Significance
Herbivore attack on plants is known to elicit defensive responses. Such environmentally induced responses can also be expressed by the offspring of attacked plants via DNA methylation—an epigenetic response—but little is known about if and how epigenetic induction varies with plant ontogeny (e.g., seedlings, reproductive plants). Here, we report that herbivory by caterpillars induced changes in the plant epigenome and chemical and physical defenses within and across generations in wild radish. We show that herbivore offense in a plant’s generation affects its progeny’s deployment of defenses throughout its life cycle and that herbivory operates as an important and nuanced driver of phenotypic diversity in plant populations.
Keywords: ecology, epigenetics, evolution, herbivory
Abstract
As they develop, many plants deploy shifts in antiherbivore defense allocation due to changing costs and benefits of their defensive traits. Plant defenses are known to be primed or directly induced by herbivore damage within generations and across generations by long-lasting epigenetic mechanisms. However, little is known about the differences between life stages of epigenetically inducible defensive traits across generations. To help fill this knowledge gap, we conducted a multigenerational experiment to determine whether defense induction in wild radish plants was reflected in chromatin modifications (DNA methylation); we then examined differences between seedlings and reproductive plants in current and transgenerational plasticity in chemical (glucosinolates) and physical (trichomes) defenses in this species. Herbivory triggered genome methylation both in targeted plants and their offspring. Within one generation, both defenses were highly inducible at the seedling stage, but only chemical defenses were inducible in reproductive plants. Across generations, herbivory experienced by mother plants caused strong direct induction of physical defenses in their progeny, with effects lasting from seedling to reproductive stages. For chemical defenses, however, this transgenerational induction was evident only in adults. Transgenerational priming was observed in physical and chemical defenses, particularly in adult plants. Our results show that transgenerational plasticity in plant defenses in response to herbivore offense differs for physical and chemical defense and changes across plant life stages.
Plants can sense natural enemies in their surrounding environment (1, 2) and, through epigenetic mechanisms, modify their own phenotype (3) and, potentially, the phenotype of their lineage across generations (3, 4). Phenotypic modifications resulting from environmental stresses can be manifested in several ecologically relevant traits (5), including induction of defenses against herbivory (6). Despite recent discoveries concerning the ecological and evolutionary implications of transgenerational effects (6), the consequences of epigenetic effects on plant defenses across different life stages remain unexplored (7).
Plants optimize their defensive resource allocation in response to the changing herbivory environment at different times in the life of an individual (ontogeny) and across generations. Effective resistance relies on the combination of multiple, sometimes genetically correlated, resistance traits (e.g., chemical and physical defenses) and strategies [e.g., constitutive and induced plastic defenses (2)]. Plastic allocation to defense is a cost-saving strategy that relies on the plants’ ability to perceive the risk of herbivore attack. The costs and benefits of plant defenses are expected to change throughout ontogeny and across generations. Inducibility of defenses in plants is generally greater in seedlings and juveniles of herbaceous species than in mature reproductive individuals (8). However, there have not been explicit tests of whether plant defensive allocation may be modified across life stages by the herbivore offense experienced by former generations through epigenetic transgenerational effects.
Based on resource allocation theory (8, 9), we hypothesize that the effect of induction (the ability to produce defenses in response to an initial herbivore offense), transgenerational induction (herbivory experienced by the mother plant leading to increased defenses in the progeny), and transgenerational priming (herbivory experienced by the mother plant leading to increased inducibility in the progeny) will be more evident in seedlings than in adult reproductive plants. This hypothesis is based on prior knowledge of plant development, whereby the seedling stage is a more vulnerable stage of the plant’s life cycle (9). Due to the plasticity of offspring in response to their current environment as they develop, transgenerational effects would be expected to be strongest during early life than later in ontogeny.
We analyze these changes in antiherbivore physical and chemical defenses at two ontogenetic stages (seedlings, adult plants) as a function of the herbivory suffered by plants in a current generation and their mothers. Determining whether transgenerational induction and priming of physical and chemical defenses occurs only at the seedling stage or continues throughout adult plant life should improve our understanding of plant development and of the effects of transgenerational plasticity on the herbivory-driven evolutionary ecology of plants.
