Significance
Since Darwin’s ground-breaking monograph on carnivorous plants, scientists have recognized only 11 independent origins of plant carnivory. We report the discovery of a new lineage of carnivorous plants, represented by the North American flowering plant Triantha occidentalis. Among monocots, Triantha represents the only instance of a sticky-trap mechanism and a clearly documented case of holocarnivory, marked by enzymatic secretion consistent with prey digestion. Its trap is unique among carnivorous plants and, unexpected based on theory, in placing all of its prey-capture sites next to its insect-pollinated flowers. Given the existence of Triantha in close proximity to major urban centers on the Pacific coast, our study serves as a vivid reminder that other cryptic carnivores may yet remain to be discovered.
Keywords: enzymatic digestion, holocarnivory, isotopic analysis, monocotyledons, predation/pollination conflict
Abstract
Carnivorous plants consume animals for mineral nutrients that enhance growth and reproduction in nutrient-poor environments. Here, we report that Triantha occidentalis (Tofieldiaceae) represents a previously overlooked carnivorous lineage that captures insects on sticky inflorescences. Field experiments, isotopic data, and mixing models demonstrate significant N transfer from prey to Triantha, with an estimated 64% of leaf N obtained from prey capture in previous years, comparable to levels inferred for the cooccurring round-leaved sundew, a recognized carnivore. N obtained via carnivory is exported from the inflorescence and developing fruits and may ultimately be transferred to next year’s leaves. Glandular hairs on flowering stems secrete phosphatase, as seen in all carnivorous plants that directly digest prey. Triantha is unique among carnivorous plants in capturing prey solely with sticky traps adjacent to its flowers, contrary to theory. However, its glandular hairs capture only small insects, unlike the large bees and butterflies that act as pollinators, which may minimize the conflict between carnivory and pollination.
Carnivorous plants, which overturn the usual trophic relationship between plants and animals, have long fascinated humans (1) and have been the subject of wide-ranging scientific research since the seminal monograph by Charles Darwin (2). Carnivorous plants fix carbon from photosynthesis but absorb mineral nutrients, especially N and P, from animal prey, helping them to survive and compete successfully in low-nutrient environments (3, 4). Eleven independent origins of carnivory are currently recognized, including ∼800 species in six orders, 13 families, and 20 genera of flowering plants, with eight origins in the eudicots and three in the monocot order Poales (5, 6). Members of other eudicot lineages have been suggested previously as being carnivorous, but these proposals lacked adequate experimental support for nutrient uptake or resulting increases in plant growth, competitive ability, and/or reproduction (1, 4, 7). The high energetic costs of structures and enzymes related to carnivory may limit carnivorous plants primarily to sunny, moist, nutrient-poor habitats where they can obtain an advantage in growth, survival, or reproduction (3, 4, 8), which may explain why carnivory occurs in only ∼0.2% of angiosperm species (5).
Over the past 2 decades, only one novel example of holocarnivory (9)—in which plants produce enzymes to digest prey—has been documented (Philcoxia, Plantaginaceae) (10). Carnivory is unknown in monocots outside order Poales (5). All three carnivorous lineages in Poales use leaf rosettes to accumulate water and drown insects in pitfall traps and (except possibly in Brocchinia reducta, Bromeliaceae) accomplish enzymatic degradation of prey primarily via microbial or insect partners, rather than through their own digestive enzymes (11).
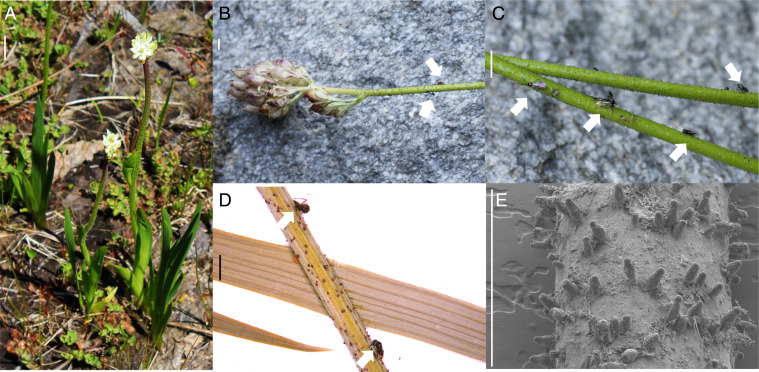
Triantha occidentalis (in the monocot family Tofieldiaceae, Alismatales; Fig. 1A) is a rhizomatous perennial herb found along the west coast of North America, from CA to AK (12). The four species of Triantha are native to North America and Japan. They occur in open nutrient-poor habitats, especially along streambanks and in wetlands, including bogs, marly shorelines, and calcareous spring-fed fens. In bogs, T. occidentalis is commonly found with recognized carnivorous plants such as Drosera rotundifolia (Droseraceae) and Pinguicula vulgaris (Lentibulariaceae). During the summer flowering season, T. occidentalis produces leafless erect flowering stems up to 80 cm tall (12). These scapes have sticky glandular hairs, especially on their upper portions, a feature distinguishing Triantha from other genera of Tofieldiaceae (Fig. 1). Small insects are often found trapped by these hairs; herbarium specimens are frequently covered in insects (Fig. 1D). Their glandular hairs bear shiny secretions and have a conspicuous reddish color (Fig. 1C), similar to those found in sundews and other sticky-trap carnivorous plants where they may play a role in luring prey (1, 3).
