Abstract
Introduction
Inflammatory bowel disease (IBD) is increasingly common among patients with other comorbid chronic conditions, particularly diabetes mellitus (DM). Yet, studies that explored the impact of comorbid diabetes on the outcomes of IBD are scanty. Therefore, this study aims to examine the outcomes of inflammatory bowel disease among hospitalized patients with diabetes mellitus.
Methods
Using the Nationwide Inpatient Sampling (NIS) database from 2016 to 2018, we identified patients' records with a diagnosis of IBD using the International Classification of Diseases, Tenth Revision codes (ICD-10). The overall study population was further stratified by diabetes mellitus status. We matched patients with IBD and diabetes mellitus (IBD DM) with IBD cohorts using a greedy propensity score matching (PSM) ratio of 1:1 and compared in-hospital outcomes between the two cohorts. Conditional logistic regression was performed to estimate the odds of outcomes.
Results
Out of the 192,456 hospitalizations for IBD, 34,073 (7.7%) had comorbid IBD DM and 158,383 (92.3%) had no diabetes mellitus (IBD only). Patients with IBD DM are likely to be older. They have higher rates of hypertension, hyperlipidemia, coronary artery disease, obesity, peripheral vascular disease, congestive heart failure, chronic kidney disease, chronic lung disease, chronic liver disease, and stroke than the IBD cohort. After propensity score matching, IBD DM was associated with a lower adverse outcome [odds ratio (OR): 0.96, confidence interval (CI): 0.93 - 0.99, p < 0.01], IBD-related complications (intestinal or rectal fistula, intra-abdominal abscess, toxic colitis, intestinal perforation, intestinal obstruction, toxic megacolon, abscess of the abdomen, and perianal abscess), (OR: 0.76, CI: 0.72 - 0.80, P <0.01), IBD-related surgery (intestinal resections, incision, and excisions of intestine and manipulations of the rectosigmoid, rectal and perianal) (OR: 0.90, CI: 0.85 - 0.95, P <0.01). Furthermore, IBD DM was associated with a higher sepsis complication than the IBD-only cohort (OR: 1.24, CI: 1.19 - 1.30, P <0.01).
Conclusion
Our results highlight the extent to which diabetes mellitus impacts IBD outcomes and prognosis. Additionally, they emphasize the clinical awareness needed in the management of those with comorbid diseases.
Keywords: inflammatory bowel disease, diabetes mellitus, adverse clinical outcomes, inflammatory bowel disease association with diabetes, complications of inflammatory bowel disease
Introduction
Inflammatory bowel disease (IBD) is a chronic non-infectious inflammation of the gastrointestinal tract, associated with significant morbidities and poor quality of life [1-3]. IBD comprises Crohn's disease and ulcerative colitis, which constitute a significant clinical and public health burden affecting about 7-million people worldwide [4]. The United States contributes a quarter of the total global patients with IBD with an age-standardized prevalence rate of 464.5 per 100,000 population in 2017 [4]. IBD results from a complex interaction of the genetic and environmental factors that disrupt the immune mechanisms of the gastrointestinal tract and result in inflammation [5-6]. While IBD affects mainly the gastrointestinal tract, the associated chronic systemic inflammation leads to several extraintestinal manifestations affecting the body's major organs [7-8]. In addition, with the rise in the aging population living with IBD, there is an increased likelihood of being affected with other comorbid conditions, including cardiovascular disease or diabetes mellitus (DM) [9]. These concomitant chronic diseases modify the progression of IBD and might lead to long periods of intermittent relapses and exacerbations [10].
DM is one of the most common comorbid conditions in the United States. DM is a chronic metabolic disorder characterized by persistent impaired blood glucose metabolism [11]. According to the National Diabetes Statistics Report, 34.2-million people currently live with diabetes in the United States [12]. As a chronic disease that affects most body organs, DM can modify disease outcomes and lead to other complications. Published studies have reported IBD DM to have a higher incidence of complications, including exacerbations requiring prolonged hospitalizations and the need for surgical interventions [10]. Also, prior studies have reported DM as a covariate associated with increased risk of hospitalizations, infectious complications, colorectal cancers, and mortality [13- 14]. However, studies that describe the outcomes of IBD among diabetic patients are minimal. Understanding the hospital outcomes of comorbid IBD DM is vital for improving management and reducing morbidity and mortality. Therefore, this study sought to investigate the role of DM on IBD using a national hospital database.
Materials and methods
Study data
This study utilized data from the Nationwide Inpatient Sample (NIS) 2016-2018 to perform a retrospective cohort study. The NIS is the largest hospital database in the United States, containing discharge records of about 8-million hospital stays annually. The NIS is a stratified, clustered database that samples discharge records from 20 percent of non-federal community hospitals. Each discharge record contains diagnoses and procedures coded using the International Classification of Disease, 10th revision (ICD-10). Institutional Review Board approval was not required for this study since the data has been de-identified.
