Significance
Circular RNAs (circRNAs) play increasingly appreciated gene-regulatory roles in the process of human tumorigenesis. Our results demonstrated that circURI1 expression levels were significantly increased in gastric cancer (GC) compared to paraGC, and the depletion of circURI1 promoted metastasis in vitro and in vivo. CircURI1 directly interacted with hnRNPM and modulated alternative splicing by sequestering the hnRNPM protein. Our study provides a reported association of circRNA, alternative splicing, and metastasis and sheds light on the molecular mechanisms of metastatic GC.
Keywords: circURI1, gastric cancer, metastasis, hnRNPM, alternative splicing
Abstract
Circular RNAs (circRNAs) have emerged as key regulators of human cancers, yet their modes of action in gastric cancer (GC) remain largely unknown. Here, we identified circURI1 back-spliced from exons 3 and 4 of unconventional prefoldin RPB5 interactor 1 (URI1) from circRNA profiling of five-paired human gastric and the corresponding nontumor adjacent specimens (paraGC). CircURI1 exhibits the significantly higher expression in GC compared with paraGC and inhibitory effects on cell migration and invasion in vitro and GC metastasis in vivo. Mechanistically, circURI1 directly interacts with heterogeneous nuclear ribonucleoprotein M (hnRNPM) to modulate alternative splicing of genes, involved in the process of cell migration, thus suppressing GC metastasis. Collectively, our study expands the current knowledge regarding the molecular mechanism of circRNA-mediated cancer metastasis via modulating alternative splicing.
Gastric cancer (GC) is the fifth most-common malignant tumor and the third leading cause of cancer death worldwide (1, 2). Although diverse treatment options are available, the prognosis for GC patients remains poor, largely due to the presence of metastatic spread in patients (3). Cancer metastasis is a multifactorial, multistep, and complex process influenced by environmental and genetic factors (1). The outlook for metastatic GC patients is very poor, with a median overall survival of ∼8 mo (2). Understanding GC metastasis at the molecular and cellular levels will help to identify potential biomarkers for diagnosis and therapeutic targets for intervention.
Circular RNAs (circRNAs) are covalent, closed, single-stranded RNA molecules generated by back-splicing or other RNA circularization mechanisms (4–7). In contrast to linear RNAs, circRNAs lack 5′ and 3′ ends and are resistant to RNA exonuclease, providing them with promising features to serve as potential biomarkers or therapeutic targets (4). Accumulating lines of evidence have illustrated the ectopic expression patterns and fundamental regulatory functions of circRNAs in biological processes, including the cell cycle, cell growth, and metastasis (8–11). Some cytoplasmic circRNAs serve as microRNA (miRNA) sponges to lift the inhibitory effects of miRNAs on their targets (12–15). Another mechanism is that exon–intron circRNAs (EIciRNAs) promote gene expression by binding to the U1 small nuclear ribonucleoprotein complex in the nucleus (16). Furthermore, a small subset of circRNAs undergoes cap-independent translation under certain circumstances, even though the vast majority of circRNAs are thought to be noncoding (17, 18). In GC, circPVT1 promotes cell proliferation by acting as a miR-125b sponge (19). As a nuclear down-regulated noncoding RNA, circHuR suppresses HuR expression and GC progression by inactivating CNBP (20). However, the biological functions and underlying mechanisms of circRNAs in GC progression remain largely elusive.
Alternative splicing gives rise to diverse messenger RNA (mRNA) isoforms by the different arrangements of exon organization from precursor mRNAs (pre-mRNAs), leading to encoding structurally and functionally distinct protein variants (21, 22). As a gene expression regulation event in eukaryotes, alternative splicing controlled by splicing factors such as hnRNP proteins plays fundamental roles in the progression of human cancers (23–27). For instance, PTBP1 (hnRNP I) mediates alternative splicing of MEIS2 and PKM to promote lymphatic metastasis and proliferation of bladder cancer (26). Another hnRNP protein hnRNPM is known to regulate breast cancer metastasis via modulating alternative splicing of CD44 (27); actually, the only known molecular role of hnRNPM is to modify alternative splicing (28).
To investigate the functional roles of circRNAs in GC, we performed RNA sequencing (RNA-seq) of five-paired GC and the corresponding nontumor adjacent specimens (paraGC) to identify promising circRNA candidates. We found that circURI1 expression levels were remarkably increased in GC compared with paraGC. With a series of molecular, cellular, and biochemical experiments, we demonstrated the roles of circURI1 in the prevention of GC metastasis and further illustrated an elegant molecular pathway, in which circURI1 served as a decoy of hnRNPM to modulate alternative splicing of a subset of genes related to cell migration.
Results
CircRNA Profiling of GC.
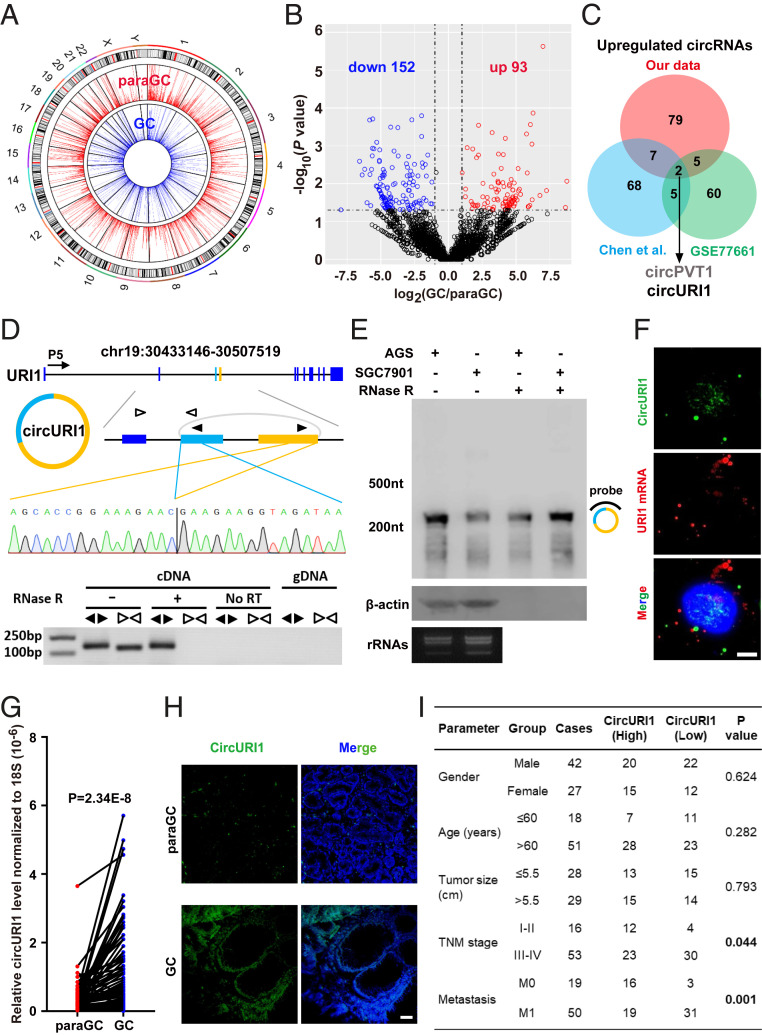
To systematically characterize the genome-wide landscape of circRNAs in GC, we performed ribosomal RNA (rRNA)–depleted RNA-seq analysis of five-paired GC and paraGC specimens to de novo identify circRNA transcripts. Principal component analysis plots revealed that the five biological replicates clustered together, while the GC and paraGC groups were clearly separated (SI Appendix, Fig. S1A). Back-spliced reads per million (BRPM) were used to evaluate circRNA expression levels. A subset of circRNAs was identified only in a fraction of 10 samples, whereas others were extensively expressed in most samples with higher expression levels (SI Appendix, Fig. S1 B and C). Circos plots globally displayed 4,485 and 5,008 circRNAs in GC and paraGC groups, respectively (Fig. 1A). Venn diagram analysis revealed the overlap of circRNAs in GC and paraGC groups, and ∼60% of all 5,757 circRNA candidates were detected in both groups (SI Appendix, Fig. S1D). We further categorized the genomic distribution of circRNAs and found that more than 85% of the circRNAs consisted of protein-coding exons, whereas ∼15% aligned to noncoding RNAs, intergenic regions, untranslated regions, antisense to known transcripts, and unannotated regions of the genome (SI Appendix, Fig. S1E). Simultaneously, 277 readthrough circRNAs, which are generated by exons from more than one coding gene and are relatively enriched in tumor cells (8), were annotated from our dataset (SI Appendix, Fig. S1E). Volcano plots and hierarchical cluster analysis were executed for all expressed circRNAs, in which 245 candidates were significantly dysregulated (152 down-regulated and 93 up-regulated) in GC (Fig. 1B and SI Appendix, Fig. S1F and Table S1). Our circRNA profiling expanded the understanding of transcriptome complexity in GC.
Fig. 1.
