Abstract
Currently, there have been more than one hundred million confirmed cases of coronavirus disease 2019 (COVID-19), with two million deaths worldwide. This has caused a huge medical burden. Severe COVID-19 patients can experience multi-organ damage, including cardiac injury, kidney injury, and liver injury. About 2.0%–4.9% of COVID-19 cases involve patients with preexisting liver diseases. Additionally, preexisting liver diseases were reported and associated with severity (odds ratio (OR) or risk ratio (RR) = 1.48–1.70) and mortality (OR or RR = 1.08–2.65) among COVID-19 patients. Furthermore, the prevalence of liver injury was 16%–29% in COVID-19 patients. Higher prevalence of liver injury may worsen prognosis in patients (severity: OR or RR = 1.9–2.6; mortality: OR or RR = 1.1–4.0). The mechanisms of this association between liver injury and severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) infection are complex, including direct cholangiocyte damage induced by SARS-COV-2, cytokine storm, and drug-induced liver injury. In particular, drug-induced liver injury may be the most important reason. This review discusses the epidemiology of COVID-19 and liver dysfunction as well as potential mechanisms underlying the association between COVID-19 and liver dysfunction or other preexisting liver diseases. However, the association between preexisting liver diseases and COVID-19 prognosis and potential mechanisms underlying these associations require further prospective studies.
Keywords: Liver dysfunction, COVID-19, Systematic review
Introduction
Coronavirus disease 2019 (COVID-19) has become a major public health problem. According to the World Health Organization (WHO), there have been more than one hundred million confirmed cases of COVID-19, with two million deaths worldwide [1]. The COVID-19 pandemic is a major threat to public health, and its common symptoms include fever and dry cough, which are secondary to lung involvement [2]. Severe patients may present a systemic and multi-organ disease [2].
Current studies have shown that COVID-19 patients may present with gastrointestinal symptoms including diarrhea, nausea, and vomiting [3], [4], [5]. Additionally, growing evidence shows that hepatic dysfunction is common among COVID-19 patients, which should be of concern [6]. One meta-analysis found that the rate of liver dysfunction among COVID-19 patients was 27.4% [7]. Severe patients may present with higher rates of liver dysfunction, and patients with abnormal liver function may have higher risks of progressing to severe disease [8]. This review introduces epidemiological aspects of COVID-19 and discusses its underlying mechanisms. Finally, associations between COVID-19 prognosis and liver dysfunction/preexisting liver diseases are discussed to provide a reference for better patient management according to recent available information.
COVID-19
According to the World Health Organization (WHO) dashboard (as of March 5, 2021), there were 115,289,961 cumulative reported cases of COVID-19 globally, with the vast majority from the Americas (44%), Europe (34%), and Southeast Asia (12%) as of [1]. Meta-analysis showed that a higher proportion of infected patients were male 53.3%–57.8% [9], [10], [11], [12]. Until March 5, 2021, nearly half of the deaths occurred in the Americas (1,227,085; 48%), followed by Europe (878,731; 34%) and Southeast Asia (209,729; 8%) [1]. The prevalence rate of mortality among hospitalized COVID-19 patients was 18.88% [13]. It was reported that men were more likely to have severe pneumonia than women [13,14]. Additionally, older age, obesity, and preexisting diseases were risk factors for mortality among hospitalized COVID-19 patients [13]. For example, a population study found that patients who were diagnosed with nonalcoholic steatohepatitis were at higher risk to infection with COVID-19 [15]. Although there have been relative reductions in case incidences in several countries recently, the ongoing and prolonged high rates of new infections continue to strain health systems in countries around the world [1].
COVID-19 patients can be asymptomatic or present with multiple clinical symptoms. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can be transmitted by asymptomatic patients [16]. There were 1.2% asymptomatic cases among 44,672 patients with COVID-19 in China [17]. From April 1, 2020 to May 14, 2020, a total of 1,303 asymptomatic infections from the Chinese mainland were reported, 3.3% of which progressed to symptomatic cases during this period [18]. Approximately 97.5% of people infected with COVID-19 will develop symptoms within 11.5 days [19].
COVID-19 infection, which has various clinical manifestations, can be classified into three stages: stage I (early infection), stage II (pulmonary phase), and stage III (hyperinflammation phase)[20]. Patients may have mild and nonspecific symptoms (e.g., malaise, fever, and dry cough), and then develop viral pneumonia with cough, fever, and possibly hypoxia. Finally, the disease may manifest as shock, respiratory failure, and even cardiopulmonary collapse [20]. In a study of 44,672 patients with COVID-19 in China, 81% of patients had mild manifestations, 14% had severe manifestations, and 5% had critical manifestations (defined by respiratory failure, septic shock, and/or multiple organ dysfunction) [17]. Children with COVID-19 usually have mild symptoms limited to the upper respiratory tract, except for patients younger than one year of age [21]. In contrast, older patients with underlying comorbidities may progress to severe cases more often [22,23]. Polymerase chain reaction testing via nasal swab is a typical diagnosis method for COVID-19 infection, and clinical, laboratory, and imaging findings may also be used to make a presumptive diagnosis [23].
Pathogenic mechanisms mainly include two steps. (1) Direct invasion: during early infection, SARS-CoV-2 enters target cells through the viral structural spike (S) protein, which binds to the angiotensin-converting enzyme 2 (ACE2) receptor [24]. This receptor is present in nasal and bronchial epithelial cells, pneumocytes, epithelia of the gastrointestinal tract, vascular endothelium, and the liver [25]. Additionally, the type 2 transmembrane serine protease (TMPRSS2), which exists in host target cells (particularly alveolar epithelial type II cells) promotes viral uptake [24]. (2) Cytokine storm: a minority of infected patients may experience extrapulmonary systemic hyperinflammation syndrome [8]. Levels of several cytokine types increase, including interleukin (IL)-2, IL-6, IL-7, IL-10, and tumor necrosis factor (TNF) α [5]. Other inflammatory biomarkers, including granulocyte-colony stimulating factor, interferon (IFN)-γ inducible protein 10, and monocyte chemoattractant protein 1, also increase [26]. Importantly, severe disease will present with significantly elevated cytokine levels [27]. A meta-analysis found that IL-6 and IL-10 and serum ferritin are strong discriminators for severe COVID-19 disease [28].
