Abstract
Background
There are acute settings where assessing the anticoagulant effect of direct oral anticoagulants (DOACs) can be useful. Due to variability among routine coagulation tests, there is an unmet need for an assay that detects DOAC effects within minutes in the laboratory or at the point of care.
Methods
We developed a novel dielectric microsensor, termed ClotChip, and previously showed that the time to reach peak permittivity (Tpeak) is a sensitive parameter of coagulation function. We conducted a prospective, single-center, pilot study to determine its clinical utility at detecting DOAC anticoagulant effects in whole blood.
Results
We accrued 154 individuals: 50 healthy volunteers, 49 rivaroxaban patients, 47 apixaban, and 8 dabigatran patients. Blood samples underwent ClotChip measurements and plasma coagulation tests. Control mean Tpeak was 428 seconds (95% confidence interval [CI]: 401–455 seconds). For rivaroxaban, mean Tpeak was 592 seconds (95% CI: 550–634 seconds). A receiver operating characteristic curve showed that the area under the curve (AUC) predicting rivaroxaban using Tpeak was 0.83 (95% CI: 0.75–0.91, p < 0.01). For apixaban, mean Tpeak was 594 seconds (95% CI: 548–639 seconds); AUC was 0.82 (95% CI: 0.73–0.91, p < 0.01). For dabigatran, mean Tpeak was 894 seconds (95% CI: 701–1,086 seconds); AUC was 1 (p < 0.01). Specificity for all DOACs was 88%; sensitivity ranged from 72 to 100%.
Conclusion
This diagnostic study using samples from “real-world” DOAC patients supports that ClotChip exhibits high sensitivity at detecting DOAC anticoagulant effects in a disposable portable platform, using a miniscule amount of whole blood (<10 μL).
Keywords: anticoagulation, monitoring, direct oral anticoagulants, point of care, coagulation tests
Introduction
Direct oral anticoagulants (DOACs) are a new class of medications designed to inhibit coagulation factor IIa (dabigatran) or factor Xa (rivaroxaban, apixaban, and edoxaban), and are rapidly replacing warfarin as anticoagulants of choice. These newer agents present several advantages over vitamin K antagonists including predictable pharmacokinetic profile, a wide therapeutic window, and a shorter half-life. However, there are several clinical settings where assessment of the anticoagulant effect of DOACs may be useful, such as on-treatment bleeding, emergency surgery, breakthrough thrombosis, suspected non-compliance, and prior to administration of reversal agents. To date, no assays developed to measure DOAC activity have been approved by the Food and Drug Administration. Conventional coagulation tests such as prothrombin time (PT), activated partial thromboplastin time (aPTT), and thrombin time are all plasma-based assays that exhibit variation between instrument and reagents, and normal results may not exclude clinically relevant levels of anticoagulation.1 Specialized coagulation tests can be used to measure DOACs with high accuracy; however, these assays are not widely available. Therefore, there is an unmet clinical need for a sensitive assay that can rapidly detect DOAC anticoagulant effects in the laboratory or at the point of care (POC). We developed a novel dielectric microsensor, termed ClotChip, which allows for the comprehensive assessment of blood hemostasis using a disposable microfluidic sensor at the POC (►Fig. 1A, B). We previously showed that the time to reach a peak in permittivity (Tpeak) is a sensitive parameter to assess coagulation function (►Fig. 1C).2,3 We hypothesized that Tpeak can reliably detect the anticoagulant effect of DOACs and conducted a pilot clinical study to determine its diagnostic sensitivity in whole blood.
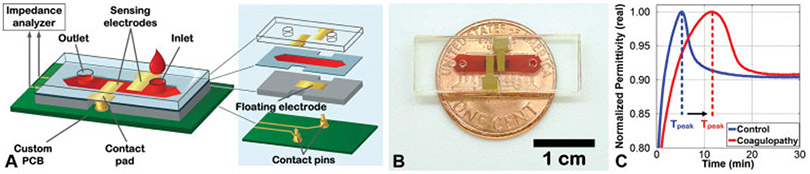
Fig. 1.
ClotChip fabrication and testing. (A) The ClotChip assembly procedure in which a polymethyl methacrylate (PMMA) cap with gold sensing electrodes was attached to a PMMA substrate with a gold floating electrode using a 250-μm double-sided adhesive (DSA) film. The DSA film was laser micromachined to form a microfluidic channel with a total sample volume of 9 μL. Contact openings were placed in the PMMA substrate and DSA film to allow for electrical connection between the sensing electrodes and an impedance analyzer. (B) Photograph of the ClotChip sensor filled with human whole blood in the microfluidic channel. (C) Representative ClotChip readout curves for human whole blood samples undergoing coagulation from a healthy volunteer (blue) and a patient with coagulopathy (red). The time to reach a permittivity peak (Tpeak) was an indicator of coagulation time of the blood sample.
Methods
Study Population
Healthy participants for this study were accrued from primary care clinics at the VA Medical Center. These individuals were primarily seen for their annual visit. Prior to study initiation, we held a face-to-face education session with all primary care staff and provided them with the International Society on Thrombosis and Haemostasis/Scientific and Standardization Committee Bleeding Assessment Tool (BAT), a questionnaire to assess the hemostatic capacity of potential study participants.4 The BAT score is ideal when excluding bleeding disorders rather than detecting mild bleeding disorders as it was validated by comparison with normal or never-referred subjects. A score of zero was required for individuals to be accrued for the study. In addition to BAT, the medication profile and laboratory results from each potential participant were reviewed. Eligible subjects in the healthy (control) group were 18 years or older; had never had abnormal hemostasis and were never prescribed anticoagulation; were not on selective serotonin reuptake inhibitors (SSRIs), nonsteroidal anti-inflammatory drugs (NSAIDs), antiplatelet agents (aspirin, clopidogrel, dipyridamole, ticagrelor, prasugrel, cilostazol) or herbal supplements (saw palmetto, St. John’s Wort, Ginkgo biloba, ginseng, Echinacea, quinine); and were without diagnosis of an acute illness in the past 4 weeks. Since variations in hematocrit number and platelet count can affect whole-blood assays,5,6 all accrued individuals had normal complete blood count profiles [normal range for: white blood cell (WBC) count: 3.6–11 K/cm3; hematocrit: 40–51%; platelet count: 150–400 K/cm3]. Exclusion criteria in this group included pregnancy, abnormal hematocrit levels (<40% or >51%), and laboratory parameters that can interfere with standard photo-optical coagulation tests [extreme lipemia (triglyceride levels > 1,000 mg/dL), hyperbilirubinemia (total bilirubin > 1.5 mg/dL)].
In the DOAC group, eligible patients were on anticoagulation with treatment doses of rivaroxaban (20 mg oral daily), apixaban (5 mg oral twice daily), or dabigatran (150 mg oral twice daily) for at least 3 months at the time of enrollment. Key exclusion criteria were concurrent use of antiplatelet medications, NSAIDs, SSRIs, or herbal supplements, and the afore-mentioned laboratory exclusion criteria applied for healthy participants.
Study Procedures and Oversight
This was a prospective, single-center, pilot clinical study conducted in accordance with the principles of the Declaration of Helsinki. All participants provided written informed consent. The protocol, amendments, and informed consent forms were approved by the institutional review board. A data monitoring committee oversaw the study and periodically assessed safety. All research laboratory members performing coagulation and ClotChip measurements were blinded for each sample.
