Abstract
Purpose
Noninvasive ventilation (NIV) is often required for patients with acute exacerbation of chronic obstructive pulmonary disease (AECOPD), and it can significantly reduce the need for endotracheal intubation. Currently, there is no standard method for predicting successful weaning from NIV. Therefore, we aimed to evaluate whether a weaning index can predict NIV outcomes of patients with AECOPD.
Methods
This study was conducted at a single academic public hospital in northern Taiwan from February 2019 to January 2021. Patients with AECOPD admitted to the hospital with respiratory failure who were treated with NIV were included in the study. Univariate and multivariate logistic regression analyses were used to identify independent predictors of successful weaning from NIV. Receiver operating characteristic curve methodology was used to assess the predictive capacity.
Results
A total of 85 patients were enrolled, 65.9% of whom were successfully weaned from NIV. The patients had a mean age of 75.8 years and were mostly men (89.4%). The rapid shallow breathing index (RSBI) (P < 0.001), maximum inspiratory pressure (P = 0.014), and maximum expiratory pressure (P = 0.004) of the successful group were significant while preparing to wean. The area under the receiver operating characteristic curve for the RSBI was 0.804, which was considered excellent discrimination.
Conclusion
The RSBI predicted successful weaning from NIV in patients with AECOPD with hypercapnic respiratory failure. This index may be useful for selecting patients with AECOPD that are suitable for NIV weaning.
Keywords: Chronic obstructive pulmonary disease, Noninvasive ventilation, Weaning, Weaning index
Introduction
Acute exacerbation of chronic obstructive pulmonary disease (AECOPD), a common cause of respiratory failure during hospital admission and readmission, is a public health problem that is associated with increased medical costs. AECOPD is characterized by persistent airflow limitation and develops when significant exposure to irritants causes an inflammatory response in the lungs [1]. The World Health Organization reported that COPD was the fifth most common disease globally in 2020 [2], with a prevalence of 7.8–19.7% in adults [3]. Approximately, 60% of patients with COPD admitted with hypercapnic respiratory failure die in hospital [4, 5].
The 1990s saw a wealth of research emerge on the characteristics of spontaneous breathing trials and how they relate to trial performance. Studies showed that noninvasive ventilation (NIV) was successful as first-line treatment for patients admitted to hospital with hypercapnic respiratory failure due to AECOPD [6–9]. COPD treated with NIV has lower inpatient mortality rates, shorter lengths of stay, and lower costs than treatment with invasive mechanical ventilation (IMV) [9, 10].
The weaning index for IMV is the most appropriate method to determine readiness for weaning training. However, no standard method has been shown to be a good predictor of weaning success with NIV, and there is no consensus among researchers regarding the extent and nature of weaning from NIV. It is possible that delayed weaning may expose the patient to unnecessary discomfort, increased risk of complications, and increased cost of care. The rapid shallow breathing index (RSBI) was introduced by Yang and Tobin in 1991. They found that there was a higher probability of weaning success if the RSBI was ≤ 105 and spontaneous breathing trials were successful [11, 12]. Once NIV therapy is initiated, however, the timing of withdrawal must be considered. Thus, we conducted a prospective observational study to investigate the use of a weaning index to predict weaning outcomes for patients with AECOPD requiring NIV.
Materials and Methods
Study Design and Population
To identify predictors of successful weaning from NIV for patients with AECOPD, we conducted a prospective, noninterventional study at the National Taiwan University Hospital Hsin-Chu Branch, a 637-bed academic public hospital in Hsinchu City, Taiwan. Consecutive patients (aged ≥ 20 years) hospitalized with AECOPD between February 2019 and January 2021 who were treated with NIV during hospitalization using a bi-level positive airway pressure mode (VPAP III ST-A; ResMed, UK) and a full facemask were enrolled. NIV was initiated based on the patient’s respiratory status and indications, including respiratory acidosis (partial pressure of CO2 ≥ 45 mmHg; arterial pH ≤ 7.35), severe dyspnea with clinical signs of respiratory muscle fatigue, or persistent hypoxemia despite supplemental oxygen therapy [13]. Subsequent ICU admission for identified cases depended on current ICU availability and the clinician’s discretionary judgement, and medical treatment was provided by a respiratory therapist. The exclusion criteria were cardiovascular instability, lack of patient cooperation or a Glasgow Coma Scale (GCS) score < 13, increased aspiration risk, recent facial trauma, upper airway obstruction, increased sputum secretion, or respiratory arrest. Patients who required NIV post-extubation, were pregnant, were transferred from another acute care facility, required long-term continuous NIV, or signed do-not-resuscitate orders were also excluded [14–16].
Weaning Criteria and Protocol
After admission, NIV was administered 24 h/day, except during meals and for expectoration. The initial inspiratory (IPAP) and expiratory (EPAP) positive airway pressures were determined based on achieving acceptable arterial blood gas (ABG) parameters, a respiratory rate (RR) < 25 breaths/minute, and patient tolerance and comfort. All patients had an initial IPAP set at 12 cmH2O, which was gradually increased by 2–3 cmH2O, as tolerated, but did not exceed 25 cmH2O. The EPAP was initially set at 5 cmH2O and then gradually increased by 1–2 cmH2O, as needed, to improve hypoxemia [17]. Decisions regarding the duration of NIV and whether to progress to endotracheal intubation were made by the clinical team, based on the attending physician’s judgement.
The criteria and weaning protocols by Duan and Momii were modified for use in this study [18, 19]. The weaning criteria used were adequate mentation, oxygen saturation (SpO2) ≥ 90% on a fraction of inspired oxygen (FiO2) ≤ 0.4, pH ≥ 7.30, a systolic blood pressure of 90–180 mmHg without vasopressor support, a body temperature of 36–38 °C, and a heart rate of 50–120 bpm [18]. Patients who met these criteria were enrolled in the weaning protocol.
The weaning protocol was performed by decreasing the IPAP and EPAP by 3 cmH2O every 30 min, with close monitoring for worsening SpO2 and/or RR. When the IPAP and EPAP were reduced to 15 and 5 cmH2O, respectively, with a satisfactory SpO2 ( ≥ 90%) on a FiO2 ≤ 0.4, NIV was withdrawn and 3 L/min oxygen was administered via a nasal cannula for an additional 30 min [19]. The primary outcome was successful weaning from NIV. Weaning failure was determined by objective or subjective determination of respiratory failure based on the need for repeated NIV, intubation, high-flow nasal cannula oxygen therapy, or long-term NIV support. Weaning failure was also determined in the case of patient death within 48 h of NIV weaning [20].