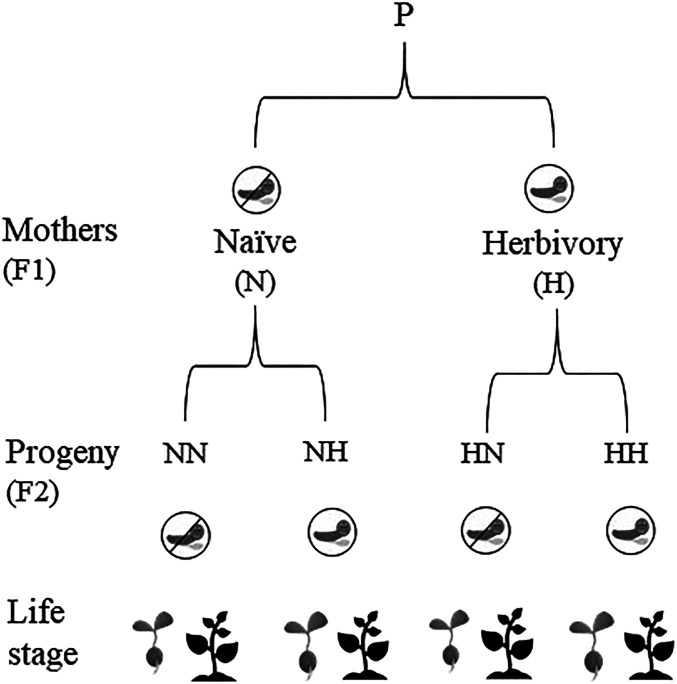
We performed a manipulative experiment (Fig. 1) with Raphanus sativus (wild radish) and its specialized prevalent herbivore, the caterpillar of the cabbage butterfly (Pieris rapae), to examine the ontogenetic trajectories of the plasticity of chemical and physical defenses. After testing whether herbivory triggered chromatin changes (via DNA methylation), we addressed three specific questions: i) to understand the patterns of inducibility across plant development (ontogeny), we studied whether, and how, the expression of chemical (glucosinolates) and physical (trichomes) defenses changes after herbivore attack, between life stages—seedlings and adult plants; ii) to understand the ontogenetic trajectory of transgenerational induction, we studied how the constitutive expression of trichomes and glucosinolates in nonattacked (naïve) plants at seedling and reproductive stages depends on whether their mothers experienced herbivory or not; and iii) to understand the ontogenetic trajectory of transgenerational priming, we studied how the expression of the defensive phenotype (trichomes and glucosinolates) in attacked plants depends on whether their mothers had experienced herbivory or not, and how this maternal effect on attacked plants differs between seedlings and adult plants. We show, firstly, that herbivory triggered chromatin modifications in wild radishes and their offspring. We then show that transgenerational defenses are found in both seedlings and adult plants, although they vary with plant life stage and type of defense. We conclude that the expression of plant defenses throughout plant ontogeny reflects both natural selective pressures across the plants’ life cycle and the effects of transgenerational plasticity.
Fig. 1.
Depiction of the full factorial experimental design of herbivore induction across two generations to study current and transgenerational effects of herbivory on plant defenses during ontogeny. First, an F1 generation of half-sib plants from a noninduced grandmaternal family (P) was produced. Mother plants (F1) were then either subjected to herbivory by P. rapae (H and caterpillar icon) or never exposed to herbivory (N, and icon crossed out). We then grew their progenies (F2), which were also subjected to herbivory or kept naïve to study plant responses to herbivory (inducibility, transgenerational induction, and transgenerational priming) at seedling (gray plant icons) and reproductive (black plant icons) stages.
Results
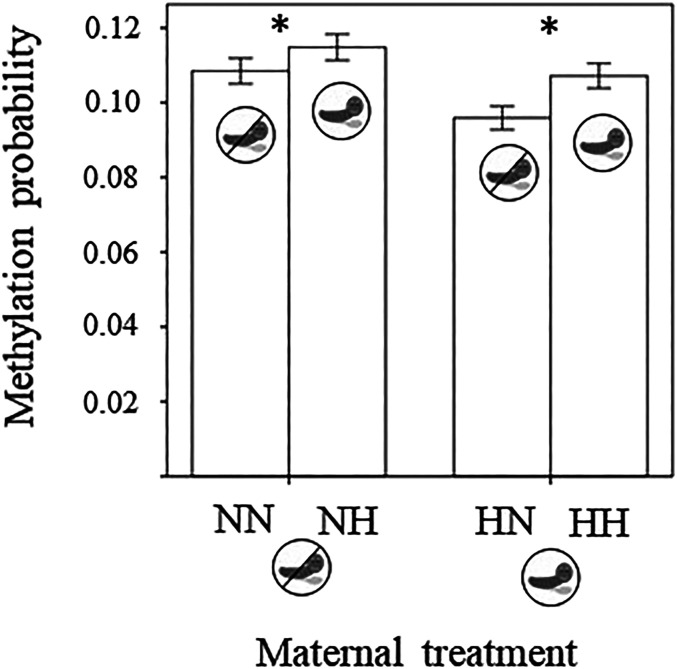
Both current and maternal exposure to herbivory by cabbage butterfly caterpillars significantly affected leaf genome methylation, in both maternal and progeny plant generations (Table 1). Methylation probability per locus was higher in seedlings subjected to herbivory than naïve seedlings, irrespective of whether mother plants had suffered herbivory or not (Table 1 and Fig. 2). However, methylation probability per locus was lower in the seedlings from attacked rather than naïve mothers (Table 1 and Fig. 1). This is consistent with the fact that only nonmethylated loci can undergo methylation after the plant experiences herbivory. If more loci have been methylated ahead of the treatment within an individual, this individual genome will acquire less methylation during the current herbivory treatment. Therefore, if there are transgenerational methylations, we expect that individuals coming from induced mothers will have a smaller number of new methylations after treatment. Thus, our result aligns with the expectation that some herbivory-sensitive loci were already methylated in plants descended from induced mothers due to transgenerational effects.
