Significance
Our findings represent a fundamental advance in general understanding of the mechanisms of exocytosis in polarized epithelial cells that are crucial for the establishment and maintenance of epithelial cell polarity. Moreover, our data also improve the knowledge on the machinery regulating polarized trafficking of glycosylphosphatidylinositol-anchored proteins, a class of lipid-associated proteins playing diverse vital functions, unraveling an unexpected role of calcium in their apical sorting.
Keywords: GPI-anchored proteins, protein sorting, calcium, protein clustering, polarized epithelial cells
Abstract
Glycosylphosphatidylinositol-anchored proteins (GPI-APs) are lipid-associated luminal secretory cargoes selectively sorted to the apical surface of the epithelia where they reside and play diverse vital functions. Cholesterol-dependent clustering of GPI-APs in the Golgi is the key step driving their apical sorting and their further plasma membrane organization and activity; however, the specific machinery involved in this Golgi event is still poorly understood. In this study, we show that the formation of GPI-AP homoclusters (made of single GPI-AP species) in the Golgi relies directly on the levels of calcium within cisternae. We further demonstrate that the TGN calcium/manganese pump, SPCA1, which regulates the calcium concentration within the Golgi, and Cab45, a calcium-binding luminal Golgi resident protein, are essential for the formation of GPI-AP homoclusters in the Golgi and for their subsequent apical sorting. Down-regulation of SPCA1 or Cab45 in polarized epithelial cells impairs the oligomerization of GPI-APs in the Golgi complex and leads to their missorting to the basolateral surface. Overall, our data reveal an unexpected role for calcium in the mechanism of GPI-AP apical sorting in polarized epithelial cells and identify the molecular machinery involved in the clustering of GPI-APs in the Golgi.
Glycosylphosphatidylinositol (GPI)-anchored proteins (GPI-APs) are localized on the apical surface of most epithelia, where they exert their physiological functions, which are regulated by their spatiotemporal compartmentalization.
In polarized epithelial cells, the organization of GPI-APs at the apical surface is driven by the mechanism of apical sorting, which relies on the formation of GPI-AP homoclusters in the Golgi apparatus (1, 2). GPI-AP homoclusters (containing a single GPI-AP species) form uniquely in the Golgi apparatus of fully polarized cells (and not in nonpolarized cells) in a cholesterol-dependent manner (1, 3, 4). Once formed, GPI-AP homoclusters become insensitive to cholesterol depletion, suggesting that protein–protein interactions stabilize them (1, 2). At the apical membrane, newly arrived homoclusters coalesce into heteroclusters (containing at least two different GPI-AP species) that are sensitive to cholesterol depletion (1). Of importance, in the absence of homoclustering in the Golgi (e.g., in nonpolarized epithelial cells), GPI-APs remain in the form of monomers and dimers and do not cluster at the cell surface (1, 5). Thus, the organization of GPI-APs at the apical plasma membrane of polarized cells strictly depends on clustering mechanisms in the Golgi apparatus allowing their apical sorting. This is different from what was shown in fibroblasts where clustering of GPI-APs occurs from monomer condensation at the plasma membrane, indicating that distinct mechanisms regulate GPI-AP clustering in polarized epithelial cells and fibroblasts (1, 6, 7). Furthermore, in polarized epithelial cells, the spatial organization of clusters also appears to regulate the biological activity of the proteins (1) so that GPI-APs are fully functional only when properly sorted to the apical surface and less active in the case of missorting to the basolateral domain (1, 8, 9). Understanding the mechanism of GPI-AP apical sorting in the Golgi apparatus is therefore crucial to decipher their organization at the plasma membrane and the regulation of their activity. The determinants for protein apical sorting have been difficult to uncover compared to the ones for basolateral sorting (10–14). Besides a role of cholesterol, the molecular factors regulating the clustering-based mechanism of GPI-AP sorting in polarized epithelial cells are unknown. Here, we analyzed the possible role of the actin cytoskeleton and of calcium levels in the Golgi. The actin cytoskeleton is not only critical for the maintenance of the Golgi structure and its mechanical properties but also provides the structural support favoring carrier biogenesis (15–18). The Golgi exit of various cargoes is altered in cells treated with drugs either depolymerizing or stabilizing actin filaments (19, 20), and the post-Golgi trafficking is affected either by the knockdown of the expression of some actin-binding proteins, which regulate actin dynamics, or by the overexpression of their mutants (12, 21–23), all together revealing the critical role of actin dynamics for protein trafficking. Only few studies have shown the involvement of actin remodeling proteins in polarized trafficking, mostly in selectively mediating the apical and basolateral trafficking of transmembrane proteins [refs. 24–26; and reviewed in ref. 27]; thus, it remains unclear whether actin filaments play a role in protein sorting in polarized cells.
On the other hand, the Golgi apparatus exhibits high calcium levels that have been revealed to be essential for protein processing and the sorting of some secreted soluble proteins in nonpolarized cells (28–31). Moreover, a functional interplay between the actin cytoskeleton and Golgi calcium in modulating protein sorting in nonpolarized cells has been shown (22).
In this study, we report that in epithelial cells, actin perturbation does not impair GPI-AP clustering capacity in the Golgi and therefore their apical sorting. In contrast, we found that the Golgi organization of GPI-APs is drastically perturbed upon calcium depletion and that the amount of calcium in the Golgi cisternae is critical for the formation of GPI-AP homoclusters. We further show that the TGN calcium/manganese pump, SPCA1 (secretory pathway Ca(2+)-ATPase pump type 1), which controls the Golgi calcium concentration (32), and Cab45, a calcium-binding luminal Golgi resident protein previously described to be involved in the sorting of a subset of soluble cargoes (33, 34), are essential for the formation of GPI-APs homoclusters in the Golgi and for their subsequent apical sorting. Indeed, down-regulation of SPCA1 or Cab45 expression impairs the oligomerization of GPI-APs in the Golgi complex and leads to their missorting to the basolateral surface but does not affect apical or basolateral transmembrane proteins. Overall, our data reveal an unexpected role for calcium in the mechanism of GPI-AP apical sorting in polarized epithelial cells and identify the molecular machinery involved in the clustering of GPI-APs in the Golgi.
Results
Calcium Levels Regulate Homoclustering of GPI-APs in the Golgi Apparatus of Polarized Epithelial Cells.
In nonpolarized cells, the calcium content of the Golgi complex is high and has been shown to regulate essential processes such as protein processing and the sorting of secreted soluble proteins (28–31). Of particular interest, some soluble cargoes cluster in a calcium-dependent manner to segregate in secretory vesicles (33–35). Based on this evidence, we analyzed the possible role of calcium levels within the Golgi complex in GPI-AP clustering and the apical sorting of GPI-APs.
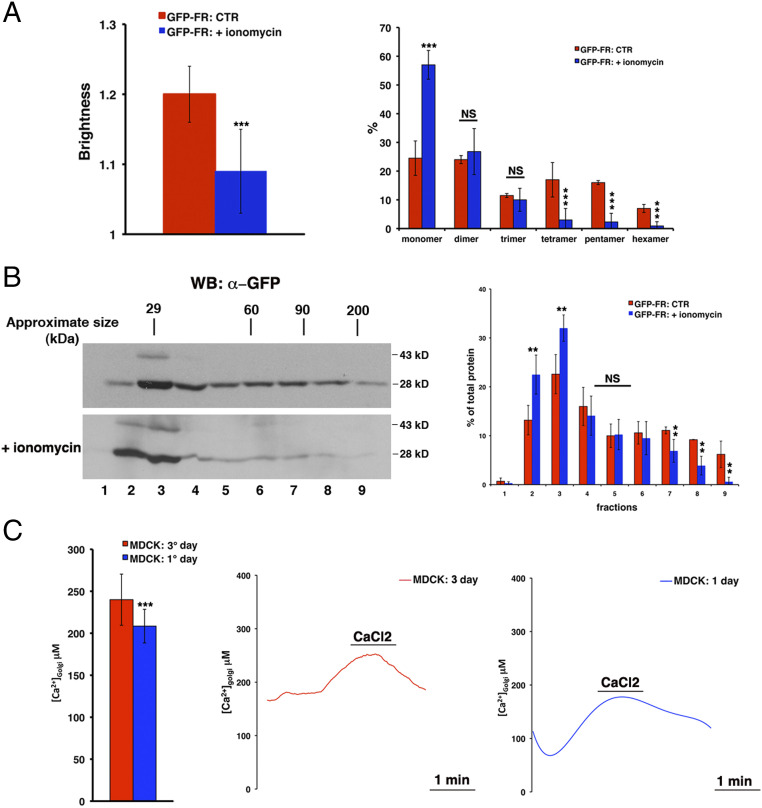
To this aim, we specifically depleted calcium ions from the Golgi by treating polarized MDCK (Madin-Darby Canine Kidney) cells with ionomycin (see Materials and Methods), an ionophore that efficiently promotes a drastic and rapid emptying of Golgi luminal calcium, with respect to other methods used for cytosol or other organelles (e.g., calcium-free medium, calcium chelators) (28, 29). By the number and brightness (N&B) technique (36, 37), we measured the aggregation state (and number of molecules) of a model apical GPI-AP, GFP-FR, in the Golgi apparatus of polarized MDCK cells (stably expressing this protein; see Materials and Methods and ref. 1) in control conditions and upon ionomycin treatment. In the control condition, the brightness of GFP-FR is ∼1.20, which corresponds to protein clusters containing three to four molecules (Fig. 1A; see also Materials and Methods and ref. 1), while upon ionomycin treatment, the brightness values of GFP-FR significantly decrease to 1.14 (P < 0.0001), indicating a shift toward monomeric/dimeric forms (Fig. 1A). To further assess the assembly of GFP-FR, we performed velocity gradient sedimentation experiments on the pool of the proteins in the Golgi apparatus (see Materials and Methods). This technique allows the proteins to sediment according to their molecular weight, thus revealing if the protein is in its monomeric form or in a high molecular weight (HMW) complex. In control conditions, the Golgi pool of GFP-FR mainly sediments in HMW complexes, indicating its capacity to cluster; while upon ionomycin treatment, the migration of the Golgi pool of GFP-FR showed a reduction in HMW complexes (Fig. 1B). These results support that the amount of calcium in the Golgi lumen regulates the formation of GPI-AP homoclusters in polarized epithelial cells.