Data Collection and Outcomes
Patient demographic data, including past medical history, physiological measurements, blood test results (including data on baseline arterial blood gases), COPD-associated therapy, hospital areas, other therapies, initial setting of NIV, and respiratory indices [maximal inspiratory (MIP) and expiratory (MEP) pressure, tidal volume (TV), RR, and RSBI], were extracted from medical records. The RSBI was measured using a Wright respirometer (“nSpire” Wright/Haloscale Respirometers; Hertford, UK). Patients were asked to breathe through the respirometer for 1 min, RR and TV were measured, and RR was divided by TV to calculate the RSBI. MIP and MEP were measured with a respiratory pressure force meter (“MTC” Gas Pressure Gauge, Taoyuan, ROC). MIP was measured with a maximum inspiratory effort maintained for ≥ 1 s, and MEP was measured with a maximum respiratory effort maintained for ≥ 1 s. All parameters were recorded during the first 2 h of NIV treatment and before NIV was turned off within 2 h. The primary outcome was successful weaning from NIV, which was determined by the number of patients no longer requiring NIV support during their hospital stay. The secondary outcome was the length of hospital stay (days).
Statistical Analyses
Categorical variables are expressed as N (%). Descriptive data are reported as mean ± standard deviation or median [interquartile range (IQR)]. Variables were evaluated for an association with NIV weaning outcome using Pearson’s chi-squared test (or Fisher’s exact test, when appropriate) for categorical data and the Student’s t test for variables with normal distribution (or Mann–Whitney U test as non-parametric methods) for numerical data. Univariate analysis was performed to determine predictive factors for successful weaning from NIV. Variables with P < 0.2 were entered into the multivariable logistic regression analysis to identify independent predictors of NIV weaning outcome. Odds ratios (ORs) and 95% confidence intervals (CIs) were reported for all independent predictors. Receiver operating characteristic (ROC) curve analysis was performed to determine the capacity to predict the success of NIV weaning for different variables. All statistical analyses were conducted using IBM SPSS software version 22.0 (IBM Co., Armonk, NY, USA). A two-tailed P < 0.05 was considered statistically significant.
Results
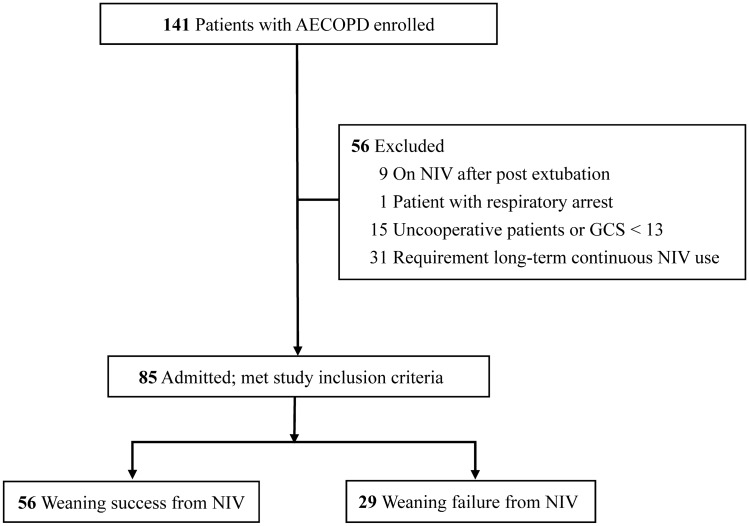
During the study period, 141 eligible patients with AECOPD received NIV treatment upon admission; 56 were excluded due to their post-extubation status, non-cooperation or GCS score (< 13), episodes of respiratory arrest, or requirement for long-term continuous NIV support (Fig. 1). Eighty-five patients met the inclusion criteria, including 56 (65.9%) in the NIV weaning success group and 29 (34.1%) in the NIV weaning failure group. The average age of the patients was 75.8 ± 10.1 years; 76 (89.4%) patients were men. Additional baseline characteristics are presented in Table 1. No significant differences were detected between the two groups in terms of sex, age, body mass index, GCS score, white blood cell count, COPD severity, Acute Physiology and Chronic Health Evaluation II score, hospital areas, C-reactive protein (CRP) level, albumin level, COPD-associated therapy, therapeutic interventions (systemic steroids and antibiotics), ABG data before the initiation of NIV, and initial setting of NIV. There were significant differences between the NIV weaning success and failure groups in terms of smoking history (50 vs. 20 cases; P = 0.020), days on NIV until weaning (2.0 vs. 3.0 days; P = 0.019), average duration of NIV treatment (5.0 vs. 13.0 days; P < 0.001), and length of hospital stay (13.0 vs. 17.0 days; P = 0.008).
Fig. 1.
Flowchart of participant recruitment. AECOPD, acute exacerbation of chronic obstructive pulmonary disease; GCS Glasgow Coma Scale; NIV noninvasive ventilation
Table 1.