COVID-19 and the liver
Relationship between preexisting liver diseases and prognosis of COVID-19 patients
According to previous studies, preexisting liver diseases in COVID-19 patients are usually comprised of chronic hepatitis B, chronic hepatitis C, metabolic associated fatty liver disease (MAFLD), alcohol related liver disease (ALD), autoimmune hepatitis, cirrhosis, and hepatocellular carcinoma (HCC) [29], [30], [31]. The prevalence of baseline preexisting liver diseases is low (ranging from 1%–11%) [32], [33], [34]. However, the prevalence of preexisting liver diseases among COVID-19 patients is 2.0%–4.9% based on recent meta-analysis [7,[29], [30], [31],[35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45]]. Furthermore, Kumar et al. found that the prevalence of hepatitis B and MAFLD was 1.76% and 3.78%, respectively, in China [29]. Richardson et al. reported that there were three (0.1%) and eight (0.1%) cases of chronic hepatitis C and B among 5,700 patients hospitalized with COVID-19 in the New York City area [46]. Additionally, there were two COVID-19 patients reported to have liver cancer [47]. Mallet et al. reported that 820 (0.32%) patients with chronic hepatitis B, 711 (2.74%) patients with chronic hepatitis C, 719 (0.28%) patients with primary liver cancer among 259,110 adult patients with COVID-19 in France [34].
Preexisting liver diseases may have adverse effects on COVID-19 prognosis, including severity, death, and mechanical ventilation. Zhou et al. demonstrated that in patients aged less than 60 years with COVID-19, MAFLD was associated with an approximately four-fold increase in the probability for severe disease after adjusting for confounders [48]. Wu et al. [39], Kumar et al. [29], Kovalic et al. [31], Dorjee et al. [43], and Barek et al.[49] all found that preexisting liver diseases were associated with severity (odds ratio (OR) or risk ratio (RR)= 1.48–1.70). Kumar et al. [29] further reported that the risk of severity among COVID-19 patients with MAFLD was 1.33 times higher than those without MAFLD (RR = 1.33, 95% CI: 0.51–3.45). A large However, most meta-analyses showed that there was no association between preexisting liver diseases and the severity of COVID-19 [29,30,35,36,[50], [51], [52], [53], [54]].
Similarly, results regarding the relationship between preexisting liver diseases and COVID-19 mortality are inconsistent [12,31,40,43,55]. Some studies showed that there was an association between preexisting liver diseases and number of COVID-19 deaths (OR/RR = 1.08–2.65) [31,40,43], while others did not [12,55]. A French national retrospective cohort study reported that patients with mild liver disease, compensated cirrhosis were not at risk for COVID-19 mortality, while patients with decompensated cirrhosis or primary liver cancer were at high risk for COVID-19 mortality [34]. These differences may be related to the included studies and different definitions of outcomes [31,53].
Currently, based on available evidence, the proportion of COVID-19 patients with baseline preexisting liver diseases is lower than other comorbidities (e.g., diabetes, hypertension, and chronic kidney disease) [44,56]. However, preexisting liver diseases may still have an impact on COVID-19 prognosis. The underlying mechanisms may be related to the fact that MAFLD is a proinflammatory hypercoagulable state that is associated with severe disease and thrombosis in COVID-19 [57]. Nevertheless, the prevalence of preexisting liver diseases was similar in populations with and without COVID-19 (p = 0.10) [31]. Given the small number of patients with preexisting liver diseases analyzed, the effects of preexisting liver diseases on COVID-19 severity require further study [51].
Additionally, liver transplantation (LT) is considered the ultimate solution for patients with end-stage chronic liver disease or acute liver failure [58,59]. Mallet et al. reported that 329 (1.27%) patients with a liver transplant among 259,110 adult patients with COVID-19 in France [34]. However, patients needed LT may are at risk of wait list mortality due to COVID-19 infection [58, 59]. The other important issue was the outcomes of LT recipient with COVID-19. A prospective Spanish study of 111 LT recipients with COVID-19 showed 18% mortality, but the mortality is similar or even lower to population without liver transplantation [60]. Another study also found that 28 (19%) recipients died, while higher mortality in the patients who had not undergone liver transplantation 167 (27%) [61]. Transplanted patients have to receive immunosuppressive therapy which may be protective against cytokine storm induced by COVID-19 [58,59]. Hence, the outcome of this population is a complex interplay of comorbidities and immunosuppression [59]. In addition, LT recipients especially COVID-19 asymptomatic recipients on immunosuppression who had more intense and prolonged shedding of the virus may had the higher risk of transmission to contacts including healthcare worker [58,59]. There should have an attention on the care of people with liver disease, especially for those after liver transplantation, the management including exposure to medical staff, appropriate immunosuppressive therapy should be cautious [58,62].
Relationship between liver injury and prognosis of COVID-19 patients
COVID-19 patients with gastrointestinal symptoms may have an increased risk of liver injury (OR=2•71, 95% CI: 1•52–4•83) [63]. Liver injury diagnosis mainly depends on biochemical indicators including alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), alkaline phosphatase (ALP), gamma-glutamyl transpeptidase (GGT), albumin (ALB), and prothrombin time (PT) [64].
Most meta-analysis showed that the prevalence of liver injury was about 16%–29% [7,9,10,38,45,53,63,[65], [66], [67], [68]]. Additionally, the prevalence of liver injury during hospitalization was higher than that at admission [30,69]. Older patients experienced liver injury at a higher prevalence [30,63,68], while meta regression showed that the prevalence of acute liver injury had no relationship with age (coefficient = 0.012, P = 0.110)[68]. For specific biochemical indicators, AST, ALT, and TBIL were common indictors. The levels of AST, ALT, TBIL, globulin, GGT, ALP, and PT increased, while levels of ALB decreased among COVID-19 patients with liver injury during COVID-19 progression. The proportion of increased AST was 15%–34% [7,29,30,35,37,63,67,[70], [71], [72], [73], [74], [75]]. Labenz et al.[72]reported that the mean AST value was 33 U/L among COVID-19 patients, and Bansal et al.[76]reported that the mean AST value was 27.28 IU/L among COVID-19 patients. The proportion of increased ALT was 15%–28% [7,29,30,35,37,63,67,[70], [71], [72], [73], [74], [75]].
