Abstract
Historically in the United States, kidneys for simultaneous liver-kidney transplantation (SLKT) candidates were allocated with livers, prioritizing SLKT recipients over much of the kidney waiting list. A 2017 change in policy delineated renal function criteria for SLKT and implemented a safety net for kidney-after-liver transplantation. We compared the use and outcomes of SLKT and kidney-after-liver transplant with the 2017 policy. United Network for Organ Sharing Standard Transplant Analysis and Research files were used to identify adults who received liver transplantations (LT) from August 10, 2007 to August 10, 2012; from August 11, 2012 to August 10, 2017; and from August 11, 2017 to June 12, 2019. LT recipients with end-stage renal disease (ESRD) were defined by dialysis requirement or estimated glomerular filtration rate <25. We evaluated outcomes and center-level, regional, and national practice before and after the policy change. Nonparametric cumulative incidence of kidney-after-liver listing and transplant were modeled by era. A total of 6332 patients received SLKTs during the study period; fewer patients with glomerular filtration rate (GFR) ≥50 mL/min underwent SLKT over time (5.8%, 4.8%, 3.0%; P = 0.01). There was also less variability in GFR at transplant after policy implementation on center and regional levels. We then evaluated LT-alone (LTA) recipients with ESRD (n = 5408 from 2012–2017; n = 2321 after the policy). Listing for a kidney within a year of LT increased from 2.9% before the policy change to 8.8% after the policy change, and the rate of kidney transplantation within 1 year increased from 0.7% to 4% (P < 0.001). After the policy change, there was no difference in patient survival rates between SLKT and LTA among patients with ESRD. Implementation of the 2017 SLKT policy change resulted in reduced variability in SLKT recipient kidney function and increased access to deceased donor kidney transplantation for LTA recipients with kidney disease without negatively affecting outcomes.
Simultaneous liver-kidney transplantation (SLKT) accounts for almost 10% of all liver transplantations (LTs) performed in the United States.(1) Nonalcoholic fatty liver disease, which is associated with risk factors for chronic kidney disease including diabetes mellitus and hypertension, will soon become the most common indication for LT in the United States.(2) As such, the prevalence of renal dysfunction at the time of LT listing is expected to rise.(3,4)
Concerns about adherence to the Organ Procurement and Transplantation Network (OPTN) Final Rule(5) and variability in the use of SLKT,(6,7) including in candidates for whom the expected benefit was unclear, led to the development of a new consensus policy by the United Network for Organ Sharing (UNOS) in 2017. Under the new policy, SLKT candidates must have chronic kidney disease, sustained acute kidney injury, or metabolic disease as diagnosed by a nephrologist. Candidates with chronic kidney disease must have glomerular filtration rates (GFRs) ≤60 mL/min for 90 days, GFR ≤30 mL/min immediately before listing, or be receiving chronic hemodialysis. Candidates with sustained acute kidney injury must have received hemodialysis for a minimum of 6 weeks or have documented GFRs of ≤25 mL/min during that time.
In addition to formalizing eligibility criteria, a core tenet of UNOS policy, the new allocation strategy also aligned regional sharing policies for SLKT and LT, which had previously been inconsistent.(8) For LT recipients who develop renal failure within the first year after LT, a “safety net” system was designed to allow prioritization on the kidney transplantation list.(9) Currently, data on outcomes of SLKT recipients under the new allocation policy are limited, but nonetheless of interest given concerns that not all patients who meet the new criteria benefit equally from SLKT.(10)
The objective of this study was to compare the utilization patterns and outcomes of SLKT and kidney-after-liver (KAL) transplantation relative to the 2017 allocation policy, with attention to the policy’s following stated goals: (1) establish medical eligibility criteria for adult candidates seeking SLKT, (2) curtail allocation of high-quality kidneys to liver candidates above highly prioritized kidney candidates, (3) clarify the rules about regional and national SLKT allocation to be consistent with deceased donor LT (DDLT), and (4) establish a safety net to address concerns about the limitations of SLKT eligibility.
Patients and Methods
Data from the UNOS Standard Transplant Analysis and Research (STAR) files (retrieved June 15, 2020) were used to identify adult patients on the LT waiting list from 2007 to 2020. To conduct analysis per patient, the most recent LT or listing was used. Patients were separated into the following 3 eras: August 10, 2007 to August 10, 2012 (historical cohort); August 11, 2012 to August 10, 2017 (to correspond with the change in SLKT listing policy); and August 11, 2017 to June 12, 2019. The recent cohort transplant date was limited to 2019 to allow sufficient follow-up for survival analysis. SLKTs were defined as patients receiving a deceased-donor kidney transplant within 7 days of receiving an LT.
To evaluate practice patterns and outcomes before and after the 2017 policy change, we defined the cohort of LT recipients with end-stage renal disease (ESRD) by dialysis requirement at listing or at transplant or estimated GFR (eGFR) <25 mL/min. This definition is intended to simulate the 2017 SLKT allocation policy criteria for kidney dysfunction using the limited data available in the UNOS STAR files and reflect the group of patients who may reasonably receive SLKT or LT alone (LTA) with an opportunity to proceed to KAL transplantation. To account for LT recipients with multiple periods on the kidney transplant waiting list, the first kidney listing or activation of an existing listing, and first kidney transplantation occurring after LT were used to define the cohort of KAL “safety net” waitlist patients and recipients. In accordance with the 2017 policy, the “safety net” listing period was defined as listing or activation within 365 days after LT. Recipients of living donor kidneys after LT were evaluated separately. “Safety net” KAL listings and transplantations were also calculated for LT recipients who did not meet the aforementioned ESRD criteria by the time of their LTA.
eGFR was calculated according to the Modification of Diet in Renal Disease (MDRD) equation and incorporated the reported creatinine at the time of listing or transplant.(11) The diagnosis of nonalcoholic steatohepatitis (NASH) was determined from primary diagnosis codes and supplemented with information from the primary free-text diagnosis in addition to the coded primary diagnosis variable. Expanded criteria donors for kidney transplantation were designated according to the UNOS definition.(12)
Chi-square tests were performed to examine differences in proportions for categorical variables across groups. Kruskal-Wallis analysis of variance tests were performed to test differences in medians for continuous variables. Patient and graft survival rates and KAL listing and transplant incidences were evaluated using Kaplan-Meier survival curve analysis and compared using log-rank tests. Nonparametric cumulative incidences of KAL listings and transplants were modeled stratified by era. The total number of kidneys used for SLKT and KAL transplantations were summarized per year of index transplantation.
