Abstract
Aims
Patients with heart failure (HF) have an increased risk of incident cancer. Data relating to the association of statin use with cancer risk and cancer-related mortality among patients with HF are sparse.
Methods and results
Using a previously validated territory-wide clinical information registry, statin use was ascertained among all eligible patients with HF (n = 87 102) from 2003 to 2015. Inverse probability of treatment weighting was used to balance baseline covariates between statin nonusers (n = 50 926) with statin users (n = 36 176). Competing risk regression with Cox proportional-hazard models was performed to estimate the risk of cancer and cancer-related mortality associated with statin use. Of all eligible subjects, the mean age was 76.5 ± 12.8 years, and 47.8% was male. Over a median follow-up of 4.1 years (interquartile range: 1.6–6.8), 11 052 (12.7%) were diagnosed with cancer. Statin use (vs. none) was associated with a 16% lower risk of cancer incidence [multivariable adjusted subdistribution hazard ratio (SHR) = 0.84; 95% confidence interval (CI), 0.80–0.89]. This inverse association with risk of cancer was duration dependent; as compared with short-term statin use (3 months to <2 years), the adjusted SHR was 0.99 (95% CI, 0.87–1.13) for 2 to <4 years of use, 0.82 (95% CI, 0.70–0.97) for 4 to <6 years of use, and 0.78 (95% CI, 0.65–0.93) for ≥6 years of use. Ten-year cancer-related mortality was 3.8% among statin users and 5.2% among nonusers (absolute risk difference, −1.4 percentage points [95% CI, −1.6% to −1.2%]; adjusted SHR = 0.74; 95% CI, 0.67–0.81).
Conclusion
Our study suggests that statin use is associated with a significantly lower risk of incident cancer and cancer-related mortality in HF, an association that appears to be duration dependent.
Keywords: Heart failure, Cancer, Cardio-oncology, Statin, Prevention
Graphical Abstract
See page 3060 for the editorial comment on this article (doi:10.1093/eurheartj/ehab482)
Introduction
Heart failure (HF) and cancer are two major public health challenges worldwide.1 , 2 The ageing demographics, along with increasing prevalence of antecedents, e.g. hypertension, diabetes, coronary artery disease, obesity, and atrial fibrillation,3 are driving the epidemic of HF globally. The improvement of HF management has further extended the longevity and increased the clinical relevance of non-cardiac morbidity and mortality in patients with HF. Recent epidemiological studies have demonstrated that cancer is the leading cause of non-cardiac death in patients with HF.4–6 Besides shared risk factors, such as diabetes mellitus, smoking, and dyslipidemia, it has been hypothesized that HF is an oncogenic condition, possibly related to links between neurohormonal activation to tumorigenesis, systemic pathological processes such as inflammation and oxidative stress, common genetic predisposition, and clonal hematopoiesis of cancer and HF.7 , 8 Preventive strategies to reduce the burden of cancer in HF patients is hence urgently needed.
Both experimental9 and clinical data10–12 suggest that statin may be chemoprotective through diverse potential mechanisms including inhibition of mevalonate pathway [a critical pathway (with its metabolites) integral for tumour development and growth],13 anti-inflammatory, antioxidant, and immune-modulatory properties. To date, there is a paucity of studies evaluating the association of statin use with cancer risk and cancer-related mortality in patients with HF. Accordingly, in this territory-wide cohort study, we aim to examine the relationship between the use of statin and the risk of cancer and cancer-related mortality among patients with HF.
Methods
This is a retrospective cohort study conducted with data from the Clinical Data Analysis Reporting System (CDARS), a territory-wide database developed by the Hong Kong Hospital Authority. As the statutory body and the singular provider of public healthcare services in Hong Kong, the Hospital Authority provides over 80% of inpatient services in Hong Kong, a territory with a population of 7.5 million.14 CDARS prospectively collects patient information including, but not limited to, demographic data, diagnoses, drug prescriptions, procedures, laboratory tests, and episodes of hospital visits since 1993.15 Prior studies have demonstrated a high percentage of coding accuracy in CDARS data.15–18 Diagnostic data, specifically, were determined by using the International Classification of Diseases, Ninth Revision (ICD-9), also shown to have a high coding accuracy.19 , 20
Patient data (name and Hong Kong identification number) were de-identified in CDARS and unique reference numbers were generated. The study was approved by the institutional review board of the University of Hong Kong and the West Cluster of the Hong Kong Hospital Authority.
Outcome definition and study subjects
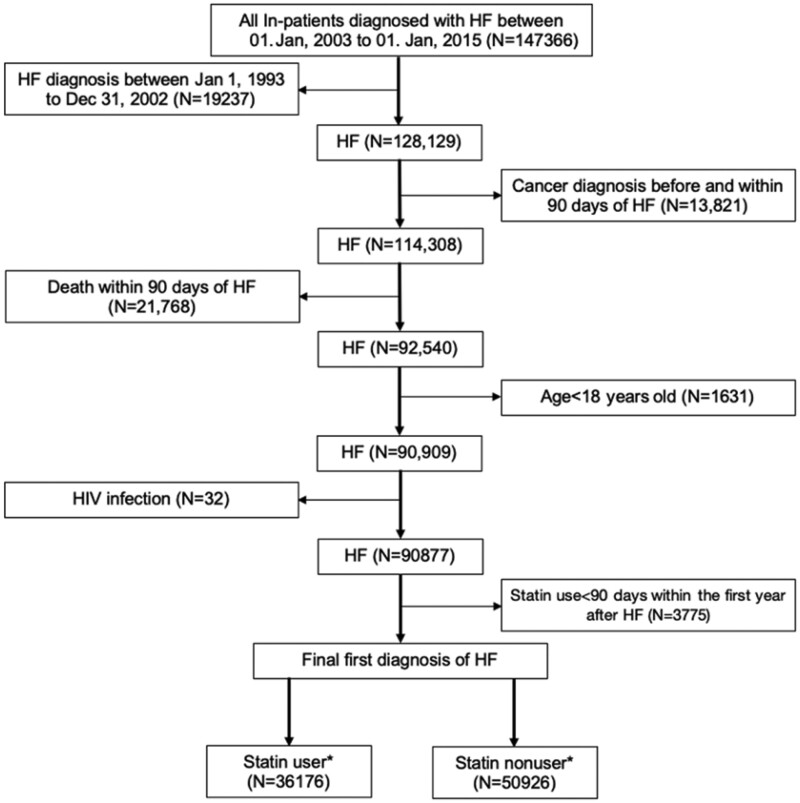
We searched for all patients aged 18 years old or above with HF (ICD-9: 402, 404, 425, 428) as a primary cause of hospitalization between 1 January 2003 and 1 January 2015. We subsequently identified all episodes of statin dispenses among the cohort, and the index date was defined as the date of first diagnosis of HF. We also excluded patients who were diagnosed with HF between 1 January 1993 to 31 December 2002 (n = 19 237) to ensure the recruited patients had no prior history of HF. Furthermore, patients who had any history of cancer or cancer incidence within 90 days after the first diagnosis of HF, death within 90 days after the first diagnosis of HF, human immunodeficiency disease (HIV), and <90 days statin use within the first year of HF diagnosis were excluded (Figure 1). The primary outcome of the study was that of incident cancer subsequent to the diagnosis of HF, for which the association with statin use was determined. Patients were followed up until a diagnosis of cancer, death, or 31 December 2018, whichever came earlier.
