Abstract
Objective
To evaluate the efficacy and safety of pegylated interferon alpha-2b (PEG IFN-α2b) administered in conjunction with the standard of care (SOC) in subjects with moderate coronavirus disease-19 (COVID-19).
Methods
In this study, adult subjects with confirmed moderate COVID-19 were randomized in a 1:1 ratio to receive either PEG IFN-α2b + SOC or SOC alone. The primary endpoint was a two-point improvement in clinical status on Day 11, measured by the World Health Organization's seven-point ordinal scale.
Results
Of 250 subjects, 120 were randomized to the PEG IFN-α2b + SOC arm and 130 were randomized to the SOC arm. The results for the PEG IFN + SOC arms vs the SOC arm for the proportion of subjects with a two-point improvement in the seven-point ordinal scale were 80.36% vs 68.18% (P=0.037) on Day 8, 91.60% vs 92.56% (P=0.781) on Day 11, and 94.12% vs 95.93% (P=0.515) on Day 15. There was a time-dependent decrease in the biomarkers in both arms, and no clinically significant changes in laboratory parameters. The safety profile was similar in both arms.
Conclusion
PEG IFN-α2b induced early viral clearance, improved the clinical status, and decreased the duration of supplemental oxygen. It provides a viable treatment option and can limit the spread of severe acute respiratory syndrome coronavirus-2.
Keywords: Pegylated interferon alpha-2b (PEG IFN-α2b), SARS-CoV-2, Phase 3, Moderate subjects
Introduction
A novel coronavirus disease caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) was identified in December 2019 in patients in Wuhan, China, and a pandemic situation has since been declared (Cucinotta and Vanelli, 2020; Spinelli and Pellino, 2020). By 6 May 2021, >154 million confirmed cases and 3.2 million deaths had been reported worldwide, with 21,077,410 confirmed cases, including 230,168 deaths, in India (World Health Organization, 2021). The clinical spectrum of SARS-CoV-2 infection appears to be wide, encompassing asymptomatic infections, mild upper respiratory tract illness, fatigue, fever, myalgias, severe life-threatening viral pneumonia which requires hospital admission, and death (Cortinovis et al., 2021).
Comorbidities, particularly hypertension, diabetes, obesity and smoking, increase the risk of severe coronavirus disease-19 (COVID-19) (Chen et al., 2021; Zheng et al., 2020). The characteristics of COVID-19 differ depending on the demographic and epidemiological profiles of each country.
To date, only dexamethasone and remdesivir have shown efficacy in randomized trials of hospitalized patients with COVID-19, and an interim analysis of monoclonal antibody infusion has shown faster viral clearance in outpatients (Beigel et al., 2020, Chen et al., 2021, Horby et al., 2021). As seen in other acute viral infections, timely initiation of antiviral therapy for COVID-19 may improve clinical outcomes (Aoki et al., 2003); however, very few studies among outpatients have been completed.
Type I interferons (IFNs) are broad-spectrum antivirals that represent the body's first natural line of defence. They are secreted subsequent to viral replication inside the cell (tenOever et al., 2016). IFNs act by binding to and activating type 1 IFN receptors, and activating the Janus kinase/signal transducer activator of transcription (JAK/STAT) pathway. Activation of the JAK/STAT pathway increases the expression of IFN-stimulated genes in multiple tissues involved in the antiviral response, including direct inhibitory effects on viral replication and supporting an immune response to clear viral infection (Lazear et al., 2019). Recent developments suggest an important association between type I IFNs and SARS-CoV-2 infection and disease (Bastard et al., 2020; Blanco-Melo et al., 2020; Hadjadj et al., 2020; Zhang et al., 2020). Levels of IFN-α decreased in direct correlation with the severity of disease (Hadjadj et al., 2020). Sera of patients with COVID-19 did not show any detectable levels of IFN-β (Blanco-Melo et al., 2020). Approximately 13.7% of patients with severe disease were found to be carrying neutralizing antibodies against IFN-α and other type I IFNs, and had low or undetectable serum levels of IFN-α2b during acute disease (Bastard et al., 2020). Another 3.5% of patients had inborn errors of type I IFN immunity (Zhang et al., 2020).
In vitro, a direct antiviral effect of IFN-α against SARS-CoV-2 has been clearly demonstrated (Lokugamage et al., 2020; Mantlo et al., 2020). In the authors’ studies, a half maximal inhibitory concentration of 15.6 fM was established for PEG IFN-α2b in a set-up where Vero E6 cells were pretreated with the drug before being challenged with SARS-CoV-2. In an exploratory study with 77 patients hospitalized with COVID-19, given IFN-α2b either alone or with arbidol (a broad-spectrum antiviral compound), it was observed that those who received IFN-α2b demonstrated a significant reduction in the duration of detectable virus in the upper respiratory tract and reduced blood levels of inflammatory markers, interleukin-6 (IL-6) and C-reactive protein (CRP) (Zhou et al., 2020). Similarly, in a retrospective analysis of 446 patients hospitalized with COVID-19, it was observed that those who were given IFN-α2b demonstrated reduced in-hospital mortality not associated with hospital discharge (Wang et al., 2020). The synthetic glucocorticoid dexamethasone has been shown to reduce deaths due to COVID-19 by suppression of an overactive immune response; however, this takes place at the cost of suppression of IFN-α2b, a crucial component of the antiviral response. Among patients receiving glucocorticoids, early IFN therapy was associated with earlier hospital discharge [adjusted hazard ratio (HR) 1.68, 95% confidence interval (CI) 1.19–2.37] and symptom relief (adjusted HR 1.48, 95% CI 1.06–2.08), and lower prevalence of prolonged viral shedding (adjusted odds ratio 0.24, 95% CI 0.10–0.57) (Lu et al., 2021). A crucial role of type I IFNs such as IFN-α2b in the protective immunity against SARS-CoV-2 is becoming clear.