Analysis was performed using Stata 14 (StataCorp, College Station, TX) and SAS (SAS Institute, Cary, NC). A P value < 0.05 was considered statistically significant. This study was determined exempt by the Duke University Institutional Review Board; no patient consent was obtained.
Results
SLKT DONOR AND RECIPIENT CHARACTERISTICS
A total of 6332 patients received SLKTs during the study period, with no decrease following policy implementation in 2017. The proportion of patients who received transplants for alcohol-related cirrhosis and NASH increased over time, whereas the proportion of patients with a primary diagnosis of hepatitis C virus (HCV) decreased. Although the proportion of patients on dialysis did not change across the 3 cohorts (2007–2012, 68.9%; 2012–2017, 69.6%; 2017–2019, 71.3%; P = 0.47), the median GFR at transplant among those not on dialysis decreased over time (median, 27.0 mL/min [interquartile range, IQR, 18.8–40.4], 25.7 [IQR, 17.2–36.5], 22.8 [IQR, 16.9–32.5]; P = 0.02). There was also a statistically significant reduction in the proportion of patients undergoing SLKT with GFR ≥25 mL/min over time (Table 1). In the era immediately preceding the policy change, patients receiving SLKT with GFRs >60 mL/min had at least 8 distinct kidney diagnosis codes (data not shown). After the policy change, the diagnosis for kidney allocation in SLKT for recipients with GFRs >60 mL/min was exclusively hepatorenal syndrome (n = 5). The proportions of patients receiving SLKT by GFR category at transplant before and after the policy are shown in Supporting Fig. 1. The median Kidney Donor Profile Index (KDPI) at transplant for SLKT grafts increased from 29% (IQR, 12–51) to 31% (IQR, 14–53), but this trend was not statistically significant (P = 0.07).
TABLE 1.
Donor and Recipient Characteristics of SLKT Recipients Over 3 Eras
| Characteristic | August 10, 2007-August 10, 2012 (n = 1974) | August 11, 2012-August 10, 2017 (n = 3124) | August 11, 2017-June 12, 2019 (n = 1234) | P Value |
|---|---|---|---|---|
| Donor age, years | 34 (22–47) | 33 (23–46) | 34 (25–46) | 0.45 |
| ECD | 193 (9.8) | 255 (8.2) | 88 (7.1) | 0.02 |
| KDPI, % | 30 (12–54) | 29 (12–51) | 31 (14–53) | 0.02 |
| KDPI >85% | 85 (4.6) | 101 (3.4) | 34 (2.8) | 0.03 |
| KDRI-Rao | 1.05 (0.88–1.33) | 1.04 (0.87–1.29) | 1.06 (0.89–1.31) | 0.01 |
| Recipient age, years | 56 (50–61) | 58 (51–63) | 58 (51–64) | <0.001 |
| Female recipient | 706 (35.8) | 1139 (36.5) | 493 (40.0) | 0.04 |
| Recipient race/ethnicity* | 0.01 | |||
| White | 1266 (64.0) | 2001 (64.0) | 757 (61.4) | |
| African American | 294 (14.9) | 468 (15.0) | 182 (14.8) | |
| Hispanic | 310 (15.7) | 520 (16.6) | 218 (17.7) | |
| Diagnosis | <0.001 | |||
| HCV | 493 (26.9) | 638 (21.0) | 155 (13.0) | |
| Alcohol-related cirrhosis | 292 (15.9) | 589 (19.4) | 303 (25.3) | |
| NASH | 186 (7.9) | 501 (16.5) | 269 (21.7) | |
| HCC | 172 (9.4) | 302 (10.0) | 97 (8.1) | |
| Recipient BMI ≥35 kg/m2 | 216 (10.9) | 355 (11.4) | 146 (11.8) | 0.74 |
| Dialysis while on waiting list | 1364 (68.9) | 2177 (69.6) | 880 (71.3) | 0.47 |
| GFR <25 mL/min at transplant | 274 (14.5) | 456 (15.2) | 197 (16.3) | P = 0.01 |
| GFR ≥25 and <50 mL/min at transplant | 231 (12.3) | 348 (11.6) | 121 (10.0) | |
| GFR ≥50 mL/min at transplant | 109 (5.8) | 144 (4.8) | 36 (3.0) | |
| GFR at transplant, mL/min | 27.0 (18.8–40.4) | 25.7 (17.2–36.5) | 22.8 (16.9–32.5) | 0.02 |
| Graft travel distance, miles | 42 (5–118) | 72 (9–195.5) | 77 (12–216) | <0.001 |
| Cold ischemic time, hours | 6.4 (5.0–8.0) | 6.1 (4.9–7.8) | 5.9 (4.7–7.3) | <0.001 |
NOTE: Data are shown as n (%) or median (IQR).
Race/ethnicity summary categories do not include Asian/Pacific Islander, Native American, Other.
In the cohort immediately preceding the policy change (2012–2017), SLKT grafts trended toward being shared less regionally and more likely to be shared nationally than DDLT grafts. After the policy, the rates of regional and national sharing of SLKT versus LTA grafts were not significantly different. Supporting Table 1 summarizes the local, regional, and national sharing of SLKT grafts with compared with contemporaneous deceased-donor LTA among all recipients. The procurement travel distance increased over time (median nautical miles: 42 [IQR, 5–118] versus 72 [IQR, 9–195.5] versus 77 [IQR, 12–216]). However, the cold ischemic time of liver grafts decreased (median hours: 6.4 [IQR, 5.0–8.0] versus 6.1 [IQR, 4.9–7.8] versus 5.9 [IQR, 4.7–7.3]; Table 1).