Figure 1.
Flow chart of the study cohort. HF, heart failure; HIV, human immunodeficiency virus. *Statin user was defined by filled prescription for at least 90 consecutive days use of statin after the index date; statin nonuser was defined as never use of statin or <90 consecutive days of statin use after the index date.
Exposure definition
We used an intention-to-treat design in the study, where statin exposure was defined as ≥90 days consecutive use of statin beginning within the first year after the index date, as defined in previous publications.17 , 21 In further analysis, we modelled statin use as a time-varying exposure to assess duration response. To evaluate duration, we summed the duration of all filled prescriptions (in days) and updated these data at each yearly interval of follow-up. Patients who received statin for a period of <90 consecutive days within the first year of HF diagnosis were excluded (n = 3775). The types of statins that were available in the public sector during the study period include simvastatin, atorvastatin, and rosuvastatin. Accordingly, we identified 36 176 statin users and 50 926 statin nonusers after the index date.
Statin users
As different indications for statins could potentially define different sub-populations of patients with HF, the presence of an indication for statin use was classified into: atherosclerotic disease (n = 21 894, 60.5%) defined based on ICD coding (coronary artery disease, ICD-9: 410–414/peripheral vascular disease, ICD-9: 440–444, 447/stroke, ICD-9: 430–438); hypercholesterolaemia (n = 8326, 23.0%) based on ICD coding (dyslipidemia, ICD-9: 272, 272.1, 272.2, 272.3, 272.4) and LDL >2.6 mmol/L;22 and undefined (n = 5956, 16.5%), where the exact indication was uncertain due to the lack of corresponding ICD coding or valid lipid baseline profile. We further evaluated the lipid control of statin users by calculating the time-weighted mean of LDL level (defined by time-weighted mean of LDL level from 3 months following initiation of statin until endpoint).23 Among all statin users, 31 454 (86.9%) patients had post-statin LDL level available and were subsequently categorized according to the time-weighted mean of LDL <1.8 mmol/L (n = 9879, 31.4%), LDL 1.8–2.6 mmol/L (n = 15 319, 48.7%), and LDL >2.6 mmol/L (n = 6256, 19.9%).
Study covariates
We traced patient records to 3 years prior to the index date and collected data including age at index date, sex, comorbidities (diabetes, obesity, hypertension, dyslipidemia, arrhythmias, coronary heart disease, vascular diseases, stroke, cirrhosis, chronic renal failure, Parkinson disease, ankylosing spondylitis, rheumatoid arthritis, systemic sclerosis, systemic lupus erythematosus), and drug history (baseline use of aspirin, antihypertensives, anti-diabetics, beta-blockers, statin) as well as lifestyle factors (alcoholism and smoking).22 Baseline drug use was defined as ≥90 days of consistent use before the index date. Details of ICD-9 codes used are in Supplementary material online, Table S1.
Statistical analysis
To address biases in the allocation of treatment due to lack of randomization, a propensity score approach was used. Covariates that were considered prognostically significant as well as those that influenced treatment selection were logistically regressed to the probability of receiving treatment.24 An inverse propensity of treatment weighting (IPTW) was used, allowing a pseudo-population to be created through assigning individuals with weights that corresponded to the inverse of their probability of receiving treatment given observed covariates. The differences in the prevalence of covariates between statin users and nonusers were considered insignificant if the standardized mean difference was ≤0.10. Cox proportional-hazards modelling was used, and statin exposure was further entered as a time-dependent variable to determine the effect of statin use, including covariates used in calculating the propensity score in ‘doubly robust estimation’.25 A Fine and Gray model was used to adjust for competing risks, with the competing events being all-cause mortality and non-cancer-related death.24 Associations were considered significant if the P-value was <0.05.
We further performed conventional Cox regression without competing risks or without considering propensity score for comparison to previous cohort studies;13 , 26 , 27 as well as subgroup analyses by age, sex, alcohol, smoke, comorbidities (diabetes and hypertension), and baseline drug use (aspirin, angiotensin-converting enzyme inhibitor, angiotensin receptor blockers, metformin) after accounting for competing risk. Several sensitivity analyses were conducted including: (i) models excluding persons with a history of alcohol abuse (n = 1486) or smoking (n = 9617); (ii) using an alternative 1:1 propensity score matched design; (iii) analyses excluding patients with any history of statin use (n = 18 131); (iv) analyses excluding patients with a diagnosis of incident cancer or death within 3 years after the index date (n = 46 701), to minimize reverse causation;24 and (v) analyses of the relationship between statin use and incident gastrointestinal bleeding (excluding those with a prior history of gastrointestinal bleeding and simultaneous digestive cancer), as a falsification endpoint. All statistical analyses were performed using R v4.0.2.28–31
Results
Patient characteristics
We identified 87 102 patients who developed incident HF between 2003 and 2015, 56 045 (64%) were 75 years or older, 41 639 (48%) were men, more than half had hypertension (n = 44 241, 51%) and nearly one-third had coronary artery disease (n = 30 195, 35%). There were a total of 50 926 statin nonusers and 36 176 statin users (Figure 1). The baseline characteristics of the entire cohort are shown in Table 1. Upon adjustment by IPTW, patient characteristics were well balanced (Table 1 and Supplementary material online, Table S2). During a median follow-up of 4.1 years [interquartile range (IQR): 1.6–6.8], with a total of 404 924 person-years, 11 052 (12.7%) patients were newly diagnosed with cancer. Cancer-related mortality occurred in 3864 (4.4%) patients (Supplementary material online, Table S3). The commonest type of cancer and cancer-related mortality was colorectal, stomach, lung, and liver/biliary system (Supplementary material online, Table S4).
Table 1.