PEG IFN-α2b is a covalent conjugate of a recombinant type I IFN, IFN-α2b, with monomethoxy polyethylene glycol. Both IFN-α and PEG IFN-α2b have been used clinically to treat hepatitis B and C viruses for several years. A phase 2 randomized study of 40 patients with moderate COVID-19 demonstrated that a single dose of PEG IFN-α2b given along with the standard of care (SOC) led to an early negative reverse transcription polymerase chain reaction (RT-PCR) result along with improvement in clinical symptoms by Day 15 compared with patients who were given SOC alone (Pandit et al., 2021). However, this study was limited by its small sample size and the fact that quantitative RT-PCR was not performed. This article presents the findings from a phase 3 study involving 250 patients with moderate COVID-19.
Materials and methods
Study design
This phase 3, multi-centric, randomized, comparator-controlled, open-label study evaluated the efficacy and safety of a single dose of PEG IFN-α2b in the treatment of adult patients diagnosed with SARS-CoV-2. The study was undertaken at 20 study centres across India. Eligible subjects were assigned at random in a 1:1 ratio to receive either PEG IFN-α2b + SOC or SOC alone.
This study was initiated after obtaining approval from Ethics Committees (ECs) and the Drugs Controller General of India (DCGI) (dated 14 December 2020); safety, efficacy and study conduct were overseen by an independent data safety monitoring board. This study was conducted in accordance with the applicable local regulations and registered with Clinical Trial Registry - India CTRI (CTRI/2020/12/029855).
Study populations
Individuals with suspected COVID-19 were recruited from 20 study centres across India from 16 December 2020 to 25 March 2021. Key inclusion criteria were age ≥18 years, RT-PCR-confirmed SARS-CoV-2 infection, pneumonia with no signs of severe disease, respiratory rate ≥24 breaths/min, SpO2 90–94%, and a negative pregnancy test (for female patients of child-bearing potential). Key eligibility criteria are provided in the online supplementary material.
Interventions
Eligible subjects were randomized in a 1:1 ratio to either PEG IFN-α2b (1 µg/kg subcutaneous injection, single dose) + SOC or SOC alone. SOC treatments [i.e. antipyretics, cough suppressants, antibiotics, steroids, vitamins, anticoagulants, hydroxychloroquine and antivirals (e.g. remdesivir)] were administered as per the COVID-19 clinical management guidelines of the Ministry of Health, Government of India and the practices of the individual institutions. Randomization was generated using SAS Version 9.4. Each vial of PEG IFN-α2b was reconstituted with 0.7 mL of water for injection for administration of up to 0.5 mL of solution. Each 0.5 mL of solution for subcutaneous injection delivers 100 μg of PEG IFN-α2b. All subjects were hospitalized, with RT-PCR tests using a pharyngeal swab performed at screening, Day 7, Day 11, Day 15 and Day 29.
Assessments
The primary outcome was to evaluate the clinical efficacy of PEG IFN-α2b based on change in the ordinal scale at Day 11 (two-point improvement in the WHO seven-point ordinal scale) (World Health Organization Solidarity Trial Consortium, 2021). The scale consists of the following categories: 1, not hospitalized, no limitation of activities; 2, not hospitalized, limitation of activities; 3, hospitalized, does not require supplemental oxygen; 4, hospitalized, requires supplemental oxygen; 5, hospitalized, requires non-invasive ventilation or on high flow oxygen devices; 6, hospitalized, requires invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO); and 7, death.
The secondary efficacy endpoints were to evaluate the clinical efficacy of PEG IFN-α2b based on change in the ordinal scale at Days 8, 11 and 15; the proportion of subjects with adverse events (AEs) that occurred on or after the first dose of PEG IFN-α2b; qualitative polymerase chain reaction for SARS-CoV-2 using a pharyngeal swab; occurrence and duration of supplemental oxygen and mechanical ventilation; time to resolution of clinical signs and symptoms; duration of hospitalization; change in white blood cell count, haemoglobin, platelets, creatinine, glucose, total bilirubin, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) from baseline to Day 29; and change in CRP, D-dimer, ferritin and IL-6 from baseline to Day 29.
Safety assessments were based on physical examinations, vitals, laboratory tests, and the incidence and severity of AEs.