Identification of circURI1 in GC. (A) Circos plots showing the distribution of circRNAs in the human genome (hg19). CircRNAs with BRPM greater than 0.2 were chosen for analysis. The outer tracks represent the cytoband ideogram of the chromosome. (B) Volcano plots illustrating differential changes of circRNAs in GC versus paraGC samples. Blue and red dots represent significantly down-regulated and up-regulated circRNAs, respectively. (C) Venn diagram revealing the overlap of up-regulated circRNAs from two published datasets and our RNA-seq data in GC. Chen et al. (19) identified circRNA transcripts from three paired normal and cancerous gastric tissues, whereas GSE77661 contained RNA profiling data of one paired cancer specimen and adjacent normal tissue from a GC patient. Datasets from both Chen et al. and GSE77661 were sequenced on a 100-bp paired-end run, and our dataset was generated on a 150-bp paired-end run. (D) The genomic loci and validation of circURI1. The putative unique back-spliced junction fragment of circURI1 (divergent primers) was performed in AGS cells by RT-PCR and validated by Sanger sequencing. P5 indicates the direction of transcription. gDNA, genomic DNA. (E) Northern blot analysis showing circURI1 in AGS and SGC7901 cells. The hybridized probe against the back-spliced junction site is indicated on the right of the blot image. β-actin mRNA was the positive control for RNase R treatment. rRNA bands are presented to indicate equal loading. (F) RNA FISH of circURI1 (green) with the probe antisense to the back-spliced junction indicated in the same region of Northern Blot (D) and URI1 mRNA (red) in AGS cells. Nuclei (blue) were stained with 4, 6-diamidino-2-phenylindole. (Scale bars, 20 μm.) (G) RT-qPCR analysis of circURI1 in 69-paired GC and paraGC samples. P value was calculated by two-tailed Student’s t test. (H) RNA FISH of circURI1 (green) in GC and paraGC tissues. (Scale bars, 1 μm.) (I) Relationship between circURI1 expression and clinicopathologic factors of GC patients. P values were calculated by χ2 test. Error bars indicate SEM from three independent experiments.
Integrative analysis of the previously reported circRNA profiling and our RNA-seq data in GC identified two up-regulated circRNAs (circPVT1 and circURI1) in all three datasets (14, 19) (Fig. 1C). CircPVT1 has previously been shown to function as a miR-125b sponge to promote cell proliferation in GC (19); therefore, we focused on circRNA circURI1 for further investigations.
Characterization of circURI1 in GC.
The genomic structure showed that circURI1 (annotated as hsa_circ_0000921 in circBase), which is composed of the third and fourth exons from the human URI1 gene, consisted of 215 nucleotides (nt) (Fig. 1D), flanked by two long introns containing reverse complementary Alu elements that promote the generation of circRNAs in either side (5, 16). Subsequently, two GC cell lines (AGS and SGC7901) were selected for further investigations due to the highest circURI1 expression levels in five GC cell lines (SI Appendix, Fig. S2A). The putative back-spliced junction fragment of circURI1 was validated by PCR amplification with divergent primers from complementary DNA (cDNA), but not from genomic DNA, and further confirmed by Sanger sequencing (Fig. 1D). Northern blot analysis confirmed the existence and full length of circURI1 with a probe against the back-spliced junction in AGS and SGC7901 cells (Fig. 1E). RNase R exonuclease assay by RT-qPCR verified that circURI1 was resistant to digestion, also demonstrating its circular nature (SI Appendix, Fig. S2B). In addition, ∼115 and ∼80 circURI1 copies per cell were estimated in AGS and SGC7901 cells, respectively (SI Appendix, Fig. S2C).
Exon sequences of URI1 between humans and mice are highly conserved (∼81.1%) (SI Appendix, Fig. S2D), and we sought to examine the existence of circURI1 derived from the same genomic region in mice. Back-spliced junction fragment of exons 3 and 4 from the murine homolog of the URI1 gene was not detected in mouse stomach tissues (SI Appendix, Fig. S2E), and the corresponding flanking introns of the murine URI1 gene contained no reverse complementary sequences (SI Appendix, Fig. S2F).
The function of noncoding RNA is tightly and closely associated with its subcellular location pattern (29–31); thus, we examined the cellular localization of circURI1. Fluorescence in situ hybridization with a probe against the back-spliced junction of circURI1 and RT-qPCR analysis of nuclear and cytoplasmic RNAs (Fig. 1F and SI Appendix, Fig. S2 G–I) were conducted, demonstrating that circURI1 was preferentially localized within the nucleus in both AGS and SGC7901 cells. Then, we examined circURI1 expression levels in 69-paired GC and paraGC clinical samples by RT-qPCR and found that circURI1 was pervasively and significantly up-regulated in GC compared with paraGC (Fig. 1G and SI Appendix, Fig. S3A). URI1 mRNA expression levels were also significantly increased in GC compared with paraGC (SI Appendix, Fig. S3B). CircURI1 was dramatically increased in GC compared to paraGC via fluorescence in situ hybridization (FISH) analysis of tissues (Fig. 1H). Simultaneously, the decreased circURI1 expression levels were significantly associated with advanced tumor–node–metastasis stage (III through IV) tumors and metastasis in GC patients (Fig. 1I). All these results indicated that circURI1 has the potential to act as a predictive biomarker or therapeutic target for GC.
CircURI1 Inhibits GC Metastasis In Vitro and In Vivo.
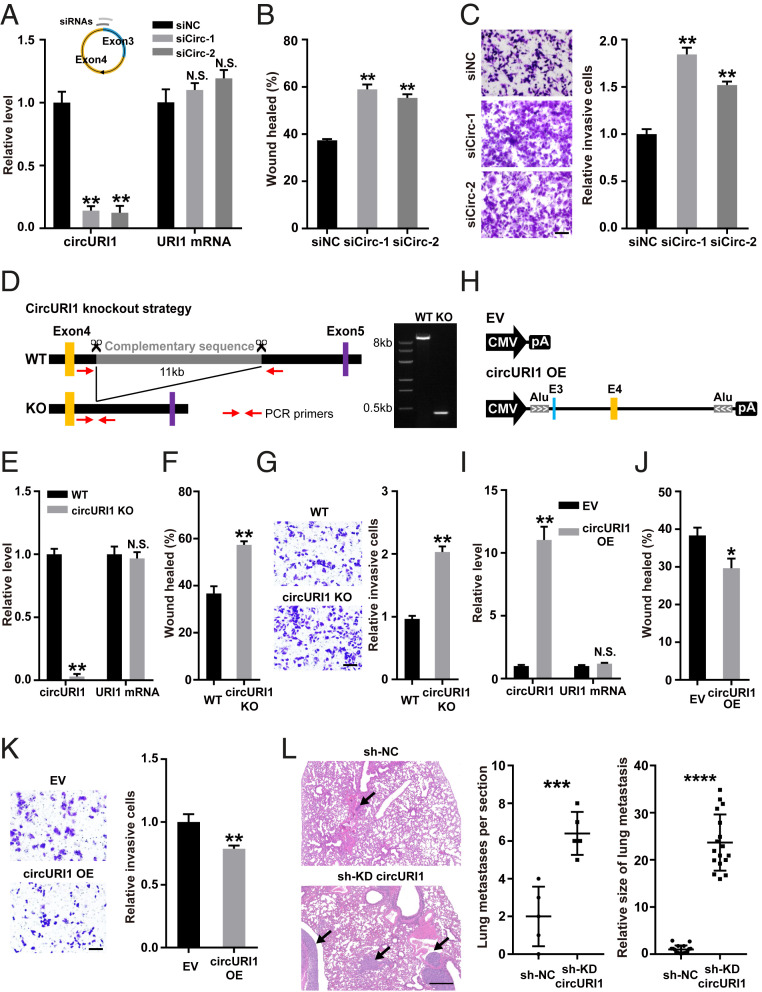
RNA interference was applied to explore the function of circURI1. Targeting circURI1 with two independent short interfering RNAs (siRNAs) against the back-spliced junction site resulted in effective knockdown of circURI1, whereas no detectable effects on URI1 mRNA expression levels were observed in AGS and SGC7901 cells (Fig. 2A and SI Appendix, Fig. S4A). CircURI1 knockdown with siRNAs had no significant change in the proportion of apoptotic AGS and SGC7901 cells or the cell cycle analyzed by flow cytometry (SI Appendix, Fig. S3 C and D). Wound-healing assays revealed that circURI1 knockdown with either siRNA improved the migratory ability in AGS and SGC7901 cells (Fig. 2B and SI Appendix, Fig. S4B). Silencing of circURI1 also enhanced the invasive ability of AGS cells via Transwell assays, as indicated by the increased number of invaded cells (Fig. 2C). Alternatively, antisense oligonucleotides, which target RNA for degradation via an RNase H–mediated mechanism (32–35), were applied to deplete circURI1, and we observed promotive effects on AGS and SGC7901 cell migration and invasion (SI Appendix, Fig. S4 C–E). Furthermore, the clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 technique was employed to generate circURI1 knockout (KO) AGS cells, in which the complementary repeat sequences including six Alu elements in the fourth intron of human URI1 were deleted, but functional intronic elements such as the splicing sites and pyrimidine tract remained unchanged (Fig. 2D). URI1 mRNA expression levels were unaltered in circURI1 KO AGS cells compared to wild-type AGS cells (Fig. 2E). Wound-healing and Transwell assays revealed that the migratory and invasive abilities were promoted in circURI1 KO cells compared with wild-type (Fig. 2 F and G). In contrast, we generated a circURI1 overexpression construct with its endogenous flanking sequences including complementary Alu element pairs, and circURI1 overexpression repressed AGS cell migration and invasion (Fig. 2 H–K).
Fig. 2.