Fig. 1.
T. occidentalis (Tofieldiaceae) scapes bearing dense glandular hairs, several with trapped insects (arrows), including flies and beetles. (A) Whole flowering plants growing in a bog at Cypress Provincial Park, BC. Image credit: Danilo Lima (Universidade Estadual de Feira de Santana, Feira de Santana, Brazil). (B and C) Fresh field specimens from North Cascades National Park, WA, with a close-up view (C) showing sticky reddish glandular hairs. (D) Herbarium specimen (L. P. Janeway, collection no. 7318, Chico State Herbarium, CHSC) from Trinity, CA, with captured insects (arrows). (E) SEM of upper stem, plant from Cypress Provincial Park, BC. (Scale bar: A, 1 cm; B–E, 1.5 mm.)
In a study of plastid phylogenomics and molecular evolution of Alismatales, Ross et al. (13) found a novel loss in Triantha of genes involved in the plastid NADH dehydrogenase-like (NDH-1) complex, a ferredoxin-plastoquinone reductase thought to regulate photosynthetic electron flow, mitigate effects of PS I inhibition, and fine-tune photosynthesis in dynamic light conditions (14–16). Ross et al. (13) conjectured that Triantha might be cryptically carnivorous, in part because ndh gene loss has also been found in some carnivorous Lentibulariaceae (17) [this loss is otherwise uncommon outside heterotrophic plants (13)]. While a possible association between carnivory and loss of ndh genes is speculative, the observation by Ross et al. (13) aroused our curiosity and helped motivate this study, along with the shared habitat of T. occidentalis with, and its similar morphology to, sticky-trap carnivorous plants. We therefore investigated its potential status as a holocarnivorous plant, following procedures applied in other studies demonstrating carnivory (10, 18). If Triantha is carnivorous, it likely is so only during the flowering season, when glandular inflorescences are present. Catching insects using sticky inflorescence stems is rare; plants that do this include Silene (1) (Caryophyllaceae) and Stylidium (Stylidiaceae) (7), but these taxa have not been demonstrated to involve botanical carnivory.
Here we test the hypothesis that T. occidentalis is carnivorous by doing a field experiment with 15N-labeled insects to demonstrate nutrient uptake. Experiments using stable isotopes have frequently been used to investigate plant carnivory (18–20), in which significant accumulation of a heavier or lighter isotope in plant tissue provides clear evidence of nutrient transfer from dead animals to plants. We also conducted assays for phosphatase to see whether T. occidentalis produces this digestive enzyme, consistent with it being holocarnivorous.
Results and Discussion
Isotopic Data Demonstrate That Triantha Is Carnivorous.
The natural leaf N concentration of T. occidentalis at our field site in coastal British Columbia was 1.20 ± 0.14%, comparable to that of carnivorous D. rotundifolia (Droseraceae), and lower than noncarnivorous Erigeron peregrinus (Asteraceae) and Nephrophyllidium crista-galli (Menyanthaceae), all at the same site (Table 1). This is consistent with the differences typically seen between carnivorous and noncarnivorous plants in various habitats (8, 21). Carnivorous Philcoxia has higher leaf N concentrations than noncarnivores in a tropical white-sand habitat (10), but this may be because many of the latter species belonged to groups characterized by low leaf N content (C4 herbs or woody plants with very long-lived foliage). In contrast, a comparison of multiple carnivores (Drosera, Sarracenia, and Utricularia) and noncarnivores in a nutrient-poor bog in WI found no significant differences in leaf N concentration (22), similar to what we observed in BC for Drosera and Triantha. Unmanipulated leaves of both Drosera and Triantha also have significantly higher values of δ15N (P < 0.02; SI Appendix, Table S1 and Fig. S1) than similar leaves of the noncarnivores (Table 1), which is consistent with increased discrimination for 15N in moving up the trophic ladder, taken as a hallmark of N uptake from prey in other carnivorous plants (8, 23, 24).
Table 1.
