Abstract
Objectives
The world is fighting with a COVID-19 pandemic, some of the uncertainties have been revealed. To figure out an estimation of asymptomatic patients and seropositive SARS-COV-2 blood donors in Iran, a national survey was conducted to find the prevalence of asymptomatic blood donors with positive SARS-COV-2 IgM/IgG test results at the end of May 2020.
Material and methods
From all 31 provinces, 1339 blood donors were included. At first, data was collected with an interview containing demographic data, risk factors and possible signs and symptoms held for each donor by a trained medical expert. Then, SARS-COV-2 serologic rapid tests were conducted. Subsequently, the test results were observed and recorded; all of their photos were checked by one single expert. We corrected the prevalence rates for sensitivity and weighted them by the last year rate of blood donation of each province.
Results
The corrected prevalence rates of positive serological test results for sensitivity in provinces were between zero and 38.24%. The national prevalence was calculated 14.45% after weighting. Out of 161 positive donors, only 43 cases reported related signs or symptoms during the defined period of time, while 118 (73.29%) seropositive cases had not reported any related signs or symptoms. Some signs or symptoms were reported more frequent in the SARS-COV-2 serologic rapid test positives. The highest OR (10.19) was linked to ageusia.
Conclusions
This study has shown the prevalence of seropositive results to be around 14% in target population in which around ¾ had not reported any signs or symptoms.
Keywords: COVID-19, Prevalence, Blood donors, Serologic rapid tests
Résumé
Objectif
Le monde se bat contre une pandémie de COVID-19, certaines incertitudes ont été révélées. Pour déterminer une estimation des patients asymptomatiques et des donneurs de sang SARS-COV-2 séropositifs en Iran, une enquête nationale a été menée pour trouver la prévalence des donneurs de sang asymptomatiques avec des résultats positifs aux tests IgM/IgG SARS-COV-2 à la fin du mois de mai 2020.
Matériels et méthodes
De toutes les 31 provinces, 1339 donneurs de sang ont été inclus. Dans un premier temps, les données ont été collectées au moyen d’un entretien contenant des données démographiques, des facteurs de risque et des signes et symptômes possibles détenus pour chaque donneur par un expert médical qualifié. Ensuite, des tests sérologiques rapides sur SARS-COV-2 ont été effectués. Par la suite, les résultats des tests ont été observés et enregistrés; toutes leurs photos ont été vérifiées par un seul expert. Nous avons corrigé les taux de prévalence pour la sensibilité et les avons pondérés par le taux de don de sang de la dernière année dans chaque province.
Résultats
Les taux de prévalence corrigés des résultats de tests sérologiques positifs pour la sensibilité dans les provinces se situaient entre zéro et 38,24 %. La prévalence nationale a été calculée à 14,45 % après pondération. Sur 161 donneurs positifs, seulement 43 cas ont signalé des signes ou des symptômes associés au cours de la période définie, tandis que 118 (73,29 %) séropositifs au SARS-COV-2 n’avaient signalé aucun signe ou symptôme associé. Certains signes ou symptômes ont été rapportés plus fréquemment dans les tests sérologiques rapides positifs au SARS-COV-2. Le OR le plus élevé (10,19) était lié à l’agueusie.
Conclusions
Cette étude a montré que la prévalence des résultats séropositifs d’environ 14 % dans la population cible dans laquelle environ ¾ n’avaient signalé aucun signe ou symptôme.
Mots clés: COVID-19, Prévalence, Donneurs de sang, Tests sérologiques rapides
1. Introduction
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The virus was first reported in December 2019 in Wuhan, China. The World Health Organization (WHO) declared the outbreak as a Public Health Emergency of International Concern on 30 January 2020 [1]. Till 27th of May 2020, when samples of this study were included, the virus spread to 216 countries with more than 5,400,000 reported positive cases while the death tolls reached nearly 350,000 [2]. In Iran, the first confirmed cases were reported on 19 February 2020 in the city of Qom. By the end of May 2020, nearly 149,000 cases reported confirmed positive resulting in 7700 deaths [3].