KAL LISTING AND TRANSPLANTATION
We then evaluated LTA recipients with ESRD (n = 5408 [19.7%] 2012–2017, n = 2321 [19.2%] after the policy). The characteristics of LTA recipients with ESRD versus SLKT recipients with ESRD are shown in Table 2. The progression of LT candidates with ESRD through transplantation, kidney listing, and kidney transplantation after the policy change is shown in Fig. 1.
TABLE 2.
Characteristics of LTA and SLKT Recipients With ESRD
| 2012–2017 |
2017–2019 |
|||
|---|---|---|---|---|
| Characteristic | LTA With ESRD (n = 5408) | SLKT With ESRD (n = 2618) | LTA With ESRD (n = 2321) | SLKT With ESRD (n = 1095) |
| Donor age, years | 38 (26–52) | 33 (24–46) | 38 (27–51) | 34 (26–46) |
| ECD | 1028 (19.0) | 227 (8.5) | 403 (17.4) | 83 (7.6) |
| Recipient age, years | 56 (48–62) | 58 (51–63) | 55 (46–62) | 58 (52–64) |
| Female recipient | 2416 (44.7) | 1016 (37.2) | 1055 (45.5) | 453 (41.3) |
| Recipient race/ethnicity* | ||||
| White | 3795 (70.2) | 1700 (63.4) | 1588 (68.4) | 666 (60.8) |
| African American | 417 (7.7) | 402 (15.0) | 140 (6.0) | 162 (14.8) |
| Hispanic | 915 (16.9) | 461 (17.2) | 455 (19.6) | 198 (18.1) |
| Diagnosis | ||||
| HCV | 880 (16.6) | 568 (21.6) | 117 (5.1) | 142 (13.3) |
| Alcohol-related cirrhosis | 1213 (22.8) | 543 (20.6) | 745 (32.7) | 280 (26.1) |
| NASH | 833 (15.7) | 453 (17.2) | 450 (19.7) | 231 (21.6) |
| HCC | 453 (8.5) | 236 (9.0) | 163 (7.1) | 83 (7.8) |
| Dialysis while on waiting list | 3333 (61.6) | 2175 (81.1) | 1545 (66.6) | 880 (80.4) |
| GFR <25 mL/min at transplant | 1743 (32.2) | 456 (17.0) | 640 (27.6) | 197 (18.0) |
NOTE: Data are shown as n (%) or median (IQR).
Race/ethnicity summary categories do not include Asian/Pacific Islander, Native American, Other.
FIG. 1.
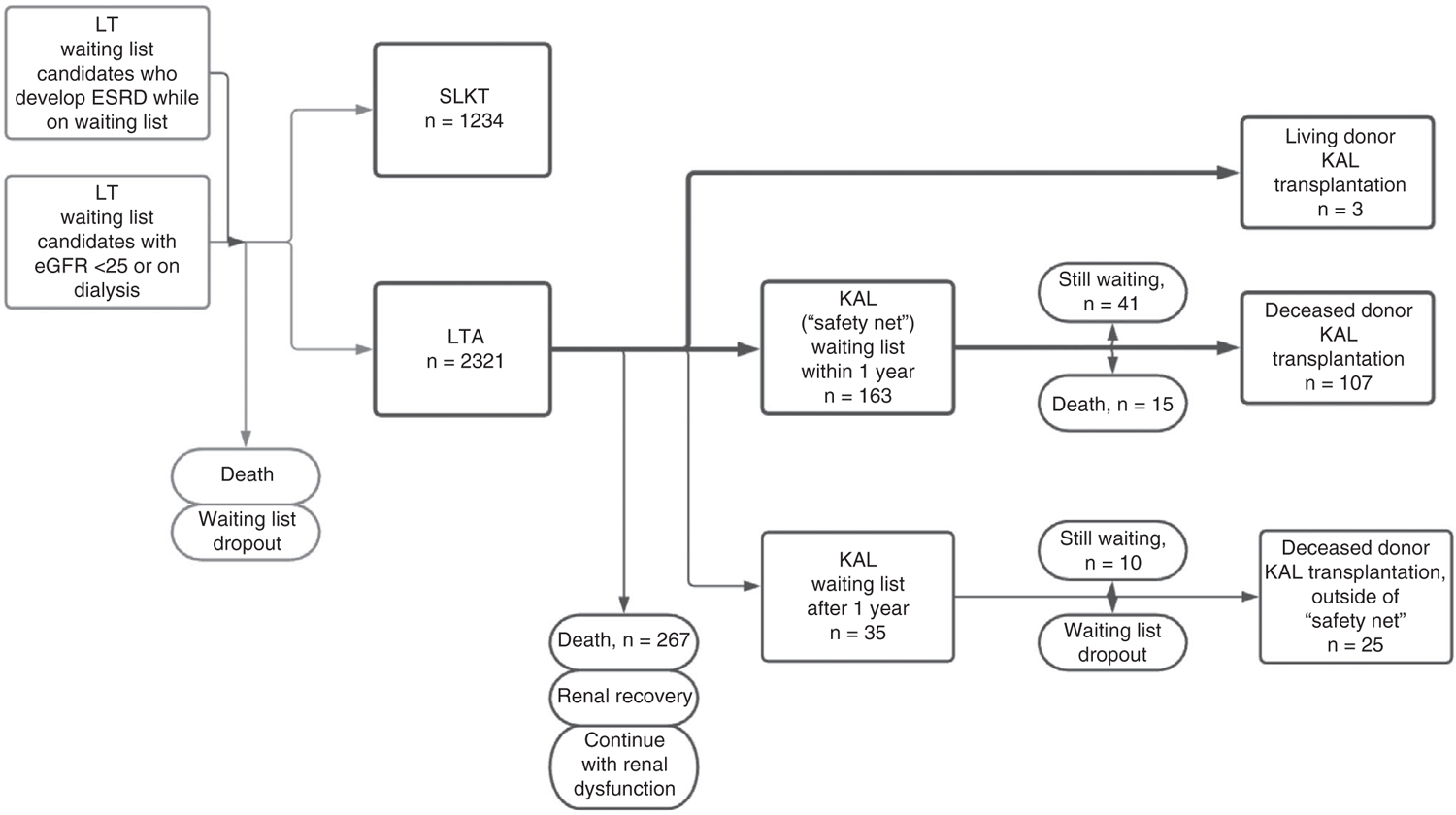
Postpolicy progression of LT candidates with ESRD.