Table 1.
Results of the Generalized Linear Mixed Model analyzing methylation probability per marker as affected by herbivory experienced by progeny (herbivory) and their mother plants (maternal herbivory) of wild radish
| Response variable | Effects | Variance | SD | Estimate | SE | Z value | P value |
| Random | |||||||
| Marker | 0.523 | 0.723 | |||||
| Individual | 0.001 | 0.030 | |||||
| Fixed | |||||||
| Current herbivory | 0.112 | 0.0364 | 3.073 | 0.002 | |||
| Methylation event | Maternal herbivory | −0.094 | 0.0365 | −2.578 | 0.009 | ||
Significant P values are given in bold.
Fig. 2.
Effect of herbivory experienced by mother plants and their offspring on the methylation probability per locus in 402 genome makers of 94 wild radishes after the occurrence of a 2 wk herbivory event. The first letter refers to the maternal treatment and the second letter the current or progeny treatment. For example, HN refers to a naïve offspring from a mother that was attacked by herbivory (“H,” caterpillar icon). Asterisks indicate statistically significant results of pairwise contrasts (with P < 0.05). Bars indicate SE.
Constitutive Defenses.
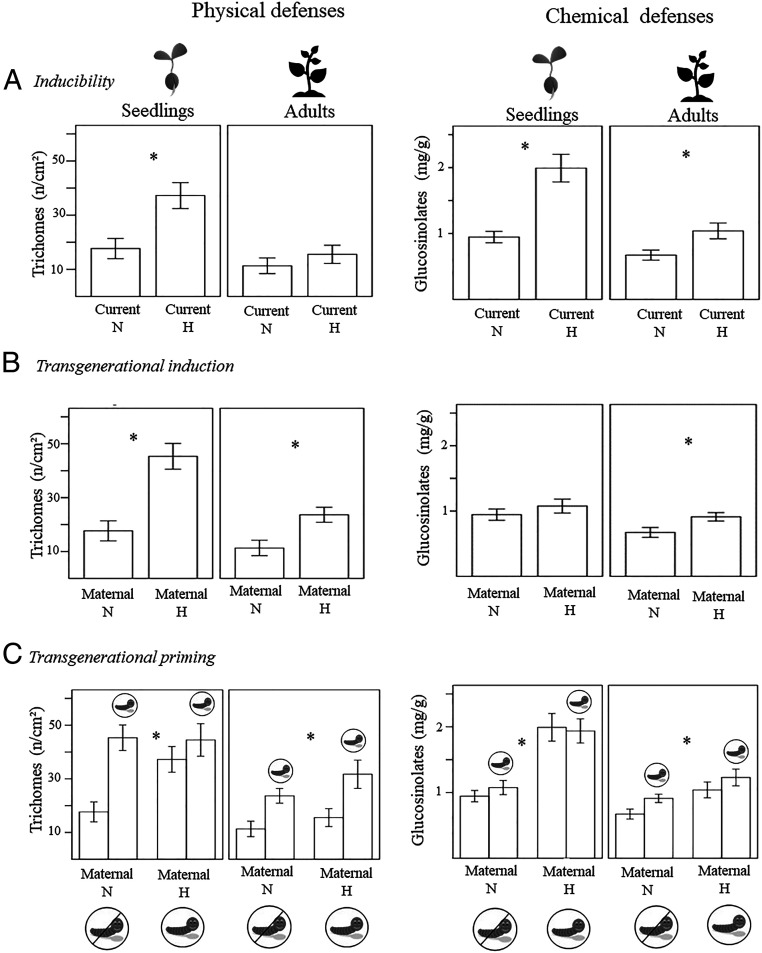
Naïve seedlings showed significantly greater levels of constitutive physical and chemical defenses than naïve reproductive adults (Table 2, “Age” effect). The specific comparison between nonattacked seedlings and adult plants (i.e., “Current N” in Fig. 3A) shows that this response was similar for both trichome density and glucosinolate concentration.
