Abstract
Purpose:
To investigate the natural history of dark adaptation (DA) function as measured by the change in rod intercept time (RIT) over 4 years and to correlate RIT change with age-related macular degeneration (AMD) severity.
Design:
Longitudinal, single-center, observational study.
Participants:
A total of 77 participants aged ≥50 years with a range of AMD severities.
Methods:
Participants each contributing a single study eye to the analysis were assigned into person-based AMD severity groups based on fundus characteristics (drusen, pigmentary changes, late AMD, and subretinal drusenoid deposits [SDDs]). The DA function was assessed in study eyes at baseline and 3,6, 12, 18, 24, 36, and 48 months. Mean change in DA function over time was calculated using the slope of linear regression fits of longitudinal RIT data. Patient-reported responses on a Low Luminance Questionnaire (LLQ) were obtained at baseline and yearly. Nonparametric statistical testing was performed on all comparisons.
Main Outcome Measure:
The RIT, defined as the time taken after a photobleach for visual sensitivity to recover detection of a 5×10−3 cd/m2 stimulus (a decrease of 3 log units), was monitored in study eyes over 4 years, and the mean rate of change was computed.
Results:
Longitudinal analysis of 65 study eyes followed on the standard testing protocol (mean age, 71±9.3 years; 49% were female) revealed that higher rates of RIT prolongation were correlated with AMD severity group assignment at baseline (P = 0.026) and with severity group assignments at year 4 (P = 0.0011). Study eyes that developed SDD during follow-up demonstrated higher rates of RIT prolongation relative to those that did not (P < 0.0001). Overall, higher rates of RIT prolongation were significantly correlated with greater 4-year decreases in LLQ scores (total mean score, P = 0.0032).
Conclusions:
Longitudinal decline in DA function, which correlated with patient-reported functional deficits, was accelerated in eyes with greater AMD severity and especially in eyes with SDD both at baseline and at 4 years. The RIT prolongation as a measure of changing DA function may be a functional outcome measure in AMD clinical studies.
Age-related macular degeneration (AMD) is a major cause of blindness in the elderly worldwide, projected to affect as many as 196 million people by 2020 and 288 million people by 2040.1 With this global burden, there is great impetus for investigating new therapies. However, because of the slowly progressive nature of the disease, the currently used outcomes of vision acuity loss and progression to late AMD require veiy long and large clinical trials. End point development reflecting disease progression over earlier stages could potentially increase the feasibility of clinical trials and allow for the identification of eyes with more severe cell dysfunction within early and intermediate categories of AMD.
Visual acuity has long been used as the primary end point for clinical trials in AMD.2 However, it has limitations as a functional outcome because it is generally stable in the earlier stages of the disease.3 Additionally, in some cases of late AMD with noncentral geographic atrophy, visual acuity can be spared. Night vision, however, is often affected earlier in the disease process.4–6 As such, rod-mediated dark adaptation (DA) has the potential to be a functional biomarker for AMD progression.3,7–11
We, and other investigators, have shown in cross-sectional analyses that impaired DA is associated with increasing AMD severity and presence of reticular pseudodrusen (RPD),11 now more generally referred to as “subrclinal drusenoid deposits” (SDD).3,8,9,12–19 This result implies that DA functioning worsens over time in eyes with AMD. In addition, a recent report on eyes with intermediate AMD finds that on average, DA functioning worsens over a 2-year follow-up.20 The purpose of this study is to report on 4-year longitudinal DA data in participants with a range of AMD severities and investigate whether changes in DA correlate with AMD disease severity. Information about the change of DA parameters over time will be useful in understanding disease pathogenesis and necessary to gauge its plausibility as a functional outcome measure for monitoring AMD progression.
Methods
Study Population
Participants included adults aged 50 years and older both with and without AMD recruited from the eye clinic at the National Eye Institute, National Institutes of Health. Bethesda, Maryland, between May 2011 and January 2014. Patients were excluded for (1) late AMD (defined as central geographic atrophy [GA] or choroidal neovascularization [CNV]) in both eyes at baseline visit; (2) any other active ocular or macular disease that may confound the progression of AMD (i.e., glaucoma, diabetic retinopathy, Stargardt disease); (3) a condition preventing compliance with the study assessment; (4) cataract surgery within 3 months before enrollment; (5) history of vitamin A deficiency; (6) high oral intake of vitamin A palmitate supplement (≥ 10 000 international units per day); and (7) active liver disease or history of liver disease. Study eyes were required to have a best-correctcd visual acuity (BCVA) of 20/100 or better.
The study was approved by the Institutional Review Board of the National Institutes of Health, and the tenets of the Declaration of Helsinki were followed. The study is registered on clinicaltrials.gov (identifier NCT01352975). All participants provided informed consent after the nature and possible consequences of the study were explained.
Examination and Imaging
All participants underwent a complete ophthalmoscopic examination, including measurement of BCVA with the Early Treatment Diabetic Retinopathy Study chart, measurement of intraocular pressure, slit-lamp examination, and dilated fundus examination. Presence of AMD features (drusen, pigmentary change, pigment epithelial detachment, CNV, central geographic atrophy) and other ocular findings (e.g., phakic status) were documented. Color fundus photographs (50 degrees) and fundus autofluorescencc (FAF) (50 degrees, excitation wavelength 575 nm) images were acquired with the TRC-50DX retinal camera (Topcon Medical Systems, Tokyo, Japan). Infrared reflectance (IR), FAF images (30 degrees, excitation wavelength 488 nm), and spectral-domain (SD) OCT scans were acquired with the Heidelberg Spcctralis (Heidelberg Engineering, Heidelberg, Germany). Each set of SD OCT scans consisted of 37 B-scans, each of which comprised 24 averaged scans, obtained within a 30° × 15° rectangle centered on the fovea with the Automated Real Time Averaging mode set to 25 images averaged and a minimum scan quality measure of 20 decibels or greater with most scans ranging from 30 to 40 decibels. In addition, enhanced depth imaging OCT scans were acquired for improved visualization of the choroid in a single horizontal scan centered at the fovea obtained over a distance of 30° consisting of 100 averaged scans.