Study population
The study population consisted of adults aged 18 and older with a diagnosis of IBD from January 1, 2016, to December 31, 2018, identified using the ICD 10: K50-K51. After excluding patients with missing age, mortality, and sex variables, we categorized the total study population into two groups: patients with IBD DM and those with IBD only. We defined DM status using ICD-10. Comparative analyses were conducted between the two groups regarding demographics such as age, sex (male and female), race/ethnicity (Whites, Blacks, Hispanics, and other races), income status (categorized into four according to the average household income of the zip-code), hospital bed size (small, medium, and large hospitals), and comorbidities (hypertension, hyperlipidemia, coronary artery disease, obesity, peripheral vascular disease, chronic heart failure, chronic kidney disease, chronic lung disease, chronic liver disease, stroke).
Study outcomes
The outcomes of this study were in-hospital adverse events, a composite of in-hospital mortality, IBD-related complications (development of fistula, abscess, colitis, perforation, intestinal obstruction, toxic megacolon), intestinal surgery (intestinal resections, incision, and excisions of intestine and manipulations of the rectosigmoid, rectal and perianal), sepsis, and septicemia, clostridium difficile infections, colorectal cancer, and resources utilization measures (length of stay and cost of hospitalizations).
Statistical analysis
Weighted values are generated to obtain a nationally representative estimate of the hospitalized patients and then produce median values and percentages for the variables. Continuous variables were expressed as weighted median values with interquartile range and compared between the cohorts using independent t-tests. In contrast, categorical variables were expressed as percentages and compared using the chi-square test. Patient demographics, comorbidities, hospital characteristics, and in-hospital outcomes were compared between males and females. We used the cost-to-charge ratio files provided by the Healthcare Cost and Utilization Project (HCUP) to convert the hospital charges to more accurate hospital costs for cost calculation. A p-value of < 0.05 was considered statistically significant.
A propensity matching method was implemented to derive two cohorts of matched samples to control potential confounding factors. A propensity score was derived for each observation via a multivariate logistic regression that models the odds of DM and the baseline characteristics. A nearest-neighbor with a ratio of 1:1, balanced propensity matching was made using a caliper width cut-off <0.2 of the standard deviation of the propensity score. A p-value of < 0.05 was considered statistically significant. After outcomes were compared between propensity score-matched subjects (IBD subjects with and without DM). The paired t-test was used to compare continuous variables and the McNemar test for categorical variables between the matched cohorts.
Finally, we developed many conditional generalized logistic regression models accounting for the matching pair, with diabetes as a primary predictor. Each of the outcomes is the dependent variable in the models. The negative binomial model was used for continuous outcomes for the length of stay and gamma distributions mode for cost. Data manipulation and statistical analyses were performed using SAS 9.4 software (SAS Institute Inc., Cary, NC). Propensity score matching (PSM) was performed using the Matchit package in R statistical software (version 3.5; R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics of the study population
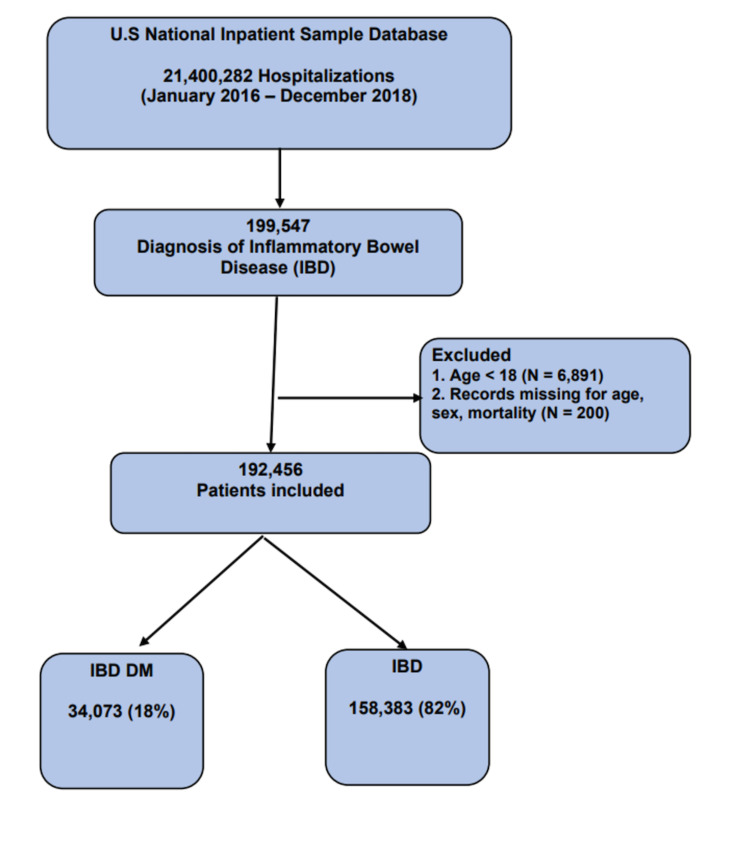
From January 1, 2016, until December 31, 2018, a total of 21,400,282 hospitalizations were present in the NIS database. Of these hospitalizations, 192,456 had a diagnosis of IBD. DM was present in 7.7% (34,073 records), as seen in Figure 1.
