Significance
Differences in immune functioning stem from multiple factors, including sex and aging. However, the specific roles of these variables in immunity remain elusive. We profiled immunocytes from young and old males and females at single-cell resolution and constructed a precise atlas of blood-circulating immunocytes. T cell– and B cell–activated signals were higher in young females than males, while aging increased the sex-related differences in immunocytes, cellular composition, and inflammatory signaling. Additionally, males showed a higher accumulation of inflammatory factors during aging, whereas cell–cell communication analysis revealed different trends in gene expression between females and males with aging. These findings might aid in the understanding of the mechanisms underlying sex-based differences in immunity and disease susceptibility across the lifespan.
Keywords: sex, aging, single-cell sequencing, immune responses, cell–cell communication
Abstract
Sex and aging influence the human immune system, resulting in disparate responses to infection, autoimmunity, and cancer. However, the impact of sex and aging on the immune system is not yet fully elucidated. Using small conditional RNA sequencing, we found that females had a lower percentage of natural killer (NK) cells and a higher percentage of plasma cells in peripheral blood compared with males. Bioinformatics revealed that young females exhibited an overrepresentation of pathways that relate to T and B cell activation. Moreover, cell–cell communication analysis revealed evidence of increased activity of the BAFF/APRIL systems in females. Notably, aging increased the percentage of monocytes and reduced the percentage of naïve T cells in the blood and the number of differentially expressed genes between the sexes. Aged males expressed higher levels of inflammatory genes. Collectively, the results suggest that females have more plasma cells in the circulation and a stronger BAFF/APRIL system, which is consistent with a stronger adaptive immune response. In contrast, males have a higher percentage of NK cells in blood and a higher expression of certain proinflammatory genes. Overall, this work expands our knowledge of sex differences in the immune system in humans.
Sex and aging are biological variables that influence immune system composition, development, and the repertoire of responses to pathogenic insults. The type and severity of immune disorders differ greatly between males and females (1). For instance, in the United States, ∼80% of the autoimmune diseases, including Sjögren syndrome and systemic lupus erythematosus (SLE), affect females (2). At the same time, males are twice as vulnerable as females to fatal malignancies (3). Additionally, sex-based differences in the responses to many vaccines have been reported for children and adults (4). Females are generally more susceptible to experiencing adverse side effects from vaccinations and generate more antibodies than men, which has led to the recommendation of different dose protocols for men and women (5). Moreover, males are more affected by severe viral, bacterial, and fungal infections than females (6). Studies have demonstrated a higher COVID-19–related fatality rate in males than in females, particularly in aged males (7). Sex-related differences in susceptibility to immunity disorders originate from the differential composition and signaling pathways between male and female immune compartments (8, 9). Sex and aging affect the composition of the immune microenvironment, thereby contributing to sex-based differences in the response to infection and development of autoimmune disease and cancer.
Seminal studies have yielded important insights into the sex- and age-related differences in immune responses. However, these studies have focused on detecting developmental and cell lineage markers of limited cell types, obscuring more complex hierarchies (1, 8, 10–12). Furthermore, the interactive and overlapping effects of sex and aging require further elucidation. Thus, a comprehensive immune cell atlas that encompasses the influences of sex and aging at a single-cell resolution is needed to reveal how the immune system integrates numerous interconnected components, pathways, and cell types in sex and aging.
To this end, we profiled the transcriptomes of peripheral immune cells sampled from men and women of two distinct age groups. Then, we investigated sex- and age-related differences in PBMC immune cell composition, molecular programs, and communication network. Extensive differences in all of these aspects were observed between sexes, particularly in aging. Compared with males, females exhibited a higher expression of T cell (TC)– and B cell (BC)–activated signaling at the transcriptional level. Overall, this work expands our knowledge of sex differences in immune cells.
Results
Study Design and Analysis of Single Immune Cell Profiling by Sex and Aging.
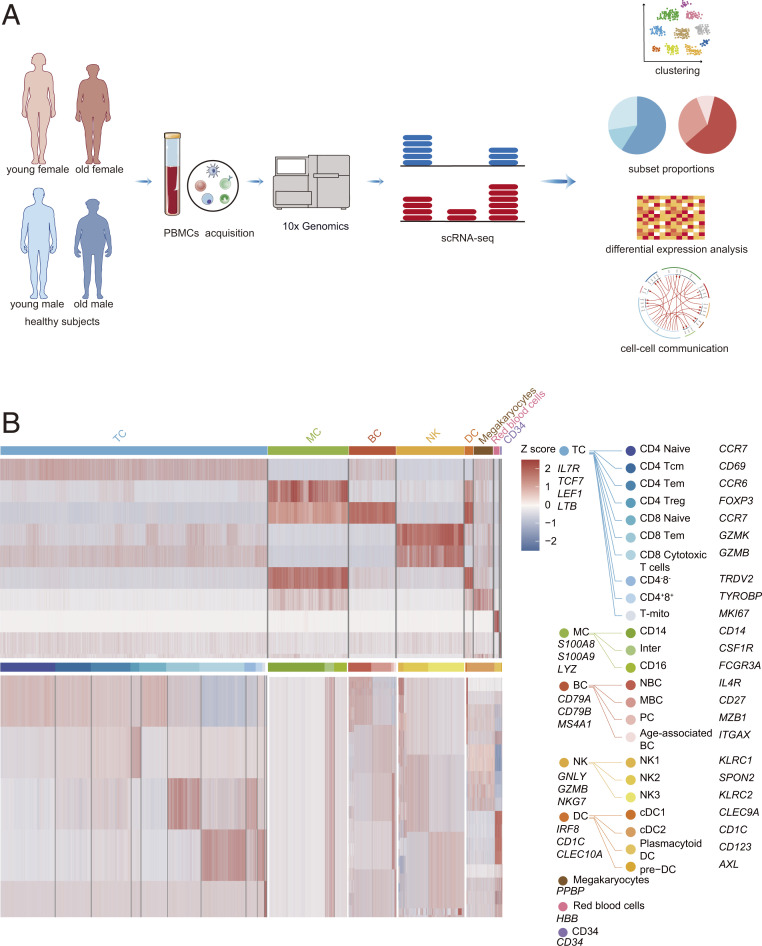
To study the effects of sex and aging on the immune microenvironment, we conducted single-cell RNA sequencing (scRNA-seq) analysis on the peripheral blood mononuclear cells (PBMCs) of five young females (YF), five old females (OF), five young males (YM), and five old males (OM) (Fig. 1A and SI Appendix, Table S1 A and B). The age range of the young group was 20 to 30 y, while that of the old group was 60 to 80 y. The single-cell suspensions were converted to barcoded scRNA-seq libraries using 10× Genomics. The sequencing data were processed by CellRanger software and passed stringent, high-quality filtering. In total, 20 samples were sequenced, and 174,684 cells (YM, 39,828 cells; YF, 43,552 cells; OM, 47,101 cells; and OF, 44,203 cells) were collected for subsequent analyses.
Fig. 1.
Overview of the approach and clustering strategy used for analyzing the circulating immune cell profiling of aging and sex. (A) Schematic of the experimental design for scRNA-seq. PBMCs were collected from YF, YM, OF, and OM then processed by scRNA-seq using the 10× Genomics platform. (B) Heatmaps showing scaled expression of discriminative genes for each cell type and subset.