Assessment of Subretinal Drusenoid Deposits
To identify study eyes with SDD, masked grading of color photographs, FAF, and IR images obtained at baseline and year 4 visits was performed. Originally, SDDs, which we had previously referred to as RPD lesions,11 were described on color fundus photographs as “round, oval, or slightly elongated and lobulatcd yellowish spots with ill-defined edges, 125 to 250 μm...in size” and an “interlacing network with intervening spaces of background color of 125 μm” that had enhanced visibility in blue light.21 Other studies have reported improved sensitivity and specificity with IR and FAF imaging, especially for detecting SDD present in the perifovea.22 Subretinal drusenoid deposits on IR imaging have been defined as hyporeflectant lesions against a background of mild hyperreflectance,23 and on FAF imaging, they are defined as hypofluorescent lesions against a background of mildly elevated autofluorcscence.24,25
Subretinal drusenoid deposits were recorded as being present if ≥2 of 3 graders (C.A.C., T.K., K.G.C.) identified a >1 disc area of a typical appearance of SDD on en face imaging21–25 and if those areas on SD OCT revealed hyperreflective material between the retinal pigment epithelium (RPE) and the photoreceptor ellipsoid zone.26
Study Eye Categorization
Participants were separated into groups based on their fundus grading at baseline, which was reassessed at year 2 and year 4. After identifying eyes with SDD (which we previously described and called RPD11) and placing them in a separate group, the remaining eyes were grouped according to increasing order of AMD severity. We based our severity groupings on the presence of large drusen (≥125 μm in diameter), late AMD, or both. The control group, Group 0, consisted of participants without any large drusen or late AMD (CNV or central geographic atrophy) in either eye. Group 1 consisted of participants with large drusen in 1 eye only and no late AMD in either eye. Group 2 included participants with large drusen in both eyes without any late AMD. Group 3 included participants with large drusen in 1 eye and late AMD in the other eye (central geographic atrophy or CNV).
Age-Related Eye Disease Study Severity Scale Grading
In addition to the severity groups described, we also use the 9-step Age-Related Eye Disease Study (AREDS) Severity Scale27 to grade AMD severity. Color fundus images of study eyes obtained at baseline, 2 years, and 4 years were presented at random to graders masked to participant identification/information, and a consensus AREDS 9-step severity scale step grade (defined as the grade demonstrating agreement between ≥2 of 4 graders [C.A.C., J.A.A., K.G.C., C.P.]) was obtained for each study eye image. We used the AMD severity grades at baseline, 4 years, and the change in AMD severity grade over 4 years in our analyses with changes in DA testing.
Dark Adaptation Testing
Dark adaptation was measured using a prototype of the AdaptDx dark adaptometer (MacuLogix, Hummelstown, PA). Each participant had only 1 study eye assigned to undergo DA testing. In participants without any large drusen, either eye could be designated the study eye. In participants with large drusen in 1 eye only, the eye with large drusen was the study eye. In participants with large drusen bilaterally, either eye was designated as the study eye. In participants with late AMD in 1 eye, the nonadvanccd eye was the study eye. Details about the testing procedure have been documented by Jackson and Edwards.12 In brief, after dilation of the study eye and while the participant was asked to focus on a fixation light, a photoflash producing an 82% focal bleach centered at 5° on the inferior visual meridian was performed (termed “standard protocol”). Threshold measurements were made at the same location with a 1.7° diameter, 500-nm wavelength circular test spot using a 3-down/l-up modified staircase threshold estimate procedure. The initial stimulus intensity was 5 cd/m2. Threshold measurements were continued until the patient’s visual sensitivity recovered to be able to detect a dimmer stimulus intensity of 5 × 10−3 cd/m2 (a decrease of 3 log units) or until a maximum test duration of 40 minutes was reached, whichever occurred first. The time to this event was defined as the rod intercept time (RIT). A measurement used in previous DA studies,8 the RIT corresponds to the time to reach a threshold within the second component of rod-mediated DA and is estimated by linear interpolation of the sensitivity responses. Tests that did not reach this threshold by 40 minutes were reported as “no rod intercept” by the machine and were conservatively defined to have an RIT of 40 minutes. Participants who reached this test ceiling were transitioned to a modified DA protocol at subsequent study visits and followed over time with reduced flash intensity equivalent to 76% focal bleach centered on a more eccentric location of 12° on the inferior visual meridian (termed “modified protocol”).8,28 The modified protocol used the same methodology of staircase threshold estimates continuing until the same target threshold was reached.
Low Luminance Questionnaire
The Low Luminance Questionnaire (LLQ) is a 32-item questionnaire designed to assess the degree of difficulty experienced by participants at night and in other low light environments.29 As previously described, each item is scored on a scale of 0 to 100, with 0 representing the greatest difficulty and 100 representing the least. Items are assigned to 1 of 6 subscalcs: dim lighting, driving, emotional distress, extreme lighting, mobility, and peripheral vision. Item scores are averaged to give 1 score per subscalc. Each subscale is then weighted by number of items and averaged to yield a total mean LLQ score.30 The questionnaire was administered before DA testing by a staff member masked to participants’ DA testing performance.
Follow-up Testing
Patients were followed for a 4-year study period with study visits occurring at baseline and 3, 6, 12, 18, 24, 36, and 48 months. A ±3-week window was permitted for each visit. The DA was measured in the study eye at every visit. Medical and ophthalmic history including concomitant medication information and ocular adverse events were also collected at every visit. Visual acuity measurements were conducted for both eyes at every visit with manifest refractions for BCVA done every 12 months and during visits in which a visual acuity change in excess of 10 Early Treatment Diabetic Retinopathy Study letters was observed. A complete ophthalmic examination including slit-lamp, dilated fundus examination, and intraocular pressure measurement was performed at every visit. Administration of the LLQ was conducted yearly. Color fundus photographs, FAF, IR images, and SD OCT scans were also obtained yearly.
Participants
Of 111 participants who enrolled in the study over 4 years ago, 77 participants had data to contribute to the 4-year analyses. Of the 34 participants who did not have 4-year data, 11 progressed to advanced disease in the study eye (central geographic atrophy or CNV), 2 were lost to follow-up, 3 missed the 4-year visit, 3 died, 4 withdrew from the study, 3 were unable to perform the testing, and 8 had cataract extraction and intraocular lens (CE/IOL) placement in their study eye (Fig S1, available at www.aaojournal.org). We excluded the 8 participants who underwent CE/IOL placement in their study eye because we noted large changes in RIT across the study visits just preceding and after cataract extraction that exceeded the RIT change rates measured for the time intervals before or after the surgical intervention. Although lens effects on DA measurements arc thought to be small, our data suggested that lens removal may have confounded the RIT measurements, prompting us to exclude them in the analyses. Changes in RIT spanning the CE/IOL surgery in study eyes are listed in Table S1 (available at www.aaojournal.org).
Of the 77 participants analyzed, 65 had testing on the standard 5° DA testing protocol (82% focal bleach, test spot at 5°) throughout the 4 years of follow-up (Baseline Group 0 [n = 341, Group 1 [n = 9], Group 2 [n = 161, Group 3 [n = 5], Group SDD [n = 1]). These participants constituted the primary analysis performed. Twelve other participants reached the test ceiling at baseline on the standard testing protocol and were followed longitudinally on the modified reduced bleach 12° protocol (Baseline Group 0 [n = 0], Group 1 [n = 1], Group 2 [n = 1], Group 3 [n = 3], Group SDD [n = 7]). These participants were analyzed separately in a secondary analysis.