Statistical analysis
The primary efficacy endpoint was the proportion of subjects who showed an improvement in condition (clinical status) using the WHO seven-point ordinal scale for clinical improvement during the dosing period, and was presented descriptively as frequency and percent. Treatment effect was assessed using Fisher's exact test for active treatment (PEG IFN-α2b + SOC) vs SOC. Non-parametric Wilcoxon rank sum test was used to assess the change in score from baseline within the group.
Of the secondary endpoints, qualitative RT-PCR, requirement for/duration of supplemental oxygen and mechanical ventilation, and time to resolution of signs and symptoms were analysed using non-parametric Wilcoxon rank sum test. CRP, IL-6, D-dimer and ferritin were compared between treatment groups using an analysis of covariance model, with treatment as the fixed effect and baseline value as the covariate.
Statistical significance was tested using a two-sided P-value of 0.05. The results are presented as mean and standard deviation (SD).
Post-hoc analysis was performed for those patients with a two-point improvement in the WHO seven-point ordinal scale at Day 8, and the data were analysed in the subgroups of patients ‘with and without remdesivir’ and ‘with and without steroids’.
The populations and sample size calculation are provided in the online supplementary material.
Results
Subject disposition and characteristics
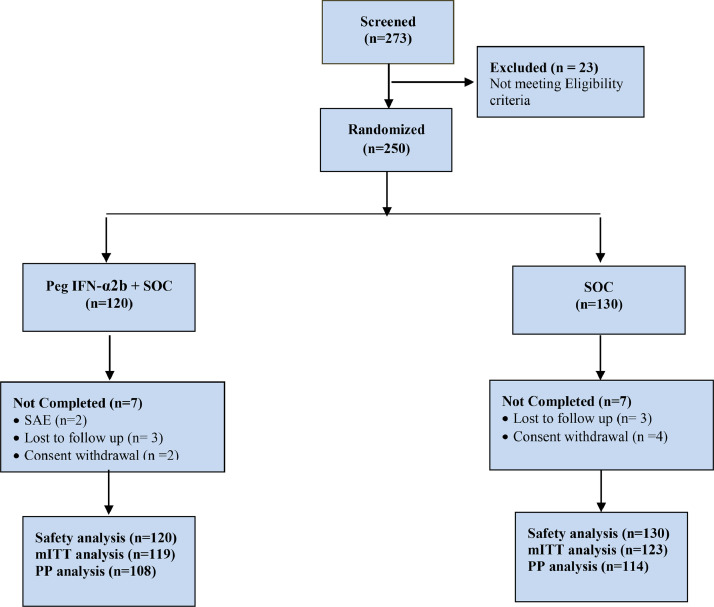
In total, 273 subjects were screened; of these, 23 subjects were not enrolled into the study, and 250 subjects were administered the study medication as per randomization (120 subjects were administered the test medication and 130 subjects were administered the reference medication).
Of the 250 subjects, 14 subjects were discontinued from the study: seven subjects from each of the treatment arms. The reasons for discontinuation from the study were as follows: serious AE (two subjects), lost to follow-up (six subjects), and consent withdrawn (six subjects).
Of the 250 subjects randomized, 242 (96.80%) subjects comprised the modified intent-to-treat (mITT) population and 250 (100.00%) subjects comprised the safety population. One subject was not administered the reference medication.
Of the 250 subjects, 177 (70.80%) were male and 73 (29.20%) were female. The mean age was 49.60 (SD 14.98) years in the PEG IFN-α2b + SOC group and 50.14 (SD 15.61) years in the SOC group. Overall, demographic characteristics of the study subjects were comparable across the treatment groups (Table 1 ). Figure 1 shows the study flow chart.
Table 1.
Summary of demographic characteristics (safety population).
| Pegylated IFN-α2b + SOC (N=120) |
SOC (N=130) |
Overall (N=250) |
P-valuea |
|
|---|---|---|---|---|
| Age (years) | ||||
| Mean ± SD | 49.60 ± 14.98 | 50.14 ± 15.61 | 49.88 ± 15.28 | 0.7814 |
| Range | 20–80 | 20–88 | 20–88 | |
| Gender, n (%) | ||||
| Female | 33 (27.50) | 40 (30.77) | 73 (29.20) | 0.5701 |
| Male | 87 (72.50) | 90 (69.23) | 177 (70.80) | |
| Weight (kg) | ||||
| Mean ± SD | 68.56 ± 12.82 | 68.55 ± 12.27 | 68.55 ± 12.51 | 0.9938 |
| Range | 40–118 | 40–98 | 40–118 | |
| Height (cm) | ||||
| Mean ± SD | 163.60 ± 10.16 | 163.88 ± 9.35 | 163.75 ± 9.73 | 0.8213 |
| Range | 127–193 | 145–189 | 127–193 | |
| BMI (kg/m2) | ||||
| Mean ± SD | 25.70 ± 4.89 | 25.56 ± 4.54 | 25.63 ± 4.70 | 0.8164 |
| Range | 14.70–48.50 | 16.60–39.80 | 14.70–48.50 |
BMI, body mass index; SD, standard deviation; SOC, standard of care; N, number of subjects on specified treatment; n, number of subjects with non-missing values.
aP-values for categorical variables were calculated using Chi-squared test, P-values for continuous variables were calculated by analysis of variance.