Using t-distributed stochastic neighbor embedding (t-SNE), we identified red blood cells, megakaryocytes (MEGA), CD34+ cells, and five major immune cell lineages (TCs, natural killer [NK] cells, BCs, monocytes [MCs], and dendritic cells [DCs]) via expression of canonical lineage markers gene expression (Fig. 1B and SI Appendix, Figs. S1 A–C and S2–S7). For the t-SNE of each individual subject, the number of cells and the mean reads per cell could be seen in SI Appendix, Fig. S8. Differentially expressed genes (DEGs) of each individual subject are shown in Dataset S1. The detailed marker genes used can be found in our previous studies (SI Appendix, Table S2) (13, 14). The gene expressions of canonical lineage markers in individual cell clusters on t-SNE plots are shown in SI Appendix, Fig. S1C. The four groups exhibited characteristic immune cell types based on t-SNE (SI Appendix, Fig. S1B), suggesting consistency in cell type definition across sex and aging. Next, we analyzed the gene expression database and subclustered immune cells into transcriptionally classical subtypes (SI Appendix, Figs. S9 and S10).
The Cellular Composition Changes in the Cellular Sex and Aging Ecosystem.
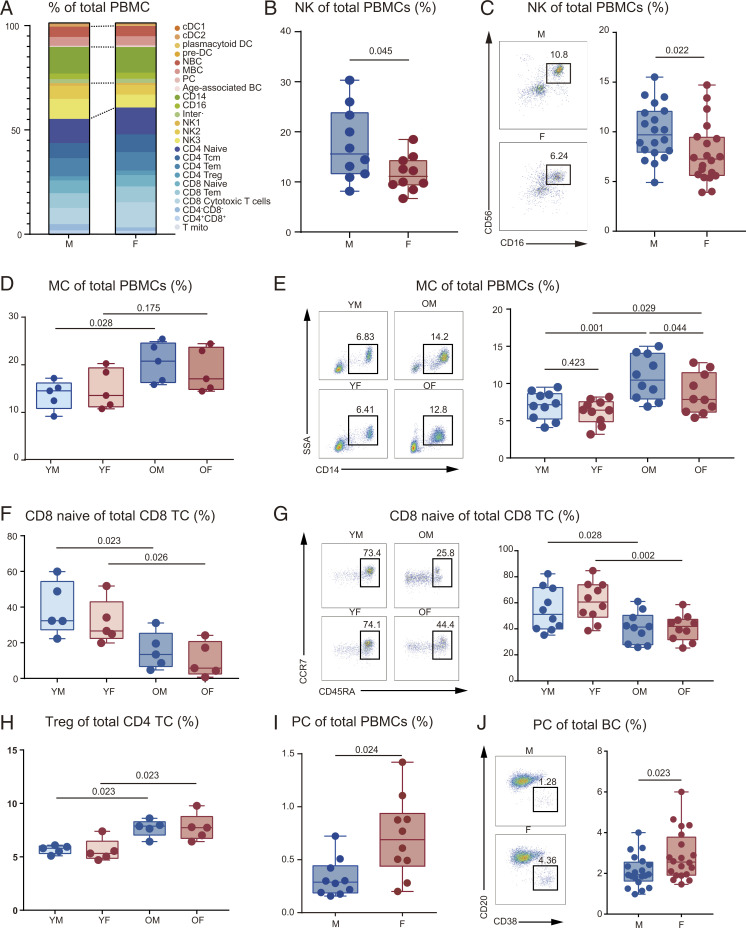
To determine the differences between male and female PBMC immune cell composition, we matched the proportion of immune cells in each group subset (Dataset S2). Generally, the composition of immune cell subsets in PBMCs differed by sex (Fig. 2A) and aging (SI Appendix, Fig. S11A). By comparing the general proportions of total PBMCs, males exhibited a higher general NK count (Fig. 2B), verified by flow cytometry (Fig. 2C and SI Appendix, Fig. S11B). Consistent with a previous study (12), female TC count was higher than in males (Fig. 2A), though the difference was not significant. Also, MCs were more abundant in aged PBMCs than in young groups (SI Appendix, Fig. S11 C and D). Importantly, the scRNA data affirmed that older male PBMCs also contained elevated MC counts compared with younger males (Fig. 2D). Flow cytometry revealed that MC count increases with age in both males and females (Fig. 2E and SI Appendix, Fig. S11E).
Fig. 2.
Changes in cell proportions associated with aging and sex. (A) Relative cluster abundance in males and females. Male group includes YM (n = 5) and OM (n = 5); female group includes YF (n = 5) and OF (n = 5). (B) Percentage of NK in PBMCs between males (n = 10) and females (n = 10). (C) Flow cytometry results for the proportion of NK in PBMCs between males (n = 20) and females (n = 20). The scatter plot is a statistical representation of the results. (D) Percentage of MC in PBMCs among the four groups (n = 5, per group). (E) Flow cytometry results for the proportion of MC in PBMCs among the four groups (n = 10, per group). The scatter plot is a statistical representation of the results. (F) Percentage of naïve CD8 TC in CD8 TC among the four groups (n = 5, per group). (G) Flow cytometry results for the proportion of CD8 naïve in CD8 TC among the four groups (n = 10, per group). The scatter plot is a statistical representation of the results. (H) Percentage of Treg in CD4 TC among the four groups (n = 5, per group). (I) Percentage of PC in PBMCs between males (n = 10) and females (n = 10). (J) Flow cytometry results for the proportion of PC in BC between males (n = 20) and females (n = 20). The scatter plot is a statistical representation of the results. Two-way ANOVA was used for the differences between sexes and age groups; F statistic and P value of sex, aging, and interaction could be seen in SI Appendix, Table S3; and false discovery rate (<5%) was corrected using the Benjamini–Hochberg method.
The CD4 and CD8 TC populations in males and females varied greatly. Hence, aging-induced lymphopenia might be attributed to a decrease in CD8+ (Fig. 2 F and G) and CD4+ naïve TCs (SI Appendix, Fig. S12 A–C). Additionally, in contrast to the reduced naïve TC count, we observed an increase of Treg proportions with aging (Fig. 2H), consistent with previous research (15). Moreover, OM exhibited a higher proportion of proliferating mitotic TC (T-mito) among the TC population. Furthermore, the sex-related differences in T-mito increased with aging (SI Appendix, Fig. S12D).
Although BC populations in PBMCs were similar between the sexes, females had more plasma cells (PCs) than males (Fig. 2 I and J). However, this trend weakened with aging, indicating differential antigen–antibody responses to aging between males and females (SI Appendix, Fig. S12 E and F). No significant differences in DC counts were observed between the different groups (Dataset S2). Cumulatively, these results demonstrate that there are differences in the composition and ratio of peripheral blood immune cells between males and females.
Aging Increases Sex Differences in Circulating Immune Cells, Particularly NK Cells.