Longitudinal Rod Intercept Time Analyses
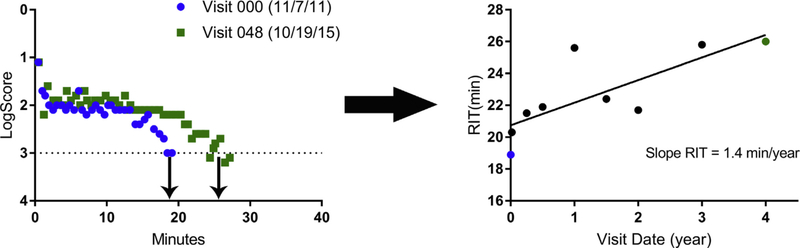
Our primary objective was to report on the change in measures of DA over time. We computed the average rate of change of RIT (termed “slope RIT”) for each study eye by performing linear regressions on all available time points (baseline, 1 week, and 3, 6, 12, 18, 24, 36, and 48 months) obtained using the same AdaptDx testing protocol (i.e., standard or modified) (Fig 1). Only eyes that completed the 4-year visit and had RIT measurements from at least 5 study visits on a single AdaptDx testing protocol were included in the analyses.
Figure 1.
Representative dark adaptation (DA) raw data for an individual participant at baseline and 4 years (left). All rod intercept time (RIT) values measured at study visits over 4 years are plotted, and the slope of the linear regression performed defines “slope RIT” (right). This measure (slope RIT) is used to represent the rate of change in RIT of a given study eye.
Statistical Analysis
All data were analyzed using nonparamctric statistics computed with GraphPad Prism 7.0 for Windows (GraphPad Software, La Jolla, CA). Continuous variables were analyzed with the Mann–Whitney test and Kruskal–Wallis test for 2 variables and more than 2 variables, respectively. For all tests, P < 0.05 was considered statistically significant.
Results
Participant Demographics
A total of 65 participants (age range, 50–91 years) followed on the standard testing protocol were included in the primary analyses. This study population was predominantly (91%) white, and 49% of the participants were female. There were no statistically significant differences between the AMD severity groups in terms of age or gender (Table 1). Mean age of these participants was 71 ±9.2 years.
Table 1.
Baseline Demographics of 65 Participants with 4-Year Follow-Up on the Standard Testing Protocol (82% Focal Bleach, Test Spot at 5°)
| Characteristic | Group 0 | Group 1 | Group 2 | Group 3 | Group SDD | P Value* |
|---|---|---|---|---|---|---|
|
| ||||||
| No. | 34 | 9 | 16 | 5 | 1 | |
| Mean age (SD), yrs | 72 (8.3) | 69 (11) | 67 (10) | 77 (5.4) | 84 (0) | 0.20 |
| Female gender, no. (%) | 19 (29) | 4 (6.2) | 6 (9.2) | 3 (4.6) | 0 (0) | 0.63 |
| Race, no. (%) | ||||||
| American Indian/Alaskan Native | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Asian | 2 (5.9) | 0 (0) | 1 (6.3) | 0 (0) | 0 (0) | |
| Black | 3 (8.8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| White | 29 (85.3) | 9 (100) | 15 (93.8) | 5 (100) | 1 (100) | |
All P values shown are from Kruskal–Wallis analyses of variances.
SD = standard deviation; SDD = subretinal drusenoid deposits.
Group SDD not included in statistical analyses because n=1.
Baseline ocular characteristics (e.g., BCVA and pseudophakic status) were also recorded by AMD severity group (Table 2). No statistically significant difference was observed for BCVA (P = 0.054), phakic status (P = 0.18), subfoveal choroidal thickness (P = 0.70), or contrast sensitivity (P = 0.87).
Table 2.
Baseline Ocular Characteristics of the 65 Study Eyes Followed on the Standard Testing Protocol (82% Focal Bleach, Test Spot at 5°)
| Characteristic | Group 0 | Group 1 | Group 2 | Group 3 | Group SDD | P Value* |
|---|---|---|---|---|---|---|
|
| ||||||
| No. | 34 | 9 | 16 | 5 | 1 | |
| Mean BCVA (SD), no. of letters | 86 (4.3) | 87 (3.2) | 82 (7.9) | 80 (7.4) | 75 (0) | 0.054 |
| Phakic status, no. (%) pseudophakic | 12 (18) | 2 (3.1) | 2 (3.1) | 0 (0) | 0 (0) | 0.18 |
| Mean SFCT (SD), μm | 221 (121)† | 248 (77) | 235 (78) | 233 (126) | 116 (0) | 0.70 |
| Mean contrast sensitivity (SD), log score | 1.6 (0.17) | 1.6 (0.079) | 1.6 (0.20) | 1.5 (0.15) | 1.4 (0) | 0.87 |
All P values shown are from Kmskal–Wallis analyses of variance.
BCVA = best-corrected visual acuity; SD = standard deviation; SDD = subretinal drusenoid deposit; SFCT = subfoveal choroidal thickness.
Group SDD not included in statistical analyses because n=1.
n=33 because 1 patient did not have baseline SFCT measurement.
Change in Visual Activity over 4 Years
We examined the change in BCVA over the 4 years of follow-up and observed small but statistically significant changes across all eyes with a mean of 1.8 letters lost (95% confidence interval, −3 to −0.57). Analysis of vision change across AMD groups revealed that the change in acuity was mostly in Group 0 and not significantly different than 0 in the other AMD groups (mean ΔBCVA: Group 0, −2.9±4.6 letters; Group 1, −2.2±4.0 letters; Group 2, −0.063±5.7 letters; Group 3, +0.2±5.9 letters; P = 0.087).
Change in Dark Adaptation over 4 Years
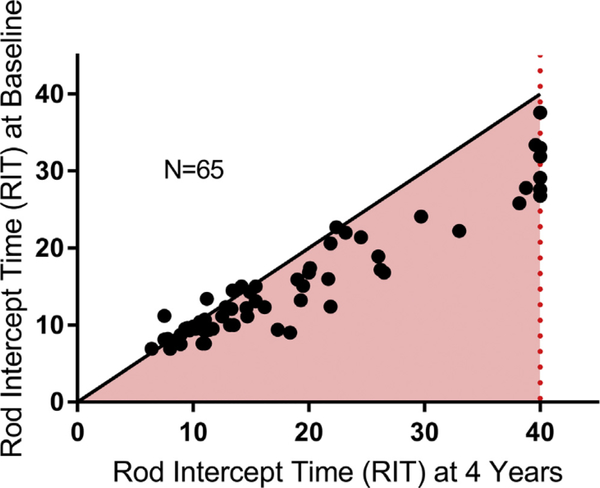
We compared RIT measurements on the standard testing protocol for individual study eyes at baseline and at 4 years in Figure 2. Eyes with baseline RITs of <15 minutes tended to have relatively similar RITs at 4 years, whereas eyes with longer baseline RITs had a higher prevalence of prolonged RITs at 4 years. Figure 2 shows that eyes with shorter RITs fall close to the line, whereas eyes with longer RITs at baseline have points that depart from the diagonal and lie below the diagonal line. These results indicate that DA function in study eyes tended to be stable or otherwise decrease over 4 years.
Figure 2.