Fig. 1.
The content of calcium within the Golgi lumen, which is higher in fully polarized than nonpolarized MDCK cells, is crucial for GFP-FR homoclustering. (A) N&B analysis of GFP-FR in the Golgi of polarized MDCK cells in control conditions or upon ionomycin treatment. Briefly, MDCK cells, grown on filters, were treated with trypsin (25 μg/mL for 25 min) exclusively at the apical side in order to remove the pool of GFP-FR already present at the plasma membrane and then incubated for 40 min at 4 °C in Krebs-Ringer–modified buffer (KRB) (CTR) or in the same buffer supplemented with ionomycin (5 μM) and 600 μM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA) (+ionomycin) and imaged at the Golgi level. (Left) Quantification of the brightness of GFP-FR in the Golgi compartment from three independent experiments either in control conditions (CTR, red bar) or upon calcium chelation (+ionomycin, blue bar). (Right) Graphical representation of the percentage of pixels falling into the different classes of B values (from monomer to hexamer) on the basis of the calibration curve (1). Values are expressed as the mean of three independent experiments, n > 25 cells. (B) MDCK cells, grown on filters and treated as in A, were lysed and run on a velocity gradient. Fractions were collected from the top (Fraction 1) to bottom (Fraction 9), trichloroacetic acid (TCA) precipitated, run on a sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel, and revealed by Western blotting with a specific anti-GFP antibody. Molecular weight markers are indicated on the top of the panels. The molecular weight of the monomeric form of GFP-FR is indicated together with the band at 43 kDa, which represents a partially denatured dimer of GFP (2). (Right) The distribution of GFP-FR in the fractions of the gradient is expressed as a percentage of the total protein. Mean values of two independent experiments are shown. (C) Golgi [Ca2+] quantification in fully polarized and nonpolarized MDCK cells. MDCK cells transiently expressing the Golgi-aequorin mutant were grown on coverslips for 1 d (nonpolarized) or for 3 d (polarized). Before carrying out the experimental procedure for aequorin reconstitution with coelenterazine (see Materials and Methods), cells were treated 5 min at 37 °C in Ca-free medium containing ionomycin (5 μM) and EGTA (2 mM) in order to completely empty the calcium from the Golgi apparatus. The coverslip with cells was placed in the thermostatic chamber of the luminometer at 37 °C and perfused with KRB supplemented with 0.1 mM EGTA. Where indicated, the EGTA was replaced with 1 mM CaCl2. The [Ca2+] in the Golgi apparatus of polarized versus nonpolarized MDCK cells is shown as a mean of three independent experiments. Representative curves are shown. Error bars, ± SD; NS, not significant; **P < 0.01; ***P < 0.001, Student’s t test.
If this hypothesis is correct, we postulated that the Golgi apparatus of nonpolarized MDCK cells, where GPI-APs do not cluster (1), would contain a lower amount of calcium compared to fully polarized MDCK cells. To test this hypothesis, we measured the calcium concentration ([Ca2+]) in the Golgi apparatus of MDCK cells grown in polarized and nonpolarized conditions by using a calcium-sensitive photoprotein (Golgi-aequorin chimera) as previously described (28, 38, 39). We found that the calcium levels in the Golgi apparatus of polarized MDCK cells are higher compared to the Golgi of nonpolarized MDCK cells (Fig. 1C), thus supporting a role for calcium in the mechanism of GPI-AP clustering in the Golgi apparatus.
The Golgi Calcium/Manganese ATPase SPCA1 Is Involved in the Calcium-Dependent Regulation of GPI-AP Homoclustering.
The calcium levels in the Golgi apparatus are achieved by the action of two groups of phosphorylation-type calcium pumps, the well-known SERCAs (sarcoendoplasmic-reticulum Ca-ATPases) and the more recently discovered SPCAs (32). Of interest, in HeLa cells, SPCA1 has been shown to regulate the sorting of some secretory soluble cargoes in secretory vesicles at the Golgi level (22, 23, 30, 33), and, differently from SERCA, its activity seems to be dependent on cholesterol and sphingomyelin (40). Thus, we first assessed whether SPCA1 regulates the concentration of calcium in the Golgi of polarized epithelial cells.
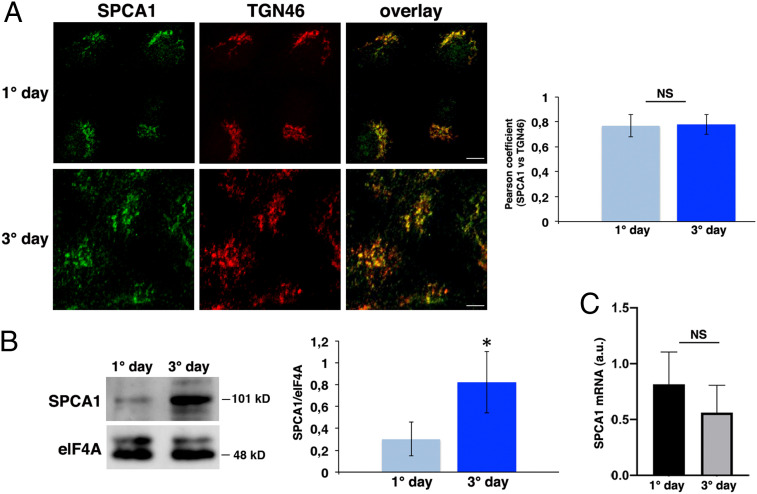
As for HeLa cells (22, 33), we found that endogenous SPCA1 localizes in the Golgi apparatus of both nonpolarized (1 d) and fully polarized (3 d) MDCK cells (Fig. 2A). In both conditions, it colocalized with the trans-Golgi marker TGN46 with a similar Pearson coefficient (0.77 ± 0.09 and 0.78 ± 0.08, respectively) (Fig. 2A). Interestingly, Western blot analysis revealed that fully polarized MDCK cells exhibit relative higher amounts of SPCA1 compared to nonpolarized MDCK cells (Fig. 2B), suggesting that the expression levels of SPCA1 may increase with the establishment of polarity. SPCA1 messenger RNA (mRNA) levels are comparable in fully polarized MDCK cells with respect to nonpolarized cells (Fig. 2C).
Fig. 2.
SPCA1 is localized at the TGN of fully polarized and nonpolarized MDCK cells, and its expression levels increase during polarization. (A) MDCK cells after 1 and 3 d were stained with SPCA1 and TGN46 antibodies and revealed by using Alexa 488 and Alexa 546, respectively. Experiments were performed three different times, and the Pearson’s coefficient for colocalization analysis was measured; n > 70 cells. (Scale bars, 10 and 5 μm [1° day and 3° day, respectively].) (B) MDCK cells grown for 1 and 3 d were tested for the expression of SPCA1 (101 kDa) by Western blotting. elF4A was used as a loading control, and the relative optical density of SPCA1 was normalized to elF4A levels. Quantification was made from eight different experiments and is expressed as a mean. (C) SPCA1 mRNA levels of MDCK cells grown for 1 or 3 d were analyzed by RT-qPCR and normalized to hypoxanthine-guanine phosphoribosyltransferase 1 (HPRT1) and Ubch5 mRNA levels; experiments were performed three independent times. Error bars, ± SD; NS, not significant; *P < 0.05, Student’s t test.
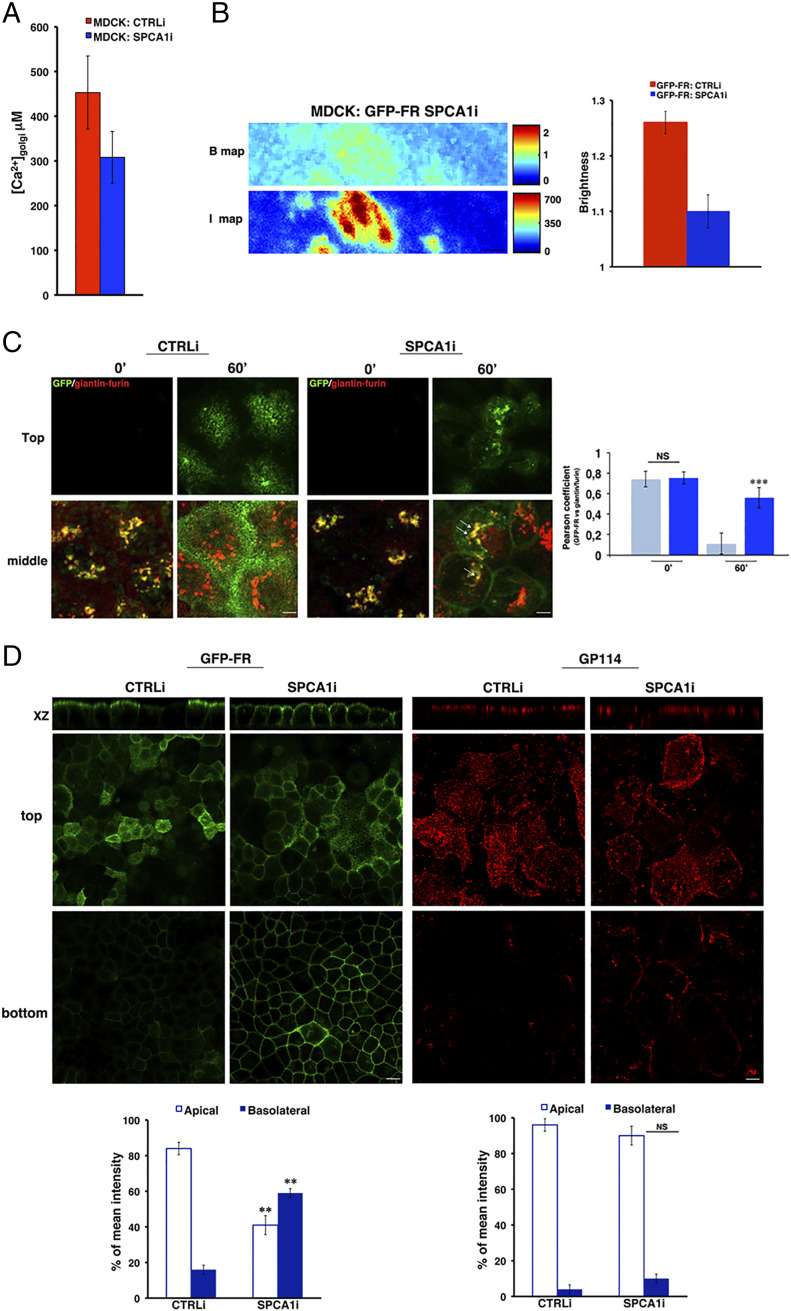
In order to investigate whether SPCA1 regulates the concentration of calcium in MDCK cells, we interfered the expression of SPCA1 by stably transfecting MDCK:GFP-FR cells with a specific short hairpin RNA (shRNA). We obtained several clones (SPCA1i) with different degrees of silencing ranging between 20 to 70% compared to scrambled interfered cells (CTRLi) as assessed by Western blotting and immunofluorescence (SI Appendix, Fig. S1). We used the clones with the highest degree of silencing for further studies. In these cells, we found a strong reduction in the concentration of calcium in the Golgi (Fig. 3A), indicating that the SPCA1 pump regulates calcium uptake into the Golgi apparatus of MDCK cells, similar to HeLa cells (22, 31). According to this hypothesis, like in wild-type MDCK cells (Fig. 1C), in control-interfered cells, calcium levels increase upon polarization (SI Appendix, Fig. S1C), whereas in SPCA1 silenced cells, the concentration of calcium in the Golgi is comparable in nonpolarized versus polarized conditions (SI Appendix, Fig. S1C).