CircURI1 represses GC metastasis in vitro and in vivo. (A) RT-qPCR analysis of circURI1 and URI1 mRNA expression in AGS cells treated with two independent siRNAs against circURI1. siNC, siRNA with scrambled sequences; siCirc-1 and siCirc-2, two siRNAs against the junction sites of circURI1. (B and C) Wound-healing and Transwell assays of AGS cells treated with siRNAs targeting circURI1. (Scale bars, 100 μm.) (D) Strategy of KO reverse complementary repeats in human URI1 intron 4 using CRISPR-Cas9 in AGS cells. Gel image of PCR products from cell genotyping is performed, and the corresponding amplifications are confirmed by Sanger sequencing. WT, wild-type; KO, deletion of the complementary repeat sequences in human URI1 intron 4. (E) RT-qPCR analysis of circURI1 and URI1 mRNA expression in WT and circURI1 KO AGS cells. (F and G) Wound-healing and transwell assays of WT and circURI1 KO cells. (Scale bars, 100 μm.) (H) Construction of circURI1 overexpression with its endogenous flanking sequences including the complementary Alu element pairs. EV, empty vector; CMV, cytomegalovirus promoter; pA, polyadenylation signal. (I) RT-qPCR analysis of circURI1 and URI1 mRNA expression upon circURI1 overexpression. EV, empty vector. (J and K) Wound-healing and Transwell assays of AGS cells after circURI1 overexpression. EV, empty vector. (Scale bars, 100 μm.) (L) Severe combined immunodeficient mice were administered an intravenous injection of the AGS stable cell line with circURI1 knockdown and the control (n = 5 per group), and sectioning of the lung followed by H&E staining was performed to visualize lung metastasis. Black arrows indicate the metastatic tumor. sh-KD circURI1, stable cell line with lentivirus shRNA to knockdown circURI1; sh-NC, negative control cells for knockdown circURI1. Error bars indicate SEM from three independent experiments. N.S., not significant; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 by two-tailed Student’s t test.
We established an AGS stable cell line with lentivirus short hairpin RNA to knockdown circURI1 and confirmed the knockdown efficiency of circURI1 (SI Appendix, Fig. S4F). We then investigated the in vivo roles of circURI1 in metastatic potential using tail vein assay of lung metastasis. Cells with stable circURI1 knockdown formed significantly more lung metastatic nodes and larger nodes than the control (Fig. 2L). These results demonstrated that circURI1 repressed GC metastasis both in vitro and in vivo.
Interaction between circURI1 and hnRNPM.
CircRNAs have been extensively reported to function as miRNA sponges in the cytoplasm (12–15), and we doubted that circURI1 might work as a miRNA sponge, as a portion of circURI1 was localized in the cytoplasm (Fig. 1F and SI Appendix, Fig. S2I). Several putative binding miRNAs of circURI1 (SI Appendix, Fig. S4G) were predicted with CircInteractome, a web tool for exploring the interaction between circRNAs and miRNAs (36). However, RNA IP (immunoprecipitation) of Ago2, the mediator of circRNA–miRNA interaction, showed significant enrichment of circHIPK3 (as a positive control) but no detectable enrichment of circURI1 in either AGS or SGC7901 cells (SI Appendix, Fig. S4H). Additionally, ribosome profiling assays confirmed no translational effect for circURI1 in AGS cells (SI Appendix, Fig. S4 I and J). All these findings strongly implied that circURI1 might function with a mechanism other than miRNA sponge and generating polypeptide.
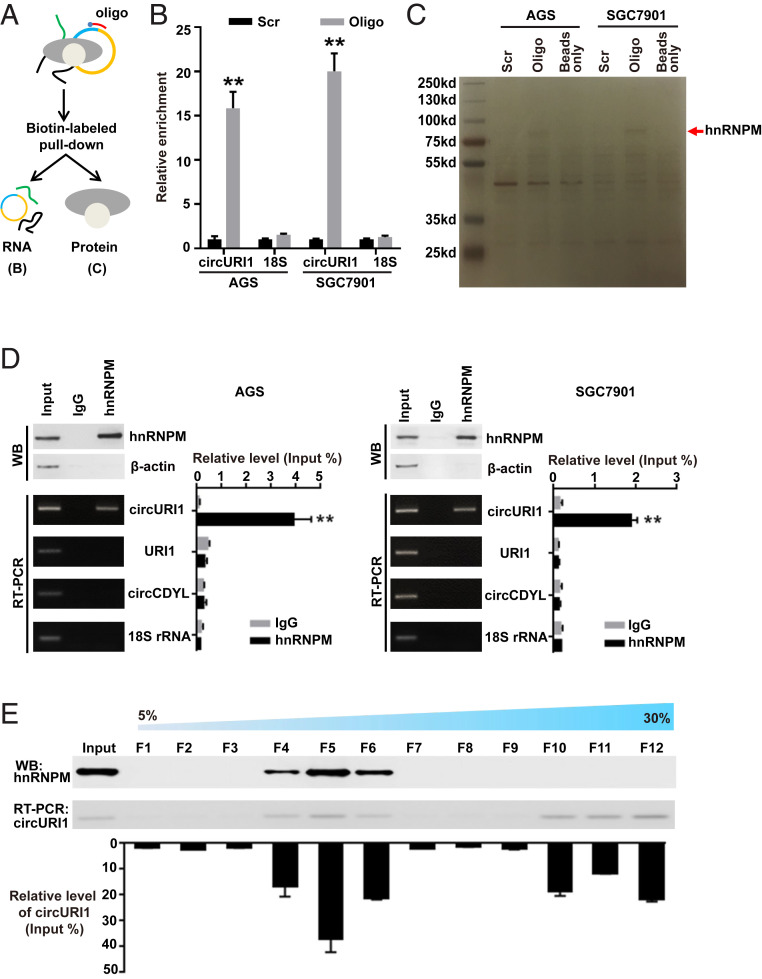
To identify the potential protein partner of circURI1 in GC cells, we designed a biotin-labeled DNA probe against the junction site and performed RNA pulldown assays (Fig. 3A). RT-qPCR indicated the effective and specific capture of circURI1 in AGS and SGC7901 cells (Fig. 3B). HnRNPM, an RNA-binding protein (RBP) (37), was later determined as the circURI1-associated protein through mass spectrometry (MS) (Fig. 3C and SI Appendix, Fig. S5A). The interaction between circURI1 and hnRNPM was further validated with antibodies against endogenous hnRNPM to examine the RNAs coimmunoprecipitated with hnRNPM (RNA IP) in AGS and SGC7901 cells (Fig. 3D). To further characterize the interaction between circURI1 and hnRNPM, we examined the sedimentation patterns of whole-cell materials from AGS cells with a 5 to 30% sucrose gradient. Briefly, total-cell lysate was loaded on sucrose gradients, and the corresponding gradients were divided into 12 fractions for later analysis after ultracentrifugation. The hnRNPM protein was mainly present in fractions 4 through 6, whereas the circURI1 levels examined by RT-qPCR were also enriched in fractions 4 through 6, with some presence in fractions 10 through 12 (Fig. 3E).
Fig. 3.
The interaction between circURI1 and hnRNPM in GC cells. (A) Illustration of the experimental procedure for the circURI1 pulldown assay with biotinylated antisense oligonucleotides. (B) Pulldown efficiency of circURI1 in AGS and SGC7901 cells. 18S rRNA was a negative control. Scr, biotin-labeled oligonucleotide with scrambled sequences; Oligo, biotin-labeled oligonucleotide with antisense sequences to the back-spliced junction site. (C) Potential circURI1-associated proteins identified via SDS-PAGE followed by silver staining. The red arrow denotes the band identified as hnRNPM by MS. (D) The specific association of hnRNPM and circURI1 demonstrated by Western blot and RT-PCR, respectively, based on hnRNPM RNA IP in AGS and SGC7901 cells. β-actin was a negative control for Western blot (WB), and URI1 mRNA, circCDYL, and 18S rRNA were used as negative controls for RT-PCR. (E) Sedimentation patterns of endogenous hnRNPM and circURI1 in 5 to 30% sucrose gradients from AGS whole-cell lysates. The components from the fractions of the gradients were determined by Western blot analysis for hnRNPM and RT-PCR for circURI1. Error bars indicate SEM from three independent experiments. **P < 0.01 by two-tailed Student’s t test.