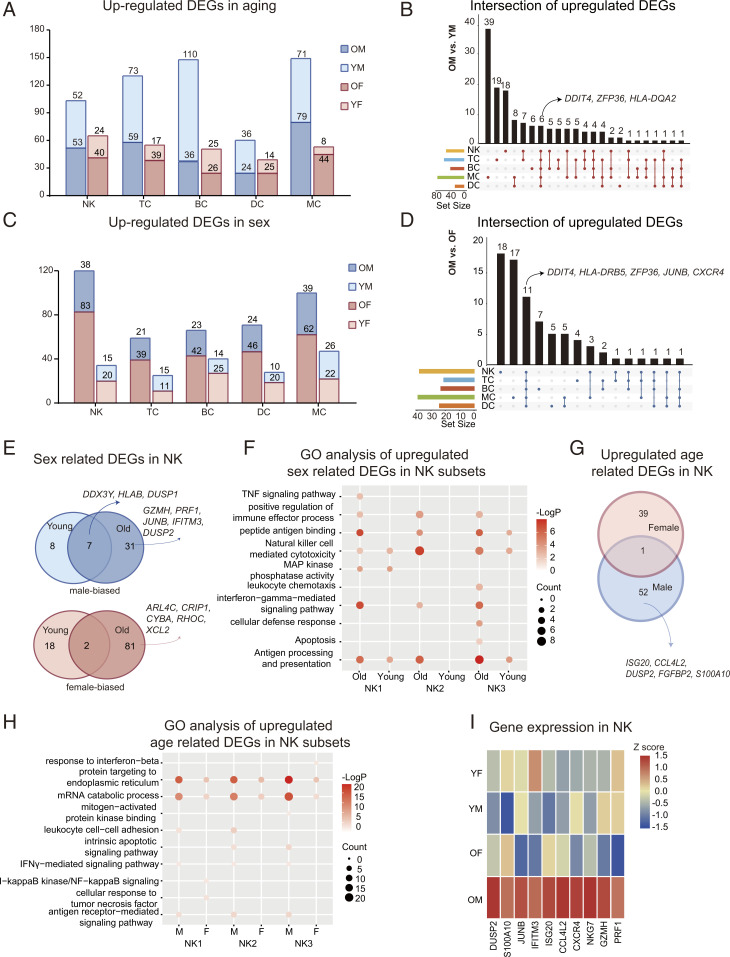
To explore the effect of sex- on age-related changes, we compared the aging DEGs between different age groups (OM: YM and OF: YF; P adjusted < 5%) in each cell type (Dataset S3). Age-related transcriptional expression changes were observed in both females and males. However, the number of DEGs was amplified in males (Fig. 3A). Moreover, in males, aging increased the expressions of aging-related genes in all immune cells (Fig. 3B). To separate the direct effects of sex on each cell type, we compared the DEGs between sexes (YM: YF and OM: OF) in each subset. Remarkably, the sex differences were widespread across immune cells and generally enhanced with aging (Fig. 3C). Specifically, based on DEG analysis, sex impacted BC and MC at a young age. Moreover, the number of sex-specific DEGs in NK cells was dramatically increased upon aging (Fig. 3C).
Fig. 3.
Changes in blood immune cell and NK cell transcriptional profiles. (A) Numbers of age-related DEGs (OM: YM and OF: YF) in the five immune cell subsets (see Dataset S4 for the gene lists). (B) Integrated comparative analysis of up-regulated DEGs in immune cell subsets between OM and YM. UpSet plots is an alternative to the Venn Diagram used to deal with more than three sets. (C) Numbers of sex-related DEGs (YM: YF and OM: OF) in the five immune cell subsets (see Dataset S5 for the gene lists). (D) Integrated comparative analysis of up-regulated DEGs in immune cell subsets between OM and OF. (E) Integrated comparative analysis of up-regulated (Top, male biased) and down-regulated (Bottom, female biased), sex-related DEGs in NK between young and old groups. (F) Representative GO terms and pathways enriched in up-regulated, sex-related DEGs, based on functional enrichment analysis in NK subsets of young and old. (G) Integrated comparative analysis of up-regulated, age-related DEGs in NK between females and males. (H) Representative GO terms and pathways enriched in up-regulated, age-related DEGs, based on functional enrichment analysis in NK subsets of males and females. (I) A heatmap representing scaled expression values of the 10 inflammatory genes in NK among groups.
We generated UpSet plots (16) of up-regulated (males) or down-regulated (females), sex-related DEGs for the elderly group (Fig. 3D and SI Appendix, Fig. S13A). Based on the number of DEGs, NK and MC genes showed the highest, sex-related differences in the elderly group (Fig. 3C). Compared with OF, OM had up-regulated DDIT4, HLA-DRB5, ZFP36, JUNB, and CXCR4 expression in all immune cells (Fig. 3D), indicating an increased oxidative stress and inflammatory state in males. Additionally, compared with OM, OF showed increased expression of CSNK2B, XIST, and EEF1G in immune cells (SI Appendix, Fig. S13A). Generally, the results highlight the importance of sex and aging in determining the immune system.
We determined the sex- and aging-related transcription signatures of NKs. Compared with females, we found that the expression of DDX3Y, HLA-B, and DUSP1 was up-regulated in YM and OM (Fig. 3E). Meanwhile, these sex differences were increased in the elderly groups, as evidenced by the increased expression of GZMH, PRF1, JUNB, IFITM3, and DUSP2 in OM compared with OF (Fig. 3E). In addition, we found ARL4C, CRIP1, CYBA, RHOC, and XCL2 expression increased in OF compared with OM (Fig. 3E). Moreover, the NK2 (CD16high CD56dim CD57low cytotoxic NK) was most influenced by aging, as the expression of sex DEGs in NK2 was dramatically increased with aging (SI Appendix, Fig. S13B).
Next, we explored the biological implications of sex DEGs (YM: YF and OM: OF) using the Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses for each NK subset. The generally up-regulated (male biased) genes across subsets were enriched in relation to NK-mediated cytotoxicity (Fig. 3F). Moreover, several pathways, such as the IFN-γ–mediated signaling pathway, and the positive regulation of immune effector processes were up-regulated in males; however, this difference was not observed in the young group (Fig. 3F). Meanwhile, the commonly down-regulated genes across subsets were enriched in genes related to cell–substrate junctions, messenger RNA (mRNA) translation initiation, and cytokine production (SI Appendix, Fig. S13C), suggesting enriched protein synthesis processes in females.
By comparing the up-regulated NK DEGs in aging individuals, in the male group, aging was associated with the up-regulation of ISG20, CCL4L2, DUSP2, and S100A10 (Fig. 3G). Subsequently, the biological implications of age-related DEGs were investigated using GO and KEGG pathway analyses for each NK subset. We found that females and males underwent different immune changes during aging, as evidenced by the increased IFN-γ–mediated signaling pathway in males (Fig. 3H). As shown in the heatmap, inflammatory genes were primarily expressed in males, particularly in OM (Fig. 3I). In summary, males could show an increased proinflammatory background and NK-mediated immunity compared with females, which increased during aging.
Females Exhibit Up-Regulated TC- and BC-Activated Signaling.