Fig. 3.
The loss of SPCA1 affects Golgi homoclustering and the sorting of GPI-APs. (A) Golgi [Ca2] quantification in MDCK:GFP-FR control-interfered (CTRLi) or SPCA1-interfered (SPCA1i) cells were measured following the same procedure described in Fig. 1C. The data represent the mean of four independent experiments performed in two knockdown clones. (B) N&B analysis of GFP-FR in the Golgi of CTRLi and SPCA1i cells. Representative B and I maps of SPCA1i cells are shown. (Scale bars, 0.9 μm.) Quantification of the brightness of GFP-FR in the Golgi compartment from three independent experiments either in CTRLi (red bar) or SPCA1i (blue bar) cells. (C) CTRLi and SPCA1i cells grown for 3 d on a coverslip were subjected to a temperature block (19.5 °C) to accumulate proteins in the TGN. Then, cells were warmed at 37 °C for the indicated times, fixed, and treated for confocal microscopy. Representative images taken at the top and at the middle of the cells are shown. Pearson’s coefficient between GFP-FR and giantin/furin is shown as mean of three different experiments (CTRLi, cyan bars; SPCA1i, blue bars), n > 60 cells. (Scale bars, 4 μm.) (D) CTRLi or SPCA1i cells grown for 4 d on a filter were imaged in live conditions or stained with anti-GP114 antibody. Mean fluorescence intensities at the apical and basolateral surface were measured and expressed as percentages of the total fluorescence. (Scale bars, 12 and 6 μm in GFP-FR and GP114 panels, respectively.) Error bars, ± SD; NS, not significant; **P < 0.01; ***P < 0.001, Student’s t test.
Of importance, we found that the brightness of GFP-FR in the Golgi was significantly reduced in SPCA1i cells compared to scramble-interfered cells (from 1.26 to 1.1, P < 0.0001; Fig. 3B). Overall, these results show that SPCA1 regulates the concentration of calcium in the Golgi of polarized MDCK cells, and its down-regulation affects GPI-AP homoclustering.
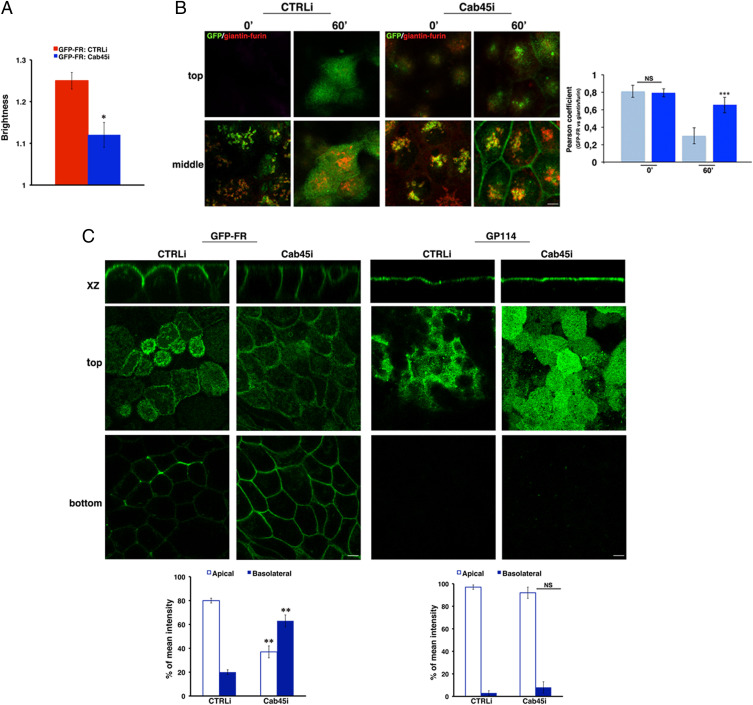
Because clustering of GPI-APs in the Golgi is crucial for their apical sorting (2, 4), we postulated that SPCA1 depletion would also alter their sorting and trafficking to the apical surface. To test this hypothesis, we monitored the transport kinetics of GFP-FR from the Golgi apparatus to the cell surface using cells grown to confluence on coverslips (Fig. 3C). We used time-lapse confocal experiments based on temperature block assays (at 19.5 °C) and subsequent warm up at 37 °C (Fig. 3C) as previously described (41). After temperature block (time 0), the protein is accumulated in the Golgi, and the Pearson’s coefficient of colocalization between GFP-FR and giantin/furin, two Golgi markers, is high in both control and SPCA1-silenced cells (Fig. 3C). As expected, in control cells 1 h after the release from the Golgi block, this colocalization decreases (Fig. 3C, Pearson coefficient of colocalization 0.64 ± 0.07 at time 0 and 0.11 ± 0.1 after 1 h; P < 0.0001), consistent with the exit of GFP-FR from the Golgi apparatus toward the surface. On the contrary, in the majority of SPCA1-silenced cells (∼70%), the colocalization between GFP-FR and giantin/furin remains high even 1 h after release from the Golgi block (Fig. 3C, Pearson coefficient of colocalization 0.75 ± 0.05 at time 0 and 0.55 ± 0.09 after 1 h; P < 0.05), indicating that a high amount of GFP-FR remains in the Golgi apparatus. In addition, it appeared that GFP-FR was also missorted to the basolateral surface (Fig. 3C), suggesting that the impairment of GFP-FR homoclustering observed in SPCA1i cells (Fig. 3B) affects both the Golgi exit and the apical sorting. In order to further investigate this hypothesis, we compared the distribution of GFP-FR at steady state in control and SPCA1-silenced cells grown on filters in fully polarized conditions. As shown in Fig. 3D and in SI Appendix, Fig. S2A, while in control cells, GFP-FR is present almost exclusively on the apical surface (80 to 85%), in the SPCA1i cells, it is found in large amounts (about 50 to 60%) at the basolateral surface.
Next, to test whether SPCA1 had a specific role in the apical sorting of GPI-APs, we analyzed the plasma membrane distribution of an endogenous apical transmembrane protein, GP114, in CTRLi and SPCA1i cells. Our data show no change in the polarized localization of this apical transmembrane protein, indicating that the loss of SPCA1 specifically affects the sorting of GPI-APs (Fig. 3D). As an additional control, we analyzed both the integrity of the monolayer and the sorting of an endogenous basolateral protein, E-cadherin. The distribution of the junctional protein ZO-1 and E-cadherin was comparable between the control and silenced cells (SI Appendix, Fig. S2 B and C), showing that SPCA1 knockdown does not alter the assembly of junctional complexes and therefore the integrity of epithelial cell monolayers and does not have a role in the basolateral sorting of transmembrane proteins.
All together, these data reveal that SPCA1 plays a specific role in regulating the calcium-dependent homoclustering of GPI-APs in the Golgi and their subsequent sorting and trafficking toward the apical surface.
Recently, a functional interplay between actin and SPCA1 via the actin filament–severing protein ADF/cofilin has been shown to promote the sorting of a subset of secretory proteins in HeLa cells (22, 23, 34). A similar mechanism could take place in polarized epithelial cells. To investigate this issue, we first asked whether perturbation of the actin cytoskeleton would affect GPI-AP protein sorting. To this aim, we measured the aggregation state of GFP-FR in the Golgi apparatus of polarized MDCK cells in control conditions and upon perturbation of the actin cytoskeleton by using latrunculin A (6 μM) as previously described (3, 42, 43) (SI Appendix, Fig. S3A). We found that, upon latrunculin treatment, the brightness values of GFP-FR did not vary compared to control cells (1.24 and 1.26, respectively; SI Appendix, Fig. S3A). Consistently, latrunculin A did not affect the migration of the Golgi pool of GFP-FR on velocity gradients, thus indicating that actin perturbation does not modulate its oligomeric status (SI Appendix, Fig. S3B). Furthermore, in agreement with the role of clustering for GPI-AP apical sorting (2, 4), upon latrunculin addition, GPI-APs were correctly sorted to the apical surface (SI Appendix, Fig. S3C).