Summary of δ15N and % nitrogen content in different species and treatments from Cypress Provincial Park, BC
| Mean ± SE δ15N | Mean ± SE. %N | No. of samples | |
| Noncarnivorous plants | |||
| Erigeron peregrinus (stem, control) | −8.94 ± 1.41 | 0.33 ± 0.03 | 5 |
| E. peregrinus (stem, 1 wk) | −8.46 ± 0.87 | 0.51 ± 0.08 | 5 |
| E. peregrinus (stem, 2 wk) | −8.84 ± 0.97 | 0.45 ± 0.02 | 5 |
| E. peregrinus (leaf, control) | −9.50 ± 0.37 | 2.03 ± 0.39 | 5 |
| Nephrophyllidium crista-galli (leaf) | −10.00 ± 1.11 | 1.76 ± 0.09 | 5 |
| Carnivorous plant | |||
| Drosera rotundifolia (leaf, control) | −0.98 ± 2.75 | 1.43 ± 0.13 | 5 |
| D. rotundifolia (leaf, 1 wk) | 416.86 ± 203.25 | 1.22 ± 0.19 | 5 |
| D. rotundifolia (leaf, 2 wk) | 550.76 ± 110.31 | 0.86 ± 0.09 | 5 |
| Putative carnivorous plant | |||
| Triantha occidentalis (leaf, control) | −2.28 ± 0.448 | 1.20 ± 0.14 | 5 |
| T. occidentalis (leaf, 1 wk) | −0.30 ± 0.548 | 1.27 ± 0.09 | 10 |
| T. occidentalis (leaf, 2 wk) | 7.49 ± 3.37 | 1.20 ± 0.14 | 10 |
| T. occidentalis (leaf, double insects) | 2.22 ± 0.85 | 1.09 ± 0.11 | 5 |
| T. occidentalis (fruit, control) | −1.66 ± 0.81 | 1.50 ± 0.35 | 5 |
| T. occidentalis (fruit, 1 wk) | 10.28 ± 5.00 | 2.60 ± 0.15 | 10 |
| T. occidentalis (fruit, 2 wk) | 19.24 ± 5.19 | 1.32 ± 0.22 | 10 |
| T. occidentalis (fruit, double insects) | 26.38 ± 5.70 | 0.94 ± 0.22 | 5 |
| T. occidentalis (stem, control) | −4.80 ± 0.85 | 0.32 ± 0.03 | 3 (+2 failed in analysis) |
| T. occidentalis (stem, 1 wk) | 14.50 ± 5.81 | 0.63 ± 0.09 | 10 |
| T. occidentalis (stem, 2 wk) | 534.83 ± 160.61 | 0.30 ± 0.02 | 8 (+2 failed in analysis) |
| T. occidentalis (stem, double insects) | 385.53 ± 130.98 | 0.23 ± 0.01 | 4 (+1 failed in analysis) |
| Insects collected at study site | 1.71 ± 0.56 | 4.38 ± 1.43 | 4 |
| Drosophila melanogaster (unlabeled) | 3.99 ± 0.08 | 9.47 ± 0.05 | 4 |
| Drosophila melanogaster (labeled) | 7,471.61 ± 224.06 | 9.15 ± 0.39 | 6 |
Plants were exposed to four 15N-labeled flies exposed for 1 or 2 wk or eight labeled flies exposed for 2 wk (double insects). Control plants were exposed to unlabeled flies for 2 wk. A subset of this table is summarized graphically in Fig. 2, with statistical analyses presented in SI Appendix.
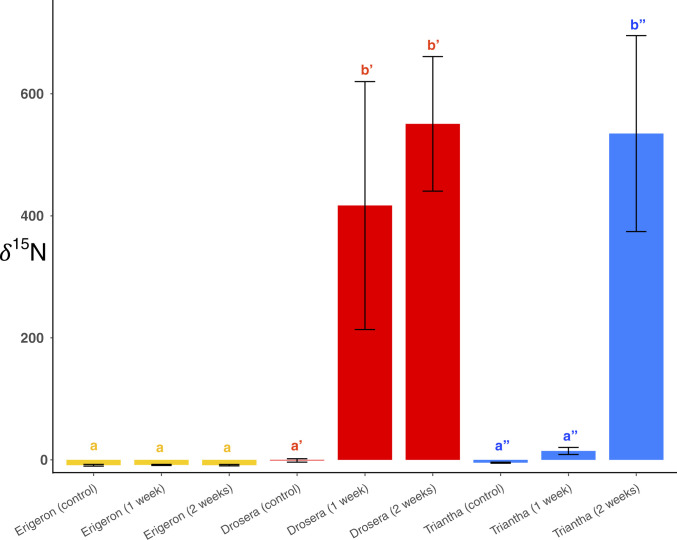
We experimentally applied 15N-labeled Drosophila to leaves of Drosera and upper stems of Erigeron and Triantha in the field (Materials and Methods). This led to no significant change in stem N content and δ15N in the noncarnivore Erigeron (Fig. 2; see also Table 1). In Drosera, feeding led to no significant increase in leaf N content but a significant (P < 1 × 10−4; SI Appendix, Table S2) and large increase of up to 550‰ in δ15N. A simple mixing model (Materials and Methods) indicates that roughly 7.4% of leaf N at the end of the short-term experiment represented N obtained from labeled prey. Measurements of leaf N concentration indicate a slight decrease in leaf %N after 2 wk of feeding (P < 0.03; SI Appendix, Table S2), suggesting that some leaf N was exported elsewhere following feeding; if some of that involved N initially present, then a simple mixing model might depart somewhat from the actual amount of leaf N provided by carnivory, depending on the fraction of initial and animal-provided N exported.
Fig. 2.
Comparison of δ15N (mean ± SE) in three species at Cypress Provincial Park, following exposure of different tissues (yellow = stems of Erigeron peregrinus, Asteraceae; red = leaves of Drosera rotundifolia, Droseraceae; blue = stems of Triantha occidentalis, Tofieldiaceae) to 15N-labeled or unlabeled fruit flies. Labeled flies were exposed to stems (Erigeron and Triantha) or leaves (Drosera) for 1 or 2 wk, and unlabeled flies (= control) were exposed for 2 wk. Significantly different values (P < 0.05) of δ 15N after exposure are noted within each species (SI Appendix, Table S2); Erigeron (no prime), Drosera (prime), and Triantha (double prime). Values of δ15N in leaves and fruit of T. occidentalis are noted in Table 1.