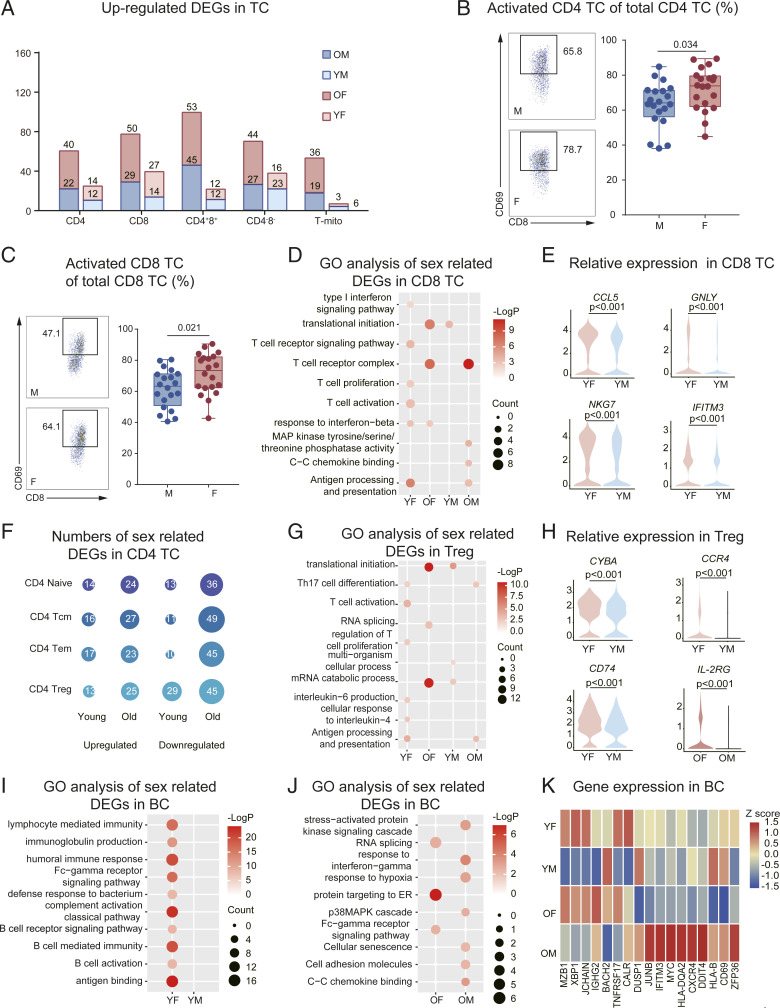
It has been reported that sex and aging play vital roles in TC development and function (17). We observed that CD4+8+ and CD8+ TC were among the subsets with the most sex-specific DEGs (Fig. 4A). Moreover, YF CD8+ TC were enriched in several processes related to TC activation. Consistent with this, flow cytometry revealed that a higher percentage of CD4+ and CD8+ TC exhibited expression of the early activation marker, CD69, compared with male counterparts (Fig. 4 B and C and SI Appendix, Fig. S14A). In contrast, older males exhibited an increased expression of genes associated with TC receptor and MAPK signaling (Fig. 4D). YF exhibited increased expression of GNLY, CCL5, NKG7, and IFITM3 in CD8+ TC (Fig. 4E). Meanwhile, older males exhibited increased expression of DUSP2, CXCR4, DDIT4, NFKBIA, and JUNB in CD4+8+ and CD8+ TC (SI Appendix, Fig. S14B).
Fig. 4.
Changes in transcriptional profiles of TC and BC. (A) Numbers of sex-related DEGs (YM: YF and OM: OF) in the TC subsets (see Dataset S6 for the gene lists). (B) Flow cytometry results for the proportion of activated CD4 TC in CD4 TC between males (n = 20) and females (n = 20). The scatter plot is a statistical representation of the results. (C) Flow cytometry results for the proportion of activated CD8 TC in CD8 TC between males (n = 20) and females (n = 20). The scatter plot is a statistical representation of the results. (D) Representative GO terms and pathways enriched in sex-related DEGs, based on functional enrichment analysis in CD8 TC of the young and old groups. (E) Distribution of normalized expression levels of selected genes in the CD8+ TC cluster between YF and YM. (F) Numbers of sex-related DEGs in CD4+ TC subsets in the young and old groups. (G) Representative GO terms and pathways enriched in sex-related DEGs, based on functional enrichment analysis in Treg of the young and old groups. (H) Distribution of normalized expression levels of selected genes in Treg clusters between YF and YM, and IL2RG between OF and OM. (I) Representative GO terms and pathways enriched in sex-related DEGs, based on functional enrichment analysis in BC of the young group. (J) Representative GO terms and pathways enriched in sex-related DEGs, based on functional enrichment analysis in BC of the old group. (K) A heatmap representing scaled expression values of the seven BC markers (on the Left) and 10 inflammatory genes (on the Right) in BC among the four groups. Two-way ANOVA was used for the differences between sexes and age groups; F statistic and P value of sex, aging, and interaction could be seen in SI Appendix, Table S3; and false discovery rate (<5%) was corrected using the Benjamini–Hochberg method.
Among CD4+ TC, Treg gene expression was most influenced by sex, with these DEGs being maintained during aging (Fig. 4F and SI Appendix, Fig. S14C). Moreover, females showed an increased enrichment of pathways that included T helper 17 cell differentiation, TC activation, and IL-6 production (Fig. 4G), which were driven by the up-regulation of CYBA, CCR4, and CD74 (Fig. 4H), compared with males. Compared with OM, OF had an increased expression of IL-2RG (Fig. 4H), an X chromosome–encoded gene that is important for TC activation and Treg homeostasis. This indicated that Treg in females might lose their immune regulatory capacity and transition into an activated state. Additionally, we found that changes in CD4+ TC caused by aging differed between females and males, which was observable as an aging-induced increase in GO pathways related to mRNA stability and protein transport and increased IFN-γ–mediated signaling in males (SI Appendix, Fig. S14D). Moreover, CD4+ TC of OM were enriched in pathways related to inflammation, compared with OF (SI Appendix, Fig. S14D).
With respect to the BC compartment, the effects of sex and aging on gene expression were analyzed using GO and KEGG analysis. YF showed an overrepresentation of pathways associated with BC-activated signaling compared with YM (Fig. 4I). Interestingly, the pathways associated with BC activity decreased with age (SI Appendix, Fig. S15A). By comparing the DEGs of OM and OF, several inflammatory and cellular senescence genes were expressed at higher levels in males (Fig. 4J). BC marker genes were primarily expressed in females, particularly in the YF group. Conversely, genes related to inflammation were increased in OM compared with the other groups (Fig. 4K).
Next, we compared the sex-related DEGs (YM: YF and OM: OF) and explored the cell subset–specific gene programs in BC subtypes. Naïve BCs (NBCs) were among the subsets with the most sex-specific DEGs (SI Appendix, Fig. S15B). Among the YF-biased DEGs, we identified a range of subtype-specific gene programs, such as IFI44L and TXNIP in NBC, LTB and ZFP36 in memory BC (MBC), and IFITM1 and BST2 in PC (SI Appendix, Fig. S15C). By comparing the NBC transcriptional differences between males and females, we found that the IFN-γ–mediated signaling pathway and the MAPK pathway were overactive in OM than those in OF, and OF showed greater up-regulation of pathways related to BCR signaling, proliferation, and activation compared with OM (SI Appendix, Fig. S15D). Furthermore, our in vitro results demonstrate that females produce more IgG following stimulation, consistent with previous studies (SI Appendix, Fig. S15E) (12). These gene expression patters suggest that NBCs of males are exposed to more proinflammatory cytokines in their in vivo niches, whereas female BC, in general, have a higher potential for activation and antibody secretion.
In summary, these results demonstrated the presence of sex differences in lymphocyte populations at single-cell resolution (i.e., females tend to have higher TC counts and up-regulated, BC-activated signaling, while males tend to exhibit a heightened inflammatory state).
Sex Differences in Myeloid Cells Are Affected by Aging.