Prior to the policy change, 2.9% and 4.7% of LTA with ESRD were listed at 1 and 2 years after transplant, respectively, compared with 8.8% and 13.0% after the policy change (log-rank P < 0.001). Of the LTA recipients with ESRD, 0.7% and 1.7% went on to receive kidney transplants at 1 and 2 years, respectively, before the policy change, and this increased to 4.0% and 11.0% after the policy change (log-rank P < 0.001; Fig. 2).
FIG. 2.
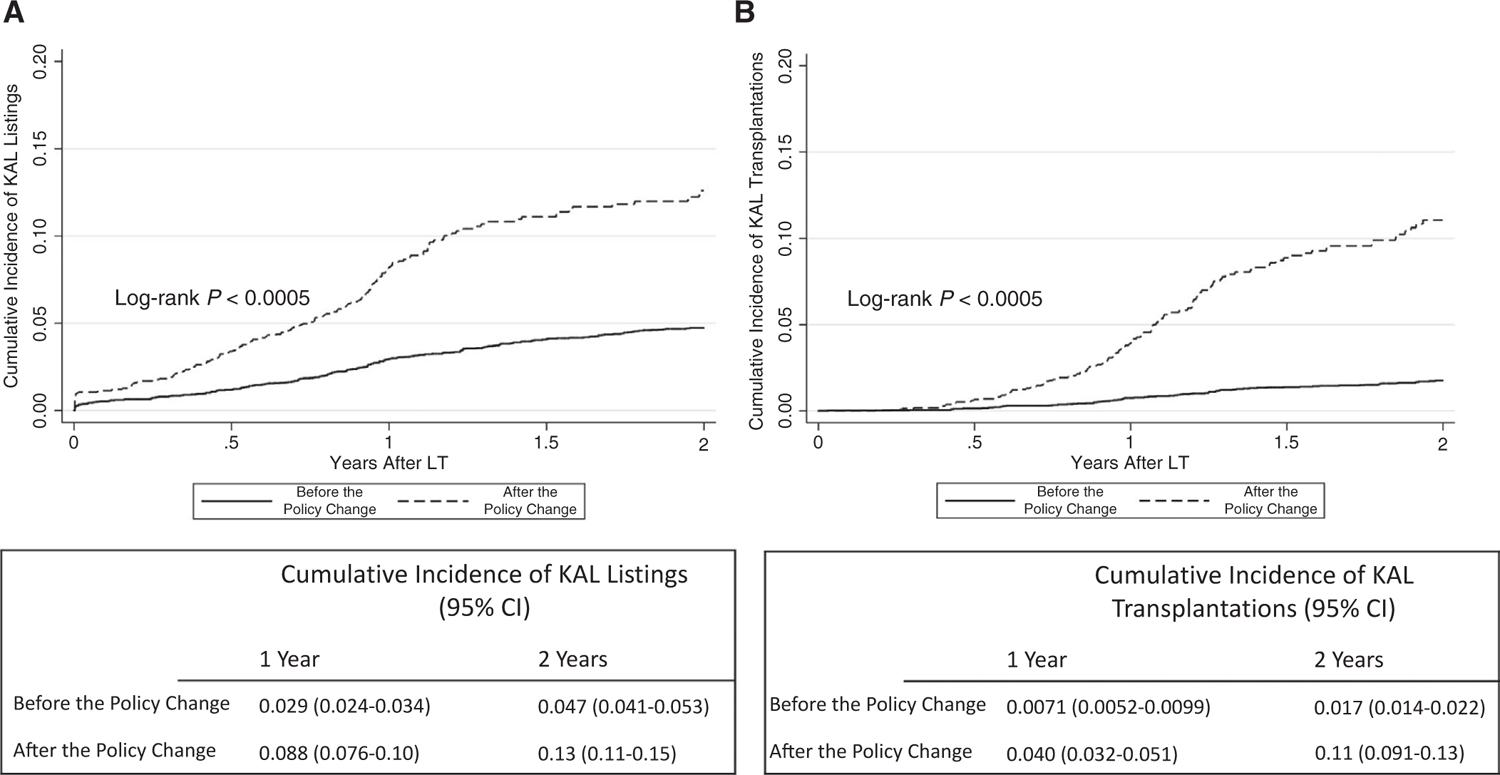
Cumulative incidence of KAL (A) listings and (B) transplantations among LTA recipients with ESRD.
Among the patients listed for kidney transplantation within 1 year after LT, the waiting time for a kidney decreased after the policy change (median, 171.5 days [IQR, 51–527 days] versus 43.5 days [IQR, 13–152 days]; P < 0.001), as did the interval between liver and kidney transplant (median, 426 days [IQR, 313–721 days] versus 336 days [IQR, 248–391 days]; P < 0.001). The median KDPI at transplant for patients receiving KAL transplantations who were listed within 1 year after LTA also increased from 40%(IQR, 24–57) to 43% (IQR, 28–58; P = 0.59). Only 6 living donor kidney transplants were performed before the policy change, amounting to 0.11% of LTA recipients with ESRD, compared with 2 after the policy change (0.09% of LTA recipients with ESRD; P = 0.76).
