Abstract
Background
Subcutaneous immunotherapy (SCIT) has been proven as an effective therapy against some allergens for seasonal allergic rhinitis (SAR) patients unresponsive to intranasal corticosteroids and/or antihistamines but carries risk of systemic allergic reactions. Dupilumab blocks the shared receptor component for interleukin-4 and interleukin-13, key and central drivers of type 2 inflammation in multiple diseases.
Objective
To evaluate the efficacy and safety of SCIT+dupilumab vs SCIT alone.
Methods
This phase 2a, multicenter, double-blind, placebo-controlled parallel-group study conducted in 103 adults with grass pollen-induced SAR (NCT03558997) randomized patients 1:1:1:1 to SCIT, dupilumab (300 mg every 2 weeks), SCIT+dupilumab, or placebo. SCIT was administered using an 8-week cluster protocol followed by 8 weeks of maintenance injections. Primary endpoint was change from pre-treatment baseline in area under the curve (AUC) in total nasal symptom score (TNSS) 0–1 h following nasal allergen challenge (NAC) with timothy grass extract at Week 17.
Results
Although 16 weeks of treatment with SCIT+dupilumab did not significantly improve TNSS AUC (0–1 h) following NAC at Week 17 vs SCIT (least squares mean −56.76% vs −52.03%), a higher proportion of SCIT+dupilumab-treated patients (61.5%) achieved SCIT maintenance dose vs SCIT (46.2%). A lower proportion of SCIT+dupilumab-treated patients (7.7%) required epinephrine rescue treatment vs SCIT (19.2%). There were significantly fewer withdrawals in the SCIT+dupilumab group than in the SCIT group (n = 2 [7.7%] vs n = 8 [30.8%]; P = 0.0216); the majority of SCIT group withdrawals were due to SCIT-related intolerability, compared with no discontinuations from the SCIT+dupilumab group.
Conclusion
In SAR patients, 16 weeks of SCIT+dupilumab may improve SCIT tolerability but did not incrementally reduce post-allergen challenge nasal symptoms compared with SCIT alone.
Clinical Study Number
Keywords: dupilumab, seasonal allergic rhinitis, subcutaneous immunotherapy, nasal allergen responses
Introduction
Allergic rhinitis (AR) is associated with a significant daily disease burden and impairment of quality of life.1 While oral and intranasal antihistamines and intranasal corticosteroids may be helpful in many patients, a significant number of patients do not adequately respond to these medications1 and require allergen immunotherapy.2 Subcutaneous immunotherapy (SCIT) is effective in several allergic disorders including AR and venom allergies.3,4 Allergen immunotherapy is also well tolerated and effective in patients with AR and mild intermittent asthma, and can reduce short-term symptoms and medication use.5 SCIT has been shown to modify the underlying disease pathogenesis and may provide long-term sustained benefit following therapy cessation after years of prolonged treatment.6,7 However, SCIT is associated with a risk of systemic allergic reactions, which is highest in patients with asthma, thus warranting administration in specialized settings with access to epinephrine and other resuscitative methods.2,8,9 Dosing regimens with accelerated cluster build-up strategies allow patients to reach their maintenance dose in a shorter amount of time and with fewer injections than conventional dosing,10 but have also been associated with more systemic reactions.11
The type 2 cytokines interleukin (IL)-4 and IL-13 play key roles in the pathophysiology of several allergic diseases, including AR.12 IL-4 is critical for the generation of CD4+ T helper type 2 cells,13 which play a key role in allergen-specific T cell responses.14 Furthermore, both IL-4 and IL-13 are critical for B cell immunoglobulin (Ig) M to IgE class switching.15 Dupilumab, a fully human monoclonal antibody that binds specifically to IL-4 receptor α (IL-4Rα), the shared receptor subunit for IL-4 and IL-13, inhibits the signaling pathways of both IL-4 and IL-13.12,16 Dupilumab is approved for patients with atopic dermatitis,17–21 asthma,22 or chronic rhinosinusitis with nasal polyps.23 Dupilumab also significantly reduced total and allergen-specific serum IgE levels during 1 year of treatment in patients with asthma and perennial AR.23–25
We hypothesized that the addition of dupilumab to SCIT would enhance the efficacy and improve the tolerability of SCIT. The current proof-of-concept trial examined the efficacy and safety of combination therapy with timothy grass (TG)–specific SCIT plus dupilumab in patients with TG-induced seasonal AR using a nasal allergen challenge (NAC) model.
Methods
Study Design
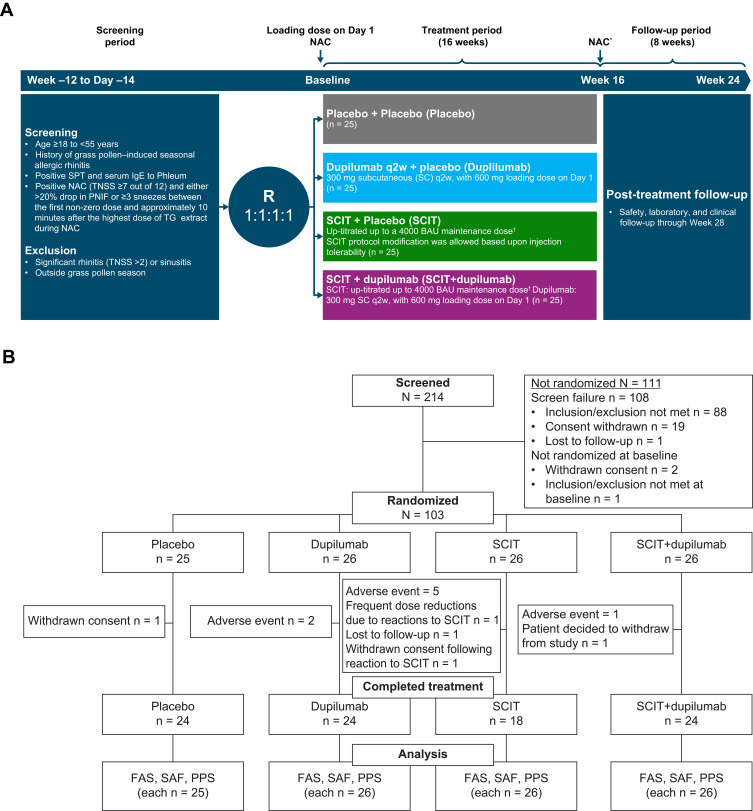
This was a phase 2a, multicenter, randomized, double-blind, placebo-controlled, parallel-group, 4-arm study of dupilumab as an adjunct to TG SCIT (NCT03558997, Figure 1). The study consisted of a 12-week screening period, a 16-week treatment period, and an 8-week post-treatment follow-up period in patients with a history of grass pollen–induced AR (Figure 1A). The study was conducted outside of the grass allergy season to prevent interference from environmental allergens with the provoked allergy symptoms by the NAC. It was conducted at 17 study centers that specialized in allergy in North America (United States and Canada) between June 7, 2018, and June 13, 2019. The complete list of participating investigators can be found in this article’s Supplementary Materials.
Figure 1.
Study information. (A) CONSORT diagram of patient disposition and (B) study design. *NAC occurred at Week 17, after 16 weeks of treatment. †SCIT dosing regimen is detailed in Supplementary Table 1 in this article’s Online Repository at https://www.dovepress.com/.
Abbreviations: BAU, bioequivalent allergy unit; FAS, full analysis set; NAC, nasal allergen challenge; PNIF, peak nasal inspiratory flow; PPS, per protocol set; q2w, every 2 weeks; R, randomization; SAF, safety analysis set; SC, subcutaneous; SCIT, subcutaneous immunotherapy; SPT, skin prick test; TG, timothy grass; TNSS, total nasal symptom score.
The main inclusion criteria included age ≥18 to <55 years at the time of study entry; a history of grass pollen–induced seasonal AR; positive skin prick test (SPT) with TG extract (mean wheal diameter at least ≥5 mm greater than negative control); positive serum TG-specific IgE (sIgE; ≥0.35 kU/L); a positive NAC with TG extract at screening with peak total nasal symptom score (TNSS; scale 0–12) ≥7 of 12; and either a >20% drop in peak nasal inspiratory flow (PNIF) or ≥3 sneezes between the first non-zero dose and approximately 10 minutes after the highest TG extract dose during NAC. TNSS is a composite symptom assessment of nasal congestion, nasal itching, rhinorrhea, and sneezing (each graded on a 0–3 scale; 0 being none, 3 being severe).
The main exclusion criteria included significant rhinitis (causing TNSS >2) or sinusitis outside of grass pollen season or due to daily contact with other allergens expected to coincide with final NAC assessments; symptoms of upper respiratory tract infection, acute sinusitis, acute otitis media, or other relevant infections at screening; or any contraindications to SCIT. Patients with a clinical history of asthma requiring chronic medication for >4 weeks per year or a history of asthma with two or more asthma exacerbations requiring hospitalizations or systemic corticosteroids in the previous year were also excluded. Use of leukotriene inhibitors, mast cell inhibitors, systemic, intranasal or inhaled corticosteroids, oral or topical decongestants, systemic or topical calcineurin inhibitors, beta blockers, long-acting beta agonists, and long-acting muscarinic antagonists were prohibited for the duration of the study. Approved standard care medications were allowed (see this article’s Supplementary Materials for details on the use of additional medication).
Randomization (1:1:1:1) was done by an interactive system. To maintain patient blinding, dupilumab-matched placebo and SCIT-matched placebo were provided (Figure 1A). With the exception of the designated study pharmacist(s)/designee at the study site who prepared and administered the SCIT, the study remained blinded to all individuals until the pre-specified unblinding. The designated study pharmacist(s)/designee at the study site who prepared and administered the SCIT was not allowed to perform the NAC or nasal and skin assessments.