Scatterplot of study eyes followed on the standard 5° dark adaptation (DA) protocol. The dashed line represents the test ceiling of rod intercept time (RIT) = 40 minutes. Points in the pink shaded region represent study eyes that have worsening of DA function at 4 years compared with baseline. Points with greater baseline RIT are farther from the diagonal demonstrating greater prolongation compared with eyes with lower baseline RITs.
Slope Rod Intercept Time and Age-Related Macular Degeneration Severity
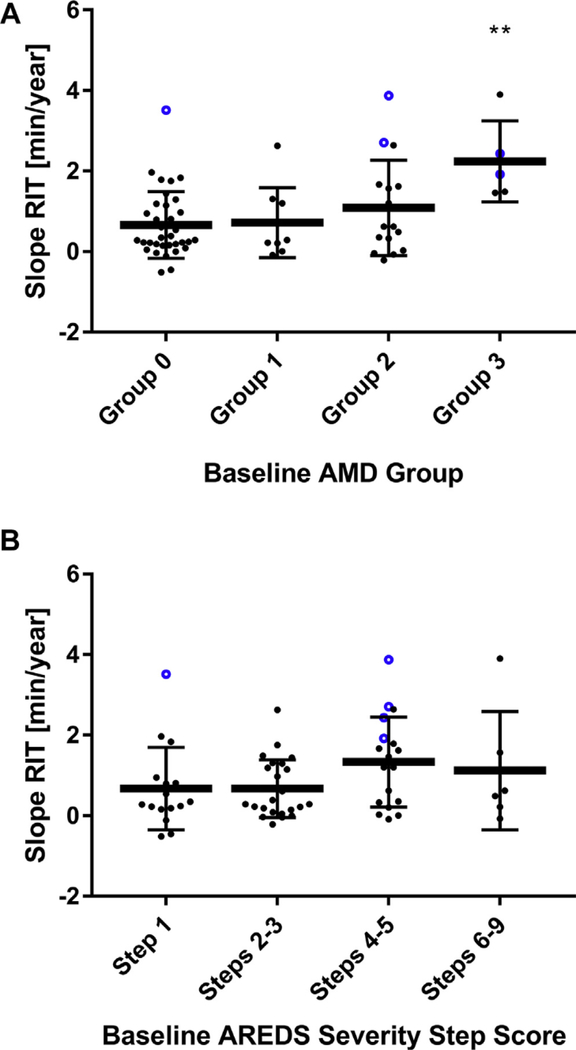
To examine the rate of change in DA over the 4 years, we computed the “slope RIT” (Fig 1) on the standard testing protocol for each study eye. Mean slope RIT for eyes across all AMD severity groups (n = 65) was +0.92 minutcs/year (95% confidence interval, 0.67–1.17), demonstrating a general prolongation of RIT over time (P < 0.0001). Slope RIT was not correlated with the change in BCVA in study eyes (P = 0.98). Slope RIT, however, increased with greater AMD severity; mean RITs for eyes in each severity group increased progressively with group stage as ascertained at baseline (Group 0, 0.67±0.83 minutes/year; Group 1, 0.72+0.87 minutcs/year; Group 2, 1.1 ± 1.2 minutes/year; Group 3, 2.2±1.0 minutes/year (Fig 3A), with that for Group 3 being statistically greater than that for Group 0 (P = 0.026).
Figure 3.
A, Scatterplot showing mean slope rod intercept time (RIT) ± standard deviation (SD) measured on the standard dark adaptation (DA) protocol by baseline age-related macular degeneration (AMD) groups. Blue points highlighted represent eyes that transition to subretinal drusenoid deposit (SDD) at 4 years. Group SDD not shown because n = 1. **P < 0.05 for comparisons with Group 0. B, Scatterplot showing mean slope RIT ± SD measured on the standard DA protocol by baseline 9-scale Age-Related Eye Disease Study (AREDS) severity step scores. Blue points highlighted represent eyes that transition to SDD at 4 years. Group SDD not shown because n = 1.
Slope RIT and baseline AMD severity were also correlated when study eyes were graded using an alternative 9-step AREDS severity scale27 for which the 9 individual step grades were grouped based on the risk of progression to late AMD27 (Step 1 [no AMD], 0.68±1.0 minutes/year; Steps 2–3, 0.67±0.72 minutes/year; Steps 4–5, 1.3±1.1 minutes/year; Steps 6–9, 1.1 ±1.5 minutes/year) (Fig 3B). Mean slope RIT for eyes in steps 4–9 was significantly greater than that for eyes in steps 1–3) (P = 0.038). Six of the 7 eyes with SDD at baseline reached the test ceiling on the standard protocol and were followed on the modified testing protocol.
Assessment of Change in Dark Adaptation Using 4-Year Grading
Wc also investigated how the rate of change in DA over the 4 years as measured by slope RIT correlated with the AMD severity of study eyes at the 4-year time point. Fundus imaging taken at 4 years was used to recategorize study eyes into 4-year AMD severity groups.
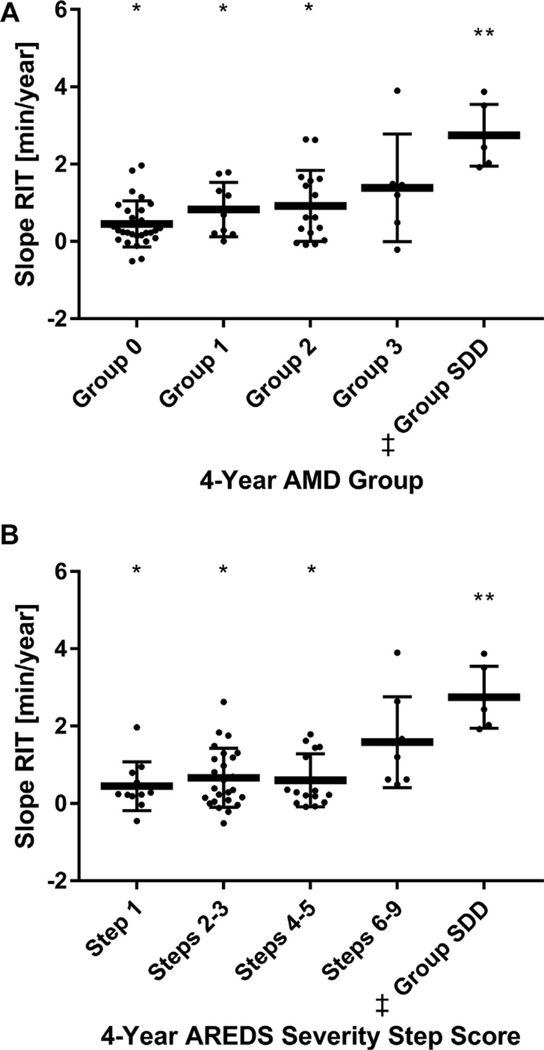
Of 65 study eyes, the grading of 4-year images identified 16 eyes that met the criteria for SDD; 6 were followed on the standard protocol and 10 reached the test ceiling on the standard protocol, and so were followed on the modified testing protocol. Slope RIT was also found to increase with AMD severity using grading of 4-year fundus images (Group 0, 0.45±0.59 minutes/year; Group 1, 0.83±0.70 minutes/year; Group 2,0.92±0.92 minutes/year; Group 3, 1.4±1.4 minutes/year). In addition, eyes in the SDD category at 4 years demonstrated the greatest mean slope RIT (2.8±0.89 minutes/year), which was significantly greater than that for Group 0 eyes (P = 0.0011) (Fig 4A). Study eyes were also recategorized using 4-year images and the 9-step AREDS severity scale, and similar associations between slope RIT and AMD severity were observed (Step 1, 0.45±0.63 minutcs/year; Steps 2–3, 0.66±0.76 minutes/year; Steps 4–5, 0.60+0.68 minutes/year; Steps 6–9, 1.6±1.2 minutes/year; Group SDD, 2.8±0.80 minutes/year; P = 0.0003) (Fig 4B).