Figure 1. Baseline characteristics of the study population.
There were significant differences in the baseline characteristics among the IBD DM and IBD cohorts. Compared to IBD patients, the IBD DM cohorts were older (median age 64.6 vs. 50.8), female (53.6% vs. 46.4%), and had a higher percentage of comorbidities than the IBD-only cohort. The baseline patient and hospital characteristics of the two groups are shown in Tables 1-2.
Table 1. Baseline characteristics of patients with inflammatory bowel disease and diabetes mellitus (Nationwide Inpatient Sampling database 2016 -2018).
Values are expressed in percentages unless otherwise stated.
WE: weighted estimates; IBD: inflammatory bowel disease; DM: diabetes mellitus; IQR: interquartile range
| Variables | Total | IBD DM | IBD | p-value |
| 192,456 | (N = 34,073) | (N =158,383) | ||
| (WE: 962,280) | (N = 170,365) | (N = 791,915) | ||
| Age (median, IQR) | 54.0 (36.7 - 68.5) | 64.6 (53.7 - 73.5) | 50.8 (34.4 - 66.5) | <0.0001 |
| Age category | ||||
| 18 -34 | 20.7 | 3.8 | 24.3 | <0.0001 |
| 35 - 54 | 29.3 | 21.7 | 30.9 | |
| 55 - 74 | 34.2 | 50.9 | 30.6 | |
| ≥ 75 | 15.8 | 23.6 | 14.1 | |
| Sex | ||||
| Male | 43.7 | 46.4 | 43.1 | <0.0001 |
| Female | 56.3 | 53.6 | 56.9 | |
| Race | ||||
| White | 76.0 | 74.7 | 76.3 | <0.0001 |
| Black | 10.9 | 12.0 | 10.6 | |
| Hispanic | 6.0 | 6.4 | 5.9 | |
| Other | 3.7 | 3.9 | 3.7 | |
| Unknown | 3.4 | 3.0 | 3.5 | |
| Comorbidities | ||||
| Hypertension | 38.2 | 63.2 | 32.9 | <0.0001 |
| Hyperlipidemia | 22.8 | 47.6 | 17.4 | <0.0001 |
| Coronary Artery Disease | 12.7 | 27.7 | 9.5 | <0.0001 |
| Obesity | 12.5 | 24.8 | 9.8 | <0.0001 |
| Peripheral Vascular Disease | 4.9 | 7.7 | 4.3 | <0.0001 |
| Chronic Heart Failure | 9.8 | 21.4 | 7.3 | <0.0001 |
| Chronic Kidney Disease | 6.5 | 14.2 | 4.8 | <0.0001 |
| Chronic Lung Disease | 20.9 | 29.8 | 19.0 | <0.0001 |
| Chronic Liver Disease | 6.8 | 10.1 | 6.1 | <0.0001 |
| Stroke | 5.8 | 10.2 | 4.9 | <0.0001 |
| Smoking | 16.9 | 13.5 | 17.6 | <0.0001 |
| Hospital Bed Size | ||||
| Small | 19.1 | 19.6 | 19.0 | <0.0001 |
| Medium | 28.1 | 29.4 | 27.8 | |
| Large | 52.9 | 51.0 | 53.3 | |
| Median Household Income | ||||
| < 25th percentile | 27.2 | 27.2 | 23.8 | <0.0001 |
| 26-50th percentile | 27.2 | 27.2 | 25.3 | |
| 51-75th percentile | 24.8 | 24.8 | 26.3 | |
| 76-100th percentile | 20.8 | 20.8 | 24.6 |
Table 2. Baseline characteristics of patients with inflammatory bowel disease and diabetes mellitus, after propensity matching.
Values are expressed in percentages unless otherwise stated.