This study was conducted in accordance with the protocol, the consensus international ethical principle guidelines, including the 2013 Declaration of Helsinki and Council for International Organizations of Medical Sciences International Ethical Guidelines and applicable International Council for Harmonization Good Clinical Practice Guidelines. All study documents were approved by the WIRB-Copernicus IRB before the study was initiated. All participants signed an informed consent form prior to undertaking any study procedure.
Treatment
This randomized, double-blind, placebo-controlled trial consisted of four treatment arms: SCIT+placebo dupilumab (SCIT), dupilumab+SCIT placebo (dupilumab), SCIT+dupilumab, or placebo for both SCIT and dupilumab (placebo) (Figure 1A).
Dupilumab 300 mg or placebo was administered subcutaneously (SC) every 2 weeks for a total of 16 weeks, after a 600 mg dupilumab loading dose or placebo on Day 1. TG-specific SCIT or placebo was administered using a rapidly escalating cluster protocol over 8 weeks, followed by a maintenance regimen for the following 8 weeks. The recommended SCIT target maintenance dose was 4000 bioequivalent allergy units (BAU), equivalent to approximately 20 µg Phleum pratense 5 (major TG allergen). The SCIT treatment protocol consisted of escalating doses of 1 to 3 SC allergen injections per weekly study visit for the first 8 weeks and then maintenance SC injections for the next 8 weeks (see Supplementary Table 1).
Modification of the SCIT protocol, with no dose escalation or dose reduction, was allowed based on injection tolerability. For all SCIT visits, patients were pre-medicated with an H1 antihistamine (loratadine 10 mg orally, supplied to study sites as a non-investigational medicinal product) 1 to 6 hours prior to each injection visit, as recommended by clinical guidelines to reduce local and systemic reactions during cluster SCIT.26 Patients were observed for ≥30 minutes following all SCIT injections. If patients developed adverse events (AEs) during the SCIT dose escalation phase, including large local reactions or grade 1–2 systemic reactions requiring dose adjustment, the investigator was permitted to reduce the planned maintenance dose of SCIT (4000 BAU) to a dose between 400 BAU and 4000 BAU in consultation with the sponsor’s medical monitor.
The severity of SCIT-related AEs (allergic reactions and anaphylaxis) was graded according to the Common Terminology Criteria for Adverse Events (CTCAE) Version 4.3.27 Only SCIT-related reactions were graded according to the CTCAE. Patients with grade 3 allergic reactions to study drug per CTCAE criteria were discontinued from study drug and were withdrawn from the study. Withdrawn patients were moved to end of treatment visit immediately, within 2 weeks of stopping study drug. If one study drug was discontinued, all study drugs were discontinued.
Participants who experienced systemic allergic reactions were treated with rescue treatment, including but not limited to intramuscular or SC epinephrine administration, as needed and determined by trained study staff. Participants were also permitted to take oral antihistamines for AR symptoms during the study as needed; however, use of oral antihistamines were not permitted within 5 days prior to or during a visit for NAC or skin testing. If a participant used oral antihistamines within 5 days prior to or during a visit for NAC or skin testing, the visit was rescheduled.
Assessment Outcomes
The primary endpoint was the percent change from pretreatment baseline in the area under the curve (AUC) at Week 17 for TNSS (0–1 h post peak TNSS), following NAC with TG extract. The change in TNSS assessment was processed to produce the AUC using the trapezoid rule (see Algorithm for calculation of AUC in this article’s Supplementary Materials).
Secondary endpoints included absolute change and percent change from pre-treatment baseline in TNSS AUC (0–1 h) following NAC with TG extract at Week 17; percent change and absolute change from baseline through Week 17 in serum TG-specific IgG4 (sIgG4) and sIgE; change from baseline through Week 17 in log-transformed value of serum sIgG4 to sIgE ratio (log [sIgG4/sIgE]); and incidence rates of treatment-emergent adverse events (TEAEs) and serious TEAEs through end of study.
All endpoints, including primary, secondary, and exploratory endpoints, and post hoc analyses are summarized in Table 1. Details on the study procedures for these endpoints are provided in this article’s Supplementary Materials.
Table 1.
Primary, Secondary, and Exploratory Endpoints and Post Hoc Analyses
| Type | Endpoint | Reported Comparison Between Groups | ||
|---|---|---|---|---|
| SCIT+dupilumab vs SCIT | Dupilumab vs Placebo | SCIT+dupilumab vs Dupilumab | ||
| Primary | % Change from pretreatment baseline in TNSS AUC (0–1 h post peak TNSS)*,†,‡,§ | X | ||
| Secondary | Absolute change from pretreatment baseline in TNSS AUC (0–1 h post peak TNSS)*,†,‡,§ | X | ||
| % Change and absolute change from pretreatment baseline in TNSS AUC (0–1 h post peak TNSS)*,†,‡,§ | X | X | ||
| % Change and absolute change from pretreatment baseline sIgG4 and sIgE† | X | |||
| Change from pretreatment baseline in log-transformed value of serum sIgG4 to sIgE ratio (log [sIgG4/sIgE])† | X | |||
| Incidence rates of TEAEs and serious TEAEs through end of study | ||||
| Exploratory | % Change and absolute change from baseline in peak TNSS‡ | X | X | |
| % Change from baseline in TOSS AUC (0–1 h post peak TNSS)§ | X | X | ||
| % Change from pretreatment baseline in PNIF AUC (0–1 h)*,§ | X | X | ||
| % Change from baseline in SPT with serial allergen titration, as measured by AUC of average wheal sizes (diameter) over allergen concentrations after the challenge (early-phase reaction)*,§ | X | X | ||
| % Change from baseline at Week 17 in wheal size diameter induced by skin TG intradermal injection 6 h after challenge (LPR) | X | X | ||
| % Change and absolute change from pretreatment baseline in sIgG† | X | |||
| Change from pretreatment baseline in (log [sIgG/sIgE])† | X | |||
| Post hoc | Analysis of completers and non-completers (patients randomized to SCIT+dupilumab and dupilumab) at Week 17 was also performed for absolute change in sIgE, sIgG4, sIgG, log (sIgG4/sIgE), and log (sIgG/sIgE) | |||
Notes: *Following NAC with timothy grass extract. †Change from pretreatment baseline at Week 17. ‡TNSS is defined as the highest TNSS attained during 0–1 h following NAC. §AUC is calculated using the trapezoid rule (see Algorithm for calculation of AUC in this article’s Online Repository at https://www.dovepress.com/).
Abbreviations: AUC, area under the curve; BL, baseline; LPR, late-phase reaction; NAC, nasal allergen challenge; PNIF, peak nasal inspiratory flow; SCIT, subcutaneous immunotherapy; sIgE, timothy grass–specific immunoglobulin E; sIgG, timothy grass–specific immunoglobulin G; sIgG4, serum timothy grass–specific IgG4; SPT, skin prick test; TEAE, treatment-emergent adverse event; TG, timothy grass; TNSS, total nasal symptom score; TOSS, total ocular symptom score.
Statistical Analysis
With an estimated 25 patients per group with a 2-sided 5% significance level, there was an estimated 80% power to detect a 29% difference between SCIT+dupilumab and SCIT for the primary endpoint. The estimate assumed the mean percent changes from baseline for SCIT+dupilumab vs placebo and SCIT vs placebo were −55% and −26%, with a common standard deviation of 35%. These assumptions were based on the GRASS trial, which reported a 34% difference between SCIT and placebo in TNSS AUC (0–1 h) at 1 year.28 A similar magnitude of improvement by adding dupilumab to SCIT was assumed for the sample size calculation.
The primary endpoint was analyzed with an analysis of covariance (ANCOVA) model as the primary analysis method. The main factor included the treatment group, with baseline value as the covariate. Multiple imputation (MI) was used for missing values as the primary analysis, with last observation carried forward (LOCF) and observed values (OC) for sensitivity analyses. The change and percent change from baseline in continuous secondary and exploratory efficacy endpoints were analyzed using the same approach as the primary endpoint, unless otherwise specified. Biomarker-related continuous endpoints were analyzed using a rank-based ANCOVA model with treatment and relevant baseline values as covariates. The proportion of patients who achieved SCIT maintenance dose (4000 BAU) during 16 weeks of treatment was compared by χ2 test between SCIT+dupilumab and dupilumab and SCIT groups. TEAEs were summarized descriptively.
All analyses were carried out using Statistical Analysis Software Version 9.4 (SAS Institute, Cary, NC, USA).
Results
Patients
Of the 214 patients screened, 103 patients were randomized and 90 patients (87.4%) completed the trial. All randomized patients were included in the efficacy and safety analyses (Figure 1B). Baseline demographics and disease characteristics were similar among groups (Table 2). The mean age ranged from 33.0 to 40.3 years across groups, and approximately half of all patients were male (38.5–61.5% across groups). Around a third of patients had a history of asthma, with 9 (36.0%), 7 (26.9%), 9 (34.6%), and 10 (38.5%) patients with a history of asthma in the placebo, dupilumab, SCIT, and SCIT+dupilumab groups, respectively.
Table 2.