Figure 1.
Study flow chart. mITT, modified intent-to-treat; PEG IFN-α2b, pegylated interferon alpha-2b; PP, per protocol; SOC, standard of care.
Primary endpoint
The primary outcome was to evaluate the clinical efficacy of PEG IFN-α2b based on changes in the ordinal scale (two-point improvement in the WHO seven-point ordinal scale) at Day 11.
In the mITT population, 90 (80.36%) and 75 (68.18%) patients had achieved clinical improvement in the PEG IFN-α2b + SOC group and the SOC group, respectively, on Day 8. There was a significant difference in clinical improvement in the PEG IFN-α2b + SOC group compared with the SOC group from Day 0 to Day 8 (P<0.05). On Day 11, 109 (91.60%) and 112 (92.56%) patients had achieved clinical improvement in the PEG IFN-α2b + SOC group and the SOC group, respectively. On Day 15, 112 (94.12%) and 118 (95.93%) patients had achieved clinical improvement in the PEG IFN-α2b + SOC group and the SOC group, respectively (Table 2 ). The per-protocol (PP) population is presented in Table S1 (see online supplementary material).
Table 2.
Analysis of proportion of subjects with clinical improvement (clinical status) from Day 0 to Day 8, Day 11 and Day 15, measured using the World Health Organization seven-point ordinal scale (modified intent-to-treat population).
| Visit | Improvement | Pegylated IFN-α2b + SOC (N=119) n (%) |
SOC (N=123) n (%) |
P-value | Risk difference (%) |
95% CI |
|---|---|---|---|---|---|---|
| Day 8 | n=112 | n=110 | ||||
| Yes | 90 (80.36) | 75 (68.18) | 0.0379 | 12.18 | (0.46 to 23.74) | |
| No | 22 (19.64) | 35 (31.82) | ||||
| Day 11 | n=119 | n=121 | ||||
| Yes | 109 (91.60) | 112 (92.56) | 0.7818 | -0.97 | (-8.38 to 6.42) | |
| No | 10 (8.40) | 9 (7.44) | ||||
| Day 15 | n=119 | n=123 | ||||
| Yes | 112 (94.12) | 118 (95.93) | 0.5150 | -1.82 | (-8.16 to 4.14) | |
| No | 7 (5.88) | 5 (4.07) |
IFN, interferon; SOC, standard of care; N, number of subjects on treatment; n, number of subjects with available data for treatment; CI, confidence interval.
Chi-squared test was used to calculate P-values, and 95% CI was calculated using risk difference.
Risk difference is defined as the difference [(pegylated IFN-α2b + SOC) - SOC)].
P<0.05 was considered to indicate statistical significance.
Subjects in the PEG IFN-α2b + SOC group achieved a greater reduction in the mean score (measured by seven-point ordinal scale) from baseline to Day 15 compared with the subjects in the SOC group. The mean change in score from baseline to Day 15 was -2.34 (SD 0.90) and -2.24 (SD 0.69) in the PEG IFN-α2b + SOC group and SOC group, respectively.
Secondary endpoints
Of the 119 subjects in the PEG IFN-α2b + SOC group, 103 (91.15%), 114 (97.44%) and 116 (98.31%) had a negative RT-PCR result at Day 7, Day 11 and Day 14, respectively (Table 3 ). Of the 123 subjects in the SOC group, 86 (78.90%), 113 (96.58%) and 119 (98.35%) had a negative RT-PCR result at Day 7, Day 11 and Day 14, respectively. There was a significant difference between the groups on Day 7, indicating that PEG IFN clears the viral load early (P<0.05). The per-protocol (PP) population is presented in Table S2 (see online supplementary material).
Table 3.
Analysis of proportion of patients with negative qualitative polymerase chain reaction for severe acute respiratory syndrome coronavirus-2 on pharyngeal swab from Day 0 to Day 7, Day 11 and Day 15 (modified intent-to-treat population).
| Visit | Result | Pegylated IFN-α2b + SOC (N=119) n (%) |
SOC (N=123) n (%) |
P-value |
|---|---|---|---|---|
| Day 7 | Negative | 103 (91.15) | 86 (78.90) | 0.0103 |
| Positive | 10 (8.85) | 23 (21.10) | ||
| Day 11 | Negative | 114 (97.44) | 113 (96.58) | 1.0000 |
| Positive | 3 (2.56) | 4 (3.42) | ||
| Day 15 | Negative | 116 (98.31) | 119 (98.35) | 1.0000 |
| Positive | 2 (1.69) | 2 (1.65) |
IFN, interferon; SOC, standard of care; N, number of subjects on treatment; n, number of subjects on treatment by available data for improvement.
P-values were calculated using Fisher's exact test for Day 7, and Chi-square test for Day 11 and Day 15.
P<0.05 was considered to indicate statistical significance.