Table 2.
Results of two Generalized Linear Models analyzing the effects of plant age, maternal herbivory, progeny herbivory (herbivory), and the interactions on the expression of antiherbivore defenses (glucosinolate concentration and trichome density) in wild radish
| Response variable | Hypothesis tested | F value | df | P value |
| Physical defenses (Trichome density) | Inducibility | |||
| Age | 23.761 | 1;147 | <0.001 | |
| Herbivory | 4.741 | 1;147 | 0.031 | |
| Age × herbivory | 0.442 | 1;147 | 0.507 | |
| Transgenerational induction | ||||
| Maternal herbivory | 34.774 | 1;147 | <0.001 | |
| Age × maternal herbivory | 0.469 | 1;147 | 0.495 | |
| Transgenerational priming | ||||
| Herbivory × maternal herbivory | 4.409 | 1;147 | 0.037 | |
| Herbivory × maternal herbivory × age | 3.706 | 1;147 | 0.056 | |
| Chemical defenses (Glucosinolate concentration) | Inducibility | |||
| Age | 19.737 | 1;149 | <0.001 | |
| Herbivory | 32.429 | 1;149 | <0.001 | |
| Age × herbivory | 3.107 | 1;149 | 0.079 | |
| Transgenerational induction | ||||
| Maternal herbivory | 3.175 | 1;149 | 0.077 | |
| Age × maternal herbivory | 2.159 | 1;149 | 0.145 | |
| Transgenerational priming | ||||
| Herbivory × maternal herbivory | 0.695 | 1;149 | 0.040 | |
| Herbivory × maternal herbivory × age | 0.084 | 1;149 | 0.775 |
Significant (<0.05) and marginally significant (<0.10) P values are given in bold. df, degrees of freedom.
Fig. 3.
Plant defense plasticity across life stages. Intra- and intergenerational defense of plants experiencing herbivory (H) or no herbivory (N). Ontogenetic changes in constitutive defenses are shown through differences between naïve seedlings and adults (A). Inducibility is shown by exposing the progeny of naïve mothers to herbivory and examining changes between seedlings and adults in induced physical and chemical antiherbivore defenses (A). Transgenerational induction is shown by comparing the progeny of both attacked and naïve mother plants (B). Transgenerational priming is examined by comparing attacked and nonattacked progeny of both attacked and naïve mother plants (C). Asterisks indicate statistically significant results of pairwise contrasts (with P < 0.05) within each model. Bars indicate means ± SE. Statistical significance of the main effects and interactions is indicated in Table 2.
Induction in the Current Generation.
We found that trichome density and glucosinolate concentration were 1.8- and 2-fold greater, respectively, in seedlings after herbivory damage by P. rapae than in nonattacked seedlings, indicating that both physical and chemical defenses are inducible in seedlings (Table 2, herbivory effect; and Fig. 3A, effect of herbivory for physical and chemical defenses). In adult plants, we found a similar pattern (Table 2 and Fig. 3A), although the effect is less marked than in seedlings.
Transgenerational Induction.
The progeny of mothers that experienced herbivory showed a 2.5-fold increase in trichome density during the seedling stage over progeny of naïve mothers (Table 2 maternal herbivory effect; Fig. 3B). This effect lasted into the reproductive stage despite defensive allocation decreasing significantly. Although we found no evidence of transgenerational induction of chemical defenses in the seedlings, we observed a significant memory effect in later stages of their progeny’s lifecycle at the reproductive stage. Adult progeny of mothers subjected to herbivory showed a 1.3-fold higher glucosinolate concentration than did progeny of naïve mothers (Fig. 3B).
Transgenerational Priming.
Phenotypic changes elicited by herbivore offense in the previous generation were also transgenerationally expressed as increased inducibility of physical defenses in the progeny after further exposure to the same herbivory challenge. After experiencing herbivore damage, the progeny of exposed mothers showed a stronger induction response, with trichome density 1.4 times higher than that of the progeny of naïve plants, but this difference was only found at the adult stage (Table 2, herbivory × maternal herbivory effect, Fig. 3C). Inducibility of chemical defenses, however, was affected by herbivore offense in the previous generation both at the seedling and at the reproductive stage (Table 2, herbivory × maternal herbivory effect, Fig. 3C).