Baseline Patient Demographics and Characteristics
| Placebo (n = 25) | Dupilumab (n = 26) | SCIT (n = 26) | SCIT+dupilumab (n = 26) | |
|---|---|---|---|---|
| Age, mean (SD), years | 34.6 (11.1) | 40.3 (11.2) | 37.8 (11.3) | 33.0 (10.6) |
| ≥18 to ≤40 years, n (%) | 16 (64.0) | 12 (46.2) | 14 (53.8) | 18 (69.2) |
| ≥40 to ≤55 years, n (%) | 9 (36.0) | 14 (53.8) | 12 (46.2) | 8 (30.8) |
| Sex, male, n (%) | 15 (60.0) | 10 (38.5) | 12 (46.2) | 16 (61.5) |
| BMI, mean (SD) | 29.3 (10.25) | 28.0 (6.64) | 26.5 (5.26) | 26.6 (5.60) |
| TNSS AUC, mean (SD) | 5.34 (2.18) | 5.03 (2.19) | 4.66 (1.99) | 4.69 (1.72) |
| TNSS peak score, mean (SD) | 9.20 (1.56) | 8.50 (1.36) | 8.30 (1.19) | 8.70 (1.29) |
| TOSS AUC, mean (SD) | 2.75 (2.53) | 2.63 (2.88) | 2.80 (2.78) | 1.59 (1.92) |
| SPT AUC, mean (SD) | 13.74 (5.12) | 10.76 (4.73) | 12.87 (4.25) | 14.32 (5.95) |
| LPR wheal diameter, mean (SD) | 52.54 (23.80) | 48.79 (16.20) | 60.77 (34.10) | 46.40 (25.68) |
| PNIF AUC (0–1 h), mean (SD) | 80.89 (52.47) | 85.40 (46.76) | 77.86 (31.59) | 80.58 (36.46) |
| PNIF AUC (1–6 h), mean (SD) | 108.28 (47.09) | 114.07 (48.36) | 111.59 (36.03) | 113.38 (40.68) |
| Sneeze count AUC, mean (SD) | 0.68 (0.80) | 0.91 (1.11) | 0.45 (0.72) | 0.96 (1.17) |
| sIgE, mean (SD), kU/L | 27.212 (45.891) | 16.201 (35.427) | 18.776 (28.168) | 44.475 (126.639) |
| sIgG4, mean (SD), mg/L | 0.186 (0.170) | 0.213 (0.282) | 0.172 (0.216) | 0.221 (0.284) |
| sIgG, mean (SD), mg/L | 2.700 (1.503) | 2.804 (1.831) | 2.623 (1.421) | 3.858 (5.276) |
| log (sIgG4/sIgE), mean (SD) | −1.877 (0.694) | −1.596 (0.569) | −1.859 (0.550) | −1.871 (0.713) |
| log (sIgG/sIgE), mean (SD) | −0.659 (0.674) | −0.357 (0.642) | −0.603 (0.612) | −0.610 (0.620) |
| Total IgE, mean (SD), IU/mL | 159.756 (173.6115) | 159.769 (141.2617) | 222.508 (235.1932) | 364.258 (643.9095) |
| TARC, mean (SD), pg/mL | 280.8 (127.37) | 363.8 (219.45) | 350.8 (185.19) | 335.8 (205.86) |
| Patients with history of asthma, n (%) | 9 (36.0) | 7 (26.9) | 9 (34.6) | 10 (38.5) |
| Ongoing asthma, n/N1 (%) | 6/9 (66.7) | 4/7 (57.1) | 5/9 (55.6) | 6/10 (60.0) |
| Asthma patient with peak flow >80% predicted, n/N1 (%) | 9/9 (100) | 7/7 (100) | 8/9 (88.9) | 9/10 (90.0) |
Abbreviations: AUC, area under the curve; BMI, body mass index; IgE, immunoglobulin E; LPR, late-phase reaction; N1, number of patients with a history of asthma; PNIF, peak nasal inspiratory flow; SCIT, subcutaneous immunotherapy; SD, standard deviation; sIgE, timothy grass–specific immunoglobulin E; sIgG, timothy grass–specific immunoglobulin G; sIgG4, serum timothy grass–specific IgG4; SPT, skin prick test; TARC, thymus and activation-regulated chemokine; TNSS, total nasal symptom score; TOSS, total ocular symptom score.
Efficacy
Total Nasal Symptom Score
Sixteen weeks of SCIT+dupilumab treatment did not significantly reduce the TNSS AUC (0–1 h) in response to NAC at Week 17 compared with SCIT, as shown by percent change in TNSS AUC (0–1 h) (primary endpoint, Table 3). The primary analysis performed with MI was supported by sensitivity analyses using LOCF and OC (Table 3).
Table 3.
Efficacy: Change in TNSS at Week 17
| Primary Endpoint | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| % Change in TNSS AUC (0–1 h) for SCIT+dupilumab vs SCIT (MI, primary analysis) LS mean difference (95% CI) | 4.73 (−21.023, 30.487) | ||||||||
| P value | 0.7185 | ||||||||
| Placebo (n =25) | Dupilumab (n = 26) | SCIT (n = 26) | SCIT+dupilumab (n = 26) | SCIT+dupilumab vs SCIT | Dupilumab vs Placebo | SCIT+dupilumab vs Dupilumab | SCIT vs Placebo | SCIT+dupilumab vs Placebo | |
| LS Mean (SE) | LS Mean Difference (95% CI) | ||||||||
| Secondary Endpoints | Pre-Specified Analyses | Post Hoc Analyses | |||||||
| % Change in TNSS AUC (0–1 h) (MI, primary analysis) | −28.82 (8.427) | −21.57 (8.415) | −56.76 (9.687) | −52.03 (8.462) |
7.25 (−16.028, 30.530) |
−30.45 (−53.874, −7.034) |
−27.94 (−52.98, −2.886) |
−23.20 (−46.691, 0.284) |
|
| P value | 0.5416 | 0.0108 | 0.0289 | 0.0528 | |||||
| % Change in TNSS AUC (0–1 h) (LOCF, sensitivity analysis) | −27.6 (8.74) | −18.4 (8.53) | −43.9 (8.55) | −52.3 (8.54) |
−8.3 (−32.26, 15.61) |
9.2 (−14.97, 33.45) |
−33.9 (−57.84, −9.88) |
−16.3 (−40.64, 8.05) |
−24.6 (−48.96, −0.29) |
| P value | 0.4916 | 0.4508 | 0.0061 | 0.1871 | 0.0474 | ||||
| % Change in TNSS AUC (0–1 h) (OC, sensitivity analysis) | (n = 24) −28.8 (8.60) |
(n = 24) −20.3 (8.57) |
(n = 18) −59.3 (9.90) |
(n = 24) −53.2 (8.58) |
6.1 (−19.88, 32.15) |
8.5 (−15.63, 32.60) |
−32.9 (−56.98, −8.75) |
−30.5 (−56.64, −4.39) |
−24.4 (−48.59, −0.17) |
| P value | 0.6404 | 0.4862 | 0.0081 | 0.0226 | 0.0484 | ||||
| Absolute change in TNSS AUC (0–1 h) (MI) | −1.85 (0.323) | −1.83 (0.329) | −3.07 (0.361) | −2.77 (0.321) | 0.30 (−0.661, 1.254) |
0.03 (−0.877, 0.928) |
−0.94 (−1.848, −0.037) |
−1.21 (−2.160, −0.266) |
−0.92 (−1.812, −0.022) |
| P value | 0.5438 | 0.9559 | 0.0414 | 0.0121 | 0.0446 | ||||
| Absolute change in TNSS AUC (0–1 h) (LOCF) | −1.74 (0.384) | −1.63 (0.375) | −2.27 (0.376) | −2.62 (0.375) | −0.34 (−1.393, 0.710) |
0.11 (−0.950, 1.177) |
−0.99 (−2.042, 0.065) |
−0.53 (−1.602, 0.537) |
−0.87 (−1.943, 0.195) |
| P value | 0.5203 | 0.8324 | 0.0656 | 0.3255 | 0.1077 | ||||
| Absolute change in TNSS AUC (0–1 h) (OC) | (n = 24) −1.83 (0.331) |
(n = 24) −1.80 (0.330) |
(n = 18) −3.08 (0.381) |
(n = 24) −2.75 (0.330) | 0.33 (−0.670, 1.334) |
0.03 (−0.896, 0.961) |
−0.95 (−1.879, −0.022) |
−1.25 (−2.256, −0.244) |
−0.92 (−1.851, −0.014) |
| P value | 0.5120 | 0.9448 | 0.0449 | 0.0155 | 0.0535 | ||||
| Exploratory Endpoints | Pre-Specified Analyses | Post Hoc Analyses | |||||||
| % Change in peak TNSS (MI) | −16.14 (6.773) | −29.91 (6.765) | −34.29 (7.760) | −39.62 (6.646) | −5.33 (−25.771, 15.113) |
−13.76 (−32.649, 5.120) |
−9.71 (−28.379, 8.960) |
−18.14 (−38.513, 2.223) |
−23.47 (−42.060, −4.887) |
| P value | 0.6090 | 0.1531 | 0.3080 | 0.0808 | 0.0133 | ||||
| % Change in peak TNSS (LOCF) | −16.4 (7.01) | −26.3 (6.74) | −29.4 (6.80) | −40.7 (6.72) | −11.2 (−30.22, 7.76) |
−9.9 (−29.31, 9.53) |
−14.4 (−33.25, 4.54) |
−13.0 (−32.70, 6.65) |
−24.3 (−43.51, −5.00) |
| P value | 0.2434 | 0.3145 | 0.1348 | 0.1921 | 0.0141 | ||||
| % Change in peak TNSS (OC) | (n = 24) −16.5 (6.89) |
(n = 24) −29.7 (6.81) |
(n = 18) −35.0 (7.87) |
(n = 24) −40.4 (6.77) |
−5.4 (−26.06, 15.25) |
−13.2 (−32.63, 6.23) |
−10.7 (−29.77, 8.43) |
−18.5 (−39.47, 2.55) |
−23.9 (−43.06, −4.68) |
| P value | 0.6042 | 0.1804 | 0.2698 | 0.0842 | 0.0154 | ||||
| Absolute change in peak TNSS (MI) | −1.6 (0.58) | −3.0 (0.58) | −3.1 (0.65) | −3.4 (0.57) | −0.4 (−2.08, 1.36) |
−1.4 (−3.01, 0.23) |
−0.5 (−2.07, 1.12) |
−1.5 (−3.23, 0.22) |
−1.9 (−3.45, −0.28) |
| P value | 0.6805 | 0.0929 | 0.5603 | 0.0871 | 0.0212 | ||||
| Absolute change in peak TNSS (LOCF) | −1.6 (0.60) | −2.6 (0.58) | −2.6 (0.58) | −3.4 (0.58) | −0.8 (−2.48, 0.78) |
−1.1 (−2.72, 0.61) |
−0.8 (−2.45, 0.80) |
−1.0 (−2.72, 0.65) |
−1.9 (−3.53, −0.23) |
| P value | 0.3060 | 0.2117 | 0.3153 | 0.2266 | 0.0262 | ||||
| Absolute change in peak TNSS (OC) | (n = 24) −1.6 (0.59) |
(n = 24) −3.0 (0.58) |
(n = 18) −3.1 (0.67) |
(n = 24) −3.5 (0.58) |
−0.3 (−2.11, 1.43) |
−1.4 (−3.03, 0.30) |
−0.5 (−2.13, 1.14) |
−1.5 (−3.32, 0.28) |
−1.9 (−3.51, −0.22) |
| P value | 0.7001 | 0.1061 | 0.5484 | 0.0970 | 0.0268 | ||||
Abbreviations: AUC, area under the curve; CI, confidence interval; LOCF, last observation carried forward; LS, least squares; MI, multiple imputation; OC, observed values; SCIT, subcutaneous immunotherapy; SE, standard error; TNSS, total nasal symptom score.