The wide range of COVID-19 severity, from asymptomatic infection to severe outcomes including respiratory failure, organ failure and death was reported. Most infected people will develop mild to moderate illness and recover without hospitalization [4], [5], [6]. In a study conducted by Chen and his colleagues, 249 patients who were monitored for five weeks, reported as the majority with no hospitalization, had mild symptoms [7]. The findings of a study performed to analyze the different clinical characteristics between children and their families infected with COVID-19 found that 66.7% children had no symptoms which made it difficult to identify their infection at early stages [8]. In many important studies including the study done in a nursing facility at New York, it was found that many persons with positive SARS-CoV-2 test results were asymptomatic at the time of testing and most likely contributed to transmission [9], [10], [11], [12], [13], [14], [15].
These findings indicate that asymptomatic persons include a significant number of patients. Asymptomatic transmission of SARS-CoV-2 is the Achilles’ heel of Covid-19 management measures which highlights the importance of an immediate change to current symptom-based screening policies [16]. In fact, the asymptomatic proportion is a useful quantity to determine the real burden of the disease and better interpret estimates of the transmission potential [17]. Kenji Mizumoto et al. analyzed 3711 passengers of a ship that had been quarantined for two weeks and reported 17% (CI = 95%, 15/5 -20/2) were asymptomatic [17]. Estimating the number of asymptomatic patients and its prevalence in the society contribute significantly to better control of COVID-19. This gains more importance to know that most asymptomatic or pre-symptomatic cases who do not seek care and their infections remain undetected which causes wider spread of disease.
A study in Japan has predicted the peak period of COVID-19 through reports of susceptible, exposed, infective and removed individuals, and those who were in contact with patients [18]. Moreover, the susceptible-infected-recovered (SIR) model was used to compute the trend of infectious diseases [19].
Few studies have been so far conducted in Iran to estimate the rate of asymptomatic patients or recovered COVID-19 persons with no symptoms. In a study, it was reported that 8 incidentally detected cases of COVID-19 were found in chest computed tomography (CT) scan [20]. To fill the gap of data and have an estimation of asymptomatic COVID-19 patients in the society, Iranian Blood Transfusion Organization was going to conduct a national survey to find the prevalence of asymptomatic blood donors with positive SARS-COV-2 IgM/IgG test results. The findings of the study will improve our understanding of COVID-19 transmission and the spectrum of disease in Iran.
2. Patients
In our descriptive study, to have precision of 0.05 and confidence interval of 0.95 considering the result of the study done in the Diamond Princess Cruiser, the calculated sample size was 945.
Quota sampling from 31 provinces over the country was used. To reach the sample size, we took samples in proportion to a total population of blood donors in each province. However, to make discussion possible for each province, we collected at least 30 samples from each province from May 18th until May 27th. Thus, the sample size of 1262 participants was calculated. However, 1339 cases were included finally. Consecutive sampling has been used to collect samples in each Quota. Since our target population was the blood donors of the country, we weighted the seropositive result of the provinces by the last year rate of blood donations of each province to calculate prevalence of SARS-COV-2 serologic test in the blood donors of the country. The study has been approved by the Medical Ethic Committee of High Institute for Research and Education in Transfusion Medicine.
Donors who were accepted for blood donation were asked to participate in our study, if they were not diagnosed COVID-19 with PCR, CT Scan or any clinical feature detected by a physician. Then they read and approved the study consent form. Screening and pre-donation prosses of the participant donors who approved to sign the consent form were 10 to 15 minutes more than other donors.