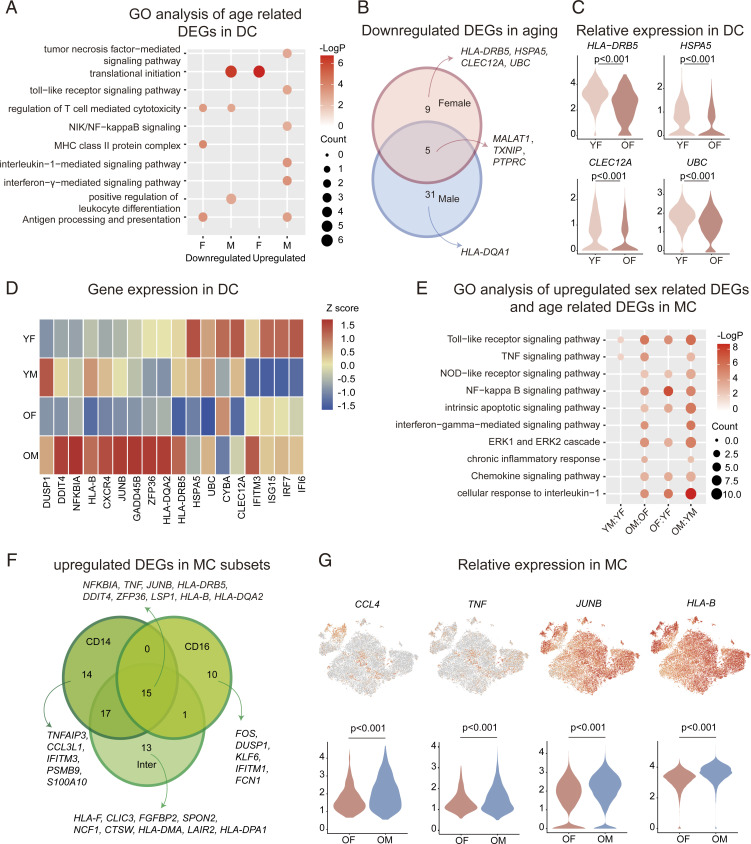
Human-circulating myeloid cells (such as DC and MC) are the primary cells that mediate antigen presentation and inflammatory activity. As reported, the capacity of DC to present antigens gradually deteriorates with age, and this is accompanied by the up-regulation of proinflammatory gene programs (14). To explore the sex-specific, aging-induced changes, we studied the biological implication of aging DEGs in DC (OM: YM and OF: YF), by conducting GO pathway analysis, and found that aging decreased pathways involved in MHC II antigen processing and presentation in females, while decreasing pathways involved in the positive regulation of leukocyte differentiation in males (Fig. 5A). Upon comparing the down-regulated, aging DEGs, we found that TXNIP and MALAT1 were down-regulated with aging in both males and females (Fig. 5B). In addition, several antigen presentation-related genes were down-regulated during female aging (Fig. 5C). Interestingly, the main effect of aging on males was represented by increased inflammatory pathways, including TNF, IFN-γ, and IL-1 pathways (Fig. 5A). These results indicate that the aging process might differentially influence DC in males and females.
Fig. 5.
Changes in DC and MC transcriptional profiles. (A) Representative GO terms and pathways enriched in age-related DEGs, based on functional enrichment analysis in DC of females and males. (B) Integrated comparative analysis of down-regulated, age-related DEGs in BC between females and males. (C) Distribution of normalized expression levels of selected genes in the DC cluster between YF and OF. (D) Heatmap representing scaled expression values of the 10 inflammatory genes (on the Left) and eight DC markers (on the Right) in DC among all the groups. (E) Representative GO terms and pathways enriched in up-regulated, sex-related DEGs and age-related DEGs, based on functional enrichment analysis in MC. (F) Integrated comparative analysis of up-regulated (male biased), sex-related DEGs in MC subsets. (G) Expression of selected genes was projected onto the t-SNE plot and are shown as violin plots. Violin plots are showing the distribution of normalized expression levels of selected genes in the MC cluster between OF and OM.
To explore the sex differences in DC-related genes or inflammatory genes at the transcriptional level, we compared their expression among the four groups. We found that inflammatory genes were primarily expressed in OM, while DC-related genes were expressed in YF (Fig. 5D). Moreover, OM showed an up-regulation in pathways related to p38/MAPK and cellular senescence in classical DC2 (cDC2) cells compared with OF (SI Appendix, Fig. S15F), with higher GADD45B and JUNB expression in the OM versus the other three groups (Fig. 5D). Hence, sex-specific differences in DC function occur that are further influenced by aging. MC are primarily involved in innate immunity and mediate inflammation (14). Although the proportion of MC was similar between sexes, differential transcriptional states were observed for MC. Specifically, in relation to females, males exhibited increased pathways related to innate immunity and inflammation; this was observed in both YM and OM. Meanwhile, the aging process further enhanced these patterns, as evidenced by up-regulated NF-κB pathway, cellular response to IL-1 pathway, and chronic inflammatory response pathway in OM compared with OF (Fig. 5E). Additionally, aging-related changes in MC showed the differences between females and males with the inflammatory responses and pathways enhanced by aging (Fig. 5E).
Next, we analyzed the sex DEGs in the old group and observed that all MC subtypes exhibited elevated expression of TNF, JUNB, and DDIT4 in OM (Fig. 5F). A range of subtype-specific patterns were identified, including IFITM3 and S100A10 in CD14+ MC, FOS and IFITM1 in CD16+ MC, and HLA-F and NCF1 in intermediate MC (Fig. 5F). Subsequently, we calculated the average expression of select genes (CCL4, TNF, JUNB, and HLA-B) in the OM versus OF (Fig. 5G) and observed a higher expression of these genes in OM, further reinforcing that certain inflammatory programs may be enhanced more in males with aging.
Enhanced BAFF/APRIL System in the Cell–Cell Cross-talk of Young Females.
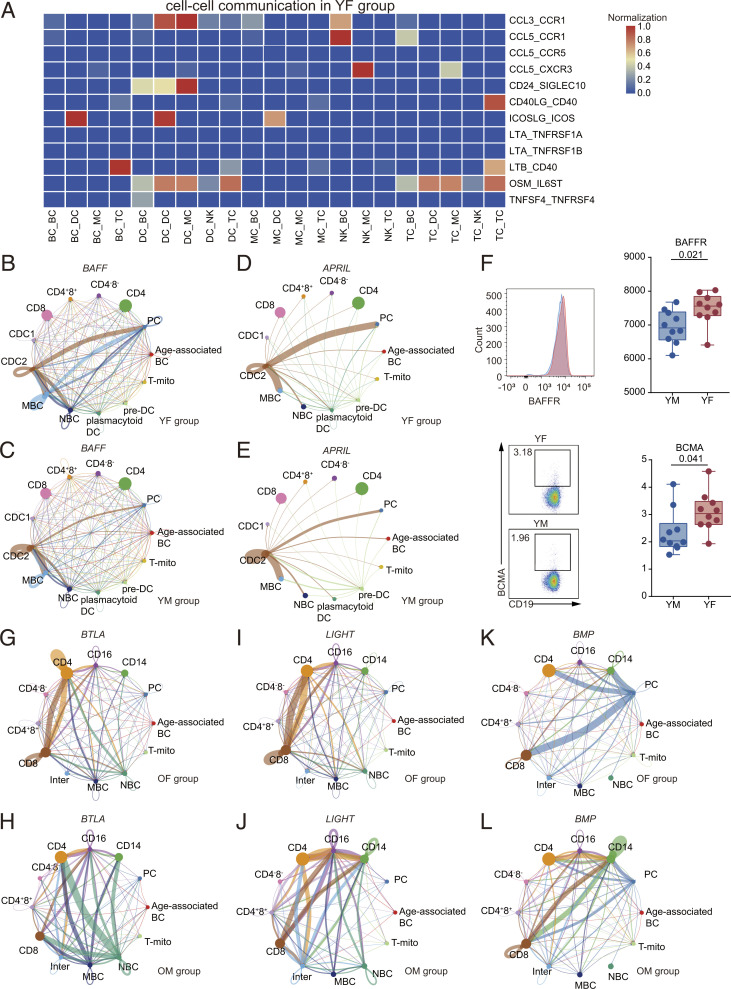
Complex cellular responses are initiated by ligand–receptor binding and the subsequent activation of specific signaling pathways. To better understand the effect of sex and age on cellular communication, using our dataset, we conducted a bioinformatics analysis of cell–cell communication using iTALK and CellChat (18, 19), which could quantitatively characterize and compare the inferred cell–cell communication, based on the average expression of the ligands and receptors in cell populations. We compared the number of interactions between cells in all groups (SI Appendix, Fig. S16A) and found that YF had increased cell–cell interactions between DC, TC, BC, and MC, compared with the other three groups. Moreover, in the old group, the number of interactions between MC, TC, DC, and BC were higher in males compared with females.