Overall, these results suggest that the actin cytoskeleton is not involved in Golgi GPI-AP clustering and apical sorting in polarized MDCK cells.
The Golgi Calcium-Binding Protein Cab45 Is Involved in the Regulation of GPI-AP Homoclustering.
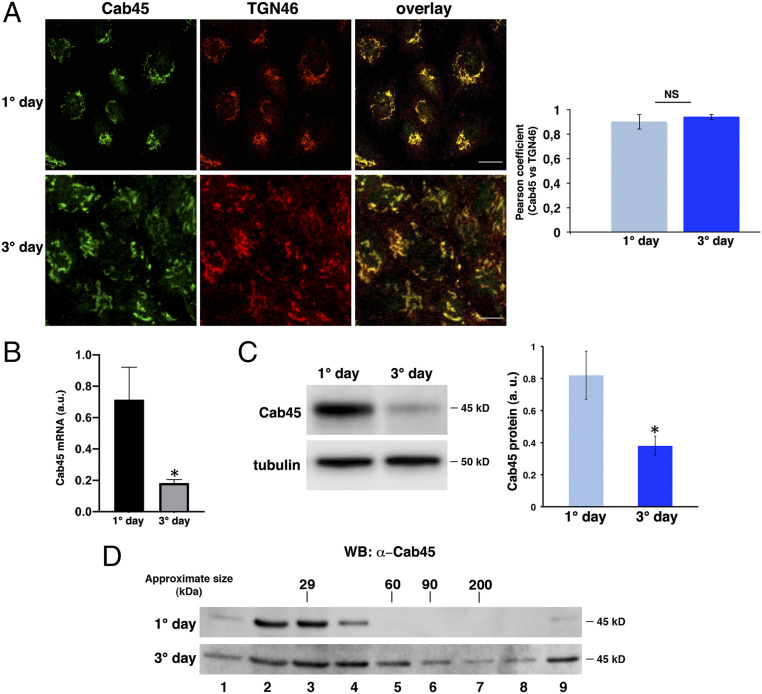
SPCA1-dependent calcium levels in the Golgi apparatus have been previously shown to regulate the segregation into secretory vesicles and export from the Golgi of a subset of secretory proteins (cartilage oligomeric matrix protein and lysozyme) in HeLa cells (22, 33). This was shown to be dependent on Cab45, a Golgi luminal protein, which oligomerizes upon calcium binding (44) and selectively interacts with these soluble secretory cargoes, allowing their export (33, 34, 45, 46). These data prompted us to investigate whether Cab45 could be involved in the regulation of apical sorting of GPI-APs in polarized cells. We first analyzed the expression and localization of Cab45 in both polarized and nonpolarized MDCK cells, and we found that, similar to HeLa cells, Cab45 is enriched in the TGN and colocalizes with the TGN46 marker in both conditions (Fig. 4A; Pearson coefficient: 0.9 ± 0.06 and 0.94 ± 0.02 in nonpolarized and polarized cells, respectively). qRT-PCR and Western blot analyses showed that mRNA and protein levels of Cab45 are higher in nonpolarized MDCK cells compared to polarized conditions (Fig. 4 B and C). Interestingly, by purification on velocity gradients, we observed that Cab45 is mostly monomeric in nonpolarized MDCK cells, while it forms an HMW complex in polarized MDCK cells (Fig. 4D), supporting a correlation between Cab45 clustering and the higher levels of calcium in the Golgi apparatus in polarized versus nonpolarized conditions (Fig. 1C). To understand the function of Cab45 in protein sorting in polarized MDCK cells, we generated stable knockdown Cab45 MDCK:GFP-FR cells (Cab45i). After infection with lentiviral particles containing a specific shRNA sequence targeted against Cab45, we selected MDCK GFP-FR clones exhibiting a decrease in Cab45 expression (SI Appendix, Fig. S4 A and B).
Fig. 4.
Cab45 localizes in the TGN and forms oligomers in polarized MDCK cells. (A) MDCK cells after 1 and 3 d were stained with Cab45 and TGN46 antibodies and revealed by using Alexa 488 and Alexa 546, respectively. Experiments were performed three different times, and the Pearson’s coefficient for colocalization analysis is shown; n > 65 cells. (Scale bars, 10 and 5 μm [1° day and 3° day, respectively].) (B) Cab45 mRNA levels of MDCK cells grown for 1 and 3 d were analyzed by RT-qPCR and normalized to HPRT and Ubch5 mRNA levels. Experiments were performed three independent times. (C) Cells were also tested for the expression of Cab45 (45 kDa) by Western blotting. Tubulin was used as a loading control, and the relative optical density of Cab45 was normalized to tubulin levels. The mean of four different experiments is shown. (D) Cells were lysed and run on velocity gradients; samples were analyzed as described in Fig. 1B. Molecular weight markers are indicated on top of the panels. The molecular weight of the monomeric form of Cab45 is indicated. Error bars, ± SD; NS, not significant; *P < 0.05, Student’s t test.
Next, we analyzed the aggregation state of GFP-FR and found that the brightness of GFP-FR is significantly reduced in the Golgi of Cab45i cells in comparison to CTRLi (Fig. 5A), indicating a reduction of GFP-FR homoclustering in the knockdown cells. By performing time-lapse microscopy, we monitored the trafficking of GFP-FR toward the surface in control and Cab45-silenced cells (Fig. 5B). Strikingly, while GFP-FR is similarly enriched in the Golgi apparatus in both CTRLi and Cab45i cells, after temperature block (time 0), we monitored a strong delay in GFP-FR Golgi exit in Cab45i cells as shown by a high Pearson’s coefficient of colocalization with giantin/furin after a 1-h release of the Golgi block at 37 °C (Fig. 5B, Pearson coefficient of colocalization 0.65 ± 0.09 and 0.2 ± 0.092 in Cab45i and CTRLi cells, respectively). In addition, it appeared that the majority of GFP-FR was missorted to the basolateral membrane in Cab45i cells (Fig. 5B), indicating that the impairment of GFP-FR homoclustering observed in Cab45i cells (Fig. 5A) affects both the Golgi exit and the apical sorting. These data were sustained by the analysis of the protein distribution at steady state in cells grown on filters in fully polarized conditions in which we observed more than 50% of GFP-FR at the basolateral surface in Cab45i cells compared to the apical enrichment in control cells (Fig. 5C and SI Appendix, Fig. S2A). Overall, these data show that in the absence of Cab45, GPI-APs do not cluster in the Golgi, and they are missorted to the basolateral surface. Importantly, we observed that the polarized distribution of apical and basolateral transmembrane proteins, GP114 and E-cadherin, respectively, was unaffected in Cab45 knockdown cells (Fig. 5C and SI Appendix, Fig. S4C), further supporting the specificity of the role of Cab45 in the apical sorting of GPI-APs. The effects of knockdown of Cab45 expression mimic the ones of calcium lowering, indicating that Cab45 might be the calcium-dependent modulator of GPI-AP clustering and apical sorting.
Fig. 5.
Cab45 is essential for apical sorting of GPI-APs. (A) N&B analysis of GFP-FR in the Golgi of polarized scrambled and Cab45-silenced MDCK cells. Quantification of the brightness of GFP-FR in the Golgi compartment from three independent experiments either in CTRLi (red bar) or Cab45i (blue bar) clones. (B) CTRLi and Cab45i cells grown for 3 d on a coverslip were subjected to a temperature block assay as described in Fig. 3C. Representative images taken at the top and at the middle of the cells are shown. Pearson’s coefficient between GFP-FR and giantin/furin is shown as mean of three different experiments (CTRLi, cyan bars; Cab45i, blue bars). (Scale bars, 4 μm.) (C) MDCK:GFP-FR CTRLi or Cab45i cells grown for 4 d on a filter were imaged in live conditions or stained with anti-GP114 antibody. Mean fluorescence intensities at the apical and basolateral surface were measured and expressed as percentages of the total fluorescence. (Scale bars, 6 μm.) Error bars, ± SD. NS, not significant; *P < 0.05; **P < 0.01; ***P < 0.001, Student’s t test.
Besides Cholesterol, Calcium Plays a Key Role in Apical Sorting of GPI-APs.
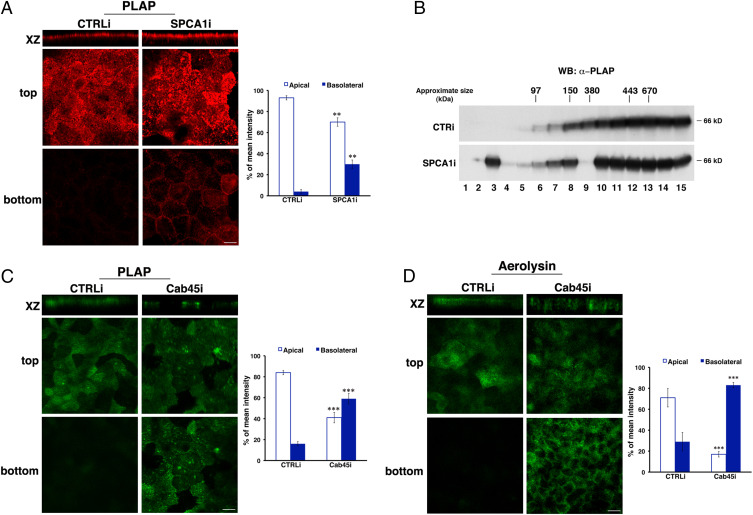
The above data have revealed the crucial role of calcium in homoclustering and the apical sorting of the model GPI-AP GFP-FR. To further corroborate these findings, we analyzed whether the suppression of SPCA1 or Cab45 impacts the sorting of a native GPI-AP, PLAP (placental alkaline phosphatase), and of endogenous proteins. To this aim, we generated knockdown MDCK:PLAP cells for SPCA1 (SI Appendix, Fig. S5) or Cab45 by using the same methods aforementioned. In agreement with data obtained for GFP-FR, we observed that PLAP (about 30 to 35%) is basolaterally missorted in SPCA1-silenced cells, unlike 3 to 5% of PLAP delivered to the basolateral side in control-interfered cells (Fig. 6A). Consistently, SPCA1 knockdown affects the PLAP homoclustering as shown by the reduction of HMW complexes on velocity gradients and a shift toward monomeric and dimeric forms (Fig. 6B). Moreover, comparable results were obtained in Cab45-silenced cells in which about 55 to 60% of PLAP is localized on the basolateral surface (Fig. 6C), further highlighting the essential role of Cab45 as a regulator of apical GPI-AP sorting.