Study Workflow
Blood samples were drawn by venipuncture into collection tubes containing 3.2% sodium citrate (ratio of blood to anticoagulant, 9:1). Healthy volunteers underwent blood sampling in untimed sessions (►Fig. 2A). For patients on rivaroxaban, blood draws were performed at two different time points; in the first group, blood was collected between 1.5 and 6 hours after the daily rivaroxaban dose, which corresponds to the time-to-peak plasma drug concentration (Tmax; ►Fig. 2B).7 In the second group of patients, blood was collected 9.5 to 16.5 hours after daily rivaroxaban dose, which corresponds to trough rivaroxaban levels (►Fig. 2B).7 For patients on apixaban and dabigatran, blood was collected between 2.5 and 4 hours and 1.5 and 4 hours after the morning dose, respectively, each corresponding to peak plasma drug levels (►Fig. 2C, D).8-10
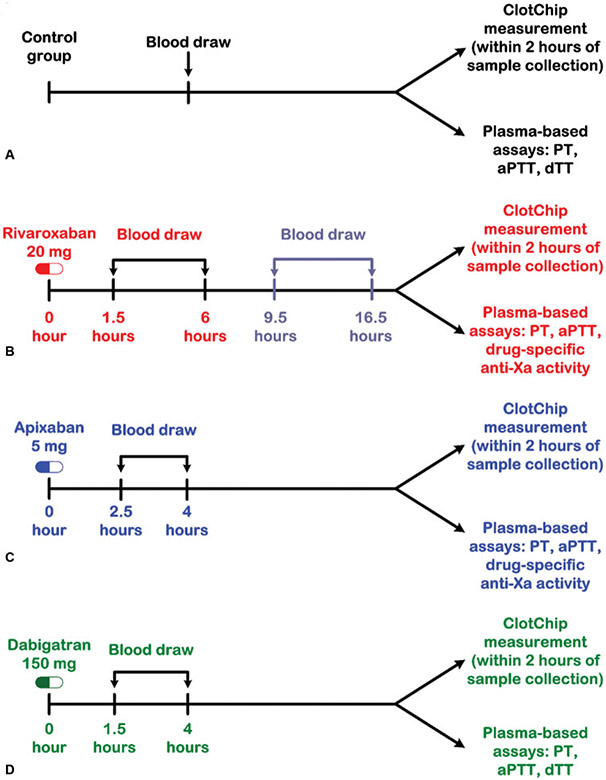
Fig. 2.
Study workflow. (A) Healthy volunteers underwent a single blood draw in an untimed manner. Whole blood was used for ClotChip measurements within 2 hours of sample collection and for plasma-based coagulation tests including prothrombin time (PT), activated partial thromboplastin time (aPTT), and dilute thrombin time (dTT). (B) Patients on rivaroxaban were accrued at two different time points; in the first group, blood was collected between 1.5 and 6 hours after daily rivaroxaban dose, corresponding to peak plasma drug concentrations. In the second group of patients, blood was collected 9.5 to 16.5 hours after daily rivaroxaban dose, which corresponded to trough plasma rivaroxaban levels. ClotChip measurements were run within 2 hours from blood collection. Plasma-based assays were also performed, including PT, aPTT, and rivaroxaban-specific anti-Xa assay. (C) For patients on apixaban, blood was collected between 2.5 and 4 hours after the morning apixaban dose and similarly processed. (D) In patients taking dabigatran, blood was collected between 1.5 and 4 hours after the morning dose. Whole blood was used for ClotChip measurements, and plasma was separated for PT, aPTT, and dTT assays.
Measurements
ClotChip Fabrication, Preanalytical Testing, and Measurements
The ClotChip operation is based on the electronic technique of dielectric spectroscopy (DS) to assess whole blood coagulation ex vivo. DS is the measurement of dielectric permittivity versus frequency, and ClotChip features a parallel-plate capacitive sensing structure that extracts the dielectric permittivity of whole blood in a microfluidic channel.2 A double-sided adhesive layer was laser-micromachined to form the microfluidic channel and was used to attach a pair of polymethyl methacrylate (PMMA) substrates incorporating gold electrodes, thereby forming a three-dimensional capacitive sensing area (►Fig. 1A,B). As blood undergoes coagulation in this area, the sensor impedance changes based upon its dielectric permittivity. For measurements in the MHz-frequency range, the dielectric permittivity of whole blood is sensitive to red blood cell (RBC) dynamics through the interfacial polarization of the RBC membrane and the surrounding plasma.11,12 We have previously shown that it is the aggregation and deformation of RBCs during clot development that gives rise to changes in dielectric permittivity during blood coagulation13,14 and that the maximum sensitivity to the blood coagulation process occurs at 1 MHz.3 The ClotChip readout is therefore taken as the temporal variation in the real part of blood dielectric permittivity at 1 MHz (►Fig. 1C). The microsensor fabrication and construction using biomedical-grade materials (PMMA and gold electrodes) incurred a material cost of <$1 per sensor, therefore making it a cost-effective, single-use, disposable cartridge.
We previously assessed the effect of preanalytical factors on the ClotChip readout.3 To test sample stability, we performed repeated measurements at 60-minute intervals for whole blood samples from healthy volunteers. A significant difference in Tpeak was found for samples assessed at 3.5 hours (p < 0.005) and 4.5 hours (p < 0.005) compared with samples that underwent ClotChip measurements at 30 minutes, 1.5 hours, and 2.5 hours after blood draw.3 Based on these validation results demonstrating that ClotChip readout exhibits repeatable characteristics for citrated whole blood when tested within 2.5 hours from blood draw, all ClotChip measurements in the present study were performed within 2 hours from the time of blood collection. Coagulation was initiated by adding calcium chloride (CaCl2, 20 mM) to citrated blood and injecting 10 μL of the mixture into the ClotChip. Measurements were immediately recorded inside a thermostatic chamber set at 37°C.
Quality control (QC) for ClotChip involved daily electronic calibration of the test setup. This entailed a custom printed-circuit board in the form of the ClotChip sensor containing an equivalent circuit model of whole blood to ensure accurate electrical measurement of blood samples.11 The second aspect of QC involved testing of a healthy sample each day that a patient sample was studied to ensure that the control Tpeak fell within our pre-established normal range. Healthy blood samples were procured from Case Western Reserve University Biorepository bank. Each donor was rigorously screened to exclude hemostatic defects with a questionnaire for history of abnormal bleeding and review of their active medications. Throughout the study, the degree of imprecision for ClotChip calibration where healthy samples yielded a Tpeak above 530 seconds was 0%.
Coagulation Assays
All assays were run in duplicate. The aPTT was performed by mixing 50 μL of citrated platelet-poor plasma with 50 μL of prewarmed aPTT reagent (Helena Laboratories) in disposable cuvettes and incubated for 3 minutes at 37°C. The reaction was initiated by adding 50 μL of 25 mM CaCl2. The endpoint clotting time was determined by a photo-optical Cascade M4 analyzer (Helena Laboratories). The PT assay was similarly performed by the addition of 100 μL of prewarmed PT reagent (Helena Laboratories) to 50 μL of citrated plasma. Rivaroxaban and apixaban anti-Xa activities were measured using BIOPHEN DiXal (Hyphen Biomed) drug-specific assays. Dabigatran concentration was measured using BIOPHEN DTI chromogenic assay. Dedicated calibrators and controls were used for each DOAC, per manufacturer instructions. Measurements were obtained on a STA-R analyzer.
