Abstract
The lethality of estrogen receptor alpha positive (ER+) breast cancer, which is often considered to have better prognosis than other subtypes, is defined by resistance to the standard of care endocrine treatment. Relapse and metastasis are inevitable in almost every patient whose cancer is resistant to endocrine treatment. Therefore, understanding the underlying causes of treatment resistance remains an important biological and clinical focus of research in this area. Growth factor receptor pathway activation, specifically HER2 activation, has been identified as 1 mechanism of endocrine treatment resistance across a range of experimental model systems. However, clinical trials conducted to test whether targeting HER2 benefits patients with endocrine treatment–resistant ER+ breast cancer have consistently and disappointingly shown mixed results. One reason for the failure of these clinical trials could be the complexity of crosstalk between ER, HER2, and other growth factor receptors and the fluidity of HER2 activation in these cells, which makes it challenging to identify stratifiers for this targeted intervention. In the absence of stratifiers that can be assayed at diagnosis to allow prospective tailoring of HER2 inhibition to the right patients, clinical trials will continue to disappoint. To understand stratifiers, it is important that the field invests in key understudied areas of research including characterization of the tumor secretome and receptor activation in response to endocrine treatment, and mapping the ER–HER2 growth factor network in the normal and developing mammary gland. Understanding these mechanisms further is critical to improving outcomes for the hard-to-treat endocrine treatment–resistant ER+ breast cancer cohort.
Keywords: Breast cancer, receptor crosstalk, HER2 activation, DNA damage
Estrogen receptor alpha positive (ER+)/HER2-negative (HER2–, ie, without amplification or mutation of HER2) breast cancer is 1 of the most frequently diagnosed cancers in women worldwide with a new diagnosis made every 19 seconds (1). Inhibiting the function of ER through endocrine treatment remains the standard of care for ER+/HER2– breast cancer patients (2). While most ER+/HER2– breast cancer patients respond to endocrine treatment, both intrinsic (occurring in ~15% of patients) and acquired (evolving in ~40% of all patients) resistance can occur resulting in relapse, metastasis, and death (3, 4). Preclinical and clinical evidence indicate that the dysregulation of ER signaling is a major mechanism of endocrine treatment resistance (5–8). However, recent studies have shown that the mechanism underlying endocrine treatment resistance in ~50% of patients with advanced ER+ breast cancer remains unknown (4).
Activation of HER2 is well supported by preclinical data as a mechanism underlying endocrine treatment resistance in ER+ breast cancer. HER2 is a member of the HER family of mitogenic receptors, comprising ErbB1/HER1/EGFR, ErbB2/HER2, ErbB3/HER3, and ErbB4/HER4. Both amplification and mutation of HER2 have been causally implicated in endocrine treatment resistance, and treating patients with such tumors with HER2 or pan-HER inhibitors has greatly improved survival (9, 10). However, in the majority of cases, where ER+ tumors are HER2–, ie, they do not exhibit HER2 amplification or mutation, there is little detectable clinical benefit to treating patients with HER inhibitors (11–14). This is surprising given the large body of preclinical literature attesting to a role for HER2 signaling as a mechanism to escape endocrine treatment in ER+/HER2– breast cancer cells (15–19). This lack of translation between findings in the laboratory and results from clinical trials suggests that the mechanisms by which HER2 can be activated in ER+/HER2– breast cancer, which largely appear to be catalyzed by exposure to endocrine treatment, need to be more carefully parsed in order to design biologically informed clinical trials. It is likely that understanding these mechanisms will reveal HER2 activation as a key driver in a higher proportion of endocrine treatment resistant ER+/HER2– breast cancer than previously thought.
In this review, we discuss genomic and nongenomic mechanisms by which HER2 can be activated in ER+ breast cancer cells, identify areas requiring further investigation, and outline the challenges to identifying predictive biomarkers that can stratify the subset of patients who are most likely to activate this escape mechanism in response to endocrine treatment.
Mechanisms of HER2 Activation in ER+/HER2– Breast Cancer
Genomic Activation of HER2
Receptor tyrosine kinases constitute a class of receptors expressed on the cell membrane that, in response to environmental cues, initiates a mitogenic signaling cascade in both normal and malignant cells. Ligands, such as epidermal growth factor (EGF), amphiregulin, betacellulin, heregulin (HRG), bind to these receptors and induce homo- or heterodimerization and autophosphorylation which activate downstream signaling through MAPK, PI3K/Akt, and JAK/STAT pathway (20, 21). HER2 (ERBB2) is 1 of the best studied of these receptors in breast cancer as it is amplified in 20% to 30% of ER+ breast tumors and is known to be an alternate mechanism for these cancer cells to survive endocrine treatment (9). The discovery of HER2 amplified ER+ breast cancer as a distinct subset by Slamon and colleagues and the subsequent identification of an antibody that could target amplified HER2 in tumors from this subset of patients (22, 23) was a turning point in the characterization of breast cancer and the treatment of breast cancer patients (9). These discoveries transformed the HER2 amplified subset of ER+ breast cancer from one with strikingly poor survival outcomes to one of the more curable forms of breast cancer in a relatively short period of time (9, 24).