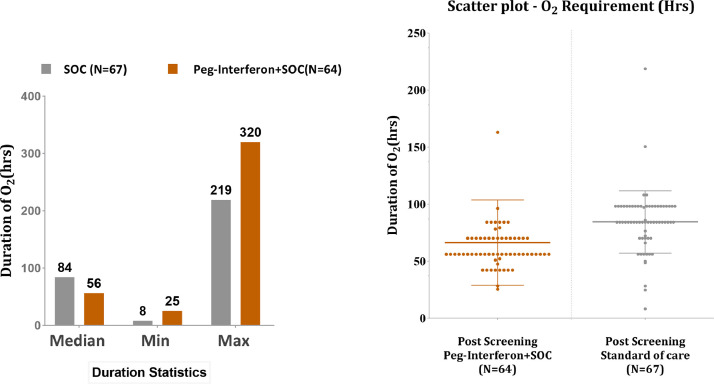
In the mITT population, 131 of the 242 subjects required oxygen support: 64 (61.54%) subjects in the PEG IFN-α2b + SOC group and 67 (59.29%) subjects in the SOC group. Subjects in the PEG IFN-α2b + SOC group had a shorter duration of supplemental oxygen than subjects in the SOC group (Figure 2 ) (median 56 h vs 84 h; P<0.05). The PP population is presented in Table S3 (see online supplementary material).
Figure 2.
Occurrence and duration of supplemental oxygen.
Of the 242 subjects, two (1.69%) subjects in the PEG IFN-α2b + SOC group and one (0.81%) subject in the SOC group required mechanical ventilation. There was no significant difference in requirement for mechanical ventilation between the two groups in this study. The population is presented in Table S4 (see online supplementary material).
Subjects in the PEG IFN-α2b + SOC group had a shorter duration to resolution of signs and symptoms than subjects in the SOC group (median 5 days vs 6 days; P<0.05). The PP population is presented in Table S5 (see online supplementary material).
The duration of hospitalization was similar in both the treatment groups for the mITT population (median 9 days; P˃0.05). Similar results were obtained in the PP population (Table S6, see online supplementary material).
Serial laboratory measurements of blood levels for CRP, IL-6, D-dimer and ferritin were also conducted. There was a decrease in both groups, but no significant differences were found between the treatment groups for any of these parameters during the study.
Post-hoc analysis
In the analysis of patients with a two-point improvement in the WHO seven-point ordinal scale at Day 8 for the subgroups of patients ‘with and without remdesivir’, the following results were observed. For the ‘with remdesivir’ analysis, the improvement was seen in 15 patients (60%) in the PEG IFN-α2b + SOC + remdesivir group (n=25) and 18 patients (66.67%) in the SOC + remdesivir group (n=27) (P=0.617). For the ‘without remdesivir’ analysis, the improvement was seen in 75 patients (86.21%) in the PEG IFN-α2b + SOC group (n=87) and 57 patients (68.67%) in the SOC group (n=83) (P=0.0061) (Tables S7 and S8, see online supplementary material).
In the analysis of patients with a two-point improvement in the WHO seven-point ordinal scale at Day 8 for the subgroups of patients ‘with and without steroids’, the following results were observed. For the ‘with steroids’ analysis, the improvement was seen in 61 patients (85.92%) in the PEG IFN-α2b + SOC + steroids group (n=71) and 50 patients (67.57%) in the SOC + steroids group (n=74) (P=0.0091). For the ‘without steroids’ analysis, the improvement was seen in 29 patients (70.73%) in the PEG IFN-α2b + SOC (n=41) group and 25 patients (69.44%) in the SOC group (n=36) (P=0.902) (Tables S9 and S10).
Safety
Of the 250 subjects, 21 (8.47%) subjects had at least one treatment emergent adverse event (TEAE) during the treatment period: eight (6.67%) subjects in the PEG IFN-α2b + SOC group and 13 (10.00%) subjects in the SOC group (Table 4 ). None of the subjects were discontinued from the study due to AEs in any of the treatment groups. All AEs were followed up until the subject was ‘recovered’ or ‘recovered with sequelae’, or until the end of post-treatment follow-up, whichever came first. Two deaths were reported in the test arm during the study. Of these, one subject had sudden cardiopulmonary arrest, but the event was not related to the study drug (declared by investigator); and one subject had COVID-19, which was not related to the study drug (declared by sponsor and investigator).
Table 4.