3. Methods
Data was collected with two methods. Firstly, an interview containing demographic data, risk factors and possible signs and symptoms was done for each donor by a trained medical expert (a blood donation physician). The demographic data included the frequency of donation, address, gender, job, the number of family members living together, BMI and age. Interview included some questions about risk factors like blood groups, history of underlying disease, close contact with a confirmed COVID-19 positive person, presence in general clinics, laboratory examination centers, family private clinics, in which COVID-19 patients were under treatment, presence in schools/universities, sanatoriums, conferences, work, public places/gatherings, smoking cigarette and drinking alcohol. Moreover, questions about the possible signs and symptoms of the COVID-19 included fever, malaise, weakness, cough, red eye, diarrhea, nausea, vomiting, GI bleeding, runny nose, sore throat, headache, dyspnea, chest pain, anosmia, ageusia, “decreased level of consciousness or seizures”, arthralgia, chilling and skin symptoms that were asked during an interview. Finally, SARS-COV-2 serologic rapid tests were done according to the manufacturer's protocol. The history, interview and test results were recorded by the trained colleagues. Moreover, the images of the bands appearing on dipsticks were sent to IBTO Research Center to be checked by one single expert. An online training webinar for physicians who have collaborated in the research was held and the flowchart of the procedures and the forms were sent to them.
SARS-COV-2 IgG/IgM Rapid Test Dipstick made by ALLTEST® Hangzhou AllTest Biotech Co China, was used for this research. The company reported relative specificity and sensitivity of 98% and more than 99.9% for IgG, and 97% and 90.9% for IgM, respectively. Regardless of the manufacturer's claim about the sensitivity and the specificity, 20 true positive samples (approved by both PCR and clinical manifestations) and 30 true negative samples (plasma samples taken a year ago) were used to assess the sensitivity and specificity of the tests.
All prevalence rates and CIs were calculated using the frequencies extracted by SPSS. The crude prevalence of SARS-COV-2 serologic test was corrected considering the sensitivity and specificity of the test based on what we tested in our laboratories. Statistical analyses were performed by SPSS software.
4. Results
All 30 plasma samples from one year ago were negative by the rapid test. However, 17 samples tested positive with the rapid test from 20 confirmed COVID-19 positive plasma units. Therefore, the sensitivity and specificity of the test were calculated 85% and 100%, respectively.
At the final point of collecting data, 1339 blood donors without any past history of clinical or laboratory manifestation of COVID-19 were included in the study on 27th May 2020. Their demographic data can be found in Table 1 .
Table 1.
Demographic data of the study population.
| Serologic SARS-COV-2 Positive | Serologic SARS-COV-2 Negative | |
|---|---|---|
| Frequency | 161 | 1171 |
| Age, Median (IQR) Year | 36 (30–43) | 36 (30–45) |
| Gender, Male | 96.27% | 95.73% |
| BMI, Median (IQR) | 27.76 (25.15–30.66) | 27.72 (25.14–30.67) |
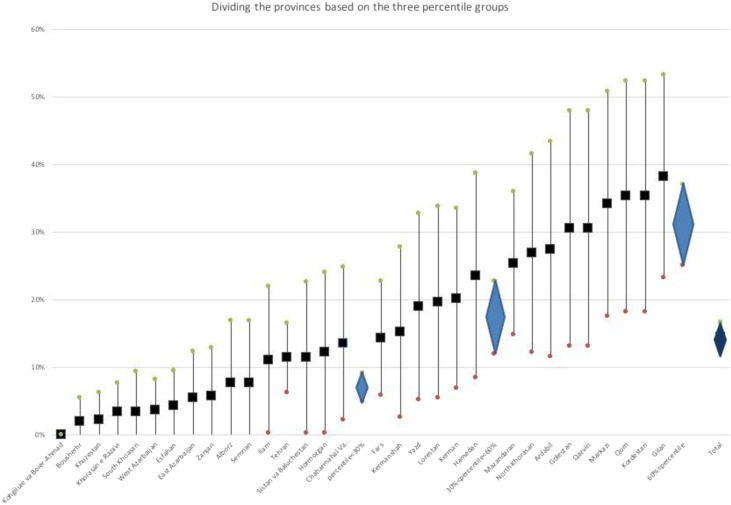
The crude prevalence of SARS-COV-2 positive serologic test was calculated 12.09% with 95% Confidence Interval (CI) between 10.34% and 13.84%. Then, the numbers of prevalence rates for each province were corrected considering the sensitivity of the test. The prevalence rates in Gilan province with 38.24% (95% CI 23.18%–53.30%) and Kohgiluyeh and Boyer-Ahmad province without any positive result were the highest and the lowest, respectively (Fig. 1 ). As the Fig. 2 shows, the variation between the provinces is high and if we divide them into three groups based on the percentile of the prevalence (0%-30%, 31%-60%, and 61%-100%), the three groups will have significantly different weighted subtotal prevalences. Finally, the prevalence of positive serological SARS-COV-2 test was calculated 14.45% (95% CI 12.27%–16.63%) in the blood donors with no known history of infection in the country. Regarding the type of antibody, IgM-only, IgG-only and IgM + IgG contained 33.54%, 58.39% and 8.07% of positive results, respectively (Fig. 3 ).