Figure 4.
A, Scatterplot showing mean slope rod intercept time (RIT) ± SD measured on the standard dark adaptation (DA) protocol by 4-year age-related macular degeneration (AMD) groups. ‡One Patient included in group suhretinal drusenoid deposit (SDD) at 4 years had SDD at baseline hut was not shown in Figure 3A. *P < 0.05 for comparisons with group SDD. **P < 0.05 for comparisons with Group 0. B, Scatterplot showing mean slope RIT ± SD measured on the standard DA protocol by 4-year groupings using the 9-scale AREDS severity step score. ‡One patient included in group SDD at 4 years had SDD at baseline but was not shown in Figure 3B. *P < 0.05 for comparisons with group SDD. **P < 0.05 for comparisons with Step 1.
Assessment of Fundus Change on the Age-Related Eye Disease Study Severity Scale
We also investigated whether eyes that progressed in AMD severity during the study as defined by longitudinal fundus grading on the 9-step AREDS scale exhibited greater rates of DA decline. We compared AMD severity grades in study eyes at baseline and 4 years and categorized eyes into separate groups: eyes that remained unchanged or decreased in step score (n = 37), eyes that increased by only 1 step (n = 15), eyes that increased by ≥2 steps (n = 7), and eyes that developed new-onset SDD (n = 5). Table 3 shows the mean slope RITs for each of these groups. Eyes demonstrating ≥2 AREDS step scale change had higher mean slope RITs (0.85 min/year) compared with those demonstrating an unchanged or decreased step score (0.60 min/year), but the difference was not statistically significant (P = 0.17), although the number of eyes with ≥2 AREDS step scale change is small (n = 7). Eyes developing new-onset SDD (4 eyes with baseline AREDS severity step scale 4 and 1 eye with baseline AREDS severity step scale 1) had the highest mean slope RIT (2.9 min/year), which was significantly greater when compared with the other groups (analysis of variance, P = 0.0027) (Table 3). Because eyes that develop new-onset SDD had different baseline AMD severity grades, we investigated whether these eyes that developed SDD (highlighted in blue in Fig 3) had different mean slope RITs from eyes with similar baseline fundus grading but did not develop SDD. We found that eyes that developed SDD demonstrated a significantly greater mean slope RIT than eyes that had a comparable AMD severity but that did not develop SDD (P < 0.0001, Tables 3 and 4). Eyes that developed SDD also demonstrated greater baseline RITs compared with eyes with comparable AMD severity but that did not develop SDD (P = 0.0001) (Table 4).
Table 3.
Mann–Whitney Unpaired Tests and Kruskal–Wallis Analysis of Variance for All Nonreticular Pseudodrusen Participants with AREDS Step Grading at Baseline on Standard 5° Testing Protocol (82% Focal Bleach, Test Spot at 5°) Comparing Participants Who Had No Change, Decreased, or Increased AREDS Step at 4 Years
| Decrease or No Change in AREDS Step | Increase in AREDS Step (1 Step) | Increase in AREDS Step (≥2 Steps) | Transitioned to SDD | |
|---|---|---|---|---|
|
| ||||
| No. | 37 | 15 | 7 | 5 |
| Median slope† (IQR), min/yr | 0.28 (0.07–1.2) | 0.69 (0.16–1.8) | 0.62 (0.29–1.4) | 2.7 (2.2–3.7) |
| Mean slope† (SD), min/yr | 0.60 (0.71) | 1.0 (1.2) | 0.85 (0.55) | 2.9 (0.80) |
| Mann–Whitney P value‡ | 0.24 | 0.17 | <0.0001* | |
| <0.0001§ | ||||
| Kruskal–Wallis analysis of variance P value | 0.27∥ | 0.0027*,¶ | ||
AREDS = Age-Related Eye Disease Study; IQR = interquartile range; RIT = rod intercept time; SD = standard deviation; SDD = subretinal drusenoid deposits.
Statistically significant at P value < 0.05.
Slope RIT = linear regression of RIT values (minimum 5 data points).
Compared with control group defined as eyes with a decrease or no change in AREDS step.
Eyes that do not transition to SDD (decrease or no change in AREDS step + increase in AREDS step [1 step] + increase in AREDS step [≥2 steps]) compared with eyes with transition to SDD.
Comparing decrease or no change in AREDS step versus increase in AREDS step (1 step) versus increase in AREDS step (≥ 2 steps).
Comparing decrease or no change in AREDS step versus increase in AREDS step (1 step) versus increase in AREDS step (≥ 2 steps) versus transition to SDD.
Table 4.
Mean Group Baseline Rod Intercept Times and Slope Rod Intercept Times on the Standard 5° Dark Adaptation Protocol for Patients Who Develop Subretinal Drusenoid Deposit by 4 Years
| Baseline AMD Group | No. of Eyes that Developed SDD by 4 Years | N (Never RPD) | Mean Baseline RIT (New SDD) | Mean Baseline RIT (Never SDD) | Slope RIT (New SDD) | Slope RIT (Never SDD) |
|---|---|---|---|---|---|---|
|
| ||||||
| Group 0 | 1 | 33 | 27.60 | 11.92 | 3.52 | 0.58 |
| Group 1 | 0 | 9 | - | 14.58 | - | 0.72 |
| Group 2 | 2 | 14 | 30.60 | 16.78 | 3.30 | 0.77 |
| Group 3 | 2 | 3 | 27.60 | 20.9 | 2.18 | 2.28 |
| Total New | 5 | 59 | 28.80 | 13.94 | 2.89 | 0.75 |
| P = 0.0001 | P < 0.0001 | |||||
AMD = age-related macular degeneration; RIT = rod intercept time; RPD = reticular pseudodrusen; SDD = subretinal drusenoid deposit.