Discussion
This work shows that the patterns of defense deployment through plant ontogeny are partly shaped by herbivore-induced plasticity not only in the current generation but across generations too. Such influence of herbivory in the former generation can be directly expressed (direct induction) or retained as priming, a hidden potential, and thus expressed only in response to the appropriate herbivore cues early or late in the plant’s life. Additionally, these trajectories depend on the nature of the defense considered.
We found that physical and chemical defenses were highly inducible in response to herbivore attack at the seedling stage but less so in reproductive plants. This result agrees with the meta-analysis by Barton and Koricheva (8), who also found increased inducibility at younger rather than older stages in plant development. Other studies have found no evidence of inducibility of defenses in adult plants, leading to the conclusion that induced responses are age dependent (10). However, we uncovered results regarding transgenerational effects of herbivory in adult plants. Herbivory experienced by mother plants produced transgenerational induction of physical defenses (progeny with an increased constitutive density of trichomes), with effects lasting from seedling through reproductive stages. However, transgenerational induction of chemical defenses was apparent only later at the reproductive stage, and not at the seedling stage. We also found that herbivory experienced in the maternal generation resulted in progeny with a primed ability to induce physical and chemical defenses, and these effects of transgenerational priming are more visible at the adult stage.
Additionally, we found notable amounts of plasticity related to epigenetic mechanisms even with a background of low genetic variation (about 10% in methylated loci per individual within 2 wk), and thus we suggest that a large part of this plasticity functions independently of genomic variation. It is worth noting that the effect of herbivore induction in the maternal generation reduced methylation probability of herbivore-sensitive loci in the progeny, which would be expected if such methylation is transgenerationally transmitted. This apparently counterintuitive effect arises from the fact that loci that were already methylated due to transgenerational effects in the progeny of induced mothers could not undergo methylation again.
Plastic changes in wild radish plants in response to herbivory, such as increased foliar trichome density and glucosinolate concentration, can be regarded as ecologically beneficial because they are known to operate as effective, fitness-enhancing antiherbivore defenses (9). Inducibility of defenses is a cost-saving strategy, because defenses are expressed only when required; that is, when the risk of damage is confirmed either by plants or by theirs mothers. We previously demonstrated that transgenerational effects of herbivory in the wild radish are cumulative through multiple generations, negatively impacting seedling palatability for generalist slugs but not affecting palatability for specialist caterpillars (11). We now show that some of these cumulative transgenerational effects, with associated ecological benefits, are not limited to the seedling stage but are retained over a plant’s lifetime.
Adult, reproductive-stage wild radishes invested fewer resources than seedlings per quantity of tissue in chemical and physical defenses. However, they retained some memory of their mothers’ environment and had stronger chemical and physical defenses when they were the progeny of induced rather than noninduced mothers. Plants suffering herbivory were readier to express higher levels of defenses when their mothers had also suffered herbivory. This finding indicates that part of the transgenerational effect remains hidden as a priming effect in adults, which can readily express a stronger induced response if the attack cue is the same as that experienced by their mothers.
Induced transgenerational changes in the progeny’s phenotype are known to affect plant fitness (12), and the benefits derived may be at least partially independent of the genetic ancestry of maternal lineages (13). Thus, transgenerational plasticity may influence the course of evolution (14), potentially providing a shortcut to evolutionary change when these environmentally induced changes become stably inherited (15). Plastic transgenerational phenotypic variation, a direct result of perceived environmental conditions by previous generations, creates variation on which natural selection can act through its effect on ecological interactions (16).
Expression of a phenotypic variant may have advantages in one environment and costs in another. Thus, when environmental conditions vary from one generation to another or between ontogenetic stages, it may be beneficial to have the potential to develop a wide phenotypic spectrum. In this scenario, the selected trait is plasticity per se—the ability to express the plastic response (17). Our work shows that the ontogenetic trajectories of plant defenses can be shaped, at least in part, by transgenerational plastic responses. In other words, we found that ontogenetic trajectories of plant defenses are partly shaped by transgenerational effects induced by herbivory and related to epigenetic mechanisms, lasting from juveniles to adults, but depending on the considered defensive trait.
Although more research is needed to assess the generality of our findings, the results highlight the extent to which intra- and intergenerational plastic variation can operate as an important yet nuanced component of phenotypic diversity. This component holds potentially significant bearings on plant ecological and evolutionary processes.
Methods
System Natural History, Experimental Layout, Plant Culturing, and Herbivore Induction.