Similar to the primary endpoint, 16 weeks of treatment with SCIT+dupilumab did not significantly reduce other TNSS endpoints such as absolute change in TNSS AUC (0–1 h), percent change in peak TNSS, and absolute change in peak TNSS compared with SCIT (Table 3). Given the small size of the study and the high degree of withdrawals from the SCIT arm (Figure 2), post hoc analyses were conducted to more fully understand the potential treatment effect of SCIT+dupilumab vs SCIT, which directly compared the treatment effects of the SCIT+dupilumab and SCIT groups to the placebo group. Due to the higher number of withdrawals from the study in the SCIT group compared with other treatment groups, the LOCF method is the most informative to account for missing data. In these post hoc analyses, 16 weeks of treatment with SCIT+dupilumab vs placebo showed significant improvement in peak TNSS endpoints compared with SCIT vs placebo (Table 3). Furthermore, 16 weeks of SCIT+dupilumab treatment showed a greater reduction from baseline in nasal symptoms after NAC vs placebo (least squares [LS] mean percent change in TNSS AUC [0–1 h]: −24.6%; 95% confidence interval [CI]: −48.96, −0.29), whereas there was no significant difference for SCIT vs placebo (LS mean percent change in TNSS AUC [0–1 h]: −16.3%; 95% CI: −40.64, 8.05). In addition, 16 weeks of SCIT+dupilumab treatment resulted in a reduction in peak TNSS compared with placebo (an improvement, −1.9; 95% CI: −3.53, −0.23; P = 0.0262). Similar improvements in peak TNSS were not seen when comparing SCIT with placebo (−1.0; 95% CI: −2.72, 0.65; P = 0.2266), suggesting the addition of dupilumab to SCIT, but not SCIT alone, may provide a greater reduction in peak TNSS post NAC (Table 3).
Figure 3.
Continue.
Figure 3.
Continue.
Figure 3.
Continue.
Figure 3.
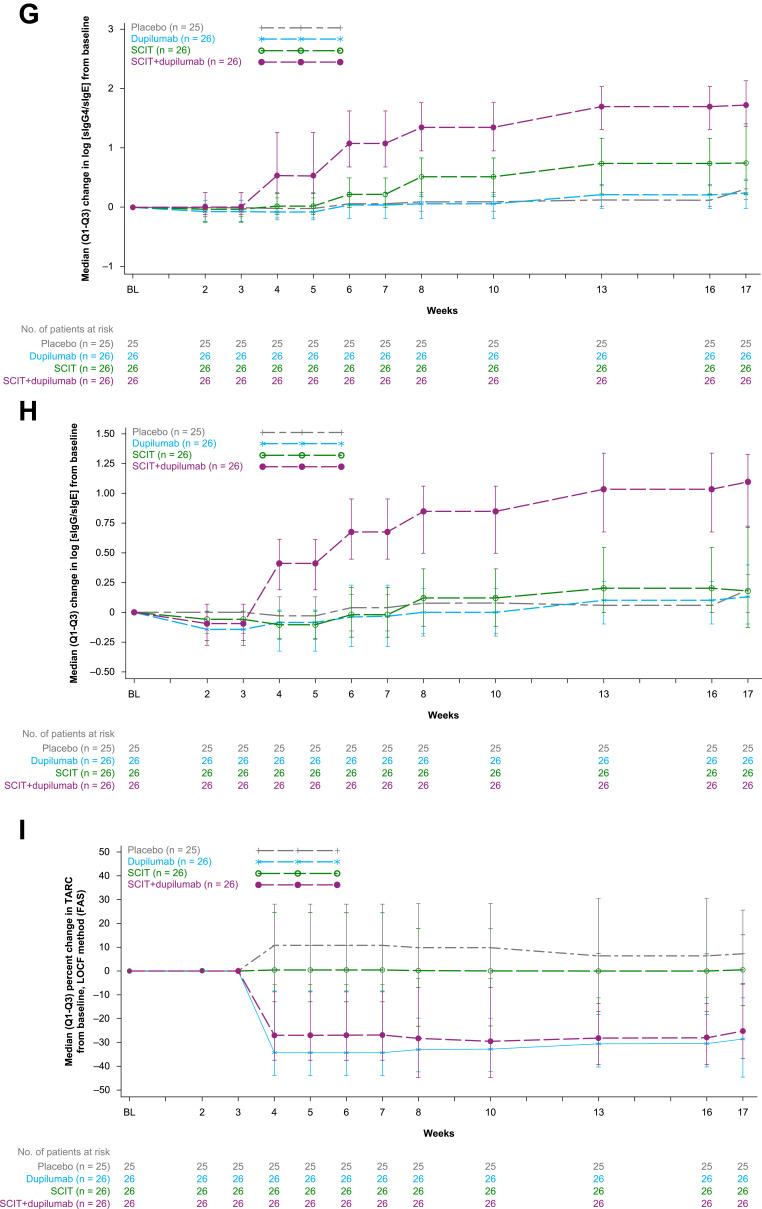
Exploratory endpoints and changes in biomarkers. (A) LS mean percent change from baseline in PNIF AUC (0–1 h) after challenge at Week 17, LOCF method (FAS). (B) Titration SPT, early-phase reaction LS mean (SE) of percent change from pre-treatment baseline in average wheal sizes (diameter) AUC over allergen concentrations in SPT after the challenge (early-phase reaction) at Week 17, LOCF method (FAS). (C) Intradermal skin challenge test, LPR LS mean (SE) of percent change from pre-treatment baseline in wheal sizes (diameter) induced by skin TG intradermal injection 6 hours after the challenge (LPR) at Week 17, LOCF method (FAS). (D) Median (Q3-Q1) percent change in sIgE from baseline, LOCF method (FAS). (E) Median (Q3-Q1) percent change in sIgG4 from baseline, LOCF method (FAS). (F) Median (Q3-Q1) percent change in sIgG from baseline, LOCF method (FAS). (G) Median (Q3-Q1) change in log-transformed sIgG4/sIgE ratio from baseline, LOCF method (FAS). (H) Median (Q3-Q1) change in log-transformed sIgG/sIgE ratio from baseline, LOCF method (FAS). (I) Median (Q3-Q1) percent change in TARC from baseline, LOCF method (FAS).
Abbreviations: AUC, area under the curve; BL, baseline; FAS, full analysis set; LOCF, last observation carried forward; LPR, late-phase reaction; LS, least squares; PNIF, peak nasal inspiratory flow; Q1, first quartile; Q3, third quartile; SCIT, subcutaneous immunotherapy; SE, standard error; sIgE, timothy grass–specific immunoglobulin E; sIgG, timothy grass–specific immunoglobulin G; SPT, skin prick test; TARC, thymus and activation-regulated chemokine; TG, timothy grass.
Figure 2.
Kaplan–Meier estimate of time to withdrawal from study treatment. Numbers below each week represent the number of patients remaining in the treatment period (16 weeks ± 1-week treatment window). Patient numbers drop after Week 15 through Week 17 due to the ±1-week treatment window at 16 weeks of study treatment. At Week 18, all subjects progressed to the follow-up period; therefore, none remained in the treatment period.
Abbreviations: BL, baseline; SCIT, subcutaneous immunotherapy.
Sixteen weeks of treatment with dupilumab alone did not lead to a significant reduction in TNSS AUC (0–1 h) in response to NAC with TG at Week 17 compared with placebo, as shown by percent change in TNSS AUC (0–1 h) (secondary endpoint, Table 3).