Fig. 6.
Calcium is critical for clustering and apical sorting of GPI-APs. MDCK:PLAP CTRLi or SPCA1i cells, grown for 4 d on filters, were subjected to different assays. (A) Cells were stained with anti-PLAP antibody and revealed with Alexa 546 secondary antibody. Mean fluorescence intensities at the apical and basolateral surface were measured and expressed as percentages of the total fluorescence. (Scale bar, 6 μm.) (B) Cells were lysed and run on velocity gradients. Fractions were collected from the top (Fraction 1) to bottom (Fraction 15), TCA precipitated, run on an SDS-PAGE gel, and revealed by Western blotting with anti-PLAP antibody. Molecular weight markers are indicated on top of the panels. The molecular weight of the monomeric form of PLAP is indicated. (C) Cells were processed as in A. (Scale bar, 10 μm.) (D) MDCK CTRLi or Cab45i cells, grown on a filter for 4 d, were incubated with ASSP (aerolysin mutant) conjugated to Alexa 488 before fixation. (Scale bar, 10 μm.) Mean fluorescence intensities at the apical and basolateral surface were expressed as percentages of the total fluorescence. Error bars, ± SD. **P < 0.01; ***P < 0.001, Student’s t test.
Taking advantage of the use of a fluorescent-conjugated version of the bacterial toxin aerolysin, which binds with high affinity GPI-APs (47), we analyzed the surface distribution of endogenous GPI-APs upon Cab45 knockdown (Fig. 6D). As expected in CTRLi cells, about 70% (±9%) of GPI-APs are localized on the apical surface (Fig. 6D), while in Cab45-silenced cells, they are largely missorted to the basolateral surface (82% ± 3%), strengthening the role of a calcium-dependent mechanism in homoclustering and apical sorting of GPI-APs.
Importantly, we could show that the polarized distribution of a basolateral GPI-AP, GFP-PrP, was unaffected in Cab45 knockdown cells (SI Appendix, Fig. S6), indicating that this calcium-dependent mechanism is specific for GPI-AP apical sorting, and it does not work for the basolateral pathway.
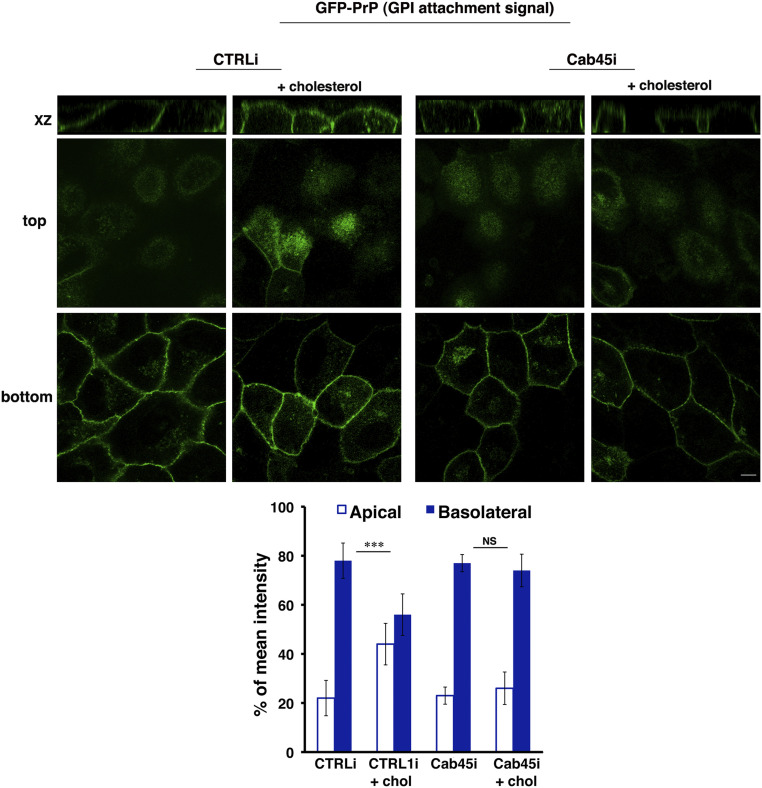
Next, to gain more insight into this mechanism, we analyzed whether Cab45 suppression affects the sorting of the chimeric GPI-AP in which the GFP protein is exclusively fused to the GPI attachment signal of PrP (3, 4). We have previously shown that this protein is basolaterally sorted, and the exogenous addition of cholesterol is sufficient to determine its oligomerization and redirect it to the apical surface (3, 4). We generated stable Cab45 knockdown MDCK:GFP-PrP (GPI attachment signal) cells, and we analyzed the impact of cholesterol addition.
As expected, in CTRLi cells upon cholesterol addition, a larger amount of GFP-PrP (GPI attachment signal) is sorted to the apical surface (44 versus 23%, treated versus untreated cells) (Fig. 7). On the contrary, the exogenous cholesterol addition is not sufficient to redirect GFP-PrP (GPI attachment signal) to the apical surface (23 versus 25%, treated versus untreated cells) in Cab45i cells (Fig. 7), further supporting the critical role of Cab45 as a calcium-dependent modulator in GPI-AP apical sorting.
Fig. 7.
Cholesterol is not sufficient to redirect the basolateral GFP-PrP to the apical surface upon Cab45 knockdown. MDCK:GFP-PrP (GPI attachment signal) CTRLi and Cab45i cells, grown for 4 d on a filter, were incubated or not for 1 h at 37 °C with water-soluble cholesterol (3, 4) before fixation. Mean fluorescence intensities at the apical and basolateral surface were expressed as percentages of the total fluorescence. Error bars, ± SD. NS, not significant; ***P < 0.001, Student’s t test.
Overall, these data indicate that both cholesterol and calcium are key regulators of Golgi GPI-AP clustering and apical sorting and are both crucial for these processes.
Discussion
Proper protein sorting and trafficking to the cell surface is essential for the establishment and maintenance of epithelial properties and function of epithelial cells.
GPI-APs are selectively localized at the apical surface in the majority of epithelia (reviewed in refs. 8, 27, and 48) and are sorted at the TGN (41, 49), the major protein-sorting station (50). It has been demonstrated that protein oligomerization is the key step to determine the apical sorting of GPI-APs in epithelial cells of different origins (2, 51). In particular, clusters of single GPI-AP species (named homoclusters) form in the Golgi apparatus of fully polarized cells when proteins traverse the medial Golgi (2, 51). The formation of GPI-AP homoclusters in the Golgi is dependent on the cholesterol concentration, while afterward, the formed homoclusters become insensitive to cholesterol depletion (1, 2, 4). It has been shown that the mechanisms responsible for homoclustering and apical sorting of GPI-APs regulate their subsequent organization in heteroclusters at the apical plasma membrane and their functional activity (1). This is important, as this mechanism would ensure that only correctly sorted proteins to the apical membrane are organized in functional clusters, while the missorted ones remain monomeric/dimeric and inactive (1, 8, 48). This implies that in epithelial cells, the functional cluster organization of GPI-APs at the apical plasma membrane is strictly linked with the acquisition of epithelial polarity (1). Hence, in nonpolarized conditions, GPI-APs are not clustered at the cell surface due to a lack of clustering in the Golgi (1).
This is very different from what occurs in fibroblasts, where GPI-APs are organized in clusters in response to surface cues (6, 52–57). The mechanism that would assure a rapid change in the Golgi of polarized cells compared to nonpolarized conditions remains unclear.
Lipidomic analyses have shown that epithelial cells undergo drastic changes in lipid compositions during cell polarization, among which is an increase in cholesterol (58). Previously, we have shown that the levels of cholesterol in the Golgi of polarized and nonpolarized cells are different (1); however, while high cholesterol concentration is necessary for the clustering of GPI-APs, this is not sufficient for their apical sorting (4).
Here, we discovered that another difference between the Golgi of polarized and nonpolarized MDCK cells is the calcium concentration, which increases substantially during the acquisition of the fully polarized phenotype (Fig. 1C). This was never reported before; nonetheless, the calcium content of the Golgi complex is known to be high (28, 29, 59) and has been shown to regulate protein processing and the sorting of secreted soluble proteins in nonpolarized cells (30, 31). High calcium concentrations in the TGN promote the selective aggregation of secretory granule cargoes such as granins, which is a key step for the sorting of regulated proteins (60–62). Therefore, we assessed whether calcium had a role in the mechanism of GPI-AP clustering occurring in the Golgi of polarized MDCK cells prior to their apical sorting. We show that ionomycin, an agent perturbing/lowering the calcium levels, impaired the homoclustering of GPI-APs in the Golgi, thus indicating that calcium levels are critical for this process.