Summary of adverse events (safety population).
| Preferred term | Pegylated IFN-α2b + SOC (N=120) n (%) |
SOC (N=130) n (%) |
Overall (N=250) n (%) |
|---|---|---|---|
| Number of subjects with at least one treatment emergent adverse event | 8 (6.67) | 13 (10.00) | 21 (8.40) |
| Chest pain | 1 (0.77) | 1 (0.83) | 2 (0.80) |
| Constipation | 0 (0.00) | 2 (1.67) | 2 (0.80) |
| Diarrhoea | 0 (0.00) | 1 (0.83) | 1 (0.40) |
| Gastritis | 0 (0.00) | 1 (0.83) | 1 (0.40) |
| Nausea | 0 (0.00) | 1 (0.83) | 1 (0.40) |
| Asthenia | 1 (0.77) | 1 (0.83) | 2 (0.80) |
| Back pain | 0 (0.00) | 1 (0.83) | 1 (0.40) |
| Myalgia | 1 (0.77) | 0 (0.00) | 1 (0.40) |
| Headache | 3 (2.31) | 0 (0.00) | 3 (1.20) |
| Cough | 0 (0.00) | 1 (0.83) | 1 (0.40) |
| Respiratory distress | 1 (0.77) | 2 (1.67) | 3 (1.20) |
| Pruritus | 1 (0.77) | 1 (0.83) | 2 (0.80) |
IFN, interferon; SOC, standard of care; N, number of subjects on treatment; n, number of subjects in specified category.
%=(n/number of subjects in safety population for whom specific safety endpoint data is available)*100.
If a subject had multiple occurrences of an adverse event, the subject is only included once for the corresponding adverse event.
The reported preferred terms (PTs) of TEAEs in the PEG IFN-α2b + SOC group were: chest pain, 1 (0.77%); asthenia, 1 (0.77%); myalgia, 1 (0.77%); headache, 3 (2.31%); respiratory distress, 1 (0.77%); and pruritus, 1 (0.77%). In the SOC group, the reported PTs of TEAEs were: chest pain, 1 (0.83%); constipation, 2 (1.67%); diarrhoea, 1 (0.83%); gastritis, 1 (0.83%); nausea, 1 (0.83%); asthenia, 1 (0.83%); back pain, 1 (0.83%); cough, 1 (0.83%); respiratory distress, 2 (1.67%); and pruritus, 1 (0.83%).
Two cases of death were reported in the test arm, both due to the progression of the underlying COVID-19 infection. The causality assessment performed by the study investigators and evaluated by the ECs and the Data Safty Monitoring Board (DSMB) found both deaths to be unrelated to the study drug.
No apparent difference was observed in any of the biomarkers and laboratory parameters between the treatment groups. No clinically relevant findings from clinical examination, vital signs and electrocardiogram evaluations were attributed to PEG IFN-α2b. Overall, a single dose of PEG IFN-α2b was found to be safe and well tolerated in this study.
Discussion
IFN-α2b is a type I IFN, known to be poorly expressed after infection with SARS-CoV-2. The use of IFN-α2b through intranasal and subcutaneous delivery routes has been studied in the treatment of COVID-19, and has been shown to help in the alleviation of disease. On the basis of these observations, this study investigated the use of a long-acting form of IFN-α2b, PEG IFN-α2b, for the treatment of patients with moderate COVID-19.
In this study, patients with moderate COVID-19 who received a single dose of PEG IFN along with SOC showed a significant (P=0.0379) two-point improvement on the WHO seven-point ordinal scale on Day 8 compared with patients who received SOC alone. This difference between the two treatment arms was not significant on Days 11 and 14. The significant difference on Day 8 in the ordinal scale correlated very well with the significant difference (P=0.01) in the qualitative RT-PCR data for the two groups on Day 7.
Type I IFNs such as IFN-α2b are produced as a first response to most viral infections by affected cells. In contrast to other respiratory viruses, SARS-CoV-2 infection does not seem to produce a strong early type I IFN response (Blanco-Melo et al., 2020), and therefore, intervention with PEG IFN may have filled this gap in the early response to COVID infection, leading to a reduction in viral load, followed by a clinical improvement on the WHO ordinal scale as well as the time to resolution of clinical signs and symptoms. In this study, patients who received PEG IFN showed a significantly reduced requirement for supplemental oxygen, as well as earlier resolution of signs and symptoms, compared with patients who did not receive PEG IFN. The early reduction in viral load mediated by PEG IFN may have helped the lungs to perform their function better due to a reduced level of lung infection, although this was not studied directly in this clinical trial. Nevertheless, the phenomenon appeared real, as a similar observation was also made in the authors’ phase 2 clinical trial involving a much smaller group of patients with moderate COVID-19 (Pandit et al., 2021).
The lack of difference between the two groups in terms of duration of hospitalization may have been influenced by the discharge decisions of the various hospitals during the pandemic, being dependent upon a variety of reasons other than the overall health status of the patient.
Biomarkers are important parameters to evaluate the safety of a product and predict the severity/progression of disease. In this study, no apparent difference was observed for any of the biomarkers (CRP, IL-6, D-dimer and ferritin) or laboratory parameters between the treatment groups. During the study, safety evaluations were performed using different parameters such as clinical examination, vital signs, electrocardiogram and monitoring AEs. No significance difference was found between the treatment arms.
In accordance with the results of the WHO Solidarity Trial, drugs such as hydroxychloroquine, remdesivir, lopinavir and IFN-β did not show an improvement in hospitalized patients with COVID-19 when measured by mortality, initiation of ventilation, and duration of hospital stay (World Health Organization Solidarity Trial Consortium, 2021).