Peak Nasal Inspiratory Flow, Total Ocular Symptom Score, Early-Phase Reaction, and Late-Phase Reaction
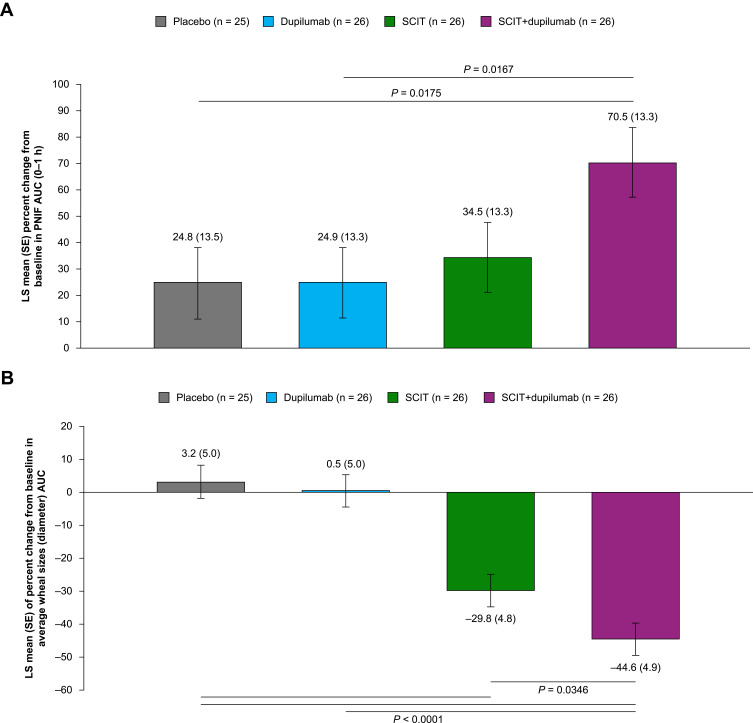
SCIT+dupilumab showed a trend towards a larger increase (improvement) in mean percent change from baseline in PNIF AUC (0–1 h) compared with SCIT at Week 17 (Figure 3A), indicating better nasal patency. Similar mean percent change in total ocular symptom score (TOSS) AUC (0–1 hr post peak TNSS) following NAC were observed in the SCIT and SCIT+dupilumab treatment groups (Supplementary Figure 1). The percent change from baseline in average wheal size (diameter) AUC over allergen concentration in the SPT (early-phase reaction) at Week 17 is shown in Figure 3B, with a significant reduction in wheal size in the SCIT+dupilumab vs SCIT groups. No significant difference was noted between the SCIT+dupilumab and SCIT treatment groups for percent change from baseline in wheal size induced by intradermal injection (late-phase reaction) at Week 17 (Figure 3C).
Sixteen weeks of treatment with dupilumab did not differ from placebo in mean percent change from baseline in PNIF AUC (0–1 h) at Week 17, mean percent change in TOSS AUC (0–1 h post peak TNSS) at Week 17, percent change from baseline in average wheal size AUC over allergen concentration in the SPT (early-phase reaction) at Week 17, or in percent change from baseline in wheal size induced by intradermal injection (late-phase reaction) at Week 17 (Figure 3A–C and Supplementary Figure 1).
Biomarker Analysis
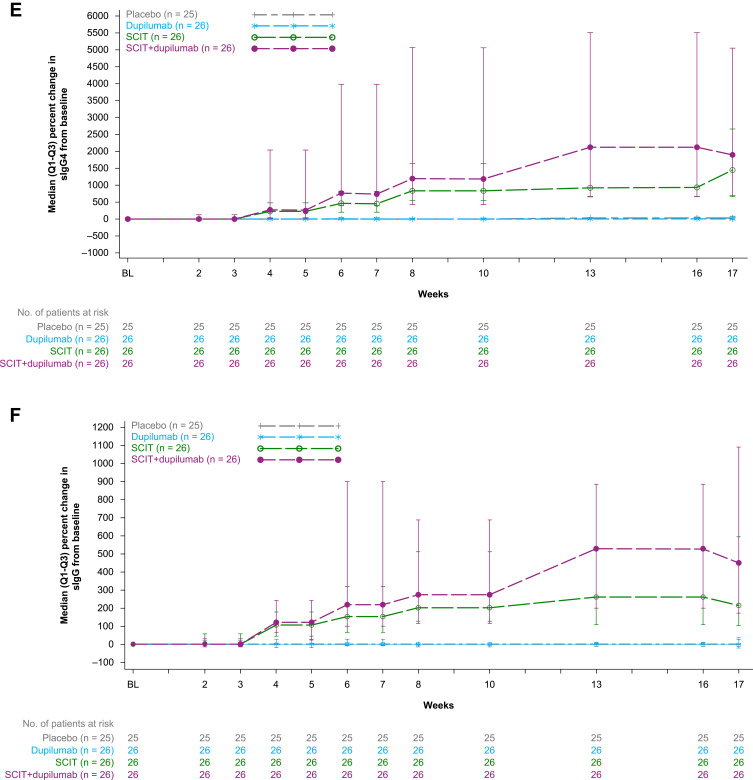
Changes from baseline in serum biomarkers are shown from baseline through Week 17 in Figure 3D–I and in Table 4.
Table 4.
Biomarker Analysis at Week 17
| Placebo (n = 25) | Dupilumab (n = 26) | SCIT (n = 26) | SCIT+dupilumab (n = 26) | SCIT+dupilumab vs SCIT Median Difference (95% CI), P value | Dupilumab vs Placebo Median Difference (95% CI), P value | |
|---|---|---|---|---|---|---|
| sIgE | ||||||
| Median % change (LOCF) | −37.2 | −16.9 | 81.3 | −56.4 | −134.6 (−205.51, −94.98), <0.0001 | 4.6 (−15.89, 29.59), 0.7314 |
| Median absolute change (LOCF) | −1.110 | −0.585 | 6.460 | −2.990 | −13.325 (−23.9400, −8.3600), <0.0001 | 0.740 (−0.9200, 3.3100), 0.3288 |
| sIgG4 | ||||||
| Median % change (LOCF) | 19.0 | 0.0 | 1444.5 | 1896.3 | 335.3 (−551.47, 1746.55), 0.1231 | 0.0 (−30.77, 0.00), 0.4024 |
| Median absolute change (LOCF) | 0.050 | 0.000 | 1.705 | 3.550 | 0.665 (−0.7700, 3.2100), 0.1449 | 0.000 (−0.0800, 0.0000), 0.2734 |
| sIgG | ||||||
| Median % change (LOCF) | 0.0 | 0.0 | 216.1 | 454.6 | 159.5 (−43.94, 434.21), 0.0079 | 0.1 (−19.13, 23.81), 0.7295 |
| Median absolute change (LOCF) | 0.000 | 0.000 | 6.150 | 10.350 | 3.800 (−0.6000, 8.9000), 0.0112 | 0.050 (−0.5000, 0.8000), 0.7653 |
| Log (sIgG4/sIgE) | ||||||
| Median change (LOCF) | 0.310 | 0.235 | 0.745 | 1.720 | 0.840 (0.4500, 1.2700), <0.0001 | −0.060 (−0.2300, 0.1200), 0.9551 |
| Log (sIgG/sIgE) | ||||||
| Median change (LOCF) | 0.190 | 0.130 | 0.180 | 1.105 | 0.830 (0.5400, 1.1300), <0.0001 | −0.005 (−0.1900, 0.1800), 0.6032 |
Note: All P values reported are nominal.
Abbreviations: CI, confidence interval; LOCF, last observation carried forward; SCIT, subcutaneous immunotherapy; sIgE, timothy grass–specific immunoglobulin E; sIgG, timothy grass–specific immunoglobulin G: sIgG4: serum timothy grass–specific IgG4.
At Week 17, SCIT+dupilumab led to nominally significant reductions in sIgE, characterized by median percent change (Table 4 and Figure 3D) and median absolute change (Table 4) compared with SCIT. Furthermore, SCIT+dupilumab also led to numerical increases (improvements) in both percent and absolute change in sIgG and sIgG4 compared with SCIT (Table 4 and Figure 3E and F). The reductions in IgE and increases in sIgG and sIgG4 resulted in nominally significant increases (improvements) in log (sIgG4/sIgE) and log (sIgG/sIgE) compared with SCIT (Figure 3G and H). Serum thymus and activation-regulated chemokine (TARC) was suppressed (median percent change, improvement) compared with SCIT (Table 4 and Figure 3I). Comparing dupilumab monotherapy to placebo at Week 17, there were no differences in sIgE, sIgG, sIgG4, log (sIgG4/sIgE), and log (sIgG/sIgE). However, TARC was reduced in dupilumab-treated patients, characterized by median percent change, compared with placebo (Table 4 and Figure 3I).
The post hoc biomarker analyses of SCIT+dupilumab and SCIT patients who completed (completers)/did not complete the study (non-completers) are shown in Supplementary Figure 2. No association was observed between the magnitude of SCIT-induced IgE induction and study completion. At Week 17, the median percent change from baseline in sIgE in all SCIT+dupilumab–treated patients was lower compared with SCIT (Supplementary Figure 2A). For sIgG4 at Week 17, the median percent change from baseline was similar in SCIT+dupilumab and SCIT completers, and higher than in non-completers in both treatment groups (Supplementary Figure 2B). There were larger median percent increases in sIgG from baseline in SCIT+dupilumab and SCIT completers than in non-completers over the 16-week treatment period (Supplementary Figure 2C). Completers in both treatment groups had a greater improvement in sIgG4/sIgE and sIgG/sIgE ratios relative to non-completers for the same treatment groups (Supplementary Figure 2D and E).