Baseline characters before and after inverse propensity of treatment weighting
| Characteristica | All (n = 87 102) | Statin userb (n = 36 176) | Statin nonuserb (n = 50 926) | SMD before IPTW | SMD after IPTW |
|---|---|---|---|---|---|
| Age at index date (years) | 76.5 ± 12.8 | 73.7 ± 12.0 | 78.5 ± 13.0 | 0.412 | 0.025 |
| Male sex | 41 639 (47.8) | 18 650 (51.6) | 22 989 (45.1) | 0.121 | 0.013 |
| Alcohol | 1486 (1.7) | 512 (1.4) | 974 (1.9) | 0.034 | 0.006 |
| Smoke | 9617 (11.0) | 2576 (7.1) | 7041 (13.8) | 0.224 | 0.004 |
| Diabetes | 18 491 (21.2) | 9375 (25.9) | 9116 (17.9) | 0.165 | 0.019 |
| Obesity | 845 (1.0) | 511 (1.4) | 334 (0.7) | 0.076 | 0.001 |
| Hypertension | 44 241 (50.8) | 20 123 (55.6) | 24 118 (47.4) | 0.147 | 0.011 |
| Arrhythmia | 26 883 (30.9) | 10 030 (27.7) | 16 853 (33.1) | 0.110 | 0.013 |
| Coronary artery disease | 30 195 (34.7) | 18 332 (50.7) | 11 863 (23.3) | 0.533 | 0.039 |
| Peripheral vascular disease | 16 475 (18.9) | 7181 (19.9) | 9294 (18.3) | 0.009 | 0.007 |
| Stroke | 8553 (9.8) | 3885 (10.7) | 4668 (9.2) | 0.030 | 0.006 |
| Parkinson | 1180 (1.4) | 287 (0.8) | 893 (1.8) | 0.092 | 0.009 |
| Dyslipidemia | 10 975 (12.6) | 8326 (23.0) | 2649 (5.2) | 0.481 | 0.064 |
| Chronic renal failure | 9226 (10.6) | 4054 (11.2) | 5172 (10.2) | 0.006 | 0.003 |
| Ankylosing spondylitis | 8262 (9.5) | 3084 (8.5) | 5178 (10.2) | 0.062 | 0.001 |
| Rheumatoid arthritis | 451 (0.5) | 155 (0.4) | 296 (0.6) | 0.021 | <0.001 |
| Drug use | |||||
| Aspirin | 29 970 (34.4) | 15 677 (43.3) | 14 293 (28.1) | 0.284 | 0.023 |
| ACE inhibitors | 25 803 (26.9) | 12 868 (35.6) | 12 935 (25.4) | 0.209 | 0.014 |
| Angiotensin receptor blockers | 4452 (5.1) | 2803 (7.7) | 1649 (3.2) | 0.189 | 0.010 |
| Beta-blockers | 27 245 (31.3) | 14 508 (40.1) | 12 737 (25.0) | 0.313 | 0.016 |
| Calcium channel blockers | 36 362 (41.7) | 17 250 (47.7) | 19 112 (37.5) | 0.188 | 0.008 |
| Statin | 18 131 (20.8) | 15 608 (43.1) | 2523 (5.0) | 0.902 | 0.083 |
| Metformin | 13 039 (15.0) | 8021 (22.2) | 5018 (9.9) | 0.329 | 0.021 |
Values are given as median ± standard deviation, or n (%).
ACE, angiotensin-converting enzyme; IPTW, inverse probability of treatment weighting; SMD, standardized mean difference.
Clinical characteristics of the patients were defined according to validated diagnoses in the International Classification of Diseases coding system (Supplementary material online, Table S1).
Statin user was defined by filled prescription for at least 90 consecutive days of statin after the index date; statin nonuser was defined as never use of statin or <90 consecutive days of statin use after the index date.
Cancer
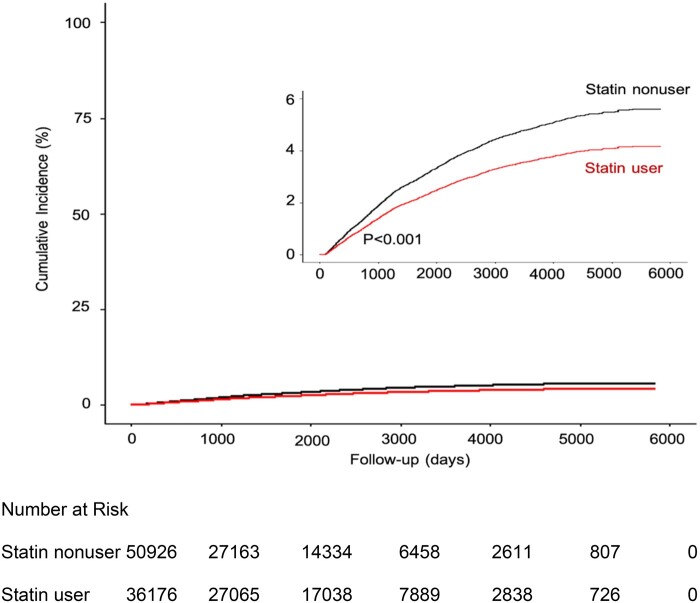
The median age at diagnosis of cancer was 79.7 years (IQR: 74.3–87.1 years) and the median time-to-diagnosis of cancer beginning from index date of HF was 3.8 years (IQR: 1.3–5.6 years). Propensity-matched statin users had a lower risk of developing cancer; the 5-year cumulative incidence of cancer was 7.9% among statin users and 10.4% among nonusers and the 10-year cumulative incidence of cancer was 11.2% among statin users and 13.2% among nonusers (Figure 2). Statin users had a 16% lower risk of cancer than nonusers after multivariable adjustment (SHR = 0.84; 95% CI, 0.80–0.89) (Table 2).
Figure 2.
Cumulative incidence of cancer between statin user and nonuser. Statin user was defined by filled prescription for at least 90 consecutive days of statin use after the index date (the date on which a patient was diagnosed as incident heart failure). Statin nonuser was defined as never use of statin or <90 consecutive days of statin use after the index date. We calculated the P-value using Gray’s test for equality of the cumulative functions between each exposure group after inverse probability of treatment weighting, accounting for competing risks of all-cause mortality. The inset shows the same data on an expanded y-axis.
Table 2.
Effect of statin use on the risk of incident cancer and cancer-related deatha
| Event and treatment group | Number with event/total number | 10-Year cumulative incidence | SHR (95% CI) |
|
|---|---|---|---|---|
| % | Unadjusted | Adjustedb | ||
| Incident cancer | ||||
| Statin nonuser | 6422/50 926 | 13.2% | 1.00 (Ref.) | 1.00 (Ref.) |
| Statin user | 4630/36 176 | 11.2% | 0.84 (0.78 to 0.87) | 0.84 (0.80 to 0.89) |
| Absolute risk difference (95% CI) | −2.0% (−2.3% to −1.7%) | |||
| Cancer-related death | ||||
| Statin nonuser | 2474/50 926 | 5.2% | 1.00 (Ref.) | 1.00 (Ref.) |
| Statin user | 1390/36 176 | 3.8% | 0.64 (0.56 to 0.72) | 0.74 (0.67 to 0.81) |
| Absolute risk difference (95% CI) | −1.4% (−1.6% to −1.2%) | |||
CI, confidential interval; SHR, subdistribution hazard ratio.