To compare the differences in immune cell communication between sexes, we combined ligand–receptors and cell pairs to form a unique identification (ID) for YF and YM groups. In YF, DC exhibited a higher expression of CCL3–CCR1 (Fig. 6A). In addition, the females exhibited an increased expression of ICOSLG, OSM, and their corresponding receptors among cells (Fig. 6A). In YM, IL18 and its receptors were highly expressed in the DC–BC and MC–BC cell interactions (SI Appendix, Fig. S16B). In addition, males showed a unique pattern of gene expression of C3–IFITM1 in MC–TC, C3–ITGAM in MC–MC, IGF1–IGF1R in BC–BC, and IL7–IL7R in NK–TC interactions (SI Appendix, Fig. S16B).
Fig. 6.
Heterogeneous, sex-based differences in cell–cell communication. (A) A heatmap depicting selected cell–cell interactions enriched in YF but absent in YM. (B) Inferred BAFF signaling networks in YF (circle plot). (C) Inferred BAFF signaling networks in YM. (D) Inferred APRIL signaling networks in YF. (E) Inferred APRIL signaling networks in YM. (F) Flow cytometry results for the proportion of BAFFR and BCMA of BC between YM and YF. The scatter plot is a statistical representation of the results. (G) Inferred BTLA signaling networks in OF. (H) Inferred BTLA signaling networks in OM. (I) Inferred LIGHT signaling networks in OF. (J) Inferred LIGHT signaling networks in OM. (K) Inferred BMP signaling networks in OF. (L) Inferred BMP signaling networks in OM.
We then explored potential, sex-based differences in cellular communication using CellChat, which could analyze intercellular communication networks and predict main signaling inputs and outputs (19). These studies suggested a potential increase in BAFF and APRIL signaling in females (Fig. 6 B–E and SI Appendix, Fig. S16 C and D), which has been shown to play a key role in the development of autoimmunity (20). Furthermore, the network centrality analysis of interactions identified cDC2 as the major contributor to the BAFF gene signal acting on MBC and PC (SI Appendix, Fig. S16 E and F). Moreover, the autocrine BAFF signaling pathway was up-regulated in MBC and PC of females versus males. Flow cytometry further demonstrated that BAFFR and BCMA (BAFF and APRIL receptors) expression was higher in YF BC compared with YM BC, supporting the notion that the BAFF/APRIL system is enhanced in YF (Fig. 6F and SI Appendix, Fig. S17A). In addition, the patterns of gene expression suggested that CD4+ and CD8+ TC had increased interaction with plasmacytoid DC and PC in females and with cDC2 and pre-DC in males, in relation to the IL-10 signaling pathway (SI Appendix, Fig. S17 B–D).
This cell–cell communication analysis highlighted potential sex differences in cell signaling pathways and immune cell interactions, pointing to increased BAFF/APRIL signaling between DC and BC in females, which may provide insights into the higher incidence of BC-mediated autoimmunity in females (21).
Sex Heterogeneity in Cell–Cell Communication During Aging.
Next, we explored the impact of age on cell–cell interactions. We found that age was associated with the up-regulation of certain CCL–CCR receptor pairings in the immune cells, which differed between females and males. While in OF, CCL3L1 expression was significantly increased in CD4+ TC compared with YF, there was no significant difference in the expression of the corresponding receptors (SI Appendix, Fig. S18A). In contrast, CCL–CCR pairing was enhanced in OM (SI Appendix, Fig. S18B). By comparing the unique ID in aging, we found that females and males shared aging-specific patterns (SI Appendix, Fig. S18C). However, several interactions were higher in OM than OF, including IL1B–IL1R2 in MC–MC and CXCL10–CXCR3 in DC–TC interactions (SI Appendix, Fig. S18C). Moreover, aging resulted in interactions in males that were absent in females. Specifically, immune cells of OM showed increased ligand–receptor pairings that suggested the potential cellular communication pathways in OM via the chemokine family (SI Appendix, Fig. S18D). Additionally, the ephrin EFNB1 and its receptor (EPH family) were dominant interactions between NK and other cells. As the largest family of receptor tyrosine kinases, EPHs play a role in the development of inflammation in optic nerve injury and atherosclerosis (22–24).
To determine if any sex-based influences were exacerbated by aging, we explored sex differences in signaling between OM and OF using CellChat. The gene expression of BTLA and LIGHT signaling pathways were up-regulated in OM compared with OF (Fig. 6 G–J and SI Appendix, Fig. S19 A and B). Known to inhibit BC and TC functions, BTLA and HVEM interactions inhibit TC activation and cytotoxic activity and promote TC survival, promoting their differentiation to memory TC. The LIGHT signaling pathway transduces proinflammatory signals to immune cells (25, 26). Our network centrality analysis of inferred BTLA and LIGHT interactions revealed that in OF CD4+ and CD8+ TC, the gene expression patterns suggested that autocrine signaling was increased compared with that in males. In OM, CD16+ MC were an important intercellular communication regulator (SI Appendix, Fig. S19 C–F).
Several pathways showed vast signaling interactions among cells, particularly MC in OM, including BMP, CD46, CD40, IL-4, IGF, and MHC II pathways (Fig. 6 K and L and SI Appendix, Figs. S19G, S20, and S21 A and B), which regulate inflammatory processes. Moreover, CellChat detected evidence of active TNF and WNT signaling pathways in immune cells of OM (SI Appendix, Fig. S21 C and D) but not OF, suggesting that these classic inflammatory signaling pathways were activated in aging males.
In summary, cell–cell communication patterns presented different trends in aging males and females. These communication patterns suggested increased proinflammatory signaling between MC, TC, and BC in aged males.
Discussion
Sex-based differences in the immune system widely impact the pathology, susceptibility, and prognosis of diseases, including autoimmune diseases and cancer. For example, females are less vulnerable to the severe acute respiratory syndrome coronavirus 2 virus and experience lower mortality compared with males, particularly in aged populations (7). However, the current understanding of the differences between the immune systems of males and females is based primarily on flow cytometry and bulk sequencing data, which can mask the slight yet key differences observable in small subsets.
The current study depicts a sex-specific immune system atlas—at a single-cell resolution—that analyzes the immunocyte compositions and the dominating pathways in both sexes. Furthermore, we explored the impact of aging on sex-specific immune responses. We observed that male PBMCs contained more NK cells than female PBMCs, whereas females had more PCs than males. Furthermore, our pathway analysis of DEGs expression suggested that females exhibit more TC and BC activity, whereas males exhibit more activation of the IFN-γ receptor and MAPK pathways compared with females. Furthermore, we revealed that age exerts a more significant impact on the immune system than sex, with aging further amplifying the observed sex-related differences.
Generally, the composition and proportion of immune cell subsets in PBMCs are roughly the same for males and females. However, we observed a number of sex-based differences between male and female PBMCs; NK cells were present at a higher percentage in males, and PCs were present at a higher percentage in females. Differences in sex hormones are the main determinants of the sex-biased NK proportion and function (27). Specifically, estrogen and progesterone repress NK cells activity; no effects of androgen treatment on NK cells are seen in mice (28). Moreover, higher estrogen levels and compromised NK cytotoxicity were observed in ovarian tumors and endometriosis (29). In addition, females taking oral contraceptives have a reduced proportion of NK in PBMCs (30). In the current study, we observed that aging was associated with a decreased TC count, increased MC counts, and markers of inflammation in PBMCs, which might account for the higher susceptibility of the elderly to COVID-19 (14). We found that the aging-induced proinflammatory genes were more apparent in older males.