Fig. 1.
The prevalence of positive serological test for SARS-COV-2 specific antibody in provinces.
Fig. 2.
The prevalence of positive serological test for SARS-COV-2 specific antibody in provinces divided based on percentile.
Fig. 3.
The estimated national prevalence of positive serological tests in the target population.
Regular, repeated and first-time blood donors made up 61%, 24% and 15% of serologic negative donors and 61%, 26% and 13% of serologic positive donors, respectively. Similarly, there were not any observed differences in the number of family members between the serologic positive and negative groups.
Some of the observed risk factors like controlled hypertension, COPD, asthma, diabetes, fatty liver, IBD, medication use, cigarette and alcohol usage, presence in COVID-19 treatment centers, clinics, schools/universities, sanatoriums, conferences, work and public places/gatherings did not show any significant differences between the serologic positive and negative groups; however, close contact with a confirmed COVID-19 case was statistically significant (Odds ratio (OR) = 4.48 (95% CI, 2.41–8.32)) (Fig. 4 ).
Fig. 4.
Contact with a confirmed COVID-19 person difference between two seropositive and seronegative groups.
Although there were no significant differences in blood groups between serologic positive and negative groups, the only blood group observed in positive donors (39%) more than negative donors (33%) was O group. Presence in COVID-19 treatment centers was observed to be statistically significantly higher in the positive serologic group (OR = 1.72, 95% CI 1.07–2.78) (Fig. 5 ).
Fig. 5.
Difference of blood group distribution between two seropositive and seronegative groups.
Out of 161 positive donors found in this study from 1339 included donors, 43 cases reported related signs or symptoms from the time of the first reported COVID-19 patient in Iran until the time of study, May 2020. Out of 95 donors who were reported only IgG positives, IgG plus IgM positives and only IgM 32.6%, 23.1% and 17.0% reported related signs or symptoms respectively. The probable signs and symptoms asses sed are reported on Table 2 . Fever, malaise, weakness, cough, diarrhea, nausea, headache, anosmia and ageusia were correlated to the result of SARS-COV-2 serologic rapid test. The highest OR linked to ageusia which was 10.19 with 95% CI between 4.39 and 23.65.
Table 2.
Differences in presentation of sign and symptoms between SARS-COV-2 positive and negative serologic test groups.