In Triantha, feeding led to no significant increase in leaf N concentration during the study period but a significant increase of up to 7.5‰ in δ15N (one-way ANOVA, df = 3, 26, P < 0.004; SI Appendix, Table S3 and Fig. S3). A simple mixing model suggests that 0.1% of postexperiment leaf N was provided by the labeled prey. However, feeding led to a significant increase (one-way ANOVA, df = 3, 26, P < 6 × 10−5; SI Appendix, Table S3 and Fig. S4) in fruit N content, from 1.5 to up to 2.6%, although after 2 wk, no significant difference remained. There was also a significant, much larger, and persistent increase in fruit δ15N, from −1.66 to 26.38‰ (one-way ANOVA, df = 3, 26, P < 0.002; Table 1). The most dramatic changes in N content and δ15N occurred in flowering stems. Stem N content nearly doubled 1 wk after feeding but then dropped to prefeeding levels after 2 wk. However, stem δ15N increased from −4.8‰ before feeding to 535‰ 2 wk after feeding; a simple mixing model suggests that carnivory accounted for 7.2% of stem N, comparable to that seen in Drosera leaves. Doubling the number of prey did not have a systematic effect on N content and δ15N of Triantha tissues (Table 1 and SI Appendix, Figs. S3 and S4).
Clearly, Triantha is acquiring N from prey, which it accumulates initially in the flower stem and fruits (and possibly the flowers themselves, which we did not measure). The apparent export of N from reproductive tissue, however, suggests that any potential advantage of carnivory would involve either maturation of more fruits at a given N content or (perhaps more likely) N retranslocation to the roots/rhizome in the current growing season, with export to leaves the following year. Assuming a mixing model with a δ15N for input from the soil comparable to that for noncarnivorous plants (i.e., δ15NB = −9.5‰ based on Erigeron), a prey δ15N input of δ15NC = 1.71‰, and a δ15NA = −2.28‰ for Triantha (Table 1), we conclude that 64% of Triantha foliar N comes from carnivory in preceding years (see equation given in Materials and Methods). A comparable calculation for Drosera implies that 76% of Drosera foliar N comes from carnivory. Both of these calculations probably overestimate annual N input from carnivory because they ignore N carryover across multiple years. However, the point is clear: isotopic data suggest that Triantha is obtaining nearly as much foliar N from carnivory as the accepted carnivorous plant D. rotundifolia. Further, N obtained from prey appears to be stored initially in reproductive tissues, affecting their N content and δ15N, with N then apparently exported to the roots/rhizome (the only available storage sites for carryover to next year’s growth) while leaving an elevated δ15N signature in the fruit and stem N pools (Table 1), presumably due to metabolic mixing of recently obtained animal N with that obtained from the soil (or belowground stores from previous years) before export from those pools. Finally, carryover from underground storage should increase the δ15N of next year’s leaves relative to carnivores. Investigating changes in photosynthetic rate and total biomass could help quantify the long-term benefit of carnivory in Triantha, as would tracing predicted N flows among organs over multiple years, but is not essential to demonstrating carnivory per se (18, 24).
Enzymatic Test in Support of Holocarnivory in Triantha.
In addition to nutrient uptake from prey, holocarnivorous genera also display high phosphatase activity from digestive enzymes secreted by glandular hairs (11, 25). We therefore tested glandular hairs for production of phosphatase, a key digestive enzyme in holocarnivores that mobilizes phosphorus from prey (26) and the most widely investigated and most easily visualized enzyme in studies of carnivory (10, 11, 25). To do this, we dipped Triantha stems in a solution containing ELF 97 phosphatase substrate (Materials and Methods). We indeed noted yellow-green fluorescence in hairs of Triantha and Drosera relative to the Erigeron control (Fig. 3), further supporting the existence of holocarnivory in T. occidentalis. This provides clear evidence in support of active enzymatic digestion, which is not known for other monocots.
Fig. 3.
Glandular hairs of three species stained with phosphatase substrate under epifluorescence and normal light microscopy (Upper vs. Lower, respectively, labeled EFM vs. LM, respectively). Yellow-green fluorescence indicates substrate hydrolysis for glandular hair from leaf of carnivorous Drosera rotundifolia and glandular hairs from the upper scape of putatively carnivorous Triantha occidentalis; glandular hairs on the stem of the noncarnivore Geranium robertianum exhibit slight blue autofluorescence but no apparent yellow-green fluorescence. (Scale bars: 2 mm.)
Conclusions and Future Directions
We conclude that T. occidentalis should be welcomed to the small but fascinating ecological guild of carnivorous plants. This species grows in sunny, wet, and infertile habitats typical of almost all carnivorous plants, in accord with cost-benefit models (3, 4). The sticky secretions of its glandular hairs trap numerous insect prey, which are then actively digested by phosphatase, and perhaps by other digestive enzymes that remain to be studied. We demonstrated that nitrogen—a key nutrient likely to be lacking in wetland habitats (4)—is transferred into plant tissue; that much of this initially accumulates in stem and fruit tissue; and that N stored temporarily in this way is later apparently exported to the roots and/or rhizome, the only organs available for storage and carryover to other tissues in ensuing years. Given 1) the substantially higher value of Triantha leaf δ15N relative to that of noncarnivorous plants in the same habitat, 2) the small within-year increment of leaf δ15N due to feeding, and 3) the ephemeral increase in the N content of reproductive tissues, we predict that most or all of the N temporarily stored in the stem and fruit tissue migrates to the roots/rhizome by the end of the season and thence to the leaves and other organs the following year. Although Triantha obtains 64% of its leaf N from carnivory, its leaf N content is still less than of many neighboring plants (Table 1). However, models for the evolution of carnivory do not predict that carnivorous plants will have higher leaf N content or photosynthetic rates than noncarnivores in the same habitat, just that they obtain an advantage in growth rate, through either decreased allocation to roots or increased rates of converting photosynthate to new leaf tissue by providing essential nutrients (3, 4). Future studies should investigate these predictions and quantify N remobilization between aboveground and belowground organs, the possible short-term storage in floral tissue, and the strong likelihood of carryover in underground organs between years.