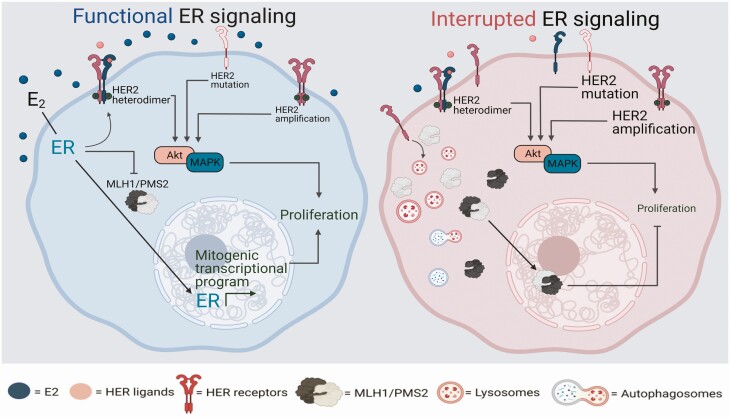
With these discoveries, the search for alternate mechanisms of HER2 activation in ER+ breast cancer gained intensity. This led to the discovery of a less common method of genomic activation of HER2 through the gain of somatic, activating mutations in the HER2 gene (10, 25). Whole genome sequencing data from 8 studies conclusively demonstrated the incidence of somatic mutations in HER2 in HER2 gene amplification-negative primary ER+ breast cancer (10). Seven out of 13 activating HER2 mutations G309A, D769H, D769Y, V777L, P780ins, V842I, and R896C were identified as recurrent (10). All of these mutations induce aggressive growth, resistance to endocrine treatment, and metastasis in ER+ breast cancer cells but induce sensitivity to specific HER2 inhibitors (10, 26–28). Additionally, metastatic ER+/HER2– breast cancer cells appear to acquire HER2 mutations in response to endocrine treatment, which then renders them endocrine treatment resistant (26, 29, 30). However, because HER2 mutations appear to be a rare event, occurring in 2% to 4% of patients with ER+/HER2– breast cancer, its potential as a predictive biomarker for more effective targeted therapy is limited (10, 26, 30, 31). Data from the BASKET trial testing the efficacy of HER inhibitors in HER2 and HER3 mutated cancers show a wide range of response to HER inhibitors, which indicates that additional research is required in this field to fully understand the potential of these mutations to serve as predictive biomarkers (29). HER2 activation can also be nongenomic, which may be more frequently invoked in ER+/HER2– breast cancer cells (Fig. 1). These mechanisms are discussed below.
Figure 1.
HER2 activation as an escape mechanism in response to endocrine therapy. Under estrogen-rich (E2) conditions, estrogen receptor (ER) signaling in ER+/HER2– breast cancer cells is the principal regulator of mitogenic signaling. In contrast, in ER+ breast cancer cells with HER2 amplification or mutation, ER and HER2 coordinately regulate mitogenic stimuli, thereby requiring simultaneous inhibition of both to completely control cell growth. An additional layer of complexity involves nongenomic ER, which can also cross-activate HER2 signaling in both HER2 wildtype and amplified conditions. In ER+/HER2– breast cancer cells in E2-depleted or ER-inhibited conditions (as during the course of endocrine treatment), if HER2 receptor activation occurs, it takes on the principal mitogenic role, thereby rendering the cells resistant to endocrine treatment. This activation is fluid and can occur through several mechanisms: (1) ligand binding to other HER family receptors which in turn heterodimerize with HER2 to activate the downstream signaling cascade mediated by PI3K Akt or MAPK signaling, and (2) protection from protein trafficking that can be induced by specific pathways, eg, defects in mismatch repair genes, MLH1 and PMS2. Other pathways regulating the mitogenic switch between ER and HER2 in ER+/HER2- breast cancer cells remain to be discovered and will likely constitute novel mechanisms causal to endocrine treatment resistance.
HER2 Activation Through Crosstalk With Nongenomic ER Signaling
ER has nuclear (genomic) and non-nuclear (nongenomic) functions (32). While its genomic functions are well studied and its transcriptional role in promoting mitogenic signaling is well understood, its nongenomic functions are less studied. Schiff and colleagues have shown that ligand-bound ER persists in the cytoplasm at low levels, where it directly activates mitogenic signaling from receptor tyrosine kinases including the HER family (Fig. 1) (17, 33, 34). This role for nongenomic ER can be initiated by binding with either estrogen or tamoxifen, a commonly used endocrine treatment in premenopausal ER+ breast cancer patients (33, 34). This cross-activation is amplified in ER+ breast cancer cells with higher RNA and protein levels of HER family members (33, 35–37). This avenue of research remains understudied especially in the HER2– subset of ER+ breast cancer, where it could potentially have the most impact in identifying predictive biomarkers for HER2 activation in response to endocrine treatment.
Evolutionarily, ER+ luminal cells in the normal mammary gland are programmed to see-saw between ER and growth factor-mediated stimuli to induce proliferation and invasion into the fat pad. Cyclical exposure to estrogen and progesterone to induce active proliferation during the estrus cycle and pregnancy/lactation is a constant through most of a woman’s lifetime. This creates a unique requirement for breast cells to be able to switch between various mitogenic and apoptotic stimuli. At key developmental stages, an interplay between ER and HER signaling is notable. Ductal invasion and establishment of the adult mammary gland during puberty is orchestrated through a fine balance of growth factors and hormones, and ER, progesterone receptor (PR, a key downstream effector of ER), HER2, and HER3 all appear essential for these processes (38–42). Studies in animal models during pregnancy and lactation also show a pattern whereby HER2/4 activation and ER activation are mutually exclusive both spatially and temporally, supporting the notion that ER+ mammary cells balance ER and HER signaling to manage mitogenesis and cell survival (43–45). In keeping with these findings, overexpression of HER2 in the mammary glands of transgenic mice inhibits ER-α–associated ductal invasion during puberty (38, 46, 47). Conversely, knocking out ER-α in HER2-overexpressing transgenic mice delays the onset of tumors although it does not inhibit tumorigenesis indicating the complex synergy between these pathways (38). Further, overexpressing HER2 renders normal mammary cells independent of growth factor stimuli, including ligands that activate the HER receptors (48). These data demonstrate the significant differences between HER2-amplified and nonamplified ER+ cells and indicate a delicate balance in the latter between various hormone and growth factor–driven mitogenic stimuli.
It is important to note that PR is a key mediator of ER signaling both in normal and malignant breast cells, and many studies have demonstrated that it is also a key mediator of nongenomic crosstalk between ER and HER receptors. Edwards and colleagues have provided abundant evidence for a role for PR bound to ER in activating the c-Src/ERK pathway which lies downstream of HER family signaling (49, 50). Seminal work from the Horwitz group showed that treating breast cancer cells with progestins upregulates HER family receptors including EGFR, HER2, and HER3 (51-53). There is also intriguing data suggesting that ligands of the HER receptors can co-opt PR signaling to drive breast cancer growth (54). While these earlier studies focused largely on EGFR and PR signaling, more recent investigations have clearly demonstrated that PR can mediate the ER–HER2 crosstalk and activation that is observed in breast cancer cells (55–57). Overall, the crosstalk between ER, PR, and HER receptors is complicated and certainly maintained in both normal and malignant breast cells. Understanding these mechanisms in the context of the normal mammary gland will shed insight into the mechanisms of ER+/HER2– breast cancer growth and treatment response.