Assessment of Low Luminance Questionnaire Scores and Dark Adaptation
Our previous analysis demonstrated that across participants, those with worse DA measures (longer RIT) reported lower scores on an LLQ.31 In the setting of our 4-year longitudinal study, we investigated the within-person association of decrements in DA testing (measured as slope RIT) and changes in patient-reported outcomes over the 4 years (measured as change in LLQ scores). Considering all 65 participants in the primary analysis as a group, we found that the changes in the overall weighted LLQ score, as well as changes in separate LLQ scores in 4 of 6 subscalcs (driving, dint lighting, mobility, and peripheral vision) were negatively and significantly correlated with slope RIT (Table 5).
Table 5.
Univariate Linear Regression P Values between Change in Low Luminance Questionnaire Score Categories (4-Year Baseline) and Slope Rod Intercept Time on Standard 5° Dark Adaptation Protocol
| LLQ Score Category | P Value |
|---|---|
|
| |
| Dim Lighting | 0.016* |
| Driving | 0.0019* |
| Emotional Distress | 0.11 |
| Extreme Lighting | 0.14 |
| Mobility | 0.0062* |
| Peripheral Vision | 0.032* |
| Total mean LLQ score | 0.0032* |
LLQ = Low Luminance Questionnaire.
Statistically significant at P value < 0.05.
Examining Eyes with Subretinal Drusenoid Deposits that Reached the Test Ceiling at Baseline
Of the 77 participants in the study, 12 had reached the test ceiling on the standard DA testing protocol at baseline. Because histologic studies have shown the earliest and maximum rod involvement occurring at 1.5 to 10 degrees from the fovea in AMD,28 in cases of severe dysfunction we tested points more eccentric to the fovea to sample an area of retina with less severe rod involvement. Eyes that reached the ceiling on the standard test were followed longitudinally on a modified testing protocol that uses a less intense bleach and tests at a point farther from the fovea (12°) to obtain a quantifiable RIT within the 40-minute duration test limit. Testing with the modified protocol also demonstrated progression of RIT with time and as RITs obtained on this protocol cannot be directly compared with those obtained on the standard protocol, these values arc denoted with the qualifier “modified.” Of these 12 study eyes, 7 had SDD present at baseline and demonstrated a mean modified slope RIT of 2.83 minutes/year, and 3 study eyes developed SDD by 4 years and had a mean modified slope RIT of 1.4 minutes/year. Overall, the high slope RIT observed in eyes with SDD on the modified protocol is consistent with the results of eyes followed on the standard testing protocol and indicate that eyes with severe deficits in DA (i.e., that reach the test ceiling on the standard protocol) continue to decline in function with time. Additionally, although the number of eyes analyzed was small, it is interesting to note that the eyes with SDD identified at baseline have a higher mean modified slope RIT than eyes that do not have SDD at baseline but develop them over the course of the study.
Discussion
Our longitudinal data demonstrate that DA, as measured by RIT, exhibits significant change over 4 years among AMD eyes and SDD eyes despite stable visual acuity. Using the rate of change in DA as measured by RIT over at least 5 time points during a 4-year follow-up period among a study population with a wide spectrum of AMD severity, we demonstrate that the measure of DA kinetics over time provides an outcome variable that captures physiologic changes that occur in the context of AMD disease. Furthermore, we demonstrate that eyes with a higher grade of AMD severity have a faster rale of decline in DA measures.
We excluded 8 study eyes from our analysis because they underwent cataract extraction and interocular lens placement (CE/IOL) during their study participation. We were unable to exclude the effects of lens removal on the study outcome because we observed large intervals of change in RIT over short time spans in study visits spanning CE/IOL (Table S1, available at www.aaojournal.org). The impact of lens replacement on RIT might have 2 or more different effects on DA. If the cataractous lens reduced the intensity of the 500 nm wavelength stimulus test spot, then we would expect replacement with the IOL to increase sensitivity of detection of the test spot; therefore, with all else being constant, the RIT would be expected to decrease after CE/IOL. If, however, the cataractous lens reduced the amount of bleaching light that reached the retina, we would expect that after CE/IOL, more bleaching light would reach the retina leading to an effectively greater bleach and therefore longer time for recovery, that is, longer RIT. Examination of the lime points immediately after the CE/IOL demonstrated that eyes had a significant prolongation in RIT in all cases, supporting the hypothesis that cataract replacement caused an effectively greater bleaching of the retina. Because cataract extraction appears to confound the assessment of disease progression on RIT, we excluded these 8 study eyes in our analyses.
Across this study, we have observed that eyes with SDD demonstrate impaired DA at baseline, demonstrated by longer RIT.11 and a greater worsening of this impairment over time, demonstrated by high slope RITs, compared with eyes with AMD or age-controlled eyes. Although SDD has been identified as a risk factor for progression to late AMD, especially GA,32–41 our data suggest that SDD is a biomarker that identifies eyes that not only have deficits in DA but also have high rates of functional decline in DA. Because the definition of SDD is based on imaging changes that can be missed, either because the assessment is difficult or the changes are transient, it is also possible that eyes that “developed” SDD during the study or even eyes classified as not having SDD would have been defined to have SDD if there was a more sensitive way to assess it.
Several pieces of data give biologic plausibility to SDD having a role in affecting DA. Eyes with SDD have coexisting pathologies in the photoreceptors, subretinal space, RPE cells, and choroid. Anatomically, eyes with SDD have a predominance of the deposits in the perifovea, a region of the retina where there is a high density of rods.17 Histopathologic study of eyes with these lesions confirms their localization to the subretinal space and notes altered photoreceptor morphology in the photoreceptors abutting the SDD, including shortened outer segments.17 In vivo imaging studies, using OCT, demonstrate a thinner outer nuclear layer thickness accompanying regions with SDD, indicating that SDD coexists with areas of altered photoreceptor integrity.18 Furthermore, in vivo studies of eyes with SDD, using adaptive optics, confirm the subretinal localization of the deposits and demonstrate an altered photoreceptor reflectivity in the involved areas.42 Studies of the retinoid cycle43 demonstrate that key steps of retinoid processing involve soluble proteins, enzymes, and other extracellular matrix components that are found in the inter-photoreceptor matrix. Relevant to DA, the transport of Vitamin A to the RPE occurs with transport from the choriocapillaris across the Bruch’s-RPE complex where RPE converts the retinoid to the active 11-cis-retinaldehyde.10,44 It is then through the inter-photoreceptor matrix that cis retinal is transported from the RPE cell to the photoreceptor outer segment where it enters into the visual cycle and through which trans retinol returns from the outer segments of the photoreceptor back to the RPE.43 Pathologic structures in the RPE-Bruch’s complex portion and in the subretinal space, such as SDD, could interfere with these dynamics and affect DA. Even beyond the abnormalities in the morphology of the photoreceptors and alterations to the subretinal space, eyes with SDD also demonstrate abnormalities to the RPE cells, with RPE degeneration observed beneath certain SDD lesions.45 These alterations in the RPE could have direct effects on the visual cycle and on the integrity of the photoreceptors. Finally, the observations that subfoveal choroidal thickness is significantly reduced in eyes with SDD also may play a role in rod function.11,46,47 Further studies are needed to determine the exact mechanisms and order of events that contribute to this DA impairment, but studying longitudinal changes of SDDs over time may better elucidate the association between SDD and DA.