Baseline characteristics of the study population
| Characteristic | Total (N = 85) | NIV weaning | ||
|---|---|---|---|---|
| Success (N = 56) | Failure (N = 29) | |||
| Sex, male (%)b | 76 (89.4) | 52 (92.9) | 24 (82.8) | 0.263 |
| Age, mean (SD), yearsa | 75.8 ± 10.1 | 76.0 ± 10.4 | 75.4 ± 9.8 | 0.786 |
| BMI, mean (SD), kg/m2a | 22.7 ± 6.5 | 22.4 ± 5.8 | 23.3 ± 7.9 | 0.595 |
| GCS, median (IQR)a | 15.0 (15.0–15.0) | 15.0 (15.0–15.0) | 15.0 (15.0–15.0) | 0.722 |
| Smoking history, N (%)b | 70 (82.4) | 50 (89.3) | 20 (69.0) | 0.020* |
| WBC, median (IQR), K/μLa | 9.4 (7.1–13.5) | 9.4 (7.4–13.9) | 9.6 (6.7–12.5) | 0.704 |
| CRP, median (IQR), mg/dLa | 3.5 (1.2–9.6) | 3.0 (0.7–9.5) | 5.2 (1.5–10.5) | 0.219 |
| Albumin, median (IQR), g/dLa | 3.2 (3.0–3.5) | 3.3 (3.1–3.5) | 3.2 (2.7–3.4) | 0.089 |
| COPD GOLD assessment (%) | 0.105 | |||
| Group A and B, N (%)b | 24 (28.2) | 19 (33.9) | 5 (17.2) | |
| Group C and D, N (%)b | 61 (71.8) | 37 (66.1) | 24 (82.8) | |
| APACHE II, median (IQR)a | 12.0 (8.5–15.0) | 12.0 (9.0–15.0) | 12.0 (7.5–15.0) | 0.824 |
| Days on NIV until weaning, median (IQR), daysa | 2.0 (2.0–4.0) | 2.0 (2.0–3.0) | 3.0 (2.0–5.0) | 0.019* |
| Average duration of NIV treatment, median (IQR), daysa | 6.0 (4.0–12.0) | 5.0 (3.0–7.0) | 13.0 (9.5–22.0) | < 0.001* |
| LOS, median (IQR), daya | 13.0 (9.5–20.5) | 13.0 (9.0–17.0) | 17.0 (12.0–26.0) | 0.008* |
| Hospital area | ||||
| Diagnosis area | 0.120 | |||
| Emergency room, N (%)b | 46 (54.1) | 33 (58.9) | 13 (44.8) | |
| General ward, N (%)b | 32 (37.6) | 17 (30.4) | 15 (51.7) | |
| Intensive care unit, N (%)b | 7 (8.2) | 6 (10.7) | 1 (3.4) | |
| Treatment area | 0.547 | |||
| Emergency room, N (%)b | 2 (2.4) | 2 (3.6) | 0 (0.0) | |
| General ward, N (%)b | 55 (64.7) | 35 (62.5) | 20 (69.0) | |
| Intensive care unit, N (%)b | 28 (32.9) | 19 (33.9) | 9 (31.0) | |
| Comorbidities | ||||
| Diabetes mellitus, N (%)b | 39 (45.9) | 29 (51.8) | 10 (34.5) | 0.129 |
| Hypertension, N (%)b | 52 (61.2) | 34 (60.7) | 18 (62.1) | 0.903 |
| Congestive heart failure, N (%)b | 11 (12.9) | 5 (8.9) | 6 (20.7) | 0.174 |
| Coronary artery disease, N (%)b | 14 (16.5) | 11 (19.6) | 3 (10.3) | 0.363 |
| Arrhythmia, N (%)b | 16 (18.8) | 9 (16.1) | 7 (24.1) | 0.367 |
| Chronic renal disease, N (%)b | 11 (12.9) | 8 (14.3) | 3 (10.3) | 0.742 |
| Benign prostatic hyperplasia, N (%)b | 16 (18.8) | 8 (14.3) | 8 (27.6) | 0.137 |
| Malignancies other than lung cancer, N (%)b | 10 (11.8) | 5 (8.9) | 5 (17.2) | 0.298 |
| Lung cancer, N (%)b | 6 (7.1) | 4 (7.1) | 2 (6.9) | > 0.999 |
| COPD therapy | ||||
| Inhaled SABA, N (%)b | 41 (48.2) | 28 (50.0) | 13 (44.8) | 0.651 |
| Inhaled SAMA, N (%)b | 28 (32.9) | 17 (30.4) | 11 (37.9) | 0.481 |
| Inhaled LABA, N (%) b | 54 (63.5) | 34 (60.7) | 20 (69.0) | 0.454 |
| Inhaled LAMA, N (%)b | 53 (62.4) | 34 (60.7) | 19 (65.5) | 0.665 |
| Inhaled corticosteroids, N (%)b | 36 (42.4) | 25 (44.6) | 11 (37.9) | 0.553 |
| Long-term oxygen therapy, N (%)b | 7 (8.2) | 3 (5.4) | 4 (13.8) | 0.223 |
| Pulmonary rehabilitation, N (%)b | 17 (20.0) | 12 (21.4) | 5 (17.2) | 0.647 |
| Therapeutic interventions | ||||
| Systemic corticosteroids, N (%)b | 74 (87.1) | 50 (89.3) | 24 (82.8) | 0.499 |
| Antibiotics, N (%)b | 74 (87.1) | 51 (91.1) | 23 (79.3) | 0.174 |
| NIV initial setting | ||||
| IPAP, median (IQR), cmH2Oa | 16.0 (15.0–18.5) | 16.0 (15.0–19.7) | 16.0 (15.0–18.0) | 0.783 |
| EPAP, median (IQR), cmH2Oa | 5.0 (5.0–6.0) | 5.0 (5.0–6.0) | 5.0 (5.0–6.0) | 0.258 |
| FiO2, median (IQR), (%)a | 35.0 (34.0–44.5) | 35.0 (33.0–40.0) | 35.0 (35.0–50.0) | 0.094 |
| Blood gas analysis (before the initiation of NIV) | ||||
| Arterial pH, mean (SD)a | 7.31 ± 0.07 | 7.30 ± 0.75 | 7.32 ± 0.88 | 0.486 |
| PaO2, median (IQR), mmHga | 77.0 (62.8–98.0) | 78.4 (61.7–100.5) | 74.1 (65.1–87.4) | 0.499 |
| PaCO2, mean (SD), mmHga | 60.3 ± 18.9 | 60.7 ± 18.7 | 59.3 ± 19.5 | 0.743 |
| HCO3, mean (SD), mEq/La | 29.1 ± 6.8 | 29.3 ± 6.7 | 28.9 ± 7.0 | 0.801 |
| BE, median (IQR), mmol/La | 2.6 (− 0.7–7.0) | 2.5 (− 0.6–6.1) | 3.2 (− 2.2–9.7) | 0.677 |
| SaO2, median (IQR), %a | 93 (90–96) | 93.5 (90.0–96.7) | 92 (89.5–95.5) | 0.316 |
APACHE II acute physiology and chronic health evaluation; BMI body mass index; BE bicarbonate; COPD chronic obstructive pulmonary disease; CRP C-reactive protein; EPAP expiratory positive airway pressure; FiO2 fraction of inspired oxygen; GCS Glasgow Coma Scale; HCO3 bicarbonate; IPAP inspiratory positive airway pressure; IQR interquartile range; LABA long acting beta agonist; LAMA long acting muscarinic antagonist; LOS length of hospital stay; MEP maximal expiratory pressure; MIP maximal inspiratory pressure; NIV noninvasive ventilation; PaO2 partial pressure of oxygen; PaCO2 partial pressure of carbon dioxide; SABA short acting beta agonist; SAMA short acting muscarinic antagonist; SaO2 arterial oxygen saturation; SD standard deviation; WBC white blood cell
aContinuous variables are expressed as mean ± SD or median (IQR)
bCategorical variables are expressed as N (%)
*P < 0.05, significant
Predictive Factors for Successful Weaning from NIV
The weaning parameters and ABG findings of the two groups are shown in Table 2. Within 2 h of initiating NIV treatment, no significant differences were detected between the two groups in any of the weaning indices or ABG data. However, immediately before NIV was turned off within 2 h, the NIV weaning success group was compared to the NIV weaning failure group, the NIV weaning success group had a lower RSBI, RR, and higher VE, TV, MIP, predict MIP, and MEP, predict MEP (P < 0.001, < 0.001, 0.016, < 0.001, 0.014, 0.013 and 0.004, 0.012, respectively). There was a significant difference between the two groups in the hospital mortality rate (3 vs. 9 cases; P = 0.002), and there were no significant differences in readmission within 60 days of discharge.