Statistical Analysis
Assuming an observed area under the curve (AUC) of 0.75 in each group and an α of 0.05, we calculated that a control group of 50 and a comparison group of 20 provided >90% power for a two-sided test of the null hypothesis (AUC = 0.5). This power calculation was performed using the pROC package in R 3.5.1 and informed recruitment goals of subjects for controls and DOAC groups. Data obtained in this study are reported as mean with 95% confidence intervals (CIs), unless stated otherwise. We used the independent samples t-test to compare mean Tpeak between control and DOAC groups using Welch’s correction assuming unequal standard deviations (SDs) after assessing normality of Tpeak values with D’Agostino–Pearson Omnibus test. AUCs are presented with a 95% DeLong CI and were evaluated for significant differences from null value of 0.5 using the Wilcoxon–Mann–Whitney test. McNemar tests were conducted to compare the sensitivity and specificity of PT and aPTT at detecting DOAC anticoagulant effects. All p-values are two-tailed; p-values less than 0.05 were considered statistically significant. In box-and-whiskers plots, the box represents the range from the first to the third quartile, the horizontal line represents the median; whiskers extend to the maximum and minimum data values, dots represent individual-patient data. Outcomes and statistical analyses were performed with R 3.5.1 software; figures were generated using GraphPad Prism 7 software.
Results
Patients
From September 2017 to August 2018, 154 individuals were accrued for the study; 50 were healthy volunteers and 104 were patients on therapeutic doses of DOACs. For this latter group, 49 were on rivaroxaban, 47 were on apixaban, and 8 were on dabigatran. Baseline demographic characteristics between groups were balanced except for race, which consisted of 50% Caucasian individuals in the control group versus 82% Caucasian patients in the cumulative DOAC group (►Table 1).
Table 1.
Demographic and baseline characteristics of accrued individuals
| Control (n = 50) | Rivaroxaban (n = 49) | Apixaban (n = 47) | Dabigatran (n = 8) | |
|---|---|---|---|---|
| Age (y) | 61.6 ± 8.7 | 66.6 ± 7.6 | 71.4 ± 9.5 | 67.3 ± 5.5 |
| Sex, no. (%) | ||||
| Male | 50 (100%) | 49 (100%) | 47 (100%) | 8 (100%) |
| Race, no. (%) | ||||
| Caucasian | 25 (50%) | 40 (82%) | 37 (79%) | 8 (100%) |
| African American | 24 (48%) | 5 (10%) | 6 (13%) | 0 (0%) |
| Other | 1 (2%) | 4 (8%) | 4 (8%) | 0 (0%) |
| Body mass index (kg/m2), mean ± SD | 29.78 ± 9.3 | 30.57 ± 5.65 | 30.59 ± 6.26 | 31.76 ± 4.51 |
| Estimated glomerular filtration rate, no. (%) | ||||
| ≥ 80 mL/min | 25 (50%) | 14 (29%) | 13 (28%) | 4 (50%) |
| 50–80 mL/min | 23 (46%) | 30 (61%) | 27 (57%) | 4 (50%) |
| 30–50 mL/min | 2 (4%) | 5 (10%) | 7 (15%) | 0 (0%) |
| Liver function, mean ± SD | ||||
| Bilirubin, total (mg/dL) | 0.5 ± 0.1 | 0.5 ± 0.2 | 0.6 ± 0.3 | 0.8 ± 0.2 |
| Aspartate aminotransferase (U/L) | 27 ± 11 | 23 ± 9 | 22 ± 10 | 26 ± 7 |
| Alanine aminotransferase (U/L) | 34 ± 13 | 32 ± 13 | 29 ± 13 | 35 ± 16 |
| Alkaline phosphatase (U/L) | 83 ± 26 | 78 ± 20 | 88 ± 24 | 77 ± 18 |
Abbreviation: SD, standard deviation.
Measurements
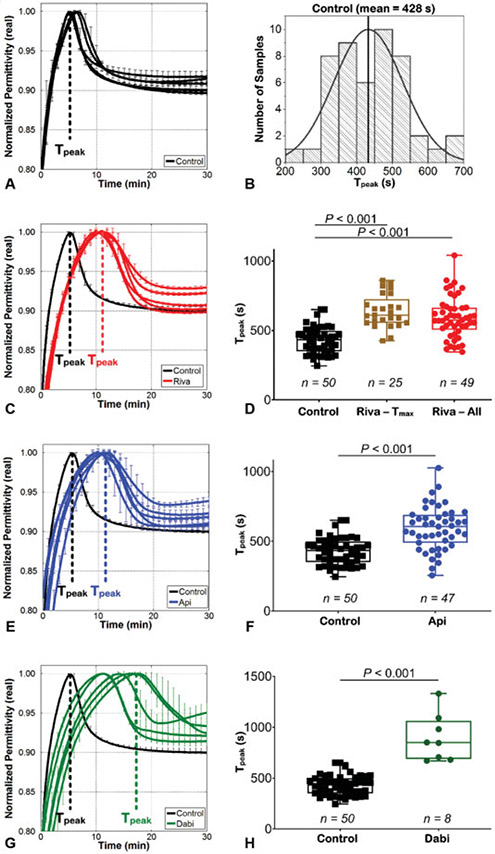
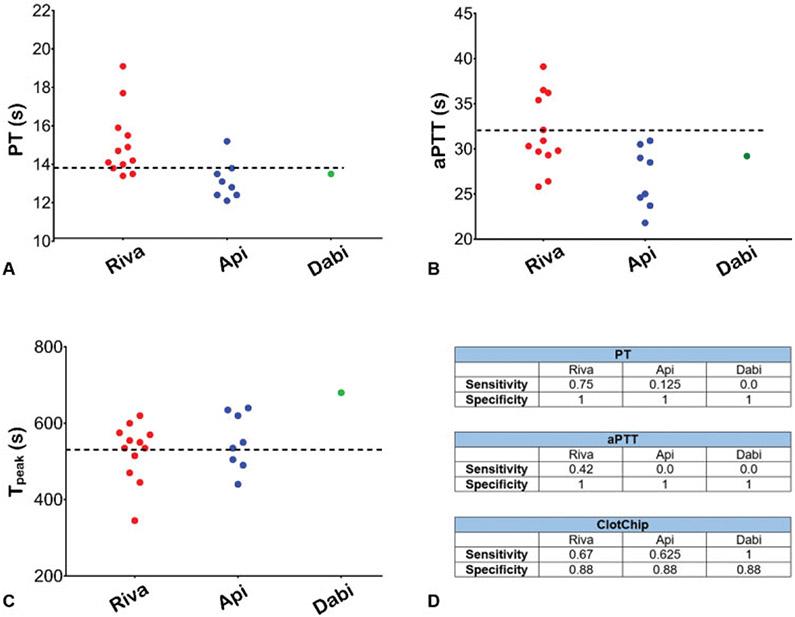
Representative ClotChip readouts from five healthy volunteers are shown in ►Fig. 3A. Measurements of the real permittivity obtained at 1 MHz were normalized to the maximum permittivity value for the entire duration of the experiment. This normalized real permittivity exhibited a repeatable characteristic rise to peak permittivity upon the start of each experiment, which is referred to as Tpeak and, based on our previous studies, is indicative of coagulation time. Mean Tpeak for the control group was 428 seconds (95% CI: 401–455 seconds; ►Fig. 3B). Coefficient of variation (CV) among duplicate ClotChip measurements was calculated as the ratio of the within-subject SD and the overall mean × 100.15 Healthy samples (control) exhibited a CV of 5.56% for duplicate ClotChip measurements. All healthy participants had normal coagulation parameters (►Fig. 4A, B); the normal PT range was 11.5 to 13.8 seconds, and the normal aPTT range was 20.6 to 32 seconds (mean ± 2 SD).