Statin user was defined by filled prescription for at least 90 consecutive days of statin use after the index date. Ten-year cumulative incidence, absolute risk difference, and hazard estimates were obtained with the use of a proportional subdistribution hazards regression model fit to the inverse probability of treatment weighted cohort that accounted for competing risks; the model was conditioned on age at index date.
A multivariable adjusted model further accounted for the following prognostic covariates: age at index date, sex, presence or absence of alcohol, smoking, comorbidities, including diabetes, obesity, hypertension, arrhythmia, coronary artery disease, peripheral vascular disease, stroke, Parkinson disease, dyslipidemia, chronic renal failure, cirrhosis, ankylosing spondylitis, rheumatoid arthritis, systemic sclerosis, systemic lupus erythematosus, and baseline use of aspirin, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, calcium channel blockers, diuretics, statin, and metformin.
To enable comparisons to prior reports, we also computed the multivariable SHR before propensity matching (0.82; 95% CI, 0.78–0.86). Without consideration of competing risks, a conventional multivariable Cox regression yielded a hazard ratio (HR) of 0.83 (95% CI, 0.80–0.87) for cancer risk (Supplementary material online, Table S5).
Cancer-related mortality
The 10-year cancer-related mortality was 3.8% (1390 episodes) among statin users and 5.2% (2474 episodes) among nonusers (Figure 3). The use of statin was significantly associated with a lower adjusted risk of cancer-related death than nonusers (SHR = 0.74; 95% CI, 0.67–0.81) (Table 2). Of interest, the 10-year all-cause mortality was 60.5% (21 886 episodes) among statin users and 78.8% (40 130 episodes) among nonusers. The use of statin was significantly associated with a lower adjusted risk of all-cause mortality than nonusers (HR = 0.62; 95% CI 0.61–0.64).
Figure 3.
Cancer-related mortality between statin user and nonuser. Statin user was defined by filled prescription for at least 90 consecutive days of statin use after the index date (the date on which a patient was diagnosed as incident heart failure). Statin nonuser was defined as never use of statin or <90 consecutive days of statin use after the index date. We calculated the P-value using Gray’s test for equality of the cumulative functions between each exposure group after inverse probability of treatment weighting, accounting for competing risks of non-cancer-related mortality. The inset shows the same data on an expanded y-axis.
Statin users
Among statin users, the crude 10-year cumulative incidence of cancer among those with atherosclerotic disease (11.34%) and hypercholesterolaemia (11.27%, as indications for statin initiation) did not differ (absolute risk difference: 0.07%, P > 0.05). Similarly, the corresponding incidence among lipid control (time-weighted mean LDL measured at least 3 months following statin initiation) groups defined by LDL <1.8, 1.8–2.6, and >2.6 mmol/L was 10.3%, 10.5%, and 10.8% (P > 0.05), respectively. After multivariable adjustment and accounting for competing risk, cancer incidence in statin users was not related to the indication for statin (atherosclerotic disease vs. hypercholesterolaemia, SHR = 1.01, 95% CI 0.81–1.26) or time-weighted LDL control (LDL 1.8–2.6 vs. LDL > 2.6 mmol/L, SHR = 1.01, 95% CI 0.91–1.14; LDL <1.8 vs. LDL > 2.6 mmol/L, SHR = 0.99, 95% CI 0.87–1.12).
Duration of statin use
The inverse relationship between statin use and the risk of cancer appeared to be duration dependent. We modelled the duration of statin use as a time-varying exposure to avoid immortal time bias.24 , 25 As shown in Table 3 where the population was restricted to statin users, compared to short-term use (from 3 months up to 2 years), the risk of cancer was significantly lower with the use of statin from 4 years up to 6 years (adjusted SHR 0.82; 95% CI, 0.70–0.97), and further lowered with long-term statin use of >6 years (adjusted SHR 0.78; 95% CI, 0.65–0.93). Similar results of duration response can be found in the association between statin use and cancer-related death. The risk of cancer-related death was significantly lower in statin use from 4 to 6 years and >6 years (adjusted SHR = 0.67; 95% CI, 0. 53–0.85 and adjusted SHR = 0.61; 95% CI, 0.46–0.82, respectively) compared with short-term use of statin, while no such association was observed in statin use from 2 to 4 years. As a sensitivity analysis, no significant difference in cancer incidence and cancer-related death was observed when comparing 90 days to 6 months, 6 months to 1 year, and 1–2 years’ use of statin (Supplementary material online, Table S6). Consequently, short-term use (defined as 3 months up to 2 years) was used as a referent.
Table 3.
Effect of duration of statin use on the risk of incident cancer and cancer-related death among statin usersa
| Event and duration of statin use | 10-Year cumulative incidence | SHR (95% CI) |
|
|---|---|---|---|
| % | Unadjusted | Adjustedb | |
| Incident cancer | |||
| 3 months to <2 years | 11.8% | 1.00 (Ref.) | 1.00 (Ref.) |
| 2 to <4 years | 11.7% | 0.98 (0.86 to 1.12) | 0.99 (0.87 to 1.13) |
| 4 to <6 years | 7.6% | 0.80 (0.68 to 0.95) | 0.82 (0.70 to 0.97) |
| ≥6 years | 5.4% | 0.74 (0.62 to 0.88) | 0.78 (0.65 to 0.93) |
| Cancer-related death | |||
| 3 months to <2 years | 4.5% | 1.00 (Ref.) | 1.00 (Ref.) |
| 2 to <4 years | 4.1% | 0.93 (0.79 to 1.10) | 0.94 (0.80 to 1.12) |
| 4 to <6 years | 2.4% | 0.64 (0.51 to 0.81) | 0.67 (0.53 to 0.85) |
| ≥6 years | 1.8% | 0.57 (0.43 to 0.75) | 0.61 (0.46 to 0.82) |
CI, confidential interval; Ref., reference; SHR, subdistribution hazard ratio.
Statin user was defined by filled prescription for at least 90 consecutive days of statin use after the index date. The cumulative duration of statin use was modelled as a time-varying exposure. Ten-year cumulative incidence and hazard estimates were obtained with the use of a proportional subdistribution hazards regression model fit to the inverse probability of treatment weighted cohort that accounted for competing risks; the model was conditioned on age at index date.
A multivariable adjusted model further accounted for the following prognostic covariates: age at index date, sex, presence or absence of alcohol, smoking, comorbidities including diabetes, obesity, hypertension, arrhythmia, coronary artery disease, peripheral vascular disease, stroke, Parkinson disease, dyslipidemia, chronic renal failure, cirrhosis, ankylosing spondylitis, rheumatoid arthritis, systemic sclerosis, systemic lupus erythematosus, and baseline use of aspirin, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, calcium channel blockers, diuretics, statin, and metformin.