The superior female adaptive immune response has been recognized in previous studies (12). We propose that the disparity in TC-mediated immunity between males and females is mediated by the down-regulation of transcriptional processes of genes associated with TC development and activity in males. ZFP36 and DUSP2 were the most noticeable male-biased DEGs in various immune cell subsets and showed the highest expression in OM. As an RNA-binding protein, ZFP36 suppresses TC activation in mice by attenuating marker gene activation, limiting TC expansion, and promoting apoptosis (31). Accordingly, the high level of ZFP36 in all OM cell subsets partly accounts for the restrained TC immunity and reduced proportions. Meanwhile, elevated expression of DUSP2 in male TC might explain the sex differences in tumor susceptibility and prognosis. Data from the Cancer Genome Atlas revealed that DUSP2 was selectively up-regulated in exhausted, tumor-infiltrating lymphocytes and associated with the poor prognosis of cancer patients. Furthermore, DUSP2high effector TC reportedly lose their proliferative and effector capacities, developing an exhausted phenotype with impaired anti-tumor functions (32). Therefore, the male-biased expression of ZFP36 and DUSP2 might account for the weaker adaptive immunity in males.
The female bias in autoimmune disease susceptibility is well documented (12). We mapped the ligand–receptor interactions that mediate cell–cell communication to depict the sex-specific immune pathways and found that the BAFF and APRIL pathways were more up-regulated in females than males. BAFF, a BC-activating factor, and APRIL, a proliferation-inducing ligand, are essential for sustaining the BC pool and humoral immunity (33). The binding of BAFF and APRIL to their respective receptors, BAFFR and BCMA, contributes to the pathogenesis of various BC-mediated autoimmune diseases, including SLE (34, 35), IgA nephropathy (36), and Sjögren’s syndrome (37). In some patients, higher serum BAFF and APRIL levels are associated with autoimmune disease severity, including elevated levels of pathogenic autoantibodies (38, 39). A genome-wide association study (40) found that the TNFSF13B variant (encoding BAFF) causes an increase in soluble BAFF, increased BC count, and up-regulated humoral immunity, which is associated with SLE. Belimumab, a BAFF inhibitor, has been approved as an add-on therapy for SLE patients (41). Atacicept is a BAFF and APRIL coinhibitor that has undergone phase IIb clinical trials in SLE patients (42). Thus, we propose that the up-regulated BAFF and APRIL pathways might be primary factors responsible for the increased incidence of certain autoimmune diseases in females than males.
As an inhibitory receptor widely expressed on lymphocytes and antigen-presenting cells, BTLA (B and T lymphocyte attenuator) provides an inhibitory signal to BC and TC upon activation by its unique ligand HVEM (43, 44). BTLA activation inhibits TC proliferation and effector function during immune responses in human and animal models (45, 46). Moreover, reduced BTLA levels hyperactivated TC in HIV patients (47). BTLA inhibited innate immune responses in patients with sepsis, thus promoting disease development and progression (48). Elevated levels of BTLA have also been observed in various carcinomas. Hepatocellular carcinoma patients’ CD4+ TCs overexpress BTLA, and the BTLA/HVEM blockade increases IFN-γ production (46). In gastric cancer patients, increased BTLA and HVEM levels correlate with increased disease development and poor prognosis (49). Additionally, in diffuse large BC lymphoma, increased BTLA levels impair TC cytotoxic functions and are associated with poor prognosis (45). Previous reports have found that BTLA blockers enhance human TC responses when used alone or in combination with antibodies against PD-1 (50). Considering the high correlation between BTLA expression and tumor development, BTLA may be a key immunotherapeutic target for treating cancer. We found that the aging process of the immune system, termed immunosenescence (51), was enhanced in older males, characterized by low-level, systemic, and chronic inflammation. DEG analysis further demonstrated that the proinflammatory genes DDIT4, HLA-DRB5, ZFP36, JUNB, and CXCR4 were the main male-biased DEGs in the older group across all immune cell subsets. Up-regulated BTLA pathways in males, accompanied by high-ZFP36 and -DUSP2 expression, might inhibit TC proliferation and activation, making males potentially more susceptible to tumorigenesis.
Taken together, the results of this study have described the sex-based differences in multiple facets of the immune system, including the cellular composition, DEGs, enriched pathways, and cell–cell communication, while also providing potential mechanisms for sex-based differences in diseases.
Materials and Methods
We recruited 20 healthy subjects at the Zhongshan Ophthalmic Center. Our study was approved by the Ethics Committee of Zhongshan Ophthalmic Center and written informed consent was obtained from all subjects. Detailed methods are provided in SI Appendix and include the following: human subjects; single-cell collection and scRNA-seq; scRNA-seq data alignment and sample aggregating; dimensionality reduction and clustering; differential analysis for clusters and groups; gene functional annotation; cell–cell communication; flow cytometry data generation and analyses; enzyme-linked immunosorbent assay data generation; statistical analysis; and data availability statement.
Supplementary Material
Acknowledgments
This study was supported by the National Key R&D Program of China (Grant 2017YFA0105804).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2023216118/-/DCSupplemental.
Data Availability
The fastq data have been deposited in the National Genomic Data Center (HRA000624). All other study data are included in the article and/or supporting information.
References
- 1.Márquez E. J., et al., Sexual-dimorphism in human immune system aging. Nat. Commun. 11, 751 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobson D. L., Gange S. J., Rose N. R., Graham N. M., Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin. Immunol. Immunopathol. 84, 223–243 (1997). [DOI] [PubMed] [Google Scholar]
- 3.Cook M. B., McGlynn K. A., Devesa S. S., Freedman N. D., Anderson W. F., Sex disparities in cancer mortality and survival. Cancer Epidemiol. Biomarkers Prev. 20, 1629–1637 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischinger S., Boudreau C. M., Butler A. L., Streeck H., Alter G., Sex differences in vaccine-induced humoral immunity. Semin. Immunopathol. 41, 239–249 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein S. L., Jedlicka A., Pekosz A., The Xs and Y of immune responses to viral vaccines. Lancet Infect. Dis. 10, 338–349 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vázquez-Martínez E. R., García-Gómez E., Camacho-Arroyo I., González-Pedrajo B., Sexual dimorphism in bacterial infections. Biol. Sex Differ. 9, 27 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scully E. P., Haverfield J., Ursin R. L., Tannenbaum C., Klein S. L., Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat. Rev. Immunol. 20, 442–447 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdullah M., et al., Gender effect on in vitro lymphocyte subset levels of healthy individuals. Cell. Immunol. 272, 214–219 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Hewagama A., Patel D., Yarlagadda S., Strickland F. M., Richardson B. C., Stronger inflammatory/cytotoxic T-cell response in women identified by microarray analysis. Genes Immun. 10, 509–516 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wertheimer A. M., et al., Aging and cytomegalovirus infection differentially and jointly affect distinct circulating T cell subsets in humans. J. Immunol. 192, 2143–2155 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peters M. J.et al.; NABEC/UKBEC Consortium , The transcriptional landscape of age in human peripheral blood. Nat. Commun. 6, 8570 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein S. L., Flanagan K. L., Sex differences in immune responses. Nat. Rev. Immunol. 16, 626–638 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Wen W., et al., Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discov. 6, 31 (2020). Correction in: Cell Discov. 6, 41 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng Y., et al., A human circulating immune cell landscape in aging and COVID-19. Protein Cell 11, 740–770 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Churov A. V., Mamashov K. Y., Novitskaia A. V., Homeostasis and the functional roles of CD4+ Treg cells in aging. Immunol. Lett. 226, 83–89 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Conway J. R., Lex A., Gehlenborg N., UpSetR: An R package for the visualization of intersecting sets and their properties. Bioinformatics 33, 2938–2940 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giefing-Kröll C., Berger P., Lepperdinger G., Grubeck-Loebenstein B., How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell 14, 309–321 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y., et al., iTALK: An R Package to characterize and illustrate intercellular communication. bioRxiv [Preprint] (2019). https://www.biorxiv.org/content/10.1101/507871v1. Accessed 29 July 2021.
