapy including radiation, chemotherapy, or checkpoint inhibition.
CRediT authorship contribution statement
Hester A. Doyle: Conceptualization, Methodology, Investigation, Formal analysis, Data curation, Writing – original draft, Writing – review & editing. Renelle J. Gee: Methodology, Investigation, Data curation. Tyler D. Masters: Investigation. Christian R. Gee: Investigation. Carmen J. Booth: Resources. Elizabeth Peterson-Roth: Conceptualization. Raymond A. Koski: Conceptualization. Stuart C. Helfand: Conceptualization. Lauren Price: Resources. Deborah Bascombe: Investigation, Resources. Dorothy Jackson: Resources. Rita Ho: Resources. Gerald R. Post: Conceptualization, Resources. Mark J. Mamula: Conceptualization, Methodology, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We thank all the owners of the patients enrolled in the study, especially Richard Kneisel (and Ranger), Mike Rossa (and Cody), Stephanie Rossner and Josh Smith (and Ruby), Laurie Shiratori (and Scout), and Cat McCrae (and Sitka). We also thank Gillian Rothchild and Anna Headrick of MedVet (Norwalk, CT) for their technical help with the canine vaccinations, blood draws, and record keeping. We thank the Yale Center for Analytical Sciences, in particular Kaitlin Maciejewski, Fangyong Li, and Dr. Constantino Kyriakides, for their statistical analysis of the data in this work. We thank Dr. Betsy Hershey, Integrative Veterinary Oncology, Phoenix, AZ for help with recruiting patients. We appreciate the help of Dr. Phillip Bergman for ongoing advice and review of this manuscript.
Funding
These studies were supported by generous donations from Ms. Alva Greenberg, Mari Maeda (Canine Cancer Research Alliance), and Ms. Stephanie Rossner and Josh Smith and Ruby (Ruby's Can For A Cure).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2021.101205.
Appendix. Supplementary materials
References
- 1.Garcia-Foncillas J., Sunakawa Y., Aderka D. Distinguishing features of cetuximab and panitumumab in colorectal cancer and other solid tumors. Front. Oncol. 2019;9:849. doi: 10.3389/fonc.2019.00849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oh D.Y., Bang Y.J. HER2-targeted therapies - a role beyond breast cancer. Nat. Rev. Clin. Oncol. 2020;17:33–48. doi: 10.1038/s41571-019-0268-3. [DOI] [PubMed] [Google Scholar]
- 3.Fischer-Colbrie J., Witt A., Heinzl H. EGFR and steroid receptors in ovarian carcinoma: comparison with prognostic parameters and outcome of patients. Anticancer Res. 1997;17:613–619. [PubMed] [Google Scholar]
- 4.Magne N., Pivot X., Bensadoun R.J. The relationship of epidermal growth factor receptor levels to the prognosis of unresectable pharyngeal cancer patients treated by chemo-radiotherapy. Eur. J. Cancer. 2001;37:2169–2177. doi: 10.1016/s0959-8049(01)00280-5. [DOI] [PubMed] [Google Scholar]
- 5.Milella M., Nuzzo C., Bria E. EGFR molecular profiling in advanced NSCLC: a prospective phase II study in molecularly/clinically selected patients pretreated with chemotherapy. J. Thorac. Oncol. 2012;7:672–680. doi: 10.1097/JTO.0b013e31824a8bde. [DOI] [PubMed] [Google Scholar]
- 6.Doyle H.A., Koski R.A., Bonafe N. Epidermal growth factor receptor peptide vaccination induces cross-reactive immunity to human EGFR, HER2, and HER3. Cancer Immunol. Immunother. 2018;67:1559–1569. doi: 10.1007/s00262-018-2218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim T.M., Yang I.S., Seung B.J. Cross-species oncogenic signatures of breast cancer in canine mammary tumors. Nat. Commun. 2020;11:3616. doi: 10.1038/s41467-020-17458-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flint A.F., U'Ren L., Legare M.E. Overexpression of the erbB-2 proto-oncogene in canine osteosarcoma cell lines and tumors. Vet. Pathol. 2004;41:291–296. doi: 10.1354/vp.41-3-291. [DOI] [PubMed] [Google Scholar]
- 9.Higgins R.J., Dickinson P.J., LeCouteur R.A. Spontaneous canine gliomas: overexpression of EGFR, PDGFRalpha and IGFBP2 demonstrated by tissue microarray immunophenotyping. J. Neurooncol. 2010;98:49–55. doi: 10.1007/s11060-009-0072-5. [DOI] [PubMed] [Google Scholar]
- 10.Sabattini S., Mancini F.R., Marconato L. EGFR overexpression in canine primary lung cancer: pathogenetic implications and impact on survival. Vet. Comp. Oncol. 2014;12:237–248. doi: 10.1111/vco.12002. [DOI] [PubMed] [Google Scholar]