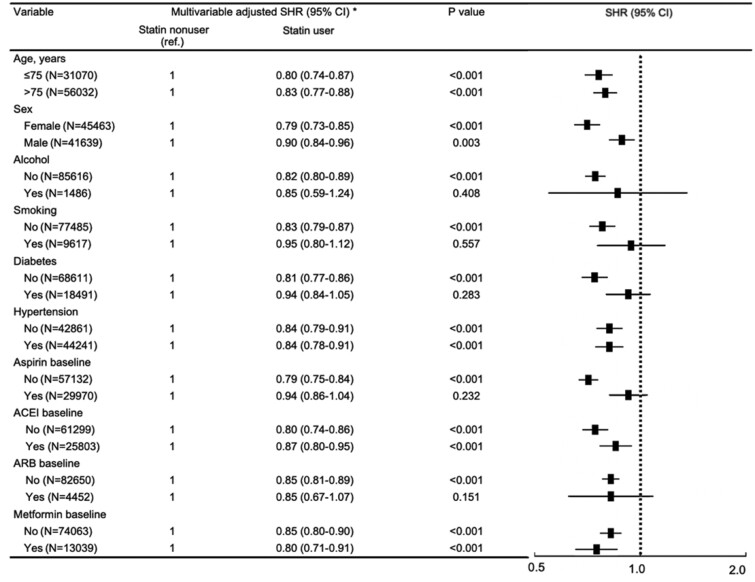
Subgroup analyses
As shown in Figure 4, the association of statin use with lower risk of incident cancer was consistent across subgroups of age, sex, alcohol use, smoking status, presence of diabetes or hypertension, and concomitant use of aspirin, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or metformin. We further assessed different types of cancer and found that statin use (compared to non-use) was associated with a lower incidence of cancers involving the colorectum, lung, liver, lymph, breast, haematological system, pancreas, and kidney; with no association for cancers involving the stomach, skin, prostate, brain, bladder, female reproductive system, head, or oesophagus (all following IPTW adjustment and accounting for competing risks). However, these results should be interpreted with caution because of small sample size in some subgroups (Supplementary material online, Table S7).
Figure 4.
Multivariable stratified analysis of the association between statin use and risk of cancer. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CI, confidence interval; SHR, subdistribution hazard ratio. *Statin use was defined by filled prescription for at least 90 consecutive days of statin use after the index date (the date on which a patient was diagnosed as incident heart failure). We calculated the P-value using Gray’s test for equality of the cumulative functions between each exposure group after inverse probability of treatment weighting, accounting for competing risks of all-cause mortality.
Sensitivity analysis
Sensitivity analyses revealed consistent results after excluding patients with a history of alcohol abuse (SHR for incident cancer, 0.82; 95% CI, 0.80–0.89) or patients with a history of smoking (SHR for incident cancer, 0.83; 95% CI, 0.79–0.87). Without propensity matching, a multivariable SHR was 0.82 (95% CI, 0.78–0.86). Without consideration of competing risks, a conventional multivariable Cox regression yielded a HR of 0.83 (95% CI, 0.80–0.87) for cancer risk (Supplementary material online, Table S5). We further created a 1:1 matched cohort using the propensity score directly without IPTW. Upon trimming 5% of the propensity score, each statin user was matched in a fixed 1:1 ratio with a statin nonuser using a ‘genetic’ algorithm with a caliper of 0.0001. Using this approach, we successfully matched 30 845 statin users to 35 735 nonusers to create a propensity score matched cohort of 66 580 patients; the multivariable adjusted SHR was 0.81 (95% CI, 0.77–0.84) after accounting for competing risk (Supplementary material online, Table S8). Furthermore, we excluded 18 131 patients with a history of baseline statin use to include only new users of statin after the index date followed by a 1:1 propensity score matching to create a matched cohort (n = 28 894). After accounting for competing risk, multivariable adjusted SHRs of the association between statin new user and risk of incident cancer and cancer-related mortality were 0.80 (95% CI, 0.75–0.86) and 0.70 (95%CI, 0.62–0.78) compared with nonuser, respectively (Supplementary material online, Table S9). After excluding patients with incident cancer diagnosed within the first 3 years after the index date, the multivariable adjusted SHR was 0.88 (95% CI, 0.81–0.96) among statin users compared with nonusers after accounting for competing risk (Supplementary material online, Table S10). We finally used gastrointestinal bleeding as a negative control, of which we excluded 20 644 patients with a prior history of gastrointestinal bleeding diagnosis before the index date of HF and 435 patients with simultaneous digestive cancer, for further analysis (Supplementary material online, Table S11). Among the remaining 66 023 patients, 7937 diagnoses of gastrointestinal bleeding were recorded between index date and patient mortality. The risk of gastrointestinal bleeding was nonetheless similar between statin users and non-users with SHR of 1.01 (95% CI, 0.96–1.06) after multivariable adjustment.
Discussion
In this territory-wide cohort study of >87 000 patients with HF, we demonstrated that statin use was independently associated with a 16% decrease in risk of developing cancer and a 26% decrease in risk of cancer-related mortality. There was some evidence of a dose–response relationship, with longer durations (4–6 and >6 years) of statin use being associated with a lower risk of cancer and cancer-related mortality compared to short-term use (<2 years). Results were consistent across clinical subgroups and in sensitivity analyses (Graphical Abstract).
Statin use is associated with a significantly lower risk of incident cancer and cancer-related mortality in patients with heart failure and results were consistent across clinical subgroups and in sensitivity analyses. The potential protective effect of statin on development of cancer merits evaluation in future randomized studies.
Advancement of treatment has greatly improved the clinical outcome of patients with HF, with a two-fold improvement of 5-year survival rates from 29.1% between 1970 and 1979 to 59.7% between 2000 and 2009.3 A decline in cardiovascular mortality was however offset by a considerable increase in non-cardiovascular mortality, with cancer-related death being the most prevalent cause.26 While one may attribute that the increased cancer-related mortality in patients with HF could be due to shared comorbidities among the two conditions, accumulating evidence has suggested that HF per se may predispose to cancer development, for example through hyper-activation of the renin-angiotensin-aldosterone system, which also promotes tumour growth.27 In a large cohort involving 9307 patients with HF, the risk of cancer was found to be 24% greater compared to patients without HF.5 In patients with myocardial infarction, those who developed HF had a 71% higher risk of developing cancer compared with those without HF.6 The increased risk of cancer in patients with HF was further confirmed by a case–control study that demonstrated a higher incidence of cancer, irrespective of diabetes control measured by glycated haemoglobin.32 Thus, beyond shared risk factors, HF itself may be an oncogenic condition, possibly related to links between neurohormonal activation to tumorigenesis, systemic pathological processes such as inflammation and oxidative stress, common genetic predisposition and clonal haematopoiesis of cancer and HF.7 , 8 These findings underscore the strong association of HF with cancer, and call for potential strategies to reduce the risk of cancer and cancer-related mortality in patients with HF. Our result corroborates prior literature suggesting an inverse association between statin use and cancer development and extends these observations for the first time to a large Asian population-based cohort. In an observational study that utilized pharmacy records of dispensing history in eight Dutch cities, regular use of statin was associated with a 20% risk reduction of cancer.28 In a nationwide study, statin users had a 15% risk reduction cancer-related mortality, regardless of the administrative dose.10 These non-randomized studies without consideration for some indications of statin, however, may be susceptible to biases in allocation of treatment, and also are limited in their consideration of confounders including co-morbidities and concurrent drug uses. The vigorous adjustment of these confounders in our study provides compelling evidence of the chemoprotective role of statin in patients with HF.