Safety
TEAEs were reported in 69–92% of the patients across treatment groups (Table 5). Serious TEAEs occurred infrequently and were reported in one patient in the placebo, dupilumab, and SCIT groups each, and in two patients in the SCIT+dupilumab group. The majority of TEAEs were mild or moderate in severity, and no deaths occurred during the study. Dupilumab-treated patients experienced similar rates of TEAEs compared with placebo-treated patients, including injection-site reactions, consistent with the safety profile of dupilumab as previously described.17–19,22,23
Table 5.
Safety Assessment
| Placebo (n = 25) | Dupilumab (n = 26) | SCIT (n = 26) | SCIT+dupilumab (n = 26) | |
|---|---|---|---|---|
| Patients with ≥1 TEAE, n (%) | 21 (84.0) | 18 (69.2) | 24 (92.3) | 22 (84.6) |
| Patients with ≥1 serious TEAE, n (%) | 1 (4.0) | 1 (3.8) | 1 (3.8) | 2 (7.7) |
| Maximum intensity of TEAE, severe, n (%) | 1 (4.0) | 1 (3.8) | 1 (3.8) | 2 (7.7) |
| Deaths | 0 | 0 | 0 | 0 |
| TEAE leading to discontinuation of study drug | ||||
| Any TEAE leading to discontinuation of study drug (dupilumab/dupilumab placebo) permanently, n (%) |
0 | 2 (7.7) | 4 (15.4) | 1 (3.8) |
| Any TEAE leading to discontinuation of study drug (SCIT/SCIT placebo) permanently, n (%) |
0 | 1 (3.8) | 5 (19.2) | 1 (3.8) |
| Any TEAE leading to discontinuation of study drug (dupilumab/dupilumab placebo or SCIT/SCIT placebo) permanently, n (%) |
0 | 2 (7.7) | 5 (19.2) | 1 (3.8) |
| Injection site reactions (HLT), n (%) | 12 (48.0) | 9 (34.6) | 18 (69.2) | 18 (69.2) |
| Patients with TEAE (≥10% by PT), n (%) | ||||
| Injection site reaction | 8 (32.0) | 8 (30.8) | 11 (42.3) | 16 (61.5) |
| Injection site erythema | 1 (4.0) | 1 (3.8) | 2 (7.7) | 4 (15.4) |
| Injection site induration | 1 (4.0) | 0 | 5 (19.2) | 4 (15.4) |
| Injection site pruritus | 1 (4.0) | 0 | 4 (15.4) | 3 (11.5) |
| Injection site swelling | 1 (4.0) | 0 | 3 (11.5) | 2 (7.7) |
| Injection site urticaria | 1 (4.0) | 0 | 3 (11.5) | 2 (7.7) |
| Nasopharyngitis | 5 (20.0) | 5 (19.2) | 1 (3.8) | 10 (38.5) |
| Viral upper respiratory tract infection | 3 (12.0) | 0 | 0 | 1 (3.8) |
| Upper respiratory tract infection | 5 (20.0) | 4 (15.4) | 1 (3.8) | 0 |
| Hypersensitivity | 0 | 0 | 5 (19.2) | 8 (30.8) |
| Headache | 3 (12.0) | 1 (3.8) | 1 (3.8) | 1 (3.8) |
| Nausea | 3 (12.0) | 0 | 2 (7.7) | 2 (7.7) |
| Nasal congestion | 1 (4.0) | 1 (3.8) | 3 (11.5) | 0 |
| Patients with ≥1 rescue medication for allergic reaction to SCIT during study, n (%) | 0 | 2 (7.7) | 14 (53.8) | 14 (53.8) |
| Patients with ≥1 epinephrine dose, n (%) | 0 | 1 (3.8) | 5 (19.2) | 2 (7.7) |
| Patients with ≥1 oral antihistamine dose, n (%) | 0 | 2 (7.7) | 12 (46.2) | 12 (46.2) |
| Patients with ≥1 oral steroid, n (%) | 0 | 0 | 5 (19.2) | 2 (7.7) |
| Patients with ≥1 topical steroid, n (%) | 0 | 0 | 2 (7.7) | 1 (3.8) |
| Patients with ≥1 SABA, n (%) | 0 | 0 | 2 (7.7) | 4 (15.4) |
| Patients achieving maintenance dose of SCIT during 16-week treatment period, n (%) | N/A | N/A | 12 (46.2) | 16 (61.5) |
Abbreviations: HLT, MedDRA high-level term; MedDRA, Medical Dictionary for Regulatory Activities; N/A, not applicable; PT, MedDRA preferred-term; SABA, short-acting beta agonist; SCIT, subcutaneous immunotherapy; TEAE, treatment-emergent adverse event.
The most commonly reported TEAEs were injection-site reactions, which were more common in the SCIT-containing treatment arms (Table 5). The proportion of patients who overall required rescue treatment for allergic reactions to SCIT was similar between the SCIT+dupilumab and SCIT regimens (Table 5). However, the requirement for rescue treatment with epinephrine was higher in the SCIT group (19.2%) than in the SCIT+dupilumab group (7.7%, Table 5). For a list of reactions and treatments in the SCIT and SCIT-placebo populations, see Supplementary Table 2. A higher proportion of SCIT+dupilumab patients (61.5%) achieved the SCIT maintenance dose during the treatment period compared with SCIT-receiving patients (46.2%, Table 5). A total of 13 (12.6%) patients withdrew from the study, with one (4.0%), two (7.7%), eight (30.8%), and two (7.7%) withdrawals in placebo, dupilumab, SCIT, and SCIT+dupilumab groups, respectively. There were significantly fewer withdrawals in the SCIT+dupilumab group than in SCIT (P = 0.0216), and although the majority of discontinuations in the SCIT group were due to SCIT-related intolerability (n = 5 [62.5%]), none of the discontinuations in the SCIT+dupilumab group were attributable to SCIT-related intolerability.
Discussion
Our results demonstrate that when directly compared, a 16-week treatment course of dupilumab plus timothy grass SCIT was not significantly more efficacious than SCIT in reducing responses to timothy grass NAC. Interpretation of efficacy data was limited by the higher than anticipated number of SCIT-related withdrawals in the SCIT arm, which affected the projected treatment effects used in the power calculations to support the study design. The primary analysis performed with MI was supported by sensitivity analyses using LOCF and OC.
Administration of dupilumab in combination with SCIT significantly reduced the response to TG allergen SPT and the number of systemic reactions to SCIT. Grass allergens have been identified as risk factors for systemic reactions during SCIT, both in studies in North America29 and in Europe.30 When compared to other allergens, the latter study showed that TG SCIT had the lowest proportion of patients reach the maintenance dose without side effects.30 The improved tolerability of SCIT in patients in the present study receiving concomitant dupilumab was supported by fewer treatment discontinuations due to adverse events with SCIT+dupilumab vs SCIT, a reduced need for epinephrine as rescue medication, and higher proportions of patients achieving the target maintenance dose of SCIT. A previous trial of SCIT plus an anti-IL-4 antibody showed no improved tolerability to SCIT.31 However, these results suggest that dual blockade of IL-4 and IL-13 may improve tolerability of SCIT and could be useful for patients in which rapid achievement of cluster SCIT maintenance dose is required. Although systemic reactions occurred in approximately 0.1% of SCIT injection visits in the United States between 2008 and 2016,32,33 a study of 441 patients receiving cluster SCIT showed systemic reactions in 10.9% of patients.29 A recent study reviewing SCIT treatment for a variety of allergens in pediatric AR patients from 2005 to 2018 found that 106 of 786 patients (13.4%) presented adverse reactions, mostly in the up-dosing phase (83/106, 78.3%).9 The number and grade of systemic reactions in the present trial are similar to those observed in the study reported by Scadding et al, where cluster SCIT was similarly utilized.28 The epinephrine administration in 19.2% (SCIT) and 7.7% (SCIT+dupilumab) of patients may be a reaction to increased SCIT fatalities in recent years32 or differences in the rates of epinephrine administration observed across different geographies. For instance, across a sublingual immunotherapy program conducted in both North America and Europe, there were 35 total epinephrine administrations with only 2 epinephrine administrations occurring in Europe.34 The number of epinephrine administrations in this trial is not unexpected given the cluster regimen and high target maintenance dose of grass allergen in this US-based trial.
As previously reported,35,36 an induction of sIgE was observed in SCIT-treated patients in this study. The increase in sIgE was suppressed in patients treated with SCIT+dupilumab. Both IL-4 and IL-13 have been shown to play key roles in B cell IgM to IgE isotype switching,15 and it has been previously shown that dupilumab suppresses total IgE over time.21–25,37 The observation that dupilumab blocks the SCIT-induced sIgE through the blockade of IL-4Rα is supportive of the hypothesis that the rise in sIgE post-allergen exposure is directly related to IL-4–mediated class switching of B cells16 and is consistent with previous studies demonstrating dupilumab suppresses IgE.21–25 Biologics against IgE, including omalizumab, have been tested in AR patients with concomitant allergic asthma and shown to reduce symptom load but not to reduce the usage of rescue medication.38,39 During a 16-week treatment period, we did not observe any significant difference in the rise of sIgG and sIgG4 between SCIT+dupilumab and SCIT treatment groups. We also observed that the SCIT-induced increases in sIgG4 were not inhibited by the dupilumab-mediated IL-4Rα blockade, a novel finding that has not been previously described.