To minimize the effect of limited follow-up in the most recent era, we additionally calculated kidney listing and transplant rates for patients receiving an LT 1 year before and 1 year after implementation of the SLKT policy. Of the 1127 patients with kidney dysfunction who received a liver in the year before the policy change, 32 (2.8%) were listed for a kidney within 1 year; 20 (62.5%) of those patients listed went on to receive kidney transplantations, with a median interval of 421 days (IQR, 329–476). Of the 1113 patients with kidney dysfunction receiving a liver in the year after the policy change, 73 (6.6%) were listed for kidney transplantation within 1 year, and 52 (71.2%) of those patients listed went on to receive a kidney transplantation, with a median interval of 327.5 days (IQR, 266.5–483).
EARLY KAL TRANSPLANTATIONS FOR LT RECIPIENTS WITHOUT ESRD
We examined the incidence of KAL listings and transplantations among LTA recipients without ESRD. The cumulative incidence of KAL transplantations among LTA recipients without ESRD increased after the policy change (Cumulative Incidence 0.0005 [95% CI, 0.0003–0.0009] before the policy change versus 0.003 [95% CI, 0.002–0.005] after the policy change; log-rank P < 0.001), as did the absolute number performed. After the policy change, 24.4% of KAL transplantation grafts were transplanted in recipients without ESRD at the time of LT versus 23.4% before the policy change. Finally, we calculated the total number of kidneys used per year for LT recipients, combining SLKT and KAL transplantations (indexed by year of LT), shown in Fig. 3.
FIG. 3.
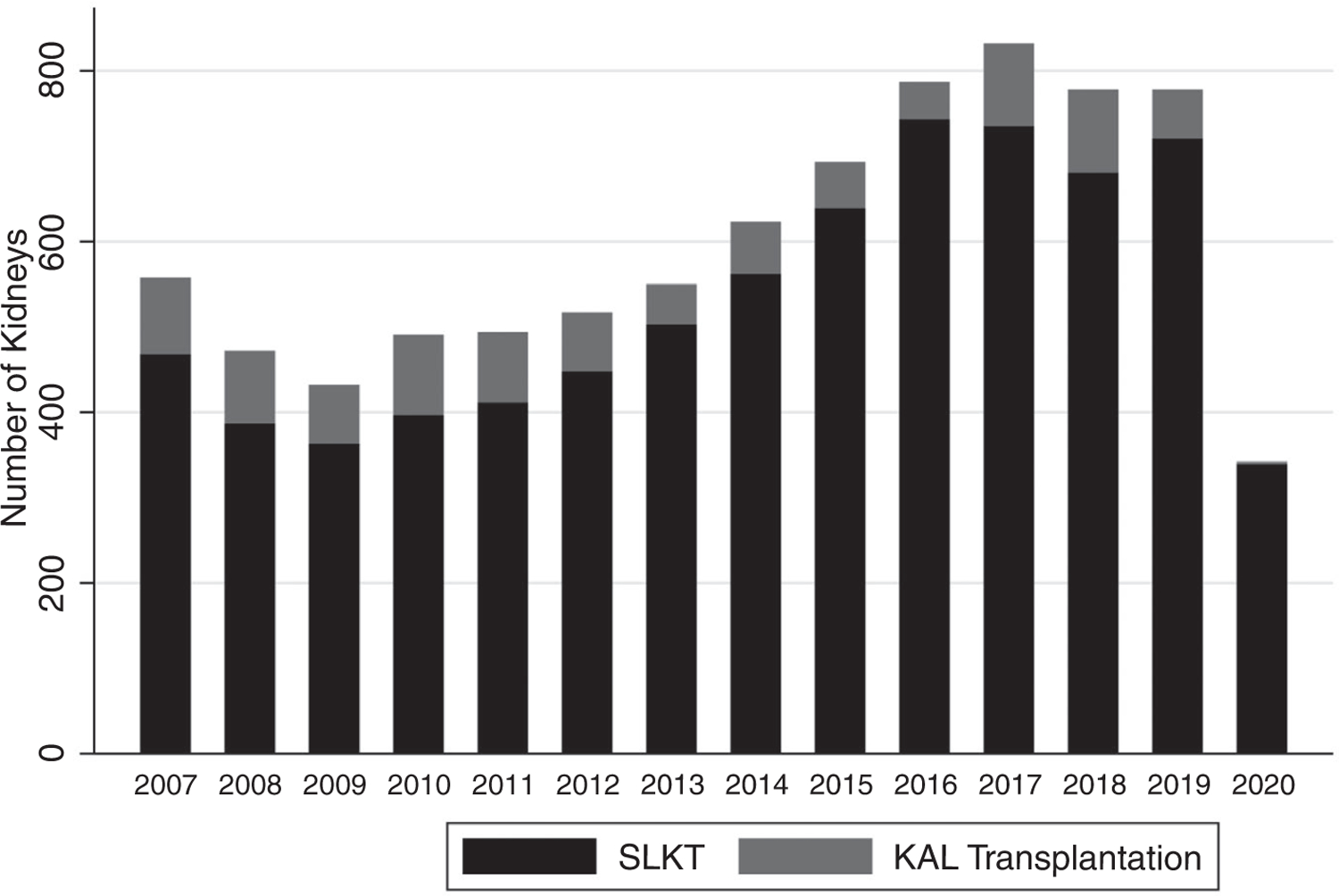
Total number of kidneys used for SLKTs and KAL transplantations by year. Data for 2020 are available only through June 11, 2020.
PATIENT AND GRAFT OUTCOMES
Among all patients receiving SLKTs, there was no difference in patient survival rates following SLKT before versus after the policy change (log-rank P = 0.18). Among patients with ESRD, patient survival rates trended toward being higher with SLKT versus LTA before the policy change (log-rank P = 0.10), but was not different after the policy change (log-rank P = 0.82; Fig. 4). Kidney graft survival rates were not different between SLKTs and KAL transplantations after the policy change (log-rank P = 0.99), between SLKTs before and after the policy change (log-rank P = 0.71), or between KAL transplantations before and after the policy change (log-rank P = 0.23; Supporting Fig. 2).
FIG. 4.