Mechanisms of statin’s chemoprotective effects in patients with HF is uncertain but can be postulated by multiple pleiotropic properties of statin, in addition to the cholesterol-lowering effect. First, the presence of escalated inflammation and oxidative stress is a common milieu in HF and cancer. For instance, proinflammatory cytokines correlate with incident HF in the general population29 and are associated with adverse outcome in HF.30 Chronic inflammation correspondingly contributes to cancer initiation and progression which result in poorer outcome.31 The prominent anti-inflammatory properties of statin may thus lessen the development of cancer in patients with HF with an increased inflammatory load. Further, HF modulation of p53 dependent pathways, not only induce cardiac apoptosis33 but has also promoted carcinogenesis. The inhibition of the mevalonate pathway by statin, up-regulated by p53, reverts the malignancy potential and reduce the invasiveness of in situ cancers.34 Finally, the potential to halt the cell-cycle progression in cancer cells, as a result of the anti-proliferative effect of statin, may further justify the observed capacity to reduce the incidence of cancer, as well as cancer-related mortality in our patients with HF.35 Our results provide further support for the pleiotropic effects of statin, independent of LDL-cholesterol, in that neither the underlying indication for statin (atherosclerotic disease vs. hypercholesterolaemia) nor the extent of lipid control (measured by time-weighted mean LDL) was associated with incident cancer among statin users. It is noteworthy that despite the presence of an established indication for statin therapy, a substantial proportion of patients with coronary artery disease (23.3%), stroke (9%), and dyslipidaemia (5.2%) did not receive statins. Although the exact reason is uncertain, we postulate that a higher rate of statin intolerance among Asians may have contributed to the observed suboptimal adherence. Indeed, Asian ethnicity is included among the list of risk factors for statin-associated muscle symptoms by the European Atherosclerosis Society Consensus Panel Statement,36 and prior studies in Asian patients with atherosclerotic cardiovascular disease have shown discontinuation rates of up to 33% within 12 months of initiating either a statin or ezetimibe.37
There are several limitations in the present study. Risk factors such as a family history of cancer were not available. Nonetheless, variables such as family history are unlikely to impact drug prescription, thus conferring minimal confounder effects in drug–cancer association studies.38 Furthermore, the left ventricular ejection fraction of our patients was not recorded in the reporting system and thus the differential chemoprotective effect in HF patients with preserved and reduced ejection fraction of statin cannot be evaluated. Studies have consistently shown that HF was associated with cancer incidence, irrespective of left ventricular ejection fraction,4 , 5 , 32 indicating that our finding can conceivably be generalizable to a wide range of HF patients. Finally, it is possible that residual confounders remain despite utilizing propensity score analytics,
Strengths of the present study include the use of a territory-wide, well-validated electronic healthcare database (CDARS) with records of all diagnoses, hospitalizations, and details of drug dispenses, allowing the collection of the relevant information required to preclude common biases in conventional observational studies such as selection and recall biases. The validity of the current result is further improved by the adjustment of potential chemoprotective agents, such as metformin19 and aspirin,24 that is commonly prescribed concomitantly with statin. Furthermore, statin users are likely to have more comorbidities than nonusers, which minimize the concern of healthy user bias. The application of IPTW to an unselected population with HF with detailed clinical and medication history provides compelling evidence regarding the potential benefits of statin in the reduction of cancer risk and cancer-related mortality. In addition, we found that statin’s chemoprotective effect is present in a duration-response manner by using a time-varying model, indicative of a potential causal relationship. The reduction of cancer incidence and cancer-related mortality with at least 4 years of statin use is consistent with the result from other observational studies.24 , 39 , 40 The current study, therefore, provides robust evidence on the relationship between statin and risk of cancer in patients with HF through a thorough consideration of potential sources of confounding and biases.
Conclusion
In this large population-based cohort of patients with HF, we demonstrated that incident cancer was not uncommon; notably, statin use was associated with a reduced risk of cancer and cancer-related mortality. These findings have major clinical implications to reduce the associated burden in HF. The potential protective effect of statin on the development of cancer merits evaluation in future randomized studies.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by Sanming Project of Medicine in Shenzhen, China [No. SZSM201911020] and HKU-SZH Fund for Shenzhen Key Medical Discipline [No.SZXK2020081].
Permission information
The authors do hereby declare that all illustrations and figures in the manuscript are entirely original and do not require reprint permission.
Conflict of interest: none declared.
Data availability
Data are available upon reasonable request to Dr Yiu Kai-Hang.
Supplementary Material
Contributor Information
Qing-Wen Ren, Cardiology Division, Department of Medicine, The University of Hong Kong Shen Zhen Hospital, No. 1 Haiyuan 1st Rd, Futian district, Shenzhen city, Guangdong province, 518009, China; Cardiology Division, Department of Medicine, The University of Hong Kong, Queen Mary Hospital, Pok Fu Lam Rd 102. Hong Kong Island, Hong Kong, 999077, China.
Si-Yeung Yu, Cardiology Division, Department of Medicine, The University of Hong Kong Shen Zhen Hospital, No. 1 Haiyuan 1st Rd, Futian district, Shenzhen city, Guangdong province, 518009, China; Cardiology Division, Department of Medicine, The University of Hong Kong, Queen Mary Hospital, Pok Fu Lam Rd 102. Hong Kong Island, Hong Kong, 999077, China.
Tiew-Hwa Katherine Teng, Duke-NUS Medical School, 8 College Road, 169857, Singapore; Department of Cardiology, National Heart Center, 5 Hospital Dr, 169609, Singapore; School of Population & Global Health, University of Western, 35 Stirling Hwy, Crawley WA 6009, Australia.
Xue Li, Cardiology Division, Department of Medicine, The University of Hong Kong, Queen Mary Hospital, Pok Fu Lam Rd 102. Hong Kong Island, Hong Kong, 999077, China.
Ka-Shing Cheung, Cardiology Division, Department of Medicine, The University of Hong Kong, Queen Mary Hospital, Pok Fu Lam Rd 102. Hong Kong Island, Hong Kong, 999077, China.
Mei-Zhen Wu, Cardiology Division, Department of Medicine, The University of Hong Kong Shen Zhen Hospital, No. 1 Haiyuan 1st Rd, Futian district, Shenzhen city, Guangdong province, 518009, China; Cardiology Division, Department of Medicine, The University of Hong Kong, Queen Mary Hospital, Pok Fu Lam Rd 102. Hong Kong Island, Hong Kong, 999077, China.