Given that the patients in this study had moderate COVID-19, most of them eventually became virus free. The significant difference in RT-PCR negativity observed at the early time point (Day 7) clearly demonstrates that treatment with PEG IFN-α2b benefited the patients. The lack of significant difference at a later time point (Day 11) suggests that the majority of patients with moderate COVID-19 eventually become virus free. Therefore, the benefit of PEG IFN-α2b therapy is in early cure of patients with moderate COVID-19, thereby minimizing their risk of severe disease.
Subgroup analysis showed that all differences between the test and reference arms were improved more significantly in the patients who also received steroids. Steroids are the most important class of drugs for the treatment of COVID-19. Steroids are known to suppress the expression of type I IFNs (Singanayagam et al., 2018). Combining PEG IFN with steroids likely eliminated this defect by supplementing this important cytokine, leading to a highly significant improvement in RT-PCR negativity, change on the WHO ordinal scale, duration of supplemental oxygenation, as well as time to resolution of clinical signs and symptoms in comparison with those patients who received steroids but not PEG IFN. These observations echo the recent observations from a retrospective analysis of hospitalized patients where those given early IFN-α therapy along with glucocorticoids showed a significant improvement in days of hospitalization, clinical symptoms and virus shedding (Lu et al., 2021).
COVID-19 is a complex disease that involves an interplay of host factors; inflammatory chemokines, cytokines, complement and various other factors have been implicated in the disease pathology, triggered by the event of viral infection and subsequent damage of infected cells (Perico et al., 2021). Therefore, apart from potentially insufficient early production of type I IFN in patients, an interplay of one or more of the above factors may play a more dominant role in the disease pathology in some patients. Beyond the observation of significant differences on Day 8, these other factors may have been responsible for the lack of difference in clinical status on Days 11 and 15, despite the fact that almost all (96.58–98.35%) of the study subjects were RT-PCR negative by this time.
Limitations of the study
This study did not use an interactive voice response system or an interactive web response system for randomization, and this led, expectedly, to an imbalance in the numbers randomized because of competitive recruitment at the study sites. Also, baseline stratification was not performed for co-morbid conditions. With respect to the quantitative RT-PCR data, there was no uniformity in reporting computed tomography values between the different pathology laboratories, and a few laboratories only reported qualitative data. As such, it was not possible to perform a meaningful analysis for the quantitative RT-PCR data. Regarding requirement for oxygen supplementation, as per the protocol, patients with moderate COVID-19 were enrolled in the study and, as per the standard COVID-19 treatment guidelines, were supposed to receive oxygen. However, individual sites followed their own institutional practices, and gave oxygen based upon the clinical judgement of the investigators as well as changes in the SpO2 level (some subjects were on oxygen support at the time of enrolment). The subgroup analysis performed in this study was a post-hoc analysis and was not defined in the study protocol. SOC was not uniform for all centres in terms of investigator discretion and different signs and symptoms of patients, although centre-wise SOC was uniform for test and reference subjects. Subjects were on multiple treatments (SOC), and hence some AEs may have been masked by these treatments.
Conclusions
Early treatment with PEG IFN-α2b induced early viral clearance and improved the clinical status of patients with moderate COVID-19. It also decreased the duration of supplemental oxygen. Treatment with PEG IFN-α2b provides a viable treatment option during the current pandemic situation. It can also limit the spread of SARS-CoV-2 in the community.
Funding
This trial was sponsored and funded by Cadila Healthcare Ltd., Ahmedabad, India. The authors would like to acknowledge Biotechnology Industry Research Assistance Council for providing a grant for the clinical development programme of PEG IFN-α2b (Proposal Reference Number: [BT/COVID0075/02/20).
Ethical approval
Written informed consent was obtained from all participants at the time of screening. This trial was initiated after obtaining approval from the ECs and DCGI, and registering the trial with CTRI. This trial was conducted in accordance with the applicable local regulations.
Author contributions
All authors were involved in conceptualization of the study, data interpretation, manuscript writing and manuscript review. Shashank Joshi, Kevinkumar Kansagra and Jatin Patel were involved in conceptualization of the study, data interpretation and manuscript writing. Manjunath Krishnappa and Richa Vellanki were involved in conducting the study, and Manjunath Krishnappa was also involved in data interpretation. Sunil Sharma and Jay Bhavsar were involved in statistical analysis; designing, programming and generating TLFs (Tables, Listings and Figures); and aided in interpretation of the results. Purav Trivedi, Anurag Parihar and Vishal Nakrani provided operational support. Shashi Bhushan, Sunil Wanve, Parshottam Koradia, Vinay Bhomia, Pravin Soni, Sisir Chakraborty, Akash Khobragade, Manoj Kumar Singh, Mahamine Kaustubh Suryakant, Nischal Yalgi, Nikhil Verma, Divyang Dalwadi, Nirav Bhalani, Sandeep Kumar Gupta and Archana Andhavarapu were study investigators. Each author contributed important intellectual content during manuscript drafting or revision, and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. All authors approved the final version of the manuscript for submission [ZRC communication number: 664]
Conflict of interest statement
Shashank Joshi has been a speaker for Abbott, Alkem, AstraZeneca, Micro, Boeringher Ingelheim, Novo Nordisk, MSD, Sanofi, PHFI, Lupin, Eli Lilly, Bayer Zydus, Zydus Cadila and DRL; and part of an advisory board for Abbott, Glenmark, Biocon, Zydus Cadila, Bayer Zydus Twin Health, Marico, Franco India, Cipla, Sun, Torrent and USV. Sanjeev Kumar Mendiratta, Kevinkumar Kansagra, Anurag Parihar, Sunil Sharma and Jatin Patel are employees of Zydus Research Centre, Cadila Healthcare Limited, Ahmedabad, India. The remaining authors report no competing interests with respect to the research, authorship and/or publication of this review.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2021.08.044.