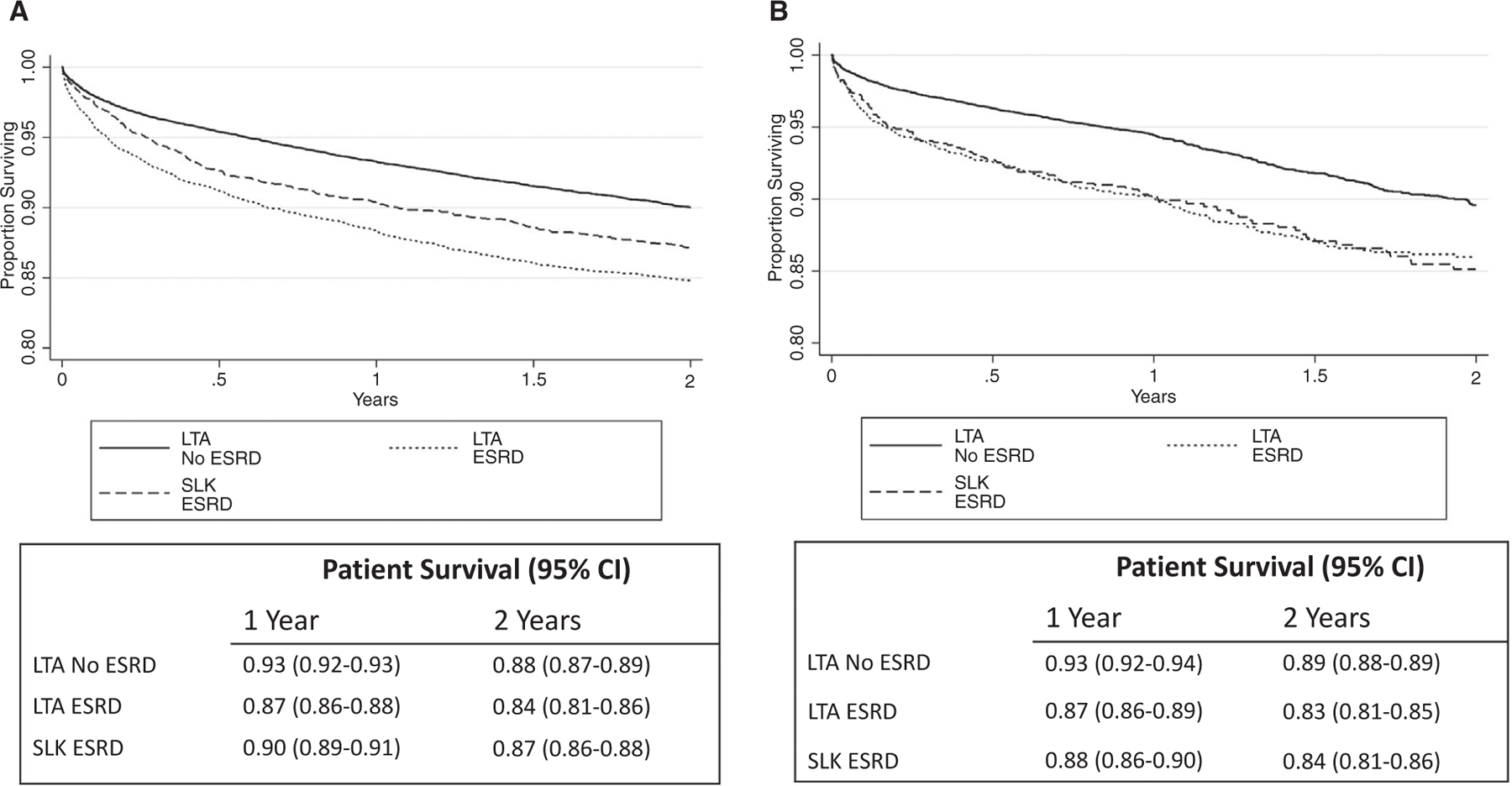
Posttransplant patient survival rates of LTA recipients with and without ESRD and SLKTs (A) before the policy change and (B) after the policy change.
Discussion
In this study, we describe the change in SLKT clinical practice and outcomes after the implementation of allocation policy that includes standardized metrics of kidney function. We find that after policy implementation, a higher proportion of SLKT recipients were on dialysis at either listing or transplant, and those not on dialysis had lower median GFRs at transplant. We also find increased utilization of the safety net for LTA recipients with ESRD, with a more than 2-fold increase in listing for deceased donor kidney transplant within the 365 days following LTA. These findings suggest that the 2017 change in SLKT allocation policy has so far demonstrated its intended effects of standardizing SLKT allocation and facilitating KAL transplantations. Within the short-term follow-up available after the policy change, outcomes for SLKT versus LTA among patients with ESRD were comparable, suggesting that LTA with the safety net may be a noninferior option for patients with end-stage liver disease with concomitant ESRD.
Historically, kidneys for SLKT candidates were allocated according to center-level protocols, intrinsically prioritizing SLKT recipients over the majority of patients on the kidney waiting list. This raised concerns about equity, as high-quality kidneys were being allocated to liver candidates who may regain renal function after LT while bypassing highly prioritized kidney candidates.(13,14) In addition, SLKT eligibility criteria were inconsistent among centers, with no consensus on a threshold for GFR or duration of dialysis. This resulted in large variations in the use of SLKT across centers and regions.(6,7) The 2017 change in SLKT allocation policy was developed to add consistency to the kidney graft distribution process and therefore be in closer compliance with the OPTN Final Rule.(5) In the following discussion, we address our findings regarding the success of policy implementation.
POLICY OBJECTIVE 1: ESTABLISH MEDICAL ELIGIBILITY CRITERIA FOR ADULT CANDIDATES SEEKING SLKT
Following the standardization of metrics of kidney function required for SLKT prioritization on the kidney waiting list, we find that the proportion of SLKT recipients on dialysis while on the waiting list did not change. However, the median GFR at transplant among those not on dialysis decreased, as did the variability of GFR at transplant (Table 1). The proportion of SLKT allocated to recipients with a GFR >50 mL/min, although small to begin with, also decreased. Center-level and regional variability in GFR at SLKT decreased as well. After the policy change, 2 regions were found to no longer perform SLKTs on recipients with GFR >50mL/min. Together, these results indicate an increasing standardization of SLKT eligibility criteria.