SMD: standardized mean differences; IBD: inflammatory bowel disease; DM: diabetes mellitus; IQR: interquartile range
| Variables | IBD DM | IBD | SMD (%) |
| Observations | (N = 33,870) | (N = 33,870) | |
| Age (median, IQR) | 64.5 (53.7 - 73.4) | 66.4 (54.6 - 76.0) | 10.3 |
| Sex | 3.0 | ||
| Male | 46.3 | 46.2 | |
| Female | 53.7 | 53.8 | |
| Race | 3.9 | ||
| White | 74.7 | 78.7 | |
| Black | 12.0 | 9.2 | |
| Hispanic | 6.4 | 4.8 | |
| Other | 3.9 | 3.3 | |
| Unknown | 3.0 | 4.0 | |
| Comorbidities | |||
| Hypertension | 63.0 | 65.6 | 5.5 |
| Hyperlipidemia | 47.3 | 46.6 | 1.5 |
| Coronary Artery Disease | 27.4 | 26.1 | 3.0 |
| Obesity | 24.5 | 21.9 | 6.1 |
| Peripheral Vascular Disease | 7.7 | 7.8 | 0.3 |
| Chronic Heart Failure | 21.1 | 19.2 | 4.7 |
| Chronic Kidney Disease | 14.0 | 12.3 | 4.9 |
| Chronic Lung Disease | 29.7 | 30.2 | 1.1 |
| Chronic Liver Disease | 10.1 | 9.8 | 1.0 |
| Stroke | 10.2 | 10.1 | 0.4 |
| Smoking | 13.6 | 13.7 | 0.3 |
| Hospital Bed Size | 3.0 | ||
| Small | 19.6 | 19.6 | |
| Medium | 29.4 | 29.2 | |
| Large | 51.0 | 51.2 | |
| Median Household Income | 1.2 | ||
| < 25th percentile | 27.1 | 26.5 | |
| 26-50th percentile | 27.2 | 27.0 | |
| 51-75th percentile | 24.9 | 25.6 | |
| 76-100th percentile | 20.8 | 20.8 |
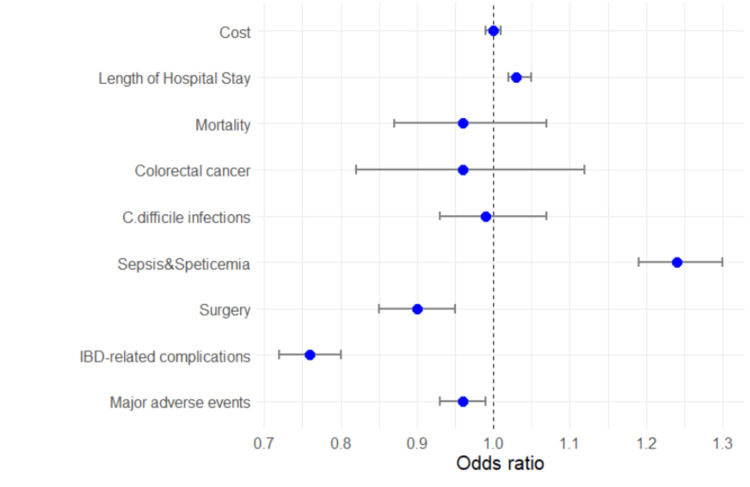
In-hospital outcomes are depicted in Table 3. Overall, the IBD DM cohorts had a lower prevalence of major in-hospital adverse events than the IBD cohorts. Compared to the IBD group, the IBD DM had lower IBD-related complications (9.3% vs. 14.5%, p < 0.01), intestinal surgeries (26.0% vs. 38.5%, p < 0.01). On the other hand, the IBD DM group had a higher prevalence of sepsis and septicemia complications (14.7% vs 10.8%, p < 0.01) and in-hospital mortality (2.2% vs 1.4%, p < 0.01). The prevalence of clostridium difficile infections and colorectal cancer are similar between the two groups. Similarly, the length of hospital stay and cost- were more prevalent among the IBD DM group than the IBD only group.
Table 3. In-hospital outcomes of inflammatory bowel disease among IBD DM vs IBD patients before propensity score matching.