Wild radish, R. sativus L. (Brassicaceae) is a self-incompatible herbaceous annual weed common in Mediterranean climates. Caterpillars of P. rapae (Lepidoptera) are specialist herbivores to the Brassicaceae family. Glucosinolates and foliar trichomes have been described as effective resistance traits for R. sativus (1).
We produced an F1 generation of half-sib plants from a noninduced maternal family (P). This F1 generation, termed “mother plants,” comprised of plants that were either never exposed to herbivory (naïve mothers) or subjected to herbivory by P. rapae for 2 wk when plants were 2 wk old (induced mothers). We then grew their progeny (F2), keeping plants naïve or subjecting them to herbivory at two stages of their life cycle: seedling stage (two-leaf status, about 1 wk after germination) and reproductive stage (after blooming). Thus, we completed a fully factorial design of herbivore induction in mother plants and their progeny to study current and transgenerational phenotypic changes elicited by herbivory (physical and chemical defenses) at two life stages of the progeny (seedlings and adults). The study comprised of 160 plants (76 seedlings and 84 adult plants). Growing conditions were those described in Neylan et al. (11). Distribution of the 160 plants was as follows: 24 adult plants with maternal and current herbivory, 26 adult plants with maternal but no current herbivory, 18 adult plants with no maternal herbivory and with current herbivory, 16 adult plants without maternal or current herbivory, 21 seedlings with maternal and current herbivory, 22 seedlings with maternal but no current herbivory, 14 seedlings with no maternal herbivory and with current herbivory, and 19 seedlings without maternal or current herbivory. Chemical defenses were analyzed in 155 plants, and physical defenses were analyzed in 157 plants due to missing values.
Two P. rapae caterpillars were placed on the leaves of each plant in the herbivory treatment and allowed to feed freely for 2 wk. At the beginning of the experiment, plants of the seedling group had two leaves, and plants at the adult group were flowering. The caterpillars were at the second instar, and the experiment lasted 2 wk, until pupation. The plants germinated 4 or 5 d after sowing the seeds, flowered around 30 d later, and ended life with mature seeds at 2 mo. This means the herbivory treatment lasted around a quarter of the plants’ lives.
The attack by caterpillars on the plants was strong, in many cases almost all leaf tissue was consumed. But the plants were capable of consistently growing new tissue, and consequently no plants were killed by caterpillars. Only fresh, recently expanded leaves were sampled both for seedlings and adult plants. Any possible induction caused by sampling was controlled by sampling all plants across herbivory and nonherbivory treatments before and after treatment. We assume that any possible induction caused by sampling would affect each treatment group similarly and therefore would not affect comparisons between the groups.
We harvested the plants 2 wk after exposure to caterpillars and analyzed the number of trichomes and the glucosinolate concentration, the main physical and chemical defenses in this species, respectively. To quantify trichome density, we sampled two 2.7 cm diameter discs taken with a cork borer from the two most recently expanded leaves of each plant (n = 4 measures per plant). The number of trichomes in digital pictures of the discs was counted with ImageJ analysis software (18). For chemical defenses, leaf discs were sampled as above and flash frozen with liquid nitrogen, freeze dried, and stored at −80 °C. Glucosinolates were analyzed by chromatography, a protocol indicated in ref. 9, and concentration was expressed as milligram per gram of dry mass.
Inducibility, Transgenerational Induction, and Transgenerational Priming.
We also studied i) the ontogenetic trajectories of the expression of induced defenses after naïve plants experienced their first herbivory challenge (i.e., inducibility), ii) the ontogenetic trajectories of the maternal effects elicited by herbivory that directly increased constitutive allocation to defenses in their progeny (i.e., transgenerational induction), and iii) the ontogenetic trajectories of the maternal effects elicited by herbivory that primed the defense system of the progeny to produce an increased response when damaged by the same herbivores (i.e., transgenerational priming).
We performed two linear models for physical and chemical defenses. Statistical analyses were performed in RStudio for R version 4.0.2 (19). Models were fitted using the function glm from the lme4 package (20). Statistical significance of fixed effects was determined by analysis of deviance, Type II. Models included the fixed effects of age (seedlings and adults), maternal herbivory (mother plants that experienced herbivory and naïve mothers), and progeny herbivory (progeny exposed to herbivory and control). Models additionally included all the interactions between effects. Variables were log transformed to achieve normality in the distribution of the residuals.