| Signs or symptoms | Seronegative group | Seropositive group | P value | Odds Ratio (95% Confidence Interval) |
|---|---|---|---|---|
| Fevera | 2.74% | 9.32% | 2.20 * 10−5 | 3.65 (1.93- 6.90) |
| Malaisea | 3.42% | 9.32% | 4.33 * 10−4 | 2.90 (1.56- 5.38) |
| Weaknessa | 2.22% | 9.32% | 1.00 * 10−6 | 4.52 (2.34–8.73) |
| Cough | 3.68% | 6.88% | 5.44 * 10−2 | 1.94 (0.98–3.83) |
| Red eye | 0.68% | 1.24% | 4.42 * 10−1 | 1.83 (0.39–8.68) |
| Diarrheaa | 1.11% | 3.73% | 8.71 * 10−3 | 3.45 (1.29–9.20) |
| Nauseaa | 0.34% | 1.86% | 4.21 * 10−2 | 5.54 (1.23–24.96) |
| Vomiting | 0.26% | 1.24% | 1.14 * 10−1 | 4.89 (0.81–29.51) |
| Runny nose | 2.83% | 4.35% | 2.89 * 10−1 | 1.56 (0.68–3.60) |
| Sore throat | 3.60% | 4.97% | 3.88 * 10−1 | 1.40 (0.65–3.05) |
| Headachea | 2.73% | 5.62% | 4.73 * 10−2 | 2.12 (0.99–4.53) |
| Dyspnea | 1.28% | 0.62% | 4.71 * 10−1 | 0.48 (0.63–3.67) |
| Chest pain | 1.03% | 1.86% | 4.12 * 10−1 | 1.83 (0.51–6.56) |
| Anosmiaa | 1.20% | 8.70% | 5.08 * 10−10 | 7.86 (3.68–16.82) |
| Ageusiaa | 0.85% | 8.07% | 4.37 * 10−11 | 10.19 (4.39–23.65) |
| Arthralgia | 0.51% | 1.24% | 2.51 * 10−1 | 2.44 (0.49–12.19) |
| Chillinga | 1.79% | 4.97% | 9.70 * 10−3 | 2.86 (1.25–6.57) |
| Skin symptoms | 0.34% | 0.62% | 4.76 * 10−1 | 1.82 (0.20–16.40) |
P value < 0.05
5. Discussion
In this national study to find the seroprevalence of positive SARS-COV-2 in the blood donors with no known history of infection at the end of May 2020, we found 14.47% (95%CI, 12.58 - 16.36) seropositive, adjusted for test sensitivity and specificity and weighted according to the blood donation of each province. This is higher than published seroprevalence rates among blood donors, respectively reported 3.3%, 1.7%, 2.6%, 2.7%, 4.9% and 3.0% for Brazil (Rio de Janeiro), Denmark, USA, the Netherlands, Mexico, and Canada (Québec) [21], [22], [23], [24], [25], [26]. The first four areas reported the first case around ten days later than Iran. However, a study in UK reported a seroprevalence of 14% by early May, also another study in India reported seroprevalence of 9.5% which were near our results [27], [28]. The reported prevalence rates were attributed to population-based immunity through natural exposure to the virus (herd immunity), the time passed since the beginning of the infection, the speed of infection spread (the real-time reproduction number), and how the population applies the protective procedures [29].
The reported confirmed cumulative incidence of COVID-19 was almost 150,000 cases at the end of May, which is less than 0.18% of the population of Iran. However, we found there were considerable amounts of seropositive people who had not been a confirmed case of COVID-19. The present findings seem to be consistent with the population-based research of Sringhini et al., which found 10.8% IgG seroprevalence and concluded for every confirmed case, there are near 12 infected cases in community [30].
The SARS-COV-2 seropositive prevalence varied substantially among provinces. Our study showed the variation of the number of susceptible and non-susceptible hosts was more than 30% as a consequence of travel ban, limited connection to other provinces or other factors. As in one of the first provinces in which COVID-19 was reported (Gilan province) with almost 38% IgG positive result, a high number of the population had immunity, while the immunity in Tehran, as the capital, was not adequate. On the other hand, there were some southern provinces like Kohgiluie and Boyer-Ahmad, Boushehr and Khuzestan in which the immunity was very low at the time of study. Three months after the first reported COVID-19 case in Iran, geographical distribution of almost 150,000 cases were the same as our study results, while we could find the undiagnosed cases. The prevalence of seropositive blood donors who had not the history of COVID-19 in each province should be added to the confirmed cases’ incidence to find if the level of herd immunity within a population was reached. Altmann, et al. reported the threshold level of herd immunity within a population is around 60% [31].