- 11.Schappa J.T., Frantz A.M., Gorden B.H. Hemangiosarcoma and its cancer stem cell subpopulation are effectively killed by a toxin targeted through epidermal growth factor and urokinase receptors. Int. J. Cancer. 2013;133:1936–1944. doi: 10.1002/ijc.28187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selvarajah G.T., Verheije M.H., Kik M. Expression of epidermal growth factor receptor in canine osteosarcoma: association with clinicopathological parameters and prognosis. Vet. J. 2012;193:412–419. doi: 10.1016/j.tvjl.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Shiomitsu K., Johnson C.L., Malarkey D.E. Expression of epidermal growth factor receptor and vascular endothelial growth factor in malignant canine epithelial nasal tumours. Vet Comp Oncol. 2009;7:106–114. doi: 10.1111/j.1476-5829.2009.00178.x. [DOI] [PubMed] [Google Scholar]
- 14.Mantovani F.B., Morrison J.A., Mutsaers A.J. Effects of epidermal growth factor receptor kinase inhibition on radiation response in canine osteosarcoma cells. BMC Vet. Res. 2016;12:82. doi: 10.1186/s12917-016-0707-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Untch M., Rezai M., Loibl S. Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010;28:2024–2031. doi: 10.1200/JCO.2009.23.8451. [DOI] [PubMed] [Google Scholar]
- 16.Gianni L., Eiermann W., Semiglazov V. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375:377–384. doi: 10.1016/S0140-6736(09)61964-4. [DOI] [PubMed] [Google Scholar]
- 17.Bristow R.G., Alexander B., Baumann M. Combining precision radiotherapy with molecular targeting and immunomodulatory agents: a guideline by the American Society for Radiation Oncology. Lancet Oncol. 2018;19:e240–e251. doi: 10.1016/S1470-2045(18)30096-2. [DOI] [PubMed] [Google Scholar]
- 18.Ricci A.D., Rizzo A., Rojas Llimpe F.L. Novel HER2-directed treatments in advanced gastric carcinoma: anotHER paradigm shift? Cancers (Basel) 2021;13:1664. doi: 10.3390/cancers13071664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klingemann H. Immunotherapy for dogs: running behind humans. Front. Immunol. 2018;9:133. doi: 10.3389/fimmu.2018.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subik K., Lee J.F., Baxter L. The expression patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67 and AR by immunohistochemical analysis in breast cancer cell lines. Breast Cancer (Auckl) 2010;4:35–41. [PMC free article] [PubMed] [Google Scholar]
- 21.Terragni R., A C.G., Sabattini S. EGFR, HER-2 and KRAS in canine gastric epithelial tumors: a potential human model? PLoS One. 2014;9:e85388. doi: 10.1371/journal.pone.0085388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Doorn E., Liu H., Huckriede A. Safety and tolerability evaluation of the use of montanide ISA51 as vaccine adjuvant: a systematic review. Hum. Vaccin. Immunother. 2016;12:159–169. doi: 10.1080/21645515.2015.1071455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith R.P., Elias S.P., Cavanaugh C.E. Seroprevalence of Borrelia burgdorferi, B. miyamotoi, and Powassan virus in residents bitten by ixodes ticks, Maine, USA. Emerg. Infect. Dis. 2019;25:804–807. doi: 10.3201/eid2504.180202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shihan M.H., Novo S.G., Le Marchand S.J. A simple method for quantitating confocal fluorescent images. Biochem. Biophys. Rep. 2021;25 doi: 10.1016/j.bbrep.2021.100916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meira D.D., Nobrega I., de Almeida V.H. Different antiproliferative effects of matuzumab and cetuximab in A431 cells are associated with persistent activity of the MAPK pathway. Eur. J. Cancer. 2009;45:1265–1273. doi: 10.1016/j.ejca.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Roskoski R. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol. Res. 2014;79:34–74. doi: 10.1016/j.phrs.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Li S., Kussie P., Ferguson K.M. Structural basis for EGF receptor inhibition by the therapeutic antibody IMC-11F8. Structure. 2008;16:216–227. doi: 10.1016/j.str.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Mason N.J., Gnanandarajah J.S., Engiles J.B. Immunotherapy with a HER2-targeting Listeria induces HER2-specific immunity and demonstrates potential therapeutic effects in a phase i trial in canine osteosarcoma. Clin Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016;22:4380–4390. doi: 10.1158/1078-0432.CCR-16-0088. [DOI] [PubMed] [Google Scholar]
- 29.Clemente C.G., Mihm M.C., Bufalino R. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77:1303–1310. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 30.Peske J.D., Woods A.B., Engelhard V.H. Control of CD8 T-cell infiltration into tumors by vasculature and microenvironment. Adv. Cancer Res. 2015;128:263–307. doi: 10.1016/bs.acr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phillips B., Powers B.E., Dernell W.S. Use of single-agent carboplatin as adjuvant or neoadjuvant therapy in conjunction with amputation for appendicular osteosarcoma in dogs. J. Am. Anim. Hosp. Assoc. 2009;45:33–38. doi: 10.5326/0450033. [DOI] [PubMed] [Google Scholar]