Triantha occur not only in nutrient-poor bogs but also in highly calcareous fens, where P and N are also likely to be limiting (26, 27). Is carnivory found elsewhere in Triantha? We also found insects on Triantha glutinosa in the wild and on herbarium specimens (e.g., D. M. Fabijan, collection no. 02879, University of Alberta Vascular Plant Herbarium, ALTA), which indicate that this species may also be carnivorous. We have not had a chance to observe fresh plants of the third North American species Triantha racemosa, but we did not find captured insects on herbarium specimens of this species, which has weaker flower stems and glandular hairs that are less sticky than other Triantha species (28). Further investigation is needed to address the extent of carnivory in other species of Triantha, at least one of which has also evidence of plastid NDH-1 loss (28), as was noted in T. occidentalis and several other carnivores (13, 17). It would also be valuable to sequence the genomes and transcriptomes of T. occidentalis and relatives to look for evidence of novel or convergent gene- or genome-level changes that may underpin or result from plant carnivory and to characterize the localization of gene expression in trap vs. nontrap organs (29–34).
Triantha is unique among plants with strong evidence of carnivory in that it captures prey solely with its inflorescence stem, close to the flowers. Almost all carnivorous plants place their flowers and traps far apart, presumably to minimize the conflict between pollination and prey capture (35). Only a very few carnivores—including some Genlisea, Pinguicula, and Utricularia and all eight species of Byblis—place any traps or digestive glandular hairs on their inflorescence stem (36, 37), and in all these (except Byblis) the inflorescence hairs are small, seem weakly adhesive, and compose only a tiny fraction of the total trap surface. The conflict between pollination and prey capture is thus uniquely acute in Triantha, which places all of its trap surface near its flowers. The glittering red glandular hairs on Triantha flowering stems may attract prey but capture only small insects—mostly flies and small beetles (38, 39). We observed floral visitation of T. occidentalis by butterflies and bees but never observed capture of these larger insects in the field or on herbarium specimens. This probably results from their much greater strength than the small insects taken as prey, although it may also reflect differences in visual modality between both groups of insects. Nonetheless, the potential for trade-offs between carnivory and pollination services in this species warrants more detailed ecological study. Trials with different-sized insects could be conducted to investigate this further, with measurements to test the adhesive force of the glandular hairs vs. the limb or lift force of pollinators and prey (40–42). Finally, the fact that T. occidentalis was hidden in plain sight as a carnivorous plant, despite its proximity to several major urban centers on the Pacific coast of North America, is remarkable. This serves as a reminder that other cryptic carnivores may yet remain to be uncovered and that much is still to be learned about the ecology of individual plant species, even in relatively well-known floras.
Materials and Methods
Field Experiments for Nutrient Uptake.
We fed 150 fruit flies (Drosophila melanogaster) with 15N-labeled amino acids (100 mg algal amino acid mixture, Sigma-Aldrich, added to 100 g standard Drosophila medium). Another 80 fruit flies were fed with normal standard Drosophila medium as a control. Fruit flies were frozen shortly before the field experiment. Field experiments were conducted in a Sphagnum-dominated bog of Cypress Provincial Park, BC (August 2018). Fruit flies were placed on leaves of D. rotundifolia (positive control) directly and upper stems of both E. peregrinus (negative control) and T. occidentalis using parafilm tape to fix them lightly for the latter two species. E. peregrinus was chosen as a negative control as it lacks features associated with carnivory such as sticky glandular hairs and has a stature similar to Triantha. Five individuals of each species were treated with four unlabeled fruit flies (two fruit flies per leaf for Drosera) for 2 wk as a control. Five individuals each of D. rotundifolia and E. peregrinus were treated with four labeled fruit flies for 1 wk, and another five plants were treated for 2 wk. Ten individuals of T. occidentalis were used instead of five for each treatment.
Leaves of D. rotundifolia and stems of T. occidentalis and E. peregrinus were measured for nutrient absorption. For T. occidentalis, we also measured N content and δ15N for leaves and fruit (measuring different plant parts from the same set of plants, for a given treatment) in addition to flowering stems. We also included a further treatment for T. occidentalis in which we applied eight fruit flies (double the other treatments) on an additional five plants for 2 wk and measured N content and δ15N for stems, leaves, and fruit of all plants for this treatment. In total, stems of five samples failed in elemental analyses due to insufficient mass of nitrogen (Table 1).