Fig. 3.
ClotChip measurements. (A) Representative ClotChip curves from five healthy volunteers. For all curves of the ClotChip readout, error bars indicate duplicate measurements. (B) Normal distribution of time-to-peak permittivity (Tpeak) for the control group (mean = 428 seconds; 95% confidence interval [CI] of the mean: 401–455 seconds; n = 50). (C) ClotChip readout from five patients on rivaroxaban showed prolonged Tpeak (representative curves in red) compared with the curve from a healthy volunteer (indicated in black). Error bars indicate duplicate measurements. (D) ClotChip Tpeak measurements from rivaroxaban samples drawn at time of peak plasma rivaroxaban concentration (defined as Tmax, n = 25) and cumulative values from all rivaroxaban samples drawn at peak and trough plasma drug levels (Riva – All, n = 49) versus control samples (n = 50). Each dot represents the mean of ClotChip measurements run in duplicate for each individual sample. Box-and-whiskers diagrams extend from each quartile to the minimum/maximum distribution. The horizontal line represents the median. *p < 0.001. (E) Representative ClotChip curves are shown for five apixaban samples drawn from patients at predicted peak plasma drug levels. Error bars indicate duplicate measurements for each sample. (F) Tpeak measurements for all apixaban samples (n = 47) versus control samples (n = 50). *p < 0.001. (G) Representative ClotChip measurements, run in duplicate, from patients on therapeutic dabigatran. (H) Quantitative representation of dabigatran Tpeak measurements (n = 8) versus control (n = 50). *p < 0.001.
Fig. 4.
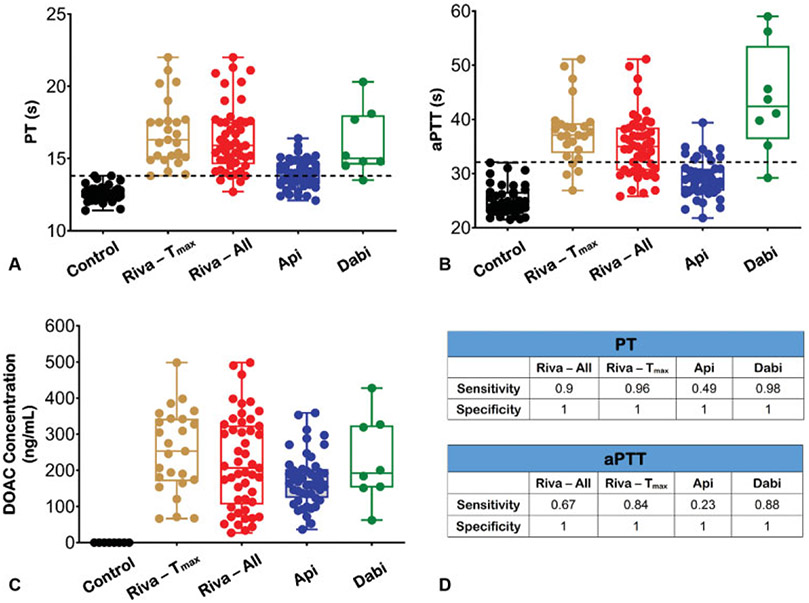
Plasma-based coagulation assays in the accrued cohort. (A) Prothrombin time (PT) results in healthy (control) individuals and patients on each DOAC agent. (B) Activated partial thromboplastin time (aPTT) results in control and DOAC individuals. Riva – Tmax refers to rivaroxaban samples drawn at time of peak plasma rivaroxaban concentration (n = 25); Riva – All refers to cumulative values from all rivaroxaban samples drawn at peak and trough plasma drug levels (n = 49); Apixaban: n = 47; Dabigatran: n = 8. The horizontal line in panels (A) and (B) represents the normal reference range for PT and aPTT, respectively. (C) Steady-state plasma drug levels for each DOAC cohort. A small number of healthy (control) samples were randomly selected for anti-Xa activity testing. Each dot in panels (A)–(C) represents the mean of duplicate measurements for each individual sample. Box-and-whiskers diagrams extend from each quartile to the minimum/maximum distribution. (D) Sensitivity and specificity of PT and aPTT assays in the DOAC cohort. DOAC, direct oral anticoagulant.
For the rivaroxaban group drawn at Tmax (n = 25), the median time since the last dose was 4.3 hours; the range was 1.5 to 6 hours. ClotChip measurements for this rivaroxaban group showed significantly prolonged mean Tpeak (637 seconds, 95% CI: 589–687 seconds) compared with control (p < 0.001; ►Fig. 3D). PT values ranged from 13.8 to 22 seconds (►Fig. 4A), and aPTT values extended from 26.9 to 51.1 seconds (►Fig. 4B). The rivaroxaban concentration ranged from 66 to 498 ng/mL (►Fig. 4C), while the mean rivaroxaban concentration was 253 ng/mL (►Fig. 4C). Measurements for all rivaroxaban samples (n = 49) showed a mean Tpeak of 592 seconds (95% CI: 550–634 seconds) that remained significantly prolonged compared with control (p < 0.001; ►Fig. 3C, D). CV for duplicate rivaroxaban measurements was 6.51%. Mean PT was 16.8 seconds (range: 12.7–22 seconds; ►Fig. 4A). Mean aPTT was 37.7 seconds (range: 26.9–51.1 seconds; ►Fig. 4B). The range of rivaroxaban concentration for all samples was 27 to 498 - ng/mL (►Fig. 4C), while the mean concentration was 221 ng/mL.
In the apixaban group, the median time since the last dose was 3.4 hours with a range of 2.5 to 4 hours. Mean Tpeak for apixaban was significantly prolonged (594 seconds, 95% CI: 548–639 seconds) compared with control (p < 0.001; ►Fig. 3E, F). CV for duplicate apixaban measurements was 6.7%. A considerable number of apixaban samples had PT (24 out of 47) and aPTT (37 out of 47) values that fell within the normal range (►Fig. 4A, B). The mean apixaban concentration was 175 ng/mL; range extended from 36.6 to 359 ng/mL (►Fig. 4C).
For the dabigatran group, the median time since last dose was 2.6 hours, range was 1.5–4 hours. Mean Tpeak was very prolonged (894 seconds, 95% CI: 701–1,086 seconds) compared with control (p < 0.001; ►Fig. 3G, H). CV for duplicate dabigatran measurements with ClotChip was 4.79%. PT values ranged from 13.5 to 20.3 seconds (►Fig. 4A), and aPTT values extended from 29.2 to 59 seconds (►Fig. 4B). The mean dabigatran concentration was 228.2 ng/mL; the range was 62 to 427.5 ng/mL (►Fig. 4C).