Additionally, Labenz et al. [72] reported that the mean ALT value was 31 unit/ liter (U/I), and Bansal et al. [76] reported that the mean ALT value was 24.44 liter international unit/ liter (IU/L). Liu et al. also found that Western populations showed abnormal indicators, including AST and ALT levels, more clearly than Eastern populations [67]. Abnormal levels of AST and ALT were reported more commonly in studies than other indictors. Additionally, some studies reported changes in biochemical indicators at admission and during hospitalization [69] as well as changes in biochemical indicators among different age populations [30,68]. Although evidence showed that there were no differences in ALT and AST abnormalities between COVID-19 patients and non-COVID-19 patients [29], considering the aforementioned information, the prevalence of liver injury and abnormal biochemical indicators were high among COVID-19 patients.
Liver dysfunction was associated with poor outcomes, including severity (OR/RR = 1.9–2.6) [11,29,38,63] and death (OR/RR = 1.1–4.0) among COVID-19 patients [38,[77], [78], [79]]. Váncsa et al. [78] found that liver failure was associated with mortality (OR = 7.59, 95% CI: 1.84–31.30). Sharma et al.[53] reported that acute liver injury (ALI) was associated with poor outcome, defined by intensive care unit (ICU) admission, oxygen saturation of <90%, invasive mechanical ventilation (IMV) utilization, severe disease, and in-hospital mortality. Additionally, Kulkarni et al. [30] found that elevated liver chemistries at initial presentation were associated with death and severe disease, while the relationship between elevated liver chemistries and death was not significant for elevated liver chemistries that developed during the illness.
Cai et al. found that 14.8% of COVID-19 patients had liver injury, which was more common among severe patients than non-severe patients (36.2% vs. 9.6%)[56]. Moreover, elevated liver enzymes were more frequent in males (OR: 1.52; 95% CI: 1.26, 1.83) with severe COVID-19 than in females [80]. Regarding specific liver biochemical indicators, AST and ALT levels were commonly reported, followed by TBIL and other indicators. Most meta-analysis showed that increased AST levels were associated with severity (OR/RR = 2.3–4.5) [7,29,32,35,50,69,73,77,78,81] and mortality (OR/RR = 4.4–10.4) [73,78,82], except for one study that found no association between increased AST and severity [82]. Furthermore, increased ALT levels were also associated with severity (OR/RR = 1.8–2.5) [7,29,32,35,69,73,78,81] and mortality (OR/RR = 1.4–2.4) [73,78,82].
While there are still contrary results [69,77,82]. Cai et al. found that serum biochemical indexes of the liver (e.g., ALT, AST, ALP, and GGT) were significantly increased among severe patients at admission and during hospitalization. Peak values of ALT, AST, TBIL, and GGT were also increased significantly among severe patients compared with non-severe patients [56]. Wang et al. [83] found that abnormalities in two or more liver function indexes were low in patients with COVID-19, but this was more likely to occur in the severe group (in this study, among 105 adult patients). According to existing evidence, liver injury and abnormal liver biochemical indictors are associated with poor prognosis among COVID-19 patients.
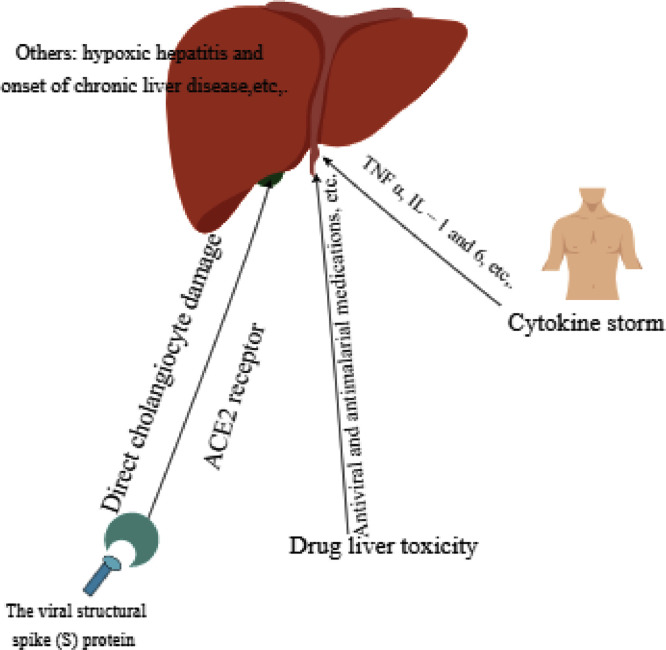
Mechanisms of liver damage in COVID-19 patients (Fig. 1)
Fig. 1.
Possible mechanisms of liver damage in COVID-19 patients. ACE2: angiotensin-converting enzyme 2; IL: interleukin; TNF: tumor necrosis factor.
Previous studies explored the mechanisms of liver damage in COVID-19 patients. The mechanisms are complex and mainly include direct cholangiocyte damage induced by SARS-COV-2, cytokine storm, and drug-induced liver injury. Drug-induced liver injury may be the most important reason ( Fig. 1 ).
Direct cholangiocyte damage of SARS-COV-2
Qi et al. found that bile duct epithelial cells highly express the ACE2 receptor, and its expression in bile duct cells is 20 times higher than in liver cells [84,85]. This suggests that SARS-COV-2 may directly infect bile duct cells, injure bile duct cells, and further lead to bile duct dysfunction. Furthermore, bile duct epithelial cells play a key role in liver regeneration and immune response. Therefore, SARS-COV-2 may invade liver cells and cause liver injury by infecting bile duct cells and causing cholestasis [84,85].
Elevated levels of ALP and GGT are sensitive indicators of bile duct epithelial cell injury [69]. Wu et al. [69] reported that the prevalence of abnormal GGT levels at admission was 35.8%. However, most studies showed that abnormal AST and ALT levels were more common than abnormal GGT and ALP levels [29,30,70,73]. Therefore, the suggestion that damage to bile duct cells caused by SARS-COV-2 further damages liver cells warrants more research. Researchers have speculated that the compensatory hyperplasia of hepatic parenchymal cells derived from bile duct epithelial cells induce the upregulation of receptor ACE2 expression in liver tissue, which may be a possible mechanism of liver injury caused by SARS-COV-2 infection of liver cells[86].
Cytokine storm
COVID-19 patients may experience extrapulmonary systemic hyperinflammation syndrome [8]. Cytokine levels and inflammatory biomarkers [e.g., IL-2, IL-6, IL-7, IL-10, interferon (IFN)-γ inducible protein 10, and tumor necrosis factor (TNF)] α increase [5,26], especially in severe cases [27]. A meta-analysis reported that IL-6 and IL-10 and serum ferritin are strong discriminators for severe disease [28,56]. However, the liver is an important immune function organ and contains a large number of cells related to the immune response. It can promote immune activity after virus infection by activating immune cells, such as Kupffer cells [87].