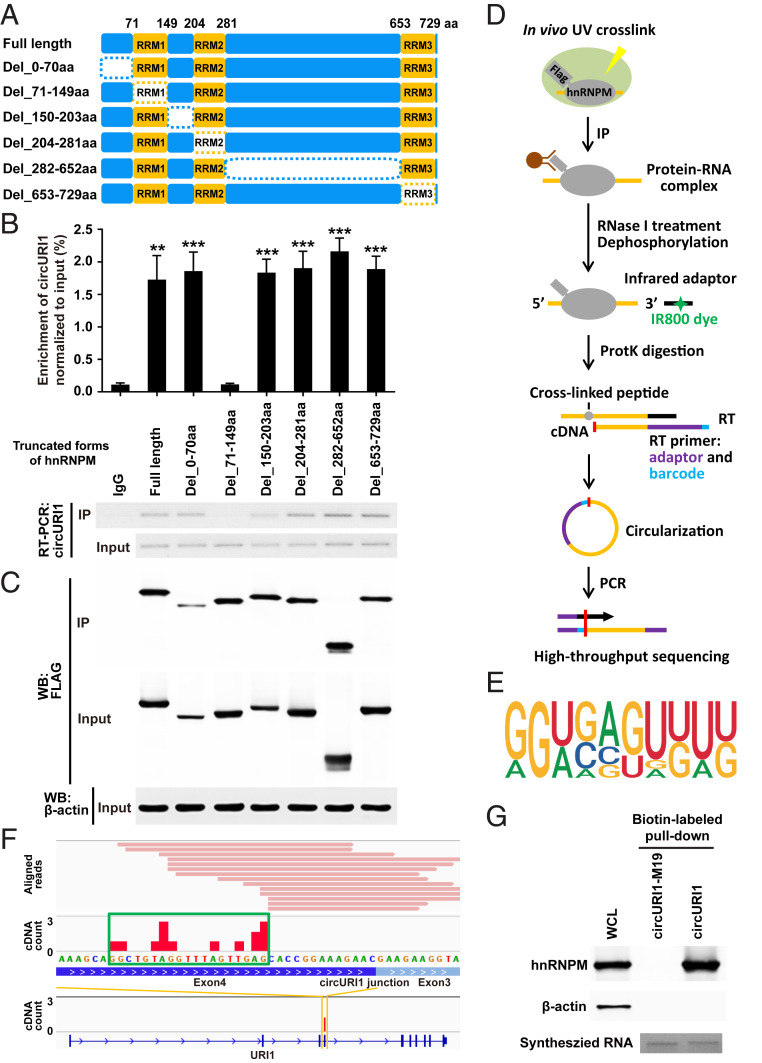
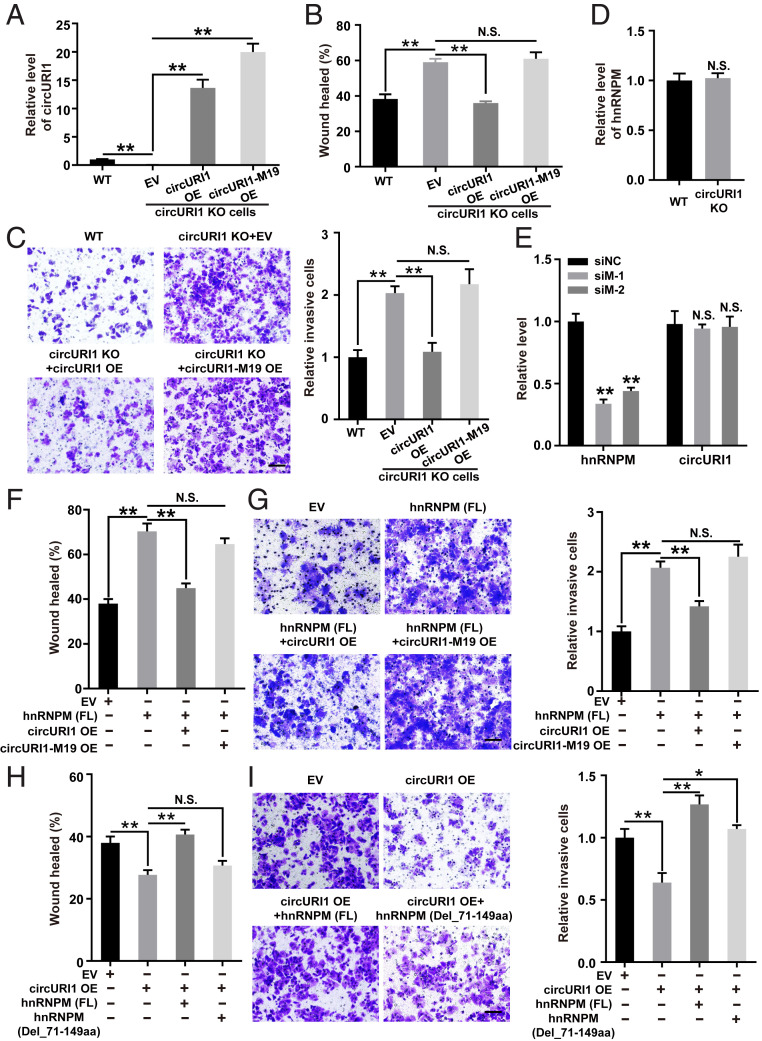
The hnRNPM protein consists of three RNA recognition motifs (RRMs) tandemly split across the structure (Fig. 4A). To depict the domain required for the interaction, full-length and a variety of truncated forms of hnRNPM were overexpressed in AGS cells. RNA IP for hnRNPM and its truncations illustrated that the RRM1 domain (71 through 149 amino acids) was responsible for the interaction with circURI1 (Fig. 4 B and C). Individual–nucleotide resolution crosslink immunoprecipitation (iCLIP) followed with RNA-seq is a powerful method to directly identify the cross-linked nucleotides of RNA bound to an RBP of interest (38-41). iCLIP-seq of FLAG-tagged hnRNPM was then performed in AGS cells, and a total of 314 hnRNPM binding targets were identified (Fig. 4D and SI Appendix, Table S2). The binding motif of hnRNPM was deduced from iCLIP-seq in AGS cells (Fig. 4E), which was inconsistent with the reported GU-rich motif often containing a UU sequence of hnRNPM–RNA interactions in 293T cells (42). HnRNPM iCLIP-seq determined that a 19-nt sequence (GGCUGUAGGUUUAGUUGAG), which was proximal to the junction site of circURI1, was the hnRNPM binding site (Fig. 4F). We also investigated the hnRNPM–circURI1 interaction with a method we termed individual-CLIP, which could be used to identify the binding site of a RBP on individual RNA target of interest (SI Appendix, Fig. S5B). The same 19-nt binding site in circURI1 was identified via hnRNPM individual-CLIP (SI Appendix, Fig. S5C and Table S3). Consistently, mutation of the 19-nt binding site in circURI1 (circURI1-M19) abolished the interaction between hnRNPM and circURI1 (Fig. 4G and SI Appendix, Fig. S5 D and E). CircURI1 but not circURI1-M19 could rescue the promotive effect of circURI1 KO on AGS cell migration and invasion (Fig. 5 A–C). Our results demonstrated that hnRNPM with its RRM1 domain interacted with circURI1 through the 19-nt RNA sequence.
Fig. 4.
The RRM1 domain of hnRNPM and the 19-nt sequence of circURI1 are required for their interaction. (A) Schematic diagram revealing full-length hnRNPM and a variety of truncated forms of hnRNPM. The dotted rectangles indicate the truncated domains. (B and C) Association of circURI1 examined by RT-PCR and RT-qPCR with RNA IP of Flag-tagged full-length and truncated hnRNPM in AGS cells. Full-length and truncated forms of hnRNPM were determined by Western blot with an anti-FLAG antibody. β-actin was a loading control for Western blot. (D) Illustration of the experimental procedure of iCLIP-seq. (E) The binding motif of hnRNPM derived from iCLIP analyzed by Hypergeometric Optimization of Motif EnRichment (HOMER). (F) Individual Flag-hnRNPM iCLIP-seq reads (in pink) were aligned to circURI1 or URI1 mRNA. iCLIP-seq cDNA counts (in red) showing the binding signals. The binding site of hnRNPM on circURI1 deduced from iCLIP-seq is depicted with the green rectangle. (G) RNA pulldown assay of biotin-labeled circURI1 and circURI1 with the mutation of the 19-nt binding site (circURI1-M19) followed by Western blot with an anti-hnRNPM antibody. CircURI1 and circURI1-M19 were circularized in vitro. Agarose gel performed in vitro synthesized biotin-labeled circURI1 and circURI1-M19. β-actin was a negative control for Western blot. WCL, whole-cell lysis. Error bars indicate SEM from three independent experiments. **P < 0.01; ***P < 0.001 by two-tailed Student’s t test.
Fig. 5.
The effects of circURI1 and hnRNPM on GC cell migration and invasion. (A) RT-qPCR analysis of circURI1 expression levels in WT or circURI1 KO AGS cells with ectopic circURI1 overexpression (OE) and circURI1 with the 19-nt mutation (circURI1-M19). (B and C) Wound-healing and Transwell assays in WT or circURI1 KO AGS cells with overexpressing circURI1 or circURI1-M19. (Scale bars, 100 μm.) (D) RT-qPCR analysis of hnRNPM expression levels in WT and circURI1 KO cells. (E) RT-qPCR analysis of knockdown efficiency of hnRNPM and circURI1 expression levels in AGS cells treated with two siRNAs against hnRNPM. siNC, siRNA with scrambled sequences; siM-1 and siM-2, two independent siRNAs against hnRNPM. (F and G) Wound-healing and Transwell assays of AGS cells transfected with full-length hnRNPM (hnRNPM [FL]) or co-overexpressed with circURI1 and circURI1-M19. (Scale bars, 100 μm.) (H and I) Wound-healing and Transwell assays of AGS cells overexpressing circURI1 or co-overexpressing hnRNPM truncated forms including hnRNPM (FL) and hnRNPM without the RRM1 domain (hnRNPM [Del_71-149aa]). (Scale bars, 100 μm.) Error bars indicate SEM from three independent experiments. N.S., not significant; *P < 0.05; **P < 0.01 by two-tailed Student’s t test.
Effects of circURI1 and hnRNPM on Cell Migration and Invasion in GC.
HnRNPM was recently proposed to modulate RNA alternative splicing in association with cancer metastasis (43, 44). Roles of hnRNPM in GC remain largely unknown. Box plots analyzed from The Cancer Genome Atlas (TCGA) datasets showed extremely higher hnRNPM expression levels in GC compared with normal tissues (SI Appendix, Fig. S6A). Knockdown of either circURI1 or hnRNPM did not change the expression level of the other interaction partner; hence, suggesting that these partners do not transcriptionally regulate or destabilize each other (Fig. 5 D and E and SI Appendix, Fig. S6 B and C). Knockdown of hnRNPM with two independent siRNAs obviously reduced cell migration in AGS and SGC7901 cells (SI Appendix, Fig. S6D). We also found that depletion of hnRNPM significantly diminished AGS cell invasion (SI Appendix, Fig. S6E), consistent with previous observations in breast cancer (27). These results provided evidence that depletion of hnRNPM impaired cell migration and invasion, which yielded the opposite effects of circURI1 inhibition.
