Corticotropin-releasing factor (CRF) is a neuropeptide critical to behavioral and physiological responses to stressors. CRF was originally discovered as an initiator of the hypothalamic-pituitary-adrenal axis, where it is released from neurons of the paraventricular nucleus of the hypothalamus into the pituitary gland. CRF was subsequently detected in the central amygdala (CeA) and bed nucleus of the stria terminalis (BNST) and was found to mediate negative valence associated with stress, anxiety, and drug withdrawal. In this role, CRF is purported to promote reward consumption by creating a negative affective state, which individuals then try to alleviate as a form of “self-medication” (1,2).
While CRF participates in neural mechanisms underlying responses to aversive stimuli, more recent evidence suggests that CRF signaling also mediates appetitive responses. Neurons that express CRF are readily detected in brain regions that mediate reward, such as the shell of the nucleus accumbens (NAc), hinting at a potential role in positive reinforcement. Indeed, microinjections of CRF directly into the NAc amplifies motivation for cues associated with rewards (3) and promotes conditioned place preference through the potentiation of dopamine release (4). These findings are at odds with the historic role for CRF in negative valence states and bring into focus the question of CRF’s role in motivated behaviors with positive valence.
Could it be that CRF has a different role on valence depending on where it is produced and mobilized? Behavioral pharmacology studies have served as valuable starting points for addressing this question. Unfortunately, these experiments suffer from a lack of cellular resolution, leaving open questions about the endogenous sources of CRF. This reckoning highlights the need for studies that directly manipulate the activity of different CRF subpopulations across the brain.
In the current issue of Biological Psychiatry, Baumgartner et al. (5) examine the role of 3 discrete populations of CRF-containing neurons (in the NAc, CeA, or BNST [NAcCRF, CeACRF, or BNSTCRF]) in incentive motivation versus aversive motivation (Figure 1). This is one of a handful of articles that examines the role of CRF neurons using transgenic Crh-Cre+ rats (6).
Figure 1.
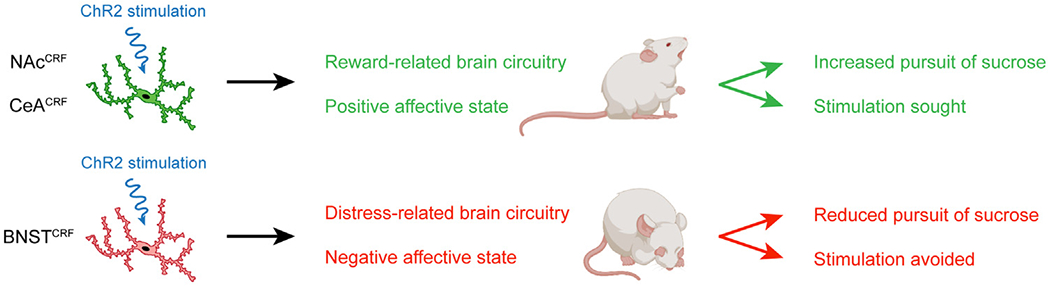
Optogenetic stimulation of corticotropin-releasing factor (CRF) neurons in the nucleus accumbens (NAc) and central amygdala (CeA) recruited reward-related brain circuits and led to reinforcement (self-stimulation) and the pursuit of sucrose. By contrast, stimulation of CRF neurons in the bed nucleus of the stria terminalis (BNST) engaged distress-related brain circuits and led to avoidance and a suppression of sucrose pursuit. These effects show the opposing impact different CRF neuron subpopulations have on incentive motivation. Figure created with images from BioRender.com. ChR2, channelrhodopsin-2.
First, the authors examined how optogenetic stimulation or inhibition of NAcCRF, CeACRF, or BNSTCRF neurons affects sucrose self-administration. Rats had to choose between responding for sucrose alone or for sucrose plus optogenetic stimulation (or inhibition) of CRF-containing neurons. They found that stimulating NAcCRF and CeACRF neurons intensified sucrose self-administration and motivation for sucrose in a progressive ratio task, whereas inhibiting these neurons had the opposite effects. Conversely, stimulating BNSTCRF neurons dampened sucrose self-administration and motivation for sucrose. These results show that stimulation of CRF neurons in the NAc and CeA enhances incentive motivation for sucrose whereas stimulation of CRF neurons in the BNST reduces it.