IBD: inflammatory bowel disease; DM: diabetes mellitus; IQR: interquartile range
| In-hospital Outcomes | Total | IBD DM | IBD | p-value |
| Major Adverse events | 40.6% | 36.1% | 41.6.1% | <0.0001 |
| IBD-related complications | 13.5% | 9.3% | 14.5% | <0.0001 |
| Surgery | 36.3% | 26.0% | 38.5% | <0.0001 |
| Sepsis/Septicemia | 11.5% | 14.7% | 10.8% | <0.0001 |
| C.difficile infections | 4.6% | 4.7% | 4.6% | 0.4205 |
| Colorectal Cancer | 0.9% | 1.0% | 0.9% | 0.9339 |
| Death | 1.5% | 2.2% | 1.4% | <0.0001 |
| Length of stay (Median (IQR) | 3.1(1.7 - 3.7) | 3.5(1.9 - 6.3) | 3.0(1.7 - 5.5) | <0.0001 |
| Cost (Median (IQR) | 8,224 (4,986 - 14,761) | 9,224 (5,582 - 16,212) | 8,036 (4,873 - 14446) | <0.0001 |
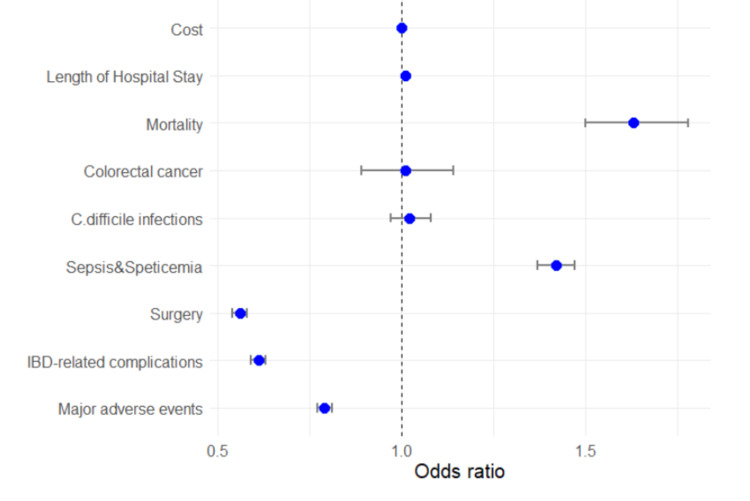
Using propensity matching, we adjusted for baseline demographics and hospital characteristics to generate matched cohorts of IBD DM and IBD groups (n = 33,870) (Table 4). We achieved the variables' standardized mean differences between the two groups to arrive at a well-matched cohort. The overall in-hospital adverse events is still lower among the IBD DM cohort as compared to the IBD group (36.2% vs. 37.2%, OR: 0.96, CI: 0.93 - 0.99, p < 0.01). Furthermore, the IBD DM cohort had a lower incidence of IBD-related complication (9.3% vs. 11.9%, OR: 0.76, CI: 0.72 - 0.80, P <0.01) and surgery (26.0% vs. 28.9%, OR: 0.90, CI: 0.85 - 0.95, P <.01). However, the DM group had a higher sepsis complication compared to the IBD group (14.7% vs. 12.2%, OR: 1.24, CI: 1.19 - 1.30, P <0.01). See Figures 2-4 and Table 5.
Table 4. In-hospital outcomes of inflammatory bowel disease among IBD DM vs IBD patients after propensity score matching.
IBD: inflammatory bowel disease; DM: diabetes mellitus; IQR: interquartile range
| In-hospital Outcomes | IBD DM | IBD | OR (95% CI) | p-value |
| Major Adverse events | 36.2 | 37.2 | 0.96 (0.93 - 0.99) | 0.0046 |
| IBD-related complications | 9.3 | 11.9 | 0.76 (0.72 - 0.80) | <0.0001 |
| Surgery | 26.0 | 28.9 | 0.90 (0.85 - 0.95) | 0.0004 |
| Sepsis/Septicemia | 14.7 | 12.2 | 1.24 (1.19 - 1.30) | <0.0001 |
| C.difficile infections | 4.7 | 4.7 | 0.99 (0.93 - 1.07) | 0.8567 |
| Colorectal Cancer | 1.0 | 1.0 | 0.96 (0.82 - 1.12) | 0.5841 |
| Death | 2.2 | 2.3 | 0.96 (0.87 - 1.07) | 0.4670 |
| Length of stay (Median IQR) | 3.5 (1.9 - 6.3) | 3.3 (1.8 - 6.1) | 1.03 (1.02 - 1.05) | <0.0001 |
| Cost (Median IQR) | 9,216 (5,578 - 16,199) | 9,147 (5,471 - 16,272) | 1.00 (0.99 - 1.01) | 0.8839 |
Table 5. Baseline characteristics of patients with inflammatory bowel disease and diabetes mellitus, after propensity matching.
Values are expressed in percentages unless otherwise stated.