Two groups of phosphorylation-type calcium pumps, the well-known SERCAs and the more recently discovered SPCAs, regulate Golgi calcium levels (32). Interestingly, SPCA1 was found mainly in detergent-resistant fractions (where GPI-APs abound), and its activity is inhibited upon cholesterol depletion, while SERCA function was unaffected (40). Moreover, recent findings have shown that SPCA1, by mediating calcium influx in the Golgi, plays a key role in the trafficking of secretory cargoes and virus maturation (23, 30, 33, 63). Here, we show that the concentration of calcium in the Golgi of MDCK cells is dependent on SPCA1. Most importantly, its protein expression increases during polarization, supporting that calcium in the Golgi lumen is a crucial determinant in the establishment of epithelial polarized phenotype. Remarkably, SPCA1 knockdown results in the loss of GPI-AP oligomerization and missorting to the basolateral surface, highlighting a role of SPCA1 in the apical sorting of GPI-APs. This appears to be specific for GPI-APs, as SPCA1 knockdown does not alter sorting of apical transmembrane proteins, further supporting that the mechanisms regulating GPI-AP and transmembrane protein sorting to the apical surface are different.
It is conceivable that Golgi calcium homeostasis is influenced by other intracellular calcium stores [e.g., endoplasmic reticulum (ER), cytosol, endosomes], or, vice versa, the SPCA1 knockdown may impact the calcium concentration of other organelles. While the initial focus centered on membrane contact sites between the ER and mitochondria (crucial for the transfer of calcium and lipids), in the last years, it is becoming clear that all organelles can contact each other, including the Golgi apparatus (64). Efficient calcium transfer was ascribed to the ER–plasma membrane contact sites and to ER–Golgi contacts (64, 65). To investigate whether the integration of calcium signals from diverse compartments may synergically regulate their homeostasis and polarized trafficking will be important in the future.
How SPCA1 would be regulated in the Golgi of polarized epithelial cells is an important question. We showed here that the actin cytoskeleton does not regulate Golgi GPI-AP clustering and apical sorting, suggesting that the SPCA1-mediated sorting of GPI-APs is independent of actin in epithelial cells. These data indicate that the mechanism regulating the activity of the SPCA1 in polarized cells and in HeLa cells is different. Interestingly, it was reported that SPCA1 is localized in cholesterol-enriched microdomains in colon adenocarcinoma and kidney cells (40) and that the SPCA1 pump’s activity seems to be regulated by the levels of cholesterol (40). Cholesterol levels in the Golgi cisternae of polarized MDCK cells are higher compared to nonpolarized MDCK cells (1, 58), and we have previously shown that high cholesterol levels in the Golgi are required but not sufficient for GPI-AP oligomerization (4). Indeed, this process needs favorable cholesterol-enriched membrane environments but depends also on protein–protein interactions of the GPI-AP ectodomains (2, 4, 27). Therefore, in light of our results, one possibility is that cholesterol would indirectly favor GPI-AP homoclustering by regulating the activity of the SPCA1, thereby controlling Golgi calcium levels.
Moreover, a recent study showed that in HeLa cells, SPCA1 activity is regulated by the levels of sphingomyelin in Golgi membranes (35). Therefore, another open question is whether sphingomyelin would also contribute to regulate SPCA1 activity in polarized MDCK cells. To date, the role of sphingolipids in GPI-AP sorting is still not clear. In thyroid polarized epithelial cells, the treatment with the ceramide synthase inhibitor fumonisin B1 leads to the basolateral missorting of GPI-APs (66) but also impairs the apical transport of transmembrane proteins, supporting a role of sphingolipids in promoting apical versus basolateral segregation rather than specifically affecting GPI-APs (66). Similar conclusions arose from studies showing that in MDCK cells, the knockdown of FAPP2 (four-phosphate-adaptor protein 2), a protein which is required for the synthesis of glycosphingolipids, resulted in delayed delivery and/or intracellular accumulation of apical raft-associated proteins (either transmembrane and GPI-AP), whereas the basolateral transport was unaffected (67). On the other hand, a recent study showing a functional link between GPI remodeling and glycosphingolipid biosynthesis (68) points to a novel critical role for sphingolipid pathways in the segregation and formation of apical GPI-AP vesicles. It will be interesting to test this hypothesis in the future.
Besides interplay between lipids and calcium, calcium per se would exert its effect on GPI-AP clustering through the binding of specific calcium-binding proteins. Several calcium-binding proteins, such as calmodulin, have been shown to mediate fusion between yeast vacuoles, the later steps of fusion vesicle trafficking and endosome fusion. At the level of the Golgi, two luminal calcium-binding proteins, Cab45 and p54/NEFA, have been identified. In nonpolarized cells, in response to SPCA1-mediated calcium influx, the Golgi resident binding protein Cab45 oligomerizes and in turn promotes oligomerization of soluble cargoes, and sequestering them within subdomains favors their export from the TGN in sphingomyelin-enriched vesicles (30, 33–35, 45, 46).
Here, we demonstrate that Cab45 localizes in the TGN of MDCK cells and forms oligomers only in fully polarized MDCK cells (and not in nonpolarized conditions), which is in good correlation with the higher levels of calcium in the Golgi of these cells. We further show that the silencing of Cab45 induces a reduction of homoclusters of GFP-FR and leads to its basolateral missorting. Importantly, we highlighted the requirement of Cab45 for the apical sorting of the native GPI-AP PLAP and of endogenous GPI-APs, further strengthening its role in GPI-AP sorting. Also in this case, as shown for SCPA1, the knockdown of Cab45 affects specifically the polarized trafficking of apical GPI-APs but not of basolateral ones as well as apical and basolateral transmembrane proteins, indicating that Cab45 is critical for Golgi clustering of GPI-APs and their apical sorting. Moreover, the fact that the mRNA of both SPCA1 and Cab45 are higher in cells expressing apical GPI-APs (SI Appendix, Fig. S7) strengthens the key role of these two factors in GPI-AP apical sorting. On the other hand, in these cells, we detected higher protein levels only for SPCA1 and not for Cab45, which may imply different mechanisms regulating the activity of these proteins.
This calcium-dependent mechanism is crucial for GPI-AP sorting because when it is inhibited, the increased Golgi cholesterol levels, which are sufficient to redirect apically a basolateral GPI-AP (3, 4), are unable to promote their apical sorting. Thus, both cholesterol and calcium are key determinants and concur to apical sorting of GPI-APs.
Overall, this study revealed in polarized epithelial cells an unexpected role for the calcium levels in mediating Golgi clustering and sorting of GPI-APs and unraveled key players of the molecular machinery regulating these processes.
It is worth noting that both SPCA1 and Cab45 undergo changes during polarization (increased protein expression and oligomerization, respectively), clearly indicating that their activity is strictly related to the acquisition of a polarized cell phenotype. Hence, the control of these two players may fall in the global epithelial polarity program (69, 70). Posttranslational rather than transcriptional mechanisms regulate SPCA1 expression. For example, SPCA1 may undergo a lower and/or slower turnover of protein levels. Its increased stability might correlate with its partitioning into lipid microdomains (40), whose main lipid components increase during cell polarization (58). Further studies will be necessary to test this hypothesis. On the other side, why Cab45 expression decreases during polarization remains an intriguing open question. One possibility is that Cab45 might be implicated in other processes important for the establishment of polarized cell phenotypes.
Moreover, another key question is through which mechanism Cab45 can sort GPI-APs. Pulldown experiments of HeLa cell lysates combined with mass spectrometry analysis suggest that Cab45 interacts with secretory proteins (34), and this also could be the case for GPI-APs. Whether and how Cab45 may recognize protein ectodomains and/or lipid anchors and whether Cab45 interacts with monomers favoring their clustering or binds GPI-AP oligomers by stabilizing them remain the most intriguing questions. The fact that Cab45 is mainly localized in the TGN of MDCK cells when the oligomers are already formed, as previously shown in pulse chase experiments (2), supports the role of Cab45 as a stabilizer of GPI-AP clusters. So far, no evidence exists showing that different GPI-APs share some structural similarities except that their C-terminal portion is characterized by four regions (including the GPI attachment signal, amino acids upstream of the omega site), all determining the efficiency of the GPI transamidation reaction (71). Moreover, posttranslational modifications and/or lipid remodeling of the GPI anchor may influence this interaction. A deep comparative analysis of protein ectodomains and lipid anchors of different GPI-APs will be the next challenge as well as that to unravel the common structural elements between GPI-APs and secreted proteins.
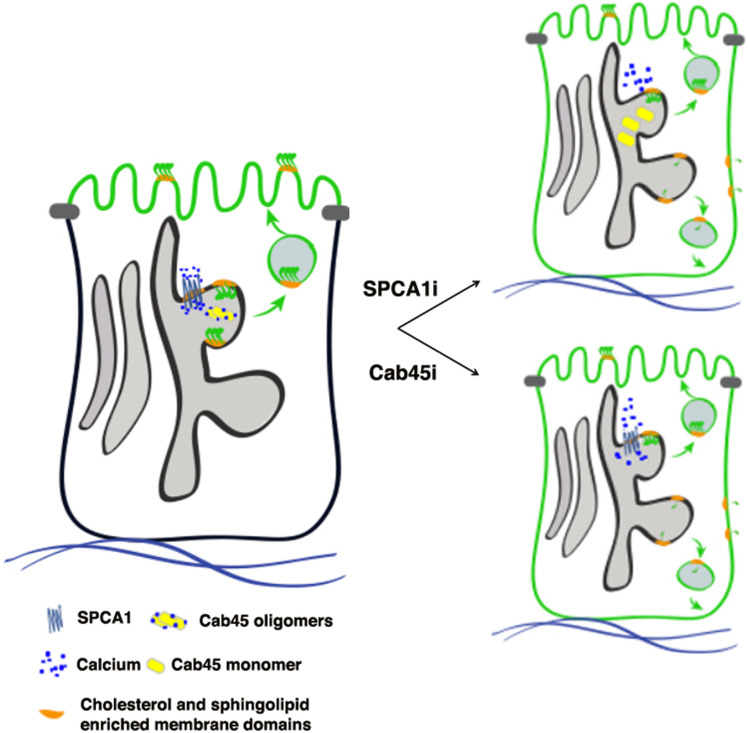
We propose a model of Golgi GPI-AP clustering in polarized MDCK cells in which the cholesterol environment could 1) allow the establishment of the lipid environment favorable for GPI-AP oligomerization and 2) regulate the activity of the pump, which in turn is important for the oligomerization of Cab45 that stabilizes GPI-AP oligomers in a cholesterol-independent manner and facilitates their segregation in apically sorted vesicles (Fig. 8).