Table 2.
Weaning parameters and arterial blood gas findings between the two groups
| Clinical outcome | Total (N = 85) | NIV weaning | P value | |
|---|---|---|---|---|
| Success (N = 56) | Failure (N = 29) | |||
| Weaning index (on NIV within 2 h) | ||||
| RSBI, mean (SD), breaths/(min mL)a | 103.1 ± 60.0 | 100.0 ± 60.4 | 109.1 ± 59.7 | 0.512 |
| VE, median (IQR), La | 6.3 (4.8–9.1) | 7.0 (5.1–9.1) | 5.1 (4.2–9.3) | 0.100 |
| TV, median (IQR), mLa | 271.0 (198.0–378.5) | 275.5 (209.2–407.0) | 242.0 (173.0–339.0) | 0.218 |
| RR, mean (SD), breaths/mina | 24.8 ± 6.9 | 24.7 ± 6.6 | 25.0 ± 7.5 | 0.877 |
| MIP, median (IQR), −cmH2Oa | 30.0 (20.0–40.0) | 34.0 (20.0–40.0) | 26.0 (17.0–38.0) | 0.205 |
| MEP, median (IQR), +cmH2Oa | 36.0 (22.0–44.0) | 36.0 (22.5–48.0) | 36.0 (21.5–41.5) | 0.263 |
| Blood gas analysis (on NIV within 2 h) | ||||
| Arterial pH, mean (SD)a | 7.31 ± 0.07 | 7.31 ± 0.06 | 7.31 ± 0.08 | 0.707 |
| PaO2, median (IQR), mmHga | 90.0 (78.5–113.1) | 91.1 (79.4–114.1) | 88.0 (75.5–114.0) | 0.777 |
| PaCO2, median (IQR), mmHga | 60.5 (48.6–67.3) | 60.4 (48.2–66.1) | 61.1 (48.8–68.4) | 0.838 |
| HCO3, mean (SD), mEq/La | 29.3 ± 6.9 | 29.5 ± 6.9 | 28.9 ± 7.0 | 0.705 |
| BE, mean (SD), mmol/La | 4.2 ± 7.7 | 4.0 ± 7.0 | 4.6 ± 8.9 | 0.733 |
| SaO2, median (IQR), (%)a | 96.0 (93.0–97.0) | 96.0 (94.0–97.0) | 94.0 (92.5–98.5) | 0.373 |
| Weaning index (before turning off NIV within 2 h) | ||||
| RSBI, median (IQR), breaths/(min mL)a | 53.5 (29.8–83.2) | 38.5 (18.7–58.9) | 84.9 (55.3–128.8) | < 0.001* |
| VE, median (IQR), La | 7.4 (5.9–9.9) | 7.9 (6.4–10.2) | 6.2 (5.5–8.1) | 0.016* |
| TV, median (IQR), mLa | 408.0 (272.5–571.0) | 444.5 (360.2–671.2) | 264.0 (219.0–395.5) | < 0.001* |
| RR, mean (SD), breaths/mina | 19.8 ± 6.8 | 17.7 ± 6.2 | 23.7 ± 6.5 | < 0.001* |
| MIP, median (IQR), −cmH2Oa | 36.0 (24.0–52.0) | 44.0 (30.5–55.2) | 30.0 (20.0–40.0) | 0.014* |
| Predict MIP, median (IQR), (%)a, c | 42.0 (29.0–61.0) | 49.0 (33.2–67.7) | 35.0 (24.0–47.0) | 0.013* |
| MEP, median (IQR), +cmH2Oa | 44 (28.0–60.0) | 48.5 (40.0–67.0) | 28.0 (20.0–50.0) | 0.004* |
| Predict MEP, median (IQR), (%)a, c | 41.0 (27.0–59.0) | 44.0 (33.2–60.5) | 30.0 (21.0–54.0) | 0.012* |
| Blood gas analysis (before turning off NIV within 2 h) | ||||
| Arterial pH, mean (SD)a | 7.41 ± 0.49 | 7.41 ± 0.50 | 7.42 ± 0.48 | 0.186 |
| PaO2, median (IQR), mmHga | 95.4 ± 34.2 | 92.3 ± 30.5 | 101.4 ± 40.6 | 0.257 |
| PaCO2, mean (SD), mmHga | 45.7 ± 10.8 | 45.4 ± 10.4 | 46.4 ± 11.6 | 0.704 |
| HCO3, median (IQR), mEq/La | 29.6 (22.8–33.6) | 29.5 (22.8–33.0) | 29.8 (22.6–34.1) | 0.765 |
| BE, mean (SD), mmol/La | 4.6 ± 6.5 | 4.0 ± 6.3 | 5.9 ± 6.9 | 0.235 |
| SaO2, median (IQR), (%)a | 96.0 (94.0–97.7) | 96.0 (93.2–97.0) | 97.0 (94.2–98.0) | 0.226 |
| Clinical outcome (after NIV treatment) | ||||
| In-hospital mortality, N (%)b | 12 (14.1) | 3 (5.4) | 9 (31.0) | 0.002* |
| Readmission after discharge within 60 days, N (%)b | 28 (32.9) | 20 (35.7) | 8 (27.6) | 0.450 |
BE bicarbonate; IQR interquartile range; MEP maximal expiratory pressure; MIP maximal inspiratory pressure; NIV noninvasive ventilation; PaO2 partial pressure of oxygen; PaCO2 partial pressure of carbon dioxide; RR respiratory rate; RSBI rapid shallow breathing index; TV tidal volume; VE minute ventilation; SaO2 arterial oxyhemoglobin saturation; SD standard deviation
aContinuous variables are expressed as mean ± SD or median (IQR)
bCategorical variables are expressed as N (%)