Although contact with the diagnosed COVID-19 person was found to be statistically significantly different between the two seropositive and seronegative groups, it is somewhat surprising that no differences were found in most of the observed risk factors. In contrast, some of them reported to be a risk factor for more morbidity or mortality in the previous studies [32], [33] while we did not find to be risk factor for catching the COVID-19. Based on the main aim of the study which was the prevalence of seropositive blood donors, we did not have so many patients and following time in whom risk factors, especially the ones related to morbidity and mortality, were required to be statistically evaluated.
Another important finding was the different frequency of blood groups between the two serological positive and negative populations which we showed in the result section; however, the differences were not statistically significant. The cases with O blood group were more common in the seronegative group of our data, which Ellinghaus et al. did a prior study about the association of genes and COVID in which they have statistically significantly reported the same results [34].
One important question investigated in the research was the sign and symptoms of COVID-19. It is interesting to note that anosmia and ageusia were found significantly more frequent in the seropositive group. This finding is compatible with Meng et al., who showed olfactory dysfunction is a characteristic sign of COVID-19 patients [35]. Despite the fact that signs and symptoms were not our main aim, the findings in mild disease were the same as the other reported articles [36], [37].
Although the transmission of the COVID-19 has not been approved by blood transfusion, transmission during the process of blood donation has to be indicated. Therefore, a high standard personal and general protection should be directed.
The methods used for this study was the same nationally in all provinces and most of the sample collections and tests were performed in two days from all over the country, however, the current study has been limited by the sample size in the small provinces. Also, the ratio of IgM+, IgG-/IgM+, IgG+ were higher than what can be expected reviewing the time duration of detection of SARS-COV-2 antibodies in blood, which could be because of the limited IgM specificity which made it hard to confidently discuss about prevalence of IgM positives. Moreover, the test results depended on the expert observation which we tried to limit, with one single expert checking all the photos.
6. Conclusion
We believe this study is the first study in its kind conducted in the region. The study population contains blood donors who are healthier than the normal population. Although this study has shown the prevalence of seropositivity around 14% in the target population in which 5% were IgM, these amounts varied between provinces and it should be considered for policy making.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgments
The authors wish to thank the donors who added to give unremunerated blood, participated in the study, as well as the colleagues in blood transfusion organization in all provinces who helped us to collect data and the colleagues in the High Institute for Research and Education in Transfusion Medicine for their cooperation. This study has been funded by the High Institute for Research and Education in Transfusion Medicine.
References
- 1.2020. Rolling updates on coronavirus disease (COVID-19) [updated 29 June 2020; cited 2020 July 14] Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen. [Google Scholar]
- 2.2020. Coronavirus disease (COVID-19) pandemic. [cited 2020 July 14]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. [Google Scholar]
- 3.CDC NCoC-EaI . 2020. Analysis of epidemic trend by provinces of Iran (till May 29) [updated May 29; cited 2020 July 15]. Available from: http://corona.behdasht.gov.ir/files/site1/files/IRAN_COVID19_Factsheet_N.43_-30May_En.pdf. [Google Scholar]
- 4.2020. Q&A on coronaviruses (COVID-19) [updated 17 April 2020; cited 2020 July 14] Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/q-a-coronaviruses#:∼:text=symptoms. [Google Scholar]