SMD: standardized mean differences; IBD: inflammatory bowel disease; DM: diabetes mellitus; IQR: interquartile range
| Variables | IBD DM | IBD | SMD (%) |
| Observations | (N = 33,870) | (N = 33,870) | |
| Age (median, IQR) | 64.5 (53.7 - 73.4) | 66.4 (54.6 - 76.0) | 10.3 |
| Sex | 3.0 | ||
| Male | 46.3 | 46.2 | |
| Female | 53.7 | 53.8 | |
| Race | 3.9 | ||
| White | 74.7 | 78.7 | |
| Black | 12.0 | 9.2 | |
| Hispanic | 6.4 | 4.8 | |
| Other | 3.9 | 3.3 | |
| Unknown | 3.0 | 4.0 | |
| Comorbidities | |||
| Hypertension | 63.0 | 65.6 | 5.5 |
| Hyperlipidemia | 47.3 | 46.6 | 1.5 |
| Coronary Artery Disease | 27.4 | 26.1 | 3.0 |
| Obesity | 24.5 | 21.9 | 6.1 |
| Peripheral Vascular Disease | 7.7 | 7.8 | 0.3 |
| Chronic Heart Failure | 21.1 | 19.2 | 4.7 |
| Chronic Kidney Disease | 14.0 | 12.3 | 4.9 |
| Chronic Lung Disease | 29.7 | 30.2 | 1.1 |
| Chronic Liver Disease | 10.1 | 9.8 | 1.0 |
| Stroke | 10.2 | 10.1 | 0.4 |
| Smoking | 13.6 | 13.7 | 0.3 |
| Hospital Bed Size | 3.0 | ||
| Small | 19.6 | 19.6 | |
| Medium | 29.4 | 29.2 | |
| Large | 51.0 | 51.2 | |
| Median Household Income | 1.2 | ||
| < 25th percentile | 27.1 | 26.5 | |
| 26-50th percentile | 27.2 | 27.0 | |
| 51-75th percentile | 24.9 | 25.6 | |
| 76-100th percentile | 20.8 | 20.8 |
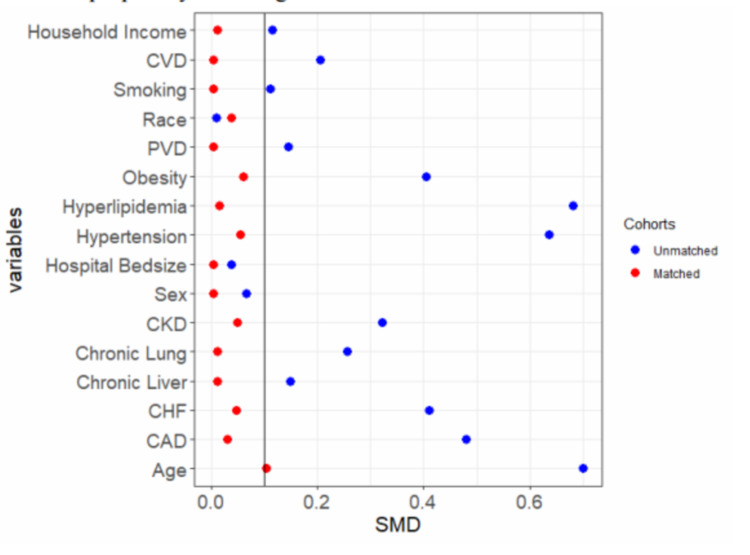
Figure 2. Dot plots showing the standardized mean differences of the baseline variables before and after propensity matching.
CVD: cerebrovascular disease; PVD: peripheral vascular disease; CKD: chronic kidney disease; CHF: congestive heart failure; CAD: coronary artery disease
Figure 3. Plots of the adjusted odds ratio of clinical outcomes of inflammatory bowel disease and diabetes mellitus after propensity matching.
Figure 4. Plot of the unadjusted odds ratio of clinical outcomes of inflammatory bowel diseases and diabetes mellitus.
Discussion
In this study, we examined the in-hospital outcomes of IBD among patients with DM. In addition, we compared IBD patients with comorbid DM to those without diabetes using a propensity score matching method. The main findings of our study are as follows: (1) IBD DM patients have a significant increase in the odds of developing sepsis and septicemia compared to the IBD only cohort ; (2) IBD DM is associated with a decrease in the odds of having IBD-related complications and surgical interventions compared to IBD cohorts; (3) there were no significant differences in the risk of mortality, clostridium difficile infections, or colorectal cancer, among IBD DM and IBD.
To date, only a few clinical or epidemiological studies had investigated the IBD outcomes among diabetic patients. A recent longitudinal cohort study conducted in the US found that comorbid IBD DM patients have a significantly increased risk of IBD-related hospitalization, complications, surgical interventions, and all-cause mortality [10]. Compared with IBD cohorts, IBD DM patients had significantly higher rates of sepsis and other infections [10]. Furthermore, a retrospective study that utilized 2810 outpatient cohorts concluded that DM is associated with worse IBD severity reflected by increased use of the emergency room and nearly double the rates of patients with gastrointestinal clinic visits [15]. While these studies have employed different databases and methodology, one consistent finding with these studies is the overall increase in the odds of infection-related complications among IBD DM patients compared to the IBD cohorts. This was consistent with our study that found an increase in the odds of having sepsis and septicemia among IBD DM patients.
Several mechanisms might explain the reason for the increased incidence of infectious complications among the IBD DM cohort. IBD and DM are both autoimmune disease conditions that disrupt the entire immune system [16-17]. These disease states are associated with dysregulation of the intestinal immune barrier, promoting local and systemic inflammation, explaining the increased susceptibility to several infections [18-19]. The dysregulation also leads to the release of multiple cytokines, including tumor necrosis factor (TNF)-alpha and interleukins [20-21]. The inhibited secretions of the interleukins in diabetic patients cause a defect in the antigen-presenting cells, monocytes, and contribute to reduced immunity [22]. Furthermore, the hyperglycemic state in DM affects the complement system and contributes to reduced neutrophil function [23]. Also, IBD patients are usually on immunosuppressive medications, which reduces the function of the overall immune system and predisposes them to an increased risk of opportunistic infections [24].