PT sensitivity for all rivaroxaban samples and for samples drawn at Tmax was 0.9 and 0.96, respectively. PT sensitivity for apixaban was determined to be 0.49, whereas for dabigatran this was 0.98 (►Fig. 4D). Similarly, aPTT sensitivity for all rivaroxaban samples was 0.67 and rose to 0.84 for those samples drawn at Tmax. The sensitivity of aPTT for apixaban samples was the lowest at 0.23 and for dabigatran this was 0.88 (►Fig. 4D). Both routine coagulation tests had specificity of 1 (►Fig. 4D). Moreover, there was no significant correlation between ClotChip Tpeak parameter and hematocrit or platelet count in the entire study population (►Supplementary Fig. S1 [available in the online version]).
Prior studies have shown a linear, concentration-dependent relationship between DOAC levels and anti-Xa activity over a wide range of concentrations when measured using a standard curve generated with drug-specific calibrators and controls (R2: 0.95–1.00),16-18 but this correlation was less robust at concentrations below 100 ng/mL.17 In this framework, we reviewed all anti-Xa and dilute thrombin time (dTT) results in the study cohort and identified samples with DOAC levels below 100 ng/mL. Twelve of these samples were from the rivaroxaban group, 8 were apixaban samples, and 1 was from the dabigatran group. The range of anti-Xa activity for rivaroxaban samples was 33 to 89 ng/mL; for apixaban samples, the anti-Xa range was 36 to 99 ng/mL; the single dabigatran sample yielded a drug level of 62 ng/mL. Imposing a threshold of 530 seconds, we determined the number of samples that were accurately captured as prolonged (true positives) by the ClotChip Tpeak parameter and compared these with the number of samples accurately detected by conventional coagulation tests (PT and aPTT). For rivaroxaban, PT was prolonged in 9/12 samples (►Fig. 5A) and aPTT was above normal range in 5/9 samples (►Fig. 5B). The ClotChip Tpeak parameter captured eight out of 12 rivaroxaban samples with anti-Xa levels below 100 ng/mL (►Fig. 5C). For apixaban samples, the ClotChip Tpeak parameter accurately captured five out of eight samples (►Fig. 5C), whereas PT was prolonged in only one out of eight samples (►Fig. 5A) and aPTT was normal in all (►Fig. 5B). For the dabigatran sample, both the PT and aPTT were within normal range (►Fig. 5A, B), whereas the ClotChip Tpeak parameter was appropriately prolonged (►Fig. 5C). Sensitivity and specificity for each assay are outlined in ►Fig. 5D. In this cohort of samples with DOAC concentrations below 100 ng/mL, we found no significant correlation between Tpeak parameter and DOAC drug levels (►Supplementary Fig. S2 [available in the online version]). Although additional studies are required to increase the number of clinical samples, these data indicate that ClotChip can detect the anticoagulant effect of low DOAC concentrations.
Fig. 5.
Diagnostic sensitivity of coagulation assays and ClotChip at low DOAC concentrations. (A) Prothrombin time (PT) results in DOAC samples with drug concentrations less than 100 ng/mL. (B) Activated partial thromboplastin time (aPTT) results in the same cohort. Rivaroxaban: n = 12; apixaban: n = 8; dabigatran: n = 1. The horizontal line in panels (A) and (B) represents the normal reference range for PT and aPTT, respectively. (C) ClotChip Tpeak levels in low DOAC concentrations. The horizontal line represents the upper limit of normal range (i.e., Tpeak = 530 seconds). Each dot in panels (A)–(C) represents the mean of duplicate measurements for each individual sample. (D) Sensitivity and specificity of PT, aPTT assays, and ClotChip Tpeak in the prespecified DOAC cohort. DOAC, direct oral anticoagulant.
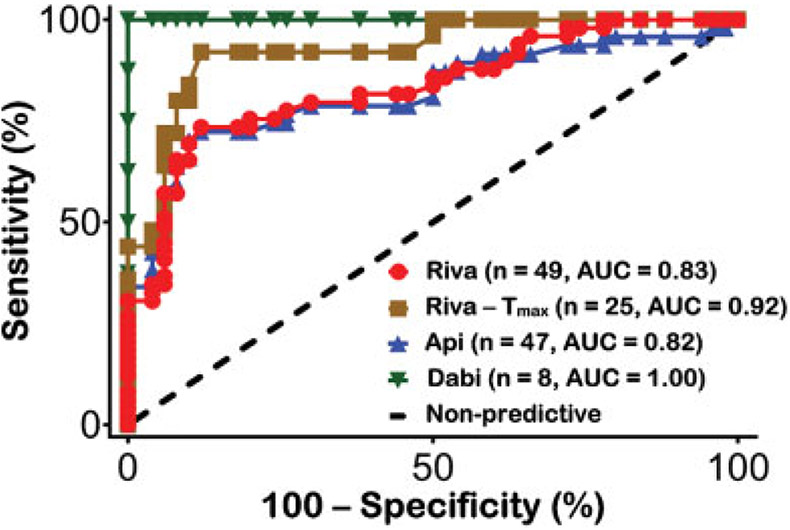
A receiver operating characteristic (ROC) curve was then generated for ClotChip using statistical software to determine the true-positive rate (sensitivity) and false-positive rate (100% – specificity) (►Fig. 6). Using all samples and imposing a diagnostic threshold of Tpeak ≥ 530 seconds conferred a specificity of 88% for any DOAC. The AUC for ClotChip Tpeak in the rivaroxaban subgroup drawn at Tmax was 0.92 (95% CI: 0.86–0.98, p < 0.01); the AUC for ClotChip Tpeak across all rivaroxaban samples was 0.83 (95% CI: 0.75–0.91, p < 0.01). The AUC for Tpeak in the apixaban group was 0.82 (95% CI: 0.73–0.91, p < 0.01) and for dabigatran was 1 (95% CI: 1–1, p < 0.01).
Fig. 6.
Receiver operator characteristic (ROC) curves of the ClotChip Tpeak parameter for detection of DOAC anticoagulant effects. ROC curves were generated using statistical software to determine the true-positive rate (sensitivity) and false-positive rate (100% – specificity). The area under the curve (AUC) for ClotChip Tpeak was 0.92 (95% CI: 0.86–0.98, p < 0.01) for rivaroxaban samples drawn at peak drug levels (n = 25, brown squares) and 0.83 (95% CI: 0.75–0.91, p < 0.01) for all rivaroxaban samples drawn at peak and trough drug levels (n = 49, red circles). For apixaban, AUC for Tpeak was 0.82 ([95% CI: 0.73–0.91, p < 0.01]; n = 47, blue triangles), whereas AUC was 1 for dabigatran ([95% CI: 1–1, p < 0.01]; n = 8, green inverted triangles). CI, confidence interval.