Then, knockdown of hnRNPM recovered the migratory ability of AGS and SGC7901 cells with circURI1 knockdown (SI Appendix, Fig. S6 F and G). Similarly, silencing hnRNPM also rescued the invasion-promoting effect of circURI1 knockdown on AGS cell invasion (SI Appendix, Fig. S6H). CircURI1 but not circURI1-M19 blocked the promoting effect of hnRNPM overexpression on AGS cell migration and invasion (Fig. 5 F and G). Additionally, full-length hnRNPM (FL) but not hnRNPM without the RRM1 domain (Del_71 through 149aa) reversed the repressive effect of circURI1 overexpression on AGS cell migration and invasion (Fig. 5 H and I). HnRNPM (FL) exhibited a more promotive effect on cell invasion than hnRNPM (Del_71 through 149aa) in circURI1 KO AGS cells, indicating that the circURI1-interacting domain RRM1 is required for the observed cellular phenotypes (SI Appendix, Fig. S6I). Taking these results together, we concluded that roles of circURI1 on cell migration and invasion were dependent on its interaction with hnRNPM.
CircURI1 Modulates Alternative Splicing via Sequestering hnRNPM.
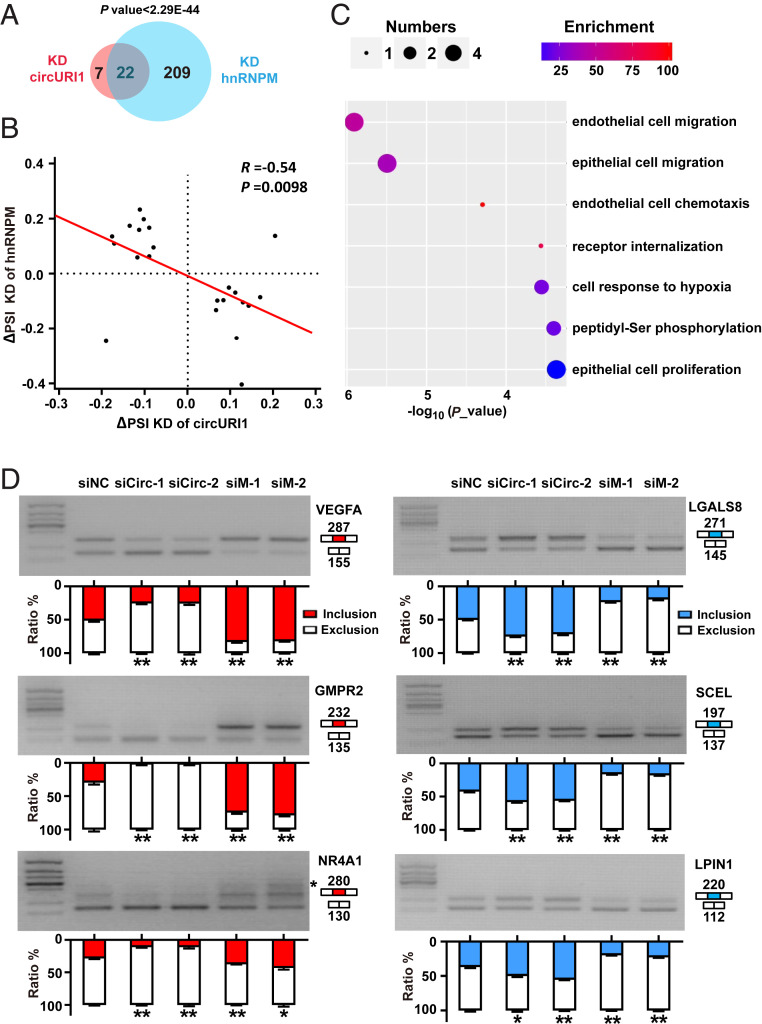
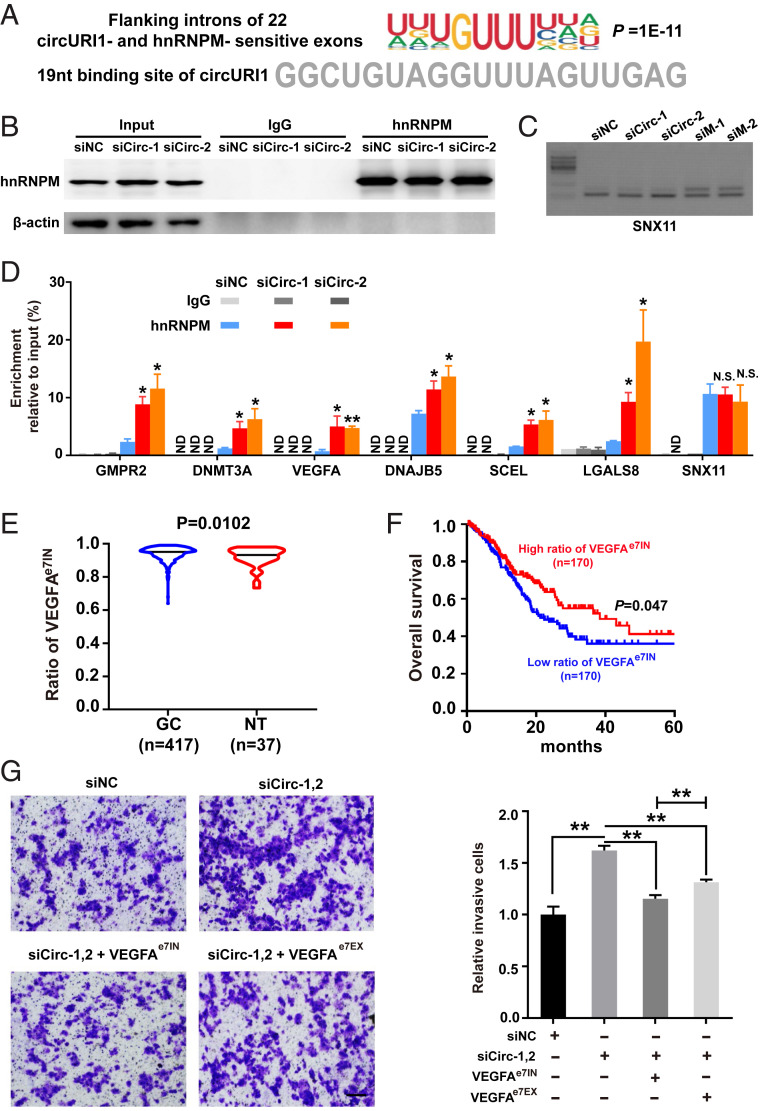
To provide insight into the mechanism of circURI1 and hnRNPM in GC, we silenced the expression levels of hnRNPM and circURI1 in AGS cells and performed RNA sequencing. HnRNPM knockdown resulted in 231 alternative splicing exons (termed hnRNPM-sensitive exons) with marked alterations in AGS cells (Fig. 6A and SI Appendix, Table S4), in line with previous findings about hnRNPM in exon inclusion or exclusion in 293T cells (44). HnRNPM iCLIP-seq showed more bindings in the flanking introns of the hnRNPM-sensitive exons than those of the insensitive exons (SI Appendix, Fig. S7A). Gene Ontology analysis for parental genes of 231 hnRNPM-sensitive exons demonstrated a biological process associated with endothelial cell migration (SI Appendix, Fig. S7B). CircURI1 knockdown led to 29 alternative splicing exons (termed circURI1-sensitive exons) with significant dysregulation (Fig. 6A and SI Appendix, Table S4). Venn diagram showed that the 22 exons were both sensitive to circURI1 and hnRNPM (Fig. 6A). The observed changes in both circURI1 and hnRNPM-sensitive exons exhibited a negative correlation (Fig. 6B). Gene Ontology analysis for parental genes of both circURI1- and hnRNPM-sensitive exons illustrated biological processes associated with cell migration, endothelial cell chemotaxis, cellular response to hypoxia, peptidyl-Ser phosphorylation, and epithelial cell proliferation (Fig. 6C). Experimental validations with semi-quantitative RT-PCR were conducted to examine the changes in alternative splicing for 10 genes (five exon inclusions and five exon exclusions) upon knockdown of circURI1 or hnRNPM (Fig. 6D and SI Appendix, Fig. S7C). The results confirmed that circURI1 could induce specific exon inclusion and exclusion. More importantly, it was clear that circURI1 influenced inclusion or exclusion in contrast to hnRNPM (Fig. 6D and SI Appendix, Fig. S7C). De novo motif analysis of flanking introns for the 22 circURI1- and hnRNPM-sensitive exons showed that the most-enriched motif was a UUGUUU sequence, similar to the 19-nt sequences in circURI1 identified by hnRNPM iCLIP-seq (Fig. 7A), which was distinct from the motifs of flanking introns for 209 circURI1 insensitive and hnRNPM-sensitive exons (SI Appendix, Fig. S7D). After circURI1 knockdown in AGS cells, hnRNPM binding to pre-mRNA of circURI1-sensitive genes was significantly increased (Fig. 7 B–D). In addition, 22 circURI1-sensitive exons are relative more sensitive to hnRNPM knockdown (SI Appendix, Fig. S7E). All these results indicated that circURI1 served as a decoy of hnRNPM to modulate alternative splicing.
Fig. 6.