Given the overlapping impairment in the immune system, concomitant DM is a significant comorbidity among patients with IBD, as reflected by increased hospitalizations [15]. While our study did not find a significant difference in the length of hospital stay and cost of hospitalization between IBD DM and IBD, we did not explore health care utilization. A study found that IBD DM patients had a higher outpatient prescription and antibiotic usage than IBD cohorts [15]. Also, IBD DM patients' increased emergency department and gastrointestinal clinic visits reflected the important burden of IBD DM comorbidity [15].
The use of propensity score matching (PSM) is one of the strengths of this study. The PSM methodology has been compared to the randomized clinical trials, as it effectively adjusts for confounders and produces estimates close to those derived from randomized clinical trials [25-26]. PSM facilitates comparability between the IBD DM and IBD cohorts, making it a valuable technique for assessing the risk factors between these groups. Therefore, PSM minimizes several biases and limitations of a large observational study like ours. The additional strengths of our study include utilizing an extensive, nationwide inpatient database, which makes the results generalizable to the entire population. Despite these strengths, our findings still needed to be interpreted in light of some limitations. The comorbidities in the nationwide inpatient sample database rely heavily on using the diagnostic coding ICD-10. Any error in coding can affect the study's validity. Thus, there might be variations, which is also a limitation that could affect the results generated. The PSM methodology was designed to minimize confounding that could bias the results from observed covariates. However, PSM cannot address unobserved factors, which may still lead to biased results. Also, our study did not explore the different outcomes regarding the variants of IBD. IBD, comprising Crohn's disease and ulcerative colitis, can have different results. The retrospective nature of this study is a limitation and the diagnosis of diabetes mellitus based on ICD-10 codes alone might be unreliable, thus some of the patients in the IBD-only group might also have diabetes mellitus but are yet to be diagnosed, which can affect the results of this study. A prospective study will be helpful to better study the complications of inflammatory bowel disease in patients with diabetes mellitus.
Conclusions
This study shows that DM is a determinant of severe disease and increased risk of severe infectious complications in hospitalized diabetic patients with IBD. The use of PSM is one of the strengths of this study, as it effectively adjusts for confounders and minimizes several biases and limitations of a large observational study. More longitudinal and prospective studies are required to further study the impact of diabetes among patients with IBD.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.The vasculature and inflammatory bowel disease: contribution to pathogenesis and clinical pathology. Hatoum OA, Binion DG. Inflamm Bowel Dis. 2005;11:304–313. doi: 10.1097/01.mib.0000160772.78951.61. [DOI] [PubMed] [Google Scholar]
- 2.Impact of inflammatory bowel disease on quality of life: results of the European Federation of Crohn's and Ulcerative Colitis Associations (EFCCA) patient survey. Ghosh S, Mitchell R. J Crohns Colitis. 2007;1:10–20. doi: 10.1016/j.crohns.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Health-related quality of life in inflammatory bowel disease patients: the role of psychopathology and personality. Vidal A, Gómez-Gil E, Sans M, Portella MJ, Salamero M, Piqué JM, Panés J. Inflamm Bowel Dis. 2008;14:977–983. doi: 10.1002/ibd.20388. [DOI] [PubMed] [Google Scholar]
- 4.The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Alatab S, Sepanlou SG, Ikuta K, et al. Lancet Gastroenterol Hepatol. 2020;5:17–30. doi: 10.1016/S2468-1253(19)30333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pathogenesis of inflammatory bowel disease and recent advances in biologic therapies. Kim DH, Cheon JH. Immune Netw. 2017;17:25–40. doi: 10.4110/in.2017.17.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geographical variability and environmental risk factors in inflammatory bowel disease. Ng SC, Bernstein CN, Vatn MH, et al. Gut. 2013;62:630–649. doi: 10.1136/gutjnl-2012-303661. [DOI] [PubMed] [Google Scholar]
- 7.Inflammatory bowel disease does not impact mortality but increases length of hospitalization in patients with acute myocardial infarction. Sinh P, Tabibian JH, Biyani PS, Mehta K, Mansoor E, Loftus EV Jr, Dave M. Dig Dis Sci. 2021;[Epub ahead of print] doi: 10.1007/s10620-020-06818-x. [DOI] [PubMed] [Google Scholar]