cIncluding data from studies in the review by Evans et al. [21]. Male MIP reference: 120−(0.41 × age); female MIP reference: 108−(0.61 × age); male MEP reference: 174−(0.83 × age); female MEP reference: 131−(0.86 × age), percentage of predict MIP or MEP of percentage = real data (MIP or MEP)/reference (MIP or MEP)
*P < 0.05, significant
Univariate Analysis
For the primary outcome of successful weaning from NIV, univariate analysis showed that higher MIP and MEP were associated with an increased rate of successful weaning [OR 1.026 (95% CI 1.001–1.052); P = 0.040 and OR 1.025 (95% CI 1.003–1.048); P = 0.026, respectively] after turning off NIV. Conversely, days on NIV until weaning and RSBI were inversely associated with successful weaning [OR 0.716 (95% CI 0.539–0.949); P = 0.020 and OR 0.970 (95% CI 0.956–0.984); P < 0.001, respectively] after turning off NIV.
Multivariate Analysis
We used a multivariable logistic model to examine selected factors, including sex, smoking history, albumin level, antibiotic use, COPD severity, MEP, RSBI, and days on NIV until weaning. MIP and MEP are highly correlated, as both are related to respiratory muscle strength. To address the issue of collinearity, we excluded MIP because it was less significant than MEP. Patients with a lower RSBI were significantly more likely to be weaned successfully [OR 0.976 (95% CI 0.959–0.993); P = 0.006] (Table 3). Hosmer and Lemeshow goodness-of-fit chi-squared test was 4.065 [P = 0.772 (> 0.05)].
Table 3.
Univariate and multivariate analyses of clinical factors associated with successful weaning
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age | 1.006 (0.963–1.052) | 0.783 | – | – |
| Sex (reference, female) | 2.708 (0.667–10.993) | 0.163 | 1.274 (0.154–10.527) | 0.822 |
| Smoking history (reference, yes) | 0.267 (0.084–0.847) | 0.025* | 0.544 (0.087–3.396) | 0.515 |
| Days on NIV until weaning, days | 0.716 (0.539–0.949) | 0.020* | 0.825 (0.562–1.211) | 0.326 |
| Albumin, g/dL | 2.561 (0.869–7.550) | 0.088 | 2.196 (0.555–8.694) | 0.262 |
| Systemic corticosteroids (reference, yes) | 1.736 (0.481–6.261) | 0.399 | – | – |
| Antibiotics (reference, yes) | 0.376 (0.104–1.358) | 0.135 | 0.405 (0.082–1.999) | 0.267 |
| COPD group (reference, C + D group) | 2.465 (0.811–7.487) | 0.112 | 1.556 (0.420–5.769) | 0.508 |
| Off NIV RSBI, breaths/(min∙mL) | 0.970 (0.956–0.984) | < 0.001* | 0.976 (0.959–0.993) | 0.006* |
| Off NIV MIP, −cmH2O | 1.026 (1.001–1.052) | 0.040* | – | – |
| Off NIV MEP; +cmH2O | 1.025 (1.003–1.048) | 0.026* | 0.996 (0.968–1.023) | 0.755 |
Obtained through enter model construction, including only significant independent predictors; OR of NIV treatment success associated with each variable in the model
CI confidence interval; COPD chronic obstructive pulmonary disease; MEP maximal expiratory pressure; MIP maximal inspiratory pressure; NIV noninvasive ventilation; OR odds ratio; RSBI rapid shallow breathing index
*P < 0.05, significant
Predictive Value of the Weaning Index
The ROC curve was analyzed by selecting the follow-up RSBI. The area under the ROC curve for NIV weaning was 0.804 (95% CI 0.706–0.901) (Fig. 2). The RSBI at a threshold of 67.4 predicted successful weaning from NIV, with a sensitivity of 82.1%, a specificity of 69.0%, a positive likelihood ratio of 2.648, and a negative likelihood ratio of 0.377 (Table 4).
Fig. 2.
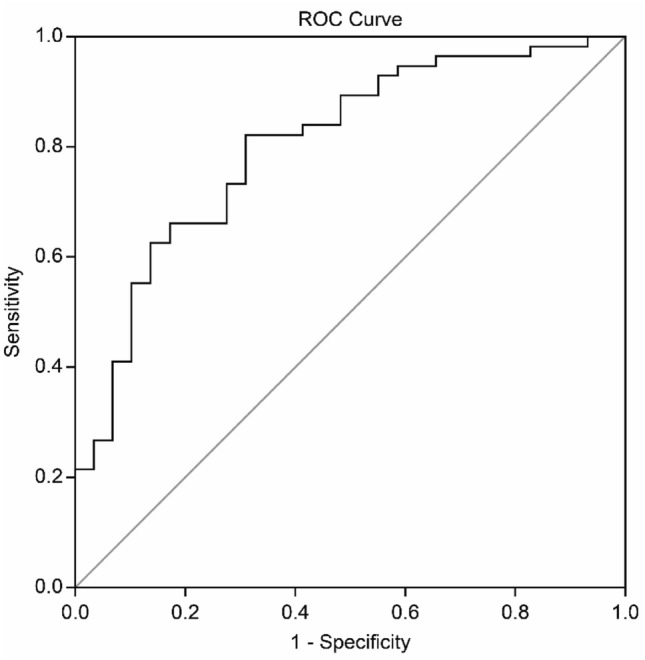
Receiver operating characteristic (ROC) curve for weaning parameters. The sensitivity and specificity of the weaning parameter for each image set a priori operation point and are indicated on the curve. They are as follows: off RSBI images: sensitivity, 69.0%; specificity, 82.1%; area under the ROC curve, 0.804
Table 4.