Furthermore, cytokine storm, including elevated TNF α, IL-1, and IL-6 levels, can be prompted nonspecific inflammation of the liver and cause extensive damage (e.g., hepatomegaly, elevated serum transaminase, hyperbilirubin, hepatic encephalopathy, or even liver failure). If the inflammatory response syndrome progresses without further control, it can develop into multiple organ function failure and death in COVID-19 patients [2,5]. Patients with severe COVID-19 had significantly higher levels of C reactive protein (CRP), TNFα, and IL-6, which suggests that enhanced inflammation may be associated with COVID-19-related liver damage [88]. However, one meta-analysis showed significantly lower absolute lymphocyte counts in the liver injury group compared with the non-liver injury group and no remarkable differences in CRP between the groups [38]. Therefore, hyperinflammation may be harmful to liver function, but liver damage may not necessarily be caused by hyperinflammation.
Drug-induced liver injury
The liver is the main organ responsible for drug metabolism in the human body. Drugs used by COVID-19 patients during the treatment process may cause liver damage. Kulkarni et al. reported that the prevalence of drug-induced liver injury was 25.4% [30]. Antimalarial medications, including chloroquine and hydroxychloroquine, have an emergency authorization for use in COVID-19 in the United States. Hydroxychloroquine is known to concentrate in the liver; thus, patients with hepatitis or other hepatic diseases or those taking other known hepatotoxic drugs, should be cautious [75].
Additionally, antiviral medications, including remdesivir, lopinavir–ritonavir, and favipiravir have been used in COVID-19 patients. One case series found that patients who were administered remdesivir had the most common hepatotoxicity, 23% of which reported elevations in hepatic enzymes associated with remdesivir [89]. Kulkarni et al. reported that the prevalence of remdesivir-induced liver injury was 15.2% [30]. The antiviral drugs lopinavir–ritonavir, which are mainly metabolized by the liver, can induce liver inflammation and lipid metabolism disorders by activating endoplasmic reticulum stress pathways in the liver. Additionally, they can cause hepatocyte apoptosis through the caspase system [90,91]. The proportion of subjects using lopinavir–ritonavir (ranging from 14%–100%) was reported in 12 studies [32].
Cai and colleagues found that compared to patients who were not administered the abovementioned drugs that may lead to liver dysfunction, patients who used lopinavir–ritonavir had a higher risk of liver injury (OR 4.44; 95% CI: 1.50–13.17) and showed much higher levels of TBIL and GGT during hospitalization [92]. Fan et al. reported that a significantly higher proportion of patients with abnormal liver function (57.8%) received lopinavir–ritonavir after admission compared with patients with normal liver function (31.3%)[90]. Yadav et al. found that the liver injury group was more frequently given lopinavir–ritonavir than the group without liver injury (OR: 4.15, 95% CI 2.36 to 7.29) [38].
Favipiravir, used in conjunction with interferon-alfa for COVID-19 treatment, was reported with a prevalence of liver injury of 2.9% in a random controlled trial [93]. Ibuprofen is one of the most commonly used nonsteroidal antiinflammatory drugs (NSAIDs) in the clinical setting, and it can induce abnormal liver function (and even acute vanishing duct syndrome) in patients [94,95]. However, Cai et al. and Yadav et al. found no significant evidence showing that the use of such drugs, including antibiotics, NSAIDs, ribavirin, herbal medications, and interferon, led to a higher risk of liver injury, except for lopinavir–ritonavir)[38,92].
Additionally, the rate of abnormal liver function in the combined medication group was higher than that in the single medication group [96]. One study [39] compared the proportion of drug products ≥ 3 kinds between patients with liver injury and those with normal liver biochemistry. It demonstrated that the proportion of drug products ≥ 3 kinds was significantly higher in patients with liver injury than in those with normal liver biochemistry (RR = 9.00, 95% CI 1.28–63.26)[69]. Wong et al.[32] found that lopinavir–ritonavir at high dosages induced higher risks of ALT elevation and hyperbilirubinemia compared with lower dosages. Mixed medications and longer durations of medication administration induced greater risk of liver damage. Therefore, it is believed that drug-induced liver injury is an important mechanism leading to liver injury in COVID-19 patients.
Considering the aforementioned information, lopinavir–ritonavir should be administered with caution. Additionally, it is necessary to take the liver injury caused by certain drugs into consideration to avoid unnecessary liver injury and clinical treatment burden, especially in severe patients with underlying liver disease [11]. Hence, lopinavir–ritonavir as a routine treatment option for patients with a history of liver dysfunction is not recommended. Clinicians should closely monitor liver function indicators, even in patients with baseline normal liver function, if they are being treated with lopinavir–ritonavir [39]. There are other possible mechanisms that affect COVID-19 prognosis, such as hypoxic hepatitis [97,98] and onset of chronic liver disease [42].
Conclusions
About 2.0%–4.9% of COVID-19 patients have preexisting liver disease. Additionally, limited evidence has shown that there is an association between preexisting liver diseases and adverse outcomes, while most evidence has not. Liver involvement during COVID-19 infection may affect about 16%–29% of patients, with higher prevalence in adult and elderly patients. The manifestations of liver damage mainly include elevated AST and ALT levels. Thus, the appearance of liver involvement during COVID-19 requires attention. While most related original studies were retrospective designs[54], they did not clearly define acute liver injury and chronic liver diseases[79].
Evidence from prospective cohort studies is still lacking. However, the underlying mechanisms are complex, including direct cholangiocyte damage induced by SARS-COV-2, cytokine storm, and drug-induced liver toxicity. Drug-induced liver toxicity may be the most likely reason; therefore, it is necessary to take the liver injury caused by certain drugs into consideration in order to avoid unnecessary liver injury, especially in severe patients with underlying liver disease.
Furthermore, liver injury and specific liver biochemical markers are associated with COVID-19 mortality and severity, so scientific management of patients with liver injury is needed. Additionally, the association between preexisting liver diseases and prognosis, as well as potential mechanisms underlying the association between COVID-19 infection and liver dysfunction/preexisting liver diseases, require further study. As this study mainly collected and analyzed epidemiological data from meta-analyses, the included original studies may have effects on the estimated results.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Acknowledgments
Author Contributions
Liu J conceived and designed the review study; Du M conducted the literature search and wrote the manuscript. Liu J, Liu M, Yang S and Du M revised the manuscript.