Activation of HER2 Through Other Growth Factor Receptors
Of the 4 members of the HER family of receptor tyrosine kinases, HER2 is the only one for which no known ligands exist. Instead, HER2 is activated by heterodimerization with its liganded family members in normal mammary cells, serving as the preferred partner for other HER family members, and especially for HER3, which lacks a tyrosine activation domain (58, 59). This heterodimerization and activation persists in breast cancer cells and is amplified as the number of HER2 molecules at the cell surface is significantly increased in cancer cells. Of note, HER2 homodimerization also occurs when HER2 is amplified or overexpressed. The patterns of HER2 heterodimerization are dictated either by the ligand composition in the cellular microenvironment or by the abundance of specific HER family receptors in the cytoplasm and cell membrane (20, 21). Heterodimerization of HER2 with EGFR or HER3 has been most commonly associated with worse outcomes in ER+ breast cancer patients, and has been shown to causally induce aggressive growth and treatment resistance in preclinical studies (58, 60–62). Using a functional protein pathway activation mapping strategy, along with targeted genomic knockdowns applied to a series of isogenic-matched pairs of lapatinib (HER2 inhibitor) sensitive and resistant cell lines, Xia et al. reported a unique mechanism of acquired resistance to HER2 inhibitors (63). Their study showed that although HER2 was inhibited in lapatinib resistant cells, EGFR tyrosine phosphorylation was not completely inhibited. Knockdown studies revealed that lapatinib-resistant cells were no longer addicted to the HER2/HER3-PI3K signaling axis, unlike lapatinib sensitive cells. Instead the lapatinib resistant cells were dependent on a HRG-driven HER3–EGFR–PI3K–PDK1 signaling axis. While lapatinib resistant cells did not show sensitivity to gefitinib and erlotinib (EGFR inhibitors) they responded to a combination of EGFR inhibition along with the irreversible pan-HER inhibitor, neratinib that inhibited HRG-dependent phosphorylation of HER3 and EGFR (63). Papadimitropoulou et al. subsequently reported that HRG alters interleukin-8 expression in ER+/HER2-breast cancer cells and drives resistance to endocrine treatment (64). These findings attest to the complexity of networking between EGFR, HER2, and HER3, and their relevance to other nongrowth factor receptor pathways of treatment resistance, which often presents a challenge to targeted interventions. In other words, the specificity of HER member inhibition, which is required for decreasing toxicity to patients can sometimes lay the groundwork for resistance through alternate HER family member activation. Intrinsic signaling networks in breast cancer cells that can regulate the balance of growth factors and ligands that they secrete to modulate the receptor landscape of ER+/HER2– breast cancer cells remains an understudied area.
A role for HER4 in heterodimerizing with HER2 to promote oncogenic signaling is less well studied than that of EGFR or HER3. During mammary development, specifically pregnancy and lactation, HER4 plays a prominent role in inducing alveolar differentiation. HER4 also activates the STAT5 pathway to promote cell survival of these alveolar differentiated cells (65, 66). In ER+/HER2– breast cancer cells, intracellular HER4 has been shown to promote growth in an ER-dependent manner (67). Overall, HER4 is one of the more interesting HER family members as it appears to serve both tumor suppressive (prodifferentiation) and tumor promoting (prosurvival) functions in the normal mammary gland. Similarly, in breast cancer samples from patients, there is conflicting evidence regarding a role for HER4 in survival outcomes: while many studies have shown associations with better outcome, a few studies suggest the opposite (68, 69). Further investigation into the role of HER4, either alone, or in combination with HER2, in regulating breast cancer phenotypes and treatment response remains to be conducted.
Lastly, HER2 signaling can also synergize with, or be compensated for, by the activity of non-HER family growth factor receptors. In HER2+ breast cancer, Fibroblast Growth Factor Receptor 1 (FGFR1) has been shown to mediate resistance to interventions targeting HER2. FGFR1 amplification is found in ~15% of ER+/HER2- metastatic breast cancer as well and is frequently associated with endocrine treatment resistance (70). While there are 2 ongoing clinical trials combining FGFR inhibitors and fulvestrant in ER+ metastatic breast cancer (NCT03238196, NCT04024436), at least 1 clinical trial has already demonstrated no benefit in administering FGFR inhibitors to endocrine treatment resistant ER+/HER2– breast cancer patients with or without FGFR1 amplification (71). More research into the role of FGFR1 as a modulator of HER2 activity and a mediator of endocrine treatment resistance in ER+/HER2– breast cancer is warranted.
Similar roles for other growth factor receptor pathways have also been reported, again primarily in the context of HER2+ breast cancer. Signaling via Insulin-like Growth Factor-1 Receptor (IGF1R) confers resistance to trastuzumab (a monoclonal antibody against HER2 used in the clinic) in HER2+ BT474 and SKBR3 breast cancer cells (72). Studies reported that the expression of IGF1R in trastuzumab-sensitive cells confers resistance to trastuzumab which is restored on treatment with recombinant IGF binding protein 3 (rhIGFBP3) (72). In vivo studies on MCF-7/HER-2-18 xenograft tumors, which overexpress HER2 also showed that the combination of rhIGFBP3 and trastuzumab has significant antitumor effect. Additionally, 1 study showed that the activation of HER2/IGF1R signaling pathways and subsequent c-MYC stabilization can upregulate the expression of peroxisome proliferator-activated receptor gamma coactivator-1-beta, an obligate cofactor for ERα activity in ER+/HER2– breast cancer cells, to induce more aggressive growth (73).