To test the hypothesis that inducibility of chemical and physical defenses in response to an initial herbivory challenge changes throughout ontogeny, we analyzed the interaction between herbivory and age. A significant herbivory × age interaction would show that the effect of herbivory-induced defense allocation changed with age. To test the hypothesis that transgenerational induction of defenses is contingent on the ontogenetic stage, we examined maternal herbivory × age. For the third hypothesis, that transgenerational priming of defenses changes during ontogeny, we examined maternal herbivory × herbivory × age interaction.
Methylation Analyses.
To verify that phenotypic changes were related to chromatin changes, DNA methylation events experienced by individual genotypes were assessed in the progeny at the seedling stage (n = 94). A simplified MSAP (Methylation Sensitive Amplification Polymorphism) method was performed using only primer combinations with the methylation-sensitive HpaII (21). We collected leaf material from the F2 generation plants before and after exposure to herbivory. We cut one 2.7 cm diameter disk with a cork borer from two leaves as before. Leaf material was kept in dry silica gel until DNA extraction. Epigenetic characterization of individual plants before and after treatment was performed by methylation-sensitive amplification fragment length polymorphism. In this technique, genomic DNA is digested by methylation-sensitive enzymes, providing an epigenetic fingerprint of the plants. Thus, we used an epigenetic fingerprint of each plant before and after induction.
By studying the epigenetic fingerprint before and after induction, we can examine the occurrence of methylation events across the genome during treatment. We compared the chromatin changes occurring in the plants exposed to herbivory and in the naïve plants not attacked by herbivores. HpaII cleaves CCGG sequences when cytosines are not methylated. Cleaving may be impaired when at least one cytosine is hemimethylated and is inactive if one or both of the cytosines are fully methylated (16). Thus, within the same genotype, polymorphism of MSAP markers reflects variation in the methylation status of the CCGG sites. A change from presence to absence implies a methylation event in a locus, and a change from absence to presence indicates a de-methylation event.
DNA was isolated using the hexadecyl-trimethyl-ammonium-bromide procedure (22) followed by MSAFLP (Methylation Sensitive Amplification Fragment Length Polymorphism) fingerprinting. We analyzed 188 samples from 94 plants in the F2 generation using two primer combinations. The selected combinations X-AC/M-AC and X-AC/M-ATC were used for fragment amplification. MSAFLP analyses were carried out by Keygene Laboratories (Netherlands).
To test whether herbivory was linked to the probability of occurrence of methylation per locus, we proceeded as follows (23). Because methylation and demethylation cannot occur at the same time, we considered nonmethylation events only when demethylation did not occur on the marker. MSAP marker scores for samples were transformed by comparison with the corresponding values (i.e., same marker and plant individual before treatment). MSAP marker scores involving a change from 1 to 0 denoted a methylation event of the marker involved. Only fragments >300 base pairs in size were included to reduce the potential impact of size homoplasy (23). A new sample (n = 94 plant individuals) by marker (n = 402) score matrix was obtained, where each element showed whether the sample involved had experienced a methylation event (score = 1) or not (score = 0) in the corresponding marker (or missing when a demethylation event occurred and therefore a methylation event was not possible).
To test whether herbivory and maternal herbivory were related to chromatin changes, a generalized linear mixed model was fitted to the data (see equivalent approaches in refs. 21 and 24). Probability of methylation per marker was analyzed using a generalized linear mixed model in which the methylation event was fitted to a binomial distribution link logit, marker and individual were added as random factors, and current and maternal herbivory were added as fixed factors. This analysis was performed for the 94 individuals in F2 and the 402 markers selected. The response variable took a value of 0 when a methylation event did not occur during treatment and a value of 1 when a methylation event occurred during treatment. In cases where loci were already methylated before treatment and therefore methylation during treatment was not possible, we treated them as missing values. The model including the interaction between current and maternal treatments detected no significant interaction and performed worse in terms of AICc (Corrected Akaike Information Criterion).
Acknowledgments
We thank Ray von Itter and Virginia Walbot for assistance with greenhouse work. Marc Feldman, Tad Fukami, and the members of the R.D. laboratory read an earlier draft and offered valuable comments. Funding for this research was provided by Stanford University (R.D., unrestricted funds).
Footnotes
The authors declare no competing interest.
Data Availability
The csv data have been deposited in DRYAD (https://datadryad.org/stash/share/MQyOYLsxhXtuf2ZcUneOLG5JgnQoIPLtu6lE4aDmOME).