Funding
Supported by the National Natural Science Foundation of China (72122001, 71934002), National Key Research and Development Project of China (2020YFC0846300, 2019YFC1710301, 2018YFC1704400), and National Science and Technology Key Projects on Prevention and Treatment of Major Infectious Diseases of China (2020ZX10001002).
Footnotes
COVID-19 and Liver Dysfunction: Epidemiology, Association, and Potential Mechanisms
Refrences
- 1.World Health Organization. WHO coronavirus disease (COVID-19) situation dashboard. 2020 [cited 6 March 2021]. Available from: https://www.who.int/
- 2.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [PMID: 32031570 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong SH, Lui RN, Sung JJ. Covid-19 and the digestive system. J Gastroenterol Hepatol. 2020;35:744–748. doi: 10.1111/jgh.15047. [PMID: 32215956 DOI:] [DOI] [PubMed] [Google Scholar]
- 4.Gao QY, Chen YX, Fang JY. 2019 Novel coronavirus infection and gastrointestinal tract. J Dig Dis. 2020;21:125–126. doi: 10.1111/1751-2980.12851. [PMID: 32096611 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [PMID: 31986264 DOI: https://doi.org/] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bangash MN, Patel J, Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5:529–530. doi: 10.1016/s2468-1253(20)30084-4. [PMID: 32203680 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wan J, Wang X, Su S, Zhang Y, Jin Y, Shi Y, et al. Digestive symptoms and liver injury in patients with coronavirus disease 2019 (COVID-19): a systematic review with meta-analysis. JGH Open. 2020;4:1047–1058. doi: 10.1002/jgh3.12428. [PMID: 33319036 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Portincasa P, Krawczyk M, Machill A, Lammert F, Di Ciaula A. Hepatic consequences of COVID-19 infection. Lapping or biting? Eur J Intern Med. 2020;77:18–24. doi: 10.1016/j.ejim.2020.05.035. [PMID: 32507608 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu J, Ji P, Pang J, Zhong Z, Li H, He C, et al. Clinical characteristics of 3062 COVID-19 patients: a meta-analysis. J Med Virol. 2020;92:1902–1914. doi: 10.1002/jmv.25884. [PMID: 32293716 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu J, Zhong Z, Ji P, Li H, Li B, Pang J, et al. Clinicopathological characteristics of 8697 patients with COVID-19 in China: a meta-analysis. Fam Med Community Health. 2020;8 doi: 10.1136/fmch-2020-000406. [PMID: 32371463 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Youssef M, H, Hussein M, Attia AS, M, Elshazli R, Omar M, Zora G, et al. COVID-19 and liver dysfunction: a systematic review and meta-analysis of retrospective studies. J Med Virol. 2020;92:1825–1833. doi: 10.1002/jmv.26055. [PMID: 32445489 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ssentongo P, Ssentongo AE, Heilbrunn ES, Ba DM, Chinchilli VM. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: a systematic review and meta-analysis. PloS one. 2020;15 doi: 10.1371/journal.pone.0238215. [PMID: 32845926 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noor FM, Islam MM. Prevalence and associated risk factors of mortality among COVID-19 patients: a meta-analysis. J Community Health. 2020;45:1270–1282. doi: 10.1007/s10900-020-00920-x. [PMID: 32918645 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu L, Mao Y, Chen G. Risk factors for 2019 novel coronavirus disease (COVID-19) patients progressing to critical illness: a systematic review and meta-analysis. Aging (Albany NY) 2020;12:12410–12421. doi: 10.18632/aging.103383. [PMID: 32575078 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghoneim S, Butt MU, Hamid O, Shah A, Asaad I. The incidence of COVID-19 in patients with metabolic syndrome and non-alcoholic steatohepatitis: a population-based study. Metabol Open. 2020;8 doi: 10.1016/j.metop.2020.100057. [PMID: 32924000 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han Y, Yang H. The transmission and diagnosis of 2019 novel coronavirus infection disease (COVID-19): a Chinese perspective. J Med Virol. 2020;92:639–644. doi: 10.1002/jmv.25749. [PMID: 32141619 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua liuxingbingxue zazhi. 2020;41:145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [PMID: 32064853 DOI:] [DOI] [PubMed] [Google Scholar]
- 18.Jia X, Chen J, Li L, Jia N, Jiangtulu B, Xue T, et al. Modeling the prevalence of asymptomatic COVID-19 infections in the Chinese Mainland. Innovation (N Y) 2020;1 doi: 10.1016/j.xinn.2020.100026. [PMID: 32914140 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577–582. doi: 10.7326/m20-0504. [PMID: 32150748 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [PMID: 32362390 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tezer H, Bedir Demirdağ T. Novel coronavirus disease (COVID-19) in children. Turk J Med Sci. 2020;50:592–603. doi: 10.3906/sag-2004-174. [PMID: 32304191 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai CC, Liu YH, Wang CY, Wang YH, Hsueh SC, Yen MY, et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths. J Microbiol Immunol Infect. 2020;53:404–412. doi: 10.1016/j.jmii.2020.02.012. [PMID: 32173241 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [PMID: 32648899 DOI:] [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and Is blocked by a clinically proven protease Inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e278 [PMID: 32142651 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [PMID: 15141377 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet (London, England) 2020;395:1033–1034. doi: 10.1016/s0140-6736(20)30628-0. [PMID: 32192578 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu H, Zhu H, Yuan C, Yao C, Luo W, Shen X, et al. Clinical and immune features of hospitalized pediatric patients with coronavirus disease 2019 (COVID-19) in Wuhan, China. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.10895. [PMID: 32492165 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58:1021–1028. doi: 10.1515/cclm-2020-0369. [PMID: 32286245 DOI:] [DOI] [PubMed] [Google Scholar]