An important question remains as to the utility of DA as an outcome variable in future clinical trials of AMD. At baseline, our study showed that impaired DA is associated with AMD severity and presence of SDDs.11 We have also demonstrated the repeatability of the psychophysical test repeated at 2 separate sittings in this aged population as being 0.02±2.26 minutes.11 Many cross-sectional studies3,8,12–14 support a correlation between impaired DA and AMD. However, few longitudinal studies exist examining DA changes over time. A small study first suggested that a minority of eyes with AMD demonstrated a worsening in DA over 12 months.9 A larger 3-year longitudinal study, limited to the study of normal aged eyes graded to be step 1 on the 9-step AREDS severity scale, concluded delayed DA at baseline was associated with development of AMD at 3 years.10 A 2-year study by Owsley et al20 reported on 23 eyes with intermediate AMD and measurable RTFs20 and reported an average RIT change of 2.55 minutes/year (5.1 minutes over 24 months),48 which is in the range of change we observe in our eyes that were graded to have AREDS severity step scores 6 to 9. The authors present data demonstrating that eyes with an RIT less than 15 minutes tend to change little over time compared with RITs greater than 15 minutes,20 which is also a finding in this study (Fig 2).
Our study adds considerably to the literature because it includes eyes with a wide spectrum of AMD disease, uses multiple modalities to define AMD and SDD, and assesses RIT changes longitudinally with multiple measurements over a 4-year follow-up period. Our longitudinal data demonstrate significant change over 4 years in DA among eyes with AMD and SDD in the setting of stable visual acuity. Interim analysis at 24 months echoes this result (Table S2, available at www.aaojournal.org). The significant correlation we observed between LLQ scores and rate of RIT progression indicate that these findings are not only statistically significant but also clinically important. We remain optimistic that measurements of DA will provide a useful outcome measure of visual function and AMD progression, especially in certain phenotypes. However, although this may be a useful outcome measure, the progression rates are generally slow, meaning that clinical trials using this as an outcome will likely still require years of follow-up. Although continued follow-up of our population and others will provide further insight into the natural history of DA changes among these eyes, larger multicenter studies may ultimately be necessary to further validate its use in interventional trials.
Supplementary Material
Acknowledgments
The authors thank Katherine Hall, RN, for her help coordinating this study and all of our study participants for their dedication to research.
National Eye Institute, National Institutes of Health, Bethesda, Maryland.
Financial Disclosure(s):
The author(s) have no proprietary or commercial interest in any materials discussed in this article.
Supported by the National Eye Institute Intramural Research Program, National Institutes of Health (NIH), Bethesda, Maryland; and the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation, the American Association for Dental Research, the Colgate-Palmolive Company, Genentech, Elsevier, and other private donors.
HUMAN SUBJECTS: Human subjects were included in this study. The human ethics committees at the National Institutes of Health approved the study. All research adhered to the tenets of the Declaration of Helsinki. All participants provided informed consent.
No animal subjects were used in this study.
Abbreviations and Acronyms:
- AMD
age-related macular degeneration
- AREDS
Age-Related Eye Disease Study
- BCVA
best-corrected visual acuity
- CE/IOL
cataract extraction and intraocular lens
- CNV
choroidal neovascularization
- DA
dark adaptation
- FAF
fundus autofluorescence
- GA
geographic atrophy
- IR
infrared reflectance
- LLQ
Low Luminance Questionnaire
- RIT
rod intercept time
- RPD
reticular pseudodrusen
- RPE
retinal pigment epithelium
- SD
spectral-domain
- SDD
subretinal drusenoid deposit
Footnotes
Supplemental material available at www.aaojournal.org.
References
- 1.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–e116. [DOI] [PubMed] [Google Scholar]
- 2.Csaky KG, Richman EA, Ferris FL 3rd. Report from the NEI/FDA Ophthalmic Clinical Trial Design and Endpoints Symposium. Invest Ophthalmol Vis Sci. 2008;49:479–489. [DOI] [PubMed] [Google Scholar]
- 3.Owsley C, Huisingh C, Clark ME, et al. Comparison of visual function in older eyes in the earliest stages of age-related macular degeneration to those in normal macular health. Curr Eye Res. 2015:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scilley K, Jackson GR, Cideciyan AV, et al. Early age-related maculopathy and self-reported visual difficulty in daily life. Ophthalmology. 2002; 109:1235–1242. [DOI] [PubMed] [Google Scholar]
- 5.Owsley C, MeGwin G, Jackson GR, et al. Effect of short-term, high-dose retinol on dark adaptation in aging and early age-related maculopathy. Invest Ophthalmol Vis Sci. 2006;47: 1310–1318. [DOI] [PubMed] [Google Scholar]
- 6.Ying GS, Maguire MG, Liu C, Antoszyk AN; Complications of Age-related Macular Degeneration Prevention Trial Research Group. Night vision symptoms and progression of age-related macular degeneration in the Complications of Age-related Macular Degeneration Prevention Trial. Ophthalmology. 2008;115:1876–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Owsley C, Huisingh C, Jackson GR, et al. Associations between abnormal rod-mediated dark adaptation and health and functioning in older adults with normal macular health. Invest Ophthalmol Vis Sci. 2014;55:4776–4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson GR, Scott IU, Kim IK, et al. Diagnostic sensitivity and specificity of dark adaptometry for detection of age-related macular degeneration. Invest Ophthalmol Pis Sci. 2014;55: 1427–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson GR, Clark ME, Scott IU, et al. Twelve-month natural history of dark adaptation in patients with AMD. Optom Vis Sci. 2014;91:925–931. [DOI] [PubMed] [Google Scholar]
- 10.Owsley C, MeGwin G Jr, Clark ME, et al. Delayed rod-mediated dark adaptation is a functional biomarker for incident early age-related macular degeneration. Ophthalmology. 2016;123:344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flamendorf J, Agron E, Wong WT, et al. Impairments in dark adaptation are associated with age-related macular degeneration severity and reticular pseudodrusen. Ophthalmology. 2015;122:2053–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson GR, Edwards JG. A short-duration dark adaptation protocol for assessment of age-related maculopathy. J Ocul Biol Dis Infor. 2008;1:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Planas MG, Biames M, Mones J. Dark adaptation impairment in patients with drusen. Invest Ophthalmol Vis Sci. 2016;57:3706. [Google Scholar]
- 14.Lains I, Miller JB, Park DH, et al. Structural changes associated with delayed dark adaptation in age-related macular degeneration. Ophthalmology. 2017;124:1340–1352. [DOI] [PubMed] [Google Scholar]