Nephrophyllidium crista-galli (Menyanthaceae) plants living in the same habitat were collected to measure natural N content and δ15N, for additional comparison. We also collected wild insects trapped by T. occidentalis using tweezers. In all cases, whole plants were collected and dried in a specimen dryer. Stems of T. occidentalis in contact with 15N-labeled fruit flies were not measured, to avoid contamination of 15N on the surface; to avoid possible contamination of falling isotopic materials from above, only sections higher than the parafilm-covered parts were used for analysis. In E. peregrinus, stems were examined (again higher than parafilm-covered parts where insects were applied), as were the leaves of the control treatment for comparison. In D. rotundifolia, different leaves from those that had insects applied were collected for measurement. All material was ground to powder and sent to the University of British Columbia (UBC) Stable Isotope Facility for isotopic analysis. The N content and δ15N were analyzed using a Vario EL Cube elemental analyzer (Elementar) interfaced with an IsoPrime isotope ratio mass spectrometer (GV Instruments). δ15N was expressed in units of per mil (‰) where δ = [(R sample /R standard) – 1] × 1,000, R = 15 N:14 N, with nitrogen in air as the standard. The precision was typically ±0.3‰ for δ15 N.
We used a mixing model to calculate the fraction p of the N content of plants or tissues derived from insect N (43):
where δ15NC is the mean δ15N of sources of outside input (which we took to be either natural insects or 15N-enriched insects, depending on the scenario), δ15NB is the mean plant δ15N without outside input (either a comparable noncarnivorous plant species, Erigeron, or the carnivorous plant not exposed to 15N-enriched insects), and δ15NA is the mean δ15N of carnivorous plants after the outside input (either the carnivorous plant without 15N-enriched insects or after exposure to 15N-enriched insects). Mixing models calculate the weighted average of isotopic sources. If a plant obtains a fraction, p, of its N from prey with δ15NC and (1-p) from soil with δ15NB (estimated from unfed carnivorous plants or comparable noncarnivores in the same habitat), then that plant should have δ15NA = p · δ15NC + (1 − p) · δ15NB. Rearrangement yields the mixing-model equation given above. All raw data can be found in SI Appendix, Table S4.
We used linear models to test for differences among species and/or treatments, using R v4.0.3 and R packages lme4, lmerTest, and emmeans (44–47). Linear models assume equal variances across groups, so we log-transformed the data to meet this assumption when necessary. After transformation, diagnostic plots indicated that the equal-variance assumption was met. To analyze control and unmanipulated plants, we used δ15N or log(%N) as the response, a mixed-effect model with the species–plant part combination as a fixed effect, and the plant (nested in species) as a random effect, as we repeatedly collected fruits and leaves from the same plant. Denominator degrees of freedom were determined with Satterthwaite’s method. Pairwise comparisons were conducted with the Tukey method from the full model, a conservative adjustment for multiple comparisons.
In analyses including treated plants, δ15N showed highly unequal variances. The lowest δ15N value was close to −12, so we used log(δ15N + 13) as the response, or log(%N) as the response. To compare the treatments in Fig. 2, we used a two-way ANOVA with treatment and species as factors. Since the treatment and species had a significant interaction effect, we conducted pairwise comparisons between treatments within each species, using the Tukey method from the full model.
To analyze the full data collected on T. occidentalis we first used a mixed-effect model with treatment and plant part as fixed effects and plant (nested in treatment) as a random effect. The variance in log(δ15N + 13) varied noticeably across plant parts. One %N value was very close to 0 (2-wk treatment, fruit), so the %N values were not log-transformed. Additionally, there was strong evidence for a treatment × plant part interaction. Therefore, we compared the treatments for each plant part individually using one-way ANOVA on the data for that plant part only (assuming equal variances within each subset only), followed by Tukey comparisons if the F test was significant. All statistical results can be found in SI Appendix.
Scanning Electron Microscope.
Preparation and observation of scanning electron microscope were performed in UBC Bioimaging Facility. We fixed 5 mm pieces of T. occidentalis upper stem in 50 mM Pipes buffer with 2% vol/vol glutaraldehyde at pH 7.0 overnight at 4 °C. After rinsing in buffer twice, samples were fixed in 2% OsO4 for 1 h; dehydrated with an ascending grade ethanol series of 30, 50, 70, 95, 100% (twice) for 20 min each; critical-point-dried in CO2 (Tousimis Autosamdri 815B Critical Point Dryer); sputter‐coated with gold‐palladium (Cressington 208HR High Resolution Sputter Coater); and examined under a Hitachi S4700 scanning electron microscope.
Determination of Enzyme Activity.
Triantha occidentalis and D. rotundifolia were collected from Cypress Provincial Park, BC (August 2018). As a control, samples of Geranium robertianum (Geraniaceae), which also has dense secretory trichomes, were collected from the campus of the UBC. Leaves of D. rotundifolia and stems of T. occidentalis and G. robertianum were incubated in a 250 μm solution of Enzyme-Labeled Fluorescence 97 phosphatase substrate (Invitrogen/MolecularProbes of Thermo Fisher Scientific) in milli-Q water at room temperature for 15 min. Samples incubated with milli-Q water were used to check for possible autofluorescence. Materials were then screened for fluorescence under an epifluorescence microscope (Leica DMR compound microscope equipped with an EBQ 100 isolated mercury lamp; excitation, 450 to 490 nm; dichromatic mirror, DM 510 nm; emission filter, 510 nm). Fluorescence was documented with a Canon EOS Rebel T5 digital camera.