- 29.Kumar MP, Mishra S, Jha DK, Shukla J, Choudhury A, Mohindra R, et al. Coronavirus disease (COVID-19) and the liver: a comprehensive systematic review and meta-analysis. Hepatol Int. 2020;14:711–722. doi: 10.1007/s12072-020-10071-9. [PMID: 32623633 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kulkarni AV, Kumar P, Tevethia HV, Premkumar M, Arab JP, Candia R, et al. Systematic review with meta-analysis: liver manifestations and outcomes in COVID-19. Aliment Pharmacol Ther. 2020;52:584–599. doi: 10.1111/apt.15916. [PMID: 32638436 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kovalic AJ, Satapathy SK, Thuluvath PJ. Prevalence of chronic liver disease in patients with COVID-19 and their clinical outcomes: a systematic review and meta-analysis. Hepatol Int. 2020;14:612–620. doi: 10.1007/s12072-020-10078-2. [PMID: 32725453 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong YJ, Tan M, Zheng Q, Li JW, Kumar R, Fock KM, et al. A systematic review and meta-analysis of the COVID-19 associated liver injury. Ann Hepatol. 2020;19:627–634. doi: 10.1016/j.aohep.2020.08.064. [PMID: 32882393 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H, Qiu P, Liu J, Wang F, Zhao Q. The liver injury and gastrointestinal symptoms in patients with coronavirus disease 19: a systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2020;44:653–661. doi: 10.1016/j.clinre.2020.04.012. [PMID: 32418852 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mallet V, Beeker N, Bouam S, Sogni P, Pol S, Demosthenes research group. Prognosis of French COVID-19 patients with chronic liver disease: a national retrospective cohort study for 2020. J Hepatol. 2021:S0168-8278(21)00329-9. doi: 10.1016/j.jhep.2021.04.052. Epub ahead of print. PMID: 33992699. [DOI] [PMC free article] [PubMed]
- 35.Dong ZY, Xiang BJ, Jiang M, Sun MJ, Dai C. The prevalence of gastrointestinal symptoms, abnormal liver function, digestive system disease and liver disease in COVID-19 infection: a systematic review and meta-analysis. J Clin Gastroenterol. 2021;55:67–76. doi: 10.1097/MCG.0000000000001424. [PMID: 33116063 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Y, Yang Q, Chi J, Dong B, Lv W, Shen L, et al. Comorbidities and the risk of severe or fatal outcomes associated with coronavirus disease 2019: a systematic review and meta-analysis. Int J Infect Dis. 2020;99:47–56. doi: 10.1016/j.ijid.2020.07.029. [PMID: 32721533 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zarifian A, Zamiri Bidary M, Arekhi S, Rafiee M, Gholamalizadeh H, Amiriani A, et al. Gastrointestinal and hepatic abnormalities in patients with confirmed COVID-19: a systematic review and meta-analysis. J Med Virol. 2020;93:336–350. doi: 10.1002/jmv.26314. [PMID: 32681674 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yadav DK, Singh A, Zhang Q, Bai X, Zhang W, Yadav RK, et al. Involvement of liver in COVID-19: systematic review and meta-analysis. Gut. 2020; gutjnl-2020-322072 [PMID: 32669289 DOI: 10.1136/gutjnl-2020-322072 ] [DOI] [PMC free article] [PubMed]
- 39.Wu T, Zuo Z, Kang S, Jiang L, Luo X, Xia Z, et al. Multi-organ dysfunction in patients with COVID-19: a systematic review and meta-analysis. Aging Dis. 2020;11:874–894. doi: 10.14336/ad.2020.0520. [PMID: 32765952 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel U, Malik P, Usman MS, Mehta D, Sharma A, Malik FA, et al. Age-adjusted risk factors associated with mortality and mechanical ventilation utilization amongst COVID-19 hospitalizations-a systematic review and meta-analysis. SN Compr Clin Med. 2020:1–10. doi: 10.1007/s42399-020-00476-w. [PMID: 32904541 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oyelade T, Alqahtani J, Canciani G. Prognosis of COVID-19 in patients with liver and kidney diseases: an early systematic review and meta-analysis. Trop Med Infect Dis. 2020;5:80. doi: 10.3390/tropicalmed5020080. [PMID: 32429038 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mantovani A, Beatrice G, Dalbeni A. Coronavirus disease 2019 and prevalence of chronic liver disease: a meta-analysis. Liver Int. 2020;40:1316–1320. doi: 10.1111/liv.14465. [PMID: 32329563 DOI:] [DOI] [PubMed] [Google Scholar]
- 43.Dorjee K, Kim H, Bonomo E, Dolma R. Prevalence and predictors of death and severe disease in patients hospitalized due to COVID-19: a comprehensive systematic review and meta-analysis of 77 studies and 38,000 patients. PloS one. 2020;15 doi: 10.1371/journal.pone.0243191. [PMID: 33284825 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baradaran A, Ebrahimzadeh MH, Baradaran A, Kachooei AR. Prevalence of comorbidities in COVID-19 patients: a systematic review and meta-analysis. Arch Bone Jt Surg. 2020;8:247–255. doi: 10.22038/abjs.2020.47754.2346. [PMID: 32733980 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahmed J, Rizwan T, Malik F, Akhter R, Malik M, Ahmad J, et al. COVID-19 and liver injury: a systematic review and meta-analysis. Cureus. 2020;12:e9424. doi: 10.7759/cureus.9424. [PMID: 32864250 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [PMID: 32320003 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang L, Zhu F, Xie L, Wang C, Wang J, Chen R, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31:894–901. doi: 10.1016/j.annonc.2020.03.296. [PMID: 32224151 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou YJ, Zheng KI, Wang XB, Sun QF, Pan KH, Wang TY, et al. Metabolic-associated fatty liver disease is associated with severity of COVID-19. Liver Int. 2020;40:2160–2163. doi: 10.1111/liv.14575. [PMID: 32573883 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barek MA, Aziz MA, Islam MS. Impact of age, sex, comorbidities and clinical symptoms on the severity of COVID-19 cases: a meta-analysis with 55 studies and 10014 cases. Heliyon. 2020;6:e05684. doi: 10.1016/j.heliyon.2020.e05684. [PMID: 33344791 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang T, Huang WS, Guan W, Hong Z, Gao J, Gao G, et al. Risk factors and predictors associated with the severity of COVID-19 in China: a systematic review, meta-analysis, and meta-regression. J Thorac Dis. 2020;12:7429–7441. doi: 10.21037/jtd-20-1743. [PMID: 33447431 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang X, Fang X, Cai Z, Wu X, Gao X, Min J, et al. Comorbid chronic diseases and acute organ injuries are strongly correlated with disease severity and mortality among COVID-19 patients: a systemic review and meta-analysis. Research (Wash D C) 2020;2020 doi: 10.34133/2020/2402961. [PMID: 32377638 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY) 2020;12:6049–6057. doi: 10.18632/aging.103000. [PMID: 32267833 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharma A, Jaiswal P, Kerakhan Y, Saravanan L, Murtaza Z, Zergham A, et al. Liver disease and outcomes among COVID-19 hospitalized patients - a systematic review and meta-analysis. Ann Hepatol. 2020;21 doi: 10.1016/j.aohep.2020.10.001. [PMID: 33075578 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu C, Yang J, Wang W, Zheng P, Tang Y. Liver injury could be associated with severe disease in COVID-19 patients: a meta-analysis. Eur J Gastroenterol Hepatol. 2020 doi: 10.1097/meg.0000000000001953. [PMID: 33031191 DOI:] [DOI] [PubMed] [Google Scholar]