Hang-Long Li, Cardiology Division, Department of Medicine, The University of Hong Kong, Queen Mary Hospital, Pok Fu Lam Rd 102. Hong Kong Island, Hong Kong, 999077, China.
Pui-Fai Wong, Cardiology Division, Department of Medicine, The University of Hong Kong, Queen Mary Hospital, Pok Fu Lam Rd 102. Hong Kong Island, Hong Kong, 999077, China.
Hung-Fat Tse, Cardiology Division, Department of Medicine, The University of Hong Kong Shen Zhen Hospital, No. 1 Haiyuan 1st Rd, Futian district, Shenzhen city, Guangdong province, 518009, China; Cardiology Division, Department of Medicine, The University of Hong Kong, Queen Mary Hospital, Pok Fu Lam Rd 102. Hong Kong Island, Hong Kong, 999077, China.
Carolyn S P Lam, Duke-NUS Medical School, 8 College Road, 169857, Singapore; Department of Cardiology, National Heart Center, 5 Hospital Dr, 169609, Singapore; University Medical Center Groningen, Department of Cardiology, Hanzeplein 1, 9713 GZ Groningen, The Netherlands.
Kai-Hang Yiu, Cardiology Division, Department of Medicine, The University of Hong Kong Shen Zhen Hospital, No. 1 Haiyuan 1st Rd, Futian district, Shenzhen city, Guangdong province, 518009, China; Cardiology Division, Department of Medicine, The University of Hong Kong, Queen Mary Hospital, Pok Fu Lam Rd 102. Hong Kong Island, Hong Kong, 999077, China.
References
- 1. Roger VL. Epidemiology of heart failure. Circ Res 2013;113:646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. The global challenge of cancer. Nat Cancer 2020;1:1–2. (Date Accessed 31 May 2021) [DOI] [PubMed] [Google Scholar]
- 3. Jones NR, Roalfe AK, Adoki I, Hobbs FDR, Taylor CJ. Survival of patients with chronic heart failure in the community: a systematic review and meta-analysis. Eur J Heart Fail 2019;21:1306–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hasin T, Gerber Y, McNallan SM, Weston SA, Kushwaha SS, Nelson TJ, Cerhan JR, Roger VL. Patients with heart failure have an increased risk of incident cancer. J Am Coll Cardiol 2013;62:881–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Banke A, Schou M, Videbaek L, Møller JE, Torp-Pedersen C, Gustafsson F, Dahl JS, Køber L, Hildebrandt PR, Gislason GH. Incidence of cancer in patients with chronic heart failure: a long-term follow-up study. Eur J Heart Fail 2016;18:260–266. [DOI] [PubMed] [Google Scholar]
- 6. Hasin T, Gerber Y, Weston SA, Jiang R, Killian JM, Manemann SM, Cerhan JR, Roger VL. Heart failure after myocardial infarction is associated with increased risk of cancer. J Am Coll Cardiol 2016;68:265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bertero E, Canepa M, Maack C, Ameri P. Linking heart failure to cancer: background evidence and research perspectives. Circulation 2018;138:735–742. [DOI] [PubMed] [Google Scholar]
- 8. de Boer RA, Hulot JS, Gabriele Tocchetti C, Aboumsallem JP, Ameri P, Anker SD, Bauersachs J, Bertero E, Coats AAJ, Čelutkienė J, Chioncel O, Dodion P, Eschenhagen T, Farmakis D, Bayes-Genis A, Jäger D, Jankowska EA, Kitsis RN, Konety SH, Larkin J, Lehmann L, Lenihan DJ, Maack C, Moslehi J, Müller OJ, Nowak-Sliwinska P, Piepoli MF, Ponikowski P, Pudil R, Rainer PP, Ruschitzka F, Sawyer D, Seferovic PM, Suter T, Thum T, van D, Meer P, Van Laake LW, von Haehling S, Heymans S, Lyon AR, Backs J. Common mechanistic pathways in cancer and heart failure. A scientific roadmap on behalf of the Translational Research Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail 2020;22:2272–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Juneja M, Kobelt D, Walther W, Voss C, Smith J, Specker E, Neuenschwander M, Gohlke B-O, Dahlmann M, Radetzki S, Preissner R, Von Kries JP, Schlag PM, Stein U. Statin and rottlerin small-molecule inhibitors restrict colon cancer progression and metastasis via MACC1. PLoS Biol 2017;15:e2000784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med 2012;367:1792–1802. [DOI] [PubMed] [Google Scholar]
- 11. Larsen SB, Dehlendorff C, Skriver C, Dalton SO, Jespersen CG, Borre M, Brasso K, Nørgaard M, Johansen C, Sørensen HT, Hallas J, Friis S. Postdiagnosis statin use and mortality in Danish patients with prostate cancer. J Clin Oncol 2017;35:3290–3297. [DOI] [PubMed] [Google Scholar]
- 12. Simon TG, Duberg AS, Aleman S, Hagstrom H, Nguyen LH, Khalili H, Chung RT, Ludvigsson JF. Lipophilic statins and risk for hepatocellular carcinoma and death in patients with chronic viral hepatitis: results from a nationwide Swedish population. Ann Intern Med 2019;171:318–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mullen PJ, Yu R, Longo J, Archer MC, Penn LZ. The interplay between cell signalling and the mevalonate pathway in cancer. Nat Rev Cancer 2016;16:718–731. [DOI] [PubMed] [Google Scholar]
- 14.Hospital Authority Statistical Report. 2018. https://www3.ha.org.hk/data/HAStatistics/StatisticalReport/2018-2019 (accessed 31 May 2021).