POLICY OBJECTIVE 2: CURTAIL ALLOCATION OF HIGH-QUALITY KIDNEYS TO LIVER CANDIDATES ABOVE HIGHLY PRIORITIZED KIDNEY CANDIDATES
We find that kidneys allocated to SLKT recipients trended toward higher KDPI after the policy change, whereas the utilization of kidneys with KDPI >85% decreased (Table 1). Similar to before the policy change,(15) >50% of kidney grafts allocated to SLKT recipients after the policy change had KDPI <35%. However, the total number of kidneys used for SLKTs and KAL transplantations combined may be decreasing (Fig. 3). We did not evaluate kidney-alone allocation and therefore do not fully address this policy objective.
POLICY OBJECTIVE 3: CLARIFY THE RULES ABOUT REGIONAL AND NATIONAL SLKT ALLOCATION TO BE CONSISTENT WITH DDLT
Compared to a discrepancy in prepolicy regional and national sharing of SLKT grafts when compared with DDLT grafts, we find that regional and national sharing are no longer significantly different between SLKT and DDLT grafts after the policy change (Table 2). Although travel distance for SLKT grafts increased, cold ischemic time did not.
POLICY OBJECTIVE 4: ESTABLISH A “SAFETY NET” TO ADDRESS CONCERNS ABOUT LIMITATIONS OF SLKT ELIGIBILITY CRITERIA
We demonstrate an increased rate of KAL listing since the policy change in 2017 (2.9% versus 8.8% within 1 year; log-rank P < 0.001). We similarly demonstrate a dramatic increase in the number of KAL recipients (Fig. 4) and a decreased waiting time for those kidneys. We believe these findings reflect successful implementation of the safety net. Although the safety net was designed to allow early access to KAL transplantation for those liver recipients who did not meet the new SLKT listing criteria, the policy has only the 2 following requirements: (1) registration on the waiting list prior to 1-year anniversary of LT and (2) at a date 60 to 365 days after LT, the candidate is on dialysis or has an eGFR <20 mL/min. As such, it is possible that an LT recipient may qualify for the safety net with de novo kidney failure arising after his or her LT. We demonstrate a significant increase in KAL transplantations for de novo kidney failure after policy implementation, with 24.4% of KAL transplantations performed in LTA recipients without pre-LT ESRD.
EFFECT OF SLKT POLICY ON RECIPIENT OUTCOMES
Despite an initial concern that the policy change would result in sicker SLKT recipients at time of transplant and poor outcomes, short-term outcomes are similar prepolicy and postpolicy implementation.(10,16,17) Among patients with ESRD, there was no difference in 2-year survival rates between SLKT versus LTA after the policy change (Fig. 4). One prior study estimated lower survival rates in the LTA-ESRD group compared with SLKT,(18) although the study was performed using data from 2007 to 2014, which largely precedes the cohort currently under discussion. In contrast, a recent single-center report of LTA performed from 2006 to 2015 suggests that the majority of LTA patients who would have been eligible for SLKT under the 2017 criteria experienced similar posttransplant graft and patient survival rates to patients with normal renal function.(19) We do not attempt to address the predictors of SLKT versus LTA benefit as previously described,(20) although we highlight this as an important area of future investigation.
LIMITATIONS
There are several important limitations to this study. Prior attempts at recreating SLKT eligibility criteria have used a creatinine cutoff value of 1.5 mg/dL(21) and extrapolated dialysis duration based on time spent on the waiting list.(10) We used conservative selection criteria, although this approach likely results in the inclusion of patients with transient renal dysfunction who were not being considered for SLKT. Although this approach decreases the calculated proportion of patients falling into the safety net, we anticipate its effect to be randomly distributed across our comparison groups and therefore unlikely to bias our findings. We are limited by the follow-up available after the recent policy change, compounded by delays in data reporting to UNOS: true rates of safety net kidney transplants will likely not be evident for another few years because of this intrinsic lag time and therefore may be higher than what we report. Finally, without a reliable method of identifying the cohort of LT candidates who would be eligible for SLKT allocation under the 2017 policy change, it is impossible to accurately model the outcomes of the comparable group who would receive LTA with intent to list for KAL transplantations. Although prior work has attempted a calculation of the benefit of SLKTs versus KAL transplantations for LT candidates with kidney dysfunction, these studies are limited by the statistical approach and the use of historical cohorts.(22,23) A more nuanced understanding of this benefit is essential for further discussion of utility versus beneficence and equity.
In conclusion, we demonstrate that following the 2017 change in SLKT listing criteria, there has been a noticeable standardization in SLKT recipient kidney function, and the rate of SLKT for high-GFR patients has fallen significantly. Although long-term outcomes are not yet available, short -term outcomes for SLKT before and after the policy change are similar, as are outcomes for LTA-ESRD and SLKT after the policy change. There is widespread and effective utilization of the safety net, with concomitantly higher rates of KAL transplantations. The total number of kidneys used for SLKTs and KAL transplantations has plateaued. These preliminary data suggest that LTA and safety net prioritization may be a noninferior alternative to SLKT in the LT candidate with ESRD. Additional work is necessary to verify this hypothesis and in the interest of optimally allocating scarce and quality resources.
Supplementary Material
Acknowledgments
The salary of Mariya L. Samoylova is supported by 5T32CA093245–13. The salary of Samantha E. Halpern is supported by TL1TR002555. The salary of Lisa M. McElroy is supported by 1U54 MD012530 and the Robert Wood Johnson Foundation. The remaining authors have no financial support relevant to this work to disclose.