Given the large amount of preclinical data, these heterodimerizing/synergistic growth factor receptor partners of HER2 have been proposed as candidates for predictive biomarkers for endocrine treatment resistance and sensitivity to receptor tyrosine kinase inhibitors in ER+/HER2– breast cancer. However, there are 2 challenges to be addressed before these receptors can be seriously considered as biomarkers: (1) changes in activity of these partnering proteins are often induced by endocrine treatment and are therefore not detectable in the tumor at diagnosis, circumscribing usefulness as a clinical diagnostic, (2) dimerization and crosstalk between these receptors is not a binary equation, and due to the fluidity of these associations has not proved clinically viable in predicting either resistance to endocrine treatment or response to specific tyrosine kinase inhibitors. Clinical data support this conclusion since circulating tumor cells in advanced ER+/HER2– breast cancer patients can rapidly gain and lose HER2 activation (74) highlighting the challenges to using gene expression levels of these receptors as predictive biomarkers. This also raises the notion that ER+/HER2– breast cancer cells likely activate HER2 through various other mechanisms that we have yet to uncover.
Activation of HER2 Through Loss of DNA Damage Repair
In breast cancer, the emphasis of investigations into DNA damage repair has been mainly on germline variants of BRCA1/2 genes which belong to the homologous recombination pathway (75, 76). The role of somatic inactivation of other DNA damage repair pathways and how they affect patient outcome has only recently come under investigation. These studies have shown that defects in DNA damage repair genes belonging to single stranded break repair pathways, primarily mismatch and excision repair, constitute a new casual mechanism observed in ~1/3 of intrinsically endocrine therapy resistant ER+/HER2– breast cancer patients (77, 78). Surprisingly, loss of the MutL complex (MLH1/PMS2) of the mismatch repair pathway in ER+/HER2– breast cancer cells activates HER2 signaling upon endocrine treatment to drive resistance to endocrine therapies (79). This activation is not linked to increased incidence of activating mutations in HER2, which might appear to be an intuitive mechanism and a natural consequence of loss of mismatch repair function. Rather, MutL loss prevents the targeting of HER2, which is upregulated in response to endocrine treatment in ER+/HER2– breast cancer cells, to lysosomal trafficking and degradation (Fig. 1). Of note, this response remains consistent independent of the type of endocrine therapy used. This finding suggests a novel avenue of research into whether and how defects in DNA damage repair pathways (perhaps, others beyond mismatch repair function similarly) in ER+/HER2– breast cancer can nongenomically activate HER2 signaling in response to endocrine treatment, to induce resistant growth. These recent findings complement years of investigation into a role for HER2 in modulating DNA damage repair. Cytosolic HER2 has been shown to regulate repair of damaged DNA from chemotherapies and radiation in breast cancer cells, and to interact with DNA damage repair proteins (80, 81). Intriguingly, this association between HER2 and DNA damage repair appears to be restricted by context of cell stemness, adding a further layer of complexity (19, 82). Finally, dysregulation of other types of DNA repair, including the APOBEC pathway have been consistently shown to be enriched in the HER2+ breast cancer context (83). Thus, the recent findings of HER2 modulation by single stranded DNA damage repair proteins provide a new facet to this field of research, while complementing a well-established paradigm of a robust relationship between HER2 activation and DNA repair signaling.
The mechanism by which DNA damage repair genes regulate protein trafficking to activate HER2 remains uncertain. It is possible that loss of the mismatch repair complex increases cytosolic DNA or activates stress pathways that are known to regulate both autophagy and growth factor receptor signaling (84). Conversely, loss of this mismatch repair complex might activate HER2 signaling by altering the secreted ligands from the cell and therefore, heterodimerization patterns. In either case, the clinical relevance of this finding has great potential. Because loss of DNA damage repair genes, like MLH1 and PMS2, is detectable at diagnosis, they are more likely candidates to serve as predictive biomarkers that can pro-actively indicate a combinatorial therapeutic strategy of endocrine therapy and HER2 inhibition for patients with ER+/HER2– breast cancer who are most likely to be resistant to standard care. Indeed they may serve as a first-in-class predictive biomarker since other candidates that have been proposed, including intermediate expression of HER2, PR loss and Ki67 positivity, have not shown promise in clinical trials, likely because these associations are not causal, but, rather, correlative (37, 85).
Conclusion: The Translational Relevance of HER2 Activation in ER+/HER2– Endocrine Treatment–resistant Breast Cancer
Targeting HER2 signaling in breast cancer has been very successful for breast tumors that overexpress or amplify HER2 with multiple clinical trials showing long-term efficacy in both the metastatic and early-stage setting (86, 87). Given the success of targeting the HER2 pathway in HER2+ disease, and the strong preclinical data linking HER2 activation to endocrine treatment resistance in preclinical models, some groups hypothesized that HER2 signaling may remain important in HER2– disease. Thus, several clinical trials were undertaken to determine if agents targeting the HER2 pathway exhibited any efficacy in the HER2– context, but so far they have shown mixed results (88). The phase III EGF30001 clinical trial tested the small-molecule inhibitor of HER2 signaling (lapatinib) in combination with paclitaxel compared to paclitaxel alone in HER2– metastatic breast cancer patients (13). While the overall trial showed no benefit to adding lapatinib, in subset analysis they found that ~10% of ER+/HER2– patients showed benefit with this combination. Multivariate analysis identified that low PR levels in patient biopsies associated with an improved event-free survival (7.3 vs 2.4 months) for patients treated with lapatinib. When considering HER2 targeting in combination with endocrine treatment, the phase III clinical trial (CALGB 40302) of 295 patients compared the ER antagonist fulvestrant in combination with lapatinib vs fulvestrant plus a placebo in postmenopausal women with ER+ advanced breast cancer. The investigators in this trial found no difference in overall or progression-free survival between the lapatinib and control arms with approximately 80% of patients being HER2- (14). Interestingly, however, Johnston and colleagues conducted another large phase III clinical trial looking at lapatinib combined with endocrine treatment as first-line therapy in metastatic breast cancer patients with ER+ disease (11). Though patients with HER2– disease did not show improvement in progression-free survival as a whole, again, a subset of HER2– patients were found to respond to the combination of lapatinib and endocrine treatment. However, no biomarkers were found at that time to stratify these patients.