- 8.Extraintestinal manifestations and complications in inflammatory bowel diseases. Rothfuss KS, Stange EF, Herrlinger KR. https://doi.org/10.3748/wjg.v12.i30.4819. World J Gastroenterol. 2006;12:4819–4831. doi: 10.3748/wjg.v12.i30.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inflammatory bowel disease in the baby to baby boomer: pediatric and elderly onset of IBD. Afzali A, Katz S. Curr Treat Options Gastroenterol. 2018;16:289–305. doi: 10.1007/s11938-018-0188-9. [DOI] [PubMed] [Google Scholar]
- 10.Comorbid diabetes in inflammatory bowel disease predicts adverse disease-related outcomes and infectious complications. Kumar A, Teslova T, Taub E, Miller JD, Lukin DJ. Dig Dis Sci. 2021;66:2005–2013. doi: 10.1007/s10620-020-06439-4. [DOI] [PubMed] [Google Scholar]
- 11.Diabetes mellitus: the epidemic of the century. Kharroubi AT, Darwish HM. https://doi.org/10.4239/wjd.v6.i6.850. World J Diabetes. 2015;6:850–867. doi: 10.4239/wjd.v6.i6.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Estimates of diabetes and its burden in the United States. [May;2021 ];https://www.cdc.gov/diabetes/data/statistics-report/index.html 2020
- 13.Effect of ulcerative colitis on incidence of colorectal cancer: results from the Nationwide Population-Based Cohort Study (2003-2013) Choi JK, Kim DW, Shin SY, Park EC, Kang JG. J Cancer. 2016;7:681–686. doi: 10.7150/jca.14264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Impact of metabolic syndrome on the hospitalization rate of Crohn's disease patients seen at a tertiary care center: a retrospective cohort study. Fitzmorris PS, Colantonio LD, Torrazza Perez E, Smith I, Kakati DD, Malik TA. Digestion. 2015;91:257–262. doi: 10.1159/000380763. [DOI] [PubMed] [Google Scholar]
- 15.Disease characteristics and severity in patients with inflammatory bowel disease with coexistent diabetes mellitus. Din H, Anderson AJ, Ramos Rivers C, et al. Inflamm Bowel Dis. 2020;26:1436–1442. doi: 10.1093/ibd/izz305. [DOI] [PubMed] [Google Scholar]
- 16.Inflammation and type 2 diabetes. Calle MC, Fernandez ML. Diabetes Metab. 2012;38:183–191. doi: 10.1016/j.diabet.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Inflammatory bowel disease, colorectal cancer and type 2 diabetes mellitus: the links. Jurjus A, Eid A, Al Kattar S, et al. BBA Clin. 2016;5:16–24. doi: 10.1016/j.bbacli.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gut microbiota in the pathogenesis of inflammatory bowel disease. Nishida A, Inoue R, Inatomi O, Bamba S, Naito Y, Andoh A. Clin J Gastroenterol. 2018;11:1–10. doi: 10.1007/s12328-017-0813-5. [DOI] [PubMed] [Google Scholar]
- 19.The gut microbiota in the pathogenesis and therapeutics of inflammatory bowel disease. Zuo T, Ng SC. Front Microbiol. 2018;9:2247. doi: 10.3389/fmicb.2018.02247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Targeting inflammation through a physical active lifestyle and pharmaceuticals for the treatment of type 2 diabetes. Knudsen SH, Pedersen BK. Curr Diab Rep. 2015;15:82. doi: 10.1007/s11892-015-0642-1. [DOI] [PubMed] [Google Scholar]
- 21.Exercise and type 2 diabetes: focus on metabolism and inflammation. Karstoft K, Pedersen BK. Immunol Cell Biol. 2016;94:146–150. doi: 10.1038/icb.2015.101. [DOI] [PubMed] [Google Scholar]
- 22.Infections in patients with diabetes mellitus: a review of pathogenesis. Casqueiro J, Casqueiro J, Alves C. Indian J Endocrinol Metab. 2012;16 Suppl 1:0–36. doi: 10.4103/2230-8210.94253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hyperglycemia enhances coagulation and reduces neutrophil degranulation, whereas hyperinsulinemia inhibits fibrinolysis during human endotoxemia. Stegenga ME, van der Crabben SN, Blümer RM, et al. Blood. 2008;112:82–89. doi: 10.1182/blood-2007-11-121723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Risk for overall infection with anti-TNF and anti-integrin agents used in IBD: a systematic review and meta-analysis. Shah ED, Farida JP, Siegel CA, Chong K, Melmed GY. Inflamm Bowel Dis. 2017;23:570–577. doi: 10.1097/MIB.0000000000001049. [DOI] [PubMed] [Google Scholar]
- 25.Demystifying propensity scores. Okoli GN, Sanders RD, Myles P. https://doi.org/10.1093/bja/aet290. Br J Anaesth. 2014;112:13–15. doi: 10.1093/bja/aet290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Why to use propensity score in observational studies? Case study based on data from the Czech clinical database AHEAD 2006-09 [Article in Czech] Littnerova S, Jarkovsky J, Parenica J, Pavlik T, Spinar J, Dusek L. https://doi.org/10.1016/j.crvasa.2013.04.001 Cor et Vasa. 2013;55:0–90. [Google Scholar]