- 32.Bergman P.J., MacEwen E.G., Kurzman I.D. Amputation and carboplatin for treatment of dogs with osteosarcoma: 48 cases (1991 to 1993) J. Vet. Intern. Med. 1996;10:76–81. doi: 10.1111/j.1939-1676.1996.tb02031.x. [DOI] [PubMed] [Google Scholar]
- 33.Hernandez I., Bott S.W., Patel A.S. Pricing of monoclonal antibody therapies: higher if used for cancer? Am. J. Manag. Care. 2018;24:109–112. [PubMed] [Google Scholar]
- 34.Doyle H.A., Mamula M.J. Autoantigenesis: the evolution of protein modifications in autoimmune disease. Curr. Opin. Immunol. 2012;24:112–118. doi: 10.1016/j.coi.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doyle H.A., Yang M.L., Raycroft M.T. Autoantigens: novel forms and presentation to the immune system. Autoimmunity. 2014;47:220–233. doi: 10.3109/08916934.2013.850495. [DOI] [PubMed] [Google Scholar]
- 36.Shlomchik M.J., Craft J.E., Mamula M.J. From T to B and back again: positive feedback in systemic autoimmune disease. Nat. Rev. Immunol. 2001;1:147–153. doi: 10.1038/35100573. [DOI] [PubMed] [Google Scholar]
- 37.Liang B., Mamula M.J. Molecular mimicry and the role of B lymphocytes in the processing of autoantigens. Cell. Mol. Life Sci. 2000;57:561–568. doi: 10.1007/PL00000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mamula M.J. Epitope spreading: the role of self peptides and autoantigen processing by B lymphocytes. Immunol. Rev. 1998;164:231–239. doi: 10.1111/j.1600-065x.1998.tb01223.x. [DOI] [PubMed] [Google Scholar]
- 39.Brightman S.E., Naradikian M.S., Miller A.M. Harnessing neoantigen specific CD4 T cells for cancer immunotherapy. J. Leukoc. Biol. 2020;107:625–633. doi: 10.1002/JLB.5RI0220-603RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schoenberger S.P. Is it possible to develop cancer vaccines to neoantigens, what are the major challenges, and how can these be overcome? Targeting the right antigens in the right patients. Cold Spring Harb. Perspect. Biol. 2018;10 doi: 10.1101/cshperspect.a028837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaumaya P.T.P., Guo L., Overholser J. Immunogenicity and antitumor efficacy of a novel human PD-1 B-cell vaccine (PD1-Vaxx) and combination immunotherapy with dual trastuzumab/pertuzumab-like HER-2 B-cell epitope vaccines (B-Vaxx) in a syngeneic mouse model. Oncoimmunology. 2020;9 doi: 10.1080/2162402X.2020.1818437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bekaii-Saab T., Wesolowski R., Ahn D.H. phase i immunotherapy trial with two chimeric HER-2 B-cell peptide vaccines emulsified in montanide ISA 720VG and Nor-MDP adjuvant in patients with advanced solid tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019;25:3495–3507. doi: 10.1158/1078-0432.CCR-18-3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singer J., Weichselbaumer M., Stockner T. Comparative oncology: erbB-1 and ErbB-2 homologues in canine cancer are susceptible to cetuximab and trastuzumab targeting. Mol. Immunol. 2012;50:200–209. doi: 10.1016/j.molimm.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tabernero J. The role of VEGF and EGFR inhibition: implications for combining anti-VEGF and anti-EGFR agents. Mol. Cancer Res. MCR. 2007;5:203–220. doi: 10.1158/1541-7786.MCR-06-0404. [DOI] [PubMed] [Google Scholar]
- 45.Angles J.M., Kennedy L.J., Pedersen N.C. Frequency and distribution of alleles of canine MHC-II DLA-DQB1, DLA-DQA1 and DLA-DRB1 in 25 representative American Kennel Club breeds. Tissue Antigens. 2005;66:173–184. doi: 10.1111/j.1399-0039.2005.00461.x. [DOI] [PubMed] [Google Scholar]
- 46.Kennedy L.J., Barnes A., Happ G.M. Extensive interbreed, but minimal intrabreed, variation of DLA class II alleles and haplotypes in dogs. Tissue Antigens. 2002;59:194–204. doi: 10.1034/j.1399-0039.2002.590303.x. [DOI] [PubMed] [Google Scholar]
- 47.Khanna C., Fan T.M., Gorlick R. Toward a drug development path that targets metastatic progression in osteosarcoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2014;20:4200–4209. doi: 10.1158/1078-0432.CCR-13-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fan T.M., Roberts R.D., Lizardo M.M. Understanding and modeling metastasis biology to improve therapeutic strategies for combating osteosarcoma progression. Front. Oncol. 2020;10:13. doi: 10.3389/fonc.2020.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.