Targeting HER2 in ER+ disease was also studied in early-stage breast cancers in small presurgical clinical trials. In the first trial, the LPS trial, lapatinib was given to 31 treatment-naïve HER2– patients with early-stage disease (89). Biopsies were taken before and after treatment to assess proliferation by Ki-67 staining. Only 4/31 (~13%) patients showed a decrease in Ki-67 >50% after treatment with lapatinib, suggesting that only a small minority of HER2– patients respond to HER2-targeted therapy. The larger phase II MAPLE trial enrolled women with primary breast cancer that was either HER2+ or HER2– and randomized them 3:1 between preoperative lapatinib or placebo (12). Pre- and post-treatment biopsies were analyzed for Ki67 levels. Of the 121 patients enrolled in the study, 78/121 (64%) were HER2– and 98/121 (81%) were ER+. In contrast to the LPS trial, there was a significant Ki-67 decrease (–27% mean reduction) in HER2– breast cancer with 14% of HER2– breast cancers demonstrating >50% Ki67 reduction with lapatinib, again indicating that HER2 targeted agents address a particular subset of HER2– patients.
Taken together, these clinical trials demonstrate that the majority of ER+/HER2– patient tumors will not respond to HER2-directed therapy in either the early or metastatic setting, however there is a significant minority (10-15%) that can respond despite not overexpressing HER2. This number is consistent with the proportion of patients (~20%) where HER2 may be activated in response to endocrine treatment. At present, we do not have clinically validated biomarkers to identify this subset of patients that may benefit from HER2-directed therapy limiting the ability to design appropriate clinical trials for these patients.
To better stratify ER+/HER2– breast cancer patients for this promising targeted therapeutic combination, mechanistic understanding of how ER+ breast cancer cells activate HER2 in response to endocrine treatment is instrumental. Currently existing predictive biomarker candidates are neither specific to HER2 activation nor sensitivity to HER2 inhibitors. A role for defects in DNA damage repair genes in activating HER2 in response to endocrine treatment in ER+/HER2– breast cancer cells suggests potential for use as a first-in-class predictive marker that is causal and specific for response to HER2 inhibition and can be assayed at diagnosis. Further biological investigation into these pathways, and other such that are as yet undiscovered, and clinical validation of their use as predictive biomarkers is the next frontier of research into endocrine treatment resistant ER+ breast cancer.
Acknowledgments
Financial Support: The study were funded by Department of Defense Breakthrough (W81XWH-18-1-0034) and Susan G. Komen Career Catalyst (CCR18548157) awards (to S.H.). Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers P30CA030199 and CA229613 (to S.H.).
Glossary
Abbreviations
- EGF
epidermal growth factor
- ER+
estrogen receptor alpha positive
- FGFR1
Fibroblast Growth Factor Receptor 1
- HRG
heregulin
- IGF1R
Insulin-like Growth Factor-1 Receptor
- PR
progesterone receptor
Additional Information
Disclosure Statement: Authors declare conflicts of interest as follows: Dr. Haricharan has a provisional patent (US 63/106777) for using DNA repair genes as a biomarker for patient outcome. Dr. Shiao receives research funding (translational science support) from Merck and (trial support) from Syndax and from 4dx.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Curtis C, Shah SP, Chin SF, et al. ; METABRIC Group . The genomic and transcriptomic architecture of 2000 breast tumours reveals novel subgroups. Nature. 2012;486(7403):346-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ellis MJ, Perou CM. The genomic landscape of breast cancer as a therapeutic roadmap. Cancer Discov. 2013;3(1):27-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hanker AB, Sudhan DR, Arteaga CL. Overcoming endocrine resistance in breast cancer. Cancer Cell. 2020;37(4):496-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Razavi P, Chang MT, Xu G, et al. The genomic landscape of endocrine-resistant advanced breast cancers. Cancer Cell. 2018;34(3):427-438.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li S, Shen D, Shao J, et al. Endocrine-therapy-resistant ESR1 variants revealed by genomic characterization of breast-cancer-derived xenografts. Cell Rep. 2013;4(6):1116-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lei JT, Shao J, Zhang J, et al. Functional annotation of ESR1 gene fusions in estrogen receptor-positive breast cancer. Cell Rep. 2018;24(6):1434-1444.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gu G, Tian L, Herzog SK, et al. Hormonal modulation of ESR1 mutant metastasis. Oncogene. 2021;40(5):997-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barone I, Cui Y, Herynk MH, et al. Expression of the K303R estrogen receptor-alpha breast cancer mutation induces resistance to an aromatase inhibitor via addiction to the PI3K/Akt kinase pathway. Cancer Res. 2009;69(11):4724-4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177-182. [DOI] [PubMed] [Google Scholar]
- 10. Bose R, Kavuri SM, Searleman AC, et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 2013;3(2):224-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johnston S, Pippen J Jr, Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2009;27(33):5538-5546. [DOI] [PubMed] [Google Scholar]
- 12. Leary A, Evans A, Johnston SR, et al. Antiproliferative effect of lapatinib in HER2-positive and HER2-negative/HER3-high breast cancer: results of the presurgical randomized MAPLE Trial (CRUK E/06/039). Clin Cancer Res. 2015;21(13):2932-2940. [DOI] [PubMed] [Google Scholar]