- 5.Pollán M., Pérez-Gómez B., Pastor-Barriuso R., Oteo J., Hernán M.A., Pérez-Olmeda M., et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet (London, England) 2020;396:535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stefanelli P., Bella A., Fedele G., Pancheri S., Leone P., Vacca P., et al. Prevalence of SARS-CoV-2 IgG antibodies in an area of North-eastern Italy with a high incidence of COVID-19 cases: a population-based study. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J., Qi T., Liu L., Ling Y., Qian Z., Li T., et al. Clinical progression of patients with COVID-19 in Shanghai, China. Journal of Infection. 2020 doi: 10.1016/j.jinf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su L., Ma X., Yu H., Zhang Z., Bian P., Han Y., et al. The different clinical characteristics of corona virus disease cases between children and their families in China–the character of children with COVID-19. Emerging microbes & infections. 2020;9:707–713. doi: 10.1080/22221751.2020.1744483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arons M.M., Hatfield K.M., Reddy S.C., Kimball A., James A., Jacobs J.R., et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020 doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishiura H., Kobayashi T., Miyama T., Suzuki A., Jung S-m, Hayashi K., et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19) International journal of infectious diseases. 2020;94:154. doi: 10.1016/j.ijid.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lavezzo E., Franchin E., Ciavarella C., Cuomo-Dannenburg G., Barzon L., Del Vecchio C., et al. Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo’. Nature. 2020 doi: 10.1038/s41586-020-2488-1. [1–] [DOI] [PubMed] [Google Scholar]
- 12.Gao Z., Xu Y., Sun C., Wang X., Guo Y., Qiu S., et al. A systematic review of asymptomatic infections with COVID-19, Journal of Microbiology. Immunology and Infection. 2020 doi: 10.1016/j.jmii.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ing A.J., Cocks C., Green J.P. COVID-19: in the footsteps of Ernest Shackleton. Thorax. 2020 doi: 10.1136/thoraxjnl-2020-215091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.An P., Song P., Wang Y., Liu B. Asymptomatic Patients with Novel Coronavirus Disease (COVID-19) Balkan medical journal. 2020;37:229–230. doi: 10.4274/balkanmedj.galenos.2020.2020.4.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y.J., Chen P., Liu Z.S., Li Y., Du H., Xu J.L. [Clinical features of asymptomatic or subclinical COVID-19 in children] Zhongguo dang dai er ke za zhi=Chinese journal of contemporary pediatrics. 2020;22:578–582. doi: 10.7499/j.issn.1008-8830.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gandhi R.T., Lynch J.B., del Rio C. Mild or moderate COVID-19. N Engl J Med. 2020 doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 17.Mizumoto K., Kagaya K., Zarebski A., Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Eurosurveillance. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [2000180] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuniya T. Prediction of the Epidemic Peak of Coronavirus Disease in Japan, 2020. Journal of clinical medicine. 2020;9 doi: 10.3390/jcm9030789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shu P., Wang W., Tang M., Do Y. Numerical identification of epidemic thresholds for susceptible-infected-recovered model on finite-size networks. Chaos (Woodbury, NY) 2015;25:063104. doi: 10.1063/1.4922153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samsami M., Zebarjadi Bagherpour J., Nematihonar B., Tahmasbi H. COVID-19 Pneumonia in Asymptomatic Trauma Patients; Report of 8 Cases. Archives of academic emergency medicine. 2020;8:e46. [PMC free article] [PubMed] [Google Scholar]
- 21.Amorim Filho L., Szwarcwald C.L., Mateos S.O.G., Leon A., Medronho R.A., Veloso V.G., et al. Seroprevalence of anti-SARS-CoV-2 among blood donors in Rio de Janeiro, Brazil. Revista de saude publica. 2020;54:69. doi: 10.11606/s1518-8787.2020054002643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erikstrup C., Hother C.E., Pedersen O.B.V., Molbak K., Skov R.L., Holm D.K., et al. Estimation of SARS-CoV-2 infection fatality rate by real-time antibody screening of blood donors. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slot E., Hogema B.M., Reusken C.B., Reimerink J.H., Molier M., Karregat J.H., et al. Herd immunity is not a realistic exit strategy during a COVID-19 outbreak. 2020 [Google Scholar]