Analysis of NIV treatment
| AUC (95% CI) | Optimal cutoff | Sensitivity (%) | Specificity (%) | P at difference | |
|---|---|---|---|---|---|
| Off NIV RSBI, breaths/(min mL) | 0.804 (0.706–0.901) | 67.4 | 69.0 | 82.1 | < 0.001* |
Data are presented as AUC (95% CI); *P < 0.05, predicted outcome of a significant difference in weaning success
AUC area under the receiver operating characteristic curve; CI confidence interval; NIV noninvasive ventilation; RSBI rapid shallow breathing index
*P < 0.05, significant
Discussion
NIV is now the mainstay of therapy before endotracheal intubation and mechanical ventilation for patients with acute respiratory distress or failure due to AECOPD [22]. NIV is now recommended as a direct alternative for the management of patients with acute respiratory failure, particularly during the course of AECOPD, to preclude tracheal intubation and subsequent complications [6, 23, 24]. This is derived from the fact that, in many cases, patient outcomes (both short- and long-term) are better with NIV than with IMV [25, 26]. There is strong evidence to suggest that this benefit is due to a reduction in the rate of ventilator-associated pneumonia and avoidance of intensive care unit admission [27, 28]. Thus far, however, there is uncertainty regarding the best method of evaluating weaning from NIV, which generally requires the cooperation of the patient during the AECOPD recovery phase. Delayed weaning may potentially expose the patient to unnecessary discomfort and increase the risk of complications [29].
Although the RSBI was not a significant predictor of successful NIV [30], our results suggest that it is a reasonably good predictor of success for weaning a patient from not only IMV [31] but also NIV. The RSBI has been found to be an accurate index [32] that is calculated by dividing RR by TV in liters. Clinicians have successfully used this ratio in most mechanical ventilation weaning protocols. In a study by Berg et al. [15], patients were divided into two groups according to their RSBI. The assisted RSBI (aRSBI) is a criterion for weaning from IMV. The authors recorded it based on the initial level of support: high (aRSBI ≥ 105) or low (aRSBI < 105). The RSBI may be useful for titrating the NIV settings, as an aRSBI ≥ 105 is associated with the need for intubation and increased in-hospital mortality. According to Sellarer et al. [33], NIV can be discontinued immediately, without the need for a weaning period, allowing rapid discontinuation of NIV, if the patient’s condition improves. However, our results suggest that a weaning period is essential for this to succeed.
In our study, using ROC curve analysis, an RSBI ≤ 67.4 was associated with the highest sensitivity and specificity for determining successful weaning from NIV. In cases where chronic hypercapnia persists during the night for patients with AECOPD, NIV can be beneficial because it can provide gas exchange and improve night-time hypoventilation. It is not clear, however, whether long-term NIV can be used for stable chronic COPD patients with chronic comorbidities or pneumonia [34–36]. Patients with moderate-to-severe COPD frequently have persistent low-grade systemic inflammation with elevated levels of circulating inflammatory cascade molecules, such as CRP [37, 38]. Furthermore, some authors have reported that high CRP levels in patients with COPD are associated with worse short- and long-term clinical outcomes [39, 40]. In our study, we found that CRP levels were not significantly associated with weaning outcomes, indicating that the mechanism of successful weaning from NIV is very complex. This suggests that NIV weaning outcomes are not entirely mediated by modulation of the immune response, or determined by the effect of lung inflammation itself, in patients with COPD. Although CRP and B-type natriuretic peptide (BNP) have high diagnostic potency for COPD patients combined with cor-pulmonale, and are positively correlated with cardiac function classification [41], CRP level may not be useful in assessing weaning outcomes for COPD patients with NIV support [42].
Our study is limited by its small sample size and observational nature. Our hospital also does not have a distinct protocol outlining when to commence NIV. Dyspnea, one of the NIV initiation criteria, may also not be clearly defined. In the future, it may be possible to use a more objective assessment scale, such as the modified Medical Research Council dyspnea or Borg scale. In addition, cor-pulmonale and ejection fraction parameters were not evaluated in this cohort study, which may affect RSBI, MEP, and MIP when weaning from NIV. Both left ventricular and right ventricular functions may interact with intra-thoracic pressure in a complex and often unpredictable fashion. In future studies, these parameters may be included and collected for adjustment additional. Our patients may differ from those treated with NIV at other hospitals, even those with AECOPD. Finally, data were not collected after discharge. Therefore, an analysis of long-term outcomes was not possible.
Conclusion
We evaluated the use of potential factors and various respiratory indices for predicting successful weaning from NIV in patients with AECOPD with hypercapnic respiratory failure. A lower RSBI immediately before turning off NIV was more likely to be associated with successful weaning. Thus, RSBI is a single clinical parameter that can be easily measured at the bedside to help predict the likelihood of successful NIV weaning in patients with AECOPD. However, additional studies with longer follow-up periods and larger sample sizes should be conducted to further evaluate the predictive ability of the RSBI, or other potential weaning indices, for NIV in populations other than patients with COPD.
Acknowledgements
We are grateful for the continued support of the Department of Thoracic Medicine, National Taiwan University Hospital Hsin-Chu Branch, and to National Yang Ming Chiao Tung University, Taiwan for editing the manuscript.
Author Contributions
List the names across each section below: Category 1; Conception and design of study: JY, MRL, CKH, YTL. Acquisition of data: JY, MRL. Analysis and/or interpretation of data: JY, MRL, CKH. Category 2; Drafting of the manuscript: JY, CTC, CKH. Revising the manuscript critically for important intellectual content: JY, MRL, CTC, CKH.
Funding
The authors did not receive support from any organization for the submitted work.
Data Availability
Data are available from the Thoracic Medicine Unit of the National Taiwan University Hsin-Chu Hospital, Taiwan.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the hospital institutional review board.
Informed Consent
Informed consent was obtained from all individual participants included in the study (or their next of kin).