- 13. Di Leo A, Gomez HL, Aziz Z, et al. Phase III, double-blind, randomized study comparing lapatinib plus paclitaxel with placebo plus paclitaxel as first-line treatment for metastatic breast cancer. J Clin Oncol. 2008;26(34):5544-5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burstein HJ, Cirrincione CT, Barry WT, et al. Endocrine therapy with or without inhibition of epidermal growth factor receptor and human epidermal growth factor receptor 2: a randomized, double-blind, placebo-controlled phase III trial of fulvestrant with or without lapatinib for postmenopausal women with hormone receptor-positive advanced breast cancer-CALGB 40302 (Alliance). J Clin Oncol. 2014;32(35):3959-3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johnston SRD. Clinical trials of intracellular signal transductions inhibitors for breast cancer--a strategy to overcome endocrine resistance. Endocr Relat Cancer. 2005;12(Suppl 1):S145-157. [DOI] [PubMed] [Google Scholar]
- 16. Johnston SRD. Enhancing the efficacy of hormonal agents with selected targeted agents. Clin Breast Cancer. 2009;9(Suppl 1):S28-S36. [DOI] [PubMed] [Google Scholar]
- 17. Morrison G, Fu X, Shea M, et al. Therapeutic potential of the dual EGFR/HER2 inhibitor AZD8931 in circumventing endocrine resistance. Breast Cancer Res Treat. 2014;144(2):263-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leary AF, Drury S, Detre S, et al. Lapatinib restores hormone sensitivity with differential effects on estrogen receptor signaling in cell models of human epidermal growth factor receptor 2-negative breast cancer with acquired endocrine resistance. Clin Cancer Res. 2010;16(5):1486-1497. [DOI] [PubMed] [Google Scholar]
- 19. Ithimakin S, Day KC, Malik F, et al. HER2 drives luminal breast cancer stem cells in the absence of HER2 amplification: implications for efficacy of adjuvant trastuzumab. Cancer Res. 2013;73(5):1635-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Révillion F, Lhotellier V, Hornez L, Bonneterre J, Peyrat JP. ErbB/HER ligands in human breast cancer, and relationships with their receptors, the bio-pathological features and prognosis. Ann Oncol. 2008;19(1):73-80. [DOI] [PubMed] [Google Scholar]
- 21. Sundaresan S, Roberts PE, King KL, Sliwkowski MX, Mather JP. Biological response to ErbB ligands in nontransformed cell lines correlates with a specific pattern of receptor expression. Endocrinology. 1998;139(12):4756-4764. [DOI] [PubMed] [Google Scholar]
- 22. Hudziak RM, Lewis GD, Winget M, Fendly BM, Shepard HM, Ullrich A. p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol Cell Biol. 1989;9(3):1165-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17(9):2639-2648. [DOI] [PubMed] [Google Scholar]
- 24. Pegram MD, Lipton A, Hayes DF, et al. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol. 1998;16(8):2659-2671. [DOI] [PubMed] [Google Scholar]
- 25. Siegel PM, Dankort DL, Hardy WR, Muller WJ. Novel activating mutations in the neu proto-oncogene involved in induction of mammary tumors. Mol Cell Biol. 1994;14(11):7068-7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nayar U, Cohen O, Kapstad C, et al. Acquired HER2 mutations in ER+ metastatic breast cancer confer resistance to estrogen receptor-directed therapies. Nat Genet. 2019;51(2):207-216. [DOI] [PubMed] [Google Scholar]
- 27. Kloth M, Ruesseler V, Engel C, et al. Activating ERBB2/HER2 mutations indicate susceptibility to pan-HER inhibitors in Lynch and Lynch-like colorectal cancer. Gut. 2016;65(8):1296-1305. [DOI] [PubMed] [Google Scholar]
- 28. Kavuri SM, Jain N, Galimi F, et al. HER2 activating mutations are targets for colorectal cancer treatment. Cancer Discov. 2015;5(8):832-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hyman DM, Piha-Paul SA, Won H, et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature. 2018;554(7691):189-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Croessmann S, Formisano L, Kinch LN, et al. Combined Blockade of Activating ERBB2 Mutations and ER Results in Synthetic Lethality of ER+/HER2 Mutant Breast Cancer. Clin Cancer Res. 2019;25(1):277-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee JW, Soung YH, Seo SH, et al. Somatic mutations of ERBB2 kinase domain in gastric, colorectal, and breast carcinomas. Clin Cancer Res. 2006;12(1):57-61. [DOI] [PubMed] [Google Scholar]
- 32. Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62:233-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schiff R, Massarweh SA, Shou J, Bharwani L, Mohsin SK, Osborne CK. Cross-talk between estrogen receptor and growth factor pathways as a molecular target for overcoming endocrine resistance. Clin Cancer Res. 2004;10(1 Pt 2):331S-336S. [DOI] [PubMed] [Google Scholar]
- 34. Santen RJ, Fan P, Zhang Z, Bao Y, Song RX, Yue W. Estrogen signals via an extra-nuclear pathway involving IGF-1R and EGFR in tamoxifen-sensitive and -resistant breast cancer cells. Steroids. 2009;74(7):586-594. [DOI] [PubMed] [Google Scholar]
- 35. Massarweh S, Osborne CK, Creighton CJ, et al. Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function. Cancer Res. 2008;68(3):826-833. [DOI] [PubMed] [Google Scholar]
- 36. Nicholson RI, Hutcheson IR, Harper ME, et al. Modulation of epidermal growth factor receptor in endocrine-resistant, estrogen-receptor-positive breast cancer. Ann N Y Acad Sci. 2002;963:104-115. [DOI] [PubMed] [Google Scholar]
- 37. Kim MH, Kim GM, Kim JH, et al. Intermediate HER2 expression is associated with poor prognosis in estrogen receptor-positive breast cancer patients aged 55 years and older. Breast Cancer Res Treat. 2020;179(3):687-697. [DOI] [PubMed] [Google Scholar]
- 38. Hewitt SC, Bocchinfuso WP, Zhai J, et al. Lack of ductal development in the absence of functional estrogen receptor alpha delays mammary tumor formation induced by transgenic expression of ErbB2/neu. Cancer Res. 2002;62(10):2798-2805. [PubMed] [Google Scholar]