- 55.Biswas M, Rahaman S, Biswas TK, Haque Z, Ibrahim B. Association of sex, age, and comorbidities with mortality in COVID-19 patients: a systematic review and meta-analysis. Intervirology. 2020: 1-12 [PMID: 33296901 DOI: 10.1159/000512592 ] [DOI] [PMC free article] [PubMed]
- 56.Cai Q, Huang D, Ou P, Yu H, Zhu Z, Xia Z, et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742–1752. doi: 10.1111/all.14309. [PMID: 32239761 DOI:] [DOI] [PubMed] [Google Scholar]
- 57.Ji D, Zhang M, Qin E, Zhang L, Xu J, Wang Y, et al. Letter to the editor: obesity, diabetes, non-alcoholic fatty liver disease and metabolic dysfunction associated fatty liver disease are proinflammatory hypercoagulable states associated with severe disease and thrombosis in Covid-19. Metabolism. 2021;115 doi: 10.1016/j.metabol.2020.154437. [PMID: 33220249 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.El Kassas M, Alboraie M, Al Balakosy A, Abdeen N, Afify S, Abdalgaber M, et al. Liver transplantation in the era of COVID-19. Arab J Gastroenterol. 2020 Jun;21(2):69–75. doi: 10.1016/j.ajg.2020.04.019. Epub 2020 May 12. PMID: 32439237; PMCID: PMC7214343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choudhary NS, Dhampalwar S, Saraf N, Soin AS. Outcomes of COVID-19 in patients with cirrhosis or liver transplantation. J Clin Exp Hepatol. 2021 doi: 10.1016/j.jceh.2021.05.003. Epub ahead of printPMID: 33994708; PMCID: PMC8112901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Colmenero J, Rodríguez-Perálvarez M, Salcedo M, Arias-Milla A, Muñoz-Serrano A, Graus J, et al. Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J Hepatol. 2021;74(1):148–155. doi: 10.1016/j.jhep.2020.07.040. Epub 2020 Aug 1. PMID: 32750442; PMCID: PMC7395653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Webb GJ, Marjot T, Cook JA, Aloman C, Armstrong MJ, Brenner EJ, et al. Outcomes following SARS-CoV-2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol Hepatol. 2020;5(11):1008–1016. doi: 10.1016/S2468-1253(20)30271-5. Epub 2020 Aug 28. PMID: 32866433; PMCID: PMC7455160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boettler T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Cornberg M, et al. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. 2020;2(3) doi: 10.1016/j.jhepr.2020.100113. Epub 2020 Apr 2. PMID: 32289115; PMCID: PMC7128473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667–678. doi: 10.1016/s2468-1253(20)30126-6. [PMID: 32405603 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xing QQ, Dong X, Ren YD, Chen WM, Zeng DY, Cai YY, et al. Liver chemistries in patients with COVID-19 who discharged alive or died: a meta-analysis. Hepatol Commun. 2020;5:12–23. doi: 10.1002/hep4.1585. [PMID: 32838104 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Samidoust P, Samidoust A, Samadani AA, Khoshdoz S. Risk of hepatic failure in COVID-19 patients. A systematic review and meta-analysis. Infez Med. 2020;28:96–103. [PMID: 32532945] [PubMed] [Google Scholar]
- 66.Merola E, Pravadelli C, de Pretis G. Prevalence of liver injury in patients with coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Acta Gastroenterol Belg. 2020;83:454–460. [PMID: 33094594] [PubMed] [Google Scholar]
- 67.Liu X, Li X, Sun T, Qin H, Zhou Y, Zou C, et al. East-West differences in clinical manifestations of COVID-19 patients: a systematic literature review and meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.26737. [PMID: 33325107 DOI:] [DOI] [PubMed] [Google Scholar]
- 68.Khateri S, Mohammadi H, Khateri R, Moradi Y. The prevalence of underlying diseases and comorbidities in COVID-19 patients; an updated systematic review and meta-analysis. Arch Acad Emerg Med. 2020;8:e72. [PMID: 33134968] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu Y, Li H, Guo X, Yoshida EM, Mendez-Sanchez N, Levi Sandri GB, et al. Incidence, risk factors, and prognosis of abnormal liver biochemical tests in COVID-19 patients: a systematic review and meta-analysis. Hepatol Int. 2020;14:621–637. doi: 10.1007/s12072-020-10074-6. [PMID: 32710250 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wijarnpreecha K, Ungprasert P, Panjawatanan P, Harnois DM, Zaver HB, Ahmed A, et al. COVID-19 and liver injury: a meta-analysis. Eur J Gastroenterol Hepatol. 2020 doi: 10.1097/meg.0000000000001817. [PMID: 32639420 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Parasa S, Desai M, Thoguluva Chandrasekar V, Patel HK, Kennedy KF, Roesch T, et al. Prevalence of gastrointestinal symptoms and fecal viral shedding in patients with coronavirus disease 2019: a systematic review and meta-analysis. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.11335. [PMID: 32525549 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Labenz C, Toenges G, Wörns MA, Sprinzl MF, Galle PR, Schattenberg JM. Liver injury in patients with severe acute respiratory syndrome coronavirus-2 infection: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2020 doi: 10.1097/meg.0000000000001827. [PMID: 32796355 DOI:] [DOI] [PubMed] [Google Scholar]
- 73.Del Zompo F, De Siena M, Ianiro G, Gasbarrini A, Pompili M, Ponziani FR. Prevalence of liver injury and correlation with clinical outcomes in patients with COVID-19: systematic review with meta-analysis. Eur Rev Med Pharmacol Sci. 2020;24:13072–13088. doi: 10.26355/eurrev_202012_24215. [PMID: 33378061 DOI:] [DOI] [PubMed] [Google Scholar]