Discussion
DOACs have positively affected patients on anticoagulation.19-23 Despite the lower incidence of bleeding with DOACs, the increasing number of patients prescribed these anticoagulants24-26 suggests that a higher absolute number of patients are at risk.27 With rising DOAC use, efforts have turned to measuring DOAC levels or surrogate markers of coagulation. Considerations for DOAC monitoring would fall in two broad categories. One category is emergency situations, and the other category is monitoring for patients with characteristics that do not conform to the normal range (body mass index > 40, suboptimal liver or renal function, interfering medications). Acute clinical scenarios such as major bleeding, need for emergency surgery, or decision making for DOAC reversal would utilize a one-time measurement to determine if a DOAC is present. In these situations, DOAC testing should consist of simple sample processing, rapid turnaround times, and considerable sensitivity to be useful. The gold-standard liquid chromatography/tandem mass spectrometry is generally unavailable outside of research settings, and drug levels do not correlate with clinical outcomes.28,29 Specialized coagulation tests including dTT, ecarin chromogenic assay (ECA), and drug-specific anti-Xa assays are not widely available. For the majority of cases, the only coagulation test available at all times is the aPTT and PT/international normalized ratio. A limitation of these tests is the preparation of plasma, which under ideal conditions takes approximately 35 minutes.30 Moreover, standard coagulation tests have inadequacies when used to monitor DOACs due to significant variability in methodology and reagents used.1,31-37 Therefore, a coagulation test that can be procured and processed at the POC, provides a binary yes or no answer for the presence of DOAC anticoagulant effect, exhibits improved reliability and sensitivity over standard coagulation tests, and is cost effective would be of significant benefit.
We developed a novel dielectric microsensor, termed ClotChip, which performs dielectric coagulometry on a miniscule volume of whole blood. We previously showed that two distinct parameters of the ClotChip readout, Tpeak and Δεr,max, provide independent information on hemostatic functions arising from noncellular (coagulation factor) and cellular (platelet) components, respectively.2,3 In the present study, we evaluated the clinical utility of ClotChip in detecting the anticoagulant effect of DOACs. We found that the Tpeak parameter was significantly prolonged in all three DOAC groups compared with healthy controls (►Fig. 3). Diagnostic specificity across all DOACs was 88%; sensitivity for rivaroxaban was 73% (with 92% sensitivity observed in the Tmax subgroup), 72% for apixaban, and 100% for dabigatran. We found the sensitivity and diagnostic accuracy of PT and aPTT for DOACs to be improved compared with prior reports (►Fig. 4D). A cogent explanation for these results is the optimal conditions in which routine coagulation tests were performed, i.e., a single individual completed all testing, samples were run on a single coagulometer, and the same lot of reagents was used throughout the study. In contrast to the results shown here, previously published studies reported the sensitivity of PT for rivaroxaban to range from 59 to 98% among thromboplastin reagents.1,33,34,38-41 For apixaban, across in vitro and ex vivo samples for a variety of reagents, the correlation between PT and on-treatment apixaban levels was modest, with R2 values of 0.36 to 0.41.33,34 As with PT, commercial aPTT reagents differ in their sensitivity to dabigatran. The aPTT of plasma spiked with dabigatran (120 ng/mL) ranged from 26 to 91.9 seconds in a cross-validation study of nine different aPTT methods.1,41 The least sensitive reagents required a dabigatran concentration of 400 ng/mL to produce a twofold prolongation in the aPTT value over control.41 In comparison, ClotChip exhibited consistently high sensitivity across all three DOAC agents, results were available within 30 minutes and were reproducible among duplicate measurements, and no sample preprocessing or specialized reagents were required.
The fact that standard coagulation tests may falsely miss therapeutic DOAC concentrations can have significant implications. In the REVERSE-AD trial, only 8.3% of patients treated with idarucizumab had normal dTT or ECA, requiring no reversal; however, 130 patients had normal aPTT results.42 In the ANNEXA-4 trial, a portion of treated patients were eventually excluded, because DOAC activity at enrollment was found to be too low.43 Although additional studies are warranted, ClotChip can be incorporated into the emergency patient workflow in all these scenarios. Results can be performed at the POC and acted on, with administration of an antidote, if positive, or not, if negative. Similarly, ClotChip can also be used effectively for DOAC patients presenting with interval thrombotic events, when thrombolytics are considered.
Strengths of our study include the comprehensive characterization of coagulation parameters in “real-world” DOAC patients, large sample size, time-specific sample collection, and wide range of DOAC activity in plasma similar to levels reported in pivotal phase 3 trials. In contrast to our study, the majority of studies evaluating the relationship between DOACs and coagulation parameters used spiked pooled plasma or spiked healthy donor plasma rather than ex vivo patient samples, which considerably reduced inter-sample variability.44
Other devices have been developed for DOAC monitoring at the POC. The urine-based dipstick approach provides a qualitative assessment of DOAC exposure,45,46 but the degree of correlation between urine and plasma DOAC levels is presently unknown. Moreover, personnel training is required, and urine color, timing of drug ingestion to urine sampling, and renal insufficiency can all potentially interfere with the interpretation of the test results.46 Alternative microfluidic devices are being developed47,48; however, results are based on in vitro spiking of samples or limited patient information, and readouts are based on fluorescence detection and optical imaging, which require additional sample processing and bulky equipment that may hamper development of a miniaturized system. In contrast, ClotChip is a fully electronic blood-clotting assay that utilizes a small amount of whole blood (<10 μL) and provides rapid results using objective readout thereby minimizing the need for technical training or result interpretation. All of these are ideal features for a portable screening device.
There are limitations in this study. First, the accrual of male participants only, which can influence coagulation parameters.49 Second, despite the overall large number of participants, there were a limited number of samples in the dabigatran cohort. Finally, samples were collected at expected peak and trough levels only for the rivaroxaban group. To address these limitations, we will soon be opening a multicenter clinical study in collaboration with civilian centers, which is expected to improve our accrual of female participants. This study will also include peak and trough blood draws for apixaban- and dabigatran-treated patients.
In conclusion, the present study evaluated the clinical utility of a device, termed ClotChip, in detecting the anticoagulant effect of DOACs. Across patients on rivaroxaban, apixaban, and dabigatran, ClotChip exhibited clinically relevant detection sensitivity compared with standard coagulation tests. A major obstacle for implementing DOAC testing in clinical laboratories is reliability, performance, and cost. ClotChip represents a sensitive whole-blood assay that can be performed cost-effectively at the POC with rapid turnaround times, showcasing its potential to guide patient care in defined scenarios with DOACs.
Supplementary Material
What is known about the topic?
There are acute settings where assessing the anticoagulant effect of direct oral anticoagulants (DOACs) can be useful.
Due to variability among routine coagulation tests, there is an unmet need for an assay that rapidly detects DOAC effects in the laboratory or at the point of care (POC).
A novel dielectric microsensor, termed ClotChip, has previously been shown to feature a readout parameter, i.e., time to reach peak permittivity (Tpeak) that is sensitive to coagulation function.
What does this paper add?
This study evaluates the clinical utility of ClotChip in detecting the anticoagulant effect of three different DOACs (rivaroxaban, apixaban, and dabigatran). ClotChip exhibits consistently high sensitivity across all three DOAC agents.
ClotChip results are available within 30 minutes and are reproducible among duplicate measurements, and no sample preprocessing or specialized reagents are required.
ClotChip represents a sensitive whole-blood assay that can be performed cost-effectively at the POC with rapid turnaround times, showcasing its potential to guide patient care in defined scenarios with DOACs.