The effects of circURI1 and hnRNPM on alternative splicing. (A) Venn diagram illustrating the overlap of altered alternative splicing events upon depleting circURI1 or hnRNPM in AGS cells. P value was calculated by χ2 test. (B) Correlation plot of ∆PSI for exons sensitive to both circURI1 and hnRNPM. The PSI was used to calculate the changes in alternative splicing. R represents Spearman’s correlation coefficient, and the P value was calculated by Spearman’s correlation test. (C) Gene Ontology analysis of both circURI1 and hnRNPM-sensitive targets analyzed by Gorilla. (D) Semi-quantitative RT-PCR validation of both circURI1 and hnRNPM-sensitive exons upon knockdown of circURI1 or hnRNPM in AGS cells. For NR4A1, an unspecific band is also amplified (indicated with a star). siNC, siRNA with scrambled sequences; siCirc-1 and siCirc-2, two siRNAs against the junction sites of circURI1; siM-1 and siM-2, two independent siRNAs against hnRNPM. Error bars indicate SEM from three independent experiments. *P < 0.05; **P < 0.01 by two-tailed Student’s t test.
Fig. 7.
CircURI1 modulates alternative splicing via sequestering hnRNPM. (A) The deduced motif of introns flanked by 22 both circURI1 and hnRNPM-sensitive exons analyzed by HOMER (-len 10). The 19-nt sequence of circURI1 required for the interaction of hnRNPM is indicated below. (B) RNA IP efficiency of hnRNPM demonstrated by Western blot after circURI1 silencing in AGS cells. β-actin was a negative control for RNA IP. siNC, siRNA with scrambled sequences; siCirc-1 and siCirc-2, two siRNAs against the junction sites of circURI1. (C) Semi-quantitative RT-PCR validation of alternatively spliced exon in sorting nexin 11 (SNX11), a hnRNPM-sensitive gene not sensitive to circURI1, upon silencing circURI1 or hnRNPM in AGS cells. siM-1 and siM-2, two independent siRNAs against hnRNPM. (D) RNA IP of hnRNPM showing the binding for the corresponding pre-mRNAs of circURI1-sensitive targets upon circURI1 knockdown in AGS cells. SNX11 was a negative control. ND, no detection. (E) Violin plots depicting the ratio of VEGFAe7IN in GC and normal patients analyzed from TCGA database. NT, normal tissue. VEGFAe7IN, exon 7 inclusion of VEGFA. (F) Kaplan–Meier analysis of overall survival for GC patients with VEGFAe7IN from TCGA database. The red curve represents survival in GC patients with a high ratio of VEGFAe7IN, and the blue line represents survival in patients with a low ratio of VEGFAe7IN. (G) Transwell assays of AGS cells treated with siRNAs targeting circURI1 or co-overexpressing VEGFAe7IN or VEGFAe7EX. siNC, siRNA with scrambled sequences; siCirc-1,2, two siRNAs (siCirc-1, siCirc-2) against the junction sites of circURI1. (Scale bars, 100 μm.) Error bars indicate SEM from three independent experiments. In D, E, and G, N.S., not significant; *P < 0.05; **P < 0.01 by two-tailed Student’s t test. In F, P value by the log-rank test.
Alternative Splicing of VEGFA Is a Functional Target of circURI1.
To further elucidate the clinical relevance of alternative splicing mediated by circURI1 and hnRNPM, we evaluated the association between the changes in alternative splicing of the four genes (VEGFA, LGALS8, AKT1, and NR4A1) involved in the process of cell migration and clinicopathological features of GC. Our results demonstrated that circURI1 could promote exon 7 inclusion of VEGFA (VEGFAe7IN), exon 8 exclusion of LGALS8 (LGALS8e8EX), exon 2 inclusion of AKT1 (AKTe2IN), and exon 2 inclusion of NR4A1 (NR4A1e2IN) (Fig. 6 and SI Appendix, Table S4). Violin analysis of TCGA database concertized that the ratios of VEGFAe7IN and LGALS8e8EX were significantly higher in GC compared with normal tissues, while AKTe2IN and NR4A1e2IN had no significances between GC and normal tissues (Fig. 7E and SI Appendix, Fig. S7F). Furthermore, Kaplan–Meier survival curve analysis of TCGA database revealed that the overall survival of GC patients with a high ratio of VEGFAe7IN was obviously longer compared with that of GC patients with a low ratio of VEGFAe7IN (Fig. 7F). Meanwhile, LGALS8e8EX, AKTe2IN, and NR4A1e2IN had no significant correlation to the survival of GC patients (SI Appendix, Fig. S7G). Finally, VEGFAe7IN isoform possessed a greater ability to recover the promotive effect of circURI1 knockdown on AGS cell invasion than VEGFAe7EX (Fig. 7G). We concluded that alternative splicing of VEGFA is a functional target of circURI1.
Discussion
Here, we uncovered that circURI1, a predominantly nuclear circRNA, had significantly higher expression levels in GC versus paraGC tissues and repressive effects on GC metastasis in vitro and in vivo, indicating that circURI1 exerted a potent anti-metastatic activity. Mechanistically, circURI1 exhibited its protecting roles in cancer metastasis via sequestering hnRNPM protein to modulate alternative splicing in a subset of metastasis-related genes such as VEGFA. The identification of a circRNA playing anti-metastasis roles through modulating alternative splicing as a protein decoy is a breakthrough in cancer research.
Many circRNAs, such as CDR1as and circBoule, are conserved across species (45–47). Our results demonstrated that circularization of human circURI1 was facilitated through the flanking reverse complementary Alu elements (Fig. 2 D and H) and not regulated by hnRNPM (Fig. 5 D and E), which was recently reported to control circRNA biogenesis (48). However, we could not exclude the possibility that other RBPs might be responsible for the generation of circURI1. CircURI1 is not detected in mice, which may be due to the lack of reverse complementary sequences in the corresponding flanking introns of the murine URI1 gene (SI Appendix, Fig. S2 E and F), demonstrating that circURI1 is not evolutionarily conserved.
CircURI1 expression level is increased in GC compared to paraGC, although it seems that this molecule with repressive roles needs to be down-regulated for the metastasis. Two long noncoding RNAs (lncRNAs), TFPI2AS1 and LINC00460, behave like circURI1 in other cancer types (49, 50). TFPI2AS1 is markedly up-regulated in non–small-cell lung cancer and suppresses cell proliferation and migration (49), and LINC00460 is significantly up-regulated in colorectal cancer (CRC) and yet exhibits inhibitory effects on CRC proliferation (50). Coding genes such as ERK3 have been shown to be transcriptionally up-regulated in non-melanoma skin cancers, and higher ERK3 expression also suppresses cell migration (51, 52). In addition to the up-regulation in GC, circURI1 was also detected in diverse 30 samples including cancer cells and multiple tissues employed in circBase database, indicating its importance beyond GC.
Considering that URI1 functions as an oncogene in ovarian cancer cells (53), our series of results excluded the cis roles of circURI1 on URI1 mRNA in GC cells. Sucrose density gradient centrifugation showed two potential circURI1-associated complexes in AGS cells. Consistent with this result, circURI1 was localized in both the nucleus and cytoplasm in GC cells (Fig. 1F). The light complex was consistent with hnRNPM and very likely localized within the nucleus and probably required for modulating alternative splicing of genes involved in the process of cell migration. It is appealing to suspect that the circURI1-associated high molecular weight complex might also play essential roles in GC as well. Understanding the molecular and cellular roles of the circURI1-associated high molecular weight complex and cytoplasmic circURI1 would further extend our knowledge about the functions of circURI1 in the pathogenesis of GC.
We identified 29 alternative splicing exons as the core of circURI1 targets through RNA-seq and overlapped with the targets of hnRNPM to obtain 22 exons sensitive to both circURI1 and hnRNPM. The parental genes for the 22 exons were enriched for several functions, including endothelial cell migration and epithelial cell migration. It is well known that angiogenesis is an important initial step in cancer metastasis (54). Alternative splicing of VEGFA, which plays key roles in vascular development in the four genes involved in the process of cell migration, is significantly associated with patient survival (Fig. 7F). The VEGF-signaling pathway is a clinically validated therapeutic target for several pathological cancers, and targeting VEGFR-2 with the antibody ramucirumab proved useful in second-line treatment of advanced GC (55–57). Given that VEGFA is frequently amplified in the chromosome instability subtype of GC (58) and VEGFAe7IN is promoted by circURI1, it would be interesting to assess whether higher circURI1 expression copes with the increased requirement of alternative splicing of VEGFA during the progression of GC and has an effect on clinical outcomes of patients receiving ramucirumab. We could not rule out the probability that other targets in addition to VEGFA, such as LGALS8 and AKT1, together contribute to GC metastasis of the circURI1/hnRNPM axis.
Noncoding RNAs play crucial roles in the regulation of alternative splicing with several mechanisms (59, 60). For instance, lncRNA NEAT1 regulates the phosphorylation status of splicing factors to affect alternative splicing (59). LncRNA 5S-OT coordinates with U2AF65 to regulate alternative splicing via RNA:RNA pairing (60). In the present study, we demonstrated that circURI1 sequestered hnRNPM protein to modulate alternative splicing to repress GC metastasis. Similarly, a recent investigation revealed that circHomer1a regulated alternative splicing of disease-associated genes with an unknown mechanism (61). Based on our data, we suspected that the binding affinity of circURI1 and hnRNPM was greater than that of circURI1-sensitive exons’ flanking introns and hnRNPM, leading to the competition of circURI1 to hnRNPM to modulate alternative splicing.