The combination of dupilumab, which causes a reduction in sIgE, plus SCIT, which leads to elevated sIgG4, results in an increase in the ratio of sIgG4/sIgE compared to patients treated with SCIT. In a setting in which IgG4 memory is already established, as in individuals with seasonal grass allergy, IL-4 is not required for IgG4 plasma cell differentiation/antibody production. In individuals with peanut allergy, human IgE-switched cells exhibit a plasmablast/plasma cell phenotype, while IgG4-switched cells exhibited a B cell phenotype.40 IL-10 can enhance the differentiation of B-cells that are already IgG4–committed to produce antibody,41 and this process was shown to be independent of IL-4.42 In fact, IL-4 was shown to suppress the expression of Blimp-1, a transcription factor that drives the terminal differentiation of B-cells to plasma cells.42 Together, these data demonstrate IgG4 memory B-cells exist, and that in the presence of antigen their subsequent differentiation to IgG4-producing cells is IL-4–independent. Augmentation of the sIgG/sIgE ratio by the SCIT+dupilumab combination therapy may have contributed to the reduction in both SPT responses and systemic reactions to SCIT. These data suggest the use of dupilumab in combination with immunotherapy for patients undergoing oral immunotherapy for other allergens to improve tolerability warrants further study.
Although 16 weeks of monotherapy with dupilumab had no significant impact on AR symptoms induced by NAC, dupilumab has previously been shown to reduce perennial AR symptoms in patients with perennial AR and comorbid asthma,24,25 and perennial AR and comorbid atopic dermatitis.43 Future studies are needed to assess whether dupilumab monotherapy affects the symptoms of seasonal AR during the natural season.
The overall safety of dupilumab and SCIT in the SCIT+dupilumab group was consistent with the known safety profile of dupilumab17–19,22,23 and SCIT.2,28 The most important limitations of our study included the small number of patients in each treatment group, the high rate of withdrawal in one study arm (SCIT), and the short 16-week treatment duration. The NAC model may also not be the most appropriate model to test the effect of dupilumab on AR, as the NAC provokes mainly early allergic responses.44
Conclusions
SCIT+dupilumab did not reduce post-allergen challenge TNSS compared with SCIT after 16 weeks of treatment. However, our data suggest that dupilumab may improve the tolerability of SCIT and may be useful for patients with severe AR and/or allergic asthma who may benefit from immunotherapy but are immunotherapy intolerant. A higher proportion of SCIT+dupilumab patients achieved the SCIT maintenance dose and fewer of these patients required epinephrine rescue treatment compared to SCIT-only patients. Dupilumab as an adjuvant to SCIT significantly reduced sIgE levels and increased the log sIgG4/sIgE and sIgG/sIgE ratios compared with SCIT alone, which may be a possible mechanism to improve the tolerability of SCIT updosing. The effect of SCIT+dupilumab on AR symptoms may not be fully captured using the NAC AR provocation model in the short, 16-week duration of treatment, and longer-term studies are needed to further evaluate whether the addition of dupilumab improves the efficacy and safety of SCIT in patients with grass pollen–induced seasonal AR.
Acknowledgments
This research was sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. Medical writing and editorial assistance provided by Julian J. Freen-van Heeren, PhD, of Excerpta Medica, funded by Sanofi Genzyme and Regeneron Pharmaceuticals, Inc.
Funding Statement
This work was supported by Sanofi and Regeneron Pharmaceuticals, Inc. ClinicalTrials.gov Identifier: NCT03558997. Medical writing/editorial assistance was provided by Julian J. Freen-van Heeren, PhD, of Excerpta Medica, funded by Sanofi Genzyme and Regeneron Pharmaceuticals, Inc.
Abbreviations
AE, adverse event; ANCOVA, analysis of covariance; AR, allergic rhinitis; AUC, area under the curve; BAU, bioequivalent allergy unit; CI, confidence interval; CTCAE, Common Terminology Criteria for Adverse Events; Ig, immunoglobulin; IL, interleukin; IL-4Rα, interleukin-4 receptor α; LS, least squares; LOCF, last observation carried forward; MI, multiple imputation; NAC, nasal allergen challenge; OC, observed values; PNIF, peak nasal inspiratory flow; SAR, seasonal allergic rhinitis; SC, subcutaneously; SCIT, subcutaneous immunotherapy; SD, standard deviation; sIgE, serum timothy grass–specific immunoglobulin E; sIgG, timothy grass–specific immunoglobulin G; sIgG4, serum timothy grass–specific immunoglobulin G4; SPT, skin prick test; TARC, thymus and activation-regulated chemokine; TEAE, treatment-emergent adverse event; TG, timothy grass; TNSS, total nasal symptom score; TOSS, total ocular symptom score.
Data Sharing Statement
Qualified researchers may request access to study documents (including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan) that support the methods and findings reported in this manuscript. Individual anonymized participant data will be considered for sharing once the product and indication has been approved by major health authorities (eg, FDA, EMA, PMDA, etc), if there is legal authority to share the data and there is not a reasonable likelihood of participant re-identification. Submit requests to https://vivli.org/.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosures
JC reports research grants from and consultancy for AstraZeneca, Genentech, Novartis, Regeneron Pharmaceuticals, Inc., and Sanofi; and speaker fees from AstraZeneca, Genentech, Novartis, Optinose, and Teva. SSS reports research grants from NIH, Novartis, and Regeneron Pharmaceuticals, Inc.; and consultancy for Allakos, Gbio, Genentech, GI Innovations, MedImmune, Novartis, Ono Pharma, and Regeneron Pharmaceuticals, Inc. RG reports research grants from ALK, AstraZeneca, BioCryst, DBV Technologies, Genentech, GreenCross, Novartis, Regeneron Pharmaceuticals, Inc., Sanofi, and Shire; consultancy for ALK, AstraZeneca, Pfizer, and Sanofi; and speaker fees from Novartis. MHM reports advisory board membership for Regeneron Pharmaceuticals, Inc. GS reports personal fees from Pfizer, Anaphylaxis Canada, Allergy, Asthma, and Immunology Society of Ontario, and Canadian Hereditary Angioedema Network; research grants from Aimmune, DBV Technologies, Genentech, AstraZeneca, CSL Behring, Merck, Leo Pharma Inc, Stallergens, ALK, Pfizer, Novartis, Regeneron Pharmaceuticals, Inc., and Sanofi; and consultancy from Amgen, Genentech, Novartis, Nuvo Pharmaceuticals, and Sanofi. JJ reports contracted research, consultant fees, and speaker fees from AstraZeneca, Allakos, Biocryst, CSL Behring, Genentech, Novartis, Regeneron Pharmaceuticals, Inc., Sanofi Genzyme, Takeda, and Teva. EL is an employee and may hold stock and/or stock options in Sanofi Genzyme. ESC, TC, YS, JM, JDH, MR, CQW, and MPO are employees and shareholders of Regeneron Pharmaceuticals, Inc and have a pending patent application on this study. The patent application number is PCT/US2020/044958. It is in the name of Regeneron Pharmaceuticals, Inc. and Sanofi Biotechnology. The authors report no other conflicts of interest in this work.
References
- 1.Seidman MD, Gurgel RK, Lin SY, et al. Clinical practice guideline: allergic rhinitis. Otolaryngol Head Neck Surg. 2015;152(Suppl 1):S1–S43. doi: 10.1177/0194599814561600 [DOI] [PubMed] [Google Scholar]
- 2.Durham SR, Penagos M. Sublingual or subcutaneous immunotherapy for allergic rhinitis? J Allergy Clin Immunol. 2016;137(2):339–349.e10. doi: 10.1016/j.jaci.2015.12.1298 [DOI] [PubMed] [Google Scholar]
- 3.Kucuksezer UC, Ozdemir C, Cevhertas L, Ogulur I, Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy and allergen tolerance. Allergol Int. 2020;69(4):549–560. PMID: 32900655. doi: 10.1016/j.alit.2020.08.002 [DOI] [PubMed] [Google Scholar]
- 4.Sturm GJ, Varga EM, Roberts G, et al. EAACI guidelines on allergen immunotherapy: hymenoptera venom allergy. Allergy. 2018;73(4):744–764. doi: 10.1111/all.13262 [DOI] [PubMed] [Google Scholar]
- 5.Dhami S, Kakourou A, Asamoah F, et al. Allergen immunotherapy for allergic asthma: a systematic review and meta-analysis. Allergy. 2017;72(12):1825–1848. doi: 10.1111/all.13208 [DOI] [PubMed] [Google Scholar]
- 6.Durham SR, Walker SM, Varga EM, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341(7):468–475. doi: 10.1056/NEJM199908123410702 [DOI] [PubMed] [Google Scholar]
- 7.Penagos M, Eifan AO, Durham SR, Scadding GW. Duration of allergen immunotherapy for long-term efficacy in allergic rhinoconjunctivitis. Curr Treat Options Allergy. 2018;5(3):275–290. doi: 10.1007/s40521-018-0176-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amin HS, Liss GM, Bernstein DI. Evaluation of near-fatal reactions to allergen immunotherapy injections. J Allergy Clin Immunol. 2006;117(1):169–175. doi: 10.1016/j.jaci.2005.10.010 [DOI] [PubMed] [Google Scholar]