Fig. 8.
Model of GPI-AP clustering and sorting in MDCK cells. The scheme depicts the role of calcium in the Golgi clustering of GPI-APs (for simplicity, only one GPI-AP is shown). A GPI-AP oligomerizes (forming a homocluster) after its association to lipid microdomains in the medial Golgi. The Golgi homoclustering regulates apical sorting of GPI-APs. Calcium uptake in the TGN, governed by SPCA1, is essential for GPI-AP clustering. In control cells, SPCA1 allows the uptake of calcium within the Golgi that would lead to oligomerization of Cab45, which in turn stabilizes GPI-AP clustering and apical sorting. Upon SPCA1 knockdown, less calcium is uptaken by the Golgi, leading to the impairment of GPI-AP clustering that results in their missorting. Similarly, upon Cab45 knockdown, Golgi GPI-AP clustering is impaired, leading to their basolateral missorting.
Likely, this mechanism may operate in epithelial cells derived from different tissues and/or organs since the mRNA expression of both SPCA1 and Cab45 is quite comparable in organs composed of epithelial tissues [such as colon, kidney, liver, lung, thyroid, etc. (44, 72, 73)]; higher mRNA and protein expression have been observed in rat brain and testis as compared to other tissues (74). Interestingly, higher mRNA levels of SPCA1 were observed in fetal organs with respect to the adult counterpart (73). This is in agreement with our data and suggests that a higher level of the SPCA1 pump is critical in the early phases of epithelial differentiation.
Among epithelia, hepatocytes are peculiar because they sort the majority part of apical proteins, including GPI-APs, through transcytosis. Whether and at which level of the transcytotic route the calcium mechanism plays a role in the polarized trafficking of GPI-APs to liver canaliculi will be very interesting to study in the future.
Materials and Methods
Cell Cultures, Transfections, and Antibodies.
MDCK cells were grown in Dulbecco’s Modified Eagle Medium (Sigma-Aldrich) containing 5% fetal bovine serum. MDCK cells stably expressing the GPI-APs GFP-FR, PLAP, GFP-PrP full length, or GFP-PrP carrying exclusively the GPI attachment signal of PrP were generated previously (2–4).
Cells were stably knocked down using either shRNAs or lentiviral vector (for details, see SI Appendix).
The antibodies used were listed in SI Appendix.
Velocity Gradients.
Velocity gradients allowing purifying proteins according to their molecular weight independently of their association with membrane domains were performed as previously described (75, 76); for details, see SI Appendix.
Calcium Measurements.
We measured the concentration of calcium in the Golgi apparatus by using the chimeric photoprotein Golgi-aequorin following a previously described protocol (28, 39); for details, see SI Appendix.
Immunofluorescence.
All details regarding the immunofluorescence assays, image acquisition, and analyses are in SI Appendix.
N&B Experiments.
The N&B method, a technique based on moment analysis for the measurements of the average number of molecules and brightness in each pixel in fluorescence microscopy images (37), provides the state of aggregation of molecules in living cells with high spatial and temporal resolution. N&B experiments were carried out as previously described (1), and all details are in SI Appendix.
Statistical Analysis.
In N&B experiments, we used a two-tailed Student’s t test for statistical analysis as well as to quantify all immunofluorescence mean intensities; in RT-qPCR, one-way ANOVA using Prism was employed.
Further information (such as the perturbation of the actin cytoskeleton or cellular calcium content, temperature block, deglycosylation assay, RT-qPCR, and the labeling of endogenous GPI-APs) is in SI Appendix.
Supplementary Material
Acknowledgments
We want to thank Sara Cruz, a master student, for her contribution on some SPCA1 experiments, Lucrezia Zerillo for her help in some biochemical experiments, Patricia Chastagner and Christel Brou for their help for RT-qPCR, Silvia Parisi, who provided shRNAs against SPCA1, and Gisou Van Der Goot for the mutant aerolysin ASSP coupled to Alexa 488. We thank C. Brou and Michael Henderson for the critical reading of the manuscript. This work is supported by research grants from Agence Nationale de la Recherche ANR-16-CE16-0019-01 and Equipe Fondation Recherche Médicale 2014 (DEQ20140329557) to C.Z. D.L. is supported by the China Scholarship Council and University-Paris Saclay. P.P. is grateful to Camilla degli Scrovegni for continuous support, and P.P.’s laboratory is supported by the Italian Ministry of University and Research (Co-financing no. 20129JLHSY_002, Futuro in Ricerca no. RBFR10EGVP_001), Telethon (GGP15219/B), and Italian Association for Cancer Research (IG-14442).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2014709118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Paladino S., et al., Golgi sorting regulates organization and activity of GPI proteins at apical membranes. Nat. Chem. Biol. 10, 350–357 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paladino S., et al., Protein oligomerization modulates raft partitioning and apical sorting of GPI-anchored proteins. J. Cell Biol. 167, 699–709 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lebreton S., Paladino S., Zurzolo C., Selective roles for cholesterol and actin in compartmentalization of different proteins in the Golgi and plasma membrane of polarized cells. J. Biol. Chem. 283, 29545–29553 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paladino S., et al., Different GPI-attachment signals affect the oligomerisation of GPI-anchored proteins and their apical sorting. J. Cell Sci. 121, 4001–4007 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Hannan L. A., Lisanti M. P., Rodriguez-Boulan E., Edidin M., Correctly sorted molecules of a GPI-anchored protein are clustered and immobile when they arrive at the apical surface of MDCK cells. J. Cell Biol. 120, 353–358 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma P., et al., Nanoscale organization of multiple GPI-anchored proteins in living cell membranes. Cell 116, 577–589 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Raghupathy R., et al., Transbilayer lipid interactions mediate nanoclustering of lipid-anchored proteins. Cell 161, 581–594 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lebreton S., Zurzolo C., Paladino S., Organization of GPI-anchored proteins at the cell surface and its physiopathological relevance. Crit. Rev. Biochem. Mol. Biol. 53, 403–419 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Zurzolo C., Simons K., Glycosylphosphatidylinositol-anchored proteins: Membrane organization and transport. Biochim. Biophys. Acta 1858, 632–639 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Cao X., Surma M. A., Simons K., Polarized sorting and trafficking in epithelial cells. Cell Res. 22, 793–805 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prydz K., Tveit H., Vedeler A., Saraste J., Arrivals and departures at the plasma membrane: Direct and indirect transport routes. Cell Tissue Res. 352, 5–20 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Stoops E. H., Caplan M. J., Trafficking to the apical and basolateral membranes in polarized epithelial cells. J. Am. Soc. Nephrol. 25, 1375–1386 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weisz O. A., Rodriguez-Boulan E., Apical trafficking in epithelial cells: Signals, clusters and motors. J. Cell Sci. 122, 4253–4266 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez-Boulan E., Kreitzer G., Müsch A., Organization of vesicular trafficking in epithelia. Nat. Rev. Mol. Cell Biol. 6, 233–247 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Guet D., et al., Mechanical role of actin dynamics in the rheology of the Golgi complex and in Golgi-associated trafficking events. Curr. Biol. 24, 1700–1711 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Lázaro-Diéguez F., et al., Actin filaments are involved in the maintenance of Golgi cisternae morphology and intra-Golgi pH. Cell Motil. Cytoskeleton 63, 778–791 (2006). [DOI] [PubMed] [Google Scholar]
- 17.Miserey-Lenkei S., et al., Coupling fission and exit of RAB6 vesicles at Golgi hotspots through kinesin-myosin interactions. Nat. Commun. 8, 1254 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miserey-Lenkei S., et al., Rab and actomyosin-dependent fission of transport vesicles at the Golgi complex. Nat. Cell Biol. 12, 645–654 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Hirschberg K., et al., Kinetic analysis of secretory protein traffic and characterization of golgi to plasma membrane transport intermediates in living cells. J. Cell Biol. 143, 1485–1503 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lázaro-Diéguez F., et al., Variable actin dynamics requirement for the exit of different cargo from the trans-Golgi network. FEBS Lett. 581, 3875–3881 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Kessels M. M., Dong J., Leibig W., Westermann P., Qualmann B., Complexes of syndapin II with dynamin II promote vesicle formation at the trans-Golgi network. J. Cell Sci. 119, 1504–1516 (2006). [DOI] [PubMed] [Google Scholar]
- 22.von Blume J., et al., ADF/cofilin regulates secretory cargo sorting at the TGN via the Ca2+ ATPase SPCA1. Dev. Cell 20, 652–662 (2011). [DOI] [PubMed] [Google Scholar]
- 23.von Blume J., et al., Actin remodeling by ADF/cofilin is required for cargo sorting at the trans-Golgi network. J. Cell Biol. 187, 1055–1069 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen D., Müsch A., Rodriguez-Boulan E., Selective control of basolateral membrane protein polarity by cdc42. Traffic 2, 556–564 (2001). [DOI] [PubMed] [Google Scholar]
- 25.Kroschewski R., Hall A., Mellman I., Cdc42 controls secretory and endocytic transport to the basolateral plasma membrane of MDCK cells. Nat. Cell Biol. 1, 8–13 (1999). [DOI] [PubMed] [Google Scholar]