In conclusion, we performed circRNA profiling and identified circURI1 as a significantly up-regulated circRNA in GC compared with paraGC. CircURI1 inhibited GC metastasis by sequestering hnRNPM protein to modulate alternative splicing of a small subset of genes involved in cell motility, intimating a potential self-preservation mechanism against tumor metastasis. To our knowledge, circURI1 is the only circRNA to manipulate alternative splicing involved in cancer metastasis, expanding the vital roles of circRNAs in human health.
Methods
CircRNA Identification.
For circRNA prediction, we identified the circRNA candidates with find_circ (13), and the junction reads were calculated as BRPM. In brief, the adapters were first trimmed with cutadapt to obtain clean reads, and the left reads were then aligned to the human genome (hg19) with bowtie2 allowing one mismatch. The reads continuously aligned to the reference genome were filtered out, and the remaining reads were subjected to following analysis. The 20-mers from both ends were extracted and aligned independently to find unique anchor positions. Finally, we extended the anchor alignments to detect the breakpoints flanked by GU/AG splice sites. The differentially expressed circRNAs with the genomic length ≤100kb (SI Appendix, Table S1) were determined by DEseq2 with the criterion of P ≤ 0.05, BRPM ≥ 0.2, and |log2(fold change)| ≥ 1.
Analysis of Alternative Splicing.
Alternative splicing analysis was performed with the previously described pipeline (60), and the percentage spliced in (PSI) denotes the fraction of mRNAs that represent the inclusion isoform. Briefly, the continuous reads aligned to the human genome (hg19) were filtered out with Bowtie, allowing two mismatches. The left unmapped reads were aligned to a custom library of exon–exon junctions (EEJs) with Bowtie (version 2-m 20-best) with at least a 4-nt overhang. The custom EEJ library was generated by using existing RNA-seq data, expressed sequence tag and cDNA evidence, gene annotations, and evolutionary conservation. For the case of single-exon kipping events, we generated EEJs for E1AS, ASE2, and E1E2 (AS represents the alternative exon, and E1 and E2 represent the neighboring constitutive exons).
∆PSI was calculated by subtracting the PSI of the knockdown group from that of the control, and P values were generated with χ2 test. The dysregulated alternative splicing (SI Appendix, Table S4) was determined with the criterion of P ≤ 0.05 and |∆PSI | ≥ 0.05.
Northern Blotting.
Northern blotting was carried out as previously described (62). For probe preparation, antisense digoxigenin-labeled RNA probes were synthesized with a DIG Northern Starter Kit (Roche) according to the manufacturer’s protocol. The corresponding PCR fragments were used as templates for T7 transcription in vitro. The primers for the amplification of the antisense probe were listed in SI Appendix, Table S5.
Ribosome Profiling.
Ribosome profile analysis was carried out as previously described (63). A total of 107 cells were treated with ice-cold phosphate-buffered saline or culture medium containing 100 mg/mL cycloheximide (Sigma) for 10 min followed by lysis in ribosome lysis buffer (10 mM Tris⋅HCl [pH 7.4], 5 mM MgCl2, 100 mM KCl, 1% Triton X-100, 3 mM dithiothreitol [DTT], 100 mg/mL cycloheximide, 5 U/mL RNase inhibitor [Promega], and 1× Protease-inhibitor mixture [Roche]). Polysomes were separated on a 20 to 50% linear sucrose gradient containing 20 mM Tris (pH 7.4), 5 mM MgCl2, 100 mM KCl, 3 mM DTT, 100 mg/mL cycloheximide, and 1 U/mL RNase inhibitor (Promega) and centrifuged at 38,000 rpm for 4 h in a Beckman SW41 Ti rotor. The gradients were fractionated with optical scanning at 254 nm using a Gradient Fractionator (BioComp).
In Vivo Tumor Metastasis Assay.
The animal studies were approved by the Institutional Animal Care and Use Committee of Hefei Institutes of Physical Science, Chinese Academy of Sciences. BABL/c 6-wk-old male nude mice were maintained under specific pathogen-free conditions, with individually ventilated cages and in a 12-h light-dark cycle with ad libitum access to food and water. For metastasis studies, 106 AGS cells were injected into tail veins of nude mice. At ∼6 wk after injection, the animals were euthanized, and the lungs were isolated and weighed. The number and size of lung metastases detected by hematoxylin–eosin (H&E) staining were assessed.
Western Blotting.
For Western blots, samples were separated on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels and then transferred to polyvinylidene fluoride membranes (Millipore). Membranes were processed according to the ECL Western blotting protocol (GE Healthcare). The following antibodies were used in Western blots: anti-FLAG (Proteintech, 20543-1-AP), hnRNPM (Proteintech, 26897-1-AP), and anti–β-actin (TransGen, HC201). Antibody validation is provided on the manufacturers’ websites.
Wound-Healing and Transwell Assays.
For the wound-healing assay, after transfection for 48 h, cells were seeded in 6-well plates with Culture-Insert 2 Well (µ-Dish 35 mm, high ibiTreat) according to the manufacturer’s instruction and then incubated in medium containing 2% fetal bovine serum (FBS). The width of the scratch was measured at 24 h using a light microscope. For the transwell assay, matrigel (BD Biosciences) was diluted with serum-free medium (1:8), mixed, and used to coat the insert chamber membrane. In total, 5 × 105 cells with 48-h transfection in serum-free medium were added to the upper chamber, and medium containing 10% FBS was added to the lower chamber. After incubation for 24 h, the remaining cells in the upper compartment were completely removed, and then the migrated cells on the membrane were stained by 0.1% crystal violet for 10 min. Finally, the number of cells that invaded into the lower chambers was counted under an inverted microscope (Olympus).
RNA Pulldown and RNA Immunoprecipitation.
RNA pulldown with 5′-biotinylated antisense oligos and RNA IP were performed as previously described (60). The following antibodies were used: anti-FLAG (Sigma, F1804), anti-AGO2 (Sigma, SAB4200085), and anti-hnRNPM (OriGene, TA301557).
MS.
MS was performed as previously described with minor modifications (60). In brief, specific and obvious silver-stained band was cut, cleaned, and digested in-gel with sequencing grade-modified trypsin (Promega) in the digestion buffer (100 mM NH4HCO3, pH 8.5). After extraction and purification, the peptide samples were analyzed by a Nano liquid chromatography electrospray ionization–MS/MS system. The mass spectrometric data were searched against the UniProt protein database with ProtTech’s ProtQuest software suite.
Circularization In Vitro.
The synthesis of circRNAs in vitro was carried out as previously described with minor modification (64). Briefly, a biotin-labeled circRNA precursor was synthesized with a Biotin RNA labeling kit (Epicentre). After DNase treatment, additional GTP was added to a final concentration of 2 mM along with a circularization buffer including magnesium (50 mM Tris [pH 7.5], 15 mM MgCl2, and 1 mM DTT,) and then the reaction was heated at 55 °C for 20 min. To enrich for circRNA, RNase R-digested RNA was separated on 5% Urea PAGE gel. Bands corresponding to circRNA were excised from the gel and eluted overnight in elution buffer (20 mM Tris [pH 7.5], 250 mM NaOAc, 1 mM ethylene diamine tetraacetic acid, and 0.25% SDS). The eluted RNA was then purified using phenol/chloroform (pH 4.5). The circRNAs in vitro synthesized could be examined from the endogenous circRNAs due to a small stretch of added sequences (29 nt) involved in SI Appendix, Table S5.
Sucrose Density Gradients.
Sucrose density gradients were carried out as previously described, with several modifications (65). Sucrose gradients (5 to 30%) were poured using the Biocomp gradient station model 153 (BioComp) and contained 50 mM Tris (pH 8.5), 150 mM NaCl, and 1 mM EDTA. Total AGS cell lysates were loaded in 13 mL 5 to 30% sucrose gradients and centrifuged for 4 h at 40,000 rpm in a Beckman SW41 Ti rotor. Following centrifugation, fractions (1 mL) were collected manually from the top. All fractions were further used for protein and RNA analysis.
Statistical Analysis.
In all experiments, Student’s t tests and χ2 tests were used to calculate P values, as indicated in the figure legends. For Student’s t tests, the values reported in the graphs represent averages of three independent experiments, with error bars showing SEM. After ANOVA with F-tests, the statistical significance and P values were evaluated with Student’s t tests.
Supplementary Material
Acknowledgments
This study was support by the National Key Research and Development Program of China (2019YFA0802600 and 2018YFC1004500), National Natural Science Foundation of China (81972191, 81902525, 81672647, 31930019, 31725016, and 91940303), Science and Technology Major Project of Anhui Province (Grant 18030801140), the Strategic Priority Research Program “Biological basis of aging and therapeutic strategies” of the Chinese Academy of Sciences (XDB39010400), and the 100-Talent Program of Chinese Academy of Sciences. We thank Shenglin Huang (Department of Gastric Cancer and Soft Tissue Sarcomas, Fudan University Shanghai Cancer Center) for discussing the results. We also thank Ao Xu (Department of Pathology, Anhui Provincial Hospital) for pathological evaluation.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. S.K. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2012881118/-/DCSupplemental.
Data Availability
RNA-seq data have been deposited in the Gene Expression Omnibus (GEO) database under accession number GSE152309. HnRNPM iCLIP-seq data have been deposited in the GEO database under accession number GSE178223. All other study data are included in the article and/or SI Appendix.