Consent for Publication
All participants have consented to the submission of the manuscript to the journal.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Qureshi H, Sharafkhaneh A, Hanania NA. Chronic obstructive pulmonary disease exacerbations: latest evidence and clinical implications. Ther Adv Chronic Dis. 2014;5:212–227. doi: 10.1177/2040622314532862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ansari SF, Memon M, Brohi N, Tahir A. Noninvasive positive pressure ventilation in patients with acute respiratory failure secondary to acute exacerbation of chronic obstructive pulmonary disease. Cureus. 2019;11:e5820. doi: 10.7759/CUREUS.5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zwerink M, Brusse-Keizer M, van der Valk PD, Zielhuis GA, Monninkhof EM, van der Palen J, Frith PA, Effing T. Self management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014 doi: 10.1002/14651858.CD002990.PUB3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lightowler JV, Wedzicha JA, Elliott MW, Ram FS. Non-invasive positive pressure ventilation to treat respiratory failure resulting from exacerbations of chronic obstructive pulmonary disease: cochrane systematic review and meta-analysis. BMJ. 2003;326:185. doi: 10.1136/BMJ.326.7382.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847–852. doi: 10.1136/THORAX.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brochard L, Mancebo J, Wysocki M, Lofaso F, Conti G, Rauss A, Simonneau G, Benito S, Gasparetto A, Lemaire F, Isabey D. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333:817–822. doi: 10.1056/NEJM199509283331301. [DOI] [PubMed] [Google Scholar]
- 7.Nicolini A, Ferrera L, Santo M, Ferrari-Bravo M, Del Forno M, Sclifò F. Noninvasive ventilation for hypercapnic exacerbation of chronic obstructive pulmonary disease: factors related to noninvasive ventilation failure. Pol Arch Med Wewn. 2014;124:525–531. doi: 10.20452/PAMW.2460. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez D, Smith G, Piper A, Rolls K. Non–invasive ventilation guidelines for adult patients with acute respiratory failure: a clinical practice guideline (Version 1) Chatswood, NSW: Agency for Clinical Innovation NSW Government; 2014. [Google Scholar]
- 9.Hill NS, Brennan J, Garpestad E, Nava S. Noninvasive ventilation in acute respiratory failure. Crit Care Med. 2007;35:2402–2407. doi: 10.1097/01.CCM.0000284587.36541.7F. [DOI] [PubMed] [Google Scholar]
- 10.Ambrosino N, Vagheggini G. Noninvasive positive pressure ventilation in the acute care setting: where are we? Eur Respir J. 2008;31:874–886. doi: 10.1183/09031936.00143507. [DOI] [PubMed] [Google Scholar]
- 11.Karthika M, Al Enezi FA, Pillai LV, Arabi YM. Rapid shallow breathing index. Ann Thorac Med. 2016;11:167–176. doi: 10.4103/1817-1737.176876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McConville JF, Kress JP. Weaning patients from the ventilator. N Engl J Med. 2012;367:2233–2239. doi: 10.1056/NEJMra1203367. [DOI] [PubMed] [Google Scholar]
- 13.Mirza S, Clay RD, Koslow MA, Scanlon PD. COPD guidelines: a review of the 2018 GOLD report. Mayo Clin Proc. 2018;93:1488–1502. doi: 10.1016/J.MAYOCP.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 14.Soleimanpour H, Taghizadieh A, Salimi R, Golzari SE, Mahmoodpoor A, Safari S, Mehdizadeh Esfanjani RM, Heshmat Y. Rapid shallow breathing index survey, a predictor of non-invasive ventilation necessity in patients with chronic obstructive pulmonary disease exacerbation: an analytical descriptive prospective study. Iran Red Crescent Med J. 2014;16:e13326. doi: 10.5812/IRCMJ.13326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berg KM, Lang GR, Salciccioli JD, Bak E, Cocchi MN, Gautam S, Donnino MW. The rapid shallow breathing index as a predictor of failure of noninvasive ventilation for patients with acute respiratory failure. Respir Care. 2012;57:1548–1554. doi: 10.4187/RESPCARE.01597. [DOI] [PubMed] [Google Scholar]
- 16.Megahed MM, Habib TN, Dwidar E. Rapid shallow breathing index as a predictor of ventilatory support necessity in patients with acute exacerbation of chronic obstructive pulmonary disease. Am J Res Commun. 2016;4:60–74. doi: 10.11648/J.JA.20170503.12. [DOI] [Google Scholar]
- 17.Carrillo A, Ferrer M, Gonzalez-Diaz G, Lopez-Martinez A, Llamas N, Alcazar M, Capilla L, Torres A. Noninvasive ventilation in acute hypercapnic respiratory failure caused by obesity hypoventilation syndrome and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186:1279–1285. doi: 10.1164/RCCM.201206-1101OC. [DOI] [PubMed] [Google Scholar]
- 18.Duan J, Tang X, Huang S, Jia J, Guo S. Protocol-directed versus physician-directed weaning from noninvasive ventilation: the impact in chronic obstructive pulmonary disease patients. J Trauma Acute Care Surg. 2012;72:1271–1275. doi: 10.1097/TA.0b013e318249a0d5. [DOI] [PubMed] [Google Scholar]
- 19.Momii H, Tashima Y, Kadokami T, Narita S, Yoshida M, Ando SI. Experience of step-wise protocol using noninvasive positive pressure ventilation for treating cardiogenic pulmonary edema. Eur J Emerg Med. 2012;19:267–270. doi: 10.1097/MEJ.0b013e32834ada48. [DOI] [PubMed] [Google Scholar]
- 20.Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, Pearl R, Silverman H, Stanchina M, Vieillard-Baron A, Welte T. Weaning from mechanical ventilation. Eur Respir J. 2007;29:1033–1056. doi: 10.1183/09031936.00010206. [DOI] [PubMed] [Google Scholar]
- 21.Evans JA, Whitelaw WA. The assessment of maximal respiratory mouth pressures in adults. Respir Care. 2009;54:1348–1359. [PubMed] [Google Scholar]