Acknowledgments
The authors thank all participating patients, research staff, and the Anticoagulation Clinic at Louis Stokes Cleveland VA Medical Center for their critical assistance with the study. Erica Woodrum, biomedical artist, designed the ClotChip artwork shown in ►Fig. 1A.
Funding
This work was supported by an American Heart Association Grant-in-Aid award (17GRNT33661005 to M.A.S., P.M., and E.X.S.), by XaTek Inc. and the Oscar D. Ratnoff Endowed Professorship (to E.X.S.). The contents do not represent the views of the US Department of Veterans Affairs or the United States Government.
Footnotes
Conflict of Interest
D.M., P.M., M.A.S., and E.X.S. are inventors of intellectual property that has been licensed by Case Western Reserve University to XaTek Inc. D.M. has a patent 9,995,701 with royalties paid by XaTek Inc., and a patent PCT/US2017/013797 with royalties paid by XaTek Inc. M.A.S. reports grants and personal fees from XaTek Inc. during the conduct of the study. In addition, M.A.S. has a patent 9,995,701 with royalties paid by XaTek Inc., and a patent PCT/US2017/013797 with royalties paid by XaTek Inc. P.M. reports grants and personal fees from XaTek Inc. during the conduct of the study. In addition, P.M. has a patent 9,995,701 with royalties paid by XaTek Inc., and a patent PCT/US2017/013797 with royalties paid by XaTek Inc. E.X.S. has a patent PCT/US2017/013797 with royalties paid by XaTek Inc. and reports grants from XaTek Inc. during the conduct of the study. A.O., B.M.W. and K.L.B. report no conflict.
References
- 1.Cuker A, Siegal DM, Crowther MA, Garcia DA. Laboratory measurement of the anticoagulant activity of the non-vitamin K oral anticoagulants. J Am Coll Cardiol 2014;64(11):1128–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maji D, Suster MA, Kucukal E, et al. ClotChip: a microfluidic dielectric sensor for point-of-care assessment of hemostasis. IEEE Trans Biomed Circuits Syst 2017;11(06):1459–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maji D, De La Fuente M, Kucukal E, et al. Assessment of whole blood coagulation with a microfluidic dielectric sensor. J Thromb Haemost 2018;16(10):2050–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodeghiero F, Tosetto A, Abshire T, et al. ; ISTH/SSC joint VWF and Perinatal/Pediatric Hemostasis Subcommittees Working Group. ISTH/SSC bleeding assessment tool: a standardized questionnaire and a proposal for a new bleeding score for inherited bleeding disorders. J Thromb Haemost 2010;8(09):2063–2065 [DOI] [PubMed] [Google Scholar]
- 5.Nagler M, Kathriner S, Bachmann LM, Wuillemin WA. Impact of changes in haematocrit level and platelet count on thromboelastometry parameters. Thromb Res 2013;131(03):249–253 [DOI] [PubMed] [Google Scholar]
- 6.Nagler M, Bachmann LM, Alberio L, et al. Variability between laboratories performing coagulation tests with identical platforms: a nationwide evaluation study. Thromb J 2013;11(01):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kubitza D, Becka M, Wensing G, Voith B, Zuehlsdorf M. Safety, pharmacodynamics, and pharmacokinetics of BAY 59-7939–an oral, direct factor Xa inhibitor–after multiple dosing in healthy male subjects. Eur J Clin Pharmacol 2005;61(12):873–880 [DOI] [PubMed] [Google Scholar]
- 8.Ezekowitz MD, Reilly PA, Nehmiz G, et al. Dabigatran with or without concomitant aspirin compared with warfarin alone in patients with nonvalvular atrial fibrillation (PETRO Study). Am J Cardiol 2007;100(09):1419–1426 [DOI] [PubMed] [Google Scholar]
- 9.van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate–a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010;103(06):1116–1127 [DOI] [PubMed] [Google Scholar]
- 10.Frost C, Nepal S, Wang J, et al. Safety, pharmacokinetics and pharmacodynamics of multiple oral doses of apixaban, a factor Xa inhibitor, in healthy subjects. Br J Clin Pharmacol 2013;76(05):776–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suster MA, Vitale NH, Maji D, Mohseni P. A circuit model of human whole blood in a microfluidic dielectric sensor. IEEE Trans Circuits Syst, II Express Briefs 2016;63(12):1156–1160 [Google Scholar]
- 12.Wolf M, Gulich R, Lunkenheimer P, Loidl A. Broadband dielectric spectroscopy on human blood. Biochim Biophys Acta 2011;1810(08):727–740 [DOI] [PubMed] [Google Scholar]
- 13.Maji D, Pourang S, Sekhon UDS, Gupta AS, Suster MA, Mohseni P. Toward diagnosis of platelet loss in trauma injury using a microfluidic dielectric sensor. Paper presented at: 2019 IEEE SENSORS; October 27–30, 2019; Montreal, Canada [Google Scholar]
- 14.Maji D, Suster MA, Mohseni P. Monitoring red blood cell aggregation dynamics in stasis and under flow using a microfluidic dielectric sensor. Paper presented at: 2018 IEEE Biomedical Circuits and Systems Conference (BioCAS); October 17–19, 2018; Cleveland, Ohio, United States [Google Scholar]
- 15.Synek V Evaluation of the standard deviation from duplicate results. Accredit Qual Assur 2008;13(06):335–337 [Google Scholar]
- 16.Mani H, Rohde G, Stratmann G, et al. Accurate determination of rivaroxaban levels requires different calibrator sets but not addition of antithrombin. Thromb Haemost 2012;108(01):191–198 [DOI] [PubMed] [Google Scholar]
- 17.Douxfils J, Tamigniau A, Chatelain B, et al. Comparison of calibrated chromogenic anti-Xa assay and PT tests with LC-MS/MS for the therapeutic monitoring of patients treated with rivaroxaban. Thromb Haemost 2013;110(04):723–731 [DOI] [PubMed] [Google Scholar]
- 18.Asmis LM, Alberio L, Angelillo-Scherrer A, et al. Rivaroxaban: quantification by anti-FXa assay and influence on coagulation tests: a study in 9 Swiss laboratories. Thromb Res 2012;129(04):492–498 [DOI] [PubMed] [Google Scholar]
- 19.Chai-Adisaksopha C, Crowther M, Isayama T, Lim W. The impact of bleeding complications in patients receiving target-specific oral anticoagulants: a systematic review and meta-analysis. Blood 2014;124(15):2450–2458 [DOI] [PubMed] [Google Scholar]
- 20.Wilson D, Charidimou A, Shakeshaft C, et al. ; CROMIS-2 collaborators. Volume and functional outcome of intracerebral hemorrhage according to oral anticoagulant type. Neurology 2016;86(04):360–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raccah BH, Perlman A, Danenberg HD, Pollak A, Muszkat M, Matok I. Major bleeding and hemorrhagic stroke with direct oral anticoagulants in patients with renal failure: systematic review and meta-analysis of randomized trials. Chest 2016;149(06):1516–1524 [DOI] [PubMed] [Google Scholar]