- 74.Abdulla S, Hussain A, Azim D, Abduallah EH, Elawamy H, Nasim S, et al. COVID-19-induced hepatic injury: a systematic review and meta-analysis. Cureus. 2020;12:e10923. doi: 10.7759/cureus.10923. [PMID: 33194489 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sultan S, Altayar O, Siddique SM, Davitkov P, Feuerstein JD, Lim JK, et al. AGA institute rapid review of the gastrointestinal and liver manifestations of COVID-19, meta-analysis of international data, and recommendations for the consultative management of patients with COVID-19. Gastroenterology. 2020;159:320–334. doi: 10.1053/j.gastro.2020.05.001. e327 [PMID: 32407808 PMCID: PMC7212965 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bansal A, Prasad JB. Liver profile in COVID-19: a meta-analysis. Z Gesundh Wiss. 2020:1–6. doi: 10.1007/s10389-020-01309-9. [PMID: 32837834 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu ZH, Yang DL. A meta-analysis of the impact of COVID-19 on liver dysfunction. Eur J Med Res. 2020;25:54. doi: 10.1186/s40001-020-00454-x. [PMID: 33148326 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Váncsa S, Hegyi PJ, Zádori N, Szakó L, Vörhendi N, Ocskay K, et al. Pre-existing liver diseases and on-admission liver-related laboratory tests in COVID-19: a prognostic accuracy meta-analysis with systematic review. Front Med (Lausanne) 2020;7 doi: 10.3389/fmed.2020.572115. [PMID: 33282888 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lim MA, Pranata R, Huang I, Yonas E, Soeroto AY, Supriyadi R. Multiorgan failure with emphasis on acute kidney injury and severity of COVID-19: systematic review and meta-analysis. Can J Kidney Health Dis. 2020;7 doi: 10.1177/2054358120938573. [PMID: 32685180 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shokri Afra H, Amiri-Dashatan N, Ghorbani F, Maleki I, Rezaei-Tavirani M. Positive association between severity of COVID-19 infection and liver damage: a systematic review and meta-analysis. Gastroenterol Hepatol Bed Bench. 2020;13:292–304. [PMID: 33244371] [PMC free article] [PubMed] [Google Scholar]
- 81.Xin S, Xu J, Yu Y. Abnormal liver function tests of patients with coronavirus disease 2019 in Mainland China: a systematic review and meta- analysis. Gastrointestin Liver Dis. 2020;29:219–226. doi: 10.15403/jgld-2513. [PMID: 32530989 DOI:] [DOI] [PubMed] [Google Scholar]
- 82.Kunutsor SK, Laukkanen JA. Markers of liver injury and clinical outcomes in COVID-19 patients: a systematic review and meta-analysis. J Infect. 2021;82:159–198. doi: 10.1016/j.jinf.2020.05.045. [PMID: 32474033 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang Q, Zhao H, Liu LG, Wang YB, Zhang T, Li MH, et al. Pattern of liver injury in adult patients with COVID-19: a retrospective analysis of 105 patients. Mil Med Res. 2020;7:28. doi: 10.1186/s40779-020-00256-6. [PMID: 32507110 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020;526:135–140. doi: 10.1016/j.bbrc.2020.03.044. [PMID: 32199615 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single-cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2. Am J Respir Crit Care Med. 2020;202(5):756–759. doi: 10.1164/rccm.202001-0179LE. Erratum in: Am J Respir Crit Care Med. 2021 Mar 15;203(6):782. PMID: 32663409; PMCID: PMC7462411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Paizis G, Tikellis C, Cooper ME, Schembri JM, Lew RA, Smith AI, et al. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut. 2005;54:1790–1796. doi: 10.1136/gut.2004.062398. [PMID: 16166274 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, et al. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807–816. doi: 10.1016/j.jhep.2020.05.002. [PMID: 32437830 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Amiri-Dashatan N, Koushki M, Ghorbani F, Naderi N. Increased inflammatory markers correlate with liver damage and predict severe COVID-19: a systematic review and meta-analysis. Gastroenterol Hepatol Bed Bench. 2020;13:282–291. [PMID: 33244370] [PMC free article] [PubMed] [Google Scholar]
- 89.Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [PMID: 32275812 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, et al. Clinical features of COVID-19-related liver functional abnormality. Clin Gastroenterol Hepatol. 2020;18:1561–1566. doi: 10.1016/j.cgh.2020.04.002. [PMID: 32283325 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen P, Zhou B. Clinical characteristics of COVID-19 patients with abnormal liver tests. J Hepatol. 2020;73:712–713. doi: 10.1016/j.jhep.2020.04.028. [PMID: 32348791 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, et al. COVID-19: abnormal liver function tests. J Hepatol. 2020;73:566–574. doi: 10.1016/j.jhep.2020.04.006. [PMID: 32298767 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cai Q, Yang M, Liu D, Chen J, Shu D, Xia J, et al. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering (Beijing, China) 2020;6:1192–1198. doi: 10.1016/j.eng.2020.03.007. [PMID: 32346491 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Basturk A, Artan R, Yılmaz A, Gelen MT, Duman O. Acute vanishing bile duct syndrome after the use of ibuprofen. Arab J Gastroenterol. 2016;17:137–139. doi: 10.1016/j.ajg.2016.08.006. [PMID: 27658326 DOI:] [DOI] [PubMed] [Google Scholar]
- 95.Zoubek ME, Lucena MI, Andrade RJ, Stephens C. Systematic review: ibuprofen-induced liver injury. Aliment Pharmacol Ther. 2020;51:603–611. doi: 10.1111/apt.15645. [PMID: 31984540 DOI:] [DOI] [PubMed] [Google Scholar]
- 96.Liu M, Gao Y, Yuan Y, Yang K, Shi S, Tian J, et al. Efficacy and safety of herbal medicine (Lianhuaqingwen) for treating COVID-19: a systematic review and meta-analysis. Integr Med Res. 2021;10 doi: 10.1016/j.imr.2020.100644. [PMID: 32864332 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/s2213-2600(20)30076-x. [PMID: 32085846 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Edler C, Schroder AS, Aepfelbacher M, Fitzek A, Heinemann A, Heinrich F, et al. Dying with SARS-CoV-2 infection-an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int J Legal Med. 2020;134:1275–1284. doi: 10.1007/s00414-020-02317-w. [PMID: 32500199 DOI:] [DOI] [PMC free article] [PubMed] [Google Scholar]