- 39. Jackson-Fisher AJ, Bellinger G, Breindel JL, et al. ErbB3 is required for ductal morphogenesis in the mouse mammary gland. Breast Cancer Res. 2008;10(6):R96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jackson-Fisher AJ, Bellinger G, Ramabhadran R, Morris JK, Lee KF, Stern DF. ErbB2 is required for ductal morphogenesis of the mammary gland. Proc Natl Acad Sci U S A. 2004;101(49):17138-17143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Robichaux JP, Hallett RM, Fuseler JW, Hassell JA, Ramsdell AF. Mammary glands exhibit molecular laterality and undergo left-right asymmetric ductal epithelial growth in MMTV-cNeu mice. Oncogene. 2015;34(15):2003-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Andrechek ER, White D, Muller WJ. Targeted disruption of ErbB2/Neu in the mammary epithelium results in impaired ductal outgrowth. Oncogene. 2005;24(5):932-937. [DOI] [PubMed] [Google Scholar]
- 43. Jones FE, Stern DF. Expression of dominant-negative ErbB2 in the mammary gland of transgenic mice reveals a role in lobuloalveolar development and lactation. Oncogene. 1999;18(23):3481-3490. [DOI] [PubMed] [Google Scholar]
- 44. Williams CC, Allison JG, Vidal GA, et al. The ERBB4/HER4 receptor tyrosine kinase regulates gene expression by functioning as a STAT5A nuclear chaperone. J Cell Biol. 2004;167(3):469-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wali VB, Haskins JW, Gilmore-Hebert M, Platt JT, Liu Z, Stern DF. Convergent and divergent cellular responses by ErbB4 isoforms in mammary epithelial cells. Mol Cancer Res. 2014;12(8):1140-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shyamala G, Chou YC, Cardiff RD, Vargis E. Effect of c-neu/ ErbB2 expression levels on estrogen receptor alpha-dependent proliferation in mammary epithelial cells: implications for breast cancer biology. Cancer Res. 2006;66(21):10391-10398. [DOI] [PubMed] [Google Scholar]
- 47. Mukherjee S, Louie SG, Campbell M, Esserman L, Shyamala G. Ductal growth is impeded in mammary glands of C-neu transgenic mice. Oncogene. 2000;19(52):5982-5987. [DOI] [PubMed] [Google Scholar]
- 48. Ignatoski KM, Lapointe AJ, Radany EH, Ethier SP. erbB-2 overexpression in human mammary epithelial cells confers growth factor independence. Endocrinology. 1999;140(8):3615-3622. [DOI] [PubMed] [Google Scholar]
- 49. Boonyaratanakornkit V, Scott MP, Ribon V, et al. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol Cell. 2001;8(2):269-280. [DOI] [PubMed] [Google Scholar]
- 50. Boonyaratanakornkit V, McGowan E, Sherman L, Mancini MA, Cheskis BJ, Edwards DP. The role of extranuclear signaling actions of progesterone receptor in mediating progesterone regulation of gene expression and the cell cycle. Mol Endocrinol. 2007;21(2):359-375. [DOI] [PubMed] [Google Scholar]
- 51. Carvajal A, Espinoza N, Kato S, et al. Progesterone pre-treatment potentiates EGF pathway signaling in the breast cancer cell line ZR-75. Breast Cancer Res Treat. 2005;94(2):171-183. [DOI] [PubMed] [Google Scholar]
- 52. Lange CA, Richer JK, Shen T, Horwitz KB. Convergence of progesterone and epidermal growth factor signaling in breast cancer. Potentiation of mitogen-activated protein kinase pathways. J Biol Chem. 1998;273(47):31308-31316. [DOI] [PubMed] [Google Scholar]
- 53. Groshong SD, Owen GI, Grimison B, et al. Biphasic regulation of breast cancer cell growth by progesterone: role of the cyclin-dependent kinase inhibitors, p21 and p27(Kip1). Mol Endocrinol. 1997;11(11):1593-1607. [DOI] [PubMed] [Google Scholar]
- 54. Faivre EJ, Lange CA. Progesterone receptors upregulate Wnt-1 to induce epidermal growth factor receptor transactivation and c-Src-dependent sustained activation of Erk1/2 mitogen-activated protein kinase in breast cancer cells. Mol Cell Biol. 2007;27(2):466-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Béguelin W, Díaz Flaqué MC, Proietti CJ, et al. Progesterone receptor induces ErbB-2 nuclear translocation to promote breast cancer growth via a novel transcriptional effect: ErbB-2 function as a coactivator of Stat3. Mol Cell Biol. 2010;30(23):5456-5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Labriola L, Salatino M, Proietti CJ, et al. Heregulin induces transcriptional activation of the progesterone receptor by a mechanism that requires functional ErbB-2 and mitogen-activated protein kinase activation in breast cancer cells. Mol Cell Biol. 2003;23(3):1095-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Proietti CJ, Rosemblit C, Beguelin W, et al. Activation of Stat3 by heregulin/ErbB-2 through the co-option of progesterone receptor signaling drives breast cancer growth. Mol Cell Biol. 2009;29(5):1249-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Alimandi M, Romano A, Curia MC, et al. Cooperative signaling of ErbB3 and ErbB2 in neoplastic transformation and human mammary carcinomas. Oncogene. 1995;10(9):1813-1821. [PubMed] [Google Scholar]
- 59. Kraus MH, Fedi P, Starks V, Muraro R, Aaronson SA. Demonstration of ligand-dependent signaling by the erbB-3 tyrosine kinase and its constitutive activation in human breast tumor cells. Proc Natl Acad Sci U S A. 1993;90(7):2900-2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Luhtala S, Staff S, Kallioniemi A, Tanner M, Isola J. Clinicopathological and prognostic correlations of HER3 expression and its degradation regulators, NEDD4-1 and NRDP1, in primary breast cancer. BMC Cancer. 2018;18(1):1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dey N, Williams C, Leyland-Jones B, De P. A critical role for HER3 in HER2-amplified and non-amplified breast cancers: function of a kinase-dead RTK. Am J Transl Res. 2015;7(4):733-750. [PMC free article] [PubMed] [Google Scholar]
- 62. Huang F, Shi Q, Li Y, et al. HER2/EGFR-AKT signaling switches TGFβ from inhibiting cell proliferation to promoting cell migration in breast cancer. Cancer Res. 2018;78(21):6073-6085. [DOI] [PubMed] [Google Scholar]