- 15.Rudolf M, Malek G, Messinger JD, et al. Sub-retinal drusenoid deposits in human retina: organization and composition. Exp Eye Res. 2008;87:402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarks J, Arnold J, Ho IV, et al. Evolution of reticular pscudodrusen. Br J Ophthalmol. 2011;95:979–985. [DOI] [PubMed] [Google Scholar]
- 17.Curcio CA, Messinger JD, Sloan KR, et al. Subrctinal drusenoid deposits in non-ncovascular age-related macular degeneration: morphology, prevalence, topography, and biogenesis model. Retina. 2013;33:265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greferath U, Guymer RH, Vessey KA, et al. Correlation of histologic features with in vivo imaging of reticular pseudodrusen. Ophthalmology. 2016;123:1320–1331. [DOI] [PubMed] [Google Scholar]
- 19.Spaide RF, Ooto S, Curcio CA. Subretinal drusenoid deposits AKA pseudodrusen, Surv Ophthalmol. 2018;63(6):782–815. [DOI] [PubMed] [Google Scholar]
- 20.Owsley C, Clark ME, McGwin G Jr. Natural history of rod-mediated dark adaptation over 2 years in intermediate age-related macular degeneration. Transl Vis Sci Technol. 2017;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnold JJ, Sarks SH, Killingsworth MC, Sarks JP. Reticular pseudodrusen. A risk factor in age-related maculopathy. Retina. 1995;15:183–191. [PubMed] [Google Scholar]
- 22.Ueda-Arakawa N, Ooto S, Tsujikawa A, et al. Sensitivity and specificity of detecting reticular pseudodrusen in multimodal imaging in Japanese patients. Retina. 2013;33:490–497. [DOI] [PubMed] [Google Scholar]
- 23.Smith RT, Sohrab MA, Busuioc M, Barile G. Reticular macular disease. Am J Ophthalmol. 2009;148:733–743.e732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith RT, Chan JK, Busuoic M, et al. Autofluorescence characteristics of early, atrophic, and high-risk fellow eyes in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2006;47:5495–5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lois N, Owens SL, Coco R, et al. Fundus autofluorescence in patients with age-related macular degeneration and high risk of visual loss. Am J Ophthalmol. 2002;133:341–349. [DOI] [PubMed] [Google Scholar]
- 26.Zweifel SA, Spaide RF, Curcio CA, et al. Reticular pseudodrusen are subretinal drusenoid deposits. Ophthalmology. 2010;117:303–312.C301. [DOI] [PubMed] [Google Scholar]
- 27.Davis MD, Gangnon RE, Lee LY, et al. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch Ophthalmol. 2005;123: 1484–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curcio CA, Medeiros NE, Millican CL. Photoreceptor loss in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1996;37:1236–1249. [PubMed] [Google Scholar]
- 29.Owsley C, MeGwin G Jr, Scillcy K, Rallies K. Development of a questionnaire to assess vision problems under low luminance in age-related maculopathy. Invest Ophthalmol Vis Sci. 2006;47:528–535. [DOI] [PubMed] [Google Scholar]
- 30.Custis PH, Bressler SB, Bressler NM. Laser management of subfoveal choroidal neovascularization in age-related macular degeneration. Curr Opin Ophthalmol. 1993;4:7–18. [DOI] [PubMed] [Google Scholar]
- 31.Yazdanic M, Alvarez J, Agron E, et al. Decreased visual function scores on a low luminance questionnaire is associated with impaired dark adaptation. Ophthalmology. 2017; 124: 1332–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmitz-Valckenberg S, Alten F, Steinberg JS, et al. Reticular drusen associated with geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52: 5009–5015. [DOI] [PubMed] [Google Scholar]
- 33.Marsiglia M, Boddu S, Bearelly S, et al. Association between geographic atrophy progression and reticular pscudodrusen in eyes with dry age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013;54:7362–7369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinberg JS, Auge J, Jaffe GJ, et al. Longitudinal analysis of reticular drusen associated with geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013;54:4054–4060. [DOI] [PubMed] [Google Scholar]
- 35.Xu L, Blonska AM, Pumariega NM, et al. Reticular macular disease is associated with multilobular geographic atrophy in age-related macular degeneration. Retina. 2013;33:1850–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hogg RE, Silva R, Staurenghi G, et al. Clinical characteristics of reticular pseudodrusen in the fellow eye of patients with unilateral ncovascular age-related macular degeneration. Ophthalmology. 2014;121:1748–1755. [DOI] [PubMed] [Google Scholar]
- 37.Finger RP, Wu Z, Luu CD, et al. Reticular pscudodrusen: a risk factor for geographic atrophy in fellow eyes of individuals with unilateral choroidal neovascularization. Ophthalmology. 2014;121:1252–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finger RP, Chong E, MeGuinness MB, et al. Reticular pseudodrusen and their association with age-related macular degeneration: The Melbourne Collaborative Cohort Study. Ophthalmology. 2016; 123:599–608. [DOI] [PubMed] [Google Scholar]
- 39.Thorell MR, Goldhardt R, Nunes RP, et al. Association between subfoveal choroidal thickness, reticular pseudodrusen, and geographic atrophy in age-related macular degeneration. Ophthalmic Surg Lasers Imaging Retina. 2015;46:513–521. [DOI] [PubMed] [Google Scholar]
- 40.Zhou Q, Daniel E, Maguire MG, et al. Pseudodrusen and incidence of late age-related macular degeneration in fellow eyes in the Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology. 2016; 123: 1530–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Q, Shaffer J, Ying GS. Pseudodrusen in the fellow eye of patients with unilateral neovascular age-related macular degeneration: a meta-analysis. PLoS One. 2016;11:e0149030, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Wang X, Rivero EB, et al. Photoreceptor perturbation around subretinal drusenoid deposits as revealed by adaptive optics scanning laser ophthalmoscopy. Am J Ophthalmol. 2014;158:584–596.C581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lamb TD, Pugh EN Jr. Dark adaptation and the retinoid cycle of vision. Prog Retin Eye Res. 2004;23:307–380. [DOI] [PubMed] [Google Scholar]
- 44.Bunt-Milam AH, Saari JC, Bredberg DL. Characterization of the interstitial space: immunocytochemical and biochemical studies. Prog Clin Biol Res. 1985;190:151–170. [PubMed] [Google Scholar]
- 45.Xu X, Liu X, Wang X, et al. Retinal pigment epithelium degeneration associated with subretinal drusenoid deposits in age-related macular degeneration. Am J Ophthalmol. 2017;175:87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Querques G, Querques L, Forte R, et al. Choroidal changes associated with reticular pseudodrusen. Invest Ophthalmol Vis Sci. 2012;53:1258–1263. [DOI] [PubMed] [Google Scholar]
- 47.Garg A, Oll M, Yzer S, et al. Reticular pseudodrusen in early age-related macular degeneration are associated with choroidal thinning. Invest Ophthalmol Vis Sci. 2013;54:7075–7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MeGwin G Jr, Jackson GR, Owsley C. Using nonlinear regression to estimate parameters of dark adaptation. Behav Res Methods Instrum Comput. 1999;31:712–717. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.