Supplementary Material
Acknowledgments
We thank Jeannette Whitton and Joanna Hirner for assistance with obtaining permits for field work in Cypress Provincial Park; Wesley Gerelle, Evan Hersh, Weijie Huang, Matthias Jost, Vivienne Lam, Danilo Lima, Vlad Lungu, Weiyue Sun, and Jianqiang Zhang for assistance with field work; Shuxian Fang for statistical advice; and Dmitry Sokoloff for discussions on carnivory in Triantha japonica. Douglas Allan, Natalie Garrett, Alice Chang, Shannon Guichon, Aarya Chithran, Payel Ganguly, Rob Guy, and Santokh Singh provided help and advice on isotopic work, and the staff of the UBC Bioimaging Facility assisted with microscopy. We also thank three anonymous reviewers and David Tank and Rob Guy for comments that improved the article. This work was supported by a Natural Sciences and Engineering Research Council of Canada Discovery grant to S.W.G. and NSF Grant DEB 1557906 to T.J.G.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2022724118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Chase M. W., Christenhusz M. J. M., Sanders D., Fay M. F., Murderous plants: Victorian Gothic, Darwin and modern insights into vegetable carnivory. Bot. J. Linn. Soc. 161, 329–356 (2009). [Google Scholar]
- 2.Darwin C., Insectivorous Plants (John Murray, London, 1875). [Google Scholar]
- 3.Givnish T. J., Burkhardt E. L., Happel R. E., Weintraub J. D., Carnivory in the bromeliad Brocchinia reducta, with a cost/benefit model for the general restriction of carnivorous plants to sunny, moist, nutrient-poor habitats. Am. Nat. 124, 479–497 (1984). [Google Scholar]
- 4.Givnish T. J., Sparks K. W., Hunter S. J., Pavlovič A., “Why are plants carnivorous? Cost/benefit analysis, whole-plant growth, and the context-specific advantages of botanical carnivory” in Carnivorous Plants: Physiology, Ecology, and Evolution, Ellison A. M., Adamec L., Eds. (Oxford University Press, London, 2018), pp. 232–255. [Google Scholar]
- 5.Fleischmann A., Schlauer J., Smith S. A., Givnish T. J., “Evolution of carnivory in angiosperms” in Carnivorous Plants: Physiology, Ecology, and Evolution, Ellison A. M., Adamec L., Eds. (Oxford University Press, London, 2018), pp. 22–41. [Google Scholar]
- 6.Roberts H. R., Warren J. M., Provan J., Evidence for facultative protocarnivory in Capsella bursa-pastoris seeds. Sci. Rep. 8, 10120 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darnowski D. W., Carroll D. M., Płachno B., Kabanoff E., Cinnamon E., Evidence of protocarnivory in triggerplants (Stylidium spp.; Stylidiaceae). Plant Biol. 8, 805–812 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Ellison A. M., Nutrient limitation and stoichiometry of carnivorous plants. Plant Biol. 8, 740–747 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Joel D., “Carnivory and parasitism in plants.” in Proceedings of the 4th International Carnivorous Plant Conference, Konda M. K., Eds. (Higashi-Hiroshima University, Higashihiroshima, Japan, 2002), pp. 55–60. [Google Scholar]
- 10.Pereira C. G., et al., Underground leaves of Philcoxia trap and digest nematodes. Proc. Natl. Acad. Sci. U.S.A. 109, 1154–1158 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Płachno B. J., et al., Fluorescence labelling of phosphatase activity in digestive glands of carnivorous plants. Plant Biol. 8, 813–820 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Packer J. G., “Triantha and Tofieldia” in Flora of North America North of Mexico (Oxford University Press, New York, 2003), 26, pp. 56–64. [Google Scholar]
- 13.Ross T. G., et al., Plastid phylogenomics and molecular evolution of Alismatales. Cladistics 32, 160–178 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Yamori W., Shikanai T., Physiological functions of cyclic electron transport around photosystem I in sustaining photosynthesis and plant growth. Annu. Rev. Plant Biol. 67, 81–106 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Laughlin T. G., Savage D. F., Davies K. M., Recent advances on the structure and function of NDH-1: The complex I of oxygenic photosynthesis. Biochim. Biophys. Acta Bioenerg. 1861, 148254 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Rantala S., et al., PGR5 and NDH-1 systems do not function as protective electron acceptors but mitigate the consequences of PSI inhibition. Biochim. Biophys. Acta Bioenerg. 1861, 148154 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Wicke S., Schäferhoff B., dePamphilis C. W., Müller K. F., Disproportional plastome-wide increase of substitution rates and relaxed purifying selection in genes of carnivorous Lentibulariaceae. Mol. Biol. Evol. 31, 529–545 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Ellis A. G., Midgley J. J., A new plant-animal mutualism involving a plant with sticky leaves and a resident hemipteran insect. Oecologia 106, 478–481 (1996). [DOI] [PubMed] [Google Scholar]
- 19.Dixon K. W., Pate J. S., Bailey W. J., Nitrogen nutrition of the tuberous sundew Drosera erythrorhiza Lindl. with special reference to catch of arthropod fauna by its glandular leaves. Aust. J. Bot. 28, 283–297 (1980). [Google Scholar]
- 20.Hanslin H. M., Karlsson P. S., Nitrogen uptake from prey and substrate as affected by prey capture level and plant reproductive status in four carnivorous plant species. Oecologia 106, 370–375 (1996). [DOI] [PubMed] [Google Scholar]
- 21.Ellison A. M., Adamec L., Ecophysiological traits of terrestrial and aquatic carnivorous plants: Are the costs and benefits the same? Oikos 120, 1721–1731 (2011). [Google Scholar]
- 22.Shiba Z., BSc thesis, Mineral nutrition of carnivorous and non-carnivorous plants in a Wisconsin bog based on NPK stoichiometry and stable N isotopes. University of Wisconsin–Madison, Madison, WI (2021).