- 26.Müsch A., Cohen D., Kreitzer G., Rodriguez-Boulan E., cdc42 regulates the exit of apical and basolateral proteins from the trans-Golgi network. EMBO J. 20, 2171–2179 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lebreton S., Paladino S., Zurzolo C., Clustering in the Golgi apparatus governs sorting and function of GPI-APs in polarized epithelial cells. FEBS Lett. 593, 2351–2365 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Pinton P., Pozzan T., Rizzuto R., The Golgi apparatus is an inositol 1,4,5-trisphosphate-sensitive Ca2+ store, with functional properties distinct from those of the endoplasmic reticulum. EMBO J. 17, 5298–5308 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chandra S., Kable E. P., Morrison G. H., Webb W. W., Calcium sequestration in the Golgi apparatus of cultured mammalian cells revealed by laser scanning confocal microscopy and ion microscopy. J. Cell Sci. 100, 747–752 (1991). [DOI] [PubMed] [Google Scholar]
- 30.Kienzle C., von Blume J., Secretory cargo sorting at the trans-Golgi network. Trends Cell Biol. 24, 584–593 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Micaroni M., Calcium around the Golgi apparatus: Implications for intracellular membrane trafficking. Adv. Exp. Med. Biol. 740, 439–460 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Missiaen L., Dode L., Vanoevelen J., Raeymaekers L., Wuytack F., Calcium in the Golgi apparatus. Cell Calcium 41, 405–416 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Crevenna A. H., et al., Secretory cargo sorting by Ca2+-dependent Cab45 oligomerization at the trans-Golgi network. J. Cell Biol. 213, 305–314 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Blume J., et al., Cab45 is required for Ca(2+)-dependent secretory cargo sorting at the trans-Golgi network. J. Cell Biol. 199, 1057–1066 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deng Y., et al., Activity of the SPCA1 calcium pump couples sphingomyelin synthesis to sorting of secretory proteins in the trans-Golgi network. Dev. Cell 47, 464–478.e8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dalal R. B., Digman M. A., Horwitz A. F., Vetri V., Gratton E., Determination of particle number and brightness using a laser scanning confocal microscope operating in the analog mode. Microsc. Res. Tech. 71, 69–81 (2008). [DOI] [PubMed] [Google Scholar]
- 37.Digman M. A., Dalal R., Horwitz A. F., Gratton E., Mapping the number of molecules and brightness in the laser scanning microscope. Biophys. J. 94, 2320–2332 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aulestia F. J., Alonso M. T., García-Sancho J., Differential calcium handling by the cis and trans regions of the Golgi apparatus. Biochem. J. 466, 455–465 (2015). [DOI] [PubMed] [Google Scholar]
- 39.Bonora M., et al., Subcellular calcium measurements in mammalian cells using jellyfish photoprotein aequorin-based probes. Nat. Protoc. 8, 2105–2118 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Baron S., et al., The secretory pathway Ca(2+)-ATPase 1 is associated with cholesterol-rich microdomains of human colon adenocarcinoma cells. Biochim. Biophys. Acta 1798, 1512–1521 (2010). [DOI] [PubMed] [Google Scholar]
- 41.Paladino S., Pocard T., Catino M. A., Zurzolo C., GPI-anchored proteins are directly targeted to the apical surface in fully polarized MDCK cells. J. Cell Biol. 172, 1023–1034 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Egea G., Lázaro-Diéguez F., Vilella M., Actin dynamics at the Golgi complex in mammalian cells. Curr. Opin. Cell Biol. 18, 168–178 (2006). [DOI] [PubMed] [Google Scholar]
- 43.Morton W. M., Ayscough K. R., McLaughlin P. J., Latrunculin alters the actin-monomer subunit interface to prevent polymerization. Nat. Cell Biol. 2, 376–378 (2000). [DOI] [PubMed] [Google Scholar]
- 44.Scherer P. E., et al., Cab45, a novel (Ca2+)-binding protein localized to the Golgi lumen. J. Cell Biol. 133, 257–268 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blank B., von Blume J., Cab45-Unraveling key features of a novel secretory cargo sorter at the trans-Golgi network. Eur. J. Cell Biol. 96, 383–390 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Pakdel M., von Blume J., Exploring new routes for secretory protein export from the trans-Golgi network. Mol. Biol. Cell 29, 235–240 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fivaz M., et al., Differential sorting and fate of endocytosed GPI-anchored proteins. EMBO J. 21, 3989–4000 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paladino S., Lebreton S., Zurzolo C., Trafficking and membrane organization of GPI-anchored proteins in health and diseases. Curr. Top. Membr. 75, 269–303 (2015). [DOI] [PubMed] [Google Scholar]
- 49.Hua W., Sheff D., Toomre D., Mellman I., Vectorial insertion of apical and basolateral membrane proteins in polarized epithelial cells revealed by quantitative 3D live cell imaging. J. Cell Biol. 172, 1035–1044 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodriguez-Boulan E., Müsch A., Le Bivic A., Epithelial trafficking: New routes to familiar places. Curr. Opin. Cell Biol. 16, 436–442 (2004). [DOI] [PubMed] [Google Scholar]
- 51.Paladino S., Sarnataro D., Tivodar S., Zurzolo C., Oligomerization is a specific requirement for apical sorting of glycosyl-phosphatidylinositol-anchored proteins but not for non-raft-associated apical proteins. Traffic 8, 251–258 (2007). [DOI] [PubMed] [Google Scholar]
- 52.Brameshuber M., et al., Imaging of mobile long-lived nanoplatforms in the live cell plasma membrane. J. Biol. Chem. 285, 41765–41771 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalappurakkal J. M., et al., Integrin mechano-chemical signaling generates plasma membrane nanodomains that promote cell spreading. Cell 177, 1738–1756.e23 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sengupta P., et al., Probing protein heterogeneity in the plasma membrane using PALM and pair correlation analysis. Nat. Methods 8, 969–975 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suzuki K. G., et al., Transient GPI-anchored protein homodimers are units for raft organization and function. Nat. Chem. Biol. 8, 774–783 (2012). [DOI] [PubMed] [Google Scholar]
- 56.van Zanten T. S., et al., Hotspots of GPI-anchored proteins and integrin nanoclusters function as nucleation sites for cell adhesion. Proc. Natl. Acad. Sci. U.S.A. 106, 18557–18562 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Varma R., Mayor S., GPI-anchored proteins are organized in submicron domains at the cell surface. Nature 394, 798–801 (1998). [DOI] [PubMed] [Google Scholar]
- 58.Sampaio J. L., et al., Membrane lipidome of an epithelial cell line. Proc. Natl. Acad. Sci. U.S.A. 108, 1903–1907 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wong A. K., et al., Heterogeneity of Ca2+ handling among and within Golgi compartments. J. Mol. Cell Biol. 5, 266–276 (2013). [DOI] [PubMed] [Google Scholar]
- 60.Gerdes H. H., et al., The primary structure of human secretogranin II, a widespread tyrosine-sulfated secretory granule protein that exhibits low pH- and calcium-induced aggregation. J. Biol. Chem. 264, 12009–12015 (1989). [PubMed] [Google Scholar]
- 61.Chanat E., Pimplikar S. W., Stinchcombe J. C., Huttner W. B., What the granins tell us about the formation of secretory granules in neuroendocrine cells. Cell Biophys. 19, 85–91 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Borgonovo B., Ouwendijk J., Solimena M., Biogenesis of secretory granules. Curr. Opin. Cell Biol. 18, 365–370 (2006). [DOI] [PubMed] [Google Scholar]
- 63.Hoffmann H. H., et al., Diverse viruses require the calcium transporter SPCA1 for maturation and spread. Cell Host Microbe 22, 460–470.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scorrano L., et al., Coming together to define membrane contact sites. Nat. Commun. 10, 1287 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burgoyne T., Patel S., Eden E. R., Calcium signaling at ER membrane contact sites. Biochim. Biophys. Acta 1853, 2012–2017 (2015). [DOI] [PubMed] [Google Scholar]
- 66.Imjeti N. S., et al., N-Glycosylation instead of cholesterol mediates oligomerization and apical sorting of GPI-APs in FRT cells. Mol. Biol. Cell 22, 4621–4634 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vieira O. V., Verkade P., Manninen A., Simons K., FAPP2 is involved in the transport of apical cargo in polarized MDCK cells. J. Cell Biol. 170, 521–526 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Y., et al., Cross-talks of glycosylphosphatidylinositol biosynthesis with glycosphingolipid biosynthesis and ER-associated degradation. Nat. Commun. 11, 860 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Halbleib J. M., Sääf A. M., Brown P. O., Nelson W. J., Transcriptional modulation of genes encoding structural characteristics of differentiating enterocytes during development of a polarized epithelium in vitro. Mol. Biol. Cell 18, 4261–4278 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rodriguez-Boulan E., Macara I. G., Organization and execution of the epithelial polarity programme. Nat. Rev. Mol. Cell Biol. 15, 225–242 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eisenhaber B., Bork P., Eisenhaber F., Sequence properties of GPI-anchored proteins near the omega-site: Constraints for the polypeptide binding site of the putative transamidase. Protein Eng. 11, 1155–1161 (1998). [DOI] [PubMed] [Google Scholar]
- 72.Hu Z., et al., Mutations in ATP2C1, encoding a calcium pump, cause Hailey-Hailey disease. Nat. Genet. 24, 61–65 (2000). [DOI] [PubMed] [Google Scholar]
- 73.Vanoevelen J., et al., The secretory pathway Ca2+/Mn2+-ATPase 2 is a Golgi-localized pump with high affinity for Ca2+ ions. J. Biol. Chem. 280, 22800–22808 (2005). [DOI] [PubMed] [Google Scholar]
- 74.Wootton L. L., Argent C. C., Wheatley M., Michelangeli F., The expression, activity and localisation of the secretory pathway Ca2+-ATPase (SPCA1) in different mammalian tissues. Biochim. Biophys. Acta 1664, 189–197 (2004). [DOI] [PubMed] [Google Scholar]
- 75.Scheiffele P., Peränen J., Simons K., N-glycans as apical sorting signals in epithelial cells. Nature 378, 96–98 (1995). [DOI] [PubMed] [Google Scholar]
- 76.Tivodar S., et al., Analysis of detergent-resistant membranes associated with apical and basolateral GPI-anchored proteins in polarized epithelial cells. FEBS Lett. 580, 5705–5712 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.