- 24.Dodd R.Y., Xu M., Stramer S.L. Change in Donor Characteristics and Antibodies to SARS-CoV-2 in Donated Blood in the US, June-August 2020. JAMA. 2020 doi: 10.1001/jama.2020.18598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewin A., Therrien R., De Serres G., Grégoire Y., Perreault J., Drouin M., et al. SARS-CoV-2 seroprevalence among blood donors in Québec, and analysis of symptoms associated with seropositivity: a nested case-control study. Can J Public Health. 2021;112:576–586. doi: 10.17269/s41997-021-00531-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez-Acuña N., Avalos-Nolazco D.M., Rodriguez-Rodriguez D.R., Martinez-Liu C.G., Galan-Huerta K.A., Padilla-Rivas G.R., et al. Seroprevalence of Anti-SARS-CoV-2 Antibodies in Blood Donors from Nuevo Leon State, Mexico, during 2020: A Retrospective Cross-Sectional Evaluation. Viruses. 2021;13 doi: 10.3390/v13071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amirthalingam G., Whitaker H., Brooks T., Brown K., Hoschler K., Linley E., et al. Seroprevalence of SARS-CoV-2 among Blood Donors and Changes after Introduction of Public Health and Social Measures, London, UK. Emerging infectious diseases. 2021;27:1795–1801. doi: 10.3201/eid2707.203167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pandey H.C., Dhiman Y., S.C.C., Coshic P., Jain P. Seroprevalence of SARS-Coronavirus 2 among asymptomatic healthy blood donors from healthcare and non-healthcare settings: Implications for safety of blood donors and blood collection staff during blood donation. Transfus Apher Sci. 2021;60:103118. doi: 10.1016/j.transci.2021.103118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan J., Li M., Lv G., Lu Z.K. Monitoring transmissibility and mortality of COVID-19 in Europe. Int J Infect Dis. 2020;95:311–315. doi: 10.1016/j.ijid.2020.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stringhini S., Wisniak A., Piumatti G., Azman A.S., Lauer S.A., Baysson H., et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet (London, England) 2020;396:313–319. doi: 10.1016/S0140-6736(20)31304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Altmann D.M., Douek D.C., Boyton R.J. What policy makers need to know about COVID-19 protective immunity. Lancet (London, England) 2020;395:1527–1529. doi: 10.1016/S0140-6736(20)30985-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahmed S.I., Hasan S.M.T., Ahmed T. Obesity is a potential risk factor for covid-19 associated morbidity and mortality in urban Bangladesh. BMJ (Clinical research ed) 2020;370 doi: 10.1136/bmj.m2811. [m2811] [DOI] [PubMed] [Google Scholar]
- 33.Salacup G., Lo K.B., Gul F., Peterson E., De Joy R., Bhargav R., et al. Characteristics and clinical outcomes of COVID-19 patients in an underserved-inner city population: A single tertiary center cohort. Journal of medical virology. 2020 doi: 10.1002/jmv.26252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ellinghaus D., Degenhardt F., Bujanda L., Buti M., Albillos A., Invernizzi P., et al. Genomewide Association Study of Severe Covid-19 with Respiratory Failure. N Engl J Med. 2020 doi: 10.1056/NEJMoa2020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meng X., Deng Y., Dai Z., Meng Z. COVID-19 and anosmia: A review based on up-to-date knowledge. American journal of otolaryngology. 2020;41:102581. doi: 10.1016/j.amjoto.2020.102581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tong J.Y., Wong A., Zhu D., Fastenberg J.H., Tham T. The Prevalence of Olfactory and Gustatory Dysfunction in COVID-19 Patients: A Systematic Review and Meta-analysis. Otolaryngol Head Neck Surg. 2020;163:3–11. doi: 10.1177/0194599820926473. [DOI] [PubMed] [Google Scholar]
- 37.Chang M.C., Park Y.K., Kim B.O., Park D. Risk factors for disease progression in COVID-19 patients. BMC infectious diseases. 2020;20:445. doi: 10.1186/s12879-020-05144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]