References
- 1.Bray F., et al., Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Smyth E. C., Nilsson M., Grabsch H. I., van Grieken N. C., Lordick F., Gastric cancer. Lancet 396, 635–648 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Williams E. D., Gao D., Redfern A., Thompson E. W., Controversies around epithelial-mesenchymal plasticity in cancer metastasis. Nat. Rev. Cancer 19, 716–732 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kristensen L. S., et al., The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 20, 675–691 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Jeck W. R., et al., Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 19, 141–157 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L., Huang C., Wang X., Shan G., Circular RNAs in eukaryotic cells. Curr. Genomics 16, 312–318 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu X., et al., Identification of mecciRNAs and their roles in the mitochondrial entry of proteins. Sci. China Life Sci. 63, 1429–1449 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Vo J. N., et al., The landscape of circular RNA in cancer. Cell 176, 869–881.e13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du W. W., et al., Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res. 44, 2846–2858 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen S., et al., Widespread and functional RNA circularization in localized prostate cancer. Cell 176, 831–843.e22 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Hanniford D., et al., Epigenetic silencing of CDR1as drives IGF2BP3-mediated melanoma invasion and metastasis. Cancer Cell 37, 55–70.e15 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen T. B., et al., Natural RNA circles function as efficient microRNA sponges. Nature 495, 384–388 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Memczak S., et al., Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495, 333–338 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Zheng Q., et al., Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat. Commun. 7, 11215 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang L., et al., Engagement of circular RNA HECW2 in the nonautophagic role of ATG5 implicated in the endothelial-mesenchymal transition. Autophagy 14, 404–418 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z., et al., Exon-intron circular RNAs regulate transcription in the nucleus. Nat. Struct. Mol. Biol. 22, 256–264 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Legnini I., et al., Circ-ZNF609 is a circular RNA that can be translated and functions in myogenesis. Mol. Cell 66, 22–37.e9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y., et al., Extensive translation of circular RNAs driven by N6-methyladenosine. Cell Res. 27, 626–641 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J., et al., Circular RNA profile identifies circPVT1 as a proliferative factor and prognostic marker in gastric cancer. Cancer Lett. 388, 208–219 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Yang F., et al., Circ-HuR suppresses HuR expression and gastric cancer progression by inhibiting CNBP transactivation. Mol. Cancer 18, 158 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maniatis T., Tasic B., Alternative pre-mRNA splicing and proteome expansion in metazoans. Nature 418, 236–243 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Fu X. D., Ares M. Jr, Context-dependent control of alternative splicing by RNA-binding proteins. Nat. Rev. Genet. 15, 689–701 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dvinge H., Guenthoer J., Porter P. L., Bradley R. K., RNA components of the spliceosome regulate tissue- and cancer-specific alternative splicing. Genome Res. 29, 1591–1604 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Climente-González H., Porta-Pardo E., Godzik A., Eyras E., The functional impact of alternative splicing in cancer. Cell Rep. 20, 2215–2226 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Lokody I., Alternative splicing: Aberrant splicing promotes colon tumour growth. Nat. Rev. Cancer 14, 382–383 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Xie R., et al., Polypyrimidine tract binding protein 1 promotes lymphatic metastasis and proliferation of bladder cancer via alternative splicing of MEIS2 and PKM. Cancer Lett. 449, 31–44 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Xu Y., et al., Cell type-restricted activity of hnRNPM promotes breast cancer metastasis via regulating alternative splicing. Genes Dev. 28, 1191–1203 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geuens T., Bouhy D., Timmerman V., The hnRNP family: Insights into their role in health and disease. Hum. Genet. 135, 851–867 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rashid F., Shah A., Shan G., Long non-coding RNAs in the cytoplasm. Genomics Proteomics Bioinformatics 14, 73–80 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quinn J. J., Chang H. Y., Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 17, 47–62 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Yu B., Shan G., Functions of long noncoding RNAs in the nucleus. Nucleus 7, 155–166 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wheeler T. M., et al., Targeting nuclear RNA for in vivo correction of myotonic dystrophy. Nature 488, 111–115 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee J. S., Mendell J. T., Antisense-mediated transcript knockdown triggers premature transcription termination. Mol. Cell 77, 1044–1054.e3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lai F., Damle S. S., Ling K. K., Rigo F., Directed RNase H cleavage of nascent transcripts causes transcription termination. Mol. Cell 77, 1032–1043.e4 (2020). [DOI] [PubMed] [Google Scholar]
- 35.Song Z., et al., Antisense oligonucleotide technology can be used to investigate a circular but not linear RNA-mediated function for its encoded gene locus. Sci. China Life Sci. 64, 784–794 (2020). [DOI] [PubMed] [Google Scholar]
- 36.Dudekula D. B., et al., CircInteractome: A web tool for exploring circular RNAs and their interacting proteins and microRNAs. RNA Biol. 13, 34–42 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Datar K. V., Dreyfuss G., Swanson M. S., The human hnRNP M proteins: Identification of a methionine/arginine-rich repeat motif in ribonucleoproteins. Nucleic Acids Res. 21, 439–446 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.König J., et al., iCLIP reveals the function of hnRNP particles in splicing at individual nucleotide resolution. Nat. Struct. Mol. Biol. 17, 909–915 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zarnegar B. J., et al., irCLIP platform for efficient characterization of protein-RNA interactions. Nat. Methods 13, 489–492 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hosono Y., et al., Oncogenic role of THOR, a conserved cancer/testis long non-coding RNA. Cell 171, 1559–1572.e20 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ennajdaoui H., et al., IGF2BP3 modulates the interaction of invasion-associated transcripts with RISC. Cell Rep. 15, 1876–1883 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huelga S. C., et al., Integrative genome-wide analysis reveals cooperative regulation of alternative splicing by hnRNP proteins. Cell Rep. 1, 167–178 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Damianov A., et al., Rbfox proteins regulate splicing as part of a large multiprotein complex LASR. Cell 165, 606–619 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.West K. O., et al., The splicing factor hnRNP M is a critical regulator of innate immune gene expression in macrophages. Cell Rep. 29, 1594–1609.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ransohoff J. D., Wei Y., Khavari P. A., The functions and unique features of long intergenic non-coding RNA. Nat. Rev. Mol. Cell Biol. 19, 143–157 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao L., et al., Circular RNAs from BOULE play conserved roles in protection against stress-induced fertility decline. Sci. Adv. 6, eabb7426 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rybak-Wolf A., et al., Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol. Cell 58, 870–885 (2015). [DOI] [PubMed] [Google Scholar]
- 48.Ho J. S., et al., HNRNPM controls circRNA biogenesis and splicing fidelity to sustain cancer cell fitness. eLife 10, e59654 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao S., et al., TFPI2AS1, a novel lncRNA that inhibits cell proliferation and migration in lung cancer. Cell Cycle 16, 2249–2258 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang X., et al., Upregulated expression of long non-coding RNA, LINC00460, suppresses proliferation of colorectal cancer. J. Cancer 9, 2834–2843 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alshammari E. S., et al., ERK3 is transcriptionally upregulated by ∆Np63α and mediates the role of ∆Np63α in suppressing cell migration in non-melanoma skin cancers. BMC Cancer 21, 155 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Agrawal N., et al., Comparative genomic analysis of esophageal adenocarcinoma and squamous cell carcinoma. Cancer Discov. 2, 899–905 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Theurillat J. P., et al., URI is an oncogene amplified in ovarian cancer cells and is required for their survival. Cancer Cell 19, 317–332 (2011). [DOI] [PubMed] [Google Scholar]
- 54.Yang J., et al., Guidelines and definitions for research on epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 21, 341–352 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.English W. R., et al., Differential expression of VEGFA isoforms regulates metastasis and response to anti-VEGFA therapy in sarcoma. Cancer Res. 77, 2633–2646 (2017). [DOI] [PubMed] [Google Scholar]
- 56.Ferrara N., Kerbel R. S., Angiogenesis as a therapeutic target. Nature 438, 967–974 (2005). [DOI] [PubMed] [Google Scholar]
- 57.Fuchs C. S., et al., Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 383, 31–39 (2014). [DOI] [PubMed] [Google Scholar]
- 58.Cancer Genome Atlas Research Network , Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513, 202–209 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Romero-Barrios N., Legascue M. F., Benhamed M., Ariel F., Crespi M., Splicing regulation by long noncoding RNAs. Nucleic Acids Res. 46, 2169–2184 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu S., Wang X., Shan G., Insertion of an Alu element in a lncRNA leads to primate-specific modulation of alternative splicing. Nat. Struct. Mol. Biol. 23, 1011–1019 (2016). [DOI] [PubMed] [Google Scholar]
- 61.Zimmerman A. J., et al., A psychiatric disease-related circular RNA controls synaptic gene expression and cognition. Mol. Psychiatry 25, 2712–2727 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang X., Shan G., Nonradioactive Northern blot of circRNAs. Methods Mol. Biol. 1724, 135–141 (2018). [DOI] [PubMed] [Google Scholar]
- 63.del Prete M. J., Vernal R., Dolznig H., Müllner E. W., Garcia-Sanz J. A., Isolation of polysome-bound mRNA from solid tissues amenable for RT-PCR and profiling experiments. RNA 13, 414–421 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wesselhoeft R. A., Kowalski P. S., Anderson D. G., Engineering circular RNA for potent and stable translation in eukaryotic cells. Nat. Commun. 9, 2629 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Macias S., Cordiner R. A., Gautier P., Plass M., Cáceres J. F., DGCR8 acts as an adaptor for the exosome complex to degrade double-stranded structured RNAs. Mol. Cell 60, 873–885 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-seq data have been deposited in the Gene Expression Omnibus (GEO) database under accession number GSE152309. HnRNPM iCLIP-seq data have been deposited in the GEO database under accession number GSE178223. All other study data are included in the article and/or SI Appendix.