References
- 1.Agrawal A. A., Induced responses to herbivory and increased plant performance. Science 279, 1201–1202 (1998). [DOI] [PubMed] [Google Scholar]
- 2.Karban R., The ecology and evolution of induced resistance against herbivores. Funct. Ecol. 25, 339–347 (2011). [Google Scholar]
- 3.Verhoeven K. J., vonHoldt B. M., Sork V. L., Epigenetics in ecology and evolution: What we know and what we need to know. Mol. Ecol. 25, 1631–1638 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Jablonka E., Raz G., Transgenerational epigenetic inheritance: Prevalence, mechanisms, and implications for the study of heredity and evolution. Q. Rev. Biol. 84, 131–176 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Bossdorf O., Arcuri D., Richards C. L., Pigliucci M., Experimental alteration of DNA methylation affects the phenotypic plasticity of ecologically relevant traits in Arabidopsis thaliana. Evol. Ecol. 24, 541–553 (2010). [Google Scholar]
- 6.Holeski L. M., Jander G., Agrawal A. A., Transgenerational defense induction and epigenetic inheritance in plants. Trends Ecol. Evol. 27, 618–626 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Barton K. E., Boege K., Future directions in the ontogeny of plant defence: Understanding the evolutionary causes and consequences. Ecol. Lett. 20, 403–411 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Barton K. E., Koricheva J., The ontogeny of plant defense and herbivory: Characterizing general patterns using meta-analysis. Am. Nat. 175, 481–493 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Boege K., Dirzo R., Siemens D., Brown P., Ontogenetic switches from plant resistance to tolerance: Minimizing costs with age? Ecol. Lett. 10, 177–187 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Quintero C., Bowers M. D., Plant induced defenses depend more on plant age than previous history of damage: Implications for plant-herbivore interactions. J. Chem. Ecol. 37, 992–1001 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Neylan I. P., Dirzo R., Sobral M., Cumulative effects of transgenerational induction on plant palatability to generalist and specialist herbivores. Web Ecol. 18, 41–46 (2018). [Google Scholar]
- 12.Agrawal A. A., Induced responses to herbivory in wild radish: Effects on several herbivores and plant fitness. Ecology 80, 1713–1723 (1999). [Google Scholar]
- 13.Furrow R. E., Feldman M. W., Genetic variation and the evolution of epigenetic regulation. Evolution 68, 673–683 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Bonduriansky R., Day T., Nongenetic inheritance and its evolutionary implications. Annu. Rev. Ecol. Evol. Syst. 40, 103–125 (2008). [Google Scholar]
- 15.Bossdorf O., Richards C. L., Pigliucci M., Epigenetics for ecologists. Ecol. Lett. 11, 106–115 (2008). [DOI] [PubMed] [Google Scholar]
- 16.Alonso C., Ramos-Cruz D., Becker C., The role of plant epigenetics in biotic interactions. New Phytol. 221, 731–737 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jablonka E., Oborny B., Molnar I., Kisdi E., Hofbauer J., Czaran T., The adaptive advantage of phenotypic memory in changing environments. Philos. Trans. R. Soc. Lond. B Biol. Sci. 350, 133–141 (1995). [DOI] [PubMed] [Google Scholar]
- 18.Schneider C. A., Rasband W. S., Eliceiri K. W., NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.R. C. Team , R: A Language and Environment for Statistical Computing (R. C. Team, Vienna, Austria, 2013). [Google Scholar]
- 20.Bates D., Mächler M., Bolker B., Walker S., Fitting linear mixed-effects 388 models using lme4. J. Stat. Softw. 67, 1–48 (2015). [Google Scholar]
- 21.Herrera C. M., Pozo M. I., Bazaga P., Jack of all nectars, master of most: DNA methylation and the epigenetic basis of niche width in a flower-living yeast. Mol. Ecol. 21, 2602–2616 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Doyle J. J., A rapid total DNA preparation procedure for fresh plant tissue. Focus 12, 13–15 (1990). [Google Scholar]
- 23.Sobral M., I.P. Neylan, E. Narbona, R. Dirzo , Transgenerational plasticity in flower color induced by caterpilars. Front. Plant. Sci. 12, 10.3389/fpls.2021.618715 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrera C. M., Medrano P., Bazaga, Variation in DNA methylation transmissibility, genetic heterogeneity and fecundity related traits in natural populatuins of the perennial herb Helleborus foetidus. Mol. Ecol. 23, 1085–1095 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Vekemans X., Beauwens T., Lemaire M., Roldán-Ruiz I., Data from amplified fragment length polymorphism (AFLP) markers show indication of size homoplasy and of a relationship between degree of homoplasy and fragment size. Mol. Ecol. 11, 139–151 (2002). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The csv data have been deposited in DRYAD (https://datadryad.org/stash/share/MQyOYLsxhXtuf2ZcUneOLG5JgnQoIPLtu6lE4aDmOME).