- 22.Sinuff T, Keenan SP, Department of Medicine, McMaster University Clinical practice guideline for the use of noninvasive positive pressure ventilation in COPD patients with acute respiratory failure. J Crit Care. 2004;19:82–91. doi: 10.1016/J.JCRC.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Bott J, Carroll MP, Conway JH, Keilty SE, Ward EM, Brown AM, Paul EA, Elliott MW, Godfrey RC, Wedzicha JA, Moxham J. Randomised controlled trial of nasal ventilation in acute ventilatory failure due to chronic obstructive airways disease. Lancet. 1993;341:1555–1557. doi: 10.1016/0140-6736(93)90696-e. [DOI] [PubMed] [Google Scholar]
- 24.Brochard L, Isabey D, Piquet J, Amaro P, Mancebo J, Messadi AA, Brun-Buisson C, Rauss A, Lemaire F, Harf A. Reversal of acute exacerbations of chronic obstructive lung disease by inspiratory assistance with a face mask. N Engl J Med. 1990;323:1523–1530. doi: 10.1056/NEJM199011293232204. [DOI] [PubMed] [Google Scholar]
- 25.Confalonieri M, Parigi P, Scartabellati A, Aiolfi S, Scorsetti S, Nava S, Gandola L. Noninvasive mechanical ventilation improves the immediate and long-term outcome of COPD patients with acute respiratory failure. Eur Respir J. 1996;9:422–430. doi: 10.1183/09031936.96.09030422. [DOI] [PubMed] [Google Scholar]
- 26.Martin TJ, Hovis JD, Costantino JP, Bierman MI, Donahoe MP, Rogers RM, Kreit JW, Sciurba FC, Stiller RA, Sanders MH. A randomized, prospective evaluation of noninvasive ventilation for acute respiratory failure. Am J Respir Crit Care Med. 2000;161:807–813. doi: 10.1164/AJRCCM.161.3.9808143. [DOI] [PubMed] [Google Scholar]
- 27.Keenan SP, Gregor J, Sibbald WJ, Cook D, Gafni A. Noninvasive positive pressure ventilation in the setting of severe, acute exacerbations of chronic obstructive pulmonary disease: more effective and less expensive. Crit Care Med. 2000;28:2094–2102. doi: 10.1097/00003246-200006000-00072. [DOI] [PubMed] [Google Scholar]
- 28.Plant PK, Owen JL, Parrott S, Elliott MW. Cost effectiveness of ward based non-invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease: economic analysis of randomised controlled trial. BMJ. 2003;326:956. doi: 10.1136/BMJ.326.7396.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall JB, Wood LD. Liberation of the patient from mechanical ventilation. JAMA. 1987;257:1621–1628. doi: 10.1001/JAMA.257.12.1621. [DOI] [PubMed] [Google Scholar]
- 30.Lin MS, Guo HR, Huang MH, Chen CR, Wu CL. Predictors of successful noninvasive ventilation treatment for patients suffering acute respiratory failure. J Chin Med Assoc. 2008;71:392–398. doi: 10.1016/S1726-4901(08)70089-3. [DOI] [PubMed] [Google Scholar]
- 31.Krieger BP, Isber J, Breitenbucher A, Throop G, Ershowsky P. Serial measurements of the rapid-shallow-breathing index as a predictor of weaning outcome in elderly medical patients. Chest. 1997;112:1029–1034. doi: 10.1378/CHEST.112.4.1029. [DOI] [PubMed] [Google Scholar]
- 32.Yang KL, Tobin MJ. A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. N Engl J Med. 1991;324:1445–1450. doi: 10.1056/NEJM199105233242101. [DOI] [PubMed] [Google Scholar]
- 33.Sellares J, Ferrer M, Anton A, Loureiro H, Bencosme C, Alonso R, Martinez-Olondris P, Sayas J, Peñacoba P, Torres A. Discontinuing noninvasive ventilation in severe chronic obstructive pulmonary disease exacerbations: a randomised controlled trial. Eur Respir J. 2017;50:28679605. doi: 10.1183/13993003.01448-2016. [DOI] [PubMed] [Google Scholar]
- 34.Lindenauer PK, Stefan MS, Shieh MS, Pekow PS, Rothberg MB, Hill NS. Outcomes associated with invasive and noninvasive ventilation among patients hospitalized with exacerbations of chronic obstructive pulmonary disease. JAMA Intern Med. 2014;174:1982–1993. doi: 10.1001/JAMAINTERNMED.2014.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mas A, Masip J. Noninvasive ventilation in acute respiratory failure. Int J Chron Obstruct Pulmon Dis. 2014;9:837–852. doi: 10.2147/COPD.S42664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Storre JH, Callegari J, Magnet FS, Schwarz SB, Duiverman ML, Wijkstra PJ, Windisch W. Home noninvasive ventilatory support for patients with chronic obstructive pulmonary disease: patient selection and perspectives. Int J Chron Obstruct Pulmon Dis. 2018;13:753–760. doi: 10.2147/COPD.S154718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. 1998;279:1477–1482. doi: 10.1001/JAMA.279.18.1477. [DOI] [PubMed] [Google Scholar]
- 38.Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59:574–580. doi: 10.1136/THX.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruiz-González A, Lacasta D, Ibarz M, Martínez-Alonso M, Falguera M, Porcel JM. C-reactive protein and other predictors of poor outcome in patients hospitalized with exacerbations of chronic obstructive pulmonary disease. Respirology. 2008;13:1028–1033. doi: 10.1111/J.1440-1843.2008.01403.X. [DOI] [PubMed] [Google Scholar]
- 40.Leuzzi G, Galeone C, Taverna F, Suatoni P, Morelli D, Pastorino U. C-reactive protein level predicts mortality in COPD: a systematic review and meta-analysis. Eur Respir Rev. 2017;26:28143876. doi: 10.1183/16000617.0070-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guilian WEI, Zhang Z. Clinical value of hs-CRP and BNP detection in the diagnosis and treatment of COPD patients with pulmonary heart disease. Chin J Prim Med Pharm. 2019;12:1429–1432. [Google Scholar]
- 42.Sato Y, Yoshihisa A, Oikawa M, et al. Prognostic impact of chronic obstructive pulmonary disease on adverse prognosis in hospitalized heart failure patients with preserved ejection fraction–a report from the JASPER registry. J Cardiol. 2019;73:459–465. doi: 10.1016/j.jjcc.2019.01.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the Thoracic Medicine Unit of the National Taiwan University Hsin-Chu Hospital, Taiwan.