Next, Baumgartner et al. (5) further assessed motivational valence by examining optogenetic self-stimulation of NAcCRF, CeACRF, or BNSTCRF neurons. They tested this with operant self-stimulation as well as using real-time place testing, where one side of a compartment was paired with laser stimulation and the other was not. They found that rats self-stimulated NAcCRF and CeACRF neurons but not BNSTCRF neurons. In fact, rats avoided the side of a compartment paired with stimulation of BNSTCRF neurons. These data indicate that stimulation of NAcCRF and CeACRF neurons has a positive valence whereas stimulation of BNSTCRF neurons has a negative valence.
Finally, Baumgartner et al. (5) analyzed brainwide Fos expression evoked by stimulation of the 3 different populations of CRF neurons. They found that stimulating CRF neurons in the NAc shell and CeA recruited a similar collection of reward-related structures, including the NAc core, BNST, ventral pallidum, hypothalamus, and ventral tegmental area. On the other hand, stimulating BNSTCRF neurons activated the paraventricular nucleus of the hypothalamus, hypothalamus, basolateral amygdala, and periaqueductal gray, which are established components of stress and fear circuitry. These distinct brain activity patterns provide clues to how the different populations of CRF neurons influence incentive motivation. Furthermore, this comprehensive experiment provides deeper insight into the consequences of stimulating CRF neuron subpopulations and serves as a major strength of the article.
Baumgartner et al.’s (5) finding that stimulating BNSTCRF neurons activated distress-related brain circuitry and was aversive aligns with the large body of literature demonstrating that CRF neurons mediate behavioral states with negative valence, including fear learning, anxiety, and negative features of drug addiction [for example, see (7,8)].
The findings that stimulation of NAcCRF neurons activated reward-related brain circuitry, was reinforcing, and increased incentive motivation for sucrose are novel (Figure 1). To our knowledge, this is the first study that manipulated these neurons directly. These new findings are in line with those showing that stimulation of the NAc is reinforcing. They are also consistent with data showing that administration of CRF into the NAc is reinforcing (4) and increases incentive motivation for sucrose (3). This raises the question of whether stimulation of NAcCRF neurons promotes reinforcement and incentive motivation for sucrose by acting locally in the NAc or via projections to other structures.
Stimulation of CeACRF neurons also activated reward-related brain circuitry, was reinforcing, and increased the pursuit of sucrose (Figure 1). This contrasts with a plethora of studies showing that activation of the CeA is aversive. However, it is consistent with a study showing that stimulation of the CeA increases sucrose consumption (9) and with a previous article showing that stimulation of CeACRF neurons supports self-stimulation in mice (10). These findings highlight distinct roles of different CeA subpopulations in motivated behaviors and support the idea that CRF from the CeA is not exclusively stress-related or anxiogenic. Thus, there may be different CRF subsystems that play distinct roles in motivated behavior. Considering the diversity of responses, it is tempting to speculate that stimulation of CeACRF neurons could amplify the valence associated with the stimulation, whether negative (when stimulation of CeACRF neurons is associated with aversive settings) or positive (when it is paired with “positive” stimuli, like sucrose intake). This is reminiscent of the finding that CRF in the NAc switches from being appetitive to aversive after intense stress (4). Therefore, it follows that the effects of CRF are versatile and vary according to affective state.
The opponent process theory of addiction predicts that abstinence from repeated drug use or palatable food recruits neural substrates mediating negative valence, promoting reward consumption in an attempt to alleviate the negative state (1,2). This prediction breaks down considering the effect of stimulating BNSTCRF neurons, which did not promote sucrose intake despite producing negative valence. It would be interesting to know if this same situation holds true in animals that are dependent on drugs or exposed to previous stress, where the neural substrates mediating negative valence are recruited and a negative state emerges. For example, it has been observed that manipulating CRF signaling modifies drug or sucrose intake in dependent or stressed animals but not in nondependent or unstressed animals (8). These data further demonstrate the dynamic effects of CRF and warrant future investigation of BNSTCRF neurons in drug-dependent and stressed states.