- 9.Pavon-Romero GF, Larenas-Linnemann DE, Xochipa Ruiz KE, Ramirez-Jimenez F, Teran LM. Subcutaneous allergen-specific immunotherapy is safe in pediatric patients with allergic rhinitis. Int Arch Allergy Immunol. 2021;182(6):553–561. doi: 10.1159/000513158 [DOI] [PubMed] [Google Scholar]
- 10.Jiang Z, Xiao H, Zhang H, Liu S, Meng J. Comparison of adverse events between cluster and conventional immunotherapy for allergic rhinitis patients with or without asthma: a systematic review and meta-analysis. Am J Otolaryngol. 2019;40(6):102269. doi: 10.1016/j.amjoto.2019.07.013 [DOI] [PubMed] [Google Scholar]
- 11.Epstein TG, Liss GM, Murphy-Berendts K, Bernstein DI. AAAAI/ACAAI surveillance study of subcutaneous immunotherapy, years 2008–2012: an update on fatal and nonfatal systemic allergic reactions. J Allergy Clin Immunol Pract. 2014;2(2):161–167. doi: 10.1016/j.jaip.2014.01.004 [DOI] [PubMed] [Google Scholar]
- 12.Gandhi NA, Pirozzi G, Graham NMH. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev Clin Immunol. 2017;13(5):425–437. doi: 10.1080/1744666X.2017.1298443 [DOI] [PubMed] [Google Scholar]
- 13.Swain SL, Weinberg AD, English M, Huston GJ. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990;145(11):3796–3806. [PubMed] [Google Scholar]
- 14.Wambre E, Bajzik V, DeLong JH, et al. A phenotypically and functionally distinct human TH2 cell subpopulation is associated with allergic disorders. Sci Transl Med. 2017;9(401):eaam9171. doi: 10.1126/scitranslmed.aam9171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lebman DA, Coffman RL. Interleukin 4 causes isotype switching to IgE in T cell-stimulated clonal B cell cultures. J Exp Med. 1988;168(3):853–862. doi: 10.1084/jem.168.3.853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Floc’h A, Allinne J, Nagashima K, et al. Dual blockade of IL‐4 and IL‐13 with dupilumab, an IL‐4Rα antibody, is required to broadly inhibit type 2 inflammation. Allergy. 2020;75(5):1188–1204. doi: 10.1111/all.14151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simpson EL, Bieber T, Guttman-Yassky E, et al. Two Phase 3 trials of dupilumab vs placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335–2348. doi: 10.1056/NEJMoa1610020 [DOI] [PubMed] [Google Scholar]
- 18.Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389(10086):2287–2303. doi: 10.1016/S0140-6736(17)31191-1 [DOI] [PubMed] [Google Scholar]
- 19.de Bruin-Weller M, Thaçi D, Smith CH, et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to cyclosporine A or when this treatment is medically inadvisable: a placebo-controlled, randomized Phase III clinical trial (LIBERTY AD CAFÉ). Br J Dermatol. 2018;178(5):1083–1101. doi: 10.1111/bjd.16156 [DOI] [PubMed] [Google Scholar]
- 20.Paller AS, Siegfried EC, Thaçi D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol. 2020;83(5):1282–1293. doi: 10.1016/j.jaad.2020.06.054 [DOI] [PubMed] [Google Scholar]
- 21.Paller AS, Siegfried EC, Simpson EL, et al. A phase 2, open-label study of single dose dupilumab in children aged 6 months to <6 years with severe uncontrolled atopic dermatitis: pharmacokinetics, safety and efficacy. J Eur Acad Dermatol Venereol. 2021;35(2):464–475. doi: 10.1111/jdv.16928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486–2496. doi: 10.1056/NEJMoa1804092 [DOI] [PubMed] [Google Scholar]
- 23.Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019;394(10209):1638–1650. doi: 10.1016/S0140-6736(19)31881-1 [DOI] [PubMed] [Google Scholar]
- 24.Weinstein SF, Katial R, Jayawardena S, et al. Efficacy and safety of dupilumab in perennial allergic rhinitis and comorbid asthma. J Allergy Clin Immunol. 2018;142(1):171.e1–177.e1. doi: 10.1016/j.jaci.2017.11.051 [DOI] [PubMed] [Google Scholar]
- 25.Busse WW, Maspero JF, Lu Y, et al. Efficacy of dupilumab on clinical outcomes in patients with asthma and perennial allergic rhinitis. Ann Allergy Asthma Immunol. 2020;125(5):565.e1–576.e1. doi: 10.1016/j.anai.2020.05.026 [DOI] [PubMed] [Google Scholar]
- 26.Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127(Suppl 1):S1–S55. doi: 10.1016/j.jaci.2010.09.034 [DOI] [PubMed] [Google Scholar]
- 27.Cox L, Larenas-Linnemann D, Lockey RF, Passalacqua G. Speaking the same language: the world allergy organization subcutaneous immunotherapy systemic reaction grading system. J Allergy Clin Immunol. 2010;125(3):569–574, 574.e1–574.e7. doi: 10.1016/j.jaci.2009.10.060 [DOI] [PubMed] [Google Scholar]
- 28.Scadding GW, Calderon MA, Shamji MH, et al. Effect of 2 years of treatment with sublingual grass pollen immunotherapy on nasal response to allergen challenge at 3 years among patients with moderate to severe seasonal allergic rhinitis: the GRASS randomized clinical trial. JAMA. 2017;317(6):615–625. doi: 10.1001/jama.2016.21040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Copenhaver CC, Parker A, Patch S. Systemic reactions with aeroallergen cluster immunotherapy in a clinical practice. Ann Allergy Asthma Immunol. 2011;107(5):441–447. doi: 10.1016/j.anai.2011.06.026 [DOI] [PubMed] [Google Scholar]
- 30.Winther L, Arnved J, Malling HJ, Nolte H, Mosbech H. Side‐effects of allergen‐specific immunotherapy: a prospective multi‐centre study. Clin Exp Allergy. 2006;36(3):254–260. doi: 10.1111/j.1365-2222.2006.02340 [DOI] [PubMed] [Google Scholar]
- 31.Chaker AM, Shamji MH, Dumitru FA, et al. Short-term subcutaneous grass pollen immunotherapy under the umbrella of anti-IL-4: a randomized controlled trial. J Allergy Clin Immunol. 2016;137(2):452–461.e9. doi: 10.1016/j.jaci.2015.08.046 [DOI] [PubMed] [Google Scholar]
- 32.Epstein TG, Liss GM, Berendts KM, Bernstein DI. AAAAI/ACAAI subcutaneous immunotherapy surveillance study (2013–2017): fatalities, infections, delayed reactions, and use of epinephrine autoinjectors. J Allergy Clin Immunol Pract. 2019;7(6):1996–2003.e1. doi: 10.1016/j.jaip.2019.01.058 [DOI] [PubMed] [Google Scholar]
- 33.Sánchez-Borges M, Bernstein DI, Calabria C. Subcutaneous immunotherapy safety: incidence per surveys and risk factors. Immunol Allergy Clin North Am. 2020;40(1):25–39. doi: 10.1016/j.iac.2019.09.001 [DOI] [PubMed] [Google Scholar]
- 34.Nolte H, Casale TB, Lockey RF, et al. Epinephrine use in clinical trials of sublingual immunotherapy tablets. J Allergy Clin Immunol Pract. 2017;5(1):84–89.e3. doi: 10.1016/j.jaip.2016.08.017 [DOI] [PubMed] [Google Scholar]
- 35.Gleich GJ, Zimmermann EM, Henderson LL, Yunginger JW. Effect of immunotherapy on immunoglobulin E and immunoglobulin G antibodies to ragweed antigens: a six-year prospective study. J Allergy Clin Immunol. 1982;70(4):261–271. doi: 10.1016/0091-6749(82)90062-8 [DOI] [PubMed] [Google Scholar]
- 36.Shamji MH, Ljørring C, Francis JN, et al. Functional rather than immunoreactive levels of IgG4 correlate closely with clinical response to grass pollen immunotherapy. Allergy. 2012;67(2):217–226. doi: 10.1111/j.1398-9995.2011.02745.x [DOI] [PubMed] [Google Scholar]
- 37.Jonstam K, Swanson BN, Mannent LP, et al. Dupilumab reduces local type 2 pro-inflammatory biomarkers in chronic rhinosinusitis with nasal polyposis. Allergy. 2019;74(4):743–752. doi: 10.1111/all.13685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vignola AM, Humbert M, Bousquet J, et al. Efficacy and tolerability of anti-immunoglobulin E therapy with omalizumab in patients with concomitant allergic asthma and persistent allergic rhinitis: SOLAR. Allergy. 2004;59(7):709–717. doi: 10.1111/j.1398-9995.2004.00550.x [DOI] [PubMed] [Google Scholar]
- 39.Cavaliere C, Begvarfaj E, Incorvaia C, et al. Long-term omalizumab efficacy in allergic rhinitis. Immunol Lett. 2020;227:81–87. doi: 10.1016/j.imlet.2020.08.002 [DOI] [PubMed] [Google Scholar]
- 40.Croote D, Darmanis S, Nadeau KC, Quake SR. High-affinity allergen-specific human antibodies cloned from single IgE B cell transcriptomes. Science. 2018;362(6420):1306–1309. doi: 10.1126/science.aau2599 [DOI] [PubMed] [Google Scholar]
- 41.Jeannin P, Lecoanet S, Delneste Y, Gauchat JF, Bonnefoy JY. IgE versus IgG4 production can be differentially regulated by IL-10. J Immunol. 1998;160(7):3555–3561. [PubMed] [Google Scholar]
- 42.Bryant VL, Ma CS, Avery DT, et al. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol. 2007;179(12):8180–8190. doi: 10.4049/jimmunol.179.12.8180 [DOI] [PubMed] [Google Scholar]
- 43.Nettis E, Patella V, Lombardo C, et al. Efficacy of dupilumab in atopic comorbidities associated with moderate‐to‐severe adult atopic dermatitis. Allergy. 2020;75(10):2653–2661. doi: 10.1111/all.14338 [DOI] [PubMed] [Google Scholar]
- 44.Orban NT, Jacobson MR, Nouri-Aria KT, Durham SR, Eifan AO. Repetitive nasal allergen challenge in allergic rhinitis: priming and Th2-type inflammation but no evidence of remodelling. Clin Exp Allergy. 2021;51(2):329–338. doi: 10.1111/cea.13775 [DOI] [PubMed] [Google Scholar]