- 23.Midgley J. J., Stock W. D., Natural abundance of δ15N confirms insectivorous habit of Roridula gorgonias, despite it having no proteolytic enzymes. Ann. Bot. 82, 387–388 (1998). [Google Scholar]
- 24.Nishi A. H., Vasconcellos-Neto J., Romero G. Q., The role of multiple partners in a digestive mutualism with a protocarnivorous plant. Ann. Bot. 111, 143–150 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Płachno B. J., Adamec L., Huet H., Mineral nutrient uptake from prey and glandular phosphatase activity as a dual test of carnivory in semi-desert plants with glandular leaves suspected of carnivory. Ann. Bot. 104, 649–654 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juniper B. E., Robins R. J., Joel D. M., The Carnivorous Plants (Academic Press, London, 1989). [Google Scholar]
- 27.Stockmeier L. A., Givnish T. J., Plant distribution, stature, rarity, and diversity in a patterned calcareous fen: Tests of geochemical and leaf-height models. Am. J. Bot. 106, 807–820 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Lin Q., PhD thesis, Phylogenetics and evolution of monocot mycoheterotrophs and a newly demonstrated lineage of carnivorous monocots. University of British Columbia, Vancouver, BC, Canada (2020).
- 29.Ibarra-Laclette E., et al., Architecture and evolution of a minute plant genome. Nature 498, 94–98 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leushkin E. V., et al., The miniature genome of a carnivorous plant Genlisea aurea contains a low number of genes and short non-coding sequences. BMC Genomics 14, 476 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fukushima K., et al., Genome of the pitcher plant Cephalotus reveals genetic changes associated with carnivory. Nat. Ecol. Evol. 1, 59 (2017). [DOI] [PubMed] [Google Scholar]
- 32.Lan T., et al., Long-read sequencing uncovers the adaptive topography of a carnivorous plant genome. Proc. Natl. Acad. Sci. U.S.A. 114, E4435–E4441 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palfalvi G., et al., Genomes of the venus flytrap and close relatives unveil the roots of plant carnivory. Curr. Biol. 30, 2312–2320.e5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Vries S., de Vries J., Plant genome evolution: Meat lovers expanded gene families for carnivory and dropped the rest. Curr. Biol. 30, R700–R702 (2020). [DOI] [PubMed] [Google Scholar]
- 35.Givnish T. J., “Ecology and evolution of carnivorous plants” in Plant-Animal Interactions, Abrahamson W. G., Ed. (McGraw-Hill, New York, 1989), pp. 243–290. [Google Scholar]
- 36.Płachno B. J., Kozieradzka-Kiszkurno M., Świątek P., Functional utrastructure of Genlisea (Lentibulariaceae) digestive hairs. Ann. Bot. 100, 195–203 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kocáb O., et al., Jasmonate-independent regulation of digestive enzyme activity in the carnivorous butterwort Pinguicula × Tina. J. Exp. Bot. 71, 3749–3758 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jürgens A., Witt T., Sciligo A., El-Sayed A. M., The effect of trap colour and trap–flower distance on prey and pollinator capture in carnivorous Drosera species. Funct. Ecol. 29, 1026–1037 (2015). [Google Scholar]
- 39.Horner J. D., Płachno B. J., Bauer U., Giusto B. D., “Attraction of prey” in Carnivorous Plants: Physiology, Ecology, and Evolution, Ellison A. M., Adamec L., Eds. (Oxford University Press, London, 2018), pp. 157–166. [Google Scholar]
- 40.Gaume L., Gorb S., Rowe N., Function of epidermal surfaces in the trapping efficiency of Nepenthes alata pitchers. New Phytol. 156, 479–489 (2002). [DOI] [PubMed] [Google Scholar]
- 41.Gorb E., et al., Composite structure of the crystalline epicuticular wax layer of the slippery zone in the pitchers of the carnivorous plant Nepenthes alata and its effect on insect attachment. J. Exp. Biol. 208, 4651–4662 (2005). [DOI] [PubMed] [Google Scholar]
- 42.Gorb E. V., Purtov J., Gorb S. N., Adhesion force measurements on the two wax layers of the waxy zone in Nepenthes alata pitchers. Sci. Rep. 4, 5154 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Treseder K. K., Davidson D. W., Ehleringer J. R., Absorption of ant-provided carbon dioxide and nitrogen by a tropical epiphyte. Nature 375, 137–139 (1995). [Google Scholar]
- 44.R Core Team , R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria (2020). https://www.R-project.org/. Accessed 6 January 2021.
- 45.Bates D., Maechler M., Bolker B., Walker S., Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015). [Google Scholar]
- 46.Kuznetsova A., Brockhoff P. B., Christensen R. H. B., lmerTest package: Tests in linear mixed effects models. J. Stat. Softw. 82, 1–26 (2017). [Google Scholar]
- 47.Lenth R. V., emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.5.4 (2021). https://CRAN.R-project.org/package=emmeans. Accessed 6 January 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.