- 63. Xia W, Petricoin EF 3rd, Zhao S, et al. An heregulin-EGFR-HER3 autocrine signaling axis can mediate acquired lapatinib resistance in HER2+ breast cancer models. Breast Cancer Res. 2013;15(5):R85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Papadimitropoulou A, Vellon L, Atlas E, et al. Heregulin drives endocrine resistance by altering IL-8 expression in ER-positive breast cancer. Int J Mol Sci. Published online October 19, 2020. Doi: 10.3390/ijms21207737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Haricharan S, Li Y. STAT signaling in mammary gland differentiation, cell survival and tumorigenesis. Mol Cell Endocrinol. 2014;382(1):560-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Haricharan S, Dong J, Hein S, et al. Mechanism and preclinical prevention of increased breast cancer risk caused by pregnancy. Elife. 2013;2:e00996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhu Y, Sullivan LL, Nair SS, et al. Coregulation of estrogen receptor by ERBB4/HER4 establishes a growth-promoting autocrine signal in breast tumor cells. Cancer Res. 2006;66(16):7991-7998. [DOI] [PubMed] [Google Scholar]
- 68. Wege AK, Chittka D, Buchholz S, et al. HER4 expression in estrogen receptor-positive breast cancer is associated with decreased sensitivity to tamoxifen treatment and reduced overall survival of postmenopausal women. Breast Cancer Res. 2018;20(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Witton CJ, Reeves JR, Going JJ, Cooke TG, Bartlett JM. Expression of the HER1-4 family of receptor tyrosine kinases in breast cancer. J Pathol. 2003;200(3):290-297. [DOI] [PubMed] [Google Scholar]
- 70. Giltnane JM, Hutchinson KE, Stricker TP, et al. Genomic profiling of ER+ breast cancers after short-term estrogen suppression reveals alterations associated with endocrine resistance. Sci Transl Med. Published online August 9, 2017. Doi: 10.1126/scitranslmed.aai7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Campone M, Bachelot T, Penault-Llorca F, et al. A phase Ib dose allocation study of oral administration of lucitanib given in combination with fulvestrant in patients with estrogen receptor-positive and FGFR1-amplified or non-amplified metastatic breast cancer. Cancer Chemother Pharmacol. 2019;83(4):743-753. [DOI] [PubMed] [Google Scholar]
- 72. Browne BC, Crown J, Venkatesan N, et al. Inhibition of IGF1R activity enhances response to trastuzumab in HER-2-positive breast cancer cells. Ann Oncol. 2011;22(1):68-73. [DOI] [PubMed] [Google Scholar]
- 73. Chang CY, Kazmin D, Jasper JS, Kunder R, Zuercher WJ, McDonnell DP. The metabolic regulator ERRα, a downstream target of HER2/IGF-1R, as a therapeutic target in breast cancer. Cancer Cell. 2011;20(4):500-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jordan NV, Bardia A, Wittner BS, et al. HER2 expression identifies dynamic functional states within circulating breast cancer cells. Nature. 2016;537(7618):102-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Anurag M, Ellis MJ, Haricharan S. DNA damage repair defects as a new class of endocrine treatment resistance driver. Oncotarget. 2018;9(91):36252-36253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. King MC, Marks JH, Mandell JB; New York Breast Cancer Study Group . Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302(5645):643-646. [DOI] [PubMed] [Google Scholar]
- 77. Haricharan S, Punturi N, Singh P, et al. Loss of MutL disrupts CHK2-dependent cell-cycle control through CDK4/6 to promote intrinsic endocrine therapy resistance in primary breast cancer. Cancer Discov. 2017;7(10):1168-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Anurag M, Punturi N, Hoog J, Bainbridge MN, Ellis MJ, Haricharan S. Comprehensive profiling of DNA repair defects in breast cancer identifies a novel class of endocrine therapy resistance drivers. Clin Cancer Res. 2018;24(19):4887-4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Punturi NB, Seker S, Devarakonda V, et al. Mismatch repair deficiency predicts response to HER2 blockade in HER2-negative breast cancer. Nat Commun. 2021;12(1):2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pietras RJ, Poen JC, Gallardo D, Wongvipat PN, Lee HJ, Slamon DJ. Monoclonal antibody to HER-2/neureceptor modulates repair of radiation-induced DNA damage and enhances radiosensitivity of human breast cancer cells overexpressing this oncogene. Cancer Res. 1999;59(6):1347-1355. [PubMed] [Google Scholar]
- 81. Boone JJ, Bhosle J, Tilby MJ, Hartley JA, Hochhauser D. Involvement of the HER2 pathway in repair of DNA damage produced by chemotherapeutic agents. Mol Cancer Ther. 2009;8(11):3015-3023. [DOI] [PubMed] [Google Scholar]
- 82. Duru N, Fan M, Candas D, et al. HER2-associated radioresistance of breast cancer stem cells isolated from HER2-negative breast cancer cells. Clin Cancer Res. 2012;18(24):6634-6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kanu N, Cerone MA, Goh G, et al. DNA replication stress mediates APOBEC3 family mutagenesis in breast cancer. Genome Biol. 2016;17(1):185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bakhoum SF, Ngo B, Laughney AM, et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature. 2018;553(7689):467-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Finn RS, Press MF, Dering J, et al. Estrogen receptor, progesterone receptor, human epidermal growth factor receptor 2 (HER2), and epidermal growth factor receptor expression and benefit from lapatinib in a randomized trial of paclitaxel with lapatinib or placebo as first-line treatment in HER2-negative or unknown metastatic breast cancer. J Clin Oncol. 2009;27(24):3908-3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mayer IA. Treatment of HER2-positive metastatic breast cancer following initial progression. Clin Breast Cancer. 2009;9(Suppl 2):S50-S57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Rexer BN, Arteaga CL. Intrinsic and acquired resistance to HER2-targeted therapies in HER2 gene-amplified breast cancer: mechanisms and clinical implications. Crit Rev Oncog. 2012;17(1):1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Pernas S, Tolaney SM. Targeting HER2 heterogeneity in early-stage breast cancer. Curr Opin Oncol. 2020;32(6):545-554. [DOI] [PubMed] [Google Scholar]
- 89. Coombes RC, Tat T, Miller ML, et al. An open-label study of lapatinib in women with HER-2-negative early breast cancer: the lapatinib pre-surgical study (LPS study). Ann Oncol. 2013;24(4):924-930. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.