Abbreviations:
- BMI
body mass index
- CI
confidence interval
- DDLT
deceased donor liver transplantation
- ECD
extended criteria donor
- eGFR
estimated glomerular filtration rate
- ESRD
end-stage renal disease
- GFR
glomerular filtration rate
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- IQR
interquartile range
- LT
liver transplantation
- LTA
liver transplantation alone
- KAL
kidney after liver
- KDPI
Kidney Donor Profile Index
- KDRI-Rao
Kidney Donor Risk Index - Rao
- MDRD
Modification of Diet in Renal Disease
- NASH
nonalcoholic steatohepatitis
- OPTN
Organ Procurement and Transplantation Network
- SLKT
simultaneous liver-kidney transplantation
- STAR
Standard Transplant Analysis and Research
- UNOS
United Network for Organ Sharing
Footnotes
Yuval A. Patel consults for Intercept.
The data reported here have been supplied by the UNOS as the contractor for the OPTN. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the OPTN or the US government.
Additional supporting information may be found in the online version of this article.
REFERENCES
- 1).Ekser B, Contreras AG, Andraus W, Taner T. Current status of combined liver-kidney transplantation. Int J Surg 2020;82S: 149–154. [DOI] [PubMed] [Google Scholar]
- 2).Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic steatohepatitis: a review. JAMA 2020;323:1175–1183. [DOI] [PubMed] [Google Scholar]
- 3).Maiwall R, Gupta M. Peri-transplant renal dysfunction in patients with non-alcoholic steatohepatitis undergoing liver transplantation. Transl Gastroenterol Hepatol 2020;5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Malhi H, Allen AM, Watt KD. Nonalcoholic fatty liver: optimizing pretransplant selection and posttransplant care to maximize survival. Curr Opin Organ Transplant 2016;21:99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Procurement O, Network T. Health resources and services administration, HHS. Final rule Fed Regist 1999;64:56650–56661. [PubMed] [Google Scholar]
- 6).Nadim MK, Davis CL, Sung R, Kellum JA, Genyk YS. Simultaneous liver-kidney transplantation: a survey of US transplant centers. Am J Transplant 2012;12:3119–3127. [DOI] [PubMed] [Google Scholar]
- 7).Miles CD, Westphal S, Liapakis A, Formica R. Simultaneous liver-kidney transplantation: impact on liver transplant patients and the kidney transplant waiting list. Curr Transplant Rep 2018;5:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Formica RN Jr., Simultaneous liver kidney transplantation. Curr Opin Nephrol Hypertens 2016;25:577–582. [DOI] [PubMed] [Google Scholar]
- 9).Simultaneous liver kidney (SLK) allocation policy. OPTN/UNOS Kidney Transplantation Committee; 2016:1–92. https://optn.transplant.hrsa.gov/media/1192/0815-12_slk_allocation.pdf. AccessedJanuary 1, 2021. [Google Scholar]
- 10).Cullaro G, Hirose R, Lai JC. Changes in simultaneous liver-kidney transplant allocation policy may impact postliver transplant outcomes. Transplantation 2019;103:959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Levey AS. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 1999;130:461. [DOI] [PubMed] [Google Scholar]
- 12).Metzger RA, Delmonico FL, Feng S, Port FK, Wynn JJ, Merion RM. Expanded criteria donors for kidney transplantation. Am J Transplant 2003;3(suppl 4):114–125. [DOI] [PubMed] [Google Scholar]
- 13).Reese PP, Veatch RM, Abt PL, Amaral S. Revisiting multiorgan transplantation in the setting of scarcity. Am J Transplant 2014;14:21–26. [DOI] [PubMed] [Google Scholar]
- 14).Chang Y, Gallon L, Shetty K, Chang Y, Jay C, Levitsky J, et al. Simulation modeling of the impact of proposed new simultaneous liver and kidney transplantation policies. Transplantation 2015;99:424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Singal AK, Ong S, Satapathy SK, Kamath PS, Wiesner RH. Simultaneous liver kidney transplantation. Transpl Int 2019;32:343–352. [DOI] [PubMed] [Google Scholar]
- 16).Wadei HM, Gonwa TA, Taner CB. Simultaneous liver kidney transplant (SLK) allocation policy change proposal: is it really a smart move? Am J Transplant 2016;16:2763–2764. [DOI] [PubMed] [Google Scholar]
- 17).Asch WS, Bia MJ. New organ allocation system for combined liver-kidney transplants and the availability of kidneys for transplant to patients with stage 4–5 CKD. Clin J Am Soc Nephrol 2017;12:848–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Nagai S, Safwan M, Collins K, Schilke RE, Rizzari M, Moonka D, et al. Liver alone or simultaneous liver-kidney transplant? Pretransplant chronic kidney disease and post-transplant outcome—a retrospective study. Transpl Int 2018;31:1028–1040. [DOI] [PubMed] [Google Scholar]
- 19).Jiang DD, Roayaioo K, Woodland D, Orloff S, Scott D. Survival and renal function after liver transplantation alone in patients meeting the new United Network for Organ Sharing simultaneous liver-kidney criteria. Clin Transplant 2020;34:e14020. [DOI] [PubMed] [Google Scholar]
- 20).Habib S, Khan K, Hsu C-H, Meister E, Rana A, Boyer T. Differential simultaneous liver and kidney transplant benefit based on severity of liver damage at the time of transplantation. Gastroenterol Res 2017;10:106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Iglesias J, Frank E, Mehandru S, Davis JM, Levine JS. Predictors of renal recovery in patients with pre-orthotopic liver transplant (OLT) renal dysfunction. BMC Nephrol 2013;14:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Cheng XS, Goldhaber-Fiebert J, Tan JC, Chertow GM, Kim WR, Wall AE. Defining a willingness-to-transplant threshold in an era of organ scarcity. Transplantation 2020;104:387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Cheng XS, Stedman MR, Chertow GM, Kim WR, Tan JC. Utility in treating kidney failure in end-stage liver disease with simultaneous liver-kidney. Transplantation 2017;101: 1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