- 19.Jin S., et al., Inference and analysis of cell-cell communication using CellChat. Nat. Commun. 12, 1088 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vincent F. B., Morand E. F., Schneider P., Mackay F., The BAFF/APRIL system in SLE pathogenesis. Nat. Rev. Rheumatol. 10, 365–373 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Selmi C., Brunetta E., Raimondo M. G., Meroni P. L., The X chromosome and the sex ratio of autoimmunity. Autoimmun. Rev. 11, A531–A537 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Finney A. C., et al., EphA2 expression regulates inflammation and fibroproliferative remodeling in atherosclerosis. Circulation 136, 566–582 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coulthard M. G., et al., Eph/Ephrin signaling in injury and inflammation. Am. J. Pathol. 181, 1493–1503 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Boyd A. W., Bartlett P. F., Lackmann M., Therapeutic targeting of EPH receptors and their ligands. Nat. Rev. Drug Discov. 13, 39–62 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez-Barbosa J. I., et al., HVEM, a cosignaling molecular switch, and its interactions with BTLA, CD160 and LIGHT. Cell. Mol. Immunol. 16, 679–682 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu Y., et al., Selective targeting of the LIGHT-HVEM costimulatory system for the treatment of graft-versus-host disease. Blood 109, 4097–4104 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hao S., et al., Modulation of 17beta-estradiol on the number and cytotoxicity of NK cells in vivo related to MCM and activating receptors. Int. Immunopharmacol. 7, 1765–1775 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Hao S., Li P., Zhao J., Hu Y., Hou Y., 17beta-estradiol suppresses cytotoxicity and proliferative capacity of murine splenic NK1.1+ cells. Cell. Mol. Immunol. 5, 357–364 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeung I., Cheon K., Kim M.-R., Decreased cytotoxicity of peripheral and peritoneal natural killer cell in endometriosis. BioMed Res. Int. 2016, 2916070 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yovel G., Shakhar K., Ben-Eliyahu S., The effects of sex, menstrual cycle, and oral contraceptives on the number and activity of natural killer cells. Gynecol. Oncol. 81, 254–262 (2001). [DOI] [PubMed] [Google Scholar]
- 31.Moore M. J., et al., ZFP36 RNA-binding proteins restrain T cell activation and anti-viral immunity. eLife 7, e33057 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dan Lu, et al., The phosphatase PAC1 acts as a T cell suppressor and attenuates host antitumor immunity. Nat. Immunol. 21, 287–297 (2020). [DOI] [PubMed] [Google Scholar]
- 33.Samy E., Wax S., Huard B., Hess H., Schneider P., Targeting BAFF and APRIL in systemic lupus erythematosus and other antibody-associated diseases. Int. Rev. Immunol. 36, 3–19 (2017). [DOI] [PubMed] [Google Scholar]
- 34.McCarthy E. M., et al., Elevated B lymphocyte stimulator levels are associated with increased damage in an Irish systemic lupus erythematosus cohort. Rheumatology (Oxford) 52, 1279–1284 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Hegazy M., Darwish H., Darweesh H., El-Shehaby A., Emad Y., Raised serum level of APRIL in patients with systemic lupus erythematosus: Correlations with disease activity indices. Clin. Immunol. 135, 118–124 (2010). [DOI] [PubMed] [Google Scholar]
- 36.Xin G., et al., Serum BAFF is elevated in patients with IgA nephropathy and associated with clinical and histopathological features. J. Nephrol. 26, 683–690 (2013). [DOI] [PubMed] [Google Scholar]
- 37.Jonsson M. V., Szodoray P., Jellestad S., Jonsson R., Skarstein K., Association between circulating levels of the novel TNF family members APRIL and BAFF and lymphoid organization in primary Sjögren’s syndrome. J. Clin. Immunol. 25, 189–201 (2005). [DOI] [PubMed] [Google Scholar]
- 38.Petri M., et al., Association of plasma B lymphocyte stimulator levels and disease activity in systemic lupus erythematosus. Arthritis Rheum. 58, 2453–2459 (2008). [DOI] [PubMed] [Google Scholar]
- 39.Treamtrakanpon W., et al., APRIL, a proliferation-inducing ligand, as a potential marker of lupus nephritis. Arthritis Res. Ther. 14, R252 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steri M., et al., Overexpression of the cytokine BAFF and autoimmunity risk. N. Engl. J. Med. 376, 1615–1626 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Navarra S. V.et al.; BLISS-52 Study Group , Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: A randomised, placebo-controlled, phase 3 trial. Lancet 377, 721–731 (2011). [DOI] [PubMed] [Google Scholar]
- 42.Merrill J. T.et al.; ADDRESS II Investigators , Efficacy and safety of atacicept in patients with systemic lupus erythematosus: Results of a twenty-four-week, multicenter, randomized, double-blind, placebo-controlled, parallel-arm, phase IIb study. Arthritis Rheumatol. 70, 266–276 (2018). Correction in: Arthritis Rheumatol. 70, 467 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murphy K. M., Nelson C. A., Sedý J. R., Balancing co-stimulation and inhibition with BTLA and HVEM. Nat. Rev. Immunol. 6, 671–681 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Sedy J. R., et al., B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat. Immunol. 6, 90–98 (2005). [DOI] [PubMed] [Google Scholar]
- 45.Quan L., et al., BTLA marks a less cytotoxic T-cell subset in diffuse large B-cell lymphoma with high expression of checkpoints. Exp. Hematol. 60, 47–56.e1 (2018). [DOI] [PubMed] [Google Scholar]
- 46.Liu J., Li J., He M., Zhang G.-L., Zhao Q., Distinct changes of BTLA and HVEM expressions in circulating CD4+ and CD8+ T cells in hepatocellular carcinoma patients. J. Immunol. Res. 2018, 4561571 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Z., et al., B and T lymphocyte attenuator down-regulation by HIV-1 depends on type I interferon and contributes to T-cell hyperactivation. J. Infect. Dis. 203, 1668–1678 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shubin N. J., et al., BTLA expression contributes to septic morbidity and mortality by inducing innate inflammatory cell dysfunction. J. Leukoc. Biol. 92, 593–603 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lan X., et al., Increased BTLA and HVEM in gastric cancer are associated with progression and poor prognosis. Onco Targets Ther. 10, 919–926 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grabmeier-Pfistershammer K., et al., Antibodies targeting BTLA or TIM-3 enhance HIV-1 specific T cell responses in combination with PD-1 blockade. Clin. Immunol. 183, 167–173 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Goronzy J. J., Weyand C. M., Understanding immunosenescence to improve responses to vaccines. Nat. Immunol. 14, 428–436 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The fastq data have been deposited in the National Genomic Data Center (HRA000624). All other study data are included in the article and/or supporting information.