- 15. Wong AY, Root A, Douglas IJ, Chui CS, Chan EW, Ghebremichael-Weldeselassie Y, Siu CW, Smeeth L, Wong IC. Cardiovascular outcomes associated with use of clarithromycin: population based study. BMJ 2016;352:h6926. [DOI] [PubMed] [Google Scholar]
- 16. Cheung KS, Chan EW, Wong AYS, Chen L, Wong ICK, Leung WK. Long-term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori: a population-based study. Gut 2018;67:28–35. [DOI] [PubMed] [Google Scholar]
- 17. Cheung KS, Chen L, Chan EW, Seto WK, Wong ICK, Leung WK. Statins reduce the progression of non-advanced adenomas to colorectal cancer: a postcolonoscopy study in 187 897 patients. Gut 2019;68:1979–1985. [DOI] [PubMed] [Google Scholar]
- 18. Leung WK, Wong IOL, Cheung KS, Yeung KF, Chan EW, Wong AYS, Chen L, Wong ICK, Graham DY. Effects of Helicobacter pylori treatment on incidence of gastric cancer in older individuals. Gastroenterology 2018;155:67–75. [DOI] [PubMed] [Google Scholar]
- 19. Cheung KS, Chan EW, Wong AYS, Chen L, Seto WK, Wong ICK, Leung WK. Metformin use and gastric cancer risk in diabetic patients after Helicobacter pylori eradication. J Natl Cancer Inst 2019;111:484–489. [DOI] [PubMed] [Google Scholar]
- 20. Lau WCY, Chan EW, Cheung C-L, Sing CW, Man KKC, Lip GYH, Siu CW, Lam JKY, Lee ACH, Wong ICK. Association between dabigatran vs warfarin and risk of osteoporotic fractures among patients with nonvalvular atrial fibrillation. JAMA 2017;317:1151–1158. [DOI] [PubMed] [Google Scholar]
- 21. Coogan PF, Smith J, Rosenberg L. Statin use and risk of colorectal cancer. J Natl Cancer Inst 2007;99:32–40. [DOI] [PubMed] [Google Scholar]
- 22. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC Jr., Sperling L, Virani SS, Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;139:e1082–e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Giugliano RP, Wiviott SD, Blazing MA, De Ferrari GM, Park JG, Murphy SA, White JA, Tershakovec AM, Cannon CP, Braunwald E. Long-term safety and efficacy of achieving very low levels of low-density lipoprotein cholesterol: a prespecified analysis of the IMPROVE-IT trial. JAMA Cardiol 2017;2:547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Simon TG, Duberg A-S, Aleman S, Chung RT, Chan AT, Ludvigsson JF. Association of aspirin with hepatocellular carcinoma and liver-related mortality. N Engl J Med 2020;382:1018–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shintani AK, Girard TD, Eden SK, Arbogast PG, Moons KG, Ely EW. Immortal time bias in critical care research: application of time-varying Cox regression for observational cohort studies. Crit Care Med 2009;37:2939–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Conrad N, Judge A, Canoy D, Tran J, Pinho-Gomes AC, Millett ERC, Salimi-Khorshidi G, Cleland JG, McMurray JJV, Rahimi K. Temporal trends and patterns in mortality after incident heart failure: a longitudinal analysis of 86 000 individuals. JAMA Cardiol 2019;4:1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rasha F, Ramalingam L, Gollahon L, Rahman RL, Rahman SM, Menikdiwela K, Moustaid-Moussa N. Mechanisms linking the renin-angiotensin system, obesity, and breast cancer. Endocr Relat Cancer 2019;26:R653–R672. [DOI] [PubMed] [Google Scholar]
- 28. Graaf MR, Beiderbeck AB, Egberts AC, Richel DJ, Guchelaar HJ. The risk of cancer in users of statins. J Clin Oncol 2004;22:2388–2394. [DOI] [PubMed] [Google Scholar]
- 29. Kalogeropoulos A, Georgiopoulou V, Psaty BM, Rodondi N, Smith AL, Harrison DG, Liu Y, Hoffmann U, Bauer DC, Newman AB, Kritchevsky SB, Harris TB, Butler J, Health ABC, Study I. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol 2010;55:2129–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tsutamoto T, Hisanaga T, Wada A, Maeda K, Ohnishi M, Fukai D, Mabuchi N, Sawaki M, Kinoshita M. Interleukin-6 spillover in the peripheral circulation increases with the severity of heart failure, and the high plasma level of interleukin-6 is an important prognostic predictor in patients with congestive heart failure. J Am Coll Cardiol 1998;31:391–398. [DOI] [PubMed] [Google Scholar]
- 31. Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sakamoto M, Hasegawa T, Asakura M, Kanzaki H, Takahama H, Amaki M, Mochizuki N, Anzai T, Hamasaki T, Kitakaze M. Does the pathophysiology of heart failure prime the incidence of cancer? Hypertens Res 2017;40:831–836. [DOI] [PubMed] [Google Scholar]
- 33. Mak TW, Hauck L, Grothe D, Billia F. p53 regulates the cardiac transcriptome. Proc Natl Acad Sci U S A 2017;114:2331–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Freed-Pastor WA, Mizuno H, Zhao X, Langerød A, Moon SH, Rodriguez-Barrueco R, Barsotti A, Chicas A, Li W, Polotskaia A, Bissell MJ, Osborne TF, Tian B, Lowe SW, Silva JM, Børresen-Dale AL, Levine AJ, Bargonetti J, Prives C. Mutant p53 disrupts mammary tissue architecture via the mevalonate pathway. Cell 2012;148:244–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gauthaman K, Fong CY, Bongso A. Statins, stem cells, and cancer. J Cell Biochem 2009;106:975–983. [DOI] [PubMed] [Google Scholar]
- 36. Stroes ES, Thompson PD, Corsini A, Vladutiu GD, Raal FJ, Ray KK, Roden M, Stein E, Tokgözoğlu L, Nordestgaard BG, Bruckert E, De Backer G, Krauss RM, Laufs U, Santos RD, Hegele RA, Hovingh GK, Leiter LA, Mach F, März W, Newman CB, Wiklund O, Jacobson TA, Catapano AL, Chapman MJ, Ginsberg HN, Stroes E, Thompson PD, Corsini A, Vladutiu GD, Raal FJ, Ray KK, Roden M, Stein E, Tokgözoğlu L, Nordestgaard BG, Bruckert E, Krauss RM, Laufs U, Santos RD, März W, Newman CB, John Chapman M, Ginsberg HN, John Chapman M, Ginsberg HN, de Backer G, Catapano AL, Hegele RA, Kees Hovingh G, Jacobson TA, Leiter L, Mach F, Wiklund O; European Atherosclerosis Society Consensus Panel. Statin-associated muscle symptoms: impact on statin therapy—European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J 2015;36:1012–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nagar SP, Rane PP, Fox KM, Meyers J, Davis K, Beaubrun A, Inomata H, Qian Y, Kajinami K. Treatment patterns, statin intolerance, and subsequent cardiovascular events among Japanese patients with high cardiovascular risk initiating statin therapy. Circ J 2018;82:1008–1016. [DOI] [PubMed] [Google Scholar]
- 38. Pottegård A, Friis S, Stürmer T, Hallas J, Bahmanyar S. Considerations for pharmacoepidemiological studies of drug-cancer associations. Basic Clin Pharmacol Toxicol 2018;122:451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kang J, Jeong SM, Shin DW, Cho M, Cho JH, Kim J. Associations of aspirin, statins, and metformin with lung cancer risk and related mortality: time-dependent analysis of population-based nationally representative data. J Thorac Oncol 2020;20:30712–30717. [DOI] [PubMed] [Google Scholar]
- 40. Cheung KS, Chan EW, Wong AYS, Chen L, Seto WK, Wong ICK, Leung WK. Statins were associated with a reduced gastric cancer risk in patients with eradicated Helicobacter pylori infection: a territory-wide propensity score matched study. Cancer Epidemiol Biomarkers Prev 2020;29:493–499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request to Dr Yiu Kai-Hang.