- 22.Chai-Adisaksopha C, Hillis C, Isayama T, Lim W, Iorio A, Crowther M. Mortality outcomes in patients receiving direct oral anticoagulants: a systematic review and meta-analysis of randomized controlled trials. J Thromb Haemost 2015;13(11):2012–2020 [DOI] [PubMed] [Google Scholar]
- 23.Graham DJ, Reichman ME, Wernecke M, et al. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation 2015;131(02):157–164 [DOI] [PubMed] [Google Scholar]
- 24.Loo SY, Dell’Aniello S, Huiart L, Renoux C. Trends in the prescription of novel oral anticoagulants in UK primary care. Br J Clin Pharmacol 2017;83(09):2096–2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnes GD, Lucas E, Alexander GC, Goldberger ZD. National trends in ambulatory oral anticoagulant use. Am J Med 2015;128(12):1300–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halvorsen S, Ghanima W, Fride Tvete I, et al. A nationwide registry study to compare bleeding rates in patients with atrial fibrillation being prescribed oral anticoagulants. Eur Heart J Cardiovasc Pharmacother 2017;3(01):28–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shehab N, Lovegrove MC, Geller AI, Rose KO, Weidle NJ, Budnitz DS. US emergency department visits for outpatient adverse drug events, 2013-2014. JAMA 2016;316(20):2115–2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan NC, Hirsh J, Ginsberg JS, Eikelboom JW. Real-world variability in dabigatran levels in patients with atrial fibrillation: reply. J Thromb Haemost 2015;13(06):1168–1169 [DOI] [PubMed] [Google Scholar]
- 29.Reilly PA, Lehr T, Haertter S, et al. ; RE-LY Investigators. The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: the RE-LY Trial (Randomized Evaluation of Long-Term Anticoagulation Therapy). J Am Coll Cardiol 2014;63(04):321–328 [DOI] [PubMed] [Google Scholar]
- 30.Seiffge DJ, Traenka C, Polymeris A, et al. Feasibility of rapid measurement of Rivaroxaban plasma levels inpatients with acute stroke. J Thromb Thrombolysis 2017;43(01):112–116 [DOI] [PubMed] [Google Scholar]
- 31.Helin TA, Pakkanen A, Lassila R, Joutsi-Korhonen L. Laboratory assessment of novel oral anticoagulants: method suitability and variability between coagulation laboratories. Clin Chem 2013;59(05):807–814 [DOI] [PubMed] [Google Scholar]
- 32.Samama MM, Martinoli JL, LeFlem L, et al. Assessment of laboratory assays to measure rivaroxaban–an oral, direct factor Xa inhibitor. Thromb Haemost 2010;103(04):815–825 [DOI] [PubMed] [Google Scholar]
- 33.Barrett YC, Wang Z, Frost C, Shenker A. Clinical laboratory measurement of direct factor Xa inhibitors: anti-Xa assay is preferable to prothrombin time assay. Thromb Haemost 2010;104(06):1263–1271 [DOI] [PubMed] [Google Scholar]
- 34.Barrett YC, Wang Z, Knabb RM. A novel prothrombin time assay for assessing the anticoagulant activity of oral factor Xa inhibitors. Clin Appl Thromb Hemost 2013;19(05):522–528 [DOI] [PubMed] [Google Scholar]
- 35.Gouin-Thibault I, Flaujac C, Delavenne X, et al. Assessment of apixaban plasma levels by laboratory tests: suitability of three anti-Xa assays. A multicentre French GEHT study. Thromb Haemost 2014;111(02):240–248 [DOI] [PubMed] [Google Scholar]
- 36.Douxfils J, Mullier F, Robert S, Chatelain C, Chatelain B, Dogné JM. Impact of dabigatran on a large panel of routine or specific coagulation assays. Laboratory recommendations for monitoring of dabigatran etexilate. Thromb Haemost 2012;107(05):985–997 [DOI] [PubMed] [Google Scholar]
- 37.Hapgood G, Butler J, Malan E, Chunilal S, Tran H. The effect of dabigatran on the activated partial thromboplastin time and thrombin time as determined bythe Hemoclot thrombin inhibitor assay in patient plasma samples. Thromb Haemost 2013;110(02):308–315 [DOI] [PubMed] [Google Scholar]
- 38.Henskens YMC, Gulpen AJW, van Oerle R, et al. Detecting clinically relevant rivaroxaban or dabigatran levels by routine coagulation tests or thromboelastography in a cohort of patients with atrial fibrillation. Thromb J 2018;16:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Francart SJ, Hawes EM, Deal AM, et al. Performance of coagulation tests in patients on therapeutic doses of rivaroxaban. A cross-sectional pharmacodynamic study based on peak and trough plasma levels. Thromb Haemost 2014;111(06):1133–1140 [DOI] [PubMed] [Google Scholar]
- 40.Samama MM, Contant G, Spiro TE, et al. ; Rivaroxaban Prothrombin Time Field Trial Laboratories. Evaluation of the prothrombin time for measuring rivaroxaban plasma concentrations using calibrators and controls: results of a multicenter field trial. Clin Appl Thromb Hemost 2012;18(02):150–158 [DOI] [PubMed] [Google Scholar]
- 41.Harenberg J, Giese C, Marx S, Kramer R. Determination of dabigatran in human plasma samples. Semin Thromb Hemost 2012;38(01):16–22 [DOI] [PubMed] [Google Scholar]
- 42.Pollack CV Jr, Reilly PA, van Ryn J, et al. Idarucizumab for dabigatran reversal - full cohort analysis. N Engl J Med 2017;377(05):431–441 [DOI] [PubMed] [Google Scholar]
- 43.Connolly SJ, Gibson CM, Crowther M. Andexanet alfa for factor Xa inhibitor reversal. N Engl J Med 2016;375(25):2499–2500 [DOI] [PubMed] [Google Scholar]
- 44.Testa S, Tripodi A, Legnani C, et al. ; START-Laboratory Register. Plasma levels of direct oral anticoagulants in real life patients with atrial fibrillation: results observed in four anticoagulation clinics. Thromb Res 2016;137:178–183 [DOI] [PubMed] [Google Scholar]
- 45.Harenberg J, Du S, Wehling M, et al. Measurement of dabigatran, rivaroxaban and apixaban in samples of plasma, serum and urine, under real life conditions. An international study. Clin Chem Lab Med 2016;54(02):275–283 [DOI] [PubMed] [Google Scholar]
- 46.Harenberg J, Beyer-Westendorf J, Crowther M, et al. Working Group Members. Accuracy of a rapid diagnostic test for the presence of direct oral factor Xa or thrombin inhibitors in urine—a multicenter trial. Thromb Haemost 2020;120(01):132–140 [DOI] [PubMed] [Google Scholar]
- 47.Moll J, Meyer dos Santos S, Hils B, et al. Micro-optical prototyping of a surface acoustic wave-based point-of-care coagulation assay and first application in anticoagulated patients. Int J Clin Pharmacol Ther 2016;54(03):177–184 [DOI] [PubMed] [Google Scholar]
- 48.Harder S, Santos SMD, Krozer V, Moll J. Surface acoustic wave-based microfluidic coagulation device for monitoring anticoagulant therapy. Semin Thromb Hemost 2019;45(03):253–258 [DOI] [PubMed] [Google Scholar]
- 49.Ho P, Lim HY, Ng C, Smith CL, Donnan G, Nandurkar H. Global coagulation assays in the normal population: female gender, older age and East Asian ethnicity associated with prothrombotic parameters. Blood 2015;126(23):4678 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.