Appendix. Supplementary materials
References
- Aoki FY, Macleod MD, Paggiaro P, Carewicz O, El Sawy A, Wat C, et al. Early administration of oral oseltamivir increases the benefits of influenza treatment. J Antimicrob Chemother. 2003;51:123–129. doi: 10.1093/jac/dkg007. [DOI] [PubMed] [Google Scholar]
- Bastard P, Rosen L, Zhang Q, Michailidis E, Hoffmann H, Zhang Y, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370:eabd4585. doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of COVID-19 – final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Melo D, Nilsson-Payant B, Liu W, Uhl S, Hoagland D, Moller R, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Zhang B, MN T, Yang K, Zou Y, Zhanga S. Clinical course of severe and critically ill patients with coronavirus disease 2019 (COVID-19): a comparative study. J Inf Secur. 2021;81:e82–e84. doi: 10.1016/j.jinf.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Nirula A, Heller B, Gottlieb R, Boscia J, Morris J, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2021;384:229–237. doi: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortinovis M, Perico N, Remuzzi G. Long-term follow-up of recovered patients with COVID-19. Lancet. 2021;397:173–175. doi: 10.1016/S0140-6736(21)00039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91:157. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in hospitalized patients with COVID19 preliminary report. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear HM, Schoggins JW, Diamond MS. Shared and distinct functions of type I and type III interferons. Immunity. 2019;50:907–923. doi: 10.1016/j.immuni.2019.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokugamage KG, Hage A, de Vries M, Valero-Jimenez AM, Schindewolf C, Dittmann M, et al. Type I interferon susceptibility distinguishes SARS-CoV-2 from SARS-CoV. J Virol. 2020;94:e01410. doi: 10.1128/JVI.01410-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Liu F, Tong G, Qiu F, Song P, Wang X, et al. Clinical evidence of an interferon–glucocorticoid therapeutic synergy in COVID-19. Sig Transduct Target Ther. 2021;6:107. doi: 10.1038/s41392-021-00496-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantlo E, Bukreyeva N, Maruyama J, Paessler S, Huang C. Antiviral activities of type I interferons to SARS-CoV-2 infection. Antiviral Res. 2020;179 doi: 10.1016/j.antiviral.2020.104811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit A, Bhalani N, Bhushan BS, Koradia P, Gargiya S, Bhomia V, et al. Efficacy and safety of pegylated interferon alfa-2b in moderate COVID-19: a phase II, randomized, controlled, open-label study. Int J Infect Dis. 2021;105:516–521. doi: 10.1016/j.ijid.2021.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perico L, Benigni A, Casiraghi F, Ng LF, Renia L, Remuzzi G. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat Rev Nephrol. 2021;17:46–64. doi: 10.1038/s41581-020-00357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singanayagam A, Glanville N, Girkin JL, Ching YM, Marcellini A, Porter JD, et al. Corticosteroid suppression of antiviral immunity increases bacterial loads and mucus production in COPD exacerbations. Nat Commun. 2018;9:1–6. doi: 10.1038/s41467-018-04574-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli A, Pellino G. COVID-19 pandemic: perspectives on an unfolding crisis. J Br Surg. 2020;107:785–787. doi: 10.1002/bjs.11627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- tenOever BR. The evolution of antiviral defense systems. Cell Host Microbe. 2016;19:142. doi: 10.1016/j.chom.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Wang N, Zhan Y, Zhu L, Hou Z, Liu F, Song P, et al. Retrospective multicenter cohort study shows early interferon therapy is associated with favorable clinical responses in COVID-19 patients. Cell Host Microbe. 2020;28:455–464. doi: 10.1016/j.chom.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . WHO; Geneva: 2021. WHO coronavirus disease (COVID-19) dashboard. [Google Scholar]
- World Health Organization Solidarity Trial Consortium . WHO; Geneva: 2021. Solidarity clinical trial for COVID-19 treatments. [Google Scholar]
- Zhang Q, Bastard P, Liu Z, Pen JL, Velez MM, Chen J, et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370:eabd4570. doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, et al. Risk factors of critical and mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect Dev Ctries. 2020;81:e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Chen V, Shannon C, Wei XS, Xiang X, Wang X, et al. Interferon-α2b treatment for COVID-19. Front Immunol. 2020;11:1061. doi: 10.3389/fimmu.2020.01061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.