The current set of intriguing results lays the foundation for further dissection of CRF circuits in incentive motivation. We still do not know if CRF itself is responsible for the effects of optogenetic stimulation of CRF neurons observed by Baumgartner et al. (5). These neurons release not only CRF and GABA (gamma-aminobutyric acid), but likely also other neuropeptides (e.g., dynorphin, neurotensin, or tachykinins, depending on the resident structure). Studies combining optogenetic (or chemogenetic) stimulation with in vivo pharmacology or gene interference methods will help determine the neurotransmitters and neuropeptides mediating the effects of stimulating CRF neurons. Moreover, optical recording techniques such as fiber photometry and microendoscopic imaging will reveal unique firing properties of different CRF populations during motivational states.
Collectively, the data presented by Baumgartner et al. (5) highlight the multifaceted roles of CRF neurons in limbic circuits. They are the first to manipulate and directly compare 3 different subpopulations of CRF neurons. Future studies exploring the mechanisms by which CRF neurons affect incentive motivation will be important for understanding brain mechanisms controlling motivation in healthy and disease states, like addiction. We anticipate that the continued use of tools like Crh-Cre+ rats and other genetic and viral techniques will greatly facilitate our understanding of CRF systems and their adaptive roles in motivated behaviors.
Acknowledgments and Disclosures
This work was supported by National Institute on Drug Abuse Grant Nos. R01DA042206 and R21DA031577 (to MM) and T32DA035165 (to MBP).
We thank Robert O. Messing for his comments.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.George O, Le Moal M, Koob GF (2012): Allostasis and addiction: Role of the dopamine and corticotropin-releasing factor systems. Physiol Behav 106:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cottone P, Sabino V, Roberto M, Bajo M, Pockros L, Frihauf JB, et al. (2009): CRF system recruitment mediates dark side of compulsive eating. Proc Natl Acad Sci U S A 106:20016–20020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pecina S, Schulkin J, Berridge KC (2006): Nucleus accumbens corticotropin-releasing factor increases cue-triggered motivation for sucrose reward: Paradoxical positive incentive effects in stress? BMC Biol 4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemos JC, Wanat MJ, Smith JS, Reyes BA, Hollon NG, Van Bockstaele EJ, et al. (2012): Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature 490:402–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baumgartner HM, Schulkin J, Berridge KC (2021): Activating corticotropin-releasing factor systems in the nucleus accumbens, amygdala, and bed nucleus of stria terminalis: Incentive motivation or aversive motivation? Biol Psychiatry 89:1162–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pomrenze MB, Millan EZ, Hopf FW, Keiflin R, Maiya R, Blasio A, et al. (2015): A transgenic rat for investigating the anatomy and function of corticotrophin releasing factor circuits. Front Neurosci 9:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanford CA, Soden ME, Baird MA, Miller SM, Schulkin J, Palmiter RD, et al. (2017): A central amygdala CRF circuit facilitates learning about weak threats. Neuron 93:164–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Guglielmo G, Kallupi M, Pomrenze MB, Crawford E, Simpson S, Schweitzer P, et al. (2019): Inactivation of a CRF-dependent amygdalofugal pathway reverses addiction-like behaviors in alcohol-dependent rats. Nat Commun 10:1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson MJ, Warlow SM, Berridge KC (2014): Optogenetic excitation of central amygdala amplifies and narrows incentive motivation to pursue one reward above another. J Neurosci 34:16567–16580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim J, Zhang X, Muralidhar S, LeBlanc SA, Tonegawa S (2017): Basolateral to central amygdala neural circuits for appetitive behaviors. Neuron 93:1464–1479.e1465. [DOI] [PMC free article